Abstract
Concomitant chemo‐radiotherapy (cCRT) with 60 Gy in 30 fractions is the standard of care for stage 111 non‐small cell lung cancer (NSCLC). With a median overall survival of 28.7 months at best and maximum locoregional control rates of 70% at two years, the prognosis for these patients is still dismal. This systematic review summarizes data on dose escalation by alternative fractionation, which has been explored as a primary strategy to improve both local control and overall survival over the past three decades. A Pubmed literature search was performed according to the PRISMA guidelines. Because of the large variety of radiation regimens total doses were converted to EQD2,T. Only studies using an EQD2,T of at least 49.5 Gy, which corresponds to the conventional 60 Gy in six weeks, were included. In a total of 3256 patients, the median OS was 17 months (range 7.4–30 months). While OS was better for patients treated after the year 2000 (P = 0.003) or with a mandatory 18F‐FDG‐PET‐CT in the diagnostic work‐up (P = 0.001), treatment sequence did not make a difference (P = 0.106). The most commonly reported toxicity was acute esophagitis (AE) with a median rate of 24% (range 0%–84%). AE increased at a rate of 0.5% per Gy increment in EQD2,T (P = 0.016). Dose escalation above the conventional 60 Gy using modified radiation fractionation schedules and shortened OTT yield similar mOS and LRC regardless of treatment sequence with a significant EQD2,T dependent increase in AE.
Key points
Significant findings
Modified radiation dose escalation sequentially combined with chemotherapy yields similar outcome as concomitant treatment.
OS is better with the mandatory inclusion of FDG‐PET‐CT in the diagnostic work‐up.
The risk of acute esophagitis increases with higher EQD2,T.
What this study adds
Chemo‐radiotherapy (CRT) with modified dose escalation regimens yields OS and LC rates in the range of standard therapy regardless of treatment sequence. This broadens the database of curative options in patients who are not eligible concomitant CRT.
Keywords: Acute esophagitis; EQD2,T; modified fractionation; radiation dose escalation; systematic review
Introduction
Approximately 80% of all patients with pulmonary malignancies present with non‐small cell lung cancer (NSCLC), of which 35% are locally advanced at the time of diagnosis.1, 2 As for pathological discrimination, 70% are nonsquamous, ie, adeno‐ or large cell carcinomas.3 In the Europe‐wide estimation for 2019, NSCLC will account for 23% and 15% of all cancer deaths in men and women, respectively.4 According to the eighth edition of the TNM classification stage III NSCLC summarizes a scope of diverse subentities.
Thoracic radiotherapy (RT) combined with chemotherapy (CT) is the cornerstone in the treatment of stage III NSCLC. Historically sequential chemo‐radiotherapy (sCRT) was the first treatment strategy in the field.5 During the following 1.5 decades prospective randomized control trials showed better outcome with concomitant chemo‐radiotherapy (cCRT) compared to the sequential mode.6, 7, 8, 9 This result was corroborated by an individual patient data meta‐analysis.1 Although cCRT with 60 Gy is regarded as the standard of care (SOC), a substantial number of patients are still treated sequentially. The majority of prospectively randomized control trials6, 7, 8, 9 were performed before the routine use of 18F‐FDG PET‐CT.
With median overall survival (mOS) of 28.7 months and 65% locoregional control (LRC) in the conventional treatment arm clinical outcome has certainly improved over the past three decades. Nevertheless, a further improvement of survival by both better local and systemic control remains challenging.10 Better LC and OS by cCRT go hand in hand with potentially higher toxicity, which is one of the reasons why not all patients may be regarded as fit enough for the concurrent approach by the treating physician.11 The second reason are comorbid conditions, which allow for the inclusion of only 30% of the patient population with stage III NSCLC in concomitant treatment regimens.8 While guidelines generally propose concurrent treatment some of them, such as The National Institute for Health and Care Excellence (NICE), recommend alternative fractionation schedules (eg, CHART) for patients who are not eligible for the concurrent approach.11
Dose escalation by modified fractionation as the primary strategy to increase LC has been explored in several phase I and II studies.8, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45 In 2015 the RTOG 0617 trial, which applied conventional dose escalation, was negative for the experimental arm in terms of LC and OS.46 The increase of the number of fractions entails prolonged OTT, which leads to accelerated repopulation and may therefore not yield the best possible results.11, 47, 48 A meta‐analysis by Mauguen et al. revealed an OS benefit for patients treated with alternative fractionation as opposed to conventional therapy48 Both hyperfractionation and hypofractionation may contribute to an increase in biologically effective total dose. The latter is especially promising since it saves radiation resources and is potentially more convenient for the patient.10
Since the majority of patients die from distant metastases, an improvement in systemic treatment is necessary.3 In recent years, new systemic agents such as immune checkpoint inhibitors (ICI) have come into play. One of the underlying mechanisms of action is assumed to be radiogenic DNA damage, which activates the immune system. In addition, RT promotes PD‐L1 upregulation, which is blocked by ICI.3 With the results of the PACIFIC trial, durvalumab maintenance therapy for one year after completion of CRT is regarded as the new standard regimen.49, 50
The aim of the current review was to summarize data accumulated in the past three decades on dose escalation by alternative fractionation with a focus on treatment sequence.
Methods
Literature search
Based on the PRISMA guidelines51 a Pubmed literature search with the following terms was performed: “(lung cancer) AND (stage III) AND (radiotherapy OR irradiation OR radiation therapy OR radiation treatment) AND (hypofract* OR accelerated) AND (clinical trial) NOT (review OR case report)”. Publications before 1990, including less than 30 patients, available in abstract only as well as in languages other than English and German, were excluded. Primarily, papers were selected by title and abstract. In a second step, the references in full papers were screened and – if suitable – included in this review. The selection process is summarized in Figure 1 and the included studies are listed in Table 1.
Figure 1.
Selection process according to the PRISMA statement.
Table 1.
