Abstract
Background
We performed a systematic review and meta‐analysis to synthesize the available evidence regarding short‐term outcomes between minimally invasive esophagectomy (MIE) and open esophagectomy (OE).
Methods
Studies were identified by searching databases including PubMed, EMBASE, Web of Science and Cochrane Library up to March 2019 without language restrictions. Results of these searches were filtered according to a set of eligibility criteria and analyzed in line with PRISMA guidelines.
Results
There were 33 studies included with a total of 13 269 patients in our review, out of which 4948 cases were of MIE and 8321 cases were of OE. The pooled results suggested that MIE had a better outcome regarding all‐cause respiratory complications (RCs) (OR = 0.56, 95% CI = 0.41–0.78, P = <0.001), in‐hospital duration (SMD = −0.51; 95% CI = −0.78−0.24; P = <0.001), and blood loss (SMD = −1.44; 95% CI = −1.95−0.93; P = <0.001). OE was associated with shorter duration of operation time, while no statistically significant differences were observed regarding other outcomes. Additionally, subgroup analyses were performed for a number of different postoperative events.
Conclusions
Our study indicated that MIE had more favorable outcomes than OE from the perspective of short‐term outcomes. Further large‐scale, multicenter randomized control trials are needed to explore the long‐term survival outcomes after MIE versus OE.
Keywords: Esophageal cancer, esophagectomy, minimally‐invasive surgery
Introduction
Esophageal cancer is the seventh most common cause of cancer‐related death globally.1 The overall five‐year survival is below 20%.2, 3 The main course of treatment is surgical resection, which is usually combined with chemotherapy or chemo‐radiotherapy for locally advanced tumors.4 Conventional surgical treatment involves open esophagectomy (OE) using transthoracic or transhiatal approaches which are associated with high morbidity and mortality. Respiratory complications (RCs) are common with OE and can increase the risk of death up to 20%.5, 6, 7 In recent decades, minimally invasive esophagectomy (MIE) has become an alternative to OE. MIE encompasses a number of techniques including total MIE (tMIE), hybrid minimally invasive esophagectomy (hMIE) and robotic surgery.8
Given the technical complexity of MIE, a number of concerns exist regarding the benefits of MIE compared with OE in terms of postoperative complications and short‐term mortality. On one hand, even though a number of previously performed studies have established MIE as a relatively safe procedure in terms of post‐operative outcomes,9, 10, 11, 12, 13 on the other, studies performed by Seesing et al. and Mariette et al. state the opposite.14, 15
With a number of emerging studies regarding MIE and OE in recent years, there has been a lack of a systematic study to investigate the short‐term outcomes after MIE versus OE. Furthermore, a detailed and updated meta‐analysis concerning the two approaches might help surgeons with their surgical decisions. Ergo, the purpose of this systematic review and meta‐analysis was not only to use the latest and largest population‐based data to extensively compare and summarize the postoperative complications after MIE versus OE for esophageal cancer, but also to clarify whether MIE could improve the post‐operative outcomes and overall survival of patients with esophageal cancer.
Methods
Literature search strategy
This study was performed according to Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) guidelines. Literature was identified by searching databases including PubMed, EMBASE, Web of Science and Cochrane Library up to June 2019 without language restrictions. The search terms used for literature identifications include “esophageal carcinoma, esophageal cancer, esophagectomy, minimally invasive esophagectomy, open esophagectomy and thoracoscopic laproscopic esophagectomy”.
Eligibility criteria for literature selection
Literature included in the study had to meet the following criteria: (i) studies comparing MIE with OE; (ii) studies published in English only; (iii) studies including at least 20 or more patients; (iv) studies with assigned NOS (Newcastle‐Ottawa quality assessment scale) score of seven or higher; (iv) prospective, randomized controlled trials or retrospective studies only; and (v) studies where full text was available.
Data extraction and quality assessment
Literature included in the study was independently assessed for methodological quality purposes (N.A and D.D). First, the titles and abstracts were screened to assess the eligibility of included literature, and then the full text was reviewed. Any discrepancies were resolved in discussion with a third author (C.D). The information recorded for each study is given in Table 1.
Table 1.
Detailed characteristics of included studies
| ASA classification | TNM staging | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Authors (year) | Country or Region | Study design | Intervention | No. of cases | Sex ratio (M/F) | Median age, years (IQ range) mean ± SD | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | Pathology (adeno/squam/others) | Neoadjuvant therapy (chemo‐radio /chemo) | NOS score |
