Abstract
We used optimized DNA expression vectors to compare two gene delivery methodologies in rhesus macaques, namely direct DNA injection and in vivo adaptive constant-current electroporation via the intramuscular route. The use of in vivo electroporation increased levels of gene expression and immune responses. We used an optimized HIV gag expression plasmid to show the development of new cellular immune responses in SIV infected animals controlling viremia. Furthermore, after vaccination with SIV expression plasmids the recall responses to the SIV antigens were very high, indicating that DNA is a strong boost in the presence of antiretroviral treatment in SIV infected animals. There was substantial animal-to-animal variability in DNA expression, revealed by plasma measurements of IL-15 produced by co-injected IL-15 DNA. IL-15 expression levels correlated with peak immune responses. Electroporation led to an expansion of antigen-specific CD4+ and CD8+ T cells of both central and effector memory phenotype. These results indicate that improved gene delivery and expression by electroporation dramatically increases immunogenicity of DNA vaccines. Electroporation is thus an important method to improve the effectiveness of DNA vaccination.
Keywords: HIV, SIV, DNA vaccine, electroporation, intramuscular injection, rhesus macaque, immune response, central memory, effector memory, T cell subset
1. Introduction
Different methodologies of gene delivery for vaccine purposes are being tested in vivo, including the use of DNA expression plasmids delivered intramuscularly (IM) or intradermally. DNA delivery has resulted in various levels of protein expression and is intensely considered for many therapeutic and vaccine applications. Although efficient delivery has been achieved in small animals, naked DNA delivery in primates appears to be inefficient, and several trials of DNA vaccination in humans have shown variable, though encouraging results [1]. It appears that DNA uptake and expression is more inefficient in primates and humans compared to rodents. Methods to improve DNA gene delivery and immunogenicity for HIV include the combination of antigen expressing plasmid with vectors producing cytokines or the use of DNA as prime in combination with recombinant virus or protein boost (reviewed in [2–12]). Recent development of DNA delivery by in vivo electroporation is an important method to improve DNA delivery and is believed to have great potential to increase the results of DNA vaccination [13–17]. It has been reported that electroporation increases gene expression and it may also provide an adjuvant effect, since histological analysis has revealed local inflammation after electroporation. More experiments are needed in primates to fully explore the most optimal electroporation protocols.
In this report, we compare the efficacy of HIV gag DNA delivery in SIV-infected rhesus macaques that control viremia. Our data demonstrate that in vivo adaptive constant-current DNA delivery greatly improves expression and immunogenicity of both new and recall antigens compared to direct IM DNA injection. We also found that the recall responses after vaccination with plasmids expressing SIV antigens in SIV-infected controllers were very high. We examined the type of immune responses by multicolor flow cytometry and showed that electroporation in rhesus macaques results in both CD4 and CD8 cellular immune responses. Our results support the conclusion that electroporation has the potential to increase the efficacy of DNA vaccination in primates.
2. Materials and methods
2.1. Animals
Indian rhesus macaques (Macaca mulatta) were housed and handled in accordance with the standards of the Association for the Assessment and Accreditation of Laboratory Animal Care International. Screening for a set of MHC class I and II alleles was performed by PCR (D. Watkins, Wisconsin Regional Primate Center) and showed that all the enrolled animals except M137 were positive for the Mamu-A*01 allele. The animals were recycled from a previous study where they were vaccinated with recombinant poxvirus and challenged with SHIV89.6P [18]. After challenge, the animals were followed by measuring plasma virus levels. SIV RNA copy numbers were determined by nucleic acid sequence-based isothermal amplification (NASBA) assay having a threshold cut-off of 100 copies per ml plasma using SIVmac251-specific primers [19]. The animals controlled the SHIV challenge and 2 years later were superinfected with highly pathogenic SIVmac251 (10 AID) intravenously (prepared by R. Pal, Advanced BioScience Laboratory, [20]). One year post-infection, they were enrolled in the present DNA vaccination study.
2.2. DNA vectors
All plasmids used for DNA vaccination contain the human CMV promoter without any introns, the bovine growth hormone polyadenylation site, and the kanamycin resistance gene [21]. Plasmid MCP3-p37gag (6H) produces the HIV gag from an RNA (codon) optimized gene and was generated by introducing multiple silent point mutations not affecting the sequence of the encoded proteins, as previously described for HIV-1 gag and env [22–25] using synthetic DNAs. A secreted variant of the HIV gag was generated by N-terminal fusion with IP10-MCP3 [26], as previously described [21, 27]. The rhesus IL-15 plasmid (rmIL15t, AG65) contains the optimized IL-15 coding sequence having the native signal peptide replaced by the tissue plasminogen activator (tPA) signal and propeptide [28]. The mixture of SIV plasmids consists of the gag expression plasmids CATEgagDX (2S) and MCP3p39 (21S) that contain the beta-catenin (CATE) signal for intracellular degradation and monocyte chemotactic protein 3 (MCP3) for improved trafficking to antigen-presenting cells, respectively; and the env expression plasmids producing the native protein (99S) and the MCP3-env fusion (73S) [21, 27]. Plasmids LAMP-pol (103S) and LAMP-NTV (147S) express an inactive pol and a nef-tat-vif fusion protein (NTV), respectively, inserted between the extracellular and the cytoplasmic tail of the human lysosome-associated membrane protein-1 (LAMP) [29–31]. Plasmid rmIL15Ra (AG120) produces the rhesus IL-15 receptor alpha [32] expressed from an RNA optimized plasmid. Highly purified, endotoxin-free DNA plasmid preparations were produced using Qiagen kits (Hilden, Germany).