Selected studies
| Study | Year | PET‐CT | RT | SD (Gy) | TD (Gy) (5) | OTT (days) | EQD2,T | N | mOS (months) | LRC (%) | AP (%) (3) | AE (%) (3) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concomitant CRT | Wada et al. (1) | 2018 | No | 3D | 2 + 1.2 | 64 | 28 | 58.9 | 163 | 23.1 | 60 | n.s. | n.s. |
| Walraven et al. | 2016 | Yes | 3D/IMRT | 2.75 | 66 | 32 | 64.6 | 92 | 30 | n.s. | n.s. | n.s. | |
| Kerner et al. | 2014 | Yes | 3D | 2.40 | 60 | 35 | 55.0 | 284 | 24.6 | n.s. | 20.8 | 23.6 | |
| Maguire et al. SOCCAR | 2014 | No | 3D | 2.75 | 55 | 28 | 54.9 | 62 | 24.3 | 47 | 3.1 | 8.8 | |
| Donato et al. | 2013 | No | Tomotherapy | 2.28 | 67.95 | 42 | 59.0 | 29 | 24.1 | 74 | 10 | 7 | |
| Bearz et al. (2) | 2013 | Yes | n.s. | 2.40 | 60 | 35 | 55.0 | 33 | 24 | n.s. | 0 | 24 | |
| Chen et al. (12) | 2013 | Yes | IMRT | 2.75 | 66 | 32 | 64.6 | 171 | n.r. | n.s. | n.s. | 29 | |
| de Ruysscher et al. INDAR | 2012 | Yes | n.s. | 1.80 | 64.8 | 24 | 62.2 | 66 | n.r. | n.s. | n.s. | n.s. | |
| Casas et al. (2) | 2011 | No | 3D | 2.68 | 61.6 | 31 | 60.1 | 30 | 16.9 | 35 | 12.5 | 34.3 | |
| Curran et al. | 2011 | No | n.s. | 1.20 | 69.6 | 41 | 55.0 | 187 | 15.6 | 71 | 3 | 45 | |
| Nyman et al. (2) | 2009 | No | 3D | 1.70 | 64.6 | 27 | 60.0 | 40 | 19.6 | n.s. | 0 | 20 | |
| Uitterhoeve | 2007 | No | 3D | 2.75 | 66 | 32 | 64.6 | 56 | 16 | 56 | 27 | ||
| Belderbos et al. | 2007 | No | n.s. | 2.75 | 66 | 32 | 64.6 | 66 | 16.5 | n.s. | 9 | 17 | |
| Chen et al. | 2004 | No | 3D | 1.2 to 1.5 | 66 | 35 | 55.3 | 73 | 13 | 35 | 8 | 15 | |
| Ball et al. | 1999 | No | 3D | 2.00 | 60 | 21 | 60.0 | 41 | 15 | 75 | n.s. | 80 | |
| Schuster‐Uitterhoeve et al. | 1996 | No | 2D | 2.75 | 55 | 28 | 54.9 | 38 | 16 | n.s. | 39.5 (4) | 31.6 (4) | |
| S.equential CRT or RT alone | Cagney et al. | 2018 | No | 3D | 3.00 | 72 | 35 | 71.0 | 48 | 13.6 | 48 | 12.2 | 22.4 |
| Wurstbauer et al. DART‐bid* | 2013 / 2017† | Yes | 3D | 1.80 | 79.2 | 30 | 73.4 | 116 | 26.3 | 70 | 11.3 | 33.3 | |
| Maguire et al. SOCCAR | 2014 | No | 3D | 2.75 | 55 | 28 | 54.9 | 45 | 18.4 | 45 | 5.2 | 8.5 | |
| Cannon et al. | 2013 | No | IMRT | 2.28 | 57 | 35 | 51.3 | 37 | 16 | 61 | 21 | 48 | |
| Donato et al. | 2013 | No | Tomotherapy | 2.28 | 67.95 | 42 | 59.0 | 32 | 18.6 | 74 | 10 | 0 | |
| Din et al. | 2013 | Yes | 2D/3D | 2.75 | 55 | 28 | 54.9 | 139 | 21 | 63 | n.s. | n.s. | |
| McPartlin et al. | 2013 (13) | No | n.s. | 2.75 | 55 | 28 | 54.9 | 45 | 7.4 | n.s. | n.s. | n.s. | |
| de Ruysscher et al. INDAR | 2012 | Yes | n.s. | 1.80 | 64.8 | 24 | 62.2 | 129 | 23.6 | n.s. | n.s. | n.s. | |
| Hatton et al. INCH | 2011 | No | n.s. | 1.50 | 54 | 12 | 51.8 | 20 | 25 | n.s. | 35 (7) | 15 | |
| Jenkins et al. INCH | 2011 | No | 3D | 1.50 | 54 | 12 | 51.8 | 33 | 15.7 | 51 | 32.5 | 10 | |
| Zhu et al. (8) | 2011 | No | 3D | 2.5 + 3 | 68 | 36 | 64.0 | 34 | 19 | 61 | 28.4 | 20.6 | |
| Baumann et al. CHARTWEL | 2011 | No | 3D | 1.50 | 60.00 | 17 | 57.5 | 187 | 15 | 31 | 7.2 | 43.5 | |
| Hatton et al. INCH | 2011 | No | n.s. | 1.50 | 54 | 12 | 51.8 | 23 | 17 | n.s. | 17 (7) | 13 | |
| van Baardwijk et al. | 2010 (9) | Yes | 3D | 1.80 | 61.2 | 23 | 59.2 | 99 | 16.2–17.2 | 67 | 28.3 (10) | 26.5 (11) | |
| Kepka et al. | 2009 | No | 3D | 2.80 | 58.8 | 27 | 59.7 | 173 | 17 | 40 | 7 | 7 | |
| Uitterhoeve et al. | 2007 | No | 3D | 2.75 | 66 | 32 | 64.6 | 26 | 16 | 56 | 23 | ||
| Belderbos et al. | 2007 | No | n.s. | 2.75 | 66 | 32 | 64.6 | 76 | 16.2 | n.s. | 8 | 5 | |
| Uitterhoeve et al. | 2007 | No | 3D | 2.75 | 66 | 32 | 64.6 | 49 | 12 | 56 | 8.2 | ||
| Ishikura et al. ‡ | 2005 | No | n.s. | 1.80 | 57.6 | 12 | 56.6 | 29 | 24 | 57 | 27.5 | 62 | |
| Belani et al. | 2005 | No | 3D | 1.5 + 1.8 + 1.5 | 57.6 | 17 | 54.6 | 56 | 20.3 | n.s. | 23.2 | 73.2 | |
| Marks et al. | 2004 | No | 3D | 1.60 | 80.8 | 37 | 70.1 | 36 | 18 | 82.1 | 9 | 39 | |
| Herskovic et al. | 2000 | No | 3D | 1.10 | 79.2 | 34 | 66.8 | 32 | 10.5 | n.s. | 10.3 | 35.9 | |
| Sause et al. | 2000 | No | n.s. | 1.20 | 69.6 | 41 | 55.0 | 154 | 12 | n.s. | n.s. | n.s. | |
| Saunders et al. CHART | 1999 | No | 3D | 1.50 | 54 | 12 | 56.3 | 309 | 16.5 | 23 | 10 | 19 | |
| Ball et al. | 1999 | No | 3D | 2.00 | 60 | 21 | 60.0 | 36 | 14.4 | 75 | n.s. | 84 | |
| King et al. | 1996 | No | 2D | 1.60 | 73.6 | 31 | 66.1 | 49 | 15.3 | 64 | 0 | 61 | |
AE, acute esophagitis; AP, acute pneumonitis; EQD2,T, biologically equivalent dose in 2 Gy fractions with an assumed start of repopulation at 21 days; IMRT, intensity modulated RT; LRC, locoregional control; mOS, median overall survival; N, number of evaluable patients per study and treatment arm; n.r., not reached; n.s., not stated; OTT, overall treatment time; RT, radiation technique; SD, single dose per fraction; TD, total dose.