| 1 |
Mariette et al. (2019)15 |
France |
RCT |
hMIE OE |
103 104 |
88/15 87/17 |
59 (23–75) 62 (41–78) |
25 34 |
61 58 |
17 12 |
0 0 |
NA NA |
18 19 |
30 33 |
50 48 |
NA§ NA§ |
57/46/0 66/38/0 |
36/41 30/45 |
9 |
|
2 |
Straatman et al. (2017)16 |
The Netherlands |
RCT |
MIE OE |
59 56 |
43/16 46/10 |
61.8 ± 8.4 62.3 ± 8.4 |
10 15 |
34 32 |
14 08 |
01 01 |
0 1 |
4 4 |
26 22 |
5 4 |
NA NA |
35/24/0 36/19/1 |
52/4 54/5 |
9 |
|
3 |
Kinjo et al. (2011)17 |
Japan |
Retrospective |
TLE TE OE |
72 34 79 |
58/14 29/5 70/9 |
62.7 ± 7.4 64.2 ± 8.8 63.3 ± 8.6 |
35 15 36 |
3 19 41 |
0 0 02 |
NA NA NA |
NA NA NA |
21 11 18 |
26 7 27 |
16 9 20 |
9 7 14 |
0/71/1 3/31/0 3/71/5 |
NA NA NA |
8 |
|
4 |
Sarkaria et al. (2018)18 |
USA |
Prospective |
rMIE OE |
64 106 |
53/11 91/15 |
61 (45–82) 63 (28–83) |
NA NA |
09 15 |
51 84 |
04 07 |
13 20 |
22 25 |
15 33 |
14 27 |
NA NA |
59/4/0 98/7/1 |
47/1 85/2 |
9 |
|
5 |
Safranek et al. (2010)19 |
UK |
Prospective |
tMIE hMIE OE |
41 34 46 |
25/16 28/6 38/8 |
64 (41–74) 63 (44–76) 60 (44–77) |
NA NA NA |
NA NA NA |
NA NA NA |
NA NA NA |
2 2 0 |
7 2 6 |
17 14 11 |
15 16 29 |
NA NA NA |
23/17/1 29/3/2 43/3/0 |
0/34 0/27 0/34 |
8 |
|
6 |
Paireder et al. (2018)20 |
Austria |
RCT |
MIE OE |
14 12 |
10/4 10/2 |
64.5 (40–75) 62.5 (49–77) |
NA NA |
NA NA |
NA NA |
NA NA |
1 2 |
4 4 |
2 2 |
6 3 |
1§ 1§ |
10/4/0 11/1/0 |
0/9 0/7 |
8 |
|
7 |
Sihag et al. (2016)21 |
USA |
Retrospective |
MIE OE |
814 2966 |
658/156 2492/474 |
63.3 ± 10.7 63.2 ± 10.2 |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
7 |
|
8 |
Schoppmann et al. (2010)22 |
Austria |
Prospective |
MIE OE |
31 31 |
25/6 21/10 |
61.5 (35.7–74.8) 58.6 (33.7–76.8) |
14 15 |
13 11 |
04 05 |
NA NA |
NA NA |
10 4 |
4 9 |
14 15 |
1 2 |
17/14/0 12/19/0 |
NA NA |
8 |
|
9 |
Klevebro et al. (2018)23 |
Sweden |
Prospective |
MIE OE |
201 165 |
162/39 132/33 |
67 (33–83) 65 (36–82) |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
19 28 |
13 34 |
119 93 |
32 5 |
153/41/7 120/42/3 |
125/20 55/59 |
8 |
|
10 |
Perry et al. (2009)24 |
USA |
Retrospective |
LE OE |
21 21 |
18/3 17/4 |
69 ± 8 61 ± 9 |
(1–2 = 13)† (1–2 = 13) |
(3–4 = 8)† (3–4 = 8) |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
7 |
||
|
11 |
Seesing et al. (2017)14 |
The Netherlands |
Retrospective |
MIE OE |
433 433 |
335/58 335/58 |
64 ± 9.0 64 ± 8.7 |
80 65 |
271 287 |
82 81 |
NA NA |
NA NA |
26 24 |
86 82 |
310 311 |
11§ 17§ |
305/128/0 311/122/0 |
375/21 376/21 |
9 |
|
12 |
Mass et al. (2013)25 |
The Netherlands |
RCT |
MIE OE |
14 13 |
10/4 12/1 |
65 (56–75) 62 (52–74) |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
13/1/0 11/2/0 |
NA NA |
8 |
|
13 |
Glatz et al. (2017)26 |
Germany |
Retrospective |
hMIE OE |
60 60 |
49/11 52/8 |
61 (42–92) 61 (44–84) |
(1–2 = 36)† (1–2 = 33) |
(3–4 = 24)† (3–4 = 27) |
(0–1 = 35)‡ (0–1 = 27)‡ |
9 14 |
14 14 |
1 5 |
46/14/0 47/13/0 |
12/35 12/38 |
9 |
|||
|
14 |
Tang et al. (2018)27 |
China |
Retrospective |
MIE (nCRT) MIE (nCT) OE (nCT) |
76 42 57 |
64/12 33/9 51/6 |
61 (44–790 61 (46–730 60 (41–73) |
24 13 19 |
48 27 36 |
04 02 02 |
0 0 0 |
NA NA NA |
NA NA NA |
NA NA NA |
47 28 37 |
29§ 14§ 20§ |
NA NA NA |
NA NA NA |
7 |
|
15 |
Lee at al. (2011)28 |
Taiwan |
Prospective |
tMIE hMIE OE |
30 44 64 |
30/0 43/1 61/3 |
59.7 ± 10.32 59.7 ± 11.17 56.5 ± 11.60 |
NA NA NA |
NA NA NA |
NA NA NA |
NA NA NA |
2 12 7 |
3 13 17 |
11 14 25 |
12 5 14 |
2 1 1 |
1/29/0 1/43/0 5/59/0 |
NA NA NA |
7 |
|
16 |
Bonavina et al. (2016)29 |
Italy |
Retrospective |
TE OE |
80 80 |
46/34 71/9 |
61.5 (53–70) 63.5 (55–68) |
15 21 |
56 47 |
09 12 |
0 0 |
NA NA |
25 15 |
25 22 |
23 31 |
7 12 |
9/68/3 63/15/2 |
31¶ 17¶ |
8 |
|
17 |
Hamouda et al. (2009)30 |
UK |
Prospective |
LE OE |
26 24 |
25/1 23/1 |
62 60 |
NA NA |
NA NA |
NA NA |
NA NA |
1 0 |
0 1 |
4 1 |
19 18 |
2§ 3§ |
21/4/1 21/3/0 |
NA NA |
7 |
|
18 |
Kauppi et al. (2014)31 |
Finland |
Prospective |
MIE OE |
74 79 |
59/15 68/11 |
66 (51–85) 63 (39–82) |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
28 25 |
44 54 |
0 0 |
NA NA |
3/55 12/59 |
7 |
|
19 |
Guo et al. (2013)32 |
China |
RCT |
TE OE |
111 110 |
68/43 72/38 |
57.3 ± 11.8 60.8 ± 12.4 |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
24 31 |
80 74 |
7 5 |
NA§ NA§ |
NA NA |
NA NA |
7 |
|
20 |
Sihvo et al.33 |
Finland |
Retrospective |
MIE OE |
150 150 |
119/31 119/31 |
63.9 (9.2) 64.3 (8.9) |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
122/27/10 263/138/30 |
26/73 31/61 |
8 |
|
21 |
Pham et al. (2010)34 |
USA |
Retrospective |
TLE OE |
44 46 |
41/3 33/13 |
63 ± 8.6 61 ± 10.7 |
(1–2 = 12)† (1–2 = 17) |
(3–4 = 32)† (3–4 = 29) |
0 0 |
6 7 |
14 13 |
18 18 |
2 1 |
34/8/0 34/6/2 |
NA NA |
8 |
||
|
22 |
Scarpa et al. (2015)35 |
Italy |
Retrospective |
hMIE OE |
34 34 |
27/7 6/25 |
62 (52–70) 64 (56–70) |
5 4 |
22 17 |
07 13 |
NA NA |
(0–1‐2 = 29) (0–1‐2 = 29) |
(3–4 = 5)‡ (3–4 = 5)‡ |
24/10/0 24/10/0 |
22¶ 22¶ |
8 |
|||
|
23 |
Biere et al. (2012)36 |
The Netherlands |
RCT |
MIE OE |
59 56 |
43/16 46/10 |
62 (34–75) 62 (42–75) |
10 15 |
34 32 |
14 08 |
01 01 |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
24/35/0 36/19/1 |
54/5 52/4 |
9 |
|
24 |
Parameswaran et al. (2013)37 |
UK |
Prospective |
tMIE LE OE |
36 31 19 |
24/12 13/8 15/4 |
