Abstract
Background:
We previously operationally-defined subtle cognitive decline (SCD) in preclinical Alzheimer’s disease (AD) using total scores on neuropsychological (NP) tests. NP process scores (i.e., provide information about how a total NP score was achieved) may be a useful tool for identifying early cognitive inefficiencies prior to objective impairment seen in mild cognitive impairment (MCI) and dementia.
Objective:
We aimed to integrate process scores into the SCD definition to identify stages of SCD and improve early detection of those at risk for decline.
Methods:
Cognitively “normal” participants from the Alzheimer’s Disease Neuroimaging Initiative were classified as “early” SCD (E-SCD; >1 SD below mean on 2 process scores or on 1 process score plus 1 NP total score), “late” SCD (L-SCD; existing SCD criteria of >1 SD below norm-adjusted mean on 2 NP total scores in different domains), or “no SCD” (NC). Process scores considered in the SCD criteria were word-list intrusion errors, retroactive interference, and learning slope. Cerebrospinal fluid AD biomarkers were used to examine pathologic burden across groups.
Results:
E-SCD and L-SCD progressed to MCI 2.5-3.4 times faster than the NC group. Survival curves for E-SCD and L-SCD converged at 7-8 years after baseline. The combined (E-SCD+L-SCD) group had improved sensitivity to detect progression to MCI relative to L-SCD only. AD biomarker positivity increased across NC, SCD, and MCI groups.
Conclusions:
Process scores can be integrated into the SCD criteria to allow for increased sensitivity and earlier identification of cognitively normal older adults at risk for decline prior to frank impairment on NP total scores.
Keywords: early detection, subtle cognitive decline, neuropsychology, mild cognitive impairment, Alzheimer’s disease, dementia
Introduction
Preclinical Alzheimer’s disease (AD) is the asymptomatic phase of AD in which individuals without frank cognitive impairment are positive for AD biomarkers such as amyloid-β (Aβ) or tau proteins, including hyperphosphorylated-tau (p-tau) and total tau (t-tau). National Institute on Aging and Alzheimer’s Association (NIA-AA) criteria for preclinical AD maintain that amyloidosis (Stage 1) and neurodegeneration (Stage 2) precede subtle cognitive changes (Stage 3) [1]. We have recently shown, however, that cognitively normal participants in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) who progressed to mild cognitive impairment (MCI) or AD were just as likely to have operationally-defined subtle cognitive decline (SCD) as their first AD marker as they were to have Aβ as their first marker [2]. These results are consistent with Braak et al.’s [3] suggestion that there is likely overlap in the development of neuropathologic and cognitive changes in preclinical AD and they indicate that SCD may be just as important as other preclinical AD markers for predicting progression. Because the detection of SCD is limited by the sensitivity of cognitive tests, it is important to find sensitive measures of cognitive change that can be used alone or with traditional AD biomarkers to improve early detection of incipient AD and give the preclinical AD classification more predictive value.
Memory impairment characterized by rapid forgetting over a delay interval is one of the most sensitive indicators of early AD [4,5]. Memory test scores that reflect the amount of information learned and remembered over a delay interval may be just as important as traditional AD biomarkers for predicting progression from normal cognition to MCI or from MCI to AD dementia. A study modelling data from ADNI, for example, showed that a memory test score [Rey Auditory Verbal Learning Test (AVLT)] at baseline significantly predicted rate of progression from MCI to AD. Predictive accuracy was not substantially improved by adding baseline MRI entorhinal cortex volume or p-tau/Aβ ratio to the model [6]. Other longitudinal studies have shown that memory measures detect the earliest cognitive change in cognitively normal older adults who go on to develop AD dementia [7]. These results suggest that tests of memory may be particularly useful for detecting SCD in preclinical AD.
