Abstract
Background
Surgical resection is still the main treatment option for patients with resectable Siewert type II adenocarcinoma of the esophagogastric junction (AEG). This retrospective study evaluated the significance of minimally invasive Sweet esophagectomy (MISE) for the treatment of Siewert type II AEG.
Methods
We retrospectively evaluated 174 patients with Siewert type II AEG who received a Sweet esophagectomy in our center between October 2013 and September 2017. Of these patients, 73 underwent MISE and 101 underwent open Sweet esophagectomy (OSE). The clinicopathologic factors, operational factors and postoperative complications were compared.
Results
The two groups were similar in terms of age, sex, American Society of Anesthesiologists grade, preoperative staging and incidence of comorbidities (P > 0.05). Relative to the OSE approach, the MISE approach was associated with a significant decrease in surgical blood loss (P < 0.001), chest tube duration (P = 0.003) and postoperative admission duration (P = 0.002). The minimally invasive approach was associated with significantly less total morbidity and fewer respiratory complications than the open approach (P = 0.015 and P = 0.016, respectively). Relative to the open approach, the MISE approach was associated with a significant increase in the number of total lymph nodes removed and the locations of the total lymph nodes removed (P < 0.001 and P < 0.001, respectively).
Conclusions
Our MISE technique can be safely and effectively performed for intrathoracic anastomosis with favorable early outcomes.
Keywords: Adenocarcinoma of the esophagogastric junction, esophageal surgery, minimally invasive surgery, thoracoscopy
Introduction
Adenocarcinoma of the esophagogastric junction (AEG) is considered a challenging therapeutic problem with high morbidity and a poor prognosis. The incidence of AEG has increased worldwide.1, 2, 3, 4 The Siewert classification for these tumors is now widely accepted and the disease is divided into three types.5 In Asian countries, especially in China, most tumors in the esophagogastric junction are classified as Siewert type II and III, whereas in Western countries, type I is more prevalent.3, 6 Surgical resection is still the main treatment option for patients with resectable AEG.7, 8 For patients with Siewert type II AEG, because the tumor is located between the thoracic and abdominal cavities, cancer cells can metastasize to the abdominal or mediastinal lymph nodes through the lymphatic system. In China, most surgeons currently prefer Sweet esophagectomy for patients with Siewert type II AEG.8, 9
Because of the relatively high mortality and morbidity associated with radical surgery for esophageal cancer and AEG, there have been many efforts to reduce its invasiveness. Since 1992, when Cuschieri et al. first reported minimally invasive esophagectomy (MIE) as a thoracoscopic esophagectomy procedure,10 many institutions have described various methods for the MIE approach. Minimally invasive McKeown esophagectomy (MIME) and minimally invasive Ivor Lewis esophagectomy (MIILE) have been reported for many years,11, 12, 13 but until now, there has been no report regarding minimally invasive Sweet esophagectomy (MISE). Since October 2013, we have used laparoscopic and thoracoscopic methods with Sweet anastomosis (MISE). The objective of this retrospective study was to describe our MISE technique and compare the short‐term clinical outcomes of this approach with those for the open Sweet esophagectomy (OSE) approach.
Methods
Patients
This study was approved by the Ethics Committee of the First Affiliated Hospital of the University of Science and Technology of China. Written informed consent was obtained from all patients prior to the operation. We retrospectively evaluated 206 consecutive patients with Siewert type II AEG who underwent Sweet esophagectomy between October 2013 and September 2017. We verified and updated the clinical data from patient records through December 2017 using the database. Patients were selected based on the following eligibility criteria: (i) patients with histopathologically proven Siewert type II AEG; (ii) patients who received Sweet esophagectomy (MISE or OSE); (iii) patients who did not receive neoadjuvant therapy; (iv) patients with clinical T1‐3N0‐1M0 disease prior to operation; and (v) patients with no known distant metastasis. Patients were excluded based on the following criteria: (i) patients who received palliative resection or (ii) patients with incomplete medical records. Based on these criteria, 174 patients were enrolled for analysis in this retrospective study (Fig 1).
Figure 1.
