Abstract
Background
Although the use of radial endobronchial ultrasound (R‐EBUS) with a guide sheath has shown improved diagnostic capability in peripheral pulmonary lesions, its utility is still low due to variable performance. To overcome its limitation, we evaluated the feasibility and efficacy of R‐EBUS combined with transbronchial biopsy (TBB) under fluoroscopic guidance.
Methods
We retrospectively reviewed medical records of 74 patients with non‐small cell lung cancer (NSCLC) who underwent R‐EBUS combined with TBB or TBB alone as a diagnostic technique. Subjects were grouped according to the diagnostic modality used (R‐EBUS combined with TBB vs. TBB alone). Each group was matched for age, sex, and location of the biopsy. The chi‐square test and paired t‐test were used to compare characteristics and identify factors that affected the diagnostic yield.
Results
The mean age of the study cohort was 67.4 ± 12.8 years, with 21 (56.8%) men and 16 (43.2%) women in each group. The lesion size was significantly smaller in the R‐EBUS group (23.6 vs. 33.9, P < 0.001). The diagnostic yield with the combined use of R‐EBUS and TBB (27/37, 72.9%) was significantly higher than that with standard TBB alone (22/37, 59.4%). Lung lesions with a positive bronchus sign were associated with a higher diagnostic yield (odds ratio = 3.52 [1.17–10.62]; P = 0.025).
Conclusions
The combination of R‐EBUS with TBB resulted in a higher diagnostic yield than either technique alone. Thus, the addition of R‐EBUS biopsy would be helpful to improve the diagnostic yield of TBB.
Key points
Significant findings of the study
The combination of R‐EBUS with TBB under fluoroscopic guidance improved the diagnostic yield of PPLs compared to TBB alone. A tissue diagnosis was more likely in pulmonary lesions with the air‐bronchus sign.
What this study adds
The use of R‐EBUS could help improve the low diagnostic yield of TBB under fluoroscopic guidance without increasing the incidence of complications.
Keywords: Biopsy, bronchoscopy, fluoroscopy, lung cancer
Introduction
Lung cancer represents a major worldwide disease burden.1 According to Global Cancer Statistics 2018, lung cancer is a leading cause of newly diagnosed cancer and deaths across 20 regions of the world.2 Despite the introduction of preventive strategies such as tobacco control, new diagnostic modalities, and therapeutic agents, the incidence of cancer and cancer‐related mortality rates are expected to increase.3
Recently, with the introduction of low‐dose helical computed tomography (CT) for lung cancer screening, the detection rate of peripheral pulmonary lesions (PPLs) has increased.4 Thus, there has been an increasing need to acquire histopathological specimens from PPLs. In addition to improving the survival rate through the early diagnosis of lung cancer, histopathology of PPLs also facilitates decision‐making regarding the use of new therapeutic agents such as immunotherapy.5
Conventionally, transbronchial biopsy (TBB) under fluoroscopic guidance has been widely used in the diagnosis of PPLs since the 1970s.6 Performing biopsies under real‐time imaging of the lesions is a major advantage of TBB. However, determining the three‐dimensional location of the lesion using only a two‐dimensional fluoroscopic image remains a challenging task. In particular, when the target area of the biopsy overlaps with other pulmonary structures, it is difficult to determine the precise location. Moreover, the diagnostic yield of TBB varies from 14% to 75%, which is lower than that of transthoracic needle aspiration.7, 8
Approximately 20 years ago, advanced bronchoscopic modalities, such as endoscopic navigation bronchoscopy (ENB), thin bronchoscopy, and radial endoscopic ultrasound (R‐EBUS), were introduced to overcome the shortcomings of these conventional techniques.9, 10 The 2013 American College of Chest Physicians guidelines recommend radial EBUS as an alternative to conventional bronchoscopy if the equipment and expertise are available.11
R‐EBUS using thin bronchoscopy has a higher diagnostic yield (51%–92%) and lower complication rate than conventional bronchoscopy.12, 13 Under ultrasound guidance, the operator can perform a biopsy while observing the PPL in real‐time. However, the equipment required for this procedure is expensive. Furthermore, the ability to accurately target the lesion may be highly dependent on the skills and knowledge of bronchial anatomy of the operator.14, 15, 16
Therefore, the purpose of this study was to determine the improved diagnostic capability of R‐EBUS when combined with conventional TBB in the diagnosis of PPLs.