2.3. Vaccination
For the first study, animals (M079, M085, M089, M114, M116 and M121) were selected which controlled the SIVmac251 challenge and had virus loads in the chronic phase of less than 100 copies per ml plasma. The animals were vaccinated with a mixture of plasmids expressing HIV gag and rhesus macaque IL-15. Three animals (M079, M089, M116) were immunized via the IM route using 5 mg of each plasmid DNA in 2.5 mg/ml of citrate buffer and bupivacaine [33]. A total of 8 injections were given at 8 different sites (0.5 ml per site) on the upper and lower arms and legs. Three animals (M085, M114, M121) were immunized by IM injection and subsequent in vivo electroporation using the CELLECTRA™ adaptive constant-current electroporator (VGX Pharmaceuticals) with 250 μg of each DNA in 0.5 ml of PBS with 1% poly-L-glutamate sodium salt (LGS). Constant current electroporation was performed at 0.5 Amps, using three pulses of 52 msec pulse length, and 80 sec lag time. One 0.5 ml injection containing 250 μg DNA was given per animal. The immunization schedule included 3 vaccinations at weeks 0, 4, and 10. Immunological analysis was performed at day of the first (wk 0) and third vaccination (wk 10) and 2 weeks after vaccination 3 (wk 12). After a rest period of 14 weeks, all the animal were subjected to another cycle of 2 vaccinations using in vivo electroporation with either 250 μg of each DNA (vaccination #4, wk 24) or 500 μg of each plasmid DNA (vaccination #5, wk 26). Plasmids expressing IL-15 and IL-15 receptor alpha [28, 32] were included in the vaccination mixture. For vaccinations #4 and #5 a total of 750 μg and 1500 μg DNA in 1 ml was used, respectively. The animals received two injections of 0.5ml per site. Immunological assays were performed at the day of vaccination #4 (wk 24) and 2 weeks after vaccination #5 (wk 28).
For the second study, the selected animals (M113, M137 and M153) had virus loads of log 3.8, 3.4 and 2.3, respectively, and were subjected to antiretroviral drug treatment (ART) using a combination of two drugs ((R)-9-(2-phosphonylmethoxypropyl) adenine (PMPA) 20 mg/kg and FTC 50 mg/kg injected subcutaneously once daily. One week after ART start, PMPA was reduced to 10 mg/kg and the animals were vaccinated by the EP method using a mixture of SIV DNA plasmids. The animals were vaccinated by EP twice using the SIV DNA mixture containing 100 μg of each of the 6 plasmids in 1 ml of water with 1% LGS (as described above) starting one week after ART initiation (wk 0) and 8 weeks later.
2.4. IL-15 measurement
Rhesus IL-15 was measured daily (day 1–4 after EP #4 and EP #5) using the human IL-15 chemiluminescent immunoassay QuantiGlo kit (R&D Systems, No. D1500) in animals M079, M085, M089, M114, M116 and M121.
2.5. Flow cytometric analysis
PBMCs were resuspended at a density of 106 cells/ml in RPMI-1640 supplemented with 10% FBS, 100 U/ml penicillin/streptomycin and 2 mM L- glutamine, in the presence of a pool of HIV-1 gag 15-aa peptides overlapping by 11-aa (114 peptides at a final concentration of 1μg/ml for each peptide). Cells were treated for 6 hrs with monensin to prevent protein secretion, and cell surface staining was performed using the following antibody cocktail: CD3-APCCy7, CD4-PerCpCy5.5, CD8-AF405, CD45RA-PE and CD28-APCCy5.5 (BD Pharmingen). Cells were washed twice, fixed, and permeabilized with Cytofix/Cytoperm (BD Pharmingen) according to the manufacturer’s instructions. Intracellular cytokine detection was performed using an antibody against IFN-γ labeled with FITC (BD Pharmingen). The samples were analyzed in a FacsAria flow cytometer (BD Pharmingen) and the fluorescent data evaluated using the FlowJo platform (Tree Star, Inc.).
3. Results
3.1. Vaccination of rhesus macaques
Six Indian rhesus macaques were enrolled in a direct comparison of intramuscular DNA injection (IM) to electroporation (EP). The animals were recycled from a previous study where they were exposed to SHIV89.9P [18] and subsequently to pathogenic SIVmac251. The animals were able to control viremia to levels below the threshold of the assay (100 copies per ml) for more than 2 years. They were divided into 2 groups of 3 animals each (Fig. 1). One group (M079, M089, M116) was vaccinated by direct IM injection, whereas the other group (M085, M114, M121) was vaccinated by IM injection followed by in vivo constant-current EP. Since the animals had not been exposed to HIV-1 gag, we used an optimized expression plasmid producing HIV-1 gag as antigen, to study development of new responses. No cross-reactive cellular immune responses were found between HIV and SIV gag. A secreted variant of the HIV gag was used, which contains a N-terminal fusion with IP10-MCP3, as previously described [21, 27]. Five mg of the gag plasmid was used per animal for the direct IM injection, whereas only 250 μg of DNA per animal was used for the EP group. Blood samples were collected and analyzed on the day of first vaccination (wk 0), the day of third vaccination (wk 10), and also at weeks 12 and 24, to evaluate the long-term effect of the immunizations. At wk 24, all animals were combined into one study group (n=6) and subjected to another cycle of vaccination using in vivo EP only. For vaccination #4 (wk 24 of study) and vaccination #5 (wk 26 of study) each animal received 250 μg and 500 μg of the gag plasmid, respectively. The DNA mixtures also contained 250 μg each of optimized plasmids producing rhesus IL-15 [28] and IL-15 receptor alpha [32]. IL-15 plasma levels, measured daily (days 1–4 post-EP #4 and #5), served as reporter of the levels of gene expression after in vivo EP. Blood samples were collected 2 weeks later (wk 28) and isolated PBMC were analyzed for interferon-gamma production after stimulation with HIV gag 15-mer peptide pool using multicolor flow cytometry.
