Abstract
Local and global measurements of parasite prevalence and abundance are critical for understanding the dynamics that underlie the diversity, distribution, and evolution of infectious diseases. Here we present a dataset of gut helminths found in 1) raccoons throughout their range, based on primary literature from 1925–2017 and 2) raccoons in Santa Barbara County, CA surveyed from 2012–2015. The range-wide dataset has 1256 parasite entries from 217 literature sources across three continents and 32 states in the USA. This dataset includes a list of all recorded raccoon gut helminths (n=100) and their presence and prevalence in surveyed raccoon populations. The Santa Barbara dataset includes gut helminth data from 182 raccoons from one Southern California County. In addition to presence and abundance data for 13 parasite species, this dataset includes measurements of 7465 individual raccoon roundworms (Baylisascaris procyonis). For both range-wide and Santa Barbara datasets, we include information on parasite site of infection in host, sampling method and sample size. We also provide geographic coordinates for infected raccoon populations (range-wide database) and individuals (Santa Barbara). In the associated metadata, we include sampling methods and summary figures for both the range-wide and Santa Barbara raccoon gut helminth records.
Keywords: parasites, Procyon lotor, raccoon, Baylisascaris, roundworm, global, helminth
INTRODUCTION
Accurate records of parasite presence and abundance are critical for understanding species distribution patterns, population dynamics, and human disease risk. Most wild animals have never been surveyed for parasites and most surveys only include hosts in a subset of their range (Dobson et al., 2008). Parasite survey data are particularly limited for larger bodied mammals; however, the raccoon (Procyon lotor) provides an unusually well surveyed host with which to explore factors structuring parasite communities across a host’s range.
Raccoons (Procyon lotor) are native to North America but have been introduced to many parts of the world. Following escapes from European zoos and the Japanese pet trade, and intentional introductions from the Russian fur industry, these medium-sized carnivores (4–9kg) now have a global distribution. Historically a hunted furbearer, raccoons are now increasing in abundance due to reduced hunting pressure, loss of natural predators, and ability to thrive in human-modified habitats (Gehrt, 2003). Although they are members of Carnivora, raccoons are opportunistic omnivores. Their diets typically reflect local food availability and, in urban areas, often incorporate pet food and garbage. As a result, raccoons reach their highest densities in urban and suburban areas (Zeveloff, 2002).
Increased overlap with humans is a major concern for disease spillover. Raccoons host a variety of zoonotic diseases including rabies, distemper, and raccoon roundworm (Baylisascaris procyonis). Among raccoon helminth parasites, B. procyonis is the best studied due to the extensive pathology it causes in both humans and other animals (Kazacos, 2001). This large ascarid nematode is found throughout both the native and introduced raccoon range. In raccoons (the definitive host), B. procyonis matures in the gut and causes little pathology. Infected raccoons pass B. procyonis eggs in their feces, creating an infection risk to birds and other mammals, including humans (Kazacos, 2001). If ingested by a non-raccoon host, eggs hatch into larval worms in the gut, and although larval worms do not mature, they can undergo substantial migrations, leading to extensive damage to eyes and the nervous system (Sprent, 1952). Baylisascaris procyonis presents a human health risk, particularly to small children, with documented cases throughout North America, including one in Santa Barbara in 2002 (Schultz, 2002). This parasite is also a conservation threat, as it can severely reduce small mammal populations (Logiudice, 2003). Growing raccoon populations, availability of hosts from fur trappers and pest removal services, and increasing recognition of disease spillover risk has resulted in a large number of raccoon parasite surveys documenting both B. procyonis and other gut helminths.
Raccoons host a diverse array of gut helminths including other nematodes (roundworms), cestodes (tapeworms), trematodes (flatworms), and acanthocephalans (thorny headed worms). These parasites exhibit a range of life cycles, including simple (e.g. Placoconus lotoris) and complex (e.g. Atriotaenia procyonis). Some parasites are found throughout the entire raccoon range (e.g. B. procyonis) while others are reported only from a few localities (e.g. Profilicollis spp.). These extensive host-parasite records offer an opportunity to examine infection patterns, potentially providing insight into disease dynamics in raccoons and other terrestrial vertebrates for which such extensive invasive surveys are not possible.
CLASS I. DATA SET DESCRIPTORS
A. Data set identity:
Six files grouped into two subgroups (Range-Wide Raccoon Parasites and Santa Barbara County Raccoon Parasites) each with three files, as follows: Range-Wide Raccoon Parasites: (1) Raccoon range-wide data, (2) Literature search summary, (3) Current parasite taxonomy; and Santa Barbara County Raccoon Parasites: (4) Santa Barbara raccoon host data, (5) Santa Barbara raccoon gut helminths, (5) Santa Barbara Baylisascaris data.
B. Data Identification Code:
Range-Wide Raccoon Parasites:
-
1
RPSITE_raccoon_rangewide.csv;
-
2
RPSITE_literature_summary.csv;
-
3
RPSITE_psite_taxonomy.csv;
Santa Barbara County Raccoon Parasites:
-
4
RPSITE_SB_hosts_data.csv;
-
5
RPSITE_SB_parasites_data.csv;
-
6
RPSITE_SB_baylisascaris_data.csv
C. Data set description:
1. Originators:
Sara B. Weinstein, Jacey C. Van Wert, Mike Kinsella, Vasyl V. Tkach, and Kevin D. Lafferty
2. Abstract:
Local and global measurements of parasite prevalence and abundance are critical for understanding the dynamics that underlie the diversity, distribution, and evolution of infectious diseases. Here we present a dataset of gut helminths found in 1) raccoons throughout their range, based on primary literature from 1925–2017 and 2) raccoons in Santa Barbara County, CA surveyed from 2012–2015. The range-wide dataset has 1256 parasite entries from 217 literature sources across three continents and 32 states in the USA. This dataset includes a list of all recorded raccoon gut helminths (n=100) and their presence and prevalence in surveyed raccoon populations. The Santa Barbara dataset includes gut helminth data from 182 raccoons from one Southern California County. In addition to presence and abundance data for 13 parasite species, this dataset includes measurements of 7465 individual raccoon roundworms (Baylisascaris procyonis). For both range-wide and Santa Barbara datasets, we include information on parasite site of infection in host, sampling method and sample size. We also provide geographic coordinates for infected raccoon populations (range-wide database) and individuals (Santa Barbara). In the associated metadata, we include sampling methods and summary figures for both the range-wide and Santa Barbara raccoon gut helminth records.
CLASS II. RESEARCH ORIGIN DESCRIPTORS
A. Overall project description
1. Identity:
The diversity and distribution of raccoon gastrointestinal helminths.