Methods
Study design and patient selection
This study was conducted at the Severance Hospital, Seoul, Korea. A retrospective review of electronic medical records was conducted on consecutive lung cancer patients at the pulmonary division of the hospital from August 2017 to November 2018. During the study period, TBB was performed in 432 lung cancer patients. Among this cohort, 37 patients underwent TBB combined with R‐EBUS biopsy. They were matched with the remaining 395 patients who underwent TBB under fluoroscopic guidance alone according to age, sex, smoking status, and characteristics of the lesion, including location, type, and size. Among the 395 patients, we selected cases highly suspected of lung cancer on chest CT to reduce the possibility of including those with benign diseases. We also excluded cases with signs of infection such as fever, hypotension, leukocytosis, or elevated C‐reactive protein levels. Even if the study subjects' bronchoscopic biopsy revealed a benign pathology, they were followed up for six months. The diagnosis of lung cancer was made by using follow‐up images, multidisciplinary consultation, as well as another modality of biopsy such as CT‐guided needle aspiration biopsy or surgical biopsy.
Ethics approval
This study was approved by the institutional review board of the Severance Hospital (Approval number 4–2019‐1106).
Bronchoscopic procedures
Bronchoscopy was performed by six fellowship trainees of the pulmonology division of our institution. Each fellowship trainee underwent a training period of 4–15 months and had performed a minimum of 100 TBBs. Both TBB and R‐EBUS were performed under the supervision of an expert faculty member with previous experience with the procedure. A thin bronchoscope (EB‐P290; Olympus) with a 4.2 mm outer diameter and a 2.0 mm working channel was used in both groups. EBUS was performed using an endoscopic ultrasound system (UM‐S20‐17S; Olympus, Japan), equipped with a 20‐MHz mechanical radial‐type probe. Prior to sedation, topical anesthesia of the larynx was applied for 5 minutes using a 2% lidocaine spray. To perform bronchoscopy under deep sedation, intravenous midazolam (2–5 mg depending on patient's bodyweight) and fentanyl (50 mcg) were administered. After insertion of the bronchoscope through the mouth, the trachea and whole bronchi were first inspected. The bronchoscope was placed on the segmental bronchus close to the PPL. A radial probe was then inserted through the working channel to advance into the segmental bronchus near the PPL to localize the lesion. This process was completed under fluoroscopic guidance to facilitate visualization. After localization using EBUS, the probe was removed with the guide sheath remaining on the peripheral lesion. Subsequently, biopsy forceps (Olympus, 1.5 mm portion diameter) were introduced via the guide sheath to perform pathological examination. After performing four biopsies using R‐EBUS, both the radial probe and guide sheath were removed via the working channel. The target site was located using typical radial EBUS images that have previously been described for solid nodules, and the blizzard sign for nodules demonstrating ground‐glass opacity (GGO).17 A representative case of R‐EBUS is shown in Fig 1. Afterwards, four biopsies were done only after confirming the approximate placement of the TBB forceps (Olympus, 1.9 mm portion diameter) to the PPL under fluoroscopy. With regard to the TBB group, only TBB was completed eight times under fluoroscopic guidance without prior R‐EBUS biopsy.
Figure 1.