Studies with different treatment arms are listed separately in the Table.
Median total dose: 79.2 Gy (range 73.8–90 Gy).
Final toxicity data published 2017.
Highest single dose given.
(1) “Daily concomitant boost”.
(2) Induction chemotherapy followed by cCRT.
(3) Toxicity grade 2 or higher according to CTC if not otherwise specified.
(4) WHO toxicity criteria.
(5) If various dose levels were tested in the study, the median total dose was taken for this review.
(6) Alternating treatment schedule: chemotherapy in week 1 and 4, radiotherapy in week 2, 3 and 6, 7.
(7) The term “breathlessness” was used instead of “pneumonitis”.
(8) The first 19 patients were treated to a total dose of 65 Gy, the remaining 15 patients received 68 Gy.
(9) The average total dose was 61.2 Gy, 13.8% of the stage III patients received the maximum dose of 79.2 Gy.
(10) Dyspnea.
(11) Dysphagia.
(12) Total of 26 patients were also included in the Walraven analysis.
(13) Minimum age 80 years, range: 80.0–94.8.
Studies were included if they used alternative fractionation in at least one treatment arm throughout the radiation therapy course. Alternative fractionation was defined as hypofractionated irradiation with daily doses of >2 Gy per fraction or more than one fraction per day either normofractionated, i.e. 1.8–2 Gy, or hyperfractionated (< 1.8 Gy per fraction). For the purpose of this review, dose escalation was defined as EQD2,T > 49.5 Gy, which equals a total dose of 60 Gy in 30 fractions given within six weeks. In accordance with the ESMO guidelines concomitant chemo‐radiotherapy (cCRT) was defined as at least one cycle of systemic treatment parallel to RT.52 If a study compared several treatment schedules, only those using alternative fractionation either with or without systemic treatment were considered for this review.
EQD2,T
Because of the wide variety of radiation regimens total doses were converted to EQD2,T assuming an α/β = 10 for tumor as well as acute effects and a T ref of 21 days as the starting point for accelerated repopulation.53, 54 D, d and D prolif are defined as total physical dose, dose per fraction and time loss factor (= 0.6 Gy per day), respectively.
If various single doses were used, subtotals were calculated and finally added up to one total EQD2,T.
Toxicity
For the purpose of this analysis, toxicities were only considered clinically relevant as of grade 2 according to the CTCAE versions available at the time the study was published. If the authors used a different scoring system, this is explicitly specified.
Statistical analysis
Clinical outcome parameters such as mOS, LRC and AE were plotted as a function of EQD2,T either based on the numbers stated in the publications or derived from the respective Kaplan‐Meier‐plots therein. In order to detect differences between cCRT and sCRT the nonparametric Mann‐Whitney‐U test was used. The correlations between EQD2,T and mOS, LRC and AE were calculated with the Pearson test.
Results
Literature search
The Pubmed literature search with the above‐mentioned terminology yielded 90 items. Papers were excluded on the basis of the following criteria: palliative treatment (7), carbon ion irradiation (1), SCLC (9), stage I (3), < 30 patients (19), other tumor entities (6), total dose <60 Gy with conventional fractionation (EQD2,T < 49.5) (3), papers with focus on technical aspects of the treatment (4), studies including surgery (6), split course (7), chemotherapy dose escalation (1), stereotactic ablative body radiotherapy (SABR) (1) and late concomitant boost (4). In total, 34 studies (42 treatment arms altogether) were included either as a result of the search strategy or as the review of the references in selected publications (Table 1). These studies encompass the time span between 1996 and 2018, which marks the transition from early 3D planning to modern treatment planning with IMRT/VMAT and IGRT. A total of 16 studies used cCRT (table). In six of these,18, 20, 24, 29, 30, 32 one treatment arm was either sCRT or RT alone. A total of 18 applied nonconcomitant treatment, ie, sCRT or RT alone (Table 1). In 22 treatment arms, the daily dose was applied in one fraction of more than the conventional 2 Gy, whereas in 20 treatment arms at least two daily fractions were used. The median total EQD2,T in all studies was 59.1 Gy (range 51.3–73.4 Gy). The total number of evaluable patients was 3256 with a median of 49 (range 20–309) per treatment arm.
Overall survival
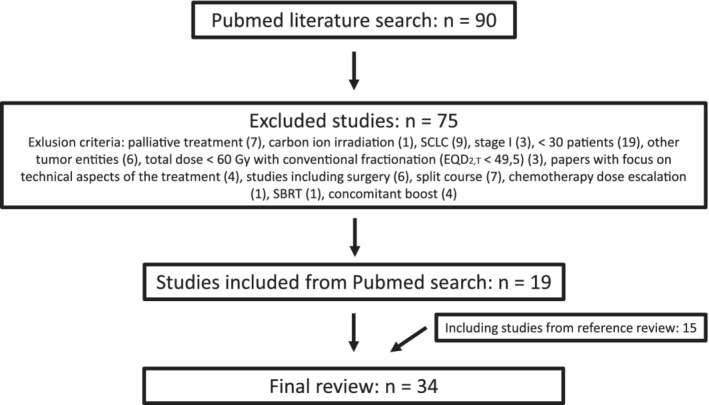
The mOS for all studies was 17 months (range 7.4–30 months, see Table 1). In two treatment arms, the median was not reached.18, 26 The correlation between EQD2,T and mOS was not significant (Pearson test P‐value = 0.437). The mOS for patients treated with cCRT was 18.3 months (range: 13–30 months), which did not differ significantly from the 16.7 months (range: 7.4–26.3 months) achieved with sCRT (Mann‐Whitney‐U test: P‐value = 0.106; Fig 2a,b). The mOS was significantly better for patients treated after the year 2000 (Mann‐Whitney‐U test: P‐value = 0.003). In the eight studies which included a mandatory 18F‐FDG‐PET‐CT scan in the diagnostic work‐up, the patients survived significantly longer than in those without (Mann‐Whitney‐U test: P‐value = 0.001; see Table 1).