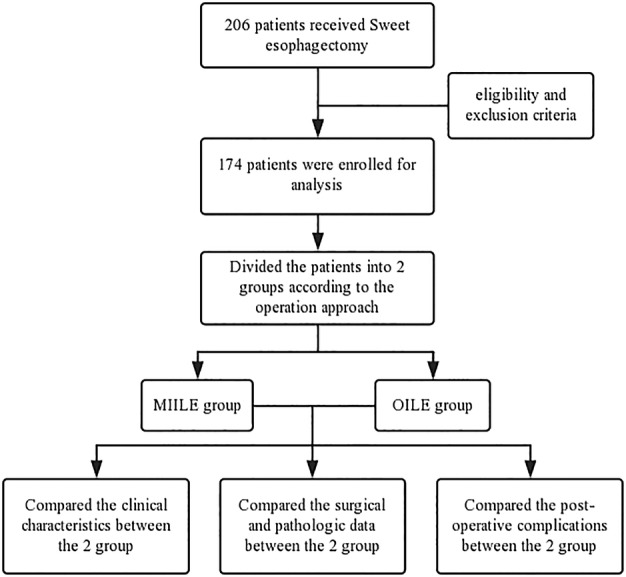
Flow diagram of the study. (MIILE = minimally invasive Ivor‐Lewis esophagectomy; OILE = open Ivor‐Lewis esophagectomy).
The routine preoperative evaluation included chest radiographs, barium swallow examinations, Doppler ultrasound examinations of the abdomen, computed tomography scans from the chest to the upper abdomen, endoscopy with biopsy, electrocardiograms, lung function tests, complete blood counts, blood biochemistry analyses and liver and renal function evaluations. All patients underwent Sweet esophagectomy with curative intent and were staged according to the TNM staging system of the American Joint Committee on Cancer (AJCC Staging Manual, eighth edition). The surgical team consisted of six thoracic surgeons trained in advanced surgical MIE techniques. Records of complications were kept according to the Clavien‐Dindo classification operative technique for MISE.
Laparoscopic phase
All procedures were performed under general anesthesia with the patient placed in a supine position. Five abdominal ports were used (Fig 2). The greater curvature of the stomach was mobilized along the anterior lobe of the transverse mesocolon, and the right gastroepiploic vessels were preserved. The left gastric vessels were then divided with a linear stapler (Ethicon Endo‐surgery, Inc., Cincinnati, OH, USA). The esophagus was then circumferentially mobilized into the mediastinum up to the level of the inferior pulmonary vein. After gastric mobilization, a linear stapler (Ethicon Endo‐surgery, Inc.) was used along the lesser curvature of the stomach (Fig 3). A jejunostomy tube was then routinely placed to secure the jejunum to the anterior abdominal wall. Finally, the five ports were closed and dressed. Of note, D2 lymph node dissection was also performed during gastric mobilization.
Figure 2.
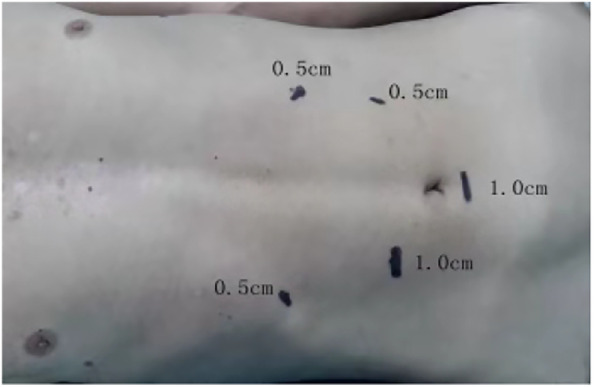
Locations of the abdominal port sites.
Figure 3.
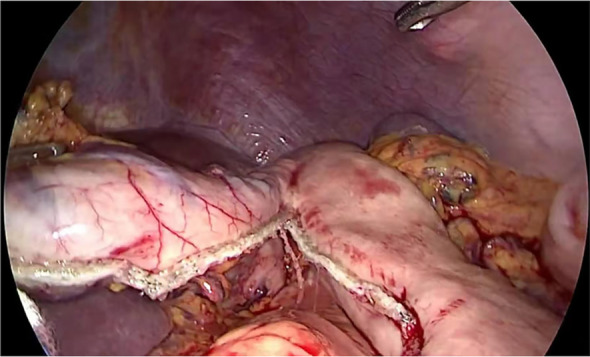
Creation of the gastric conduit.
Thoracoscopic phase
In part two of the operation, the patient was repositioned in the right lateral decubitus position with single‐lung ventilation. Two ports and a 4 cm incision were made in the left chest wall (Fig 4). The left lung was anteriorly retracted in order to expose the mediastinal esophagus. The mediastinal pleura overlying the esophagus was divided. The esophagus was then circumferentially mobilized from the esophageal hiatus below the aortic arch (Fig 5). During esophageal mobilization, the middle and lower paraesophageal lymph nodes, subcarinal nodes, nearby hilar nodes and paralymphatic fat tissues were routinely explored and completely dissected.
Figure 4.