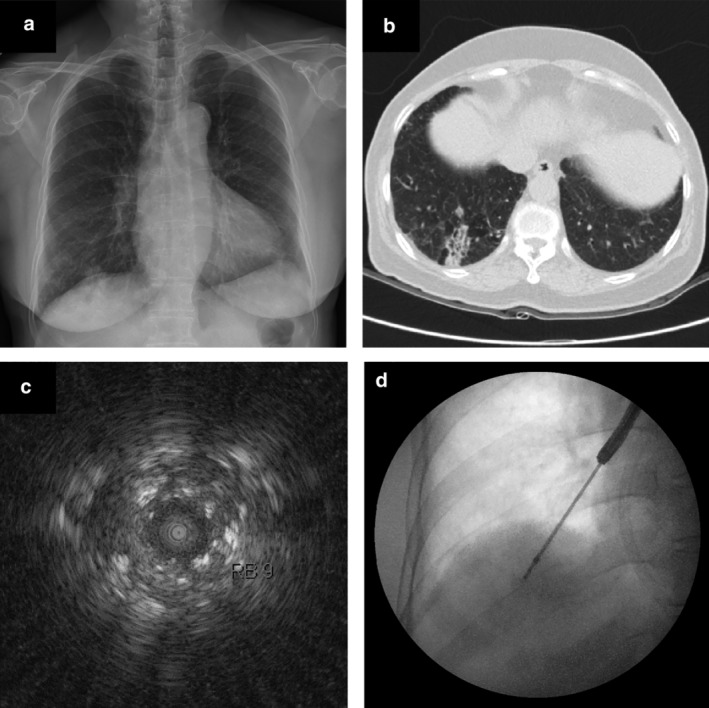
Case of a 74‐year‐old man with a consolidation in the right lower lobe.(a) Chest X‐ray showing consolidation at the right lower lobe. (b) Computed tomography (CT) scan showing a 38 mm peripheral lung mass. (c) Radial probe endobronchial ultrasound (R‐EBUS) in the lateral basilar segment of the right lower lobe revealing a mixed blizzard sign containing a heterogeneous acoustic shadow with hyperechoic dots, linear arcs, and vessels. (d) Fluoroscopic image during transbronchial biopsy through a guide sheath.
Complications
Patients were hospitalized for more than two days for the bronchoscopy procedure and monitored for complications during the hospital stay. They were specifically monitored for the development of hemoptysis, pneumothorax, fever, desaturation, chest pain, and respiratory failure. In‐hospital mortality among the study subjects was also recorded. Chest radiography was performed within three hours after the procedure to check for possible pneumothorax and TBB‐related bleeding. Desaturation was defined as a drop in oxygen saturation to <95% during or after the procedure.
Statistical analysis
Data analysis was completed on demographic and clinicopathologic variables. Descriptive statistics are reported as the mean ± standard deviation, and categorical variables as frequencies and proportions. Differences between the standard TBB and R‐EBUS with TBB groups were analyzed using the Student's t‐test, chi‐square test, or Fisher's exact test. We performed univariate and multivariate logistic regression analyses to assess the significance of lesion characteristics as an independent predictive factor for diagnostic yield. Statistical analyses were performed using the IBM SPSS software, version 25.0 (IBM Co., Armonk, NY, USA).
Results
Characteristics of study subjects and procedures
The study cohort consisted of 37 patients in each group, including 21 (56.8%) men and 16 (43.2%) women. The mean age of the study cohort was 67.4 ± 12.8 years. Comorbidities such as hypertension, diabetes, and tumor marker (carcinoembryonic antigen and Cyfra 21–1) levels were not significantly different between the two groups. The mean duration of the bronchoscopy procedure was 42.9 ± 16.4 minutes in all patients. The mean procedural time was longer in the R‐EBUS‐TBB group than in the TBB group (47.6 ± 15.3 vs. 38.1 ± 16.2 minutes); however, the difference was not statistically different (P = 0.743) (Table 1).
Table 1.
Patient and lesion characteristics
| Variable | All subjects | Standard TBB | R‐EBUS‐TBB | P‐value |
|---|---|---|---|---|
| Male sex | 42 (37.8) | 21 (56.8) | 21 (56.8) | 1.000 |
| Age | 67.4 ± 12.8 | 67.3 ± 12.8 | 67.5 ± 13.0 | 0.808 |
| Ever‐smoker | 28 (56.7) | 21 (56.8) | 17 (45.9) | 0.486 |
| Comorbidities | ||||
| Hypertension | 27 (36.4) | 14 (37.8) | 13 (35.1) | 1.000 |
| Diabetes | 12 (16.2) | 8 (21.6) | 4 (10.8) | 0.345 |
| Old CVA | 2 (2.7) | 1 (2.7) | 1 (2.7) | 1.000 |
| Old Tbc | 10 (13.5) | 7 (18.9) | 3 (8.1) | 0.308 |
| COPD | 6 (8.1) | 3 (8.1) | 3 (8.1) | 1.000 |
| Asthma | 4 (5.4) | 2 (5.4) | 2 (5.4) | 1.000 |
| Other malignancies | 16 (21.6) | 9 (24.3) | 7 (18.9) | 0.778 |
| Tumor markers | ||||
| CEA | 26.4 ± 83.8 | 35.7 ± 95.7 | 19.2 ± 73.9 | 0.447 |