Fig. 1.
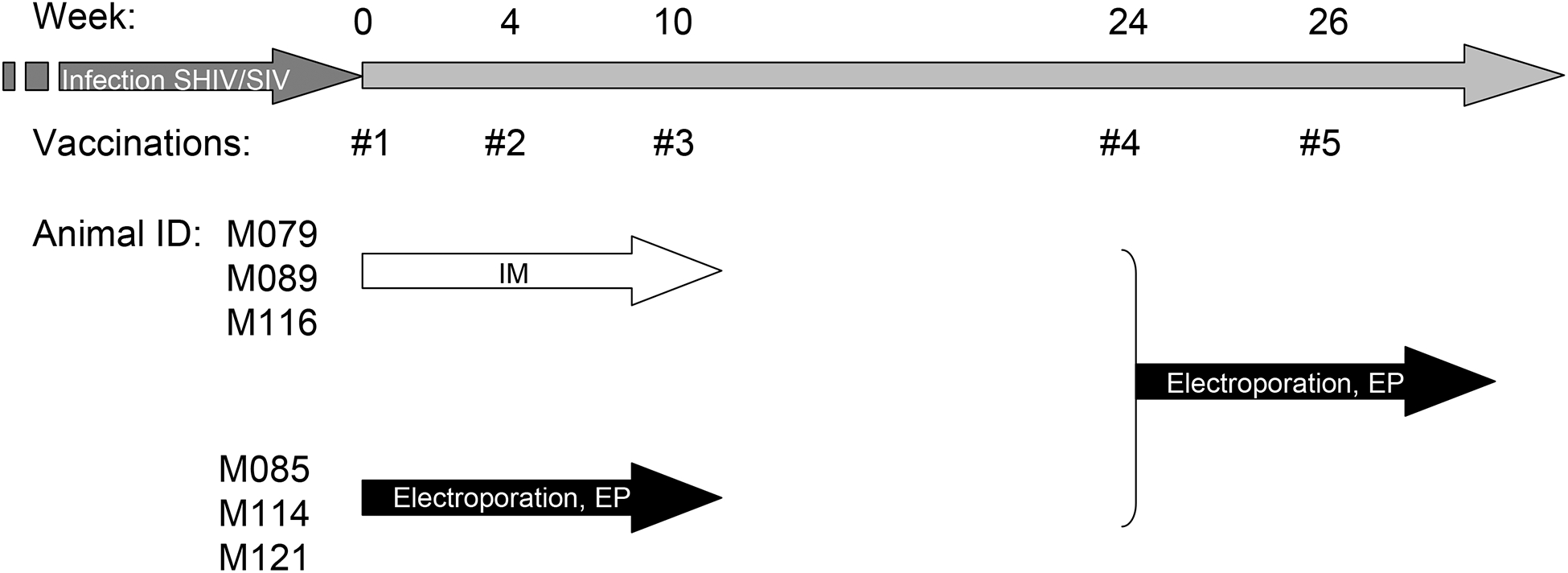
Schedule of HIV gag DNA vaccination. Two groups of 3 animals were vaccinated by DNA intramuscular injection (IM) or electroporation (EP) as indicated. After a rest period of 14 weeks, the two groups were combined and subjected to a second cycle of EP.
3.2. In vivo electroporation is more efficient than direct intramuscular injection
Fig. 2 shows a comparison of the levels of IFN-γ-producing gag-specific CD3+ cells from the 3 animals vaccinated by direct IM injection (Fig. 2A) and by in vivo EP (Fig. 2B) before vaccination (wk 0), at the time of the third vaccination (wk 10) and 2 weeks later (wk 12). The peak responses for both groups were measured 2 weeks after the third vaccination (wk 12). Interestingly, the 3 electroporated animals showed higher immune responses, which were still detected in the blood 14 weeks after the third vaccination, whereas the lower responses in the 3 IM vaccinated animals were at background levels at this time point (wk 24 of study). Thus, despite the use of 20 fold less DNA in the electroporated macaques, the immune responses generated after electroporation were higher and were detected in the circulating lymphocytes for much longer periods.
Fig. 2.

Comparison of direct IM DNA injection and adaptive in vivo constant-current DNA EP. Blood samples were analyzed on the day of first vaccination and on weeks 10, 12, 24 and 28. The frequency of HIV gag-specific CD3+ cells was measured by multicolor flow cytometry after in vitro stimulation with gag peptides and intracellular staining for IFN-γ.
After a rest period of 14 weeks, all 6 animals were vaccinated using EP (wk 24 and wk 26 of study) and the cellular immune responses were evaluated by the flow cytometry assay 2 weeks later (wk 28). EP boosted the antigen-specific immune responses in all six animals to high levels (Fig. 2). The peak responses of the 3 animals recruited from the IM group also increased dramatically and approached the levels of the EP animals, indicating that EP is a more efficient vaccination procedure and that the animals of the IM group in the first vaccination cycle were indeed capable of inducing high levels of gag-specific immune responses. Thus, in vivo EP is a more effective DNA vaccination method.
Increased immunogenicity is, at least in part, the result of higher antigen expression after EP. This was shown by measurements of IL-15 in the plasma produced by the optimized IL-15/IL-15 receptor alpha vectors included in the vaccine mixture and electroporated at the same site. Plasma levels of IL-15 were measured daily (day 1–4) after EP #4 and EP #5 and we found that the peak levels varied between 6.6 and 68 pg/ml plasma. Fig. 3 shows that there is a direct and significant correlation of the IL-15 levels measured at peak (day 3 or 4 post-EP) and the levels of gag-specific IFN-γ+ CD3+ cells measured 2 weeks after EP #5 (wk 28). Thus, these data suggest that high levels of antigen-specific immune responses is the result of more efficient gene expression obtained by in vivo EP of plasmid DNA.