Figure 2.
(a) Median overall survival. The correlation between EQD2,T and mOS was not significant (Pearson test P‐value = 0.437). (b) Median overall survival for patients treated with concomitant (cCRT) versus sequential chemo‐radiotherapy (sCRT) did not differ significantly (Mann‐Whitney‐U test: P‐value = 0.106). ( ) cCRT, (
) cCRT, ( ) sCRT or RT alone, (
) sCRT or RT alone, ( ) Linear (cCRT), (
) Linear (cCRT), ( ) Linear (sCRT or RT alone)
) Linear (sCRT or RT alone)
Locoregional control
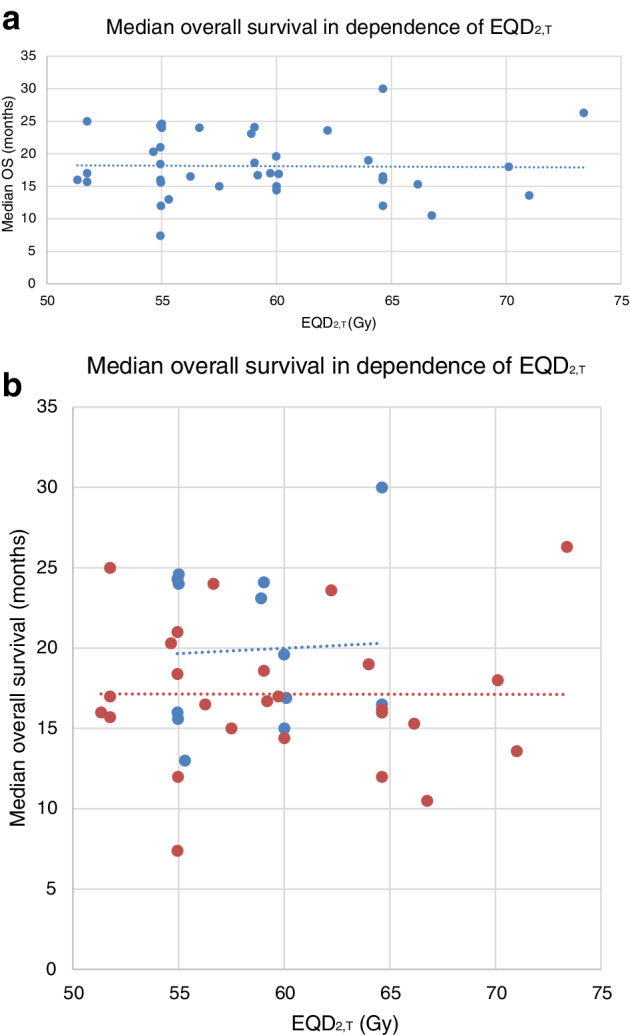
For 26 treatment arms, the median LRC was available. The overall median LRC at two years was 59% (range: 23%–82.1%, see Table 1). The correlation between EQD2,T and LRC was not significant (Pearson test P‐value = 0.371). Furthermore, no significant differences could be detected between cCRT (median: 56%, range 35%–75%) and sCRT (median: 59%; range: 23%–82.1%; Mann‐Whitney‐U test: P‐value = 0.651; Fig 3a,b). We also compared cCRT to sCRT in studies published after the year 2000 and found no differences in LRC (Mann‐Whitney‐U test: P‐value = 0.352). A comparison of the two treatment modalities in those studies with mandatory 18F‐FDG‐PET‐CT in the diagnostic work‐up revealed a similar result (Mann‐Whitney‐U test: P‐value = 0.242).
Figure 3.
Locoregional control after two years. (a) The correlation between EQD2,T and LRC was not significant (Pearson test P‐value = 0.371). (b) Locoregional control after two years for patients treated with concomitant (cCRT) versus sequential chemo‐radiotherapy (sCRT) did not differ significantly (Mann‐Whitney‐U test: P‐value = 0.651). ( ) cCRT, (
) cCRT, ( ) sCRT or RT alone, (
) sCRT or RT alone, ( ) Linear (cCRT), (
) Linear (cCRT), ( ) Linear (sCRT or RT alone)
) Linear (sCRT or RT alone)
Acute esophagitis
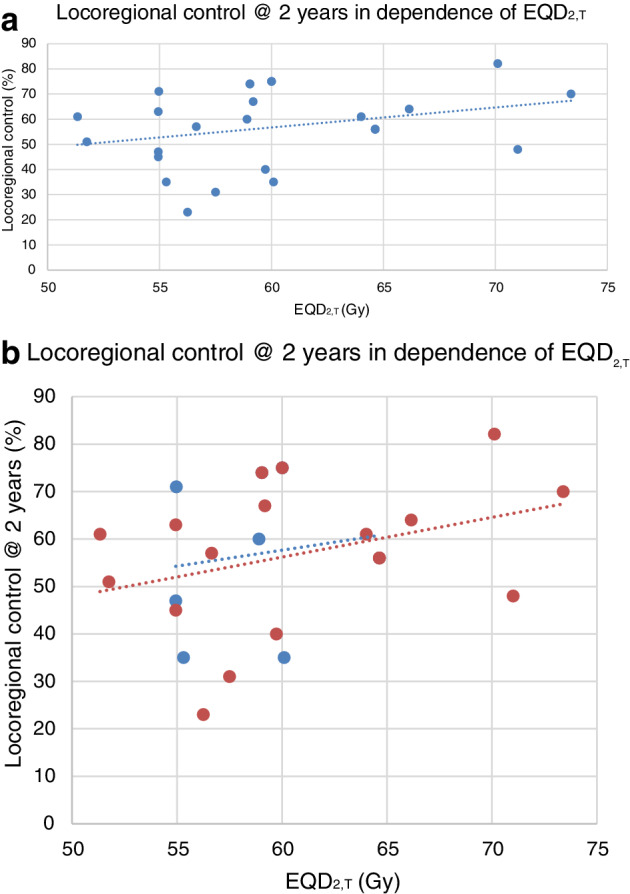
The most common toxicity was acute esophagitis (AE), which was reported in 32 treatment arms with an overall median rate of 24% (range: 0%–84%). In three study arms AE was summarized with AP under “treatment toxicity” and was not assessed in seven treatment schedules (Table 1). While the incidence of AE was not correlated with the sequence of CRT (Fig. 4,b) a significant correlation with EQD2,T could be detected. In treatment arms with EQD2,T above median (= 59.11 Gy) patients had a significantly higher incidence of AE (Pearson test P‐value = 0.016). The probability of AE increased at a rate of 0.5% per Gy increment in EQD2,T.