| Cyfra 21–1 | 3.0 ± 1.9 | 2.9 ± 1.4 | 3.1 ± 2.2 | 0.856 |
| Procedure duration (minutes) | 42.9 ± 16.4 | 38.1 ± 16.2 | 47.6 ± 15.3 | 0.743 |
| Location of PPL(s) | 1.000 | |||
| RUL | 16 (21.6) | 8 (21.6) | 8 (21.6) | |
| RML | 6 (8.1) | 3 (8.1) | 3 (8.1) | |
| RLL | 20 (27.0) | 10 (27.0) | 10 (27.0) | |
| LUL | 22 (29.7) | 11 (29.7) | 11 (29.7) | |
| LLL | 10 (13.5) | 5 (13.5) | 5 (13.5) | |
| Nodule type | 0.193 | |||
| Solid | 53 (71.6) | 26 (70.3) | 27 (73.0) | |
| Subsolid | 15 (20.2) | 6 (16.2) | 9 (24.3) | |
| GGO | 6 (8.1) | 5 (13.5) | 1 (2.7) | |
| Air‐bronchus sign | 32 (43.2) | 18 (48.6) | 14 (37.8) | 0.241 |
| Size, mean (mm) | 28.7 ± 13.2 | 34.5 ± 15.5 | 23.6 ± 7.6 | < 0.001* |
| < 20 | 20 (27.0) | 7 (18.9) | 13 (35.1) | |
| 20–30 | 28 (37.8) | 11 (29.7) | 17 (45.9) | |
| > 30 | 26 (35.1) | 19 (51.4) | 7 (18.9) | |
| Pleural distance (mm) | 12.5 ± 12.7 | 10.3 ± 10.5 | 14.8 ± 14.4 | 0.102 |
Significant differences (P < 0.05). CEA, carcinoembryonic antigen; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; GGO, ground‐glass opacity; LLL, left lower lobe; LUL, left upper lobe; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe; TBB, transbronchial biopsy; Tbc, tuberculosis.
Radiographic characteristics of pulmonary lesions
Radiographic characteristics of the target lesions are presented in Table 1. Most lesions were located in the left upper lobe (22, 29.7%) and were solid in appearance (53, 71.6%). The air‐bronchus sign was seen in 32 (43.2%) cases, with no significant difference between the two groups (P = 0.241). The mean distance from the pleura to the lesion also showed no significant difference between two groups (14.8 ± 14.4 mm vs. 10.3 ± 10.5 mm, P = 0.102). The mean size of the pulmonary lesion was 28.7 ± 13.2 mm, which was smaller in the R‐EBUS‐TBB group than in the TBB group (23.6 ± 7.6 vs. 34.5 ± 15.5 mm, P < 0.001).
Factors associated with diagnostic yield
In the R‐EBUS‐TBB group, diagnostic yield of R‐EBUS and TBB were 40.5% and 54%, respectively. When we merged both biopsy results, overall 27 patients (72.9%) were diagnosed with lung cancer. This result was much higher than the diagnostic yield of the TBB‐only group (59.4%). The diagnostic rate of TBB was slightly higher than that of the R‐EBUS‐TBB group (Fig. 2). Among the variables studied, lung lesions with the air‐bronchus sign were associated with a higher diagnostic yield (odds ratio = 3.52 (1.17–10.62); P = 0.025) (Table 2).
Figure 2.

Schematic figure of diagnostic yield in two groups. * R‐EBUS, radial probe endobronchial ultrasound; TBB, transbronchial biopsy.
Table 2.
Univariate and multivariate analysis for predictors of diagnostic yield
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | P‐value | OR (95% CI) | P‐value |
| Solid nodule | 0.49 (0.15–1.56) | 0.223 | ||
| Nodule size ≥30 mm | 0.72 (0.27–1.95) | 0.526 | ||
| Air‐bronchus sign | 3.30 (1.21–9.00) | 0.017 | 3.52 (1.17–10.62) | 0.025* |
| Cavitation | 0.82 (0.21–3.24) | 0.783 | ||
| Pleural distance >20 mm | 0.65 (0.18–2.34) | 1.000 | ||
| R‐EBUS arm | 1.27 (0.48–3.33) | 0.624 | ||
Significant differences (P < 0.05). CI, confidence interval; OR, odds ratio; R‐EBUS, radial endobronchial ultrasound.