The ability of memory tests to detect SCD in preclinical AD may be enhanced by consideration of both the amount of information learned and remembered and the processes by which learning and memory took place. Neuropsychological test process scores are the quantification of errors or other aspects of an individual’s performance that allows one to determine how and why an individual achieved a final outcome reflected by the total score on a neuropsychological measure [8]. Process score analysis of word-list learning and memory tests has demonstrated that amnestic MCI and early AD are associated with a flattened learning slope, increased susceptibility to interference, and a greater frequency of extra-list intrusion errors relative to cognitively normal individuals [4,5,9-12]. These aspects of learning and memory could be altered before to an obvious decrement in the amount of information learned and remembered. Thus, consideration of “process” features or errors on tests of learning and memory may provide additional information about early cognitive changes related to preclinical AD, MCI, and AD dementia [9,10,12,13].
We recently examined this topic using the ADNI dataset and found baseline differences in number of AVLT intrusion errors, slope of learning, and susceptibility to retroactive interference between participants who remained cognitively normal and those who progressed-to-MCI within 5 years [13]. Furthermore, we demonstrated the added utility of memory intrusion errors in predicting progression from cognitively normal to MCI and mild dementia, even after statistically controlling for known risk factors, including demographic characteristics, depressive symptoms, daily functioning, neuropsychological test total scores, APOE ε4 status, and cerebrospinal fluid (CSF) AD markers (Aβ, t-tau, p-tau). These results suggest that clinically relevant errors and subtle cognitive inefficiencies on a word-list memory test occur in older adults identified as “cognitively normal” based on standard neuropsychological test total scores, and that these subtle changes may predict the subsequent development of MCI and AD.
What remains unclear, however, is to what extent memory process scores can contribute to our understanding and detection of SCD. Thus, we aimed to incorporate these process scores into our existing operational definition of SCD [2] and to utilize process scores as a method to identify “early” and “late” stages of SCD. We hypothesized that process score impairments may emerge earlier than decline in neuropsychological test total scores in the preclinical pathogenesis of AD (i.e., in early SCD), though both early and late SCD may ultimately have similar rates of progression to MCI and dementia.
Methods
Data used in the preparation of this article were obtained from the ADNI database (adni.loni.usc.edu). ADNI was launched in 2003 as a public-private partnership. The primary goal of ADNI is to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. For up-to-date information on ADNI, see www.adni-info.org. This study was approved by the Institutional Review Boards at each of the participating institutions, and written informed consent was obtained from all participants or authorized representatives at each site.
Participants
All non-demented ADNI participants who completed a baseline neuropsychological assessment were considered for analyses. Of the 1,397 participants considered for inclusion, 57 were excluded due to the absence of follow-up data. The final sample consisted of 1,340 participants. Of these participants, 616 were determined to meet criteria for MCI based on Jak/Bondi actuarial neuropsychological diagnostic method [14,15]. Participants were classified as MCI if they 1) performed >1SD below the age/education/sex-adjusted mean on two measures within the same cognitive domain, or 2) performed >1SD below the demographically-adjusted mean on at least one measure across all three sampled cognitive domains, or 3) were rated by a study partner to have a Functional Activities Questionnaire (FAQ) score > 5, suggesting functional difficulties across at least two areas of functioning (see Figure 1). The remaining 724 participants were determined to be cognitively normal. We applied the Jak/Bondi criteria at each of the participants’ follow-up visits (6-, 12-, 24-, 36-, 48-, 60-, 72-, 84-, 96-, 108-, and 120-months after baseline) to determine whether or not, and if so at what point, they progressed to MCI. We also tracked whether participants progressed to dementia based on ADNI’s AD criteria: 1) subjective memory complaint reported by the subject, study partner, or clinician; 2) abnormal memory function defined by scoring below the education-adjusted cutoff on Story A of Logical Memory II from the Wechsler Memory Scale–Revised; 3) MMSE score < 27; 4) Clinical Dementia Rating score of 0.5 or 1.0; 4) met NINCDS/ADRDA criteria for probable AD [16]. A proportion of the sample underwent a lumbar puncture at baseline (MCI n=434; cognitively normal n=532); this subset was used for analyses involving CSF markers.
Figure 1.