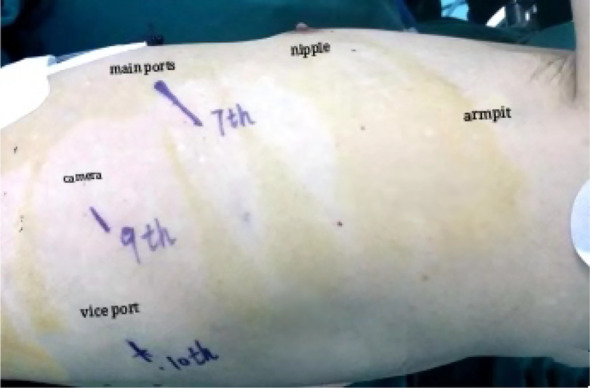
Location of the thoracoscopic ports.
Figure 5.
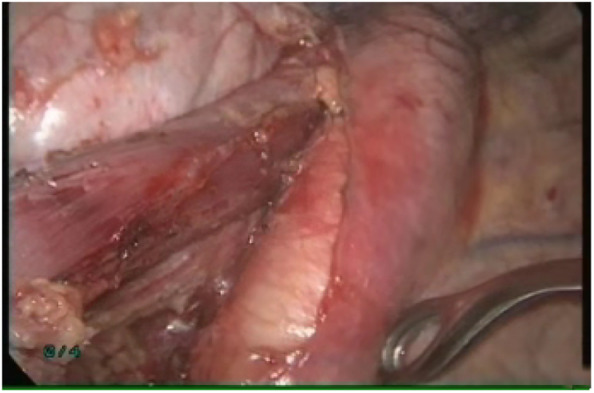
The esophagus was circumferentially mobilized from the esophageal hiatus below the aortic arch.
A purse‐string clamp device (Shanghai Medical Instruments, Shanghai, China) was inserted through the incision and secured with a purse‐string suture (Fig 6). Next, an esophagotomy was created at the level of the inferior pulmonary vein, and the anvil of a 25 mm Premium Plus CEEA device (Covidien Ltd., Dublin, Republic of Ireland) was passed into the esophagus and pushed above the level of the purse‐string suture. The purse‐string was tied in a standard fashion using a knot pusher. The distal esophagus was resected, and the patient's gastric was manipulated into the left thoracic cavity. The gastric near the cardia and the omentum majus were resected, and the specimen was removed.
Figure 6.
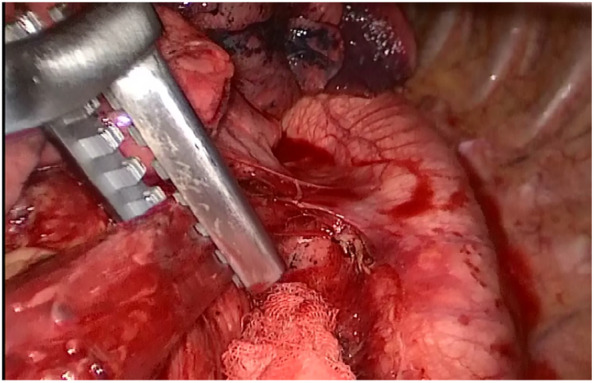
A purse‐string clamp device was inserted through the incision and secured with a purse‐string suture.
A gastrostomy was created at the tip of the distal gastric conduit. Lastly, the circular stapler was inserted into the gastric conduit and an esophagogastric anastomosis was created (Fig 7). The gastrostomy was then stapled closed with a linear stapler (Ethicon Endo‐surgery, Inc.) (Fig 8). A 32‐Fr chest tube was placed in situ for chest drainage, and a Jackson Pratt drain was placed in the esophageal bed for mediastinal drainage. All ports and incisions were closed and dressed.
Figure 7.
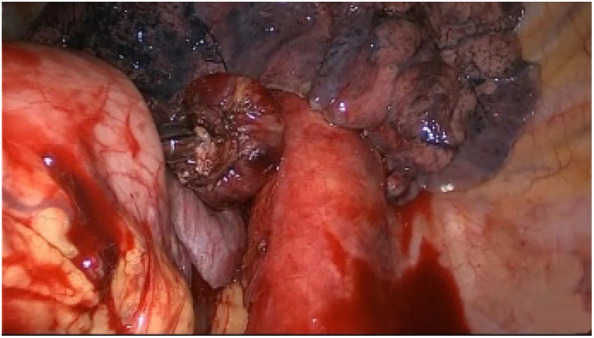
A circular stapler was inserted into the gastric conduit and an esophagogastric anastomosis was created.