Bronchoscopic diagnosis
The bronchoscopic diagnosis of study subjects is presented in Table 3. Of the 74 patients, 49 (66.2%) were diagnosed with malignancy. Among the diagnosed cases, adenocarcinoma (49, 58.1%) was the most common. Benign conditions such as nonspecific inflammation, granuloma, and pneumonia were diagnosed in six patients (8.1%). We were unable to obtain meaningful results from 11 (29.7%) cases in the TBB group and eight (21.6%) cases in the R‐EBUS‐TBB group because the biopsy forceps were unable to approach the PPL, or insufficient tissue sample collection.
Table 3.
Bronchoscopic diagnosis
| Diagnosis | Total, No. (%) | Standard TBB, No. (%) | R‐EBUS‐TBB, No. (%) |
|---|---|---|---|
| Diagnostic | 49 (66.2) | 22 (59.4) | 27 (72.9) |
| Adenocarcinoma | 43 (58.1) | 19 (51.3) | 24 (64.8) |
| Squamous cell cancer | 2 (2.7) | 1 (2.7) | 1 (2.7) |
| Small cell cancer | 1 (1.3) | 0 (0.0) | 1 (2.7) |
| Large cell cancer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Metastasis | 3 (4.0) | 2 (5.4) | 1 (2.7) |
| Nondiagnostic | 25 (33.7) | 15 (40.5) | 10 (37.0) |
| Biopsy failure | 19 (25.6) | 11 (29.7) | 8 (21.6) |
| Inflammation | 4 (5.4) | 2 (5.4) | 2 (5.4) |
| Granuloma | 1 (1.3) | 1 (2.7) | 0 (0.0) |
| Pneumonia | 1 (1.3) | 1 (2.7) | 0 (0.0) |
R‐EBUS, radial endobronchial ultrasound; TBB, transbronchial biopsy.
Complications after the procedure
Overall, complications were encountered in seven patients (9.4%). Desaturation (6, 8.1%) was the most common complication, following by chest pain (5, 6.7%), pneumothorax (3, 4.0%), and hemoptysis (3, 4.0%). There were no life‐threatening complications such as respiratory failure and no in‐hospital mortality in the study subjects. There was no significant difference in the complications between the TBB and R‐EBUS‐TBB groups (P = 0.317) (Table 4).
Table 4.
Complications of each procedure
| Complications | Total, No. (%) | Standard TBB, No. (%) | R‐EBUS‐TBB, No. (%) | P‐value |
|---|---|---|---|---|
| Hemoptysis | 2 (2.7) | 1 (2.7) | 1 (2.7) | 0.667 |
| Pneumothorax | 3 (4.0) | 1 (2.7) | 2 (5.4) | 0.750 |
| Fever | 3 (4.0) | 1 (2.7) | 2 (5.4) | 0.600 |
| Desaturation (SpO2 < 95%) | 6 (8.1) | 3 (8.1) | 3 (8.1) | 0.315 |
| Chest pain | 5 (6.7) | 3 (8.1) | 2 (5.4) | 0.500 |
| Respiratory failure | 0 (0.0) | 0 (0.0) | 0 (0.0) | ‐ |
| Death | 0 (0.0) | 0 (0.0) | 0 (0.0) | ‐ |
| Total cases | 7 (9.4) | 4 (10.8) | 3 (8.1) | 0.317 |
Significant differences (P < 0.05). R‐EBUS, radial endobronchial ultrasound; SpO2, peripheral oxygen saturation; TBB, transbronchial biopsy.
Discussion
The present study revealed that combining R‐EBUS with conventional TBB resulted in a higher diagnostic yield than TBB alone. Even though the lesion size was smaller in the R‐EBUS‐TBB group, the diagnostic yield was higher. Ultrasonographic guidance allowed the confirmation of the exact location of the lesion, thereby enabling the precise performance of biopsy. Furthermore, the acquisition of larger specimens was possible when TBB was combined with R‐EBUS.