Fig. 3.
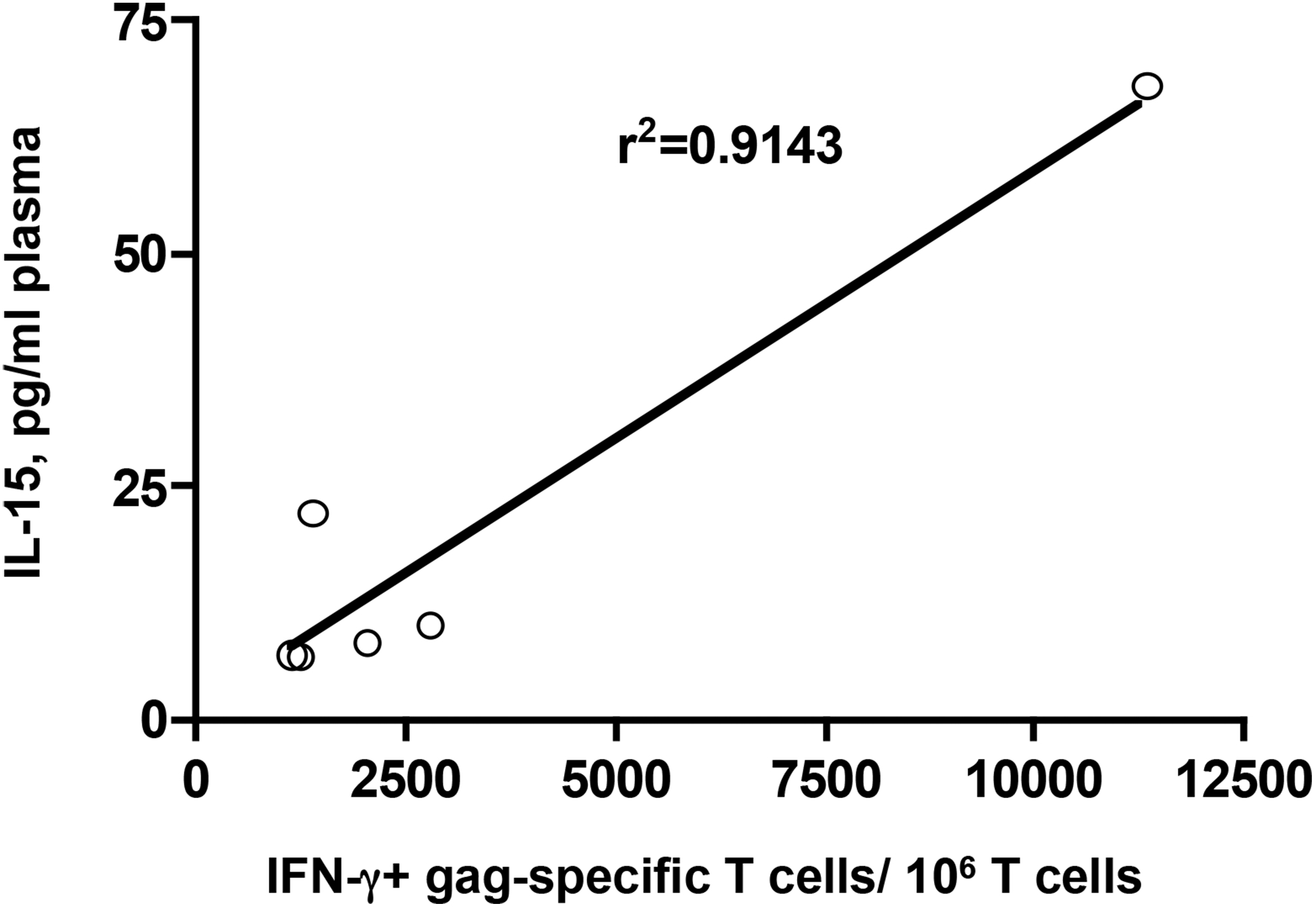
Correlation of peak IL-15 expression and antigen-specific immune response. Gag-specific immune responses were measured 2 weeks post EP #5 (wk 28) as detailed in Fig. 2. Peak IL-15 plasma levels were measured by ELISA (days 1 to 4 post-EP).
3.3. In vivo electroporation induces central and effector memory gag-specific immune responses
We further analyzed different subsets of T cells for their ability to respond to HIV gag peptides using combination of cell surface immunophenotyping and intracellular cytokine staining (Fig. 4 and Fig. 5) at 2 weeks post third vaccination (wk 12 of study). Fig. 4 shows flow cytometric analysis for total CD3+ cells, as well as for the CD4+ and CD8+ T subsets with central memory markers (CD28+CD45RA-) and of the CD8+ T subset with effector memory (CD28-) markers for the highest responder of each group. Fig. 5 shows a comparison of the different lymphocyte subsets in all animals in the two groups. In the EP animals, we found an increase in both the CD4+ and CD8+ T cell subsets. Further analysis of these cell populations revealed a significant qualitative difference in the phenotype of antigen-specific T cells. We found that the gag-specific increase in CD4+ T cells is mainly in cells that have a central memory phenotype. On the other hand, we found an expansion of the CD8+ T cells with both central memory and effector memory phenotype. These results suggest that EP expands the phenotype of the circulating gag-specific cells and results in higher numbers of CD8 cells. This may be a result of the higher levels of antigen expressed upon EP.