Figure 8.
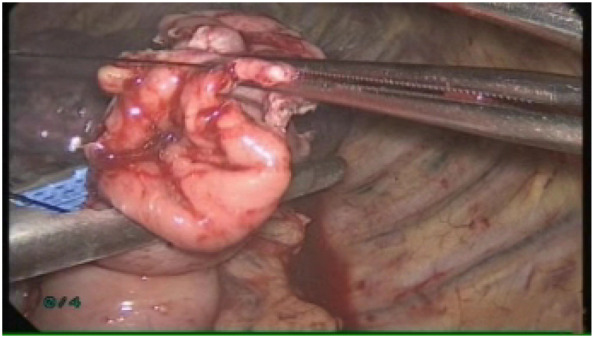
The gastrostomy was stapled closed with a linear stapler.
OSE was performed in a standard fashion as previously described.14 All procedures were carried out under general anesthesia. A posterolateral thoracotomy (15–30 cm) at the seventh intercostal space was performed, and subtotal esophagectomy with superior polar gastrectomy was adopted. Lymph node dissection was performed mainly in the middle and lower mediastinal and lymph nodes around the stomach. The location of the anastomosis was the same as the MISE approach.
Statistical analysis
Statistical analysis was performed with SPSS 16.0 (SPSS Inc., Chicago, IL, USA). All data are expressed as the mean ± standard deviation. Differences between groups with continuous variables were assessed using the χ2 test or Fisher's exact test. Statistical significance was confirmed as P < 0.05 throughout the study.
Results
Between October 2013 and September 2017, 174 patients with Siewert type II AEG underwent Sweet esophagectomy at our hospital. Of these patients, 73 underwent MISE and 101 underwent OSE. In the MISE group, four patients were converted from laparoscopic surgery to open surgery. One patient was converted because the pancreas was invaded, and the patient received total gastrectomy and partial pancreatectomy. Three patients were converted because the stomach was widely invaded; these patients received total gastrectomy. There were no conversions from thoracoscopic surgery to open surgery. In the OSE group, six patients received total gastrectomy because the stomach was widely invaded. The two groups were similar in terms of age, sex, American Society of Anesthesiologists (ASA) grade, preoperative staging and incidence of comorbidities (Table 1).
Table 1.
Patient clinical characteristics
| MISE group (n = 73) | OSE group (n = 101) | χ2 | P‐value | |
|---|---|---|---|---|
| Sex | 0.121 | 0.728 | ||
| Male | 51 (69.9%) | 73 (72.3%) | ||
| Female | 22 (30.1%) | 28 (27.7%) | ||
| Age | 0.452 | 0.501 | ||
| ≤60 years | 16 (21.9%) | 18 (17.8%) | ||
| >60 years | 57 (78.1%) | 83 (82.2%) | ||
| ASA grade | 0.440 | 0.803 | ||
| I | 30 (41.1%) | 39 (38.6%) | ||
| II | 34 (46.6%) | 46 (45.5%) | ||
| III | 9 (12.3%) | 16 (15.8%) | ||
| Preoperative T stage | 1.458 | 0.482 | ||
| T1 | 12 (16.4%) | 20 (19.8%) | ||
| T2 | 33 (45.2%) | 51 (50.5%) | ||
| T3 | 28 (38.4%) | 30 (29.7%) | ||
| Preoperative N stage | 3.265 | 0.071 | ||
| N0 | 39 (53.4%) | 40 (39.6%) | ||
| N1 | 34 (46.6%) | 61 (60.4%) | ||
| Comorbidity | ||||
| Hypertension | 9 (12.3%) | 15 (14.9%) | 0.227 | 0.634 |
| Diabetes mellitus | 8 (11.0%) | 13 (12.9%) | 0.146 | 0.702 |
| COPD | 8 (11.0%) | 9 (8.9%) | 0.202 | 0.653 |
| Arrhythmia | 6 (8.2%) | 12 (11.9%) | 0.613 | 0.414 |
| Other | 3 (4.1%) | 5 (5.0%) | 1.000 | 0.549 |
ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; Other, brain and/or peripheral vascular lesions; MIILE, minimally invasive Ivor Lewis esophagectomy; OILE, open Ivor Lewis esophagectomy.
All 174 patients were shown to have Siewert type II AEG by two pathologists and were staged according to the TNM staging system of the American Joint Committee on Cancer (AJCC Staging Manual, eighth edition). There were no significant differences in the postoperative histologic features or operation times between the two groups. However, surgical blood loss of the MISE group was less than that of the OSE group (P < 0.001), and the MISE group had a significantly shorter chest tube duration and postoperative admission duration than the OSE group (P = 0.003 and P = 0.002, respectively) (Table 2).