2. Originators:
Same authors as above
3. Period of Study:
2015–2018 (Literature search conducted), 1925–2017 (Dates of source literature), 2012–2015 (Santa Barbara raccoon collections).
4. Objectives:
To collate and summarize gastrointestinal parasite records for the raccoon (Procyon lotor) throughout its introduced and native range, with a focus on Baylisascaris procyonis and parasite communities in raccoons from Santa Barbara County, California.
5. Abstract:
See above.
6. Source(s) of funding:
Funding was provided by NSF grant numbers DGE 1144085 and DEB 1601362.
B1. “Specific subproject” description: Range-Wide Raccoon Parasites
1. Site description:
Data were collected from literature. Records in the dataset span three continents and 32 states in the USA (See Figures 1 and 2).
Figure 1:
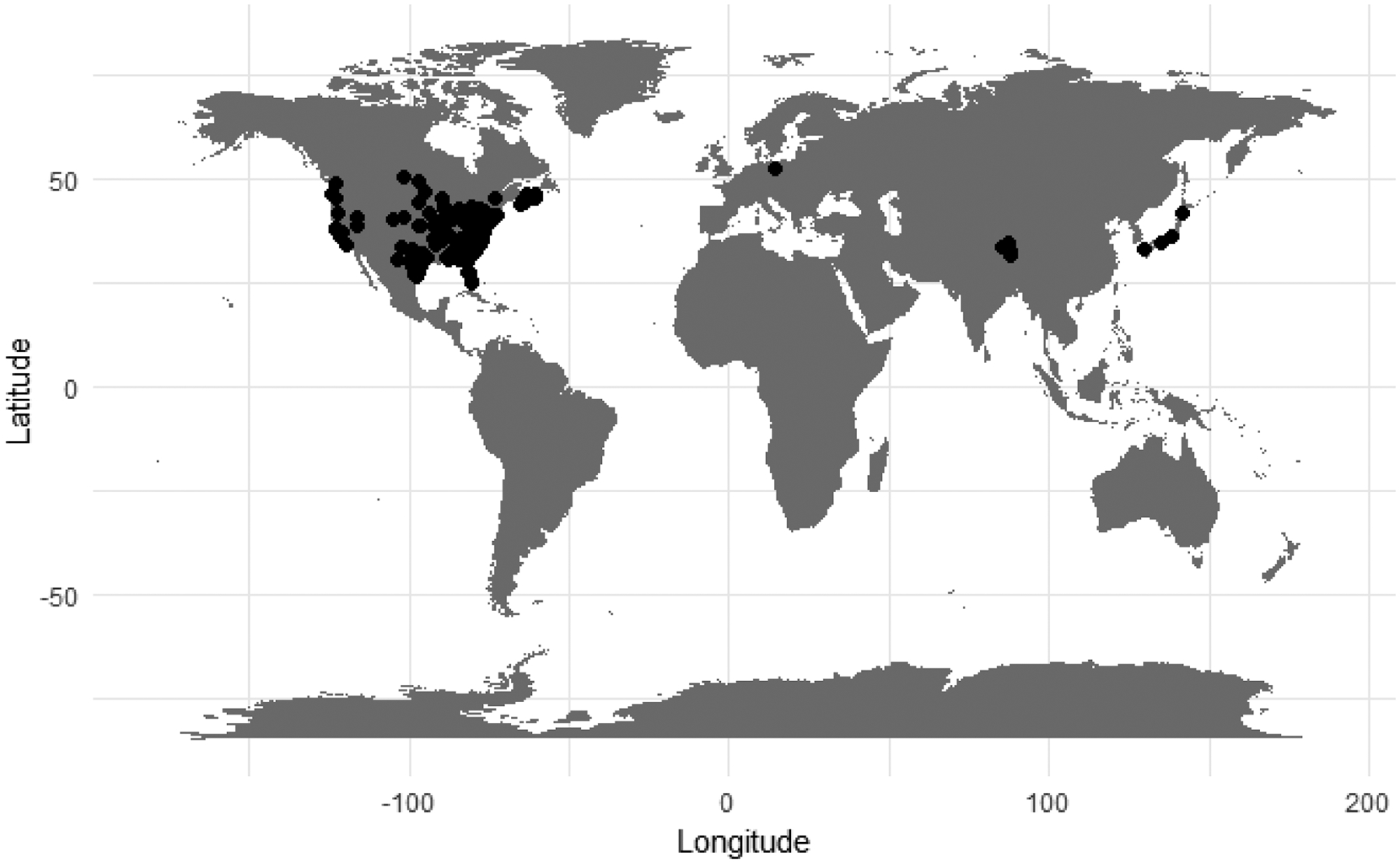
Locations of raccoon populations with parasite surveys (“included” in RPSITE_literature_summary.csv), with sufficient location data to georeference collection site.