Figure 4.
(a) Acute esophagitis (AE). The occurrence of AE depended on EQD2,T (Pearson correlation: P = 0.016). (b) Acute esophagitis in patients treated with concomitant (cCRT) versus sequential chemo‐radiotherapy (sCRT) did not differ significantly (Mann‐Whitney‐U test: P‐value = 0.640). ( ) cCRT, (
) cCRT, ( ) sCRT or RT alone, (
) sCRT or RT alone, ( ) Linear (cCRT), (
) Linear (cCRT), ( ) Linear (sCRT or RT alone)
) Linear (sCRT or RT alone)
Discussion
This systematic review demonstrated that cCRT and sCRT are equally effective in terms of mOS and LRC if alternative radiation schedules are used. OS was significantly better if patients were treated after the year 2000 with compulsory inclusion of 18F‐FDG‐PET‐CT in the diagnostic work‐up. The risk of AE increased with higher EQD2,T.
The standard of care for stage III NSCLC is cCRT with 60 Gy combined with two cycles of platinum‐based chemotherapy. Four prospectively randomized control trials published between 1999 and 2011 demonstrated the superiority of this treatment mode compared to sCRT.6, 7, 8, 9 The highest mOS and LRC rates were 17 months8 and 72%,6 respectively. As for toxicity, 18%9 to 32%7 AE and 4%6, 8, 9 to 5%7 AP were reported. A meta‐analysis based on individualized patient data showed that higher LRC achieved by cCRT translates into better OS.1 In fact, radiation dose escalation is a strategy to improve the dismal prognosis for patients with stage III NSCLC since it harbors the potential to increase LRC, which may – combined with effective systemic treatment – prolong survival.
The latest prospectively randomized phase III trial on dose escalation was the four‐armed RTOG 0617 study.46 In this randomized trial, 544 patients received platinum‐based chemotherapy concurrently with a total dose of 60 Gy or 74 Gy with or without the addition of cetuximab.46 LC at two years ranged between 61.4% and 69.3%. The mOS in the standard treatment arm with 60 Gy was 28.7 months compared to 20.3 months with dose escalation. The AP and AE rates were 7% and 44%. One of the major reasons for the unexpected outcome was the prolonged OTT of 7.5 weeks in the experimental arm.46 It also appears that enrollment policy has influenced OS in as far as patients treated in high volume centers had easier access to advanced treatment modalities, which resulted in better OS55 because of lower doses to OARs.55, 56 Although RTOG 0617 provides evidence that there is no gain in conventional dose escalation, alternative radiation fractionation schemes may achieve better OS by biological dose escalation compared to conventional RT.48
A meta‐analysis by Mauguen et al. revealed a significant absolute OS benefit of 2.5% with alternatively fractionated RT compared to the conventional 60 Gy.48 No difference between the two treatment approaches could be detected for LRC control. The pre‐requisite for inclusion in this meta‐analysis was that the patients in the included studies received a minimum total dose of 60 Gy. In terms of toxicity, the authors publish robust data only on AE, with a two‐fold increase in the experimental arms up to an average of 19% clinically relevant esophagitis. In summary, they conclude that their OS data support the rationale for increasingly aggressive radiation schedules.48 Our systematic review including more than 3200 patients in 42 treatment arms8, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45 partly revealed comparable results for mOS, LRC and AE. Since the results of the meta‐analysis by Mauguen et al. are mainly driven by one study, ie, CHART, the authors' conclusion that alternative fractionation as such leads to better mOS could be mitigated in the sense that alternative dose escalation with shortened OTT is successful.
As mentioned in the context of the RTOG 0617 trial, prolonged OTT triggers accelerated repopulation.57, 58 This is why conventional dose escalation may not be the best possible option to improve mOS and LRC. This idea is supported by Kaster et al. who found a relationship between biologically effective dose including a time factor and OS.10 The results of the current study corroborate such a correlation between EQD2,T and AE (Fig 4) with a toxicity increase of 0.5% per 1 Gy increment in total dose. This is important since toxicity is of major concern with any dose escalation strategy.10, 59 While in a substantial part of the reports radiation techniques were not explicitly mentioned, most studies used 3D used conformal RT. Based on the given data it was not possible to draw any conclusions with respect to OS and AE (see Table 1). Nevertheless, it is not counterintuitive that with modern radiation technologies such as IMRT/VMAT, protons and SABR, radiation doses to the OARs can be kept low thereby allowing for safe treatment delivery presumably with less toxicity increase per Gy dose escalation.
In recent years, immune checkpoint inhibitors (ICI) have shown their efficacy in the treatment of stage III NSCLC.49, 50 Currently, four ICIs are available for the treatment of unresectable stage III NSCLC. A http://clinicaltrials.gov search revealed 28 studies worldwide investigating the combination of radiation and ICIs. (accessed on 10 September 2019). In light of the promising results of the PACIFIC trial,49, 50 which have made CRT followed by durvalumab maintenance therapy the new SOC, its efficacy and toxicity profile in combination with alternative fractionation remains an unresolved issue. The total doses in the PACIFIC trial ranged from 54 to 66 Gy delivered in conventional fractionation. Unfortunately, details on radiation therapy were not described, which could have been rewarding since the underlying mechanism of action is – putatively to a large extent – based on radiogenic stimulation of the immune system potentiated by PD‐L1 blockage. Thus far no integration of ICI in alternative fractionation regimens has been reported. Nevertheless, this could be an interesting treatment approach since higher total radiation doses could – at least theoretically – stimulate the immune system in a more effective way. What this means in terms of toxicity, especially for AP, is yet to be resolved. Since checkpoint inhibitors themselves have a considerable pneumonitis potential, the question of supra‐additive effects of high dose irradiation combined with ICIs is of special interest to the radio‐oncological community.
