SUMMARY
It has long been known that excessive mitotic activity due to H-Ras can block keratinocyte differentiation and cause skin cancer. It is not clear whether there are any innate surveillants that are able to ensure that keratinocytes undergo terminal differentiation, preventing the disease. IKKα induces keratinocyte terminal differentiation and its reduction promotes skin tumor development. However, its intrinsic function in skin cancer is unknown. Here we found that mice with IKKα deletion in keratinocytes developed a thickened epidermis and spontaneous squamous cell-like carcinomas. Inactivation of epidermal growth factor receptor (EGFR) or reintroduction of IKKα inhibited excessive mitosis, induced terminal differentiation, and prevented skin cancer through repressing an EGFR-driven autocrine loop. Thus, IKKα serves as an innate surveillant.
INTRODUCTION
Keratinocytes constitute the stratified epidermis, the largest and most rapidly renewing organ in the body (Blanpain et al., 2007). Keratinocytes in the basal epidermis are mitotic, providing new cells to replace those that are shed. After moving to the suprabasal layers, the cells gradually differentiate and give rise to the tough, soft cornified layer at the top of the skin that protects the internal organs. Each cell renewal cycle takes approximately 4 weeks. Stem cells in the epidermis and hair follicles also provide cells needed in case of “emergent cell loss”, such as injury. Therefore, a balance between keratinocyte proliferation and differentiation is required to maintain epidermal homeostasis. Numerous extrinsic or intrinsic factors can trigger keratinocytes to produce a broad spectrum of growth factors and cytokines, influencing their differentiation and proliferation. Maintaining proper cellular responses to various stimuli is, therefore, a pre-requisite for preventing skin disorders.
The mitogenic signaling cascade from EGFR, Ras, and extracellular signal-regulated kinase (ERK) to transcription factors, activating gene expression of growth factors including EGFR ligands, has been implicated in the regulation of keratinocyte proliferation and differentiation (Dlugosz et al., 1997). Two decades ago, Yuspa and colleagues demonstrated that oncogenic v-H-Ras induced keratinocyte proliferation and transformation but blocked terminal differentiation (Yuspa et al., 1985). The pathology of Ras-induced keratinocyte-derived cancer resembles human squamous cell carcinomas (SCCs). The chemical carcinogen 7,12-dimethylbenz[a]anthracene (DMBA) activates H-Ras to initiate skin tumors (Balmain and Pragnell, 1983). The activating Ras mutations and elevated Ras-related pathways, as well as elevated activity of EGFR and its ligands, were also seen in human SCCs (Leong et al., 2004; Pierceall et al., 1991). Thus, elevated activity along this cascade provides the molecular basis for the promotion of keratinocyte proliferation, dedifferentiation, and transformation. On the other hand, inactivation of EGFR was found to inhibit Ras-mediated keratinocyte proliferation and promote differentiation in vitro, as well as prevent skin tumors induced by overexpression of a dominant form of son of sevenless (SOS-F), a guanine nucleotide exchange factor that facilitates the GDP-GTP exchange of Ras protein (Sibilia et al., 2000). Thus, EGFR may serve as a switch-point for keratinocyte proliferation and differentiation. However, it is unknown whether there are any surveillants that can antagonize the cascade activity to balance keratinocyte proliferation and differentiation.
Gene disruption studies demonstrated that the epidermis of Ikkα−/− newborn mice lacked a terminally differentiated cornified layer and exhibited marked thickening; these mice died soon after birth (Hu et al., 1999; Takeda et al., 1999). Reintroduction of IKKα or kinase inactive IKKα induced keratinocyte terminal differentiation and repressed hyperproliferation in vitro and in vivo (Hu et al., 2001; Sil et al., 2004). These findings underscore a pivotal role for IKKα in regulating keratinocyte proliferation and differentiation. Furthermore, our recent studies showed that reduced IKKα expression provided a selective growth advantage that cooperated with Ras activity to promote the formation of benign and malignant skin tumors induced by DMBA/12-O-tetradecanoylphorbol-13-acetate (TPA) (Park et al., 2007). Loss of heterozygosity, a classical tumor suppressor feature, was found in most carcinomas in Ikkα+/− mice, and somatic Ikkα mutations were detected in Ikkα+/+ carcinomas. Also, Ikkα genetic alterations and epigenetically down-regulated IKKα expression were reported to be associated with dedifferentiation, invasion, and progression of human SCCs (Liu et al., 2006; Maeda et al., 2007). Thus, IKKα may regulate mitogenic and Ras activities in keratinocyte proliferation, differentiation, and skin tumor development.
Differentiation is thought to require the withdrawal of keratinocytes from the cell cycle (Dlugosz et al., 1997; Gandarillas and Watt, 1997). Activated Ras has been suggested to increase the S phase, so that the enhanced cell progression is able to block the exit of keratinocytes from the cell cycle, thereby preventing differentiation (Dlugosz et al., 1997). We previously reported that there were more bromodeoxyuridine (BrdU)-labeled keratinocytes, an indicator for the S phase, in IKKα-null than in wild-type (WT) mouse epidermis (Hu et al., 1999). An elevated S phase and a reduced G2/M phase were detected in primary cultured, undifferentiated Ikkα−/− keratinocytes (Hu et al., 2001; Zhu et al., 2007). The re-expression of IKKα rescued these cell cycle defects. Thus, IKKα-loss-enhanced cell progression may prevent keratinocyte terminal differentiation by utilizing this cell cycle mechanism. However, the pathway that IKKα uses to regulate this switch between keratinocyte proliferation and differentiation remains to be defined.
IKKα, IKKβ, and IKKγ (NEMO) form the IKK complex that activates NF-κB through phosphorylating IκBs, the inhibitors of NF-κB (Ghosh and Karin, 2002). Although IKKα and IKKβ are highly conserved protein kinases, IKKβ shows a stronger kinase activity for IκBs than does IKKα. Mice with deletion of IKKβ or IκBα in the basal epidermis developed tumor necrosis factor receptor (TNFR)-dependent skin inflammation and mice overexpressing IκBα in the basal epidermis developed TNFR-dependent skin tumors (Lind et al., 2004; Pasparakis et al., 2002; Rebholz et al., 2007). However, mice overexpressing IKKα in the basal or suprabasal epidermis had normal skin and developed fewer SCCs and metastases induced by DMBA/TPA than did WT mice (Liu et al., 2006; Sil et al., 2004). Elevated IKKα expression was found to enhance terminal differentiation and antagonize chemical carcinogen-induced mitogenic and angiogenic activities. Conversely, reduced IKKα expression resulted in elevated mitogenic and angiogenic activities (Park et al., 2007). Thus, these physiological functions of IKKα in the skin of mice are beyond NF-κB signaling alone.
A study, however, suggested that IKKα-mediated keratinocyte differentiation was not cell autonomous (Gareus et al., 2007), which could not explain the phenotypes in IKKα deficient mice and keratinocytes described above. Also, the function of IKKα in adult mice has not been revealed. Thus, here we generated mice with IKKα deletion in keratinocytes and investigated the role of IKKα in skin homeostasis and skin cancer development.
RESULTS
IKKα Deletion in Keratinocytes Causes Thickened and Wrinkled Skin in Mice
To determine the function of IKKα in the skin of mice, we generated mice with a floxed Ikkα allele (IkkαF/F) (Figure S1A–D). Genotyping confirmed that the number of IkkαF/F homozygotes matched the expected Mendelian ratio. To study the role of IKKα in the epidermis, we crossed IkkαF/F mice with mice expressing Cre under the control of keratin 5 (K5) promoter, which is expressed in basal epidermal keratinocytes, to generate IkkαF/F/K5.Cre mice. It has been documented that K5.Cre or K14.Cre is expressed in oocytes of female mice, which leads to germline mutations (Hafner et al., 2004; Ramirez et al., 2004). The expression of paternally transmitted K5.Cre was reported in the mouse epidermis at embryonic day 15.5. As expected, the appearance, skins, and skeletons of IkkαF/F/K5.Cre mice generated from male IkkαF/F and female IkkαF/+/K5.Cre mice were identical to those of Ikkα−/− mice (Figures 1A, S2, S3) (Hu et al., 1999; Takeda et al., 1999). Western blotting showed no IKKα expression in multiple organs obtained from the IkkαF/F/K5.Cre mice (Figure 1B). IkkαF/F/K5.Cre newborns generated from female IkkαF/F and male IkkαF/+/K5.Cre mice were indistinguishable from wild-type (WT, IkkαF/F) mice (P1, Figure 1C). By day 5, however, the skin of IkkαF/F/K5.Cre mice was noticeably more wrinkled and thicker, and mice gradually showed retarded development (P11, Figure 1C). Most mutant mice died between 12 to 16 days after birth; a few survived longer but no longer than 22 days. No food was found in the stomachs of the dead mice (Figure S4A). The esophagus was smaller in mutant mice than in WT mice (Figure S4B). A similar phenotype has been described in Ikkα−/− mice, which might be relevant to the mouse deaths (Hu et al., 1999; Sil et al., 2004). Western blotting detected a low level of IKKα in the epidermis of mutant mice at day 1 after birth and a further reduction of IKKα expression was observed later (Figure 1D), which was consistent with the loss of the WT Ikkα allele in DNA isolated from the epidermis of the mice (Figure S5). IKKα expression was reduced in the tongue, esophagus, and intestine of IkkαF/F/K5.Cre mice, but was expressed normally in the dermis, liver, lung, spleen (B cells), stomach, thymus (T cells), bone, brain, heart, kidney, bone marrow (hematopoietic cells), and blood cells (hematopoietic cells) in IkkαF/F/K5.Cre mice compared with those in WT mice (Figure 1E). The level of IKKα expression depended on whether any Ikkα alleles remained (Figure 1E). These results indicate that K5.Cre deletes IKKα specifically in the cells that express K5 in IkkαF/F mice. To evaluate the effect of mouse genetic backgrounds on these phenotypes, we backcrossed IkkαF/F and K5.Cre mice with C57BL6 or FVB mice for five generations. An FVB background was found to confer a slight increase in skin thickness and slightly earlier death compared to a C57BL6 background (data not shown). Collectively, these results suggest that a low level of IKKα in the epidermis is sufficient for normal embryonic skin development, but a further reduction causes skin lesions.
Figure 1. IKKα Deletion in Keratinocytes Causes Skin Defects in Postnatal Mice.