There are several benefits and disadvantages of TBB under fluoroscopic guidance. Real‐time imaging with fluoroscopy enables visual feedback to the operator, allowing precise guidance during biopsy. However, some studies have reported that fluoroscopy does not improve specimen acquisition because it only provides two‐dimensional images. In addition, there are concerns regarding radiation exposure to the operator.18, 19
Due to the limitations of conventional bronchoscopy, efficacy of newly developed techniques of bronchoscopic biopsy, including R‐EBUS, electromagnetic navigation bronchoscopy, and thin bronchoscopy, has been reported. The overall diagnostic yield is reported to be 53.0%–82.4% using R‐EBUS. Some studies have reported increased diagnostic rates of up to 84.4% when ENB and R‐EBUS were combined.18, 20 A combination of different bronchoscopic modalities is expected to increase the diagnostic yield. However, few studies have evaluated an increase in the diagnostic yield with R‐EBUS combined with standard TBB.
Recently, a multicenter prospective study compared the diagnostic yield of standard TBB with that of R‐EBUS‐TBB21 and reported poor diagnostic yield despite combining R‐EBUS with standard TBB. This result may be explained by a high proportion of benign diseases among this study cohort. According to a recent study by Kim et al. there was no significant difference between combined use of R‐EBUS and TBB and TBB alone in the diagnosis of benign lung disease.22 It is difficult to determine the precise location of benign inflammatory lesions compared with that of cancer lesions usually accompanied by solid or subsolid nodules, which are typically clearly visualized on EBUS images. Thus, the presence of benign lung diseases among a study cohort may mask the benefits of R‐EBUS in identifying lung cancer. In the present study, we only enrolled patients with a high level of suspicion for lung cancer in CT images. Therefore, the proportion of patients with benign diseases was lower than that in the previous study, which may have contributed to a higher diagnostic yield with the concomitant use of R‐EBUS.
The fellowship trainees who performed the procedure in our study had a minimum experience of 5–20 months in the pulmonology department and had performed at least 100 fluoroscopy‐guided TBBs. Although the bronchoscopy skills of these operators were adequate to perform fluoroscopy‐guided TBB, they were less experienced with R‐EBUS.23 Because an understanding of the detailed three‐dimensional anatomical structure is essential, pathologic biopsy with R‐EBUS is a procedure that is challenging to master. A previous study had reported that over three years of experience or performance of 400 procedures is required to be able to achieve consistent success with R‐EBUS‐guided TBB.24 This means that even if the procedures are conducted under the instruction of a supervisor, the fellowship trainees may experience considerable difficulty in the process. However, our results showed a marked increase in the diagnostic yield with the combined use of R‐EBUS and fluoroscopic biopsy even though the examiners had not fully mastered their skills. This relative success may be attributed to the availability of guidance using fluoroscopy, which helped minimize mislocation of the radial probe by allowing visualization of the target anatomic location. Hence, in a training institution like our hospital, we expect that the combined use of the two modalities will have educational benefits as well.
The overall complication rate in the present study was similar to the previously reported complication rate of TBB25 and slightly higher than that reported for R‐EBUS.26 Although R‐EBUS was newly introduced in our department, the incidence of complications was not higher than that with TBB alone. We first identified the approximate location of the lesion under fluoroscopy, followed by precise positioning with the radial ultrasound probe, which resulted in fewer complications. Clear visualization of the bronchial and vascular structures was possible using R‐EBUS, thereby reducing the incidence of complications such as bleeding. Furthermore, we found that the air‐bronchus sign associated with PPLs was a significant predictive factor of a higher diagnostic yield, as reported in a previous meta‐analysis on R‐EBUS.27 If the lesion is located adjacent to the bronchus, it may be more precisely confirmed by ultrasound and contribute to a higher diagnostic yield. This combined use of fluoroscopy and the radial ultrasound probe may have contributed to the lower complication rate and higher diagnostic yield observed in the present study. Although some studies have revealed higher complication rates during the first trimester of the introduction of new techniques, our complication rate was not higher than that reported in previous studies.28
The overall procedure time in both groups was longer than that reported in a previous study.29 This was because most patients underwent EBUS‐guided transbronchial needle aspiration if mediastinal lymph nodes were noted, adding to the procedural time. However, it is noteworthy that the difference in the procedural time was not statistically significant between the groups. If the operator is skillful in TBB, a combined technique, including R‐EBUS, does not significantly prolong the duration of the procedure. This may also be a reason for the similar complication rates observed between the two groups in this study. Our findings are similar to those of a previous study by Curull et al. which reported that R‐EBUS under fluoroscopy was safe and did not increase the total duration of the procedure.30
To the best of our knowledge, the present study is the first to evaluate the impact of the introduction of R‐EBUS on the diagnostic yield in a hospital where TBB has already been implemented. We believe that this is an important finding as it offers an alternative technique that can overcome the limitations of TBB, which has a low diagnostic yield. Operators who were unfamiliar with R‐EBUS could also improve the diagnostic yield when they used it in combination with TBB.