Fig. 4.

Flow cytometric analysis of HIV gag-specific IFN-γ producing T cells at 2 weeks after the third vaccination for animals M114 and M089 receiving DNA via EP and direct IM vaccination, respectively.
Fig. 5.

Comparison of HIV gag-specific IFN-γ producing lymphocyte subsets induced by vaccination. Numbers indicate IFN-γ producing T cells after stimulation with a gag peptide pool minus spontaneous stimulation expressed per million live T lymphocytes. CM, central memory cells, defined as CD28+CD45RA-; EM, effector memory cells, defined as CD28-.
3.4. Generation of strong SIV immune responses after electroporation
We also wished to compare the responses developed for HIV gag to those generated after EP of SIV antigens, to which these infected animals were already exposed. For this, in a proof-of-concept study, 3 additional animals (Fig. 6) were subjected to ART to fully suppress SIV replication. One week after ART initiation, the animals were vaccinated by EP twice (wk 0 and 8) using a mixture of optimized SIV vectors for gag, env, pol, nef, tat and vif, as described in Materials and Methods. Measurement of SIV-specific T cells revealed the presence of high levels of immune responses (Fig. 6B) upon vaccination. The animals showed immune responses to all tested proteins included in the vaccines. The total SIV-specific T cells in one of the animals, M113, at 2 weeks post-EP #2 (wk 10) were more than 10% of the circulating T cells. We did not observe such high levels of immune responses in a previous immuntherapeutic study using direct IM vaccination of 15 macaques with SIV DNA plasmids [27]. As part of other studies (data not shown), we also showed that EP by itself or EP of IL-15 DNA does not affect the SIV-specific immune responses, demonstrating that the observed SIV–specific immune responses shown in Fig. 6 and Fig. 7 are the result of the vaccination with the SIV DNA mixture by EP.
Fig. 6.
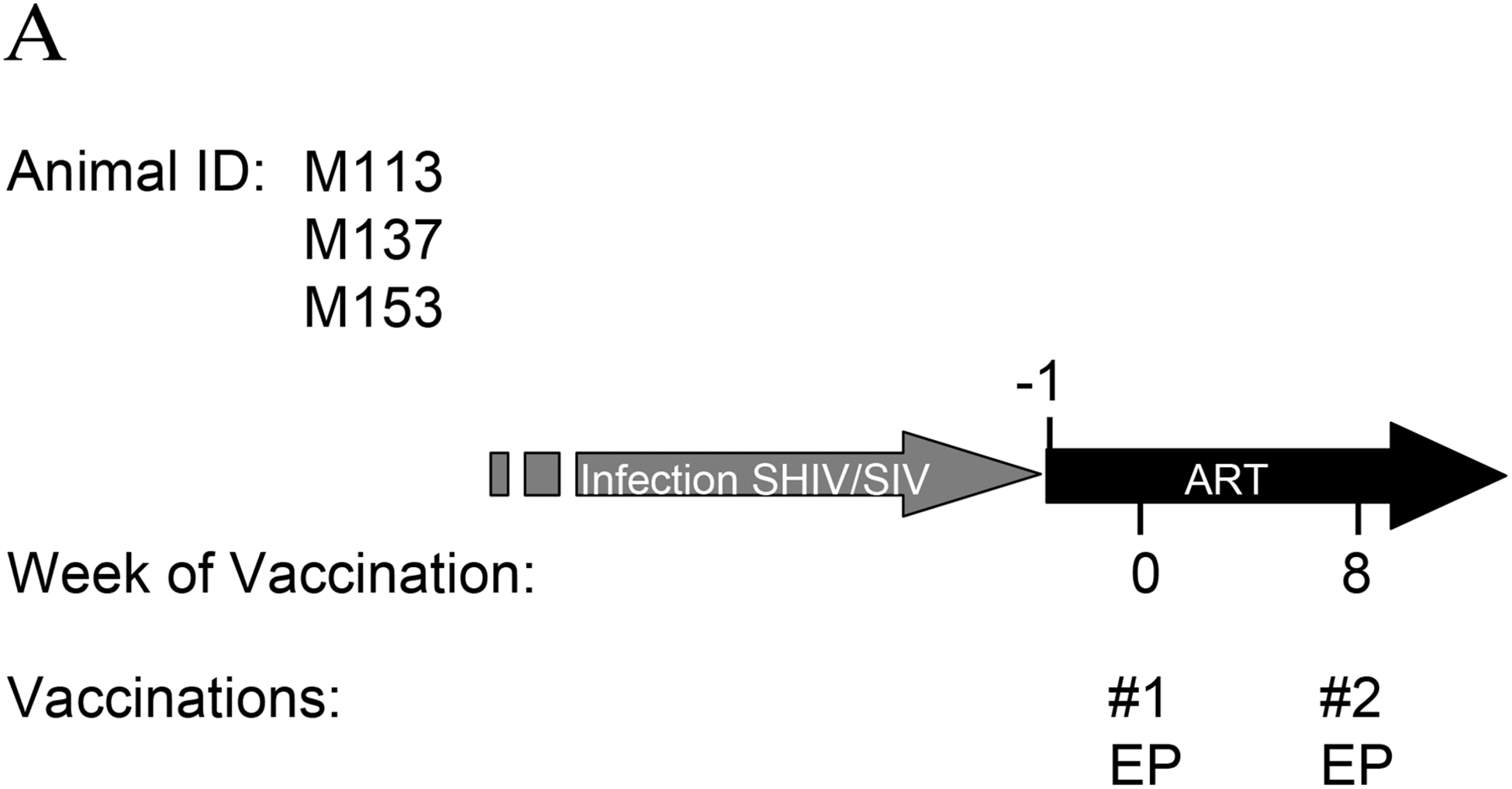

Vaccination with SIV DNA plasmids. (A) Schedule of macaques M133, M137 and M153 vaccinated with SIV DNA vectors. (B) Immune responses determined by flow cytometry to 5 SIV proteins at start of vaccination study (wk 0), at wk 4, wk 8 (second vaccination) and 2 weeks later at wk 10. Note that different scales are used for the Y-axis.
