Abstract
Background
Aspirin is a classic anti‐inflammatory drug and its anticancer effect has been previously explored in many types of cancer including colorectal cancer therapy. Programmed cell death‐ligand 1 (PD‐L1) is widely expressed in tumor cells and displays an inhibitory role in antitumor immunity. This study aimed to clarify the role of PD‐L1 in aspirin‐suppressed lung cancer.
Methods
The inhibitory effect of aspirin on lung cancer cell proliferation was assessed using an MTT cell viability assay. The role of aspirin in the modulation of PD‐L1 expression was analyzed by western blot or RT‐PCR assays. In lung cancer cells, the influence of aspirin on PD‐L1 promoter activity was detected using a luciferase reporter assay. The interaction of TAZ with PD‐L1 promoter in the cells, with or without aspirin administration, was tested via chromatin immunoprecipitation (ChIP) analysis. The function of PD‐L1 in aspirin‐mediated growth inhibition of lung cancer was examined using a cell viability assay.
Results
Following treatment with aspirin, lung cancer cell growth was markedly suppressed. Aspirin was able to markedly decrease the expression of PD‐L1 at the mRNA and protein levels in lung cancer cells. For the mechanism study, we found that the promoter of PD‐L1 was inactivated by aspirin via TAZ transcriptional coactivator in the cells. With regard to the functional investigation, aspirin was capable of resisting cell proliferation and PD‐L1 overexpression abolished aspirin‐depressed cell proliferation in lung cancer.
Conclusions
Aspirin suppressed the growth of lung cancer cells via targeting the TAZ/PD‐L1 axis.
Keywords: Aspirin, growth, lung cancer, PD‐L1, TAZ
Introduction
Serving as a traditional anti‐inflammatory reagent, aspirin has been widely utilized in anticancer investigation. Several reports have revealed that aspirin has been found to regulate certain transcription factors which affect cell apoptosis, proliferation, migration or other processes.1, 2, 3 Aspirin has also been previously reported to be effective in the prevention and treatment of colorectal cancer therapy, and its anticancer effects have since been widely adopted.4, 5, 6 In colorectal cancer, the reduced risk and metastasis is frequently revealed when patients are treated with aspirin.7 In many cancers such as ovarian, prostate, or liver cancers, the anticancer effect of aspirin has been well‐studied.8, 9, 10, 11 However, the novel targets of the anticancer function of aspirin remain a research hotspot.
Programmed cell death ligand‐1 (PD‐L1) is a vital immune checkpoint molecule which can manipulate cancer cells to escape immune surveillance via its receptor programmed cell death‐1 (PD‐1).12, 13 PD‐L1 is a transmembrane protein which is expressed in many different cancers including breast, ovarian, bladder, colon, melanoma and lung cancers.14, 15, 16, 17, 18 Previous studies have investigated PD‐L1 signaling in cancers and have focused on its implications in tumor immune evasion. Recent work shows that PD‐L1 inside tumor cells can regulate ovarian cancer and melanoma cell growth, pathogenesis and autophagy,19 promote bladder cancer proliferation, glycolysis,15 and be involved in lung cancer chemoresistance.20 However, the function of PD‐L1 in aspirin‐resisted lung cancer remains unclear.
In the present investigation, we clarified the role of PD‐L1 as a novel target in aspirin‐suppressed lung cancer and its potential regulatory mechanism. The growth of lung cancer in vitro is suppressed by aspirin. For the mechanism investigation, aspirin resisted the PD‐L1 transcription by targeting the TAZ transcriptional coactivator, resulting in the blocking of lung cancer cell growth. Therefore, our findings indicate that, therapeutically, aspirin can serve as a potential drug for use in lung cancer.
Methods
Cell lines
The lung cancer cell lines including A549 and H1299 were obtained from the American Type Culture Collection (ATCC, USA) and grown in DMEM media (Gibco, USA) adding 10% fetal bovine serum (FBS, Gibco), streptomycin (100 μg/mL) and penicillin (100 U/mL) at 37°C with 5% CO2.
Cell viability analysis
Cell viability was assessed using an MTT assay to measure the results of lung cancer cell proliferation. Cells were seeded in 96‐well plates with 3000 cells/well in at least three replicates. Then, 10 hours later, the confluent monolayers were formed, and the medium was changed to medium containing aspirin for another 24, 36 or 72 hours. We then supplemented 10 μL MTT (5 mg/mL) into each well, and after four hours incubation the medium was discarded and MTT in 150 μL DMSO was added into each well. At OD490nm, the absorbance values were measured via an absorbance reader.