Figure 2:
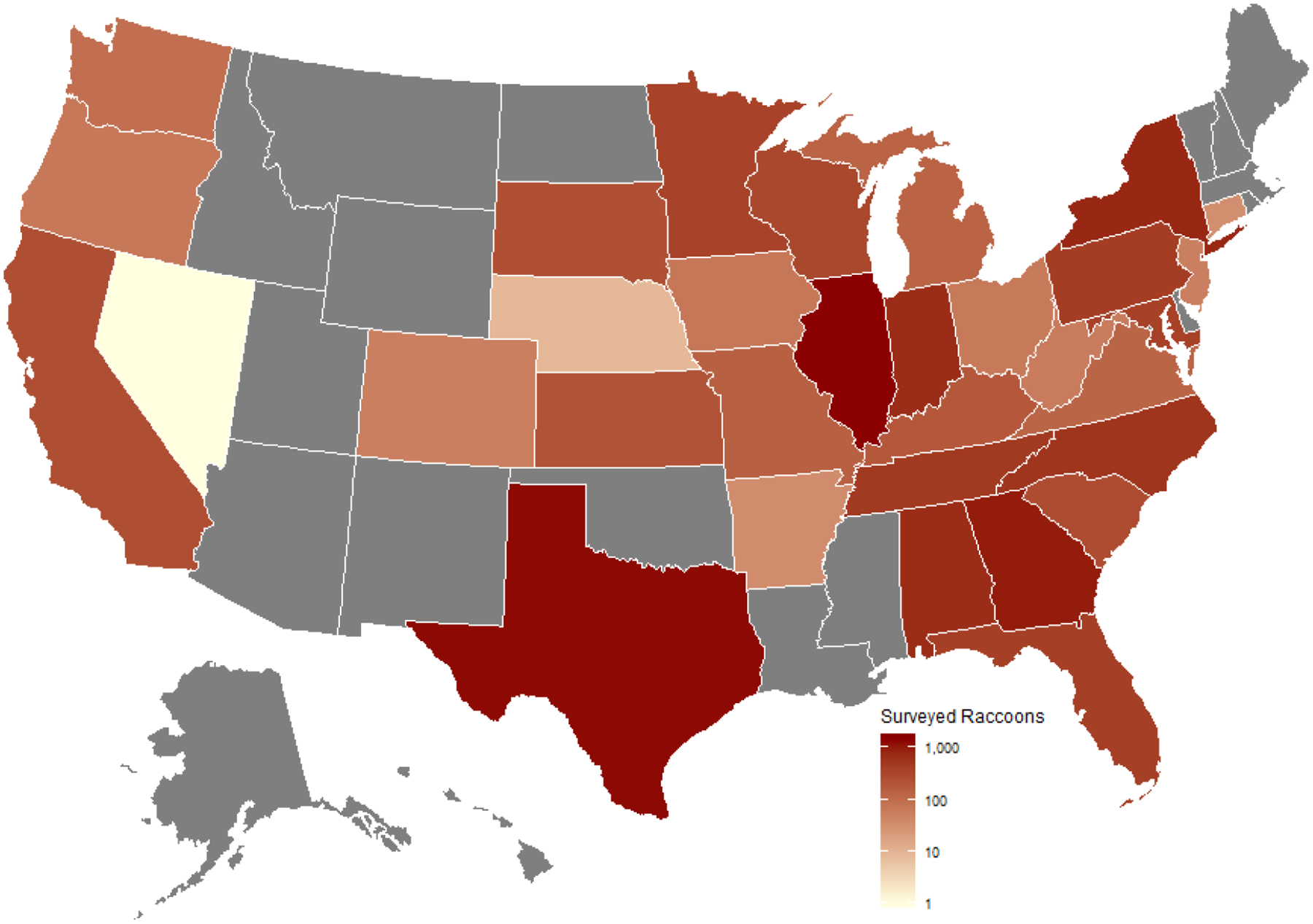
Map of the United States of America depicting the number of raccoons examined for gut helminths per state. Raccoon totals include individuals surveyed for only a subset of gut helminths (e.g. only Baylisascaris procyonis). Data deficient states are indicated in grey.
2. Experimental/sampling design:
Data were obtained from literature published between 1925 and 2017 (see Figure 3).
Figure 3.
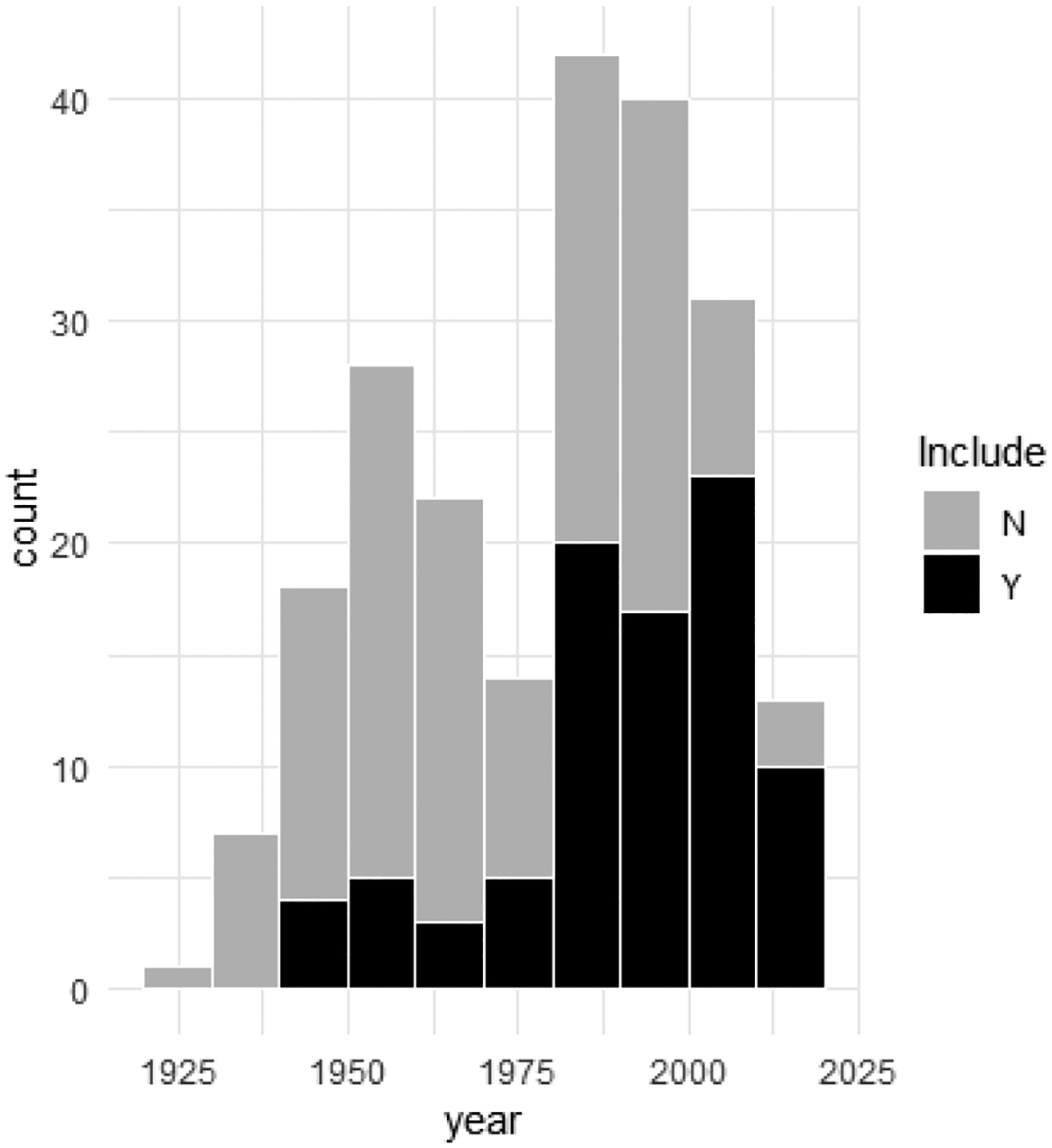
Histogram of publication year for raccoon parasites studies listed in RPSITE_literature_summary.csv. “Included” (n=91) gastrointestinal helminth studies (including studies that include only a subset of the parasite community) are differentiated from studies that do not meet criteria for inclusion as helminth surveys (n=126) raccoon parasite literature records recovered during literature searches. Figure does not include five unpublished surveys without study dates.