Table 2.
Surgical and pathologic data
| MISE group (n = 73) | OSE group (n = 101) | χ2 | P‐value | |
|---|---|---|---|---|
| T stage | χ2 = 2.017 | 0.569 | ||
| T1 | 9 (12.3%) | 18 (17.8%) | ||
| T2 | 31 (42.5%) | 46 (45.5%) | ||
| T3 | 26 (35.6%) | 27 (26.7%) | ||
| T4 | 7 (9.6%) | 10 (9.9%) | ||
| N stage | χ2 = 4.174 | 0.243 | ||
| N0 | 37 (50.7%) | 39 (38.6%) | ||
| N1 | 19 (26.0%) | 41 (40.6%) | ||
| N2 | 11 (15.1%) | 13 (12.9%) | ||
| N3 | 6 (8.2%) | 8 (7.9%) | ||
| Operation time (minutes) | 190.29 ± 36.615 | 195.42 ± 25.806 | t = 1.084 | 0.280 |
| Blood loss (mL) | 74.32 ± 25.226 | 112.23 ± 39.746 | t = 7.170 | <0.001 |
| Chest tube duration (days) | 8.67 ± 2.651 | 10.50 ± 4.780 | t = 2.963 | 0.003 |
| Postop admission duration (days) | 9.74 ± 2.824 | 11.76 ± 4.858 | t = 3.188 | 0.002 |
| Number of DN | 22.34 ± 2.631 | 15.87 ± 1.553 | t = 20.312 | <0.001 |
| Thoracic | 8.08 ± 1.351 | 8.37 ± 1.046 | t = 1.563 | 0.120 |
| Abdominal | 14.26 ± 2.433 | 7.50 ± 1.622 | t = 21.966 | <0.001 |
| Number of DNL | 11.08 ± 1.730 | 7.45 ± 1.410 | t = 15.250 | <0.001 |
Data are n (%) or median ± SD unless otherwise indicated.
DN, dissected nodes; DNL, dissected nodal locations; MIILE, minimally invasive Ivor‐Lewis esophagectomy; OILE, open Ivor‐Lewis esophagectomy; Postop, postoperative.
The MISE approach was associated with a significant increase in the number of total lymph nodes removed and the locations of the total lymph nodes removed (22.34 ± 2.631 per patient in the MISE group vs. 15.87 ± 1.553 per patient in the OSE group, P < 0.001, and 11.08 ± 1.730 per patient in the MISE group vs. 7.45 ± 1.410 per patient in the OSE group, P < 0.001) relative to the open approach. For the thoracic lymph node, there were no significant differences between the two groups (8.08 ± 1.351 per patient in the MISE group vs. 8.37 ± 1.046 per patient in the OSE group, P = 0.120). However, for the abdominal lymph node, the MISE approach was better than the OSE approach (14.26 ± 2.433 per patient in the MISE group vs. 7.50 ± 1.622 per patient in the OSE group, P < 0.001; Table 2).
No deaths occurred during surgery for either group. Complications according to the Clavien‐Dindo classification were reported in 66 patients (37.9%). Major complications (Clavien‐Dindo grade 3–5) occurred in 23 (13.2%) of the 174 patients (including three deaths), and minor complications (Clavien‐Dindo grade 1–2) occurred in 43 (24.7%) patients. The minimally invasive approach was associated with significantly less total morbidity and fewer respiratory complications than the open approach (27.4% in the MISE group vs. 45.5% in the OSE group, P = 0.015, and 9.6% in the MISE group vs. 23.8% in the OSE group, P = 0.016). There was no significant difference in the incidence of anastomotic leaks between the two groups (P = 0.625; Table 3).
Table 3.