RNA collection and PCR
Total RNA of lung cancer cell samples was extracted using TRIzol reagent. With regard to each sample, cDNA was reverse transcribed from 1 μg RNA. The levels of mRNA were analyzed through reverse transcription (RT)‐PCR using TransScript First‐Strand cDNA Synthesis SuperMix (TransGen Biotech, China). The relative quantification of the mRNA level was performed by the comparative method (2‐△△Ct), and △Ct value of each sample was the average of triplicates.
Western blot analysis
Western blot analysis was performed in lung cancer cells with different treatments. After aspirin administration, total proteins were obtained from the different cell groups and the proteins in equal measure from each group were examined. The primary antibodies used in this study, anti‐TAZ or anti‐β‐actin, were purchased from Cell Signaling Technology (USA) or Santa Cruz Biotechnology (USA). The secondary antibodies including goat anti‐rabbit (Abcam, USA) or anti‐mouse antibody (Abcam) were incubated with the blots and visualized through an enhanced ECL chemiluminescent substrate kit (Roche, Switzerland).
Luciferase reporter gene analysis
The lung cancer cell lines including A549 and H1299 (4 × 104 cells/well) were grown on 24‐well plates. The luciferase reporter pGL3‐PD‐L1 plasmid (100 ng/well) and pRL‐TK plasmid (40 ng/well) (Promega, USA) were cotransfected into the cells adding aspirin (ASA). Then, 48 hours later, all group cells were collected to analyze the luciferase activity via dual‐luciferase reporter assay system (Promega, USA). Each experiment was performed a minimum of three times.
Chromatin immunoprecipitation (ChIP) analysis
For chromatin immunoprecipitation (ChIP) analysis, EpiQuik chromatin immunoprecipitation kit from Epigentek Group Inc. (Brooklyn, NY) was applied. Anti‐TAZ or normal rabbit IgG (a negative control antibody) was utilized to immunoprecipitated the protein‐DNA complexes. DNA extractions from different groups were subsequently used for PCR analysis.
Statistical analysis
Each experiment was performed on a minimum of three occasions. A Student's t‐test was applied to assess the statistical significance comparing ± SD. The statistically significant differences were considered as follows: nonsignificant, ns; *P < 0.05; **P < 0.01; ***P < 0.001.
Results
Growth of lung cancer A549 and H1299 cells in vitro suppressed by aspirin
Aspirin has been reported to exert an anticancer role in several cancers including colorectal, breast and liver cancers.6, 21, 22, 23 However, novel targets in aspirin‐treated lung cancer to a certain extent remain undetermined. To clarify this issue, we first performed cell viability assay in lung cancer cell lines including A549 and H1299, managing the cells with saline or elevated concentrations of aspirin (2.5 mM and 5.0 mM). Our data revealed that the cell viability was obviously suppressed by the elevated concentration of aspirin (Fig 1a,b). We were therefore able to confirm effective suppression of aspirin on the growth of lung cancer cells.
Figure 1.
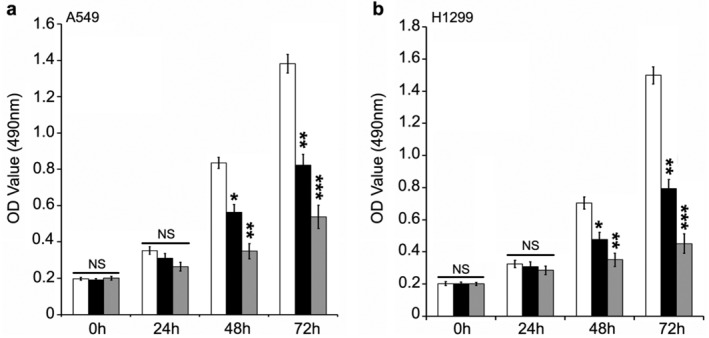
Aspirin (ASA) inhibits the proliferation of lung cancer A549 and H1299 cells in vitro. (a,b) MTT analysis was used to evaluate the effect of aspirin (ASA) treatment on the proliferation of lung cancer cells including A549 and H1299 (a) ( ) ASA 0 mM, (
) ASA 0 mM, ( ) ASA 2.5 mM, (
) ASA 2.5 mM, ( ) ASA 5.0 mM; (b) (
) ASA 5.0 mM; (b) ( ) ASA 0 mM, (
) ASA 0 mM, ( ) ASA 2.5 mM, (
) ASA 2.5 mM, ( ) ASA 5.0 mM. ***P < 0.001; **P < 0.01; *P < 0.05. ns, not significant.