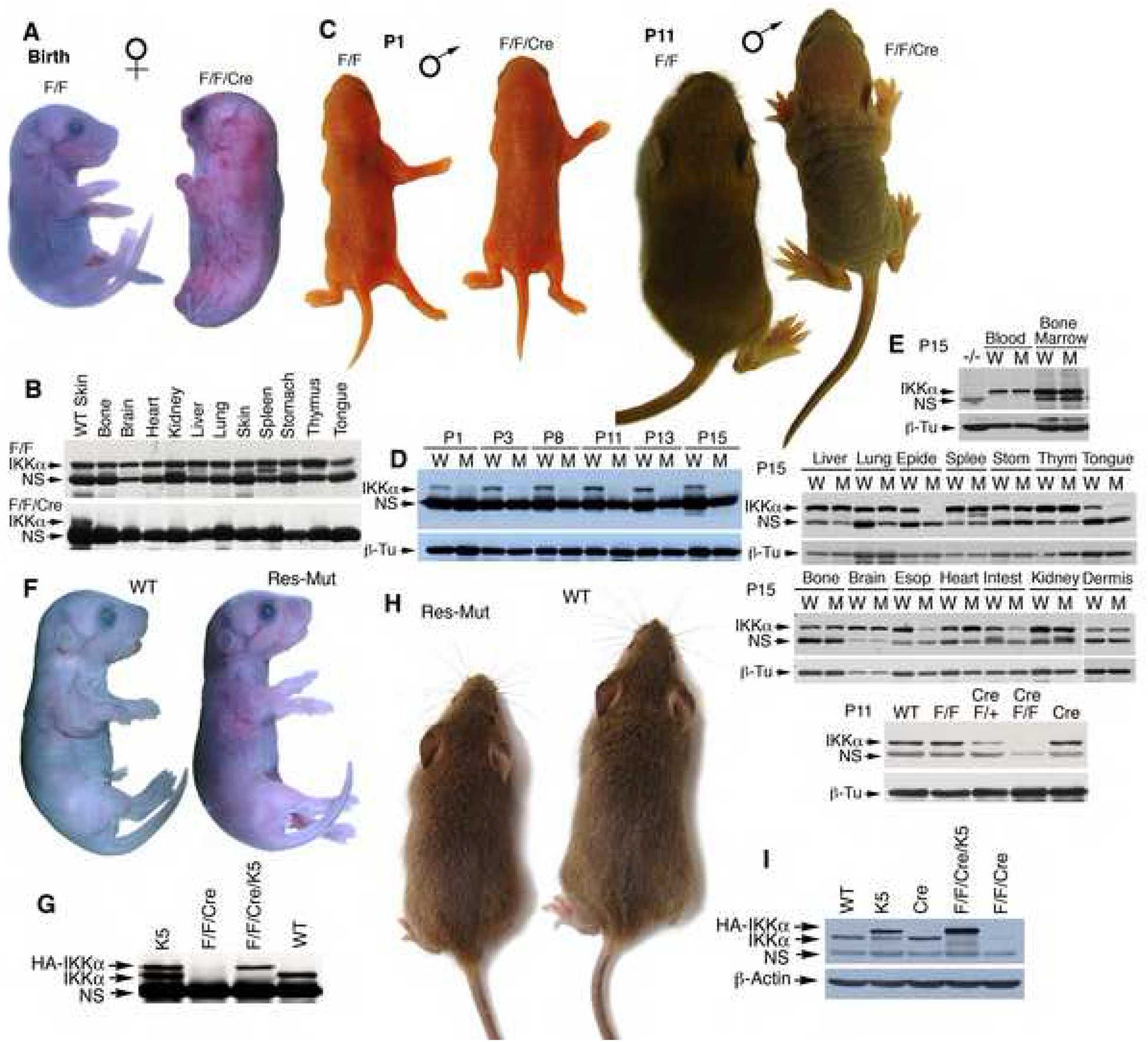
(A) Appearances of IkkαF/F (F/F) and IkkαF/F/K5.Cre (F/F/Cre) mice at birth. Female symbol, maternally transmitted K5.Cre.
(B) IKKα levels in different organs of mice (A), as detected by Western blotting. NS, non-specific band.
(C) Appearances of IkkαF/F (F/F) and IkkαF/F/K5.Cre (F/F/Cre) mice. Male symbol, paternally transmitted K5.Cre; P, postnatal day.
(D-E) IKKα levels in the epidermis of IkkαF/F (W) and IkkαF/F/K5.Cre (M) mice (C), as detected by Western blotting. NS, non-specific bands; β-Tu, β-tubulin as loading control; WT, wild-type; Epide, epidermis; Splee, spleen; Stom, stomach; Thym, thymus; Esop, esophagus; Intest, intestine; blood, blood cells.
(F) Appearances of WT and maternally transmitted IkkαF/F/K5.Cre/K5 (Res-Mut) newborns.
(G) IKKα levels in the skin of indicated mice (F), as detected by Western blotting. K5, K5.IKKα transgene; HA-IKKα, HA-tagged IKKα transgene expression.
(H) Appearances of WT and paternally transmitted IkkαF/F/K5.Cre/K5 (Res-Mut) mice at 4 weeks old.
(I) IKKα levels in the epidermis of indicated mice (H), as detected by Western blotting. β-Actin, loading control.
To verify that these phenotypes in IkkαF/F/K5.Cre mice were due to an IKKα-specific deletion in keratinocytes, we re-expressed a keratinocyte-specific K5.IKKα transgene in IkkαF/F/K5.Cre mice (Lomada et al., 2007). K5.IKKα expression rescued the skin phenotype of maternally transmitted IkkαF/F/K5.Cre mice (Figures 1F, G, S6A), but the rescued mice died soon after birth, which was consistent with observations in previous reports (Lomada et al., 2007; Sil et al., 2004). K5.IKKα expression completely rescued paternally transmitted IkkαF/F/K5.Cre mice (Figures 1H, I, S6B). No skin disorders were found in adult IkkαF/F/K5.Cre/K5.IKKα mice. Thus, IKKα deletion did cause the skin phenotypes in IkkαF/F/K5.Cre mice.
IKKα Deletion Causes Epidermal Keratinocyte Hyperproliferation and Deregulates Expression of Many Genes
Histologies of the skins from WT and IkkαF/F/K5.Cre mice were almost identical on days 1 and 3 (Figure 2A), which reflected their normal appearances. From days 6 to 22, the epidermal thickness in mutants gradually increased (Figure 2A). The total number of keratinocytes in the epidermis was significantly higher in mutants than in WT mice. Immunohistochemical staining showed that the entire mutant epidermis expressed K5 and gradually reduced K10 with increased epidermal thickness (Figure S7). The thickened mutant epidermis did not express the terminal differentiation markers loricin and filaggrin, although the markers were weakly detected on the surface of the skin (Figure S7). These results suggest that hyperproliferation may prevent terminal differentiation in keratinocytes. We also examined CD34-positive keratinocytes, which are have been identified as follicular stem cells (Blanpain et al., 2004; Morris et al., 2004). Hair follicles are still developing in mice at 1 to 16 days old; thus, the hair follicle of an adult mouse was used as a control for CD34-positive cells in the hair follicle (Figure 2B). Immunohistochemical staining showed that the number of CD34-positive keratinocytes was similar in hair follicles of WT and mutant mice although the total number of keratinocytes was greater in mutant hair follicles than in WT hair follicles (Figures 2B, S7), suggesting that IKKα deletion may have a stronger impact on proliferation of CD34 negative keratinocytes than on CD34 positive keratinocytes.
Figure 2. IKKα Deletion Causes Epidermal Hyperplasia.
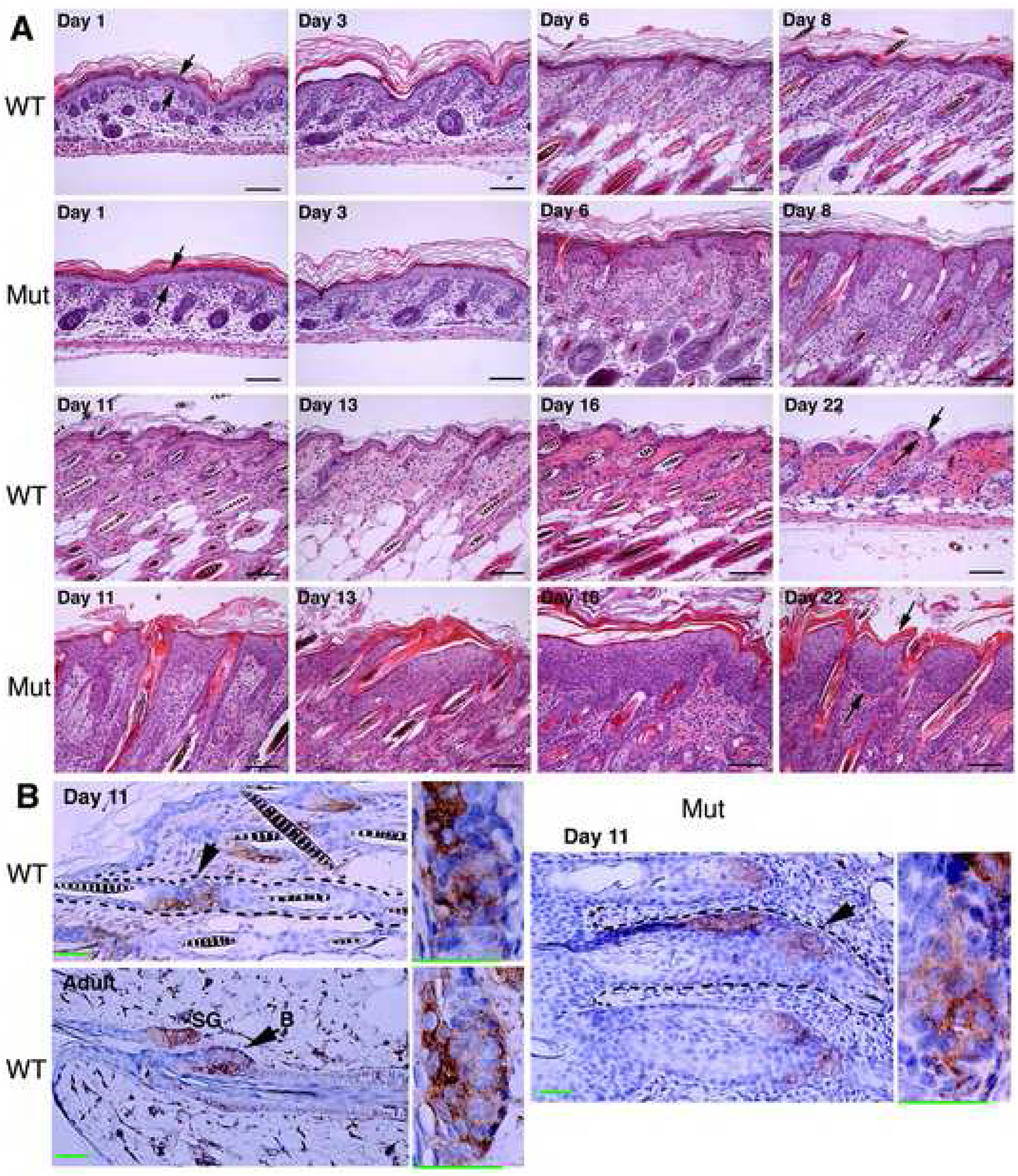
(A) Histology of the skin of indicated mice, stained with hematoxylin and eosin (H&E). WT, wild-type mice; Mut, IkkαF/F/K5.Cre mice; Day, postnatal day; arrows, indicating epidermis. Scale bars, 30 μm.
(B) Brown, CD34-positive cells indicated by arrows, enlarged in boxes; blue, nuclear counter-staining; SG, sebaceous gland; adult, control for CD34-positive cells in bulge. Scale bars, 30 μm.
Mice with keratinocyte-specific IKKα deletions by K14.Cre generated in a previous study died at birth (Gareus et al., 2007). Although it is difficult to speculate on their observation because the authors did not describe in detail how they generated those mice, we attempted to clarify the differences by generating IkkαF/F/K14.Cre mice. Paternally transmitted IkkαF/F/K14.Cre and IkkαF/F/K5.Cre mice showed similar skin phenotypes (Figure S8A). Some of the maternally transmitted IkkαF/F/K14.Cre mice recapitulated the phenotype of Ikkα−/− mice (Figure S8B) and others developed epidermal hyperplasia after birth (data not shown). Ramirez et al. (2004) observed a partial gene deletion pattern from oocytes in some cases by Cre, which might explain these different phenotypes.