3. Research methods:
a. Data sources:
We constructed a database of published and unpublished raccoon gastrointestinal helminth surveys using 1) personal reference libraries 2) relevant studies cited in these papers and 3) a Google Scholar (https://scholar.google.com/) search with key words “Procyon lotor,” “raccoon*,” “parasite*,” and “endoparasite*”. This included unpublished data sources such as BA, MSc and PhD dissertation theses. We included all English language literature that fit our criteria and noted in our database (RPSITE_literature_summary.csv) papers that we could not access. We note whether studies were full gut parasite surveys or included only a subset of the parasite community. We did not include ectoparasite or pathogen/microparasite-specific surveys. Downstream analyses (Weinstein et al., in review) exclude species descriptions, case reports, and case studies that used only moribund animals; however these studies are included in the database.
b. Data collection:
We downloaded or scanned sources and saved them to a shared database. We read each paper for details regarding parasite extraction and identification methods, site in host, host locations, and parasite prevalence for each raccoon population. Raccoon populations were recorded as defined by the authors, with limited exceptions (see RPSITE_raccoon_rangewide.csv). We also recorded additional notes that might be relevant to each host-parasite interaction (e.g. author suggests a parasite is from raccoon prey, raccoons are captive). For endoparasite surveys, we recorded survey location, parasitology methods, host sites searched, endoparasites encountered, and prevalence for each raccoon population. We also took note of non-gut parasites (i.e. lungs, bladder) and hemoparasites or ectoparasites when part of a larger survey but did not include these parasites in later analyses. We also noted parasites from ingested prey items and parasites not identified to genus or species (e.g. “unknown Trematoda”).
c. Data verification:
Following initial data collection, we checked each cell for spelling or input errors and searched for extreme or undefined values. For data discrepancies, we referred to the primary literature for confirmation. The global raccoon endoparasite database may be limited by cases of missing data, inaccessible publications, unpublished data, and language barriers and we invite updates regarding any new or missing publications.
d. Parasite taxonomy:
We constructed a database (RPSITE_psite_taxonomy.csv) with current nomenclature and associated synonyms for each raccoon gut helminth. Recorded parasites are grouped by taxonomic order (Figure 4) and references for current nomenclature are listed. The current taxonomy is based first on references in online taxonomic databases (see RPSITE_psite_taxonomy.csv for database references) and the following keys: Keys to the Cestode Parasites of Vertebrates by Khalil et al. (1994); Keys to the Trematoda (3 volumes) by Gibson et al. (2002), Jones et al. (2005), and Bray et al. (2008); CIH Keys to the Nematode Parasites of Vertebrates by Anderson et al. (1975–1983) and the Supplemental Volume by Gibbons (2010). For species not included in online databases or keys, we provide a reference distinguishing the taxon as a valid species. We used this database to check for misspellings, taxonomic revisions, and classify each parasite to order.
Figure 4:
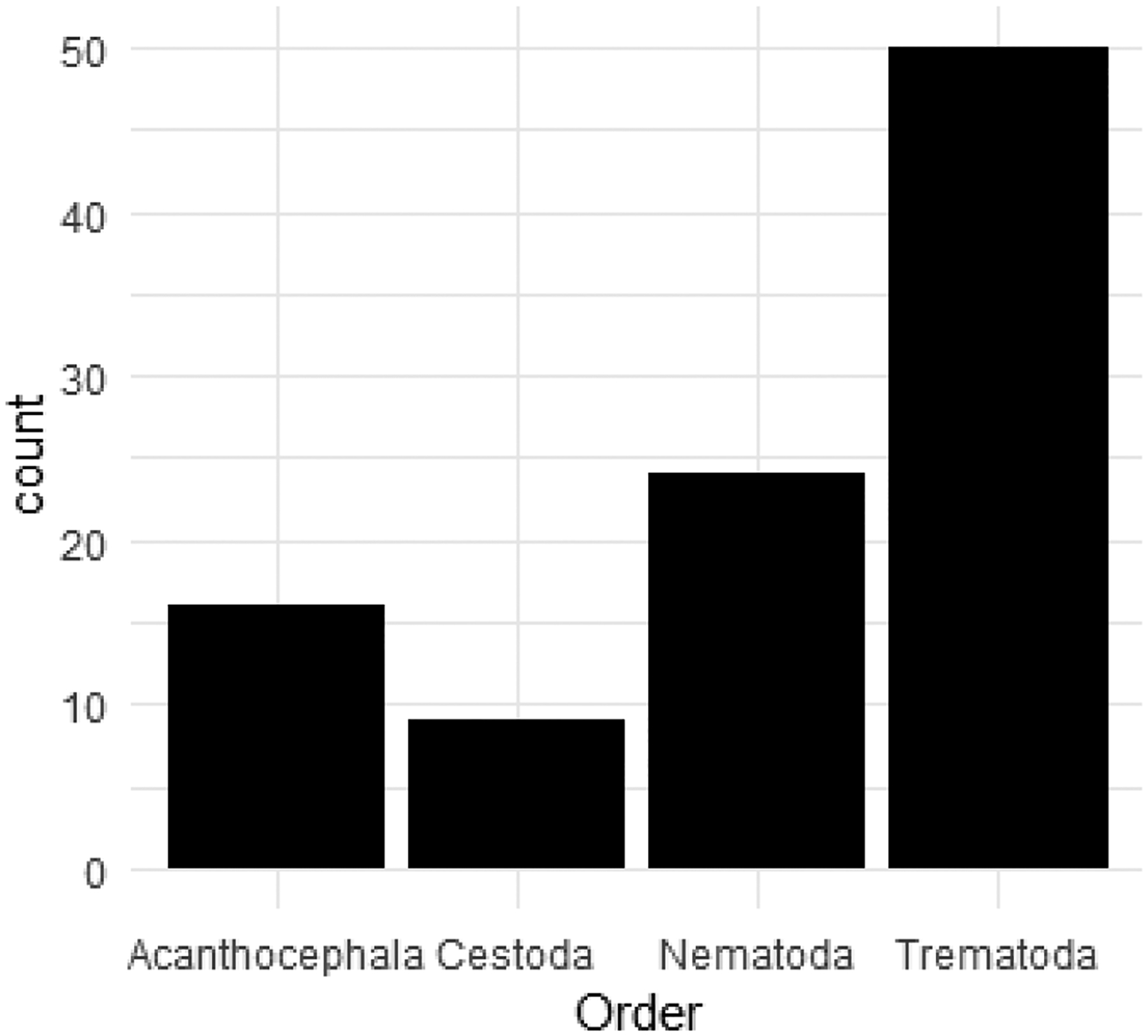
Raccoons host gut helminths belonging to four taxonomic orders: Acanthocephala, Cestoda, Nematoda, and Trematoda. Eleven parasite genera found in raccoons have only been described from surveys conducted outside the native range of the raccoon.
e. Georeferencing:
We obtained coordinates for raccoon populations either (1) by converting the author provided specific location data into decimal degree latitude/longitude coordinates, (2) using the county political center, city political center, island center, university center, zoo center, museum center using Google Maps Platform (https://developers.google.com/maps/documentation/geocoding/intro), or 3) manually selected locations on Google Earth (https://www.google.com/earth/) for populations between multiple cities, counties, or states, populations in a general area (i.e. Western NY), or populations in specific localities (forests, parks, zoos, and farms). In cases where studies lacked specific location details, but were associated with a university, we used the university address. In separate columns, we have provided the country, state or providence, and specific location according to the primary source in our RPSITE_raccoon_rangewide.csv database. In addition, we provide the longitude, latitude, and source of these coordinates.