This systematic review has several limitations. We had no access to individualized patient data, so the numbers were either taken directly from the publications or extracted from the graphs in the respective results sections. The current analysis is potentially not very reliable with respect to LRC as it was reported in only 62% (26/42) of the treatment arms. Toxicities are only partially comparable among studies since the CTC versions changed over time and some studies used different scoring systems. Finally, with systematic reviews like this, nomenclature is a problem in the sense that some publications might not have been considered simply because they were registered with keywords that were not included in our search terms.
In summary, it seems that the question of the best possible treatment strategy for stage III patients is a matter of ongoing debate, which can only be resolved by direct comparison of modified fractionation schedules to SOC within a prospective randomized control trial.2, 10, 59, 60 Such a study must include 18F‐FDG‐PET‐CT in the diagnostic work‐up and use modern radiation techniques to escalate the dose to the tumor while sparing OARs.
In conclusion, dose escalation above the conventional 60 Gy using modified radiation fractionation schedules and shortened OTT yield similar mOS and LRC regardless of treatment sequence with a significant EQD2,T dependent increase in AE. In order to counterbalance toxicity the use of modern irradiation techniques is mandatory.
Disclosure
All authors declare that they have no conflict of interest.
References
- 1. Auperin A, Le Pechoux C, Rolland E et al Meta‐analysis of concomitant versus sequential radiochemotherapy in locally advanced non‐small‐cell lung cancer. J Clin Oncol 2010; 28 (13): 2181–90. 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 2. O'Rourke N, Macbeth F. Is concurrent chemoradiation the standard of care for locally advanced non‐small cell lung cancer? A review of guidelines and evidence. Clin Oncol (R Coll Radiol) 2010; 22 (5): 347–55. 10.1016/j.clon.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 3. Melosky B, Juergens R, McLeod D et al Immune checkpoint‐inhibitors and chemoradiation in stage III unresectable non‐small cell lung cancer. Lung Cancer 2019; 134: 259–67. 10.1016/j.lungcan.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 4. Malvezzi M, Carioli G, Bertuccio P et al European cancer mortality predictions for the year 2019 with focus on breast cancer. Ann Oncol 2019; 30 (5): 781–7. 10.1093/annonc/mdz051. [DOI] [PubMed] [Google Scholar]
- 5. Dillman RO, Seagren SL, Propert KJ et al A randomized trial of induction chemotherapy plus high‐dose radiation versus radiation alone in stage III non‐small‐cell lung cancer. N Engl J Med 1990; 323 (14): 940–5. 10.1056/NEJM199010043231403. [DOI] [PubMed] [Google Scholar]
- 6. Furuse K, Fukuoka M, Kawahara M et al Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non‐small‐cell lung cancer. J Clin Oncol 1999; 7 (9): 2692–9. [DOI] [PubMed] [Google Scholar]
- 7. Fournel P, Robinet G, Thomas P et al Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non‐small‐cell lung cancer: Groupe Lyon‐saint‐Etienne d'Oncologie Thoracique‐Groupe Francais de Pneumo‐Cancerologie NPC 95‐01 study. J Clin Oncol 2005; 23 (25): 5910–7. 10.1200/JCO.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 8. Curran WJ Jr, Paulus R, Langer CJ et al Sequential vs. concurrent chemoradiation for stage III non‐small cell lung cancer: Randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011; 103 (19): 1452–60. 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zatloukal P, Petruzelka L, Zemanova M et al Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non‐small cell lung cancer: A randomized study. Lung Cancer 2004; 46 (1): 87–98. 10.1016/j.lungcan.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 10. Kaster TS, Yaremko B, Palma DA, Rodrigues GB. Radical‐intent hypofractionated radiotherapy for locally advanced non‐small‐cell lung cancer: A systematic review of the literature. Clin Lung Cancer 2015; 16 (2): 71–9. 10.1016/j.cllc.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 11. Roach MC, Bradley JD, Robinson CG. Optimizing radiation dose and fractionation for the definitive treatment of locally advanced non‐small cell lung cancer. J Thorac Dis 2018; 10 (Suppl 21): S2465–73. 10.21037/jtd.2018.01.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saunders M, Dische S, Barrett A, Harvey A, Gibson D, Parmar M. Continuous hyperfractionated accelerated radiotherapy (CHART) versus conventional radiotherapy in non‐small‐cell lung cancer: A randomised multicentre trial. CHART steering committee. Lancet 1997; 350 (9072): 161–5. [DOI] [PubMed] [Google Scholar]
- 13. Saunders M, Dische S, Barrett A, Harvey A, Griffiths G, Palmar M. Continuous, hyperfractionated, accelerated radiotherapy (CHART) versus conventional radiotherapy in non‐small cell lung cancer: Mature data from the randomised multicentre trial. CHART steering committee. Radiother Oncol 1999; 52 (2): 137–48. [DOI] [PubMed] [Google Scholar]
- 14. Belani CP, Wang W, Johnson DH et al Phase III study of the Eastern Cooperative Oncology group (ECOG 2597): Induction chemotherapy followed by either standard thoracic radiotherapy or hyperfractionated accelerated radiotherapy for patients with unresectable stage IIIA and B non‐small‐cell lung cancer. J Clin Oncol 2005; 23 (16): 3760–7. 10.1200/JCO.2005.09.108. [DOI] [PubMed] [Google Scholar]
- 15. van Baardwijk A, Wanders S, Boersma L et al Mature results of an individualized radiation dose prescription study based on normal tissue constraints in stages I to III non‐small‐cell lung cancer. J Clin Oncol 2010; 28 (8): 1380–6. 10.1200/JCO.2009.24.7221. [DOI] [PubMed] [Google Scholar]
- 16. Baumann M, Herrmann T, Koch R et al Final results of the randomized phase III CHARTWEL‐trial (ARO 97‐1) comparing hyperfractionated‐accelerated versus conventionally fractionated radiotherapy in non‐small cell lung cancer (NSCLC). Radiother Oncol 2011; 100 (1): 76–85. 10.1016/j.radonc.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 17. Hatton M, Nankivell M, Lyn E et al Induction chemotherapy and continuous hyperfractionated accelerated radiotherapy (chart) for patients with locally advanced inoperable non‐small‐cell lung cancer: The MRC INCH randomized trial. Int J Radiat Oncol Biol Phys 2011; 81 (3): 712–8. 10.1016/j.ijrobp.2010.06.053. [DOI] [PubMed] [Google Scholar]