64 (45–84) 67 (48–79) 64 (51–77) |
NA NA NA |
NA NA NA |
NA NA NA |
NA NA NA |
6 1 0 |
6 5 0 |
13 12 8 |
10 13 11 |
0 0 0 |
22/8/5 27/3/0 16/3/0 |
23¶ 27¶ 17¶ |
8 |
|
25 |
Noble et al. (2012)38 |
UK |
Prospective |
MIE OE |
53 53 |
43/10 45/8 |
66 (45–85) 64 (36–81) |
4 10 |
44 32 |
05 11 |
NA NA |
2 0 |
0 1 |
9 15 |
42 33 |
0§ 4§ |
47/4/1 48/3/0 |
1/12 2/9 |
9 |
|
26 |
Burdall et al. (2014)39 |
UK |
Retrospective |
LE MIE OE |
184 67 83 |
151/33 48/19 67/16 |
64.8 (39–79) 65.4 (36–79) 63.9 (43–77) |
NA NA NA |
NA NA NA |
NA NA NA |
NA NA NA |
6 3 1 |
32 37 12 |
25 9 10 |
119 18 60 |
2§ 0§ 0§ |
167/14/3 53/7/0 74/8/1 |
0/158 0/23 0/76 |
8 |
|
27 |
Dolan et al. (2013)40 |
USA |
Retrospective |
MIE OE |
82 64 |
65/17 55/9 |
67 (60–76) 69 (63–75) |
1 0 |
28 16 |
47 35 |
03 04 |
NA NA |
NA NA |
31 23 |
48 33 |
NA NA |
NA NA |
74¶ 39¶ |
8 |
|
28 |
Hsu et al. (2013)41 |
Taiwan |
Retrospective |
TE OE |
66 63 |
61/5 58/5 |
58.8 ± 10.4 60 ± 11.3 |
NA NA |
NA NA |
NA NA |
NA NA |
NA NA |
24 15 |
14 12 |
25 33 |
3§ 3§ |
NA NA |
0/10 0/14 |
7 |
|
29 |
Kanekiyo et al. (2017)42 |
Japan |
Retrospective |
TE OE |
65 65 |
56/9 58/7 |
66 (62–70) 66 (61–70) |
16 14 |
45 47 |
04 04 |
0 0 |
(0–1 = 24) (0–1 = 24) |
(2–3‐4 = 41)‡ (2–3‐4 = 41)‡ |
NA NA |
0/37 0/35 |
8 |
|||
|
30 |
Rinieri et al. (2016)43 |
France |
Prospective |
MIE OE |
70 70 |
59/11 54/16 |
61.1 ± 9 61 ± 9 |
9 14 |
48 40 |
13 16 |
0 0 |
15 15 |
22 23 |
15 11 |
17 20 |
1 1 |
50/20/0 55/15/0 |
NA NA |
8 |
|
31 |
Thomson et al. (2010)44 |
Australia |
Prospective |
TE OE |
165 56 |
134/31 45/11 |
68 (36–84) 65 (42–82) |
(1–2 = 120)† (1–2 = 30) |
(3–4 = 45)† (3–4 = 26) |
NA NA |
51 5 |
46 16 |
68 35 |
NA NA |
128/37/0 48/8/0 |
NA NA |
8 |
||
|
32 |
Yerokun et al. (2016)45 |
USA |
Retrospective |
MIE rMIE OE |
1077 231 2958 |
905/172 195/36 2474/484 |
57 (64–70) 57 (64–70) 57 (64–70) |
NA NA NA |
NA NA NA |
NA NA NA |
NA NA NA |
194 52 494 |
350 72 812 |
149 31 443 |
291 63 852 |
6§ 1§ 12§ |
861/216/0 186/45/0 2305/653/0 |
643/0 157/0 800/0 |
8 |
|
33 |
Zingg et al. (2009)46 |
Australia |
Prospective |
MIE OE |
56 98 |
45/11 71/27 |
66.3 (1.3) 67.8 (1.1) |
NA NA |
NA NA |
NA NA |
NA NA |
15 14 |
9 15 |
21 33 |
11 27 |
NA NA |
46/10/0 65/29/4 |
40¶ 48¶ |
8 |
adeno; adenocarcinoma; ASA, American Society of Anesthesiologists; chemo, chemotherapy; chem‐radio, chemo‐radiotherapy; hMIE, Hybrid minimally invasive esophagectomy; IQ, interquartile; LE, laparoscopic‐assisted esophagectomy; M/F, Male/Female; NA, not available; nCRT, neoadjuvant chemo‐radiotherapy; nCT, neoadjuvant chemotherapy; NOS, Newcastle‐Ottawa quality assessment scale; OE, open esophagectomy; RCT, randomized controlled trial; rMIE, robotic‐assisted minimally invasive esophagectomy; SD, standard deviation; squam; squamous cell carcinoma; TE, thoracoscopic esophagectomy; TLE, thoracoscopic laparoscopic esophagectomy; tMIE, total minimally invasive esophagectomy.
The ASA classification data for multiple stages is provided together.
The TNM staging data for multiple stages is provided together.
Mention of size or direct extension of primary tumor only.
Not specified whether the neoadjuvant therapy was chemo‐radiotherapy or chemotherapy.
Definition of study endpoints
In total, we discussed 11 endpoints in our study: one primary and 10 secondary endpoints. All‐cause respiratory complications (RCs) were chosen to be discussed as the primary endpoint. These RCs included atelectasis, pneumonia, acute respiratory distress syndrome (ARDS), pleural effusion, pneumothorax and respiratory insufficiency. The details of 10 secondary endpoints are given below. All‐cause cardiac complications (CCs) which included cardiac arrest, myocardial infarction, atrial & ventricular dysrhythmia, congestive heart failure and pericarditis; all‐cause anastomotic leakage (AL) defined as full thickness GI defect involving esophagus, anastomosis, staple line, or conduit irrespective of presentation or method of identification; total length of in‐hospital stay; total operation time; total blood loss; R0 resection; 30‐day mortality; 90‐day mortality; all‐cause in‐hospital mortality; and reoperation rate.
Statistical analysis
SPSS software was used for general data analysis. Data was extracted and entered into review manager. Continuous variables were expressed as median and interquartile ratio or range, and the mean and SD were estimated from the available data. The Mantel‐Haenszel method for dichotomous data was used. Fixed or random‐effects models were used in this study. Forest plots were provided to illustrate pooled odds ratios (ORs), and corresponding 95% confidence intervals (CIs). Cochran's Q test and Higgins I 2 were used to test the heterogeneity of different studies. A P‐value of less than 0.1 was considered significant. Heterogeneity was interpreted according to the thresholds outlined in the Cochrane Handbook. With significant heterogeneity, a pooled effect was calculated with a random‐effects model; otherwise, a fixed‐effects model was applied. The reasons for interstudy heterogeneity were explored by using subgroup analysis. We also conducted sensitivity analysis by omission of each single study to evaluate stability of the results. Publication bias was assessed by using funnel plots.