Existing criteria for SCD and MCI, and proposed SCD staging criteria. Impaired neuropsychological total and process scores were defined as >1 SD below age/education/sex-adjusted mean. FAQ=Functional Activities Questionnaire
Materials
Six neuropsychological test scores were used to determine MCI and SCD status via Jak/Bondi criteria. The scores were two measures of language: 30-item Boston Naming Test (BNT) total correct responses, Animal Fluency total score; two measures of attention/executive function: Trail Making Test (TMT), Part A and Part B times-to-completion; and two measures of memory: AVLT delayed free recall correct responses and AVLT recognition (hits minus false positives).
We also examined three neuropsychological process scores from the AVLT [17] that were not used in MCI classification to determine their added benefit for identifying and staging SCD. The AVLT is a 15-item word-list learning and memory test of semantically-unrelated words that includes 5 learning trials (List A, Trials 1-5), an interference trial with a different list (List B), a short-delay free recall trial (Trial 6) for List A, a long delay free recall trial (Trial 7) for List A after 30-minutes, and delayed recognition of List A. The three process scores from the AVLT that we examined included: intrusion errors (total number of extra-list intrusion errors across all recall trials), learning slope [(List A Trial 5 – List A Trial 1)/5], and retroactive interference, which captures the extent to which new learning inhibits previously learned information (List A Trial 6/List A Trial 5) [9]. These process scores have been previously shown to predict progression from cognitively normal to MCI [13]. CSF data included concentrations of Aβ1-42 and ratios of p-tau/Aβ and t-tau/Aβ.
Procedure
SCD classification and staging
Neuropsychological test total and process scores were converted to age-, education-, and sex-adjusted z-scores based on regression coefficients derived from a sample of ADNI’s cognitively normal participants who did not progress to MCI for the duration of their study participation (i.e., “robust” controls; N=381) [18]. All cognitively normal participants (N=724) were then classified as: “early” SCD (E-SCD; N=143), “late” SCD (L-SCD; N=106), or normal control (NC; N=475). Participants were classified as E-SCD if they either 1) performed >1 SD below the demographically-adjusted mean (i.e., an impaired score) on one total score and also had one impaired process score, or 2) had two impaired process scores. Participants were classified as L-SCD if they met our previously-published criteria for SCD [2] (i.e., had one impaired total test score in 2 different cognitive domains). If cognitively normal participants did not meet criteria for either E-SCD or L-SCD, they were classified as an NC.
AD biomarker classification
AD CSF biomarkers were processed using Roche Elecsys ® immunoassays. Biomarker positivity was determined by cut-off scores optimized for ADNI [19]: < 977 pg/ml for Aβ1-42, >.025 for p-tau/Aβ, and >.27 for t-tau/Aβ.
Statistical Analyses
Baseline demographic and clinical characteristics for each group (NC, E-SCD, L-SCD, MCI) were compared using one-way analyses of variance (ANOVAs) with Tukey correction for multiple comparisons, or chi-squared tests (for categorical variables). Logistic regression was used to examine the sensitivity and specificity of classifying participants who progressed to MCI (over 5 and 10 years) when using only the L-SCD classification only versus using both E-SCD and L-SCD classifications. Cox regression, adjusting for demographic and mood differences between groups (education, sex, and depressive symptoms), was used to determine the risk of MCI and dementia by group classification, with NC as the reference group. In these analyses, time-to-MCI and time-to-dementia were the number of months from baseline neuropsychological assessment to the assessment when the participant first met criteria for MCI of dementia. Participants who did not progress to MCI or dementia during their follow-up period were censored at their last visit. Kaplan-Meier curves were used to depict rate of progression to MCI and dementia. Log rank tests were used to determine if the Kaplan-Meier curves differed.
Results
Demographic and clinical characteristics
Table 1 shows the baseline demographic and clinical characteristics by group. There were significant differences in all baseline variables except for age.
Table 1.