The retrospective study design and small sample size constitute the limitations of our study. Furthermore, the lack of objective assessment data regarding the bronchoscopy skills of individual operators may be a limitation. Therefore, future prospective studies with larger sample sizes are required to determine the impact of operator skills on the diagnostic yield of the R‐EBUS‐TBB combination technique.
In conclusion, the combination of R‐EBUS and TBB resulted in a higher diagnostic yield than R‐EBUS or TBB alone. In particular, a tissue diagnosis was more likely in pulmonary lesions with the air‐bronchus sign. In institutions where fluoroscopic TBB has already been implemented, the introduction of the combination technique with R‐EBUS may increase the diagnostic yield of PPLs.
Disclosure
The authors have no potential conflicts of interest to disclose.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2018R1C1B5043991).
Contributor Information
Sang Hoon Lee, Email: cloud9@yuhs.ac.
Chang Hoon Han*, Email: chang122@nhimc.or.kr.
References
- 1. Cheng T‐YD, Cramb SM, Baade PD, Youlden DR, Nwogu C, Reid ME. The international epidemiology of lung cancer: Latest trends, disparities, and tumor characteristics. J Thorac Oncol 2016; 11 (10): 1653–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferlay J, Colombet M, Soerjomataram I et al Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019; 144 (8): 1941–53. [DOI] [PubMed] [Google Scholar]
- 3. Didkowska J, Wojciechowska U, Mańczuk M, Łobaszewski J. Lung cancer epidemiology: Contemporary and future challenges worldwide. Ann Transl Med 2016; 4 (8): 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gould MK, Tang T, Liu I‐LA et al Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med 2015; 192 (10): 1208–14. [DOI] [PubMed] [Google Scholar]
- 5. Ettinger DS, Aisner DL, Wood DE et al NCCN guidelines insights: Non–small cell lung cancer, version 5.2018. J Natl Compr Canc Netw 2018; 16 (7): 807–21. [DOI] [PubMed] [Google Scholar]
- 6. Radke JR, Conway WA, Eyler WR, Kvale PA. Diagnostic accuracy in peripheral lung lesions: Factors predicting success with flexible fiberoptic bronchoscopy. Chest 1979; 76 (2): 176–9. [DOI] [PubMed] [Google Scholar]
- 7. Nagai Y, Kouno Y. The Role of X‐Ray Fluoroscopy in Bronchoscopy In: Izumo T, Sasada S, Aso T, Nasu K, Arai Y. (eds). Respiratory Endoscopy. Springer, Singapore: 2017; 3–8. [Google Scholar]
- 8. Baaklini WA, Reinoso MA, Gorin AB, Sharafkaneh A, Manian P. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000; 117 (4): 1049–54. [DOI] [PubMed] [Google Scholar]
- 9. Eberhardt R, Anantham D, Ernst A, Feller‐Kopman D, Herth F. Multimodality bronchoscopic diagnosis of peripheral lung lesions: A randomized controlled trial. Am J Respir Crit Care Med 2007; 176 (1): 36–41. [DOI] [PubMed] [Google Scholar]
- 10. Shinagawa N, Nakano K, Asahina H et al Endobronchial ultrasonography with a guide sheath in the diagnosis of benign peripheral diseases. Ann Thorac Surg 2012; 93 (3): 951–7. [DOI] [PubMed] [Google Scholar]
- 11. Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2013; 143 (5 Suppl): e142S–e65S. [DOI] [PubMed] [Google Scholar]
- 12. Hsia DW, Jensen KW, Curran‐Everett D, Musani AI. Diagnosis of lung nodules with peripheral/radial endobronchial ultrasound‐guided transbronchial biopsy. J Bronchol Interv Pulmonol 2012; 19 (1): 5–11. [DOI] [PubMed] [Google Scholar]
- 13. Ali MS, Trick W, Mba BI, Mohananey D, Sethi J, Musani AI. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: A systematic review and meta‐analysis. Respirology 2017; 22 (3): 443–53. [DOI] [PubMed] [Google Scholar]