Postoperative complications
| MISE group (n = 73) | OSE group (n = 101) | χ2 | P‐value | |
|---|---|---|---|---|
| Minor complications | ||||
| Pulmonary air leak | 2 (2.7%) | 7 (6.9%) | 0.783 | 0.376 |
| Pneumonia | 3 (4.1%) | 9 (8.1%) | 0.592 | 0.442 |
| Atelectasis | 2 (2.7%) | 4 (4.0%) | 1.000 | 0.503 |
| Wound infection | 0 (0%) | 4 (4.0%) | 0.140 | 0.111 |
| Arrhythmia | 3 (4.1%) | 7 (6.9%) | 0.211 | 0.646 |
| Incomplete intestinal obstruction | 2 (2.7%) | 0 (0%) | 0.175 | 0.175 |
| Major complications | ||||
| Pneumonia | 0 (0%) | 3 (3.0%) | 0.265 | 0.193 |
| Chylothorax | 0 (0%) | 1 (1.0%) | 1.000 | 0.580 |
| Leak | 3 (4.1%) | 4 (4.0%) | 1.000 | 0.625 |
| Delayed gastric emptying | 3 (4.1%) | 4 (4.0%) | 1.000 | 0.625 |
| Reoperation for bleeding | 2 (2.7%) | 3 (3.0%) | 1.000 | 0.650 |
| Total morbidity | 20 (27.4%) | 46 (45.5%) | 5.927 | 0.015 |
| Total respiratory complications | 7 (9.6%) | 24 (23.8%) | 5.814 | 0.016 |
| Mortality, in‐hospital | 0 (0%) | 3 (3.0%) | 0.265 | 0.193 |
Minor complications, Clavien ‐ Dindo 1–2 grade; Major complications, Clavien‐Dindo 3–5 grade; MIILE, minimally invasive Ivor‐Lewis esophagectomy; OILE, open Ivor‐Lewis esophagectomy.
No deaths occurred in the MISE group, but there were three deaths in the OSE group. Two patients died from an anastomotic leak and multiorgan failure, and a third died from severe pneumonia and acute respiratory distress syndrome (ARDS). There were no significant differences in in‐hospital mortality between the two groups (0% in the MIILE group vs. 3.0% in the OILE group, P = 0.193; Table 3).
Discussion
Almost 20 years after the development of MIE, the practice of minimally invasive procedures has been used to minimize surgical trauma and reduce postoperative complication rates compared with open procedures. However, the number of single‐institution reports of MIILE and MIME continues to increase,11, 12, 13 and to our knowledge, this is the first study to report the MISE approach in patients with Siewert type II AEG. Compared with OSE, MISE has two theoretical advantages. First, this approach avoids thoracotomy and the incision of the diaphragm and has a smaller influence on postoperative respiration and pain. Second, this approach incorporates the supine position to perform an abdominal approach, which means that it is better for disection of abdominal lymph nodes. This retrospective study found that the number of abdominal lymph nodes was higher in the MISE group than those in the OSE group. In addition, the MISE approach was associated with a significant decrease in surgical blood loss, chest tube duration, postoperative stay and postoperative complications relative to the OSE approach. These data indicate that MISE may be a safe and effective approach for patients with Siewert type II AEG due to its better lymph node dissection, lower morbidity and lower mortality.
The conversion rate is typically higher for institutions that have begun to perform thoracoscopy and laparoscopy operations. Major reasons for intraoperative conversion include extensive adhesion, extensive bleeding, operational injury and tumor invasion of important organs.11, 15 We confirmed that all operators were skilled in MIME and MIILE before MISE was performed. Compared with MIILE, MISE changes the location of the anastomosis to the left thoracic cavity and enlarges the extent of the dissections of the gastric and abdominal lymph nodes. In the present study, MISE was successfully completed in 69 (94.5%) patients. Four patients required conversion to open laparotomy because the tumor was invading important organs, or because the range of the tumor was very large. We are of the opinion that the majority of pleural adhesions can be performed under thoracoscopy and that only a minority of patients with a history of severe tuberculosis require thoracotomy. Due to improvements in technology and instruments, many operational injuries and bleeding can be repaired endoscopically. We advocate the use of MISE for patients with cT1‐3N0‐1M0 Siewert type II AEG if the range of the tumor or lymph node metastasis is very wide, or if conversion to open laparotomy or open thoracotomy is needed.
To date, a number of studies have demonstrated acceptable short‐term outcomes of MIE in terms of operating time, blood loss and postoperative complications. Some studies have demonstrated that the incidence of respiratory complications was significantly lower with MIE than that with open esophagectomy (OE).12, 16, 17, 18 Straatman et al.12 conducted a randomized controlled trial to compare MIE with OE to treat patients with esophageal cancer and found significantly fewer pulmonary infections after MIE compared with those after OE. Nagpal et al. performed a meta‐analysis and showed significantly fewer respiratory complications after MIE compared with those after OE.16 In the current study, the minimally invasive approach was associated with a significant decrease in surgical blood loss, chest tube duration, postoperative admission duration, total morbidity and respiratory complications relative to the OSE approach. We believe that since the MISE approach avoids thoracotomy and the incision of the diaphragm and the integrity of the thorax and abdomen is maintained, MISE has a lesser influence on postoperative respiration and pain. Meanwhile, the minimally invasive approach avoids touching the lungs, reduces the inflammatory response, and promotes postoperative pulmonary retention, which leads to a significant reduction in postoperative respiratory complications. In addition, thoracoscopy has an amplification effect, the anatomy is more precise in the operation, minor injuries associated with the operation are reduced, and the recovery time is accelerated.