Baseline demographic and clinical characteristics [mean (SD) or %] by group.
| Normal Controls (N=475) |
Early SCD (N=143) |
Late SCD (N=106) |
MCI (N=616) |
F or χ2 | p-value | Effect size | |
|---|---|---|---|---|---|---|---|
| Demographics and emotional/functional inventories | |||||||
| Age | 73.07 (6.66) | 74.07 (7.21) | 74.74 (7.20) | 73.82 (7.20) | F=2.30 | .076 | ηp2=.005 |
| Education | 16.40 (2.73)a | 16.22 (2.61) | 16.24 (2.68) | 15.81 (2.87)d | F=4.22 | .006 | ηp2=.009 |
| Female, % | 50.6% | 45.5% | 37.7% | 40.7% | χ2=13.06 | .005 | φ=.099 |
| GDS | 1.16 (1.30)a | 1.31 (1.42) | 1.08 (1.24)a | 1.58 (1.42)bd | F=10.36 | <.001 | ηp2=.023 |
| Cognitive measures | |||||||
| MMSE | 28.91 (1.25)abc | 28.45 (1.60)ad | 28.25 (1.78)ad | 27.42 (1.87)bcd | F=76.96 | <.001 | ηp2=.147 |
| Animal Fluency (z-score) | 0.14 (0.91)abc | −0.23 (0.76)abd | −0.71 (0.96)cd | −0.94 (0.92)cd | F=132.29 | <.001 | ηp2=.229 |
| BNT (z-score) | 0.14 (0.78)ab | −0.15 (0.99)ab | −0.68 (1.12)acd | −1.36 (1.91)bcd | F=101.64 | <.001 | ηp2=.186 |
| TMT Part A (z-score) | 0.21 (0.72)ab | 0.10 (0.82)ab | −0.42 (0.98)acd | −1.05 (1.84)bcd | F=83.09 | <.001 | ηp2=.157 |
| TMT Part B (z-score) | 0.11 (0.72)ab | −0.06 (0.92)a | −0.46 (1.20)ad | −1.27 (1.80)bcd | F=98.77 | <.001 | ηp2=.183 |
| AVLT Delayed recall (z-score) | 0.27 (0.82)abc | −0.66 (0.70)ad | −0.85 (0.84)ad | −1.36 (0.85)bcd | F=354.73 | <.001 | ηp2=.444 |
| AVLT Recognition (z-score) | 0.28 (0.63)abc | −0.30 (1.03)ad | −0.54 (0.81)ad | −1.65 (1.39)bcd | F=295.48 | <.001 | ηp2=.399 |
| Process scores | |||||||
| Intrusion Errors (z-score) | 0.13 (0.85)abc | −0.76 (1.36)abd | −0.22 (1.30)cd | −0.40 (1.27)cd | F=30.27 | <.001 | ηp2=.064 |
| Learning Slope (z-score) | 0.19 (0.95)abc | −0.72 (1.03)ad | −0.47 (1.05)ad | −1.20 (1.01)bcd | F=176.91 | <.001 | ηp2=.285 |
| Retroactive Interference (z-score) | 0.23 (0.82)abc | −0.77 (1.42)ad | −0.60 (1.25)ad | −1.36 (1.49)bcd | F=141.93 | <.001 | ηp2=.242 |
Note:
significantly different from MCI
significantly different from late SCD
significantly different from early SCD
significantly different from normal controls. For the E-SCD classification, 86.7% of the participants (n=124) were classified based on 1 neuropsychological total score impairment + 1 process score impairment, and 13.3% of the participants (n=19) were classified based on 2 process score impairments. GDS= Geriatric Depression Scale; MMSE=Mini Mental Status Exam; BNT=Boston Naming Test; TMT=Trail Making Test; AVLT=Rey Auditory Verbal Learning Test.