- 18. De Ruysscher D, van Baardwijk A, Steevens J et al Individualised isotoxic accelerated radiotherapy and chemotherapy are associated with improved long‐term survival of patients with stage III NSCLC: A prospective population‐based study. Radiother Oncol 2012; 102 (2): 228–33. 10.1016/j.radonc.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 19. Wurstbauer K, Deutschmann H, Dagn K et al DART‐bid (dose‐differentiated accelerated radiation therapy, 1.8 Gy twice daily)–a novel approach for non‐resected NSCLC: Final results of a prospective study, correlating radiation dose to tumor volume. Radiat Oncol 2013; 8: 49 10.1186/1748-717X-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maguire J, Khan I, McMenemin R et al SOCCAR: A randomised phase II trial comparing sequential versus concurrent chemotherapy and radical hypofractionated radiotherapy in patients with inoperable stage III non‐small cell lung cancer and good performance status. Eur J Cancer 2014; 50 (17): 2939–49. 10.1016/j.ejca.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 21. Walraven I, van den Heuvel M, van Diessen J et al Long‐term follow‐up of patients with locally advanced non‐small cell lung cancer receiving concurrent hypofractionated chemoradiotherapy with or without cetuximab. Radiother Oncol 2016; 118 (3): 442–6. 10.1016/j.radonc.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 22. Wada K, Kishi N, Kanayama N et al Radiation dose escalation in accelerated hyperfractionated radiotherapy for stage III non‐small‐cell lung cancer. Anticancer Res 2018; 38 (10): 5951–8. 10.21873/anticanres.12941. [DOI] [PubMed] [Google Scholar]
- 23. Kerner GSMA, van Dullemen LFA, Wiegman EM et al Concurrent gemcitabine and 3D radiotherapy in patients with stage III unresectable non‐small cell lung cancer. Radiat Oncol 2014; 9: 190 10.1186/1748-717x-9-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Donato V, Arcangeli S, Monaco A et al Moderately escalated hypofractionated (chemo) radiotherapy delivered with helical intensity‐modulated technique in stage III Unresectable non‐small cell lung cancer. Front Oncol 2013; 3: 286 10.3389/fonc.2013.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bearz A, Minatel E, Abu Rumeileh I et al Concurrent chemoradiotherapy with tomotherapy in locally advanced non‐small cell lung cancer: A phase i, docetaxel dose‐escalation study, with hypofractionated radiation regimen. BMC Cancer 2013; 13: 513 10.1186/1471-2407-13-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen C, Uyterlinde W, Sonke JJ, de Bois J, van den Heuvel M, Belderbos J. Severe late esophagus toxicity in NSCLC patients treated with IMRT and concurrent chemotherapy. Radiother Oncol 2013; 108 (2): 337–41. 10.1016/j.radonc.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 27. Casas F, Vinolas N, Ferrer F et al Long‐term results of a phase II trial of induction paclitaxel‐carboplatin followed by concurrent radiation therapy and weekly paclitaxel and consolidation paclitaxel‐carboplatin in stage III non‐small cell lung cancer. J Thorac Oncol 2011; 6 (1): 79–85. 10.1097/JTO.0b013e318200e563. [DOI] [PubMed] [Google Scholar]
- 28. Nyman J, Friesland S, Hallqvist A et al How to improve loco‐regional control in stages IIIa‐b NSCLC? Results of a three‐armed randomized trial from the Swedish lung cancer study group. Lung Cancer 2009; 65 (1): 62–7. 10.1016/j.lungcan.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 29. Uitterhoeve ALJ, Koolen MGJ, van Os RM et al Accelerated high‐dose radiotherapy alone or combined with either concomitant or sequential chemotherapy; treatments of choice in patients with non‐small cell lung cancer. Radiat Oncol 2007; 2: 27 10.1186/1748-717x-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Belderbos J, Uitterhoeve L, van Zandwijk N et al Randomised trial of sequential versus concurrent chemo‐radiotherapy in patients with inoperable non‐small cell lung cancer (EORTC 08972‐22973). Eur J Cancer 2007; 43 (1): 114–21. 10.1016/j.ejca.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 31. Chen GY, Jiang GL, Qian H et al Escalated hyperfractionated accelerated radiation therapy for locally advanced non‐small cell lung cancer: A clinical phase II trial. Radiother Oncol 2004; 71 (2): 157–62. 10.1016/j.radonc.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 32. Ball D, Bishop J, Smith J et al A randomised phase III study of accelerated or standard fraction radiotherapy with or without concurrent carboplatin in inoperable nonsmall cell lung cancer: Final report of an Australian multi‐centre trial. Radiotherapy and Oncology 1999; 52 (2): 129–36. 10.1016/S0167-8140(99)00093-6. [DOI] [PubMed] [Google Scholar]
- 33. SchusterUitterhoeve ALJ, Vande Vaart PJM, SchaakeKoning CCE et al Feasibility of escalating daily doses of cisplatin in combination with accelerated radiotherapy in non‐small cell lung cancer. Eur J Cancer 1996; 32A (8): 1314–9. 10.1016/0959-8049(96)00077-9. [DOI] [PubMed] [Google Scholar]