With regard to the number of retrieved total lymph nodes, most studies have demonstrated that MIE is almost equivalent to OE.11, 13, 15 At present, the range of lymph node dissection for gastric cancer requires the removal of the omentum majus and D2 nodes. Siewert type II AEG is a special type of proximal gastric cancer. Therefore, for patients with Siewert type II AEG, the range of lymph node dissection should be equal to that of proximal stomach cancer. Because of the right lateral decubitus position, patients who received traditional OSE only had the paracardial, greater curvature, lesser curvature, suprapyloric and left gastric artery lymph nodes removed because it is difficult to remove the infrapyloric, splenic hilum and hepatoduodenal ligament lymph nodes. Most surgeons can perform D1 dissection for patients in this position. Chen et al. reported that the range of abdominal lymph node dissection for OSE was significantly lower than that for the abdominal‐transhiatal approach.9 Meanwhile, an increasing number of studies have reported that the range of lymph node dissection with the laparoscopic approach was comparable to open surgery.19, 20 The results of this study show that the numbers and locations of dissected lymph nodes were better in the MISE group than those in the OSE group. For the thoracic lymph node, there were no significant differences between the two groups; for the abdominal lymph node, however, the MISE approach was better than the OSE approach. Furthermore, the rate of lymph node metastasis was higher for the MISE group than that for the OSE group, although the difference was not significant. We believe that the main reason for this difference is that the supine position of the laparoscopic approach is better for dissecting the abdominal lymph nodes. In addition, the endoscope provides a better view of local blood vessels and lymph nodes; therefore, it is advantageous for lymph node dissection.
The surgical approach and extent of lymph node dissection in Siewert type II AEG is controversial. Some surgeons believe that Ivor Lewis esophagectomy is the best choice for patients with Siewert type II AEG. Other surgeons, however, believe that Sweet esophagectomy or the abdominal‐transhiatal approach is better. Until now, there has been no literature regarding different survival rates between these three approaches. Hulscher et al.21 conducted a randomized controlled trial to compare the Ivor Lewis approach with the abdominal‐transhiatal approach to treat patients with AEG and found no significant difference in the survival rate between the two groups. Sasako and associates22 also completed a randomized controlled trial to compare the left thoracoabdominal approach with the abdominal‐transhiatal approach for gastric cancer of the cardia or subcardia and found that the left thoracoabdominal approach did not improve the survival rate compared with the abdominal‐transhiatal approach. Chen et al. reported no significant difference between Ivor Lewis esophagectomy and Sweet esophagectomy in the five‐year survival rate.9 In addition, most studies reported that for patients with Siewert type II AEG who received Ivor Lewis esophagectomy, the range of thoracic lymph node dissection was restricted to the middle and lower mediastinum,9, 21, 22 which is similar to the range for the Sweet approach. Compared with MIILE, MISE has four advantages: (i) the approach avoids the need for exposure and injury of the upper mediastinum; (ii) the range of esophageal resection is reduced; (iii) the range of gastric resection is enlarged; and (iv) the operation is more simple, and the operation time is reduced.
This study had several limitations. First, it was subject to a potential selection bias due to its retrospective nature. Second, the MISE approach was not directly compared with MIILE. Third, there were incomplete long‐term survival data. Therefore, a multicenter, prospective, randomized controlled trial is required to further demonstrate the effectiveness of this operation.
In conclusion, the MISE approach was considered safe and feasible for patients with Siewert type II AEG. Compared with OSE, the MISE technique could result in a significant improvement in short‐term outcomes for patients with resectable Siewert type II AEG.
Disclosure
The authors do not report any conflict of interest.
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China and The Fundamental Research Funds for the Central Universities (NO.81973643 and No.WK9110000021).