Results
Selection of eligible studies
The PRISMA flowchart diagram is shown in Figure 1. In summary, our literature search strategy initially identified 150 articles. Finally, 33 articles qualified to be included in our meta‐analysis study.
Figure 1.
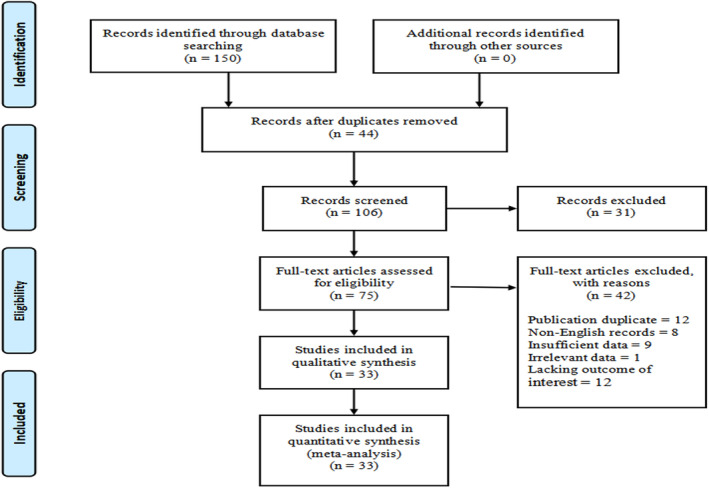
PRISMA flowchart of literature search strategy.
Characteristics of included literature
A total of 13 269 patients were included in this meta‐analysis study, out of which 4948 cases were of MIE and 8321 cases were of OE. Table 1 provides detailed characteristics of the articles included. In summary, six studies had a RCT study design, 12 had a prospective study design and the remaining 15 had a retrospective study design.
Primary outcome: All‐cause RCs
A total of 24 studies14, 15, 16, 17, 18, 19, 20, 21, 22, 24, 25, 26, 27, 28, 29, 30, 32, 34, 35, 36, 37, 38, 42 with 7117 patients were involved in the analysis of all‐cause RCs. Figure 2a shows that the patients who underwent MIE experienced less postoperative RCs as compared to those who underwent OE (OR = 0.56; 95% CI = 0.41, 0.78; P = <0.001). Test of heterogeneity showed considerable heterogeneity (I 2 = 77% and P = <0.001). Subgroup analyses were conducted to explore potential sources of that heterogeneity (Table 2). The pooled ORs of most subgroups were not markedly changed by the study characteristics. However, the subgroup analysis by intervention type showed considerable significance for tMIE/OE (P = <0.001; I 2 = 91%) as compare to hMIE/OE (P = 0.07; I 2 = 35%) which was less significant. We also noted the changes in statistical heterogeneity in the subgroup analysis of different institutes and facilities (single center, I 2 = 64%; multicenter, I 2 = 83%), initial inclusion period (<2008, I 2 = 68%; ≥2008 I 2 = 88%), study design (RCT, I 2 = 74%; prospective, I 2 = 79%; retrospective, I 2 = 54%), and NOS score (7, I 2 = 56%; 8, I 2 = 74%; 9, I 2 = 87%). Sensitivity analysis was conducted by omission of each single study to evaluate the stability of results indicating an unaffected pooled OR. The funnel plots displaying the publication bias of all cause RCs is shown in Figure S2b.
Figure 2.
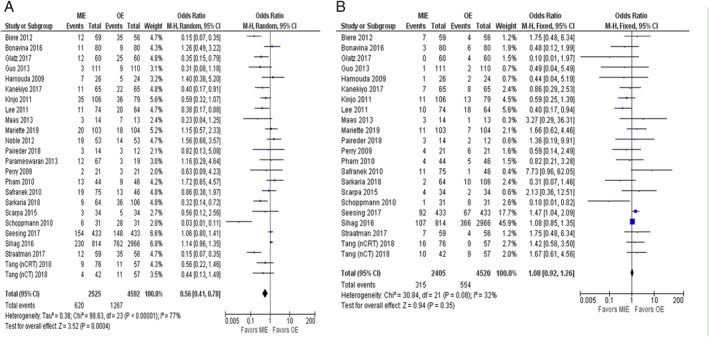
(a) Forest plot of all‐cause RCs. (b) Forest plot of all‐cause AL.
Table 2.
Subgroup analyses of all‐cause RCs of MIE and OE
| Test of association | Test of heterogeneity | |||||
|---|---|---|---|---|---|---|
| Variable | Studies | OR | 95% CI | P‐value | I2 (%) | P‐value |
| Total | 24 | 0.56 | 0.41–0.78 | <0.001 | 77 | <0.001 |
| Publication year | ||||||
| <2016 | 13 | 0.51 | 0.29–0.90 | <0.001 | 72 | 0.02 |
| ≥2016 | 11 | 0.61 | 0.42–0.90 | <0.001 | 77 | 0.01 |
| No. of cases | ||||||
| <100 | 9 | 0.52 | 0.22–1.24 | 0.001 | 69 | 0.014 |
| >100 | 15 | 0.57 | 0.40–0.81 | <0.001 | 80 | 0.002 |
| Research region | ||||||
| The Netherlands | 4 | 0.29 | 0.08–1.07 | <0.001 | 92 | 0.06 |
| The UK | 4 | 1.19 | 0.72–1.96 | 0.79 | 0.00 | 0.49 |
| The USA | 4 | 0.84 | 0.40–1.74 | 0.02 | 71 | 0.63 |
| China (Mainland) | 3 | 0.45 | 0.24–0.88 | 0.78 | 0.00 | 0.02 |
| Italy | 2 | 1.1 | 0.45–2.24 | 0.38 | 0.00 | 0.99 |
| Japan | 2 | 0.51 | 0.32–0.84 | 0.45 | 0.00 | 0.007 |
| Austria | 2 | 0.14 | 0.00–4.13 | 0.004 | 88 | 0.25 |
| Miscellaneous regions (Germany, France, Taiwan) | 3 | 0.55 | 0.25–1.21 | 0.05 | 67 | 0.14 |
| Institutes/facilities | ||||||
| Single center | 14 | 0.56 | 0.36–0.88 | 0.01 | 64 | <0.001 |
| Multicenter | 10 | 0.57 | 0.36–0.90 | 0.02 | 83 | <0.001 |
| Initial inclusion period | ||||||
| <2008 | 12 | 0.64 | 0.38–1.08 | <0.001 | 68 | 0.09 |
| ≥2008 | 12 | 0.50 | 0.32–0.77 | <0.001 | 82 | 0.002 |
| Study design | ||||||
| RCT | 6 | 0.33 | 0.14–0.79 | 0.001 | 74 | 0.01 |
| Prospective | 7 | 0.52 | 0.23–1.20 | <0.001 | 79 | 0.13 |
| Retrospective | 11 | 0.79 | 0.59–1.05 | 0.02 | 54 | 0.11 |
| Intervention | ||||||
| tMIE/OE | 7 | 0.33 | 0.16–0.68 | <0.001 | 91 | 0.002 |
| hMIE/OE | 17 | 0.68 | 0.51–0.90 | 0.07 | 35 | 0.008 |
| Neoadjuvant therapy | ||||||
| With | 13 | 0.59 | 0.37–0.92 | <0.001 | 76 | 0.02 |
| Without | 11 | 0.52 | 0.30–0.90 | <0.001 | 77 | 0.02 |
| NOS score | ||||||
| 7 | 7 | 0.66 | 0.39–1.11 | 0.03 | 56 | 0.12 |
| 8 | 10 | 0.64 | 0.39–1.06 | <0.001 | 74 | 0.08 |
| 9 | 7 | 0.48 | 0.24–0.97 | <0.001 | 87 | 0.04 |
CI, confidence interval; hMIE, hybrid minimally invasive esophagectomy; NOS, Newcastle‐Ottawa quality assessment scale; OR; odds ratio; RCT, randomized controlled trial; tMIE, total minimally invasive esophagectomy.