- 34. Cagney DN, Thirion PG, Dunne MT et al A phase II toxicity end point trial (ICORG 99‐09) of accelerated dose‐escalated Hypofractionated radiation in non‐small cell lung cancer. Clin Oncol (R Coll Radiol) 2018; 30 (1): 30–8. 10.1016/j.clon.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 35. Cannon DM, Mehta MP, Adkison JB et al Dose‐limiting toxicity after hypofractionated dose‐escalated radiotherapy in non‐small‐cell lung cancer. J Clin Oncol 2013; 31 (34): 4343–8. 10.1200/JCO.2013.51.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Din OS, Harden SV, Hudson E et al Accelerated hypo‐fractionated radiotherapy for non small cell lung cancer: Results from 4 UK centres. Radiother Oncol 2013; 109 (1): 8–12. 10.1016/j.radonc.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 37. McPartlin AJ, Chaudhry S, Swindell R et al The largest UKsingle Centre series using hypofractionated radical radiotherapy for NSCLC in the very elderly. Lung Cancer 2013; 81 (1): 144 10.1016/j.lungcan.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 38. Jenkins P, Anderson S, Wronski S, Ashton A. A phase II trial of induction chemotherapy followed by continuous hyperfractionated accelerated radiotherapy in locally advanced non‐small‐cell lung cancer. Radiother Oncol 2009; 93 (3): 396–401. 10.1016/j.radonc.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 39. Zhu ZF, Fan M, Wu KL et al A phase II trial of accelerated hypofractionated three‐dimensional conformal radiation therapy in locally advanced non‐small cell lung cancer. Radiother Oncol 2011; 98 (3): 304–8. 10.1016/j.radonc.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 40. Kepka L, Tyc‐Szczepaniak D, Bujko K. Dose‐per‐fraction escalation of accelerated Hypofractionated three‐dimensional conformal radiotherapy in locally advanced non‐small cell lung cancer. J Thoracic Oncol 2009; 2009;4 (7): 853–61. 10.1097/JTO.0b013e3181a97dda. [DOI] [PubMed] [Google Scholar]
- 41. Ishikura S, Ohe Y, Nihei K et al A phase II study of hyperfractionated accelerated radiotherapy (HART) after induction cisplatin (CDDP) and vinorelbine (VNR) for stage III non‐small‐cell lung cancer (NSCLC). Int J Radiat Oncol 2005; 61 (4): 1117–22. 10.1016/j.ijrobp.2004.07.692. [DOI] [PubMed] [Google Scholar]
- 42. Marks LB, Garst J, Socinski MA et al Carboplatin/paclitaxel or carboplatin/vinorelbine followed by accelerated hyperfractionated conformal radiation therapy: Report of a prospective phase I dose escalation trial from the Carolina conformal therapy consortium. J Clin Oncol 2004; 22 (21): 4329–40. 10.1200/Jco.2004.02.165. [DOI] [PubMed] [Google Scholar]
- 43. Herskovic A, Scott C, Demas W, Gaspar L, Trotti A. Accelerated hyperfractionation for bronchogenic cancer ‐ radiation therapy oncology group 9205. Am J Clin Oncol‐Canc 2000; 23 (2): 207–12. 10.1097/00000421-200004000-00020. [DOI] [PubMed] [Google Scholar]
- 44. Sause W, Kolesar P, Taylor S et al Final results of phase III trial in regionally advanced unresectable non‐small cell lung cancer ‐ radiation therapy Oncology group, Eastern Cooperative Oncology group, and southwest Oncology group. Chest 2000; 117 (2): 358–64. 10.1378/chest.117.2.358. [DOI] [PubMed] [Google Scholar]
- 45. King SC, Acker JC, Kussin PS, Marks LB, Weeks KJ, Leopold KA. High‐dose, hyperfractionated, accelerated radiotherapy using a concurrent boost for the treatment of nonsmall cell lung cancer: Unusual toxicity and promising early results. Int J Radiat Oncol 1996; 36 (3): 593–9. 10.1016/S0360-3016(96)00353-7. [DOI] [PubMed] [Google Scholar]
- 46. Bradley JD, Paulus R, Komaki R et al Standard‐dose versus high‐dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non‐small‐cell lung cancer (RTOG 0617): A randomised, two‐by‐two factorial phase 3 study. Lancet Oncol 2015; 16 (2): 187–99. 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fowler JF, Chappell R. Non‐small cell lung tumors repopulate rapidly during radiation therapy. Int J Radiat Oncol 2000; 46 (2): 516–7. [DOI] [PubMed] [Google Scholar]
- 48. Mauguen A, Le Pechoux C, Saunders MI et al Hyperfractionated or accelerated radiotherapy in lung cancer: An individual patient data meta‐analysis. J Clin Oncol 2012; 30 (22): 2788–97. 10.1200/JCO.2012.41.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Antonia SJ, Villegas A, Daniel D et al Overall survival with Durvalumab after Chemoradiotherapy in stage III NSCLC. N Engl J Med 2018; 379 (24): 2342–50. 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 50. Antonia SJ, Villegas A, Daniel D et al Durvalumab after Chemoradiotherapy in stage III non‐small‐cell lung cancer. N Engl J Med 2017; 377 (20): 1919–29. 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 51. Moher D, Shamseer L, Clarke M et al Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev 2015; 4: 1(2015). 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Eberhardt WEE, De Ruysscher D, Weder W et al 2nd ESMO consensus conference in lung cancer: Locally advanced stage III non‐small‐cell lung cancer. Ann Oncol 2015; 26 (8): 1573–88. 10.1093/annonc/mdv187. [DOI] [PubMed] [Google Scholar]
- 53. van Baardwijk A, Tome WA, van Elmpt W et al Is high‐dose stereotactic body radiotherapy (SBRT) for stage I non‐small cell lung cancer (NSCLC) overkill? A systematic review. Radiother Oncol 2012; 105 (2): 145–9. 10.1016/j.radonc.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 54. Fowler JF, Tome WA, Fenwick JD, Mehta MP. A challenge to traditional radiation oncology. Int J Radiat Oncol Biol Phys 2004; 60 (4): 1241–56. 10.1016/j.ijrobp.2004.07.691. [DOI] [PubMed] [Google Scholar]
- 55. Eaton BR, Pugh SL, Bradley JD et al Institutional enrollment and survival among NSCLC patients receiving Chemoradiation: NRG Oncology radiation therapy Oncology group (RTOG) 0617. J Natl Cancer Inst 2016; 108 (9): djw034. 10.1093/jnci/djw034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chun SG, Hu C, Choy H et al Impact of intensity‐modulated radiation therapy technique for locally advanced non‐small‐cell lung cancer: A secondary analysis of the NRG Oncology RTOG 0617 randomized clinical trial. J Clin Oncol 2017; 35 (1): 56–62. 10.1200/JCO.2016.69.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kerr KM, Lamb D. Actual growth‐rate and tumor‐cell proliferation in human pulmonary neoplasms. Br J Cancer 1984; 50 (3): 343–9. 10.1038/bjc.1984.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Trott KR. Cell repopulation and overall treatment time. Int J Radiat Oncol 1990; 1990;19 (4): 1071–5. 10.1016/0360-3016(90)90036-J. [DOI] [PubMed] [Google Scholar]
- 59. Mery B, Guy JB, Swalduz A et al The evolving locally‐advanced non‐small cell lung cancer landscape: Building on past evidence and experience. Crit Rev Oncol Hemat 2015; 96 (2): 319–27. 10.1016/j.critrevonc.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 60. O'Rourke N, Figuls MRI, Bernado NF, Macbeth F. Concurrent chemoradiotherapy in non‐small cell lung cancer. Cochrane Db Syst Rev 2010; 6: CD002140 10.1002/14651858.CD002140.pub3. [DOI] [PubMed] [Google Scholar]