B2. “Specific subproject” description: Santa Barbara County Raccoon Parasites
1. Site description:
Raccoons were collected from Santa Barbara County (Figure 5). Located in coastal Southern California; the sampling site includes beach, coastal scrub, chaparral, and urban/suburban areas.
Figure 5:
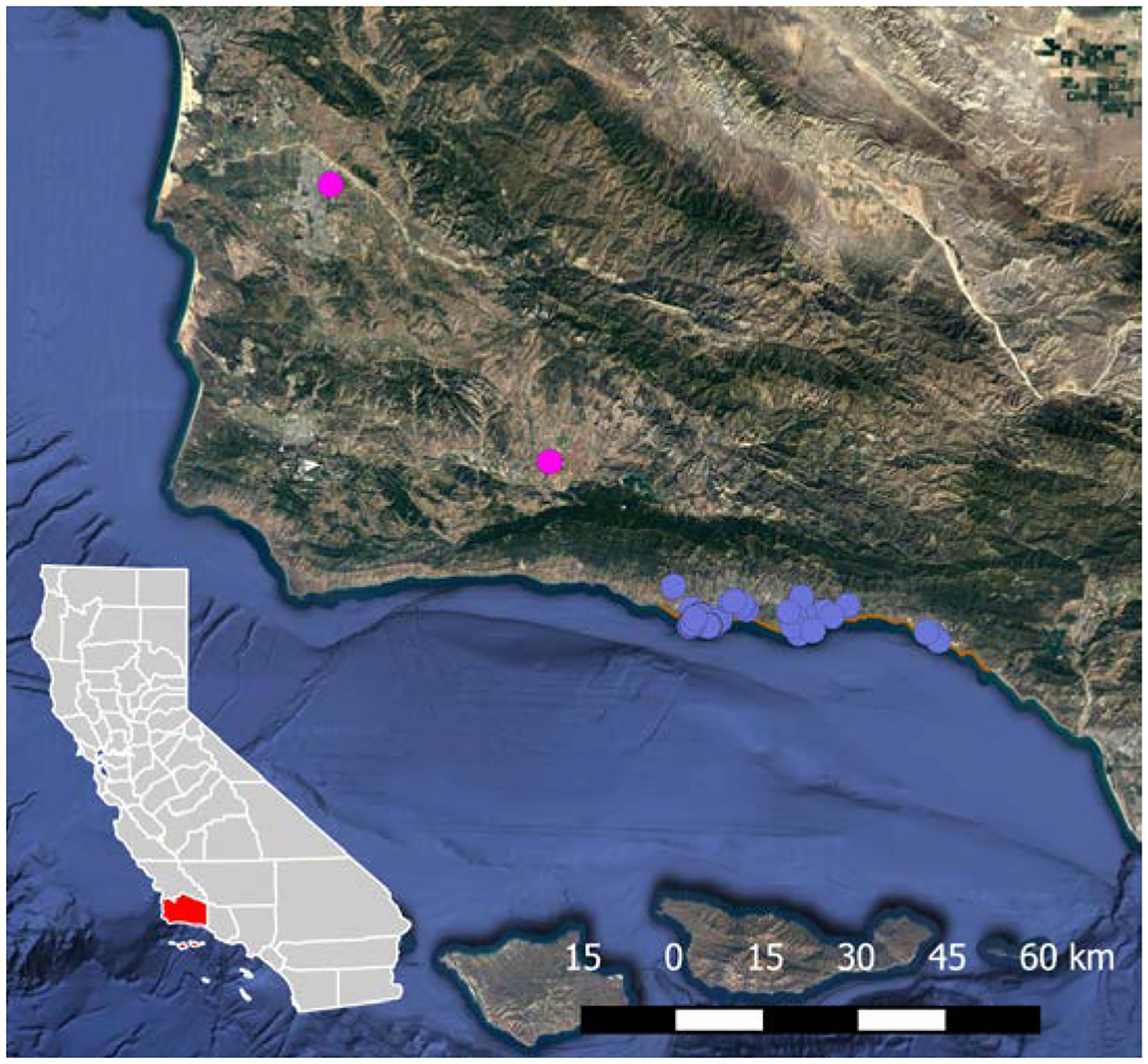
Collection sites for the 181 georeferenced raccoons with associated gut helminth community data from Santa Barbara County, California. Two animals from distinct populations in the northern part of the county are shown as pink dots and all other collection sites are purple.
2. Experimental/Sampling design:
Raccoons were received from trappers or animal control, trapped by SBW, or collected as roadkill in Santa Barbara County, California, between 2012 and 2015.
3. Research methods:
a. Laboratory:
We either dissected raccoons immediately or froze them for later necropsy. We measured and sexed each animal and then determined age based on skull morphology and dentition (see Weinstein 2016). We removed the small and large intestines and then opened them longitudinally. We first removed all visible worms, including B. procyonis, acanthocephalans, and mature cestodes. We then separated intestines into sections and scraped remaining gut contents from the mucosa and washed this material into a beaker of saline. This solution was decanted as necessary to remove fine sediment, rinsed using nearly boiled water to kill B. procyonis eggs, and then stored in 70% ethanol. We examined the intestinal contents under 12–30x magnification in ~1 mL portions placed in a petri dish. We sorted, counted, and collected parasites until the entire ethanol-preserved solution had been examined.
b. Taxonomy and systematics:
For parasite identification, we used 80% phenol or lactophenol to clear and mount nematodes and acanthocephalans. We stained cestodes and trematodes with aqueous alum carmine, cleared them in clove oil, and mounted them in Damar gum or Canada balsam. Cestodes were identified to the generic level by using the Keys to the Cestode Parasites of Vertebrates by Khalil et al. (1994); trematodes by using the Keys to the Trematoda (3 vol.) by Gibson et al. (2002), Jones et al. (2005), and Bray et al. (2008), and the monograph by Schell (1985); and nematodes by using the CIH Keys to the Nematode Parasites of Vertebrates by Anderson et al. (1975–1983) and the Supplemental Volume by Gibbons (2010). Acanthocephalan taxonomy followed the classification by Amin (1985). Identification to the specific level was made by reference to original descriptions in the literature and an attempt was made to use the most currently accepted name for each species (see RPSITE_psite_taxonomy.csv).
c. Permit history:
Animal use was in accordance with University of California, Santa Barbara IACUC protocol #850 and CA DFG permit #11188.
d. Georeferencing:
We recorded raccoon collection site to the nearest building name or cross-street, except for one individual for which location data was unavailable. We georeferenced raccoon collection sites in Google Earth 7.1.8.3036 (https://www.google.com/earth/) to decimal degree latitude/longitude coordinates.