) ASA 5.0 mM. ***P < 0.001; **P < 0.01; *P < 0.05. ns, not significant.
Decrease of oncogenic PD‐L1 in aspirin‐treated lung cancer cells
PD‐L1 is widely expressed in tumor cells and plays a crucial inhibitory role in antitumor immunity via interaction with receptor PD1 in T lymphocytes. However, whether oncogenic PD‐L1 inside the cells is involved in aspirin‐depressed lung cancer cells is unclear. Using western blot and RT‐PCR analyses, the expression of PD‐L1 at the mRNA and protein level was tested in lung cancer cell lines including A549 and H1299 with the administration of different doses of aspirin. We observed that in contrast to the control group, aspirin was able to significantly reduce the PD‐L1 expression at the protein level in A549 cells (Fig 2a). We further investigated whether the PD‐L1 transcription was affected by aspirin, and our data indicated that the transcription of PD‐L1 was apparently suppressed by treatment with aspirin in the cells (Fig 2b). The data from another lung cancer cell line H1299 was the same as the data from A549 cells (Figs 2c,d). Collectively, our results imply that aspirin was able to control the expression of PD‐L1 in lung cancer cells.
Figure 2.
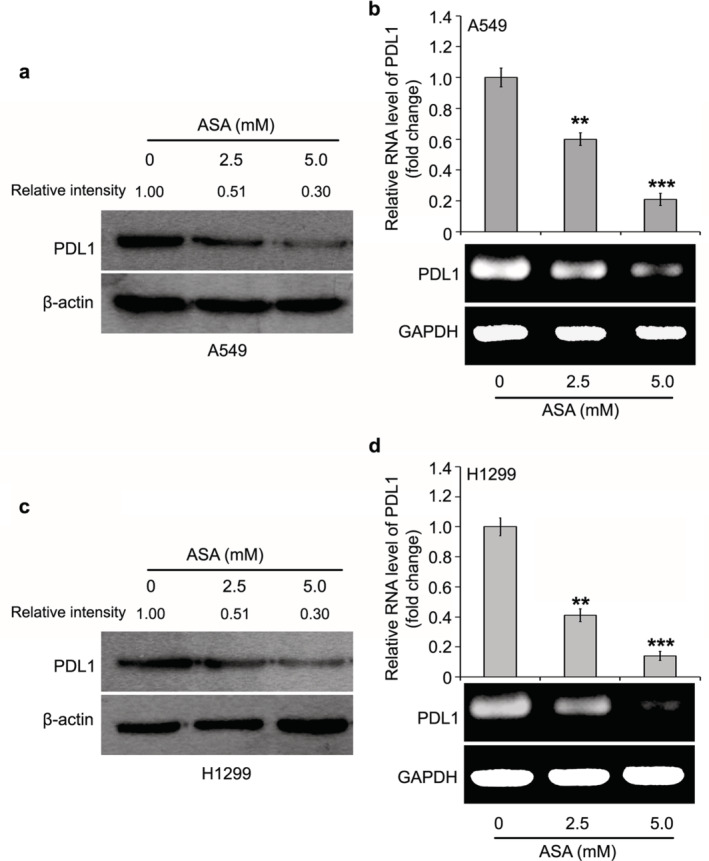
Oncogenic PD‐L1 is decreased in aspirin‐treated lung cancer cells. (a,b) Western blot and RT‐PCR assays were performed to test the expression of PD‐L1 at the mRNA and protein levels in the A549 cell line supplemented with aspirin. The quantification of immunoblotting bands was evaluated by BandScan. The statistical change from three independent experiments for RT‐PCR assay is presented. (c,d) Western blot and RT‐PCR assays were performed to test the expression of PD‐L1 at the mRNA and protein levels in H1299 cell line supplemented with aspirin. The quantification of immunoblotting bands was evaluated by BandScan. The statistical change from three independent experiments for RT‐PCR assay is presented. ***P < 0.001; **P < 0.01.