Proportion of NC, SCD, and MCI groups that progressed
Within the NC participants, 31.4% (N=149) progressed to MCI within 5 years and 36.0% (N=171) progressed to MCI within 10 years; 4.2% (N=20) and 6.5% (N=31) progressed to dementia within 5 years and 10 years, respectively. Within the E-SCD participants, 60.8% (N=87) progressed to MCI within 5 years and 63.6% (N=91) progressed to MCI within 10 years; 11.9% (N=17) and 15.4% (N=22) progressed to dementia within 5 and 10 years, respectively. Within the L-SCD participants, 72.6% (N=77) progressed to MCI within 5 years and 74.5% (N=79) progressed to MCI within 10 years; 14.2% (N=15) and 15.1% (N=16) progressed to dementia within 5 and 10 years, respectively. Within the MCI group, 41.2% (N=254) and 44.2% (N=272) progressed to dementia within 5 and 10 years, respectively. All SCD (E-SCD and L-SCD) participants who progressed to dementia were determined by ADNI to have AD. There were 6 NC participants who progressed to dementia who were not diagnosed with AD (5 other etiologies; 1 initially AD but switched to other etiology at a later visit). There were also 6 MCI participants who progressed to non-AD dementia (3 initially AD but switched to another etiology; 3 were another etiology).
Prediction of MCI status
The addition of participants that met criteria for E-SCD improved sensitivity and overall prediction of those who progress to MCI and dementia. SCD criteria based only on neuropsychological test total scores (i.e., only L-SCD) [2] accurately predicted 24.4% of the participants who transitioned to MCI (sensitivity) and 92.9% of participants who remained cognitively normal (specificity) over 5 years. Overall, 63.3% of the sample was correctly classified (Nagelkerke R2=.077). Results were similar for prediction of progression to MCI over 10 years—sensitivity was 22.9%, specificity was 92.9%, and overall classification was 60.0% (Nagelkerke R2=.068).
Criteria that incorporated neuropsychological test total scores and process scores (E-SCD + L-SCD) accurately predicted 52.4% of the participants who transitioned to MCI (sensitivity) and 79.3% of participants who remained cognitively normal (specificity) over 5 years. Overall, 67.7% of the sample was correctly classified (Nagelkerke R2=.140). Results were similar for prediction of progression to MCI over 10 years—sensitivity was 49.9%, specificity was 79.4%, and overall classification was 65.5% (Nagelkerke R2=.122).
Rate of progression to MCI
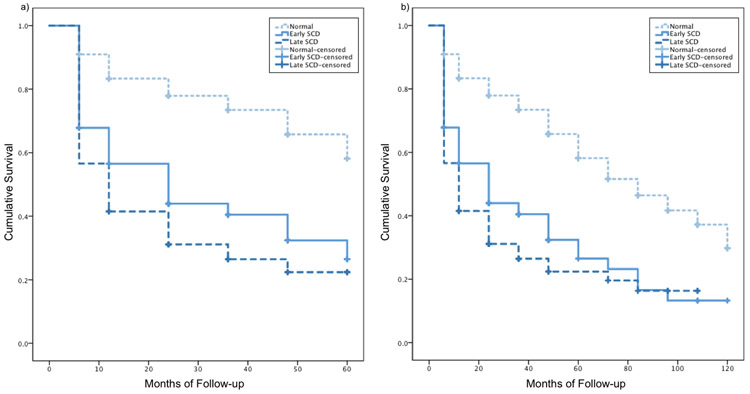
Cox regressions adjusting for education, sex, and depressive symptoms showed that both E-SCD (hazard ratio, HR: 2.60 [95% confidence interval, CI 1.99, 3.39], p<.001) and L-SCD (HR: 3.44 [95% CI 2.60, 4.55], p<.001) groups progressed to MCI faster than the NC group over 5 years. Similarly, over 10 years, both E-SCD (HR: 2.46 [95% CI 1.90, 3.18], p<.001) and L-SCD (HR: 3.17 [95% CI 2.41, 4.16], p<.001) groups progressed to MCI faster than the NC group, though the difference between the hazard ratios of the E-SCD and L-SCD groups was smaller over 10 years compared to over 5 years. Kaplan Meier curves for NC, E-SCD, and L-SCD progression to MCI over 5 and 10 years are shown in Figure 2. Log-rank tests showed that over 5 years, the NC group progressed to MCI at a slower rate than the E-SCD (χ2=65.30, p<.001) and L-SCD (χ2=102.50, p<.001) groups. The E-SCD group progressed to MCI at a slower rate than the L-SCD group (χ2=4.23, p=.040). Over 10 years, the NC group progressed to MCI at a significantly slower rate than the E-SCD (χ2=63.69, p<.001) and L-SCD groups (χ2=94.49, p<.001). The E-SCD and L-SCD groups did not significantly differ (p>.05) and the survival curves appear to converge at 7-8 years.