CLASS III. DATA SET STATUS AND AVAILABILITY
A. Status
1. Latest update:
May 5, 2019.
2. Latest archive date:
May 5, 2019.
3. Metadata status:
Last updated May 5, 2019.
4. Data Verification:
See description in Class II, Sections B1 and B2 above.
B. Accessibility
1. Storage location and medium:
In addition to Ecological Society of America Data Registry, our datasets are stored on a shared database (https://www.dropbox.com/). Southern California raccoon parasite data (RPSITE_SB_hosts_data.csv, RPSITE_SB_parasites_data.csv, RPSITE_SB_baylisascaris_data.csv) are also stored as raw data sheets with S.B. Weinstein.
2. Contact persons:
Sara B. Weinstein (batrachoseps@gmail.com) and Jacey C. Van Wert (jcvanwert@gmail.com).
3. Copyright and proprietary restrictions:
There are no copyright or proprietary restrictions for research and/or teaching purposes. We ask that the dataset is cited with the following citation:
Citation:
Weinstein, S.B., J.C. Van Wert, M. Kinsella, V.V. Tkach, and K.D. Lafferty. 201X. Southern California and Range-Wide Raccoon Gastrointestinal Helminth Database. Ecology XX: XXX.
4. Costs:
None.
Class IV: DATA STRUCTURAL DESCRIPTORS
A. Data set files
1. Identity and size:
DataS1.zip (zipped CSV files), 98 Kilobytes
In DataS1.zip:
RPSITE_raccoon_rangewide.csv: 1257 lines of data (header included), 266 kilobytes
RPSITE_literature_summary.csv: 218 lines of data (header included), 57 kilobytes
RPSITE_psite_taxonomy.csv: 101 lines of data (header included), 30 kilobytes
RPSITE_SB_hosts_data.csv: 188 lines of data (header included), 32 kilobytes
RPSITE_SB_parasites_data.csv: 436 lines of data (header included), 25 kilobytes
RPSITE_SB_baylisascaris_data.csv: 7568 lines of data (header included), 219 kilobytes
2. Format:
Comma-separated values text files (*.csv)
3. Other Information:
Header:
Describes column contents. Detailed descriptions of column headers and contents are described in Variable definitions: Tables 1–6.
Data anomalies:
When no data are available the field is empty. For some missing data, explanations are provided in the Notes column.
B. Variable definitions
Table 1:
Variable definitions for RPSITE_raccoon_rangewide.csv. Parasite records from raccoon parasite surveys conducted throughout the raccoon range. Each row is a parasite species record from a raccoon population. When data entries are restricted (e.g. “Y” or “N”), acceptable values are listed in the Definition.
| Variable | Definition | Format |
|---|---|---|
| Included_paper | Included paper for Weinstein et al. (in review) analysis? Y for yes, N for no. | text |
| Included_observation | Included observation for Weinstein et al. (in review) analysis? Y for yes, N for no. | text |
| Paper | Primary literature paper abbreviation. | text |
| Group | Nematoda, Trematoda, Acanthocephala, Cestoda, Apicomplexa, Protozoa, or Ecto (e.g.: ticks, lice). | text |
| Genus | Parasite genus as reported in original source. | text |
| Species | Parasite species as reported in original source. | text |
| Taxonomic_unit | Final, corrected taxonomic unit as genus used for Weinstein et al. (in review). Taxonomic units, associated synonyms, and nomenclature references are found in RPSITE_psite_taxonomy.csv. | text |
| Site_in_host | Organ or organ system in which parasite was collected and identified, as described by original author. ‘Feces’ indicates parasite was identified from a fecal sample. | text |
| Prevalence | Prevalence as % of surveyed raccoons in population infected. If no prevalence listed but present, value is indicated as “present”). | numeric or text |
| Raccoon_sample_size | Total number of raccoons per population (as identified by author) surveyed. | numeric |
| Country | Raccoon population country. | text |
| State_or_providence | Raccoon population state or providence. | text |
| Location_name | Location or region name of raccoon population as stated in the original source. | text |
| Latitude | Decimal degree latitude of study. In cases where many localities are provided for a single defined population, a central location is reported. Field is left blank when author-given location was vague and parasite observation was not included in Weinstein et al. (in review). | numeric |
| Longitude | Decimal degree longitude of study. In cases where many localities are provided for a single defined population, a central location is reported. Field is left blank when author-given location was vague and parasite observation was not included in Weinstein et al. (in review). | numeric |
| Geocoordinate_source | Source for latitude and longitude (i.e. directly from the paper, central location, study university location, etc.). | text |
| Notes | Additional notes on sampling, population, or parasite identification. | text |
| Method_notes | Additional sampling method notes. | text |
| Method | Whether parasite presence and prevalence was determined from necropsy, fecal samples, both necropsy and fecal, or blood. | text |
| Wild | Was raccoon population wild and un-altered or managed in some way (ex: translocated, captive, zoo, pet)? Y for yes, N for no. | text |
| Prey | Was parasite likely from raccoon prey [as stated by author or as assumed based on identification or from prey item if listed.] Y if yes, N if no. | text |
Table 2:
Variable definitions for RPSITE_literature_summary.csv. Literature survey used to assemble Weinstein et al. (in review). Each row is a paper. Additional information about the parasites is tabulated in RPSITE_raccoon_rangewide.csv. Nine columns (“All_gut_psites”, “Baylis”,” Macro”, “Acan_ex_Mac”, “Gnath”, “Atrio_Taenia”, “Nem_ex_Bay_Gnath”, “Cest_ex_Atrio_Taen”, “Trems”) summarize which parasites were included in the study and are used to calculate the number of raccoon populations surveyed for each parasite as described in Weinstein et al. (in review). When data entries are restricted (e.g. “Y” or “N”), acceptable values are listed in the Definition.