Next, we determined the mitotic activity in the epidermis using immunohistochemical staining. We found that the proliferation marker Ki67 was significantly more numerous in IkkαF/F/K5.Cre than in WT epidermis (Figure 3A). BrdU-positive cells were consistently more numerous in the mutant than in the WT epidermis (Figure S9A, B), and expression of K6, which is highly expressed in abnormally proliferating epidermis, was elevated in the mutant epidermis (Figure 3A). The abnormally proliferating epidermis may promote dermal cell proliferation or attract macrophages or lymphocytes. Thus, we found a slightly increase in macrophages (F4/80) but no increase in T (CD3) or B (CD45R) cells (Pasparakis et al., 2002) in the stroma of the mutants 6 days old (Figure S10A). Also, TNFα expression was higher in IKKα null than in WT keratinocytes (Figure S10B).
Figure 3. IKKα Deletion Causes Keratinocyte Hyperproliferation and Deregulates Expression of Many Genes.
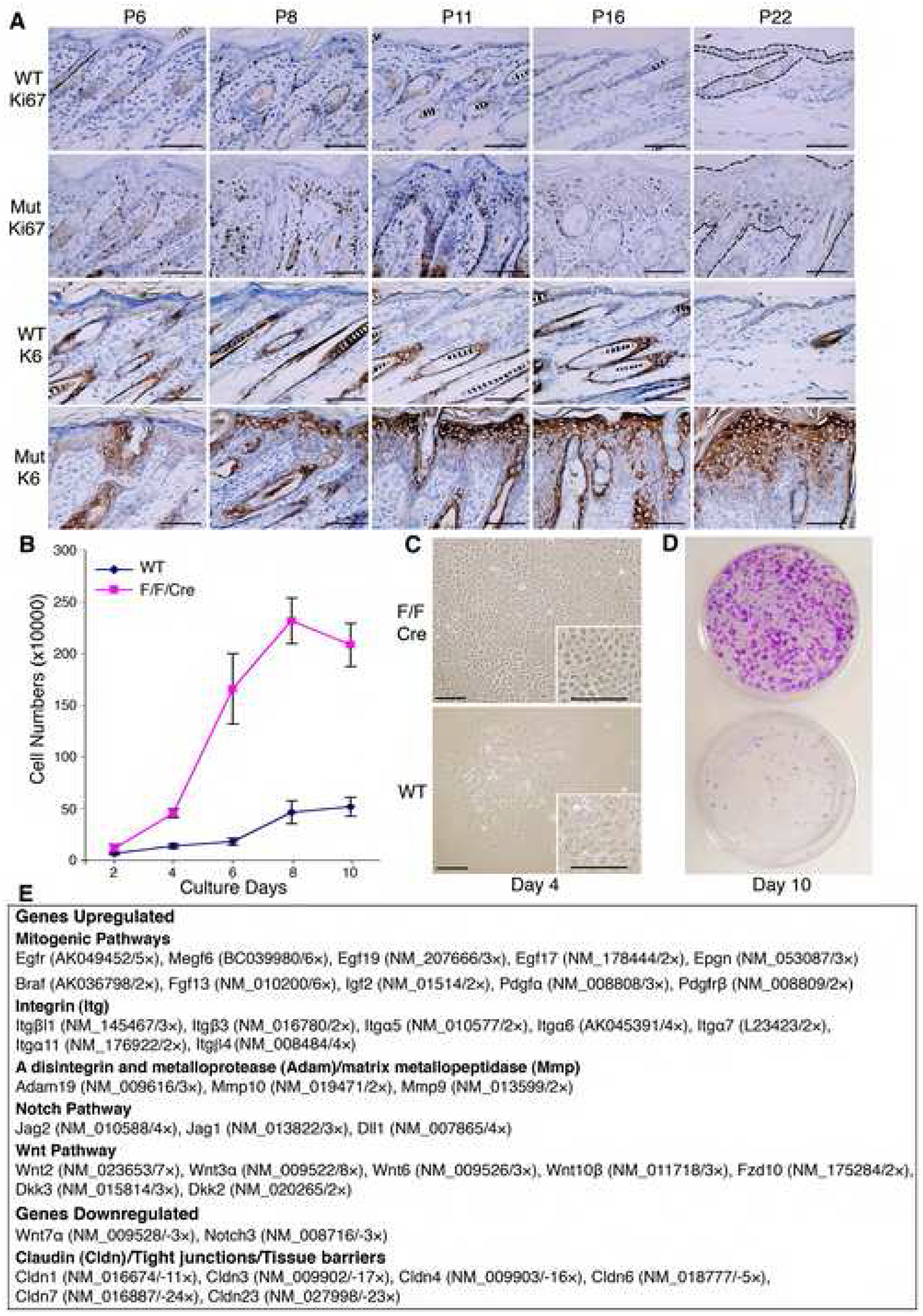
(A) Immunohistochemical skin sections stained for Ki67 and K6. Mut, IkkαF/F/K5.Cre; dark brown color, positive cells; blue color, nuclear counter-staining; P, postnatal day. Scale bars, 50 μm.
(B) Keratinocyte growth curve. Equal numbers of cells from newborns were plated in cultures and cell growth was statistically analyzed at indicated days (n=8). Error bars indicate ± SD.
(C) Keratinocyte morphologies in culture at day 4. Scale bars, 50 μm.
(D) Keratinocytes colony formation at day 10. Cell colonies were stained with 0.5% crystal violet solution.
(E) A summary of microarray analysis results for cultured keratinocytes. Genes were up-regulated and down-regulated in IkkαF/F/K5.Cre compared to WT cells. References for each gene’s functions can be found in the Systematic Name database via NCBI. Detailed results are shown in Table S1.
To determine if the hyperplastic epidermis is intrinsic to IKKα-deficient keratinocytes, we cultured keratinocytes isolated from IkkαF/F/K5.Cre and WT newborns. Cell growth curves showed that mutant cells grew more rapidly than WT cells (Figure 3B). WT keratinocytes had terminally differentiated morphologies (Hu et al., 2001), whereas mutant cells did not (Figure 3C). Also, mutant cells formed many more and larger colonies than did WT cells (Figure 3D). Thus, IKKα deletion induced keratinocyte-autonomous hyperproliferation.
To determine which signaling pathways may promote cell proliferation, we compared the transcriptional profiles of genes in WT and mutant keratinocytes by using microarray analyses (Table S1). As shown in Figure 3E, up-regulated genes in mutants include EGFR, EGFR ligands, Braf, FGF13, IGF2, and PDGFα; a group of integrin genes; ADAM19, MMP10 and MMP9; Notch ligands; Wnt inhibitors Dkk3 and Dkk2; and various Wnts. Down-regulated genes include Wnt7α, Notch3, and a group of Claudins (Figure 3E and Table S1). Such a broad collection of deregulated genes indicates that IKKα may have a broad impact on skin biology.
IKKα Downregulates an Autocrine Loop of EGFR, ERK, EGFR Ligands, and a Group of ADAMs in the Epidermis and in Keratinocytes
To determine the signaling pathways responsible for hyperproliferation and dedifferentiation in IKKα-deficient cells (Figure 3E), we examined the EGFR-driven pathway because it has been reported to regulate keratinocyte proliferation and differentiation (Dlugosz et al., 1997). Also, the activity of ERK, a downstream target of EGFR, was previously found to be elevated in IKKα deficient keratinocytes (Park et al., 2007; Sil et al., 2004). Western blotting showed more EGFR and ERK activity in mutant than in WT epidermis (Figure 4A, B). Because EGFR ligands activate EGFR and the appearance of IkkαF/F/K5.Cre mice resembles that of mice expressing increased soluble forms of heparin-binding EGF (HB-EGF) (Yamazaki et al., 2003), we examined levels of EGF and HB-EGF. Using Western blotting, we found higher levels of mature EGF and HB-EGF and lower levels of EGF and HB-EGF precursors in the mutant than in the WT epidermis (Figure 4C). It is known that ADAMs sheddases cleave EGF and HB-EGF precursors to generate their active soluble forms (Huovila et al., 2005). Figure 3E shows that ADAM19 was up-regulated in IKKα-deficient keratinocytes. We thus examined the mRNA levels of ADAM12, 10, 17, 19, and 9 in primary cultured keratinocytes by using reverse transcription polymerase chain reaction (RT-PCR) and found that their expression levels were higher in Ikkα−/− than in WT cells and that the stimulation of cell growth elevated these levels (Figure 4D). Western blotting confirmed elevated levels of ADAM12, 10, and 17 in the mutant epidermis (Figure 4E). The presence of elevated ADAM10 expression in mutant epidermis was confirmed by immunofluorescence staining (Figure S11A). Furthermore, zymography (Hall and Erickson, 2003) verified that ADAMs were more active in the mutant than in the WT epidermis, and reintroduction of IKKα repressed this activity (Figure 4F).
Figure 4. Elevated Autocrine Loop in IKKα Deficient Keratinocytes.
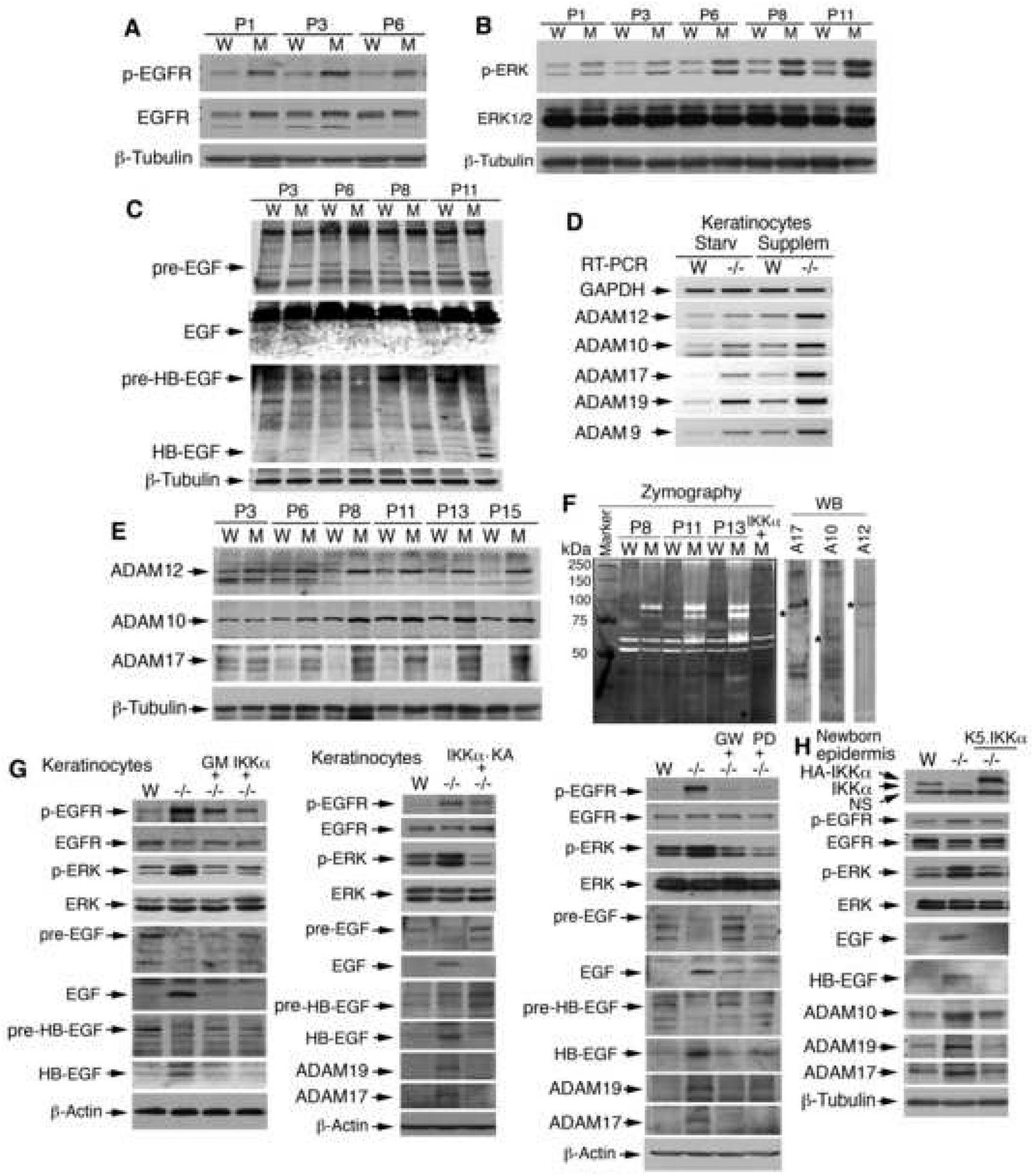
(A-C) Levels of phosphorylated (p)-EGFR and p-ERK, HB-EGF, EGF, precursor (pre)-EGF, and pre-HB-EGF in the epidermis of WT (W) and IkkαF/F/K5.Cre (M) mice, as detected by Western blotting. P, postnatal day; β-Tubulin, loading control.