Secondary endpoints
A total of 22 studies14, 15, 16, 17, 18, 19, 20, 21, 22, 24, 25, 26, 27, 28, 29, 30, 32, 34, 35, 36, 38, 42 with 6925 patients were included in the analysis of all‐cause AL, which showed low level of heterogeneity (P = 0.08, I 2 = 32%) and no statistical difference between MIE versus OE (OR = 1.08; 95% CI = 0.92, 1.26; P = 0.35) (Figs 2b, S2c). Data for all‐cause CCs was reported in 13 studies13, 14, 18, 24, 26, 27, 29, 30, 31, 35, 38, 42 with 2302 patients and showed neither heterogeneity (P = 0.99, I 2 = 0%), nor statistically significant difference between MIE or OE (OR = 0.97; 95% CI = 0.74, 1.26; P = 0.81) (Figs 3a, S2d).
Figure 3.
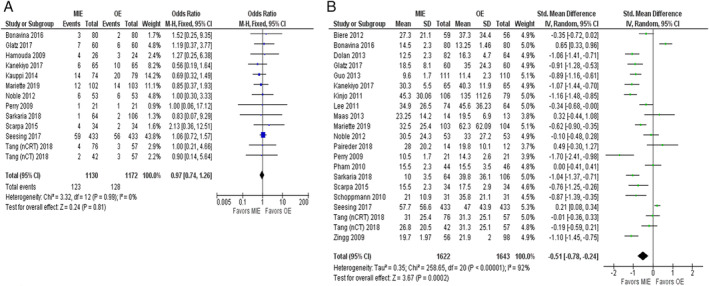
(a) Forest plot of all‐cause CCs. (b) Forest plot of in‐hospital stay.
Evaluation of data for total length of in‐hospital stay from 21 studies14, 15, 17, 18, 20, 22, 24, 25, 26, 27, 28, 29, 32, 34, 35, 36, 38, 40, 42, 46 with 3265 patients showed that patients who underwent MIE got to experience less in‐hospital duration compared with those who underwent OE (SMD = −0.51; 95% CI = −0.78, −0.24; P = <0.001) (Fig. 3b). Substantial heterogeneity (P = <0.001, I 2 = 92%) was found and subgroup analyses were performed to explore the potential source of heterogeneity as shown in Table S1. A total of 23 studies15, 16, 17, 18, 20, 22, 24, 25, 26, 27, 28, 29, 31, 32, 34, 35, 36, 38, 40, 41, 42, 46 with 2796 patients included in analyzing the data for total operation time showed that the patients who underwent MIE experienced longer operation time compared to those who underwent OE (SMD = 0.52; 95% CI = 0.16, 0.89; P = 0.005) (Fig. 4a and Table S2).
Figure 4.
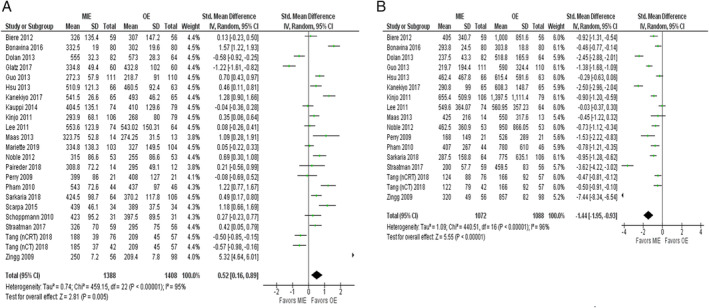
(a) Forest plot of total operation time. (b) Forest plot of blood loss.
Data for total blood loss gathered from 17 studies16, 17, 18, 24, 25, 27, 28, 29, 32, 34, 36, 38, 40, 41, 42, 46 with 2160 patients revealed that MIE resulted in less blood loss in comparison with OE (SMD = −1.44; 95% CI = −1.95, −0.93; P = <0.001) (Fig. 4b). The outcome also indicated the presence of substantial heterogeneity (P = <0.001, I 2 = 96%) which led us to perform subgroup analyses to analyze the source of heterogeneity (Table S3). Other outcomes such as R0 resection (OR = 1.47; 95% CI =1.13, 1.92; P = 0.004), 30‐day mortality (OR = 0.92; 95% CI = 0.69, 1.22; P = 0.56), 90‐day mortality (OR = 0.52; 95% CI = 0.29, 0.91; P = 0.02), in‐hospital mortality (OR = 0.73; 95% CI = 0.38, 1.41; P = 0.35), and the rate of reoperation (OR = 1.30; 95% CI = 0.85, 1.98; P = 0.22) showed no significant statistical differences between MIE and OE as shown in Figs S1a‐S1d, S2a.
Discussion
This study compared the outcomes of OE with both tMIE and hMIE. Due to the complexity of esophagectomy, different types of surgical approaches might lead to different kinds of surgical complications, but the main morbidities remain the same which include RCs, CCs, AL and the aforementioned.