| Variable | Definition | Format |
|---|---|---|
| Paper | Primary literature paper short citation. | text |
| Reference | Full reference for the paper listed in “Paper” column. | text |
| Year | Year study was published. | numeric |
| Include | Was this paper included in Weinstein et al. (in review). Y if included, N if not. | text |
| Access | Did metadata authors have direct access to primary paper. Y for yes, N for no. | text |
| Method | Did paper use necropsy, fecal samples, both necropsy and fecal samples, or blood samples. | text |
| Wild_pop | Was raccoon population wild and un-altered? If yes (Y), no (N) or some portion of the study (S). | text |
| Repeated | Was this data repeated in later studies? Yes (Y), no (N), if data is repeated in a later more detailed study (OK). | text |
| All_gut_psites | Did the survey make an attempt to find all gut parasites, including trematodes. Y for yes, N for no. | text |
| Baylis | Did paper survey for Baylisascaris procyonis? Y for yes, N for no. | text |
| Macro | Did paper survey for Macracanthorhynchus? Y for yes, N for no. | text |
| Acan_ex_Mac | Did paper survey for acanthocephalans in addition to Macracanthorhynchus? Y for yes, N for no. | text |
| Gnath | Did paper survey for Gnathosoma? Y for yes, N for no. | text |
| Atrio_Taenia | Did paper survey for Atriotaenia or Taenia? Y for yes, N for no. | text |
| Nem_ex_Bay_Gnath | Did paper survey for nematodes in addition to Baylisascaris procyonis or Gnathosoma? Y for yes, N for no. | text |
| Cest_ex_Atrio_Taen | Did paper survey for cestodes in addition to Atriotaenia or Taenia? Y for yes, N for no. | text |
| Trems | Did paper survey for trematodes? Y for yes, N for no. | text |
| Notes | Additional notes on paper and methods. | text |
Table 3:
Variable definitions for RPSITE_psite_taxonomy.csv.
| Variable | Definition | Format |
|---|---|---|
| Group | Parasite group (Trematoda, Nematoda, Acanthocephala, Cestoda). | text |
| Genus | Accepted genus as of December 2018. | text |
| Species | Accepted specific epitaph as of December 2018. | text |
| Synonyms | Synonyms for the genus and/or species found in literature on raccoon parasites. Note that this is not a complete list of synonyms. | text |
| Source | Source for current accepted nomenclature and synonyms. | text |
| Notes | Additional notes. | text |
| Reference | Full reference(s) for source material | text |
Table 4:
Variable definitions for RPSITE_SB_hosts_data.csv. Host data for approximately 200 raccoons used for parasitological studies and collected in Santa Barbara County, California from 2012 through 2015. When data entries are restricted (e.g. “F” or “M”), acceptable values are listed in the Definition.
| Variable | Definition | Format |
|---|---|---|
| SBMNH_accession | Animal accession number at Santa Barbara Museum of Natural History. Raccoons are deposited as, at minimum, a skull voucher. | numeric |
| Project_host_ID | Code assigned to animal at time of dissection. | numeric |
| Collection_date | Date animal was collected. If trapped, this is trap date. If salvaged, this is the salvage date. Date is listed as MM/DD/YYYY. | date |
| Location | Verbatim animal collection location, typically provided by trappers, unless salvaged. | text |
| Latitude | Georeferenced latitude based on written location (decimal degrees). | numeric |
| Longitude | Georeferenced longitude based on written location (decimal degrees). | numeric |
| Fresh_frozen | State of animal at the time of dissection. “Fre” for dissected fresh and never frozen, “Fzn” for frozen and thawed prior to dissection. | text |
| Dissection_date | Date of animal dissection. Date is listed as MM/DD/YYYY, or given as a range in the form of MM/DD/YYYY-MM/DD/YYYY if the exact date of dissection is unknown | date or text |
| Source | How animal was procured, either “trap”, “roadkill”, or “euthanized by animal control” | text |
| Source_detail | Additional notes about animal source. SBCAS is Santa Barbara County Animal Shelter, USDA is United States Department of Agriculture, AIPM is Associated Pest Management. | text |
| Age_min | Minimum estimated age (in months) based on skull sutures, tooth emergence, and tooth wear. | numeric |
| Age_max | Maximum estimated age in months based on skull sutures, tooth emergence, and tooth wear. | numeric |
| Age_average | Average of Age_min and Age_max, in months. | numeric |
| Whole_length | Whole animal length, in millimeters. Taken from the tip of the nose to the end of the tail bone with the animal laying, straightened on its back on a measuring tape. Asterisks indicate that the measurement is non-standard, refer to “Notes” column before using. | numeric or text |
| Tail_length | Tail length measurement, in millimeters. Measured by bending tail at a 90 degree angle up from the body and measuring from the tail base to the end of the skin/bone (not including fur). Asterisks indicate that the measurement is non-standard, refer to “Notes” column before using. | numeric or text |
| Hindfoot_length | Hind foot length measurement, in millimeters. Measured by bending foot at the ankle and measuring from end of heel to tip of longest toe nail. | numeric |
| Ear_length | Ear length measurement, in millimeters. Measured from notch to tip of pinnae. Measuring to the edge of the skin, not end of the fur. | numeric |
| Weight | Weight in kilograms. Weighed by holding the animal on step scale, then subtracting the weight of the researcher and bag containing the animal. | numeric |
| Sex | Animal sex, either F for female or M for male. Determined by dissection and identification of gonads. | text |
| Testes | For male animals only, testes status is either “Abd” for abdominal or “Scr” for scrotal. | text |
| Embryos | For female animals only, if no embryos present “0”, otherwise the number of embryos. | numeric |
| Scars | For female animals only, uterus checked for scars and number of scars is recorded as either the total number or #+# referring to the number of scars in each uterine horn. | numeric or text |
| Spleen | Spleen weight in grams. | numeric |
| EPG_feces | Baylisascaris procyonis eggs per gram feces. Calculated using 4 grams of feces in 26ml of Sheathers sugar solution and McMaster slides as described in Weinstein (2016). 2–4 aliquots were counted from each host. If no data is present, then no fecal egg count was done for that host. | numeric |
| Notes | Additional notes, includes gonad condition, uterus width (U.W.), observed ectoparasites and host condition if substandard (eg. rotten). | text |
Table 5:
Variable definitions for RPSITE_SB_parasites_data.csv. Host-parasite records for the examined raccoons in RPSITE_SB_hosts_data.csv. Each parasite species record from each host is assigned a new row. See Section V for supplemental summary table of parasite prevalence and intensity.