Fig. 7.

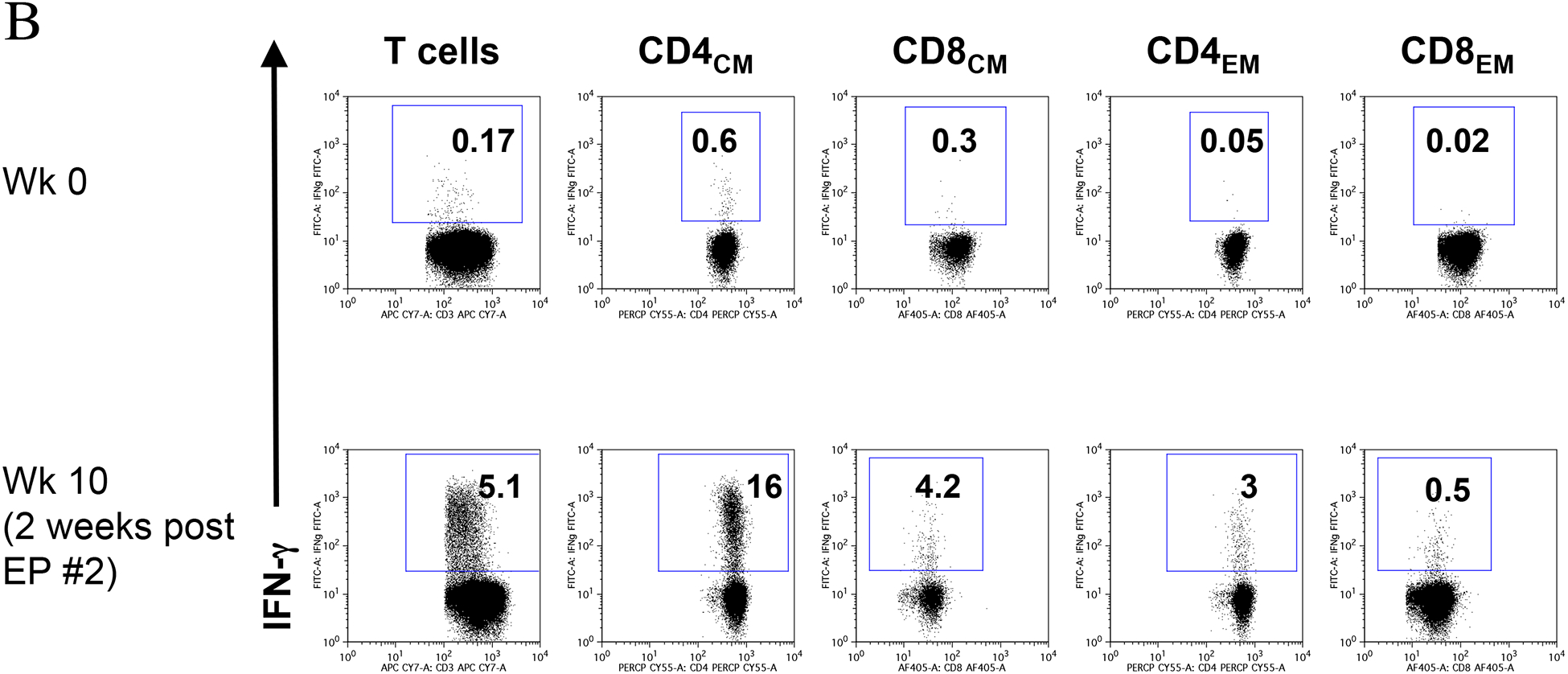
Analysis of gag-specific immune responses in animal M113. M113 was vaccinated as out-lined in Fig. 6. Gag-specific immune responses to in the CD4 and CD8 T cells (panel A) and in different subsets of CD4 and CD8 T cells (panel B) are shown at start of vaccination study (wk 0) and 2 weeks post EP vaccination #2 (wk 10). High levels of both CM and effector cells are induced by vaccination.
Focusing on the SIV gag-specific immune responses, we analyzed the T cell subsets of animal M113 at the start of the study (wk 0) and 2 weeks post EP #2 (wk 10) (Fig. 7). We found that the phenotype of gag-specific cells was both CD4 and CD8 T cells (Fig. 7A) with central and effector memory characteristics (Fig. 7B). The other two animals also showed strong gag-specific immune responses (data not shown). Therefore, DNA vaccination by EP in the presence of ART in these animals showed the triggering of high immune responses, which have not been observed in previous DNA vaccination experiments [27].