Most of the meta‐analysis studies comparing the outcomes of MIE and OE previously performed were either based on retrospective studies only, or had a small sample size.11, 47, 48, 49 Although, Lv et al. had a relatively larger sample size of 6025 patients from 20 studies, their study only included literature up to 2016.12 Since then, a considerable number of updated studies have been published, showing new findings and discrepancies in their results.9, 13, 14, 15, 16, 18, 20, 23, 26, 27, 33, 42 In contrast, we included 33 studies in total involving 13 269 patients in our meta‐analysis to provide the latest and more robust outcomes comparing MIE and OE.
Postoperative RCs are of great importance and could impact the prognosis of patients, which are also the most frequent morbidity events after esophagectomy. Some previous studies have shown contradictory results regarding the advantages of MIE over OE with respect to postoperative RCs. Two retrospective studies showed no significant differences regarding RCs between two groups.13, 50 On the other hand, two RCTs showed a significantly lower incidence of respiratory complications after MIE than OE.36, 51 Pooled data from our study also showed that patients who underwent MIE experienced fewer postoperative RCs compared to those who underwent OE (Fig. 2a). The association of MIE with fewer postoperative RCs could be explained by the elegance of the MIE operation procedure which decreases surgical trauma to the chest wall and does less harm to pulmonary tissues.
The results from our study showed that MIE was associated with a longer operative time as compared to OE. These results were consistent with other recently published studies and could be attributed to the technical difficulty in MIE and a limited operating space for surgeons to perform the delicate procedure.16, 18, 42 Data analyses also demonstrated that patients who underwent MIE experienced shorter postoperative in‐hospital stay and had less in‐operative blood loss, as compared to those who underwent OE. Both these results were in accordance with previous studies and can be associated with the less intrusive nature of MIE.23, 26, 36
Notably, pooled results and subgroup analyses from our study showed no significant correlation between neoadjuvant therapy and improvement of postoperative outcomes, either after MIE or OE.
Principle findings and limitations
Our meta‐analysis provides strong evidence for the association of MIE with overall better short‐term outcomes (Table 3). When stratified by publication year, initial inclusion period, number of cases, types of surgical intervention, and NOS quality score, the results remained mostly constant. Meanwhile, the heterogeneity in subgroup analyses was shown to be not considerable in general. In addition, with the application of some advanced statistical methods, the results have demonstrated that the outcomes tend to be much more stable with the increasing number of studies over time.
Table 3.
Summary of the final results of all primary and secondary endpoints
| Endpoints | Studies | Cases | OR/SMD | 95%CI | P‐value | I2 | P‐value | Favors |
|---|---|---|---|---|---|---|---|---|
| All‐cause RCs | 24 | 7117 | 0.56 | 0.41, 0.78 | <0.001 | 77% | <0.001 | MIE |
| All‐cause AL | 22 | 6925 | 1.08 | 0.92, 1.26 | 0.35 | 32% | 0.08 | None |
| All‐cause CCs | 13 | 2302 | 0.97 | 0.74, 1.26 | 0.81 | 0% | 0.99 | None |
| In‐hospital stay | 21 | 3265 | −0.51 | −0.78, −0.24 | <0.001 | 96% | <0.001 | MIE |
| Total operation time | 23 | 2796 | 0.52 | 0.16, 0.89 | 0.005 | 95% | <0.001 | OE |
| Blood loss | 17 | 2160 | −1.44 | −1.95, −0.93 | <0.001 | 96% | <0.001 | MIE |
| R0 resection | 13 | 2938 | 1.47 | 1.13, 1.92 | 0.004 | 0% | 0.56 | None |
| 30‐day mortality | 12 | 7976 | 0.92 | 0.69, 1.22 | 0.56 | 0% | 0.95 | None |
| 90‐day mortality | 6 | 1095 | 0.52 | 0.29, 0.91 | 0.02 | 0% | 0.91 | None |
| In‐hospital mortality | 8 | 846 | 0.73 | 0.38, 1.41 | 0.35 | 0% | 0.71 | None |
| Reoperation | 10 | 4767 | 1.30 | 0.85, 1.98 | 0.22 | 33% | 0.14 | None |
AL, anastomotic leakage; CCs, cardiac complications; CI, confidence interval; MIE; minimally invasive esophagectomy; OE, open esophagectomy; OR; odds ratio; RCs, respiratory complications; SMD, standardized mean difference.
There are several limitations to our study that should also be acknowledged. First, as shown in Table 1, the pathological TNM staging and ASA classification is missing from several included studies, which resulted in undeniable differences in their quality and strength. Second, patients of different ethnical groups were placed together into MIE or OE groups, which would also have effects on the results of this study. Third, different MIE methods (tMIE or hMIE) were used in different included studies, which makes it difficult to more specifically point out if there was any particular MIE technique that was the most beneficial for better outcomes. Fourth, there is also a possibility that patients with beneficial prognostic variables, for example, younger age and less comorbidity, were more readily selected for MIE rather than OE. Finally, even though our study included several RCTs, the lack of larger number of multi‐institutional RCTs might reduce the effectiveness of the research. Consequently, the work definitely needs to be improved when there are more RCTs. Although advanced statistical methods were applied, publication bias was inevitable as shown in Fig. S2.
In conclusion, while OE was associated with shorter operation time and a slightly better surgical clearance of the tumor (R0 resection rates) compared with MIE, MIE was associated with fewer RCs, lesser blood loss, shorter postoperative in‐hospital stay and better overall postoperative outcomes. Further large‐scale, multicenter RCTs are needed to continue to explore further long‐term survival outcomes of patients with MIE and OE.
Disclosure
The authors report that there are no conflicts of interest.
Supporting information
Figure S1. (a) Forest plot of R0 resection; (b) forest plot of 30‐day mortality; (c) forest plot of 90‐day mortality; and (d) forest plot of in‐hospital mortality.
Figure S2. (a) Forest plot of reoperation; (b) funnel plot of all‐cause RCs; (c) funnel plot of all‐cause AL; and (d) funnel plot of all‐cause CCs.
Table S1. Subgroup analysis of in‐hospital stay between MIE and OE.
Table S2. Subgroup analysis of total operation time between MIE and OE.
Table S3. Subgroup analysis of blood loss between MIE and OE.
Acknowledgments
This study was supported by the projects from Shanghai Hospital Development Center (SHDC12015116), the National Natural Science Foundation of China (81802256), Science and Technology Commission of Shanghai Municipality (15 411 968 400 and 14 411 962 600), Suzhou Key Laboratory of Thoracic Oncology (SZS201907), Suzhou Key Discipline for Medicine (SZXK201803), the Science and Technology Research Foundation of Suzhou Municipality (SYS2018063, SYS2018064) and Municipal Program of People's Livelihood Science and Technology in Suzhou (SS2019061).
