Abstract
The discovery of a mechanism that guards against a type of cell death celled ferroptosis reveals a system that regenerates a ubiquitous protective component of biological membranes, and might offer a target for anticancer drugs.
On 3 December 1956, the biochemist Frederick Crane detected, for the first time ever, a yellow substance purified from cow hearts, which had been obtained from the Oscar Mayer meat-processing factory in Madison, Wisconsin1. In the laboratory of David Green at the University of Wisconsin, Crane investigated the oily material he had discovered, and struck gold: he found a lipid molecule that has a crucial role in energy generation in cells. But there were hints that it might have another function. Now, writing in Nature, Doll et a12. and Bersuker et al3. report the discovery of this elusive role, finally revealing the missing part of the puzzle 63 years after Crane’s discovery.
When Crane analysed the molecule he had identified, he noted that it was structurally similar to certain vitamins, and it was initially named vitamin Q10. This molecule is important for respiration1−the energy-generating process that makes the molecule ATP−and acts in mitochondria, organelles that are responsible for much of the energy production in cells. It was subsequently discovered1 to be ubiquitous. It not only resides in mitochondria, but is also present elsewhere in the cell in almost all lipid membranes.
The molecule was renamed ubiquinone because of its ubiquity and because it contains a type of chemical structure known as a quinone. Ubiquinone now also goes by the name coenzyme Q10. But its function outside mitochondria has remained enigmatic, until now.
In 2012, a previously unknown type of programmed cell death called ferroptosis was described4. This iron-dependent pathway causes cells to die when a lipid modification called peroxidation degrades their membranes (Fig. 1). Ferroptosis has been linked to a range of processes. For example, it has been implicated in degenerative diseases; in damage to plants caused by climate-change-associated heat stress; and in natural functions of the immune system, such as the elimination of tumour cells by killer T cells5–7. Therapeutic approaches that harness ferroptosis are being developed for some cancers, given that the abnormalities of certain tumour cells can make them vulnerable to this type of cell death.
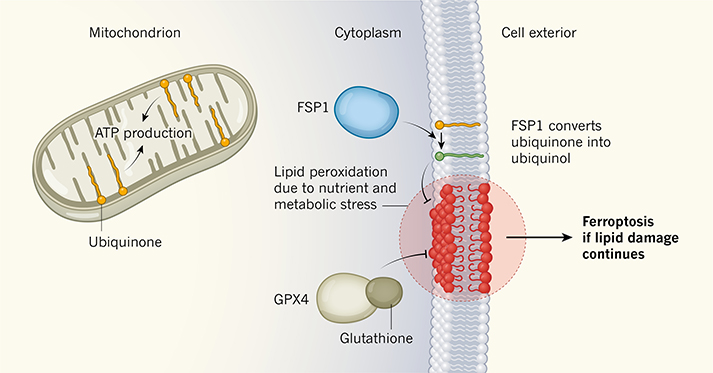
Figure 1 |. A pathway that blocks cell death.
Doll et al.2 and Bersuker et al3. report studies revealing that the FSP1 protein protects human cells from a type of cell death called ferroptosis. Some tumour cells are susceptible to ferroptosis, and this insight about FSP1 is of clinical interest. The finding has also uncovered a previously unknown role for a lipid called ubiquinone. Ubiquinone is found in lipid membranes, including those of an organelle called a mitochondrion, where it aids production of the molecule ATP, the cell’s energy carrier1. The authors report that FSP1 targets ubiquinone in the cell membrane to generate a reduced form of the molecule, called ubiquinol (green). Ferroptosis occurs if a form of lipid modification called peroxidation (red) damages the cell membrane; however, ubiquinol inhibits peroxidation and blocks ferroptosis. FSP1 acts independently of another pathway known to block lipid peroxidation and ferroptosis8, which requires the proteins GPX4 and glutathione.
The damage to cell membranes that occurs during ferroptosis can be prevented by a protein called GPX4 acting together with a small peptide called glutathione8, which confers antioxidant properties by combating lipid damage caused by peroxidation. As a consequence, approaches that inhibit GPX4 and that deplete glutathione from cells have recently been examined as potential anticancer strategies9. In 2016, researchers developed FIN56, a chemical that can induce ferroptosis, and this tool provided a clue that there might be another mechanism, besides GPX4-and glutathione-mediated repair, that protects against this process10. FIN56 was found to kill cells by perturbing a metabolic pathway called the mevalonate pathway, which acts upstream of ubiquinone synthesis; furthermore, supplementing cells with idebenone, a synthetic compound that is similar to ubiquinone, prevented FIN56 lethality, suggesting that FIN56 functions by depleting ubiquinone10. This previous work raised the question of whether ubiquinone normally functions to protect cells from ferroptosis. However, because FIN56 can deplete both ubiquinone and GPX4, it was difficult to draw any definitive conclusions.
Doll et al. and Bersuker et al. hypothesized that cells have a way of protecting themselves against ferroptosis even in the absence of GPX4. This was a heretical idea given that, during the short history of ferroptosis research, the dogma that GPX4 is essential to guard against ferroptosis in all contexts had already been established. Nevertheless, these two teams searched for other such protective mechanisms. Both groups analysed human cells grown in vitro to test whether any components block ferroptosis when GPX4 is not present, and they independently identified a gene encoding a protein that they name ferroptosis suppressor protein 1 (FSP1), which was previously called AIFM2.
Excitingly, the authors discovered that FSP1 replenishes a reduced form of ubiquinone, called ubiquinol, that acts protectively by combating the lipid peroxidation that drives ferroptosis. Further experiments revealed that this FSP1-dependent modification of ubiquinone, in locations other than mitochondria, acts to protect against ferroptosis. The comic-book superhero Green Lantern has a power ring that needs to be recharged once its protective energy becomes depleted, and, by analogy, FSP1’s role in generating protective ubiquinol could be a similarly crucial recharging process.
The identification of this FSP1-mediated process suggests that drugs that inhibit FSP1 might be developed as anticancer treatments. Doll et al. and Bersuker et al. found that the level of resistance to ferroptosis across many human cancer cell lines grown in vitro correlates with the amount of FSP1 present in the cells, suggesting that modulating FSP1 might have clinical relevance. It would also be worth investigating whether treatments that boost FSP1 activity are useful as therapies for degenerative diseases driven by ferroptosis. These two latest studies clearly suggest that the mysteries of ferroptosis continue to yield important biological and therapeutic insights.
Footnotes
The author declares competing financial interests: see go.nature.com/2bdv9eq for details.
References
- 1.Crane FL Mitochondrion 7 (Suppl.), S2–S7 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Doll S et al. Nature 10.1038/s41586-019-1707-0 (2019). [DOI] [Google Scholar]
- 3.Bersuker K et al. Nature 10.1038/s41586-019-1705-2 (2019). [DOI] [Google Scholar]
- 4.Dixon SJ et al. Cell 149, 1060–1072 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stockwell BR & Jiang X Cell Metab. 30, 14–15 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirschhorn T & Stockwell BR Free Radic. Biol. Med 133, 130–143 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stockwell BR et al. Cell 171, 273–285 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang WS et al. Cell 156, 317–331 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H, Schreiber SL & Stockwell BR Biochemistry 57, 2059–2060 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimada K et al. Nature Chem. Biol 12, 497–503 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]