(D) mRNA levels of ADAMs in WT and Ikkα−/− (−/−) primary keratinocytes cultured with no growth supplement for 1 day (Starv) or with growth supplement (Supplem), as detected by RT-PCR. GAPDH, PCR control.
(E) Levels of ADAM12, 10, and 17 in the epidermis of WT and IkkαF/F/K5.Cre (M) mice, as detected by Western blotting.
(F) Activities of sheddases in the epidermis of WT, IkkαF/F/K5.Cre (M) and IkkαF/F/K5.Cre/K5.IKKα mice (M+IKKα), as detected by gelatin zymography. WB, Western blotting; *, indicates proteins.
(G) Levels of p-EGFR, p-ERK, EGF, HB-EGF, and/or ADAMs in WT, Ikkα−/− (−/−), and Ikkα−/−keratinocytes treated with GM6001 (GM), GW2974 (GW), and/or PD98059 (PD) for 24h or with reintroduced IKKα or IKKα-KA after 48 h, as detected by Western blotting. β-Actin, loading control.
(H) Levels of indicated proteins in the skin of WT, Ikkα−/− (−/−), Ikkα−/−/K5.IKKα newborns, as detected by Western blotting. NS, non-specific band.
ERK and EGFR are believed to up-regulate ADAMs (Huovila et al., 2005). We, therefore, hypothesized that IKKα regulated EGFR-driven mitogenic signaling and ADAMs expression through the same signaling loop. To prove this hypothesis, we treated IKKα-deficient keratinocytes with inhibitors for a broad spectrum of ADAMs (GM6001), EGFR (GW2974), and/or ERK (PD98059) and re-expressed IKKα or a kinase dead full-length IKKα (a mutation at the ATP binding site within the kinase domain, IKKα-KA) in the cells. Western blotting showed reduced EGFR and ERK activities and EGF, HB-EGF and ADAMs levels but elevated EGF and HB-EGF precursors in mutant keratinocytes after these treatments (Figure 4G). Similar results were obtained for transgenic IKKα in the epidermis (Figure 4H). Taken together, these results suggest that IKKα downregulates the activity of the autocrine loop of EGFR, ERK, ADAMs, and EGFR ligands in keratinocytes.
IKKα Suppresses Promoter Activation of EGF, HB-EGF, Amphiregulin (AR), and ADAMs
To determine the mechanism of how IKKα prevents EGFR autocrine loop activity in keratinocytes, we examined whether IKKα regulated the transcription of EGFR ligand genes and ADAMs because IKKα has been shown to have transcriptional activity in the keratinocyte nucleus (Liu et al., 2006). Using the ChIP assay, we found that IKKα bound to the promoters of the EGF (−1511 to −1133 bp), HB-EGF (−1911 to −1534 bp), and AR (−3129 to −2785 bp) genes in Ikkα+/+ and Ikkα+/− primary cultured keratinocytes but not in Ikkα−/− keratinocytes (Figure 5A). The bonds were stronger in Ikkα+/+ than in Ikkα+/− cells. Treatment with TPA (30 ng/ml) attenuated binding of IKKα to these promoters, which profoundly correlated with increased expression of these corresponding genes (Figure 5B). Treatment with EGF (10 ng/ml) also reduced binding of IKKα to the promoters of the EGF, HB-EGF, AR, ADAM12 (−2733 to −2516 bp), ADAM19 (−3402 to −3182 bp), and ADAM17 (−2389 to −2073 bp) genes in WT keratinocytes (Figure 5C), and the addition of growth factors elevated the expression of these ADAMs (Figure 4D). We found that IKKα failed to bind to the proximate promoter regions of the genes described above (data not shown). These results suggest that IKKα suppresses the induced expression of EGF, HB-EGF, AR, and ADAMs by down-regulating their promoter activities.
Figure 5. IKKα Regulates Promoter Activities of EGF, HB-EGF, AR, and ADAMs.
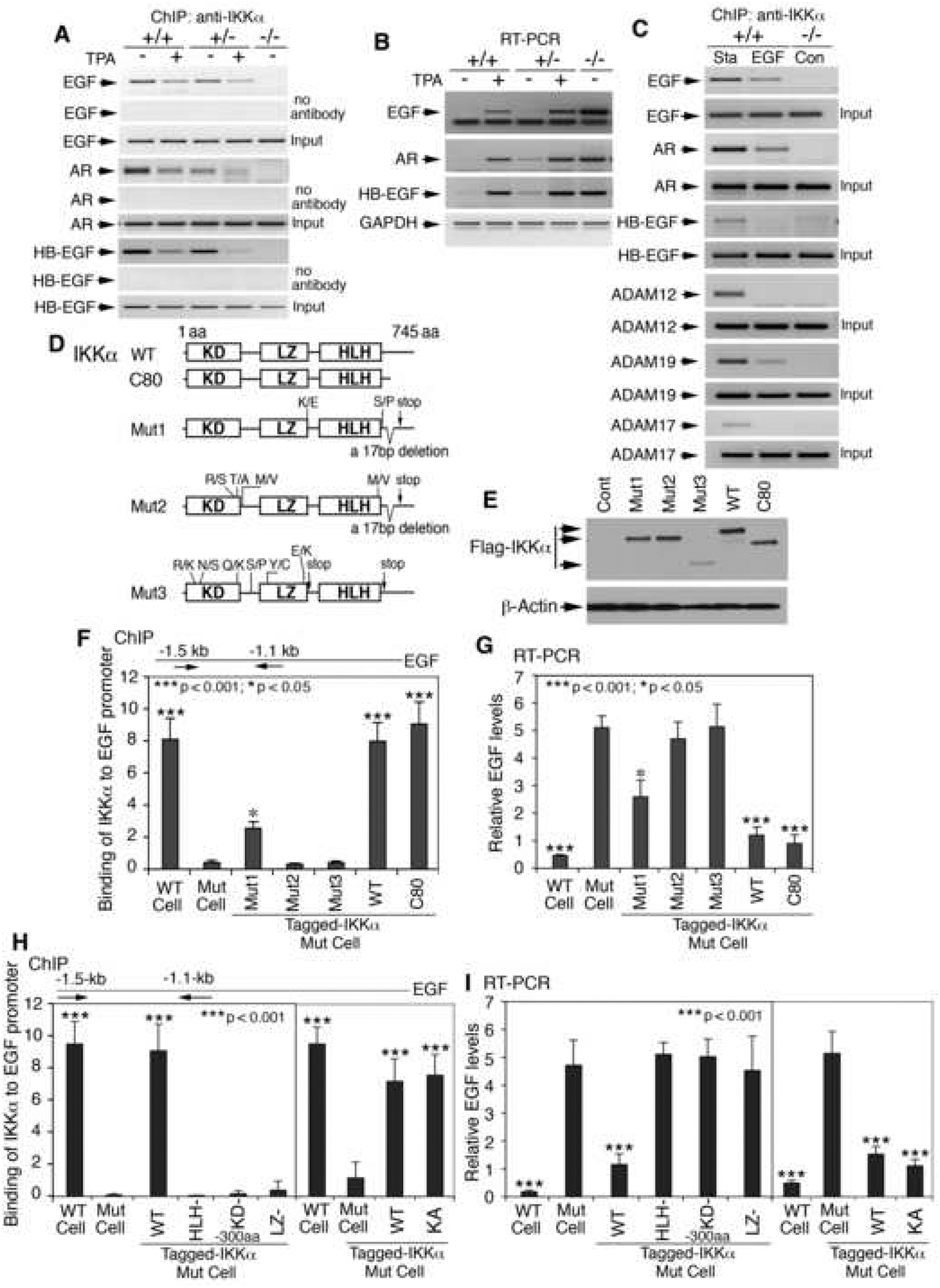
(A) Binding of IKKα to promoters of EGF, HB-EGF, and AR, detected by ChIP assay with an anti-IKKα antibody for immunoprecipitation. No antibody, negative control; Input, PCR control; TPA, 30 ng/ml; +/+, Ikkα+/+; +/−, Ikkα+/−; −/−, Ikkα−/− keratinocytes.
(B) RT-PCR for expression of indicated genes; GAPDH, mRNA loading control.
(C) Binding of IKKα to the promoters of indicated genes. Sta, cells cultured in basic keratinocyte culture medium; EGF, 10 ng/ml; Con, control.
(D) Protein structures of IKKα. aa, amino acid; R/S, T/A, M/V, K/E, S/P, R/K, N/S, Q/K, Y/C, and E/K: amino acid substitutions; R, arginine; S, serine; T, threonine; A, alanine; M, methionine; V, valine; C, cysteine; K, lysine; E, glutamic acid; P, proline; N, asparagine; Q, glutamine; Y, tyrosine; stop, stop codon; KD, kinase domain; LZ, leucine zipper; HLH, helix-loop-helix domain.
(E) Indicated IKKα protein levels in IKKα-deficient keratinocytes detected by Western blotting. Cont, no IKKα; β-Actin, loading control.
(F) Binding of different forms of IKKα to the EGF promoter detected by ChIP assay with an anti-IKKα antibody for immunoprecipitation. All the samples were compared with IKKα-deficient cells (Mut). Error bars indicate ± SD.
(G) RT-PCR for expression of EGF affected by different forms of IKKα. Error bars indicate ± SD.
(H) Binding of different forms of IKKα (see Figure S11B) to the EGF promoter detected by ChIP assay. −300aa, a 300aa-deletion. Error bars indicate ± SD.
(I) RT-PCR for expression of EGF affected by different forms of IKKα. Error bars indicate ± SD.