References
- 1. Fitzmaurice C, Akinyemiju TF, Al Lami FH et al Global burden of disease cancer collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability‐adjusted life years for 29 cancer groups, 1990 to 2016: A systematic analysis for the global burden of disease study. JAMA Oncol 2018; 4: 1553–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 3. Anderson LA, Tavilla A, Brenner H et al Survival for oesophageal, stomach and small intestine cancers in Europe 1999–2007: Results from EUROCARE‐5. Eur J Cancer 2015; 51: 2144–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet 2017; 390: 2383–96. [DOI] [PubMed] [Google Scholar]
- 5. Reichert M, Schistek M, Uhle F et al Ivor Lewis esophagectomy patients are particularly vulnerable to respiratory impairment ‐ a comparison to major lung resection. Sci Rep 2019; 9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ohi M, Toiyama Y, Omura Y et al Risk factors and measures of pulmonary complications after thoracoscopic esophagectomy for esophageal cancer. Surg Today 2018; 49: 176–86. [DOI] [PubMed] [Google Scholar]
- 7. Hayami M, Watanabe M, Ishizuka N et al Prognostic impact of postoperative pulmonary complications following salvage esophagectomy after definitive chemoradiotherapy. J Surg Oncol 2017; 117: 1251–9. [DOI] [PubMed] [Google Scholar]
- 8. Decker G, Coosemans W, De Leyn P et al Minimally invasive esophagectomy for cancer. Eur J Cardiothorac Surg 2009; 35: 13–21. [DOI] [PubMed] [Google Scholar]
- 9. Sakamoto T, Fujiogi M, Matsui H, Fushimi K, Yasunaga H. Comparing perioperative mortality and morbidity of minimally invasive esophagectomy versus open esophagectomy for esophageal cancer. Ann Surg 2019; 1: 10.1097/SLA.0000000000003500. [DOI] [PubMed] [Google Scholar]
- 10. Luketich J, Pennathur A, Awais O et al Outcomes after minimally invasive esophagectomy. Ann Surg 2012; 256: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiong W, Li R, Lei H, Jiang Z. Comparison of outcomes between minimally invasive oesophagectomy and open oesophagectomy for oesophageal cancer. ANZ J Surg 2015; 87: 165–70. [DOI] [PubMed] [Google Scholar]
- 12. Lv L, Hu W, Ren Y, Wei X. Minimally invasive esophagectomy versus open esophagectomy for esophageal cancer: A meta‐analysis. Onco Targets Ther 2016; 9: 6751–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gottlieb‐Vedi E, Kauppila J, Malietzis G, Nilsson M, Markar S, Lagergren J. Long‐term survival in esophageal cancer after minimally invasive compared to open esophagectomy. Ann Surg 2019; 270: 1005–17. [DOI] [PubMed] [Google Scholar]
- 14. Seesing M, Gisbertz S, Goense L et al A propensity score matched analysis of open versus minimally invasive transthoracic esophagectomy in The Netherlands. Ann Surg 2017; 266: 839–46. [DOI] [PubMed] [Google Scholar]
- 15. Mariette C, Markar S, Dabakuyo‐Yonli T et al Hybrid minimally invasive esophagectomy for esophageal cancer. N Engl J Med 2019; 380: 152–62. [DOI] [PubMed] [Google Scholar]
- 16. Straatman J, van der Wielen N, Cuesta M et al Minimally invasive versus open esophageal resection. Ann Surg 2017; 266: 232–6. [DOI] [PubMed] [Google Scholar]
- 17. Kinjo Y, Kurita N, Nakamura F et al Effectiveness of combined thoracoscopic–laparoscopic esophagectomy: Comparison of postoperative complications and midterm oncological outcomes in patients with esophageal cancer. Surg Endosc 2011; 26: 381–90. [DOI] [PubMed] [Google Scholar]
- 18. Sarkaria I, Rizk N, Goldman D et al Early quality of life outcomes after robotic‐assisted minimally invasive and open esophagectomy. Ann Thorac Surg 2019; 108: 920–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Safranek P, Cubitt J, Booth M, Dehn T. Review of open and minimal access approaches to oesophagectomy for cancer. Br J Surg 2010; 97: 1845–53. [DOI] [PubMed] [Google Scholar]
- 20. Paireder M, Asari R, Kristo I et al Morbidity in open versus minimally invasive hybrid esophagectomy (MIOMIE). Eur Surg 2018; 50: 249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sihag S, Kosinski AS, Gaissert HA, Wright CD, Schipper PH. Minimally invasive versus open esophagectomy for esophageal cancer: A comparison of early surgical outcomes from the Society of Thoracic Surgeons National Database. Ann Thorac Surg 2016; 101: 1281–9. [DOI] [PubMed] [Google Scholar]
- 22. Schoppmann S, Prager G, Langer F et al Open versus minimally invasive esophagectomy: A single‐center case controlled study. Surg Endosc 2010; 24: 3044–53. [DOI] [PubMed] [Google Scholar]
- 23. Klevebro F, Scandavini C, Kamiya S, Nilsson M, Lundell L, Rouvelas I. Single center consecutive series cohort study of minimally invasive versus open resection for cancer in the esophagus or gastroesophageal junction. Dis Esophagus 2018; 31: 10. [DOI] [PubMed] [Google Scholar]
- 24. Perry K, Enestvedt CK, Pham T et al Comparison of laparoscopic inversion esophagectomy and open transhiatal esophagectomy for high‐grade dysplasia and stage I esophageal adenocarcinoma. Arch Surg 2009; 144: 679–84. [DOI] [PubMed] [Google Scholar]
- 25. Maas K, Biere S, van Hoogstraten I, van der Peet D, Cuesta M. Immunological changes after minimally invasive or conventional esophageal resection for cancer: A randomized trial. World J Surg 2013; 38: 131–7. [DOI] [PubMed] [Google Scholar]
- 26. Glatz T, Marjanovic G, Kulemann B, Sick O, Hopt U, Hoeppner J. Hybrid minimally invasive esophagectomy vs. open esophagectomy: A matched case analysis in 120 patients. Langenbeck's Arch Surg 2017; 402: 323–31. [DOI] [PubMed] [Google Scholar]
- 27. Tang H, Zheng H, Tan L et al Neoadjuvant chemoradiotherapy followed by minimally invasive esophagectomy: Is it a superior approach for locally advanced resectable esophageal squamous cell carcinoma? J Thorac Dis 2018; 10: 963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee J, Cheng J, Lin M, Huang P, Chen J, Lee Y. Is there any benefit to incorporating a laparoscopic procedure into minimally invasive esophagectomy? The impact on perioperative results in patients with esophageal cancer. World J Surg 2011; 35: 790–7. [DOI] [PubMed] [Google Scholar]