| Variable | Definition | Format |
|---|---|---|
| Project_host_ID | Code assigned to the animal at time of dissection. | numeric |
| Sort_method | How gut contents were examined. “Not sorted” means that contents were not systematically sorted under a stereomicroscope and that host should not be used for parasite community or juvenile Baylisascaris analyses. “Sorted” means that all gut contents were examined under a stereomicroscope to remove all helminths greater than approximately 0.1 mm. If an asterisk is present, see Notes column. | text |
| Tissue | Parasite location, either “intestine” for intestine lumen (including acanthocephalans with proboscis anchored in wall and body in the lumen), or “stomach” for parasites found in stomach contents or attached to stomach wall. | text |
| Parasite_group | Taxonomic group of observed parasite: Nematoda, Trematoda, Acanthocephala, or Cestoda. If “0”, no helminth parasites were observed in that host. | text |
| Genus_species | Parasite genus, and species if known. If unidentifiable, the parasite is listed as “unknown”, if no parasites were present it is listed as “0”. | text |
| Count | Count of individual parasites, if less than 100 typically all individuals were counted, otherwise counts were estimated an estimated counts are indicated by either “~”, > 10 (for tens), >100 (for hundreds) or >1000 (for thousands). Includes adult and juvenile worms. | numeric or text |
| Notes | Additional notes about parasite samples and identification, including whether host should be used in parasite community analyses. | text |
Table 6:
Variable definitions for RPSITE_SB_baylisascaris_data.csv. Measurements on Baylisascaris worms recorded in RPSITE_SB_parasites_data.csv and removed from raccoons listed in RPSITE_SB_hosts_data.csv. When data entries are restricted (e.g. “M” or “F” or “U”), acceptable values are listed in the Definition.
| Variable | Definition | Format |
|---|---|---|
| Project_host_ID | Code assigned to the animal at time of dissection. | numeric |
| Worm_ID | Worm ID, each Baylisascaris worm is assigned a unique ID code which starts with the raccoon number (R#) followed by the worm number, generating a code in the from R#W#. If “0” no Baylisascaris were found in that host. | text or numeric |
| Location | Location in host small intestine, measured in centimeters from the junction with the stomach. For worms found in stomach, location is listed as stomach. These data were never used for analyses as worms can migrate after host death and it is unclear how reliable these measurements are. | text or numeric |
| Mature | Reproductive status of each worm. “A” for adult with mature gonads present, “U” for immature, without developed gonads. | text |
| Sex | Sex of worm. “M” for Male, “F” for female, “U” for uncertain. Uncertain typically used for immature worms without characteristic male and female tail morphology (or gonads) | text |
| Weight | Whole worm wet weight in grams. Worms were blotted on a paper towel and weighed on an electric balance; weight is in grams to the nearest 0.001g. Recorded only for a subset of worms. | numeric |
| Length | Full length of worm, measured to the nearest millimeter using a ruler. | numeric |
| Width | Width of worm at widest point, measured to the nearest hundredth of a millimeter using electronic calipers. Recorded only for a subset of worms. | numeric |
| Uterus_weight | Weight of uterus dissected out of female worms in grams. Recorded only for a subset of female worms | numeric |
| Notes | Additional notes | text |
Supplementary Material
Further Acknowledgments:
We would like to thank the many people who assisted with dissections and literature searches, including C. Lake, M. Luo, R. Lee, S. Hannah, E. Yee, G. Dunn, E. Lum, T. Grigsby, J. Mendez, A. Tokuyama, M. Armijo, J. Rydz and K. McKee. Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Sources Cited:
- Amin OM 1985. Classification. Pages 27–72 in Crompton DWT, and Nickol BB, editors. Biology of the Acanthocephala. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Anderson RC, Chabaud AG, and Willmott S, editors. 1975–1983. CIH keys to the nematode parasites of vertebrates. Parts 1–10. CAB International, Wallingford, UK. [Google Scholar]
- Bray RA, Gibson DI, and Jones A, editors. 2008. Keys to the Trematoda. Volume 3 CAB International, Wallingford, UK: 824 pp. [Google Scholar]
- Devleesschauwer B, Torgerson P, Charlier J, Levecke B, Praet N, Roelandt S, Smit S, Dorny P, Berkvens D, and Speybroeck N. 2014. Prevalence: tools for prevalence assessment studies. R package version 0.4.0 http://cran.r-project.org/package=prevalence. [Google Scholar]
- Dobson A, Lafferty KD, Kuris AM, Hechinger RF, and Jetz W. 2008. Homage to Linnaeus: How many parasites? How many hosts? Proceedings of the National Academy of Sciences 105:11482–11489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrt SD 2003. Raccoons and allies Pages 611–634 in Feldhamer GA, Thompson BC, and Chapman JA, editors. Wild mammals of North America: Biology, management, and conservation. The Johns Hopkins University Press, Baltimore, Maryland, USA. [Google Scholar]
- Gibbons LM 2010. Keys to the nematode parasites of vertebrates. Supplementary Volume CAB International, Wallingford, UK: 416 pp. [Google Scholar]
- Gibson DI, Jones A, and Bray RA, editors. 2002. Keys to the Trematoda. Volume 1 CAB International, Wallingford, UK: 521 pp. [Google Scholar]
- Jones A, Bray RA, and Gibson DI, editors. 2005. Keys to the Trematoda. Volume 2 CAB International, Wallingford, UK: 745 pp. [Google Scholar]
- Kazacos KR 2001. Baylisascaris procyonis and related species. Pages 301–341 in Samuel WM, Pybus MJ, and Kocan AA, editors. Parasitic diseases of wild mammals. Iowa State University Press, Ames, Iowa, USA. [Google Scholar]
- Khalil LF, Jones A and Bray RA, editors. 1994. Keys to the cestode parasites of vertebrates. CAB International, Wallingford, UK: 751 pp. [Google Scholar]
- Logiudice K 2003. Trophically transmitted parasites and the conservation of small populations: raccoon roundworm and the imperiled Allegheny woodrat. Conservation Biology 17:258–266. [Google Scholar]
- R Core Team. 2016. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Schell SC 1985. Handbook of Trematodes of North America North of Mexico. University Press of Idaho, Moscow, Idaho, USA, 263 pp. [Google Scholar]
- Schultz T 2002. “Raccoon roundworm” infection confirmed. In Santa Barbara News-Press; p. B1,3 McCaw, Wendy P. Santa Barbara, CA, USA. [Google Scholar]
- Sprent JFA 1952. On the migratory behavior of the larvae of various Ascaris species in white mice: I. Distribution of larvae in tissues. Journal of Infectious Diseases 90:165–176. [DOI] [PubMed] [Google Scholar]
- Weinstein SB 2016. Baylisascaris procyonis demography and egg production in a California raccoon population. Journal of Parasitology 102:622–628. [DOI] [PubMed] [Google Scholar]
- Weinstein SB, Van Wert JC, Kinsella M, Tkach VV, and Lafferty KD. In review Infection at an ecotone: Cross-system foraging increases satellite parasites but decreases core parasites in coastal raccoons. [DOI] [PMC free article] [PubMed]
- Zeveloff SI 2002. Raccoons: a natural history. Smithsonian Institution Press and UBC Press, Vancouver/Toronto, Canada: 240 pp. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