To further elucidate how IKKα regulates gene expression, we introduced WT IKKα, an 80-amino-acid-deletion IKKα (IKKα-C80) and three IKKα mutants (Mut1, Mut2, and Mut3) isolated from poorly differentiated SCC into IKKα-deficient keratinocytes (Figure 5D, E) (Hu et al., 2001; Liu et al., 2006; Zhu et al., 2007). ChIP assay and RT-PCR showed that IKKα and IKKα-C80 bound to the EGF promoter and suppressed EGF expression; Mut1 showed incomplete binding activity to the EGF promoter and a reduced inhibitory effect on EGF expression compared to IKKα; Mut2 and Mut3 failed to bind to the EGF promoter or suppress EGF expression (Figure 5F, G). WT keratinocytes were used as controls. Because IKKα-C80 had activity comparable to that of IKKα, the C-terminal deletion in Mut1 and Mut2 may not be a major cause of loss of IKKα function. In addition to the C-terminal deletion, a mutation occurred at the LZ motif in Mut1; Mut2 and Mut3 had multiple mutations in the HLH or LZ motif and in the region between the kinase and LZ domain. To further confirm whether these motifs of IKKα protein are required to suppress the activities of the above promoters, we introduced IKKα mutants with mutations in its LZ or HLH domains (LZ- or HLH-), IKKα-KA, and/or an N-terminal 300-amino-acid (aa) deletion (KD-) (Zhu et al., 2007) into IKKα-deficient keratinocytes (Figure S11B). ChIP assay showed that the activities of IKKα-KA in binding to the EGF promoter and repressing EGF expression were similar to those of WT IKKα, however, LZ-, HLH-, and KD- IKKα mutants were not able to bind to the EGF promoter and failed to suppress EGF expression (Figure 5H, I). These results indicate that the LZ and HLH motifs and protein conformation of IKKα are important for regulation of gene expression.
IKKα Regulates Keratinocyte Proliferation and Differentiation through the EGFR-led Pathway
To understand whether IKKα regulates keratinocyte proliferation and differentiation via the EGFR-driven loop, we treated IKKα-deficient keratinocytes with inhibitors of EGFR (AG1478, GW2974), ADAMs (GM6001), and ERK (PD98059) and also reintroduced IKKα, IKKα-C80, Mut1, Mut2, Mut3, LZ-, HLH-, KD-, and KA into these cells. We previously reported that reintroduced IKKα specifically induced larger terminally differentiated keratinocytes and resulted in the expression of the terminal differentiation markers loricrin and filaggrin in Ikkα−/− cells (Hu et al., 2001). Thus, we used the cell morphologies and filaggrin induction to identify terminally differentiated keratinocytes. The results showed that those tested inhibitors, IKKα, IKKα-KA, and IKKα-C80 inhibited proliferation and induced terminal differentiation in mutant keratinocytes; Mut1 slightly inhibited hyperproliferation and induced filaggrin to a lesser degree, but Mut2, Mut3, LZ-, HLH-, and KD-failed to inhibit cell proliferation or induce keratinocyte terminal differentiation (Figure 6A, B). We further compared levels of filaggrin induced by IKKα, IKKα-C80, and Mut1 in IKKα-deficient cells by diluting these cell lysates to ½ and ¼ and found that, compared to IKKα and IKKα-C80, Mut1 induced lower levels of filaggrin (Figure 6C). To test whether the effect of the EGFR-driven pathway on cell proliferation was related to differentiation or apoptosis in IKKα deficient cells, we further examined the markers of apoptosis in mutant keratinocytes. Using Western blotting, we found that inhibitors of EGFR, ERK, and ADAMs did not increase apoptosis in mutant keratinocytes (Figure S11C). Collectively, these results suggest that IKKα and the EGFR-driven pathway coordinately regulate keratinocyte proliferation and differentiation and that the IKKα activity requires its functional domains but not its kinase activity.
Figure 6. IKKα Switches Keratinocyte Proliferation and Differentiation via the EGFR Pathway.
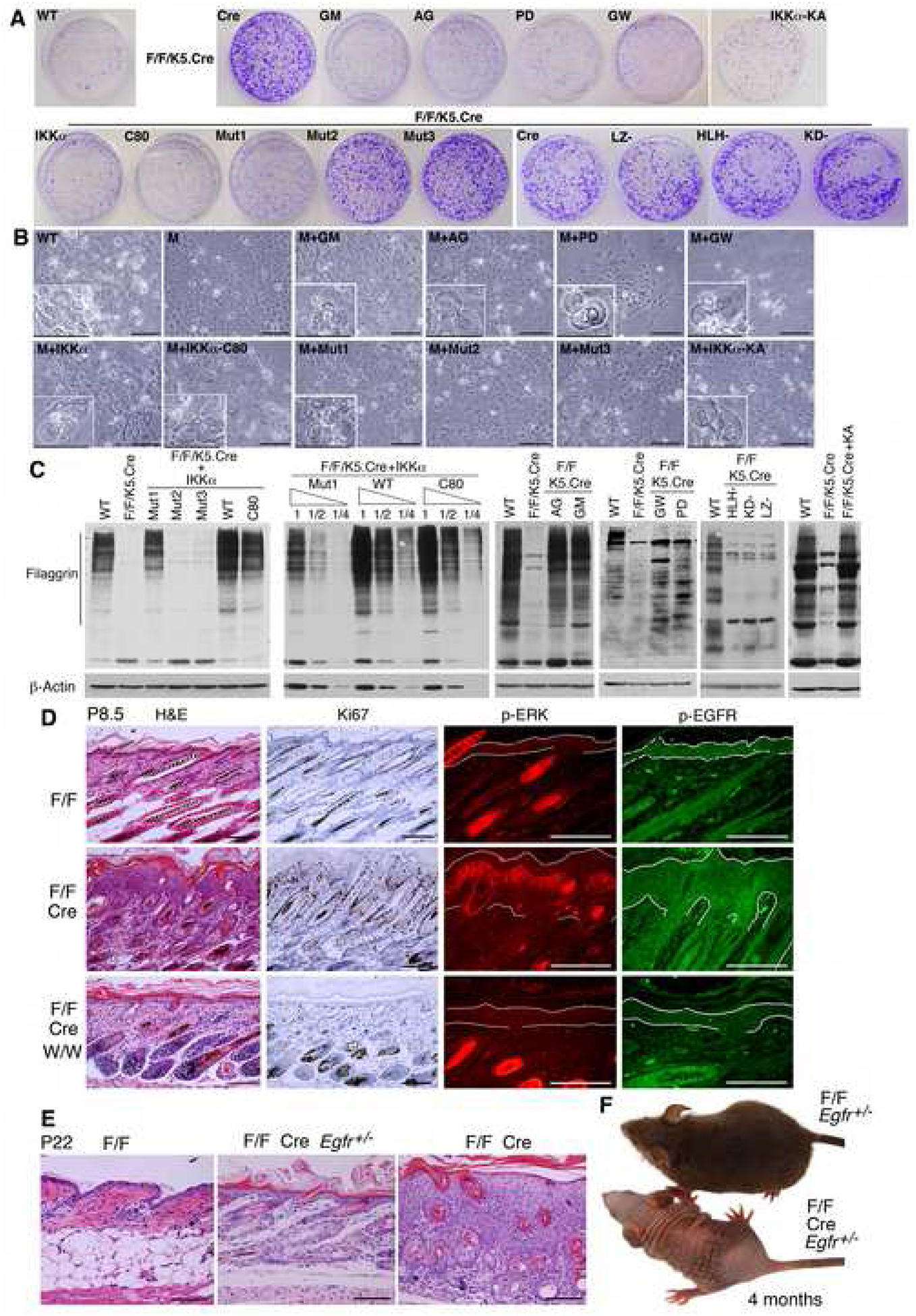
(A) Effects of different forms of IKKα and inhibitors on colony formation of indicated keratinocytes. Different IKKα forms include IKKα, C80, Mut1, Mut2, Mut3, LZ-, HLH-, KD-, and KA. AG, AG1478; GM, GM6001; GW, GW2974; PD, PD98059; keratinocyte colonies, stained with 0.5% crystal violet; WT, IkkαF/F keratinocytes; F/F/K5.Cre, IkkαF/F/K5.Cre keratinocytes.
(B) Keratinocyte morphologies in culture. Cells in small boxes represent terminally differentiated keratinocytes. M, IkkαF/F/K5.Cre keratinocytes. Scale bars, 40 μm.
(C) Filaggrin levels in indicated keratinocytes, as detected by Western blotting. 1, ½, ¼, protein dilutions, β-Actin, loading controls.
(D) The effect of inactivation of EGFR on the epidermis in the indicated mice. P, postnatal day; W/W, Egfrwa2/wa2; Cre, K5.Cre; H&E, hematoxylin and eosin; Ki67, dark brown color stained by immunohistochemistry; p-ERK, red color stained by immunofluorescence; p-EGFR, green stained by immunofluorescence; lines in p-ERK and p-EGFR, limits of the epidermis. Scale bars, 50 μm.
(E) Skin histology of indicated mice, stained with H&E. Scale bars, 60 μm.
(F) Appearances of indicated mice at 4 months of age.
Inactivation of EGFR Reverses the Hyperplastic Epidermis in IkkαF/F/K5.Cre Mice
To further determine the physiological function of the EGFR-driven loop in IkkαF/F/K5.Cre mice, we inactivated the EGFR activity in IkkαF/F/K5.Cre mice by backcrossing Egfrwa2/wa2 (a kinase inactive mutation in EGFR) (Luetteke et al., 1994) with IkkαF/F and K5.Cre mice to generate IkkαF/F/K5.Cre/Egfrwa2/wa2 mice. The epidermis of IkkαF/F/K5.Cre/Egfrwa2/wa2 mice was as thin as that of the WT control, and Ki67-positive cells and EGFR and ERK activities were decreased in the epidermis (Figure 6D). Like the IkkαF/F/K5.Cre mice, these mice died early. GW2974, given orally, also inhibited epidermal thickening in IkkαF/F/K5.Cre mice (Figure S12) (Kiguchi et al., 2005). Furthermore, we generated IkkαF/F/K5.Cre/Egfr−/− mice but these mice died within 3 days after birth (Sibilia and Wagner, 1995; Threadgill et al., 1995). Reducing the Egfr gene dosage by half was found to dramatically decrease the epidermal thickness in IkkαF/F/K5.Cre/Egfr+/− mice although their epidermis was still slightly thicker than that of WT mice (Figure 6E). Most of the IkkαF/F/K5.Cre/Egfr+/− mice still died but a small fraction survived (Figure 6F). The survived mice developed obvious defects in hair and eyes. It is known that Egfr−/− mice on the same genetic background are prone to die during a large range of ages, which underscores the importance of EGFR for individual mouse development (Sibilia and Wagner, 1995; Threadgill et al., 1995). Taken together, these results suggest that a reduction of EGFR activity can mitigate IKKα-deletion-induced excessive proliferative signals in the epidermis, thereby reversing epidermal hyperplasia.
Inactivation of IKKα kinase in IkkαK44A/K44A mice was found not to cause hyperproliferation in the epidermis (Figure S13) (Zhu et al., 2007). Thus, the elevated epidermal thickness in IkkαF/F/K5.Cre mice is IKKα kinase independent. IKKα loss also elevated TNFα expression (Figure S10B). Overexpression of TNFα can cause severe inflammation-dependent skin lesions in mice (Lind et al., 2004). To determine whether the epidermal phenotype was TNFα dependent, we generate IkkαF/F/K5.Cre/Tnfr1−/− mice and found that depleting TNFR1 did not attenuate the epidermal phenotype but did lead to the slightly earlier death of the mice (Figure S14A, B). No significant alterations in the levels of IKKβ, IKKγ, p65, p100, p52 and IκBα, and in IKK kinase activity were found in the epidermis of WT and mutant mice (Figure S15A, B). Collectively, these results show that excessive EGFR-driven loop activity, but not TNFR, is a primary trigger responsible for inducing the epidermal hyperplasia in IkkαF/F/K5.Cre mice.