Figure 2.
Kaplan Meier curves for NC, E-SCD, and L-SCD progression to MCI over a) 5 years and b) 10 years.
Rate of progression to dementia
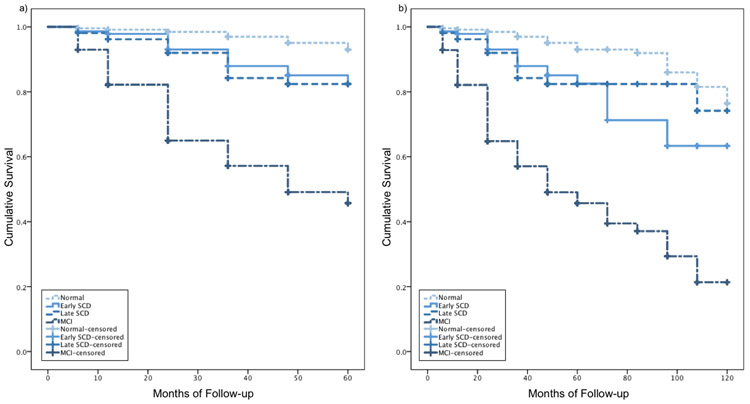
Cox regressions adjusting for education, sex, and depressive symptoms showed that the E-SCD (HR: 3.03 [95% CI 1.59, 5.79], p=.001), L-SCD (HR: 3.60 [95% CI 1.84, 7.03], p<.001), and MCI (HR: 12.88 [95% CI 8.14, 20.37], p<.001) groups all progressed to dementia faster than the NC group over 5 years. A similar pattern was found for E-SCD (HR: 2.66 [95% CI 1.54, 4.59], p<.001), L-SCD (HR: 2.60 [95% CI 1.42, 4.75], p=.002), and MCI (HR: 10.36 [95% CI 7.08, 15.14], p<.001) groups over 10 years, though, again, the difference between the hazard ratio of the E-SCD and L-SCD group was reduced compared to over 5 years such that over 10 years, the risk of progression to dementia for the E-SCD and L-SCD groups are almost identical. Kaplan Meier curves for NC, E-SCD, L-SCD, and MCI progression to dementia over 5 and 10 years are shown in Figure 3. Log-rank tests showed that over 5 years, the NC group progressed to dementia at a slower rate than the E-SCD (χ2=13.67, p<.001), L-SCD (χ2=16.61, p<.001), and MCI (χ2=224.04, p<.001) groups. The MCI group progressed to dementia at a faster rate than both E-SCD (χ2=45.76, p<.001) and L-SCD (χ2=31.56, p<.001) groups. The E-SCD and L-SCD groups did not significantly differ from each other (p>.05). Over 10 years, the NC group progressed to dementia at a slower rate than the E-SCD (χ2=15.53, p<.001), L-SCD (χ2=11.26, p=.001), and MCI (χ2=246.35, p=.001) groups. The MCI group progressed to dementia at a faster rate than both E-SCD (χ2=48.09, p<.001) and L-SCD (χ2=38.30, p<.001) groups. The E-SCD and L-SCD groups again did not significantly differ from each other (p>.05).
Figure 3.
Kaplan Meier curves for NC, E-SCD, L-SCD, and MCI progression to dementia over a) 5 years and b) 10 years.