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69 (1): 7–34. [DOI] [PubMed] [Google Scholar]
- 2. Feith M, Stein HJ, Siewert JR. Adenocarcinoma of the esophagogastric junction: Surgical therapy based on 1602 consecutive resected patients. Surg Oncol Clin N Am 2006; 15 (4): 751–64. [DOI] [PubMed] [Google Scholar]
- 3. Yamashita K, Sakuramoto S, Nemoto M et al Trend in gastric cancer: 35 years of surgical experience in Japan. World J Gastroenterol 2011; 17 (29): 3390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bai JG, Lv Y, Dang CX. Adenocarcinoma of the Esophagogastric junction in China according to Siewert's classification. Jpn J Clin Oncol 2006; 36 (6): 364–7. [DOI] [PubMed] [Google Scholar]
- 5. Rudiger SJ, Feith M, Werner M et al Adenocarcinoma of the esophagogastric junction: Results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg 2000; 232 (3): 353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kusano C, Gotoda T, Khor CJ et al Changing trends in the proportion of adenocarcinoma of the esophagogastric junction in a large tertiary referral center in Japan. J Gastroenterol Hepatol 2008; 23 (11): 1662–5. [DOI] [PubMed] [Google Scholar]
- 7. Hosoda K, Yamashita K, Moriya H, Mieno H, Watanabe M. Optimal treatment for Siewert type II and III adenocarcinoma of the esophagogastric junction: A retrospective cohort study with long‐term follow‐up. World J Gastroenterol 2017; 23 (15): 2723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sisic L, Blank S, Weichert W et al Prognostic impact of lymph node involvement and the extent of lymphadenectomy (LAD) in adenocarcinoma of the esophagogastric junction (AEG). Langenbecks Arch Surg 2013; 398 (7): 973–81. [DOI] [PubMed] [Google Scholar]
- 9. Peng J, Wang WP, Yuan Y, Hu Y, Wang Y, Chen LQ. Optimal extent of lymph node dissection for Siewert type II Esophagogastric junction adenocarcinoma. Ann Thorac Surg 2015; 100 (1): 263–9. [DOI] [PubMed] [Google Scholar]
- 10. Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb 1992; 37 (1): 7–11. [PubMed] [Google Scholar]
- 11. Yibulayin W, Abulizi S, Lv H, Sun W. Minimally invasive oesophagectomy versus open esophagectomy for resectable esophageal cancer: A meta‐analysis. World J Surg Oncol 2016; 14 (1): 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Straatman J, van der Wielen N, Cuesta MA et al Minimally invasive versus open Esophageal resection: Three‐year follow‐up of the previously reported randomized controlled trial: The TIME trial. Ann Surg 2017; 266 (2): 232–6. [DOI] [PubMed] [Google Scholar]
- 13. Xie MR, Liu CQ, Guo MF, Mei XY, Sun XH, Xu MQ. Short‐term outcomes of minimally invasive Ivor‐Lewis esophagectomy for esophageal cancer. Ann Thorac Surg 2014; 97 (5): 1721–7. [DOI] [PubMed] [Google Scholar]
- 14. Liu Q, Chen J, Wen J et al Comparison of right‐ and left‐approach esophagectomy for elderly patients with operable thoracic esophageal squamous cell carcinoma: A propensity matched study. J Thorac Dis 2017; 9 (7): 1883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luketich JD, Pennathur A, Franchetti Y et al Minimally invasive esophagectomy: Results of a prospective phase II multicenter trial‐the eastern cooperative oncology group (E2202) study. Ann Surg 2015; 261 (4): 702–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nagpal K, Ahmed K, Vats A et al Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta‐analysis. Surg Endosc 2010; 24 (7): 1621–9. [DOI] [PubMed] [Google Scholar]
- 17. Yerokun BA, Sun Z, Yang CJ et al Minimally invasive versus open esophagectomy for esophageal cancer: A population‐based analysis. Ann Thorac Surg 2016; 102 (2): 416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khan M, Ashraf MI, Syed AA, Khattak S, Urooj N, Muzaffar A. Morbidity analysis in minimally invasive esophagectomy for oesophageal cancer versus conventional over the last 10 years, a single institution experience. J Minim Access Surg 2017; 13 (3): 192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mu JW, Gao SG, Xue Q et al Comparison of short‐term outcomes and three yearsurvival between total minimally invasive McKeown and dual‐incision esophagectomy. Thorac Cancer 2017; 8 (2): 80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Puntambekar SP, Agarwal GA, Joshi SN et al Thoracolaparoscopy in the lateral position for esophageal cancer: The experience of a single institution with 112 consecutive patients. Surg Endosc 2010; 24 (10): 2407–14. [DOI] [PubMed] [Google Scholar]
- 21. Hulscher JB, van Sandick JW, de Boer AG et al Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002; 347 (21): 1662–9. [DOI] [PubMed] [Google Scholar]
- 22. Sasako M, Sano T, Yamamoto S et al Left thoracoabdominal approach versus abdominal‐transhiatal approach for gastric cancer of the cardia or subcardia: A randomised controlled trial. Lancet Oncol 2006; 7 (8): 644–51. [DOI] [PubMed] [Google Scholar]