Mice with IKKα Deletion in Keratinocytes Develop Spontaneous Skin Cancer
To determine if IKKα deletion in keratinocytes induces skin tumors, we used mouse mammary tumor virus (MMTV).Cre mice to delete IKKα. IkkαF/F/MMTV.Cre mice were distinguished from WT mice by their sparse body hair 3 weeks after birth. We found that 8 of 9 IkkαF/F/MMTV.Cre mice began to develop spontaneous tumors on their face at 1 to 5 months of age (Figure 7A, B). Because the oldest mice that we observed were 8 months old, it is possible that the tumor incidence may reach 100% as the mice get older. These tumors expressed pan-keratin and resembled SCCs pathologically (Figures 7C). None of the IkkαF/F or MMTV.Cre mice developed any spontaneous tumors. The dorsal epidermis of IkkαF/F/MMTV.Cre mice was hyperplastic, with elevated EGFR and ERK activities compared with the WT epidermis (Figure S16).
Figure 7. IKKα Deletion in Keratinocytes Causes Spontaneous Skin Cancer.
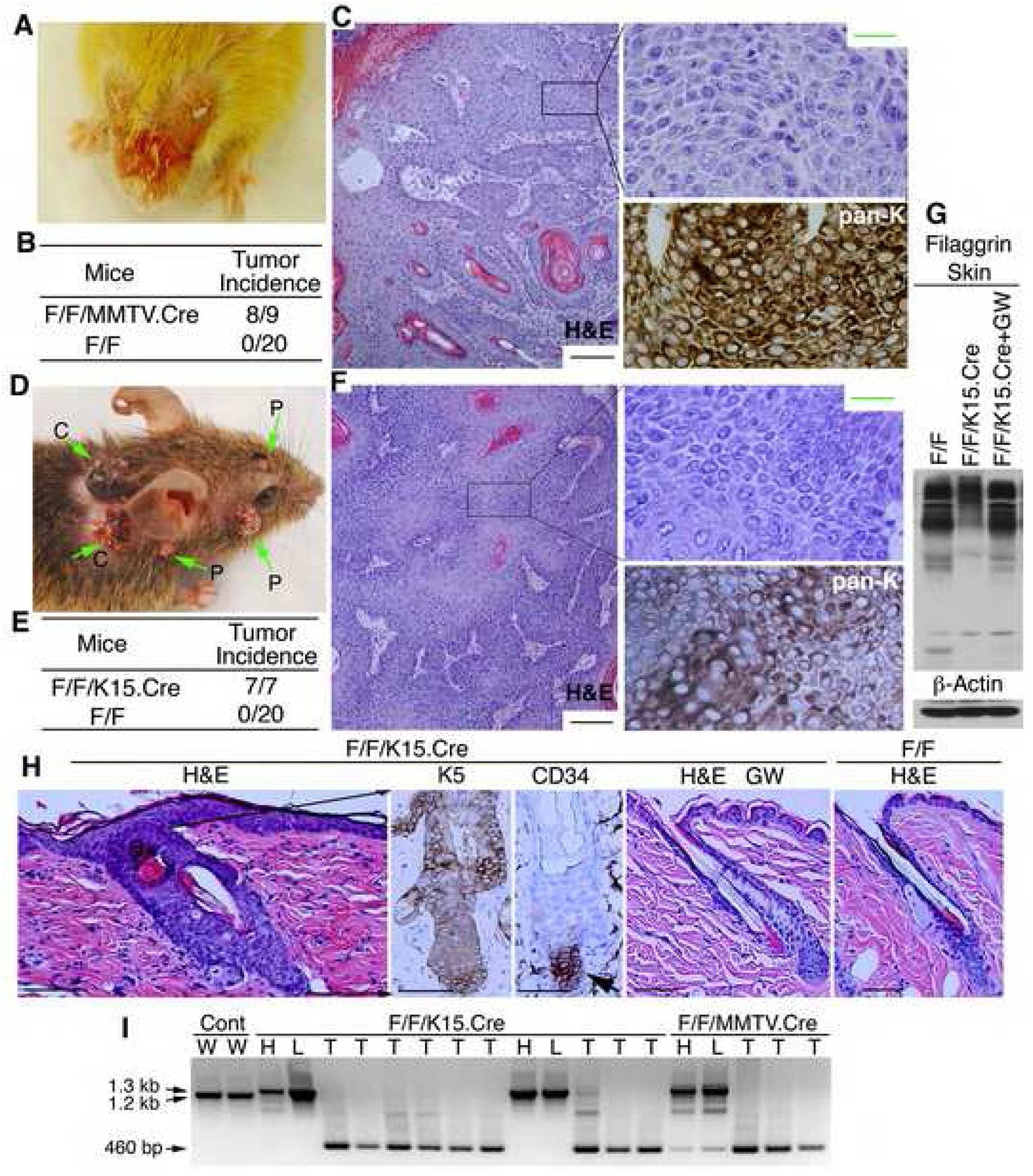
(A) Tumors in an 8-month-old IkkαF/F/MMTV.Cre mouse.
(B) Tumor incidence in IkkαF/F/MMTV.Cre (F/F/MMTV.Cre) and WT (F/F) mice.
(C) Pathologies of H&E stained tumor sections of IkkαF/F/MMTV.Cre mice. Boxes, amplified (indicated by lines) and pan-keratin staining (brown). Black scale bar, 25 μm; green scale bar, 10 μm.
(D) Tumors in an IkkαF/F/K15.Cre mouse 6 months after IKKα deletion. P, papillomas; C, carcinomas.
(E) Tumor incidence in IkkαF/F/K15.Cre (F/F/K15.Cre) and WT (F/F) mice.
(F) Pathologies of H&E stained tumor sections of IkkαF/F/K15.Cre mice. Boxes, amplified (indicated by lines) and pan-keratin staining (brown). Black scale bar, 25 μm; green scale bar, 10 μm.
(G) Filaggrin levels in indicated skins, as detected by Western blotting. β-Actin, loading control.
(H) Skin sections from indicated mice stained with H&E and antibodies against K5 or CD34 (indicated by arrow). Brown color, K5 or CD34; lines, indicate hair follicle; GW, GW2974. Scale bar, 40 μm.
(I) Genotyping. 1.2-kb, WT allele; 1.3-kb, allele containing loxp sites; 460-bp, Ikkα gene deletion; Cont, WT skin; H, heart; L, liver; T, tumor.
Because MMTV.Cre is also expressed in other types of cells (Wagner et al., 1997), we investigated whether inducible IKKα deletion specifically in keratinocytes causes spontaneous skin tumors. We used inducible K15.Cre, which is specifically expressed in keratinocytes of hair follicles (Morris et al., 2004), to delete IKKα in mice by using RU846. We treated 7 IkkαF/F/K15.Cre mice with RU846. Four months after treatment, the 7 mice began to develop skin tumors (Figure 7D, E). The appearance of these tumors was similar to that of DMBA/TPA-induced skin papillomas and malignant carcinomas (Figure 7D, F). The mouse in Figure 7D developed more than 10 tumors. The tumors resembled SCCs pathologically and expressed pan-keratin (Figure 7F).
We further examined the status of keratinocyte differentiation and proliferation in WT and IkkαF/F/K15.Cre skins. Western blotting showed that filaggrin expression was lower in IkkαF/F/K15.Cre than in WT skin and that treatment with GW2974 elevated filaggrin expression (Figure 7G). In IkkαF/F/K15.Cre skin, increased keratinocyte numbers in the hair follicles and the epidermis surrounding the hair follicles and elevated EGFR and ERK activities were detected, but CD34-positive cells were restricted in the hair follicle (Figures 7H, S17). GW2974 inhibited hyperproliferation and EGFR and ERK activities in the mutant epidermis (Figures 7H, S17). Also, 7 GW2974-treated IkkαF/F/K15.Cre mice had not developed any tumors at 6 months after treatment with RU846, suggesting that inactivation of EGFR induced terminal differentiation, inhibited proliferation, and prevented tumor development.
The Ikkα gene was deleted in all the tumors found in IkkαF/F/MMTV.Cre and IkkαF/F/K15.Cre mice (Figure 7I). Because MMTV.Cre is expressed in various types of cells, weak deletion bands were detected in the heart and liver of IkkαF/F/MMTV.Cremice (Figure 7I). Taken together, these results indicate that IKKα deletion in keratinocytes can cause skin carcinomas, which are accompanied by elevated EGFR and ERK activities (Figures S16A, S17).
In addition, we generated tamoxifen-inducible IkkαF/F/K5.Cre mice. The dorsal epidermis of the mice exhibited hyperplasia (Figure S18A). Four of the 8 inducible IkkαF/F/K5.Cre mice developed skin tumors 10 to 12 months after tamoxifen treatment (Figure S18B). Only 1–3 tumors per mouse were observed and the tumors lost IKKα. Thus, the number and incidence of skin tumors were lower in inducible IkkαF/F/K5.Cre mice than in inducible IkkαF/F/K15.Cre mice. The K5 and K15 promoters are expressed in different subtypes of keratinocytes. Keratinocytes expressing K5 are physiologically closer to suprabasal differentiating cells than hair follicle keratinocytes expressing K15. The difference may have an impact on the susceptibility to tumorigenesis in mice.
Activated Ras, a downstream target of EGFR and upstream target of ERK, is involved in keratinocyte transformation (Yuspa et al., 1985). We further found that Ras activity was significantly higher in IKKα-null than in WT keratinocytes (Figure 8A). Reintroduced IKKα, EGFR inhibitor, and dominant negative RasN17 suppressed Ras and ERK activities and cell proliferation but induced terminal differentiation in IKKα-null keratinocytes (Figure 8A, B, C). Increased Ras activity was found to suppress terminal differentiation in WT keratinocytes (Figure 8D). Thus, IKKα deletion may activate Ras via the EGFR-driven loop in keratinocytes (Figure 8E).
Figure 8. Elevated Ras Activity Incorporated with the EGFR Pathway in IKKα-null Keratinocytes.