Aspirin controls PD‐L1 transcription via TAZ transcriptional coactivator
To clarify the molecular mechanism by which aspirin modulates the PD‐L1 transcription in lung cancer, we cloned the core promoter of PD‐L124 into luciferase reporter pGL3‐basic plasmid. Our aim was to determine whether aspirin affected the promoter activities of PD‐L1 in lung cancer cells. We found that the luciferase activity of pGL3‐PD‐L1 was obviously reduced by the treatment with aspirin in A549 cells (Fig 3a). We further confirmed the results using H1299 lung cancer cells (Fig 3b). According to a previous study,24 TAZ transcriptional coactivator is able to bind to the promoter of PD‐L1. ChIP assay was used to analyze whether TAZ was involved in aspirin‐controlled PD‐L1 transcription. We found that in lung cancer cells the interaction of TAZ with PD‐L1 promoter was disrupted by aspirin (Fig 3c). Thus, we were able to conclude that aspirin restrains the PD‐L1 transcription by regulating TAZ in lung cancer.
Figure 3.
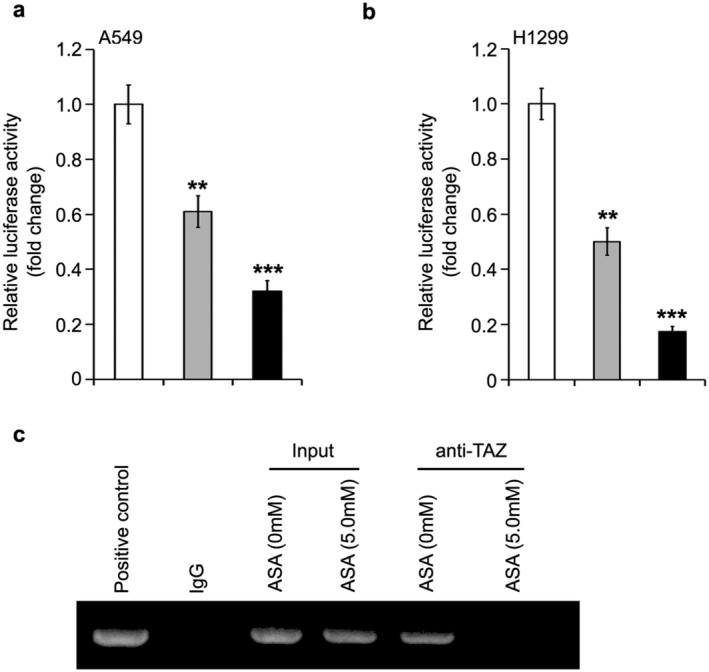
Aspirin controls PD‐L1 transcription via TAZ transcriptional coactivator. (a,b) Luciferase reporter gene analysis was used to examine the role of aspirin (ASA) in the regulation of PD‐L1 transcription in A549 and H1299 cell lines (a) ( ) ASA 0 mM, (
) ASA 0 mM, ( ) ASA 2.5 mM, (
) ASA 2.5 mM, ( ) ASA 5.0 mM; (b) (
) ASA 5.0 mM; (b) ( ) ASA 0 mM, (
) ASA 0 mM, ( ) ASA 2.5 mM, (
) ASA 2.5 mM, ( ) ASA 5.0 mM. ***P < 0.001; **P < 0.01. (c) ChIP and PCR assays was used to analyze the interaction of TAZ with PD‐L1 promoter in lung cancer cells with aspirin treatment.
) ASA 5.0 mM. ***P < 0.001; **P < 0.01. (c) ChIP and PCR assays was used to analyze the interaction of TAZ with PD‐L1 promoter in lung cancer cells with aspirin treatment.
PD‐L1 serves as a novel target in aspirin‐relieved lung cancer
Finally, we evaluated the effect of aspirin‐restrained PD‐L1 in the growth of lung cancer cells in vitro. In A549 cells, the addition of 5.0 mM aspirin was able to clearly suppress cell proliferation. Notably, overexpressed PD‐L1 sharply abolished the inhibition of A549 cell proliferation (Fig 4a). Meanwhile, the level of PD‐L1 was evaluated through western blot analysis when A549 cells were treated with aspirin and/or PD‐L1 transfection (Fig 4b). Furthermore, we confirmed that aspirin controlled cell growth and PD‐L1 introduction reversed aspirin‐mediated growth suppression (Fig 4c,d). It was therefore concluded that aspirin suppressed the growth of lung cancer cells via targeting PD‐L1.
Figure 4.