- 29. Bonavina L, Scolari F, Aiolfi A et al Early outcome of thoracoscopic and hybrid esophagectomy: Propensity‐matched comparative analysis. Surgery 2016; 159: 1073–81. [DOI] [PubMed] [Google Scholar]
- 30. Hamouda A, Forshaw M, Tsigritis K et al Perioperative outcomes after transition from conventional to minimally invasive Ivor‐Lewis esophagectomy in a specialized center. Surg Endosc 2009; 24: 865–9. [DOI] [PubMed] [Google Scholar]
- 31. Kauppi J, Räsänen J, Sihvo E, Huuhtanen R, Nelskylä K, Salo J. Open versus minimally invasive esophagectomy: Clinical outcomes for locally advanced esophageal adenocarcinoma. Surg Endosc 2014; 29: 2614–9. [DOI] [PubMed] [Google Scholar]
- 32. Guo M, Xie B, Sun X, Hu M, Yang Q, Lei Y. A comparative study of the therapeutic effect in two protocols: Video‐assisted thoracic surgery combined with laparoscopy versus right open transthoracic esophagectomy for esophageal cancer management. Chin‐German J Clin Oncol 2013; 12: 68–71. [Google Scholar]
- 33. Sihvo E, Helminen O, Gunn J, Sipilä J, Rautava P, Kytö V. Long‐term outcomes following minimally invasive and open esophagectomy in Finland: A population‐based study. Eur J Surg Oncol 2019; 45: 1099–104. [DOI] [PubMed] [Google Scholar]
- 34. Pham T, Perry K, Dolan J et al Comparison of perioperative outcomes after combined thoracoscopic‐laparoscopic esophagectomy and open Ivor‐Lewis esophagectomy. Am J Surg 2010; 199: 594–8. [DOI] [PubMed] [Google Scholar]
- 35. Scarpa M, Cavallin F, Saadeh L et al Hybrid minimally invasive esophagectomy for cancer: Impact on postoperative inflammatory and nutritional status. Dis Esophagus 2015; 29: 1064–70. [DOI] [PubMed] [Google Scholar]
- 36. Biere S, van Berge HM, Maas K et al Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: A multicentre, open‐label, randomised controlled trial. Lancet 2012; 379: 1887–92. [DOI] [PubMed] [Google Scholar]
- 37. Parameswaran R, Titcomb D, Blencowe N et al Assessment and comparison of recovery after open and minimally invasive esophagectomy for cancer: An exploratory study in two centers. Ann Surg Oncol 2013; 20: 1970–7. [DOI] [PubMed] [Google Scholar]
- 38. Noble F, Kelly J, Bailey I, Byrne J, Underwood T. A prospective comparison of totally minimally invasive versus open Ivor Lewis esophagectomy. Dis Esophagus 2012; 26: 263–71. [DOI] [PubMed] [Google Scholar]
- 39. Burdall O, Boddy A, Fullick J et al A comparative study of survival after minimally invasive and open oesophagectomy. Surg Endosc 2014; 29: 431–7. [DOI] [PubMed] [Google Scholar]
- 40. Dolan J, Kaur T, Diggs B et al Impact of comorbidity on outcomes and overall survival after open and minimally invasive esophagectomy for locally advanced esophageal cancer. Surg Endosc 2013; 27: 4094–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hsu P, Huang C, Wu Y, Chou T, Hsu W. Open versus thoracoscopic esophagectomy in patients with esophageal squamous cell carcinoma. World J Surg 2013; 38: 402–9. [DOI] [PubMed] [Google Scholar]
- 42. Kanekiyo S, Takeda S, Tsutsui M et al Low invasiveness of thoracoscopic esophagectomy in the prone position for esophageal cancer: A propensity score‐matched comparison of operative approaches between thoracoscopic and open esophagectomy. Surg Endosc 2017; 32: 1945–53. [DOI] [PubMed] [Google Scholar]
- 43. Rinieri P, Ouattara M, Brioude G et al Long‐term outcome of open versus hybrid minimally invasive Ivor Lewis oesophagectomy: A propensity score matched study. Eur J Cardiothorac Surg 2016; 273: 1–7. [DOI] [PubMed] [Google Scholar]
- 44. Thomson I, Smithers B, Gotley D et al Thoracoscopic‐assisted esophagectomy for esophageal cancer. Ann Surg 2010; 252: 281–91. [DOI] [PubMed] [Google Scholar]
- 45. Yerokun BA, Sun Z, Yang C‐FJ et al Minimally invasive versus open esophagectomy for esophageal cancer: A population‐based analysis. Ann Thorac Surg 2016; 102: 416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zingg U, McQuinn A, DiValentino D et al Minimally invasive versus open esophagectomy for patients with esophageal cancer. Ann Thorac Surg 2009; 87: 911–9. [DOI] [PubMed] [Google Scholar]
- 47. Nagpal K, Ahmed K, Vats A et al Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta‐analysis. Surg Endosc 2010; 24: 1621–9. [DOI] [PubMed] [Google Scholar]
- 48. Dantoc M, Cox MR, Eslick GD. Evidence to support the use of minimally invasive esophagectomy for esophageal cancer: A meta‐analysis. Arch Surg 2012; 147: 768–76. [DOI] [PubMed] [Google Scholar]
- 49. Sgourakis G, Gockel I, Radtke A et al Minimally invasive versus open esophagectomy: Meta‐analysis of outcomes. Dig Dis Sci 2010; 55: 3031–40. [DOI] [PubMed] [Google Scholar]
- 50. Takeuchi H, Miyata H, Gotoh M et al A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web‐based database. Ann Surg 2014; 260: 259–66. [DOI] [PubMed] [Google Scholar]
- 51. Nozaki I, Mizusawa J, Kato K et al Impact of laparoscopy on the prevention of pulmonary complications after thoracoscopic esophagectomy using data from JCOG0502: A prospective multicenter study. Surg Endosc 2017; 32: 651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. (a) Forest plot of R0 resection; (b) forest plot of 30‐day mortality; (c) forest plot of 90‐day mortality; and (d) forest plot of in‐hospital mortality.
Figure S2. (a) Forest plot of reoperation; (b) funnel plot of all‐cause RCs; (c) funnel plot of all‐cause AL; and (d) funnel plot of all‐cause CCs.
Table S1. Subgroup analysis of in‐hospital stay between MIE and OE.
Table S2. Subgroup analysis of total operation time between MIE and OE.
Table S3. Subgroup analysis of blood loss between MIE and OE.