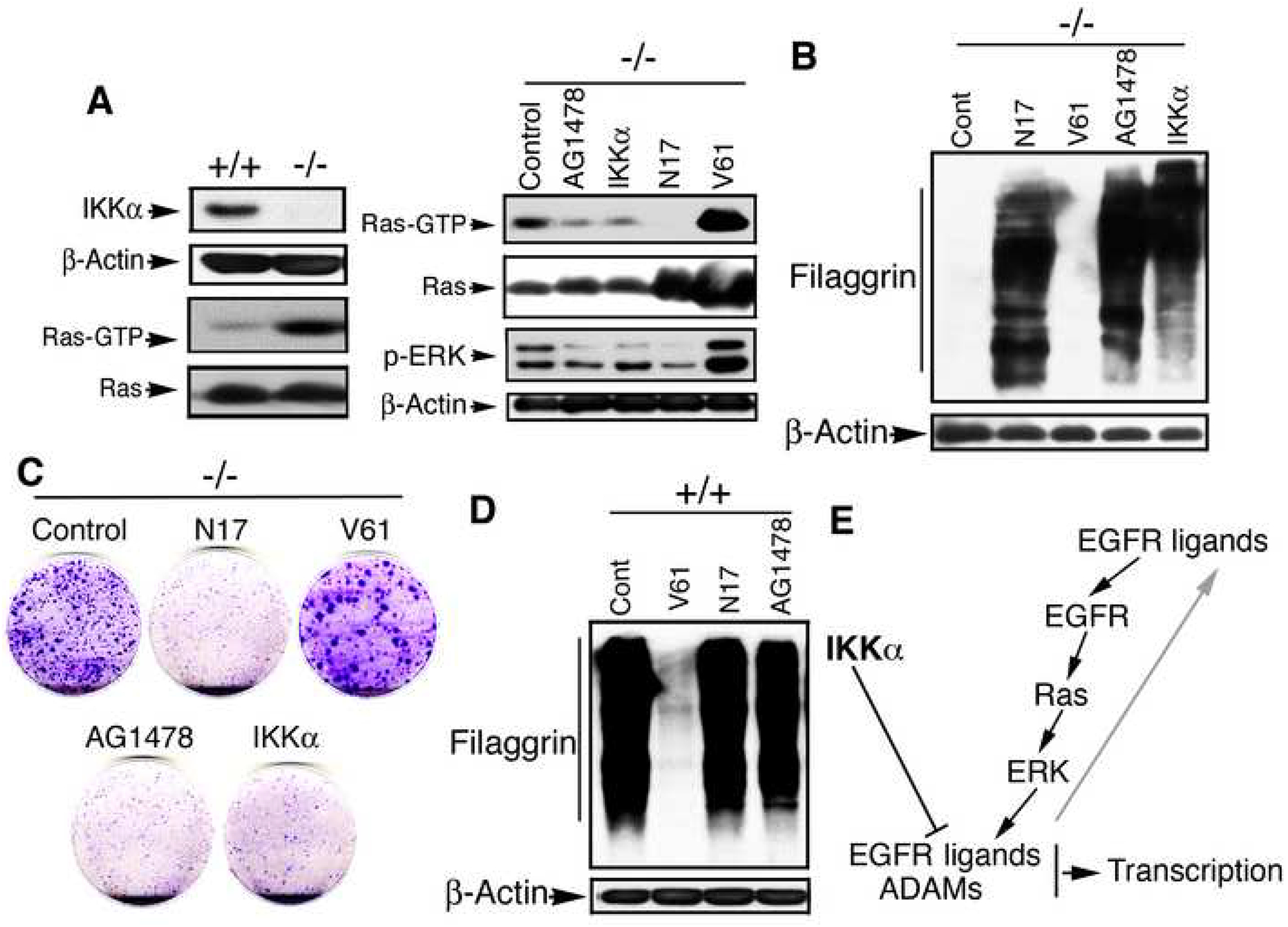
(A) Ras (GTP) activity in keratinocytes, as detected by GST-pull down and Western blotting; elevated Ras and ERK activities were down-regulated by IKKα, inactivation of Ras, and EGFR inhibitor. +/+, Ikkα+/+; −/−, Ikkα−/− keratinocytes; Ras-GTP, active form of Ras; Ras, p-ERK, and β-Actin, detected by Western blotting; AG1478, EGFR inhibitor; N17, domain negative form of Ras; V61, active form of Ras.
(B) Filaggrin expression in Ikkα−/− keratincoytes affected by indicated proteins, as detected by Western blotting. β-Actin, loading control.
(C) Colony formation of Ikkα−/− keratincoytes affected by indicated proteins, stained with 0.5% crystal violet blue.
(D) Filaggrin expression in Ikkα+/+ keratincoytes affected by indicated proteins, as detected by Western blotting.
(E) A work hypothesis of IKKα function in keratinocytes for maintaining skin homeostasis and preventing skin cancer. Arrows, promotion; line, inhibition.
DISCUSSION
IKKα Functions as a Switch for Keratinocyte Differentiation and Proliferation via the EGFR-led Pathway and Is Required for Skin Homeostasis
Our results, here, demonstrated that a low level of IKKα in keratinocytes was sufficient to maintain embryonic skin development in mice. However, a further IKKα deletion induced epidermal hyperplasia in postnatal and adult mice. Epidermal thickness in mutant mice was found to gradually increase after birth, which was accompanied by gradually elevated EGFR and ERK activities and levels of EGFR ligands and ADAMs; this also occurred in cultured mutant keratinocytes. The hyperproliferative keratinocytes in the epidermis or in cultures lacked terminal differentiation (Figures S7, 6A, C). Inactivation of EGFR by genetic and pharmacological inhibitor approaches as well as transgenic IKKα were able to suppress keratinocyte hyperproliferation and direct the cells to terminal differentiation, reversing the epidermal phenotype in mice with IKKα deletion in keratinocytes. Furthermore, IKKα and IKKα functional domains were required to suppress the transcription of EGFR’s ligands and these ligands’ activators and to suppress EGFR and ERK activities in mutant keratinocytes. Taken together, these results provide evidence that IKKα regulates keratinocyte proliferation and differentiation through the EGFR-driven loop, which is required for maintaining skin homeostasis in mice.
Quantitation assays showed that mutations in Ikkα (Mut1) impaired the capacity of IKKα to induce terminal differentiation and suppress hyperproliferation at the same time in IKKα-deficient keratinocytes (Figure 6A, C), providing further, direct evidence that IKKα is a switch for keratinocyte proliferation and differentiation; the integrity of Ikkα, but not its kinase activity, is required for this switch. Previously, we showed that the culture medium obtained from WT keratinocytes was able to temporarily induce terminal differentiation in Ikkα−/− keratinocytes (Hu et al., 2001), which was inactivated by treatment with trypsin or heat (90°C). The proteinaceous factor(s) was named keratinocyte differentiation inducible factor (KDIF). We also suggested that the grafted Ikkα−/− skin was induced to undergo terminal differentiation by KDIF from surrounding WT keratinocytes in mice (Hu et al., 2001). Our findings here further explain this transplant result. We previously placed Ikkα−/− skin in basic keratinocyte culture medium at 4°C for longer than overnight before grafting (Hu et al., 2001). Such conditions might affect the ability of the Ikkα−/− keratinocytes to proliferate, causing them instead to undergo terminal differentiation. Thus, in addition to secreted KDIF, which may include inhibitors to components in the EGFR-driven loop, intrinsic conditions also regulate the status of IKKα-deficient keratinocytes.
Although here we found that depleting TNFR was not able to prevent the development of hyperplastic epidermis in IkkαF/F/K5.Cre mice (Figure S14), the elevated TNFα may affect late phenotypes in these mice. Also inflammatory cells in the dermis (Figure S10A) may contribute to epidermal cell proliferation in mutant mice. IKKα loss was previously found to upregulate vascular endothelial growth factor expression (Liu et al., 2006). Indeed, an increase in microblood vessels was observed in the skin of IkkαF/F/K5.Cre mice (data not shown). In addition, we observed very short hair in survived IkkαF/F/K5.Cre/Egfr+/− mice and sparse hair in IkkαF/F/MMTV.Cre mice, suggesting that IKKα plays a role in hair development, although the mechanism remains to be elucidated. Moreover, our microarray analyses revealed the elevated expression of multiple integrins, various growth factors, MMPs, Notch receptor ligands, Wnts, and Wnt inhibitors in IKKα-deficient keratinocytes (Figure 3E). A group of tight-junction proteins, Claudins, which affect barrier formation and hair development, were dramatically downregulated in mutant keratinocytes (Figure 3E). Taken together, these molecular alterations may contribute to the skin phenotypes in mice with an IKKα deletion in keratinocytes. Thus, IKKα plays a broad role in maintaining normal skin.
IKKα Is an Innate Surveillant to Prevent Skin Cancer
In mouse models of skin carcinogenesis, DMBA-activated Ras or over-expressed Ras in basal or suprabasal keratinocytes can induce skin (squamous cell-like) tumors (Brown et al., 1998; Greenhalgh et al., 1993; Wang et al., 2000), indicating that uncontrolled proliferation is able to block terminal differentiation at different stages, allowing a single cell to grow into a mass. Additional alterations cause the mass to irreversibly invade and become malignant. Hair follicular keratinocytes targeted by Ras efficiently developed into malignant tumors because skin stem cells located there were thought to be targeted by the tumor initiator (Brown et al., 1998; Kangsamaksin et al., 2007).
Here we showed that IKKα deletion in keratinocytes resulted in skin carcinomas that resembled DMBA/TPA-induced tumors (Figure 7D, F). In IKKα-deficient keratinocytes, elevated Ras activity, which was incorporated into the EGFR-driven loop, and elevated expression of multiple growth factors, integrins, cytokines, and MMPs provided the molecular bases for promotion of keratinocyte proliferation and transformation in vivo (Figures 3E, 8A–D). Inactivation of EGFR or reintroduction of IKKα induced terminal differentiation, inhibited hyperproliferation in IKKα-deficient keratinocytes (as described above), and prevented tumor development. These results underscore the importance of IKKα-mediated terminal differentiation as an innate, primary mechanism for preventing skin carcinogenesis. In addition to the effect of EGFR on keratinocyte differentiation, Sibilia et al. (2000) reported that inactivation of EGFR prevented overexpressed SOS-F-induced skin tumors through a survival pathway but did not affect ERK activity, which suggests that overexpressed SOS-F and IKKα loss may affect different internal cellular pathways.
Treatment with TPA or EGF was found to attenuate binding of IKKα to the promoters of growth factors, which was accompanied by the elevated expression of their corresponding genes (Figure 5A–I). Previously, TPA, ultraviolet light radiation, and/or ETS-1, a proto-oncogenic protein, were found to up-regulate IKKα expression (Gu et al., 2004; Li and Karin, 1998; Park et al., 2007). Elevated expression of IKKα may suppress the activities of its targets to ensure that excessive numbers of keratinocytes undergo terminal differentiation, thereby preventing cell proliferation and tumor formation. Moreover, p63 regulates IKKα expression and impaired Ikkα may affect p63-related tumor development (Koster et al., 2007). DNA methylation has been shown to downregulate IKKα expression in human oral SCCs (Maeda et al., 2007). IKKα prevents DNA methylation of the 14-3-3σ gene (Zhu et al., 2007). It will be also interesting to see whether IKKα can be self-regulated in an epigenetic manner. Collectively, these results indicate that IKKα executes its role as a surveillant in preventing skin cancer through multiple avenues.
We observed that IkkαF/F/MMTV.Cre mice developed the carcinomas only on their faces, and most of the tumors in IkkαF/F/K15.Cre mice were close to neck and face. It is possible that microenvironmental conditions, such as the presence of more micro-blood vessels in the faces than in the dorsal skin, may have contributed to these phenotypes. Finally, we would like to emphasize that Western blotting using commercial antibodies against IKKα detects a strong, nonspecific band closely underneath the IKKα protein in tissue lysates but not in cultured cell lysates. Therefore, proper techniques can avoid misleading findings and help us to understand the physiological functions of IKKα.
Perspectives
Here we have identified the pathway that IKKα utilizes in its role as a keratinocyte proliferation and differentiation switch, which is required for maintaining skin homeostasis and preventing skin cancer. Although many deregulated genes were identified in IKKα-deficient keratinocytes, it remains to be elucidated how IKKα cross-talks with other pathways. It also will be important to develop new tools to identify the target cells of IKKα deletion-induced skin tumors, which will facilitate the design of efficient therapeutic drugs to battle cancer. Stem cells are thought to be targets for most skin tumors (Kangsamaksin et al., 2007). It remains to be determined whether suprabasal keratinocytes are resistant to dedifferentiation, and whether the hair follicle keratinocytes or daughters of stem cells are resistant to differentiation when IKKα is absent during carcinogenesis. Also, how the microenvironmental inflammation interacts with cancer initiation cells remains to be defined. In addition, it will be important to determine whether IKKα plays a role in stem cell maintenance, renewal, and division. Overall, the animal models provide good opportunities for understanding many fundamental biological questions and identifying new therapeutic targets for preventing cancer.
EXPERIMENTAL PROCEDURES
Animal Experiments
All the mice used in this study were cared for in accordance with the guidelines of the Animal Care and Use Committee of The University of Texas M. D. Anderson Cancer Center (animal protocol 04-01-05732).
Supplementary Material
SIGNIFICANCE.