- 14. Chen C‐H, Liao W‐C, Wu B‐R et al Endobronchial ultrasound changed the world of lung cancer patients: A 11‐year institutional experience. PLOS One 2015; 10 (11): e0142336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kurimoto N, Miyazawa T, Okimasa S et al Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004; 126 (3): 959–65. [DOI] [PubMed] [Google Scholar]
- 16. Steinfort DP, Khor YH, Manser RL, Irving LB. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: Systematic review and meta‐analysis. Eur Respir J 2011; 37 (4): 902–10. [DOI] [PubMed] [Google Scholar]
- 17. Chavez C, Sasada S, Izumo T. et al. Endobronchial ultrasound with a guide sheath for small malignant pulmonary nodules: A retrospective comparison between central and peripheral locations. J Thorac Dis 2015; 7 (4): 596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshikawa M, Sukoh N, Yamazaki K et al Diagnostic value of endobronchial ultrasonography with a guide sheath for peripheral pulmonary lesions without X‐ray fluoroscopy. Chest 2007; 131 (6): 1788–93. [DOI] [PubMed] [Google Scholar]
- 19. Steinfort DP, Einsiedel P, Irving LB. Radiation dose to patients and clinicians during fluoroscopically‐guided biopsy of peripheral pulmonary lesions. Respir Care 2010; 55 (11): 1469–74. [PubMed] [Google Scholar]
- 20. Memoli JSW, Nietert PJ, Silvestri GA. Meta‐analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012; 142 (2): 385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tanner NT, Yarmus L, Chen A. et al. Standard bronchoscopy with fluoroscopy vs thin bronchoscopy and radial endobronchial ultrasound for biopsy of pulmonary lesions: A multicenter, prospective, randomized trial. Chest 2018; 154 (5): 1035–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim EJ, Kim KC. Utility of radial probe endobronchial ultrasound‐guided transbronchial lung biopsy in diffuse lung lesions. Tuberc Respir Dis 2019; 82 (3): 201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wahidi MM, Silvestri GA, Coakley RD et al A prospective multicenter study of competency metrics and educational interventions in the learning of bronchoscopy among new pulmonary fellows. Chest 2010; 137 (5): 1040–9. [DOI] [PubMed] [Google Scholar]
- 24. Huang CT, Ruan SY, Tsai YJ, Ho CC, Yu CJ. Experience improves the performance of endobronchial ultrasound‐guided transbronchial biopsy for peripheral pulmonary lesions: A learning curve at a medical Centre. PLOS One 2017; 12 (6): e0179719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Milman N, Faurschou P, Munch E, Grode G. Transbronchial lung biopsy through the fibre optic bronchoscope. Results and complications in 452 examinations. Respir Med 1994; 88 (10): 749–53. [DOI] [PubMed] [Google Scholar]
- 26. Hayama M, Izumo T, Matsumoto Y, Chavez C, Tsuchida T, Sasada S. Complications with endobronchial ultrasound with a guide sheath for the diagnosis of peripheral pulmonary lesions. Respiration 2015; 90 (2): 129–35. [DOI] [PubMed] [Google Scholar]
- 27. Ali MS, Sethi J, Taneja A, Musani A, Maldonado F. Computed tomography bronchus sign and the diagnostic yield of guided bronchoscopy for peripheral pulmonary lesions. A systematic review and meta‐analysis. Ann Am Thorac Soc 2018; 15 (8): 978–87. [DOI] [PubMed] [Google Scholar]
- 28. Ouellette DR. The safety of bronchoscopy in a pulmonary fellowship program. Chest 2006; 130 (4): 1185–90. [DOI] [PubMed] [Google Scholar]
- 29. Eom JS, Mok JH, Kim I et al Radial probe endobronchial ultrasound using a guide sheath for peripheral lung lesions in beginners. BMC Pulm Med 2018; 18 (1): 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sánchez‐Font A, Giralt L, Vollmer I, Pijuan L, Gea J, Curull V. Endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions. A controlled study with fluoroscopy. Arch Bronco (English Edition) 2014; 50 (5): 166–171. [DOI] [PubMed] [Google Scholar]