PD‐L1 serves as a novel target in aspirin‐relieved lung cancer. (a) The role of aspirin and/or PD‐L1 overexpression (OE PD‐L1) in proliferation was analyzed by MTT assay in the A549 cell line ( ) ASA (0 mM), (
) ASA (0 mM), ( ) ASA (5.0 mM), (
) ASA (5.0 mM), ( ) ASA (5.0 mM) + OE PDL1. (b) The level of A549 cell line with the treatment of aspirin and/or PD‐L1 overexpression (OE PD‐L1) was examined using western blotting. (c) The role of aspirin and/or PD‐L1 overexpression (OE PD‐L1) in proliferation was analyzed by MTT assay in H1299 cell line (
) ASA (5.0 mM) + OE PDL1. (b) The level of A549 cell line with the treatment of aspirin and/or PD‐L1 overexpression (OE PD‐L1) was examined using western blotting. (c) The role of aspirin and/or PD‐L1 overexpression (OE PD‐L1) in proliferation was analyzed by MTT assay in H1299 cell line ( ) ASA (0 mM), (
) ASA (0 mM), ( ) ASA (5.0 mM), (
) ASA (5.0 mM), ( ) ASA (5.0 mM) + OE PDL1. (d) The level of H1299 cell line with the treatment of aspirin and/or PD‐L1 overexpression (OE PD‐L1) was examined using western blotting. **P < 0.01.
) ASA (5.0 mM) + OE PDL1. (d) The level of H1299 cell line with the treatment of aspirin and/or PD‐L1 overexpression (OE PD‐L1) was examined using western blotting. **P < 0.01.
Discussion
Accumulating evidence reveals that aspirin is widely used in colorectal cancer therapy.4, 5, 6 In other cancers such as liver, prostate, or ovarian cancers, the inhibitory effects of aspirin on cancer have also been reported.8, 9, 10, 11 However, the novel targets of aspirin in lung cancer therapy remain unexplored.
First, in our study we aimed to confirm the effect of aspirin on the growth of lung cancer. Aspirin was capable of clearly decreasing the cell viability using lung cancer cell lines including A549 and H1299. Serving as a vital immune checkpoint molecule, PD‐L1 can make cancer cells escape immune surveillance.12 Most investigations of PD‐L1 signals in cancers have focused on the extrinsic PD‐L1 roles in tumor cells, especially in immune evasion. Recent work shows that tumor‐intrinsic PD‐L1 can regulate ovarian and melanoma cancer growth, pathogenesis and autophagy,19 promote bladder cancer proliferation, glycolysis,15 and is involved in lung cancer chemoresistance.20 Yet, the function of PD‐L1 in aspirin‐resisted lung cancer remains unclear.
In this study, we were interested in the function of PD‐L1 in the growth of aspirin‐suppressed lung cancer. The sharp decrease in PD‐L1 expression at the promoter, mRNA and protein level by aspirin was demonstrated in lung cancer cells. In the next investigation, we will seek to determine how aspirin modulates PD‐L1 in lung cancer cells. A previous study has revealed the role of transcription coactivator TAZ in PD‐L1 transcription,24 indicating that TAZ could be involved in aspirin‐controlled PD‐L1 in lung cancer. ChIP assay further confirmed that the interaction of TAZ with PD‐L1 promoter could be disrupted by aspirin. Our final function investigation revealed that PD‐L1 is a novel target in aspirin‐suppressed lung cancer growth in vitro.
In conclusion, our findings identify a new mechanism of relieving lung cancer using aspirin. Aspirin has been shown to be capable of resisting the growth of lung cancer cells. PD‐L1 has been determined to be reduced in aspirin‐inhibited lung cancer. Aspirin regulates the TAZ transcriptional coactivator to suppress PD‐L1 activation, resulting in lung cancer growth blockade. The findings of our study emphasize that aspirin may become a new treatment with great potential in lung cancer therapy.
Disclosure
No conflict of interest is reported.
Acknowledgments
This study was supported by the funding of Natural Science Foundation of Liaoning Province, China (No. 20180550106).
Contributor Information
Yan Dong, Email: dongyan100@aliyun.com.
Qingkai Yang, Email: dalianmed@163.com.