5. Discussion
We report the analysis of immune response to HIV or SIV antigens in macaques previously exposed to SIV and controlling viremia. These animals were able to respond rapidly and efficiently and generate new responses to HIV antigens as well as recall responses to SIV antigens. Comparison of EP to direct IM delivery indicates that EP resulted in higher levels of expression and immune response. Animals vaccinated with SIV DNA plasmids show rapid recall immune responses, which reach very high levels (2.5% and 12% of circulating T cells for animals 153M and M113, respectively, Fig. 6B). Although we did not have a group of macaques injected IM for direct comparison to EP, we used as historical controls 15 animals injected with the same DNA vectors IM [27]. We did not observe such high levels of immune responses in our previous experiments, despite using much higher amounts of DNA. Therefore, we conclude that EP is a promising technique to increase DNA vaccination results in primates. DNA vaccination in humans has shown good results only using optimized expression vectors and the delivery of several milligrams of DNA per injection. More efficient DNA vaccination methods are highly desirable to increase efficacy and practicality and to lower the cost of this procedure. In our experiments, a 20-fold lower dose delivered by constant-current EP gave stronger immune responses than IM injection alone. These results are in agreement with other recent reports indicating superior and long-lasting immunogenicity by EP [34]. The reason for this increased immune response is at least in part the likely higher expression achieved by EP. This was verified in our experiments by measuring plasma levels of IL-15 produced by optimized DNA expression vectors included in the vaccination mixture. The peak levels of plasma IL-15 varied by one log in different animals, indicating that significant animal-to-animal differences in expression occur. Identification of the sources of such variability may lead to more optimal delivery methods. Peak IL-15 expression levels correlated with immune response at 2 wks post-EP, indicating that it is important to further optimize efficient and reproducible delivery methods for DNA vaccination. Additional factors may contribute to the superior effects achieved by EP. Local inflammation produced by EP has been reported in previous studies, and may provide an adjuvant effect. IM DNA injection does not have a strong adjuvant effect, and local inflammation may be important for the increased efficiency of this procedure.
The long-term effects of EP are not known, but our animals recovered well from the procedure without scarring or any local signs of persistent inflammation, or other systemic or local adverse effects. It is also important to assess DNA integration after EP. DNA integration and persistence needs to be examined thoroughly for the different methods of DNA delivery. Variable results have been reported on integration after EP [34–36].
In conclusion, these results show the feasibility and importance of using improved DNA delivery methods for prophylactic and therapeutic vaccination and suggest that a reproducible EP procedure is an important delivery improvement. The efficient DNA delivery using in vivo EP, resulted in quantitative and qualitative increase of HIV and SIV specific immune responses. The observed expansion of CD4 and CD8 central memory and effector memory antigen-specific T cells may provide methods to further improve DNA vaccination.
Acknowledgments
We thank P. Roth for technical support, N. Miller, NAID/NIH for support with macaques, S. Orndorff and the staff at Advanced BioScience Laboratories, Kensington, for their expert help. We thank T. Jones for editorial assistance.
Abbreviations:
- EP
electroporation
- IM
intramuscular
- ELISA
enzyme-linked immunoabsorbent assay
- LGS
poly-L glutamate sodium salt
- IFN-γ
interferon-gamma
- aa
amino acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Graham BS, Koup RA, Roederer M, Bailer RT, Enama ME, Moodie Z, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 DNA candidate vaccine. J Infect Dis 2006;194(12):1650–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].McMichael AJ. HIV vaccines. Annu Rev Immunol 2006;24:227–55. [DOI] [PubMed] [Google Scholar]
- [3].Brave A, Ljungberg K, Wahren B, Liu MA. Vaccine delivery methods using viral vectors. Mol Pharm 2007;4(1):18–32. [DOI] [PubMed] [Google Scholar]
- [4].Dale CJ, Thomson S, De Rose R, Ranasinghe C, Medveczky CJ, Pamungkas J, et al. Prime-boost strategies in DNA vaccines. Methods Mol Med 2006;127:171–97. [DOI] [PubMed] [Google Scholar]
- [5].Duerr A, Wasserheit JN, Corey L. HIV vaccines: new frontiers in vaccine development. Clin Infect Dis 2006;43(4):500–11. [DOI] [PubMed] [Google Scholar]
- [6].Girard MP, Osmanov SK, Kieny MP. A review of vaccine research and development: the human immunodeficiency virus (HIV). Vaccine 2006;24(19):4062–81. [DOI] [PubMed] [Google Scholar]
- [7].Hokey DA, Weiner DB. DNA vaccines for HIV: challenges and opportunities. Springer Semin Immunopathol 2006;28(3):267–79. [DOI] [PubMed] [Google Scholar]
- [8].Liu MA, Wahren B, Karlsson Hedestam GB. DNA vaccines: recent developments and future possibilities. Hum Gene Ther 2006;17(11):1051–61. [DOI] [PubMed] [Google Scholar]
- [9].Lori F, Weiner DB, Calarota SA, Kelly LM, Lisziewicz J. Cytokine-adjuvanted HIVDNA vaccination strategies. Springer Semin Immunopathol 2006;28(3):231–8. [DOI] [PubMed] [Google Scholar]
- [10].Lu S Combination DNA plus protein HIV vaccines. Springer Semin Immunopathol 2006;28(3):255–65. [DOI] [PubMed] [Google Scholar]
- [11].Rodriguez-Chavez IR, Allen M, Hill EL, Sheets RL, Pensiero M, Bradac JA, et al. Current advances and challenges in HIV-1 vaccines. Curr HIV/AIDS Rep 2006;3(1):39–47. [DOI] [PubMed] [Google Scholar]
- [12].Thorner AR, Barouch DH. HIV-1 Vaccine Development: Progress and Prospects. Curr Infect Dis Rep 2007;9(1):71–5. [DOI] [PubMed] [Google Scholar]