Squamous cell carcinoma (SCC) derived from epithelial cells in many organs is one of the most common malignancies in human. Downregulation of IKKα was reported in SCCs of skin, esophagus, lungs, and head and neck in humans, highlighting the importance of IKKα in human cancers. Although a reduction in IKKα expression promoted chemical carcinogen-induced SCCs, it is unknown whether IKKα deletion can be a cause of skin SCCs. Here we showed that IKKα deletion in keratinocytes induced skin SCCs through upregulating transcription of EGFR ligands and ligand activators, thus activating the EGFR-driven pathway in mice. Inactivation of EGFR prevented the development of epidermal hyperplasia and tumors. These findings shed light on therapeutic targets for preventing IKKα-related SCC development.
ACKNOWLEDGMENTS
We thank Jan Parker-Thornburg for assisting in the generation of IkkαF/F mice, Wei Zhang and Limei Hu for microarray analyses, and Pierre Chambon for providing tamoxifen-inducible K5.Cre mice. This work was supported by grants from the National Cancer Institute (CA102510 and CA117314 to YH, CA105345 to SMF, and CA16672). The authors declare that they have no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Balmain A, and Pragnell IB (1983). Mouse skin carcinomas induced in vivo by chemical carcinogens have a transforming Harvey-ras oncogene. Nature 303, 72–74. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Horsley V, and Fuchs E (2007). Epithelial stem cells: turning over new leaves. Cell 128, 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, and Fuchs E (2004). Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118, 635–648. [DOI] [PubMed] [Google Scholar]
- Brown K, Strathdee D, Bryson S, Lambie W, and Balmain A (1998). The malignant capacity of skin tumours induced by expression of a mutant H-ras transgene depends on the cell type targeted. Curr. Biol 8, 516–524. [DOI] [PubMed] [Google Scholar]
- Dlugosz AA, Hansen L, Cheng C, Alexander N, Denning MF, Threadgill DW, Magnuson T, Coffey RJ Jr., and Yuspa SH (1997). Targeted disruption of the epidermal growth factor receptor impairs growth of squamous papillomas expressing the v-ras(Ha) oncogene but does not block in vitro keratinocyte responses to oncogenic ras. Cancer Res. 57, 3180–3188. [PubMed] [Google Scholar]
- Gandarillas A, and Watt FM (1997). c-Myc promotes differentiation of human epidermal stem cells. Genes Dev. 11, 2869–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareus R, Huth M, Breiden B, Nenci A, Rosch N, Haase I, Bloch W, Sandhoff K, and Pasparakis M (2007). Normal epidermal differentiation but impaired skin-barrier formation upon keratinocyte-restricted IKK1 ablation. Nat. Cell Biol 9, 461–469. [DOI] [PubMed] [Google Scholar]
- Ghosh S, and Karin M (2002). Missing pieces in the NF-kappaB puzzle. Cell 109 Suppl, S81–96. [DOI] [PubMed] [Google Scholar]
- Greenhalgh DA, Rothnagel JA, Quintanilla MI, Orengo CC, Gagne TA, Bundman DS, Longley MA, and Roop DR (1993). Induction of epidermal hyperplasia, hyperkeratosis, and papillomas in transgenic mice by a targeted v-Ha-ras oncogene. Mol. Carcinog 7, 99–110. [DOI] [PubMed] [Google Scholar]
- Gu L, Zhu N, Findley HW, Woods WG, and Zhou M (2004). Identification and characterization of the IKKalpha promoter: positive and negative regulation by ETS-1 and p53, respectively. J. Biol. Chem 279, 52141–52149. [DOI] [PubMed] [Google Scholar]
- Hafner M, Wenk J, Nenci A, Pasparakis M, Scharffetter-Kochanek K, Smyth N, Peters T, Kess D, Holtkotter O, Shephard P, et al. (2004). Keratin 14 Cre transgenic mice authenticate keratin 14 as an oocyte-expressed protein. Genesis 38, 176–181. [DOI] [PubMed] [Google Scholar]
- Hall RJ, and Erickson CA (2003). ADAM 10: an active metalloprotease expressed during avian epithelial morphogenesis. Dev. Biol 256, 146–159. [DOI] [PubMed] [Google Scholar]
- Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M, Johnson R, and Karin M (1999). Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of IκB kinase. Science 284, 316–320. [DOI] [PubMed] [Google Scholar]
- Hu Y, Baud V, Oga T, Kim KI, Yoshida K, and Karin M (2001). IKKα controls formation of the epidermis independently of NF-κB. Nature 410, 710–714. [DOI] [PubMed] [Google Scholar]
- Huovila AP, Turner AJ, Pelto-Huikko M, Karkkainen I, and Ortiz RM (2005). Shedding light on ADAM metalloproteinases. Trends Biochem. Sci 30, 413–422. [DOI] [PubMed] [Google Scholar]
- Kangsamaksin T, Park HJ, Trempus CS, and Morris RJ (2007). A perspective on murine keratinocyte stem cells as targets of chemically induced skin cancer. Mol. Carcinog 46, 579–584. [DOI] [PubMed] [Google Scholar]
- Kiguchi K, Ruffino L, Kawamoto T, Ajiki T, and Digiovanni J (2005). Chemopreventive and therapeutic efficacy of orally active tyrosine kinase inhibitors in a transgenic mouse model of gallbladder carcinoma. Clin. Cancer Res 11, 5572–5580. [DOI] [PubMed] [Google Scholar]
- Koster MI, Dai D, Marinari B, Sano Y, Costanzo A, Karin M, and Roop DR (2007). p63 induces key target genes required for epidermal morphogenesis. Proc. Natl. Acad. Sci. U S A 104, 3255–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong JL, Loh KS, Putti TC, Goh BC, and Tan LK (2004). Epidermal growth factor receptor in undifferentiated carcinoma of the nasopharynx. Laryngoscope 114, 153–157. [DOI] [PubMed] [Google Scholar]
- Li N, and Karin M (1998). Ionizing radiation and short wavelength UV activate NF-kappaB through two distinct mechanisms. Proc. Natl. Acad. Sci. U S A 95, 13012–13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind MH, Rozell B, Wallin RP, van Hogerlinden M, Ljunggren HG, Toftgard R, and Sur I (2004). Tumor necrosis factor receptor 1-mediated signaling is required for skin cancer development induced by NF-kappaB inhibition. Proc. Natl. Acad. Sci. U S A 101, 4972–4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Park E, Zhu F, Bustos T, Liu J, Shen J, Fischer SM, and Hu Y (2006). A critical role for I{kappa}B kinase {alpha} in the development of human and mouse squamous cell carcinomas. Proc. Natl. Acad. Sci. U S A 103, 17202–17207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomada D, Liu B, Coghlan L, Hu Y, and Richie ER (2007). Thymus Medulla Formation and Central Tolerance Are Restored in IKK{alpha}−/− Mice That Express an IKK{alpha} Transgene in Keratin 5+ Thymic Epithelial Cells. J. Immunol 178, 829–837. [DOI] [PubMed] [Google Scholar]
- Luetteke NC, Phillips HK, Qiu TH, Copeland NG, Earp HS, Jenkins NA, and Lee DC (1994). The mouse waved-2 phenotype results from a point mutation in the EGF receptor tyrosine kinase. Genes Dev. 8, 399–413. [DOI] [PubMed] [Google Scholar]
- Maeda G, Chiba T, Kawashiri S, Satoh T, and Imai K (2007). Epigenetic inactivation of IkappaB Kinase-alpha in oral carcinomas and tumor progression. Clin. Cancer Res 13, 5041–5047. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, and Cotsarelis G (2004). Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol 22, 411–417. [DOI] [PubMed] [Google Scholar]
- Park E, Zhu F, Liu B, Xia X, Shen J, Bustos T, Fischer SM, and Hu Y (2007). Reduction in IkappaB kinase alpha expression promotes the development of skin papillomas and carcinomas. Cancer Res. 67, 9158–9168. [DOI] [PubMed] [Google Scholar]
- Pasparakis M, Courtois G, Hafner M, Schmidt-Supprian M, Nenci A, Toksoy A, Krampert M, Goebeler M, Gillitzer R, Israel A, et al. (2002). TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature 417, 861–866. [DOI] [PubMed] [Google Scholar]
- Pierceall WE, Goldberg LH, Tainsky MA, Mukhopadhyay T, and Ananthaswamy HN (1991). Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol. Carcinog 4, 196–202. [DOI] [PubMed] [Google Scholar]
- Ramirez A, Page A, Gandarillas A, Zanet J, Pibre S, Vidal M, Tusell L, Genesca A, Whitaker DA, Melton DW, and Jorcano JL (2004). A keratin K5Cre transgenic line appropriate for tissue-specific or generalized Cre-mediated recombination. Genesis 39, 52–57. [DOI] [PubMed] [Google Scholar]
- Rebholz B, Haase I, Eckelt B, Paxian S, Flaig MJ, Ghoreschi K, Nedospasov SA, Mailhammer R, Debey-Pascher S, Schultze JL, et al. (2007). Crosstalk between Keratinocytes and Adaptive Immune Cells in an IkappaBalpha Protein-Mediated Inflammatory Disease of the Skin. Immunity 27, 296–307. [DOI] [PubMed] [Google Scholar]
- Sibilia M, Fleischmann A, Behrens A, Stingl L, Carroll J, Watt FM, Schlessinger J, and Wagner EF (2000). The EGF receptor provides an essential survival signal for SOS-dependent skin tumor development. Cell 102, 211–220. [DOI] [PubMed] [Google Scholar]
- Sibilia M, and Wagner EF (1995). Strain-dependent epithelial defects in mice lacking the EGF receptor. Science 269, 234–238. [DOI] [PubMed] [Google Scholar]
- Sil AK, Maeda S, Sano Y, Roop DR, and Karin M (2004). IKKα acts in the epidermis to control skeletal and craniofacial morphogenesis. Nature 428, 660–664. [DOI] [PubMed] [Google Scholar]
- Takeda K, Takeuchi O, Tsujimura T, Itami S, Adachi O, Kawai T, Sanjo H, Yoshikawa K, Terada N, and Akira S (1999). Limb and skin abnormalities in mice lacking IKKα. Science 284, 313–316. [DOI] [PubMed] [Google Scholar]
- Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC, and et al. (1995). Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science 269, 230–234. [DOI] [PubMed] [Google Scholar]
- Wagner KU, Wall RJ, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L, Li M, Furth PA, and Hennighausen L (1997). Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res. 25, 4323–4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Greenhalgh DA, and Roop DR (2000). Transgenic coexpression of v-Ha-ras and transforming growth factor alpha increases epidermal hyperproliferation and tumorigenesis and predisposes to malignant conversion via endogenous c-Ha-ras activation. Mol. Carcinog 27, 200–209. [PubMed] [Google Scholar]
- Yamazaki S, Iwamoto R, Saeki K, Asakura M, Takashima S, Yamazaki A, Kimura R, Mizushima H, Moribe H, Higashiyama S, et al. (2003). Mice with defects in HB-EGF ectodomain shedding show severe developmental abnormalities. J. Cell Biol 163, 469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuspa SH, Kilkenny AE, Stanley J, and Lichti U (1985). Keratinocytes blocked in phorbol ester-responsive early stage of terminal differentiation by sarcoma viruses. Nature 314, 459–462. [DOI] [PubMed] [Google Scholar]
- Zhu F, Xia X, Liu B, Shen J, Hu Y, Person M, and Hu Y (2007). IKKalpha Shields 14-3-3sigma, a G(2)/M Cell Cycle Checkpoint Gene, from Hypermethylation, Preventing Its Silencing. Mol. Cell 27, 214–227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