References
- 1. Huo X, Zhang X, Yu C et al Aspirin prevents NF‐kappaB activation and CDX2 expression stimulated by acid and bile salts in oesophageal squamous cells of patients with Barrett's oesophagus. Gut 2018; 67: 606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benamouzig R, Uzzan B, Martin A et al Cyclooxygenase‐2 expression and recurrence of colorectal adenomas: Effect of aspirin chemoprevention. Gut 2010; 59: 622–9. [DOI] [PubMed] [Google Scholar]
- 3. Galipeau PC, Li X, Blount PL et al NSAIDs modulate CDKN2A, TP53, and DNA content risk for progression to esophageal adenocarcinoma. PLOS Med 2007; 4: e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCowan C, Munro AJ, Donnan PT, Steele RJ. Use of aspirin post‐diagnosis in a cohort of patients with colorectal cancer and its association with all‐cause and colorectal cancer specific mortality. Eur J Cancer 2013; 49: 1049–57. [DOI] [PubMed] [Google Scholar]
- 5. Li P, Wu H, Zhang H et al Aspirin use after diagnosis but not prediagnosis improves established colorectal cancer survival: A meta‐analysis. Gut 2015; 64: 1419–25. [DOI] [PubMed] [Google Scholar]
- 6. Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA 2009; 302: 649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: A study of incident cancers during randomised controlled trials. Lancet 2012; 379: 1591–601. [DOI] [PubMed] [Google Scholar]
- 8. Sitia G, Aiolfi R, Di Lucia P et al Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci U S A 2012; 109: E2165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Downer MK, Allard CB, Preston MA et al Regular aspirin use and the risk of lethal prostate cancer in the physicians' health study. Eur Urol 2017; 72: 821–7. [DOI] [PubMed] [Google Scholar]
- 10. Marshall SF, Bernstein L, Anton‐Culver H et al Nonsteroidal anti‐inflammatory drug use and breast cancer risk by stage and hormone receptor status. J Natl Cancer Inst 2005; 97: 805–12. [DOI] [PubMed] [Google Scholar]
- 11. Trabert B, Ness RB, Lo‐Ciganic WH et al Aspirin, nonaspirin nonsteroidal anti‐inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: A pooled analysis in the Ovarian Cancer Association Consortium. Journal of the National Cancer Institute 2014; 106: djt431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cai G, Karni A, Oliveira EM, Weiner HL, Hafler DA, Freeman GJ. PD‐1 ligands, negative regulators for activation of naive, memory, and recently activated human CD4+ T cells. Cell Immunol 2004; 230: 89–98. [DOI] [PubMed] [Google Scholar]
- 14. Lim SO, Li CW, Xia W et al Deubiquitination and stabilization of PD‐L1 by CSN5. Cancer Cell 2016; 30: 925–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Z, Cao D, Qi Z et al Retinoic acid‐related orphan receptor C regulates proliferation, glycolysis, and chemoresistance via the PD‐L1/ITGB6/STAT3 signaling axis in bladder cancer. Cancer Res 2019; 79: 2604–18. [DOI] [PubMed] [Google Scholar]
- 16. Ritprajak P, Azuma M. Intrinsic and extrinsic control of expression of the immunoregulatory molecule PD‐L1 in epithelial cells and squamous cell carcinoma. Oral Oncol 2015; 51: 221–8. [DOI] [PubMed] [Google Scholar]
- 17. Wang S, Li J, Xie J et al Programmed death ligand 1 promotes lymph node metastasis and glucose metabolism in cervical cancer by activating integrin beta4/SNAI1/SIRT3 signaling pathway. Oncogene 2018; 37: 4164–80. [DOI] [PubMed] [Google Scholar]
- 18. Hersey P, Gallagher S. A focus on PD‐L1 in human melanoma. Clin Cancer Res 2013; 19: 514–6. [DOI] [PubMed] [Google Scholar]
- 19. Clark CA, Gupta H, Sareddy GR et al Tumor‐intrinsic PD‐L1 signals regulate cell growth, pathogenesis, and autophagy in ovarian cancer and melanoma. Cancer Res 2016; 76: 6964–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fujita Y, Yagishita S, Hagiwara K et al The clinical relevance of the miR‐197/CKS1B/STAT3‐mediated PD‐L1 network in chemoresistant non‐small‐cell lung cancer. Mol Ther 2015; 23: 717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Choi BH, Chakraborty G, Baek K, Yoon HS. Aspirin‐induced Bcl‐2 translocation and its phosphorylation in the nucleus trigger apoptosis in breast cancer cells. Exp Mol Med 2013; 45: e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Din FV, Valanciute A, Houde VP et al Aspirin inhibits mTOR signaling, activates AMP‐activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology 2012; 142: 1504–15 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sitia G, Iannacone M, Guidotti LG. Anti‐platelet therapy in the prevention of hepatitis B virus‐associated hepatocellular carcinoma. J Hepatol 2013; 59: 1135–8. [DOI] [PubMed] [Google Scholar]
- 24. Feng J, Yang H, Zhang Y et al Tumor cell‐derived lactate induces TAZ‐dependent upregulation of PD‐L1 through GPR81 in human lung cancer cells. Oncogene 2017;pii:onc2017188. 10.1038/onc.2017.188 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