- [13].Prud’homme GJ, Glinka Y, Khan AS, Draghia-Akli R. Electroporation-enhanced nonviral gene transfer for the prevention or treatment of immunological, endocrine and neoplastic diseases. Curr Gene Ther 2006;6(2):243–73. [DOI] [PubMed] [Google Scholar]
- [14].Aihara H, Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nat Biotechnol 1998;16(9):867–70. [DOI] [PubMed] [Google Scholar]
- [15].Widera G, Austin M, Rabussay D, Goldbeck C, Barnett SW, Chen M, et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J Immunol 2000;164(9):4635–40. [DOI] [PubMed] [Google Scholar]
- [16].Rizzuto G, Cappelletti M, Maione D, Savino R, Lazzaro D, Costa P, et al. Efficient and regulated erythropoietin production by naked DNA injection and muscle electroporation. Proc Natl Acad Sci U S A 1999;96(11):6417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mathiesen I Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Ther 1999;6(4):508–14. [DOI] [PubMed] [Google Scholar]
- [18].Santra S, Sun Y, Parvani JG, Philippon V, Wyand MS, Manson K, et al. Heterologous prime/boost immunization of rhesus monkeys by using diverse poxvirus vectors. J Virol 2007;81(16):8563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Romano JW, Shurtliff RN, Dobratz E, Gibson A, Hickman K, Markham PD, et al. Quantitative evaluation of simian immunodeficiency virus infection using NASBA technology. J Virol Methods 2000;86(1):61–70. [DOI] [PubMed] [Google Scholar]
- [20].Patterson LJ, Malkevitch N, Venzon D, Pinczewski J, Gomez-Roman VR, Wang L, et al. Protection against mucosal simian immunodeficiency virus SIV(mac251) challenge by using replicating adenovirus-SIV multigene vaccine priming and subunit boosting. J Virol 2004;78(5):2212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rosati M, von Gegerfelt A, Roth P, Alicea C, Valentin A, Robert-Guroff M, et al. DNA vaccines expressing different forms of SIV antigens decrease viremia upon SIVmac251 challenge. J Virol 2005;79(13):8480–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schwartz S, Campbell M, Nasioulas G, Harrison J, Felber BK, Pavlakis GN. Mutational inactivation of an inhibitory sequence in human immunodeficiency virus type 1 results in Rev-independent gag expression. J Virol 1992;66(12):7176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schwartz S, Felber BK, Pavlakis GN. Distinct RNA sequences in the gag region of human immunodeficiency virus type 1 decrease RNA stability and inhibit expression in the absence of Rev protein. J Virol 1992;66(1):150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schneider R, Campbell M, Nasioulas G, Felber BK, Pavlakis GN. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J Virol 1997;71(7):4892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nasioulas G, Zolotukhin AS, Tabernero C, Solomin L, Cunningham CP, Pavlakis GN, et al. Elements distinct from human immunodeficiency virus type 1 splice sites are responsible for the Rev dependence of env mRNA. J Virol 1994;68(5):2986–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Biragyn A, Tani K, Grimm MC, Weeks S, Kwak LW. Genetic fusion of chemokines to a self tumor antigen induces protective, T-cell dependent antitumor immunity. Nat Biotechnol 1999;17(3):253–8. [DOI] [PubMed] [Google Scholar]
- [27].von Gegerfelt AS, Rosati M, Alicea C, Valentin A, Roth P, Bear J, et al. Long-lasting decrease in viremia in macaques chronically infected with simian immunodeficiency virus SIVmac251 after therapeutic DNA immunization. J Virol 2007;81(4):1972–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jalah R, Rosati R, Kulkarni V, Patel V, Bergamaschi C, Valentin A, et al. Efficient Systemic Expression Of Bioactive IL-15 In Mice Upon Delivery Of Optimized DNA Expression Plasmids. DNA and Cell Biol 2007;in press. [DOI] [PubMed] [Google Scholar]
- [29].Marques ET Jr., Chikhlikar P, de Arruda LB, Leao IC, Lu Y, Wong J, et al. HIV-1 p55Gag encoded in the lysosome-associated membrane protein-1 as a DNA plasmid vaccine chimera is highly expressed, traffics to the major histocompatibility class II compartment, and elicits enhanced immune responses. J Biol Chem 2003;278(39):37926–36. [DOI] [PubMed] [Google Scholar]
- [30].de Arruda LB, Chikhlikar PR, August JT, Marques ET. DNA vaccine encoding human immunodeficiency virus-1 Gag, targeted to the major histocompatibility complex II compartment by lysosomal-associated membrane protein, elicits enhanced long-term memory response. Immunology 2004;112(1):126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chikhlikar P, de Arruda LB, Maciel M, Silvera P, Lewis MG, August JT, et al. DNA encoding an HIV-1 Gag/human lysosome-associated membrane protein-1 chimera elicits a broad cellular and humoral immune response in Rhesus macaques. PLoS ONE 2006;1:e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bergamaschi C, Rosati M, Jalah R, Valentin A, Kulkarni V, Alicea C, et al. Intracellular Interaction of IL-15 with its Receptor alpha During Production Leads to Mutual Stabilization and Increased Bioactivity. submitted. [DOI] [PubMed]
- [33].Pachuk CJ, Ciccarelli RB, Samuel M, Bayer ME, Troutman RD, Zurawski DV, et al. Characterization of a new class of DNA delivery complexes formed by the local anesthetic bupivacaine. Biochim Biophys Acta 2000;1468(1–2):20–30. [DOI] [PubMed] [Google Scholar]
- [34].Luckay A, Sidhu MK, Kjeken R, Megati S, Chong SY, Roopchand V, et al. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J Virol 2007;81(10):5257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sheets RL, Stein J, Manetz TS, Duffy C, Nason M, Andrews C, et al. Biodistribution of DNA plasmid vaccines against HIV-1, Ebola, Severe Acute Respiratory Syndrome, or West Nile virus is similar, without integration, despite differing plasmid backbones or gene inserts. Toxicol Sci 2006;91(2):610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wang Z, Troilo PJ, Wang X, Griffiths TG, Pacchione SJ, Barnum AB, et al. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther 2004;11(8):711–21. [DOI] [PubMed] [Google Scholar]