AD CSF Biomarkers by group
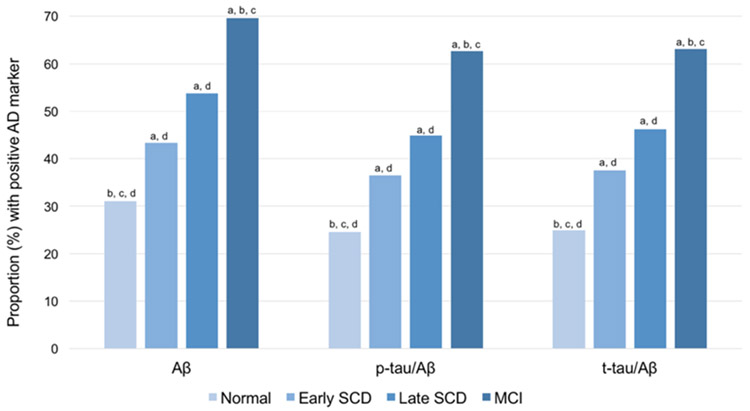
Figure 4 shows the proportion of participants with positive biomarkers in each of the groups. There were significant group differences in the proportion of positive markers for Aβ1-42 (χ2=98.75, df=3, p<.001), t-tau/Aβ (χ2=125.67, df=3, p<.001), and p-tau/Aβ (χ2=120.55, df=3, p<.001), with greater biomarker positivity in the E-SCD, L-SCD, and MCI groups compared to the NC group and greater biomarker positivity in the MCI group compared to the SCD groups.
Figure 4.
Proportion of participants with positive AD biomarkers by cognitive group. Sample size by group was: NC n=350, E-SCD n=104, L-SCD n=78, MCI n=434. Letters denote group differences (p<.05): a=significant different than NC, b=significant different than E-SCD, c=significant different than L-SCD, d=significant different than MCI.
Discussion
The current study integrated memory process scores into our previously operationalized criteria for SCD [2] and used these new criteria to identify a subset of “cognitively normal” participants with E-SCD. The E-SCD and L-SCD groups had a risk of progression to MCI that was 2.60 and 3.44 times greater than the NC group over 5 years and the difference between the risk of progression between the E-SCD and L-SCD groups narrowed slightly over 10 years at 2.46 and 3.17, respectively. This is consistent with the survival curves showing that the E-SCD and L-SCD curves converge at 7-8 years after baseline.
Notably, there were no differences between the E-SCD and L-SCD group in rate of progression to dementia, though both groups progressed significantly faster than the NC, but slower than the MCI group. A higher rate of progression to dementia in the E-SCD group than in the NC group and no differences between the E-SCD and L-SCD progression rates suggest that process scores used in the new E-SCD criteria are as equally valuable as total test scores for prognosis during the preclinical stage of AD. Process scores used in the E-SCD criteria have been shown to be present in amnestic MCI and early AD [4,10], and predict progression from cognitively normal to MCI [7,13]. These results demonstrate that subtle cognitive weaknesses captured by neuropsychological test process scores are clinically meaningful and might be a useful cognitive marker of preclinical AD.
Combining the E-SCD and L-SCD groups improved sensitivity to predict progression to MCI within 5 years relative to the L-SCD group alone (52.4% vs. 24.4%). The combined group also provided a better balance of sensitivity and specificity than the L-SCD group alone as indicated by better overall classification accuracy (67.7% vs. 63.3%) and a higher proportion of variance explained (14.0% vs. 7.7%). These results indicate that adding neuropsychological test process scores to the operational definition of SCD provides more sensitive criteria for detecting those at risk for progression to MCI without sacrificing overall classification accuracy.
The percentage of individuals with a positive AD biomarker showed the expected increase across the NC, E-SCD, L-SCD, and MCI groups for Aβ1-42, p-tau/Aβ ratio, and t-tau/Aβ ratio. These findings suggest that the early, preclinical cognitive changes in the E-SCD and L-SCD groups coincide with AD biomarker changes [3]. The Elecsys immunoassays used in this current study represent a strength given the automated processing mechanism that allows for reduced variability between laboratories and batches [19,20].
To our knowledge, this study is the first to formally integrate neuropsychological test process scores into an operational definition of SCD. Despite the inclusion of SCD in the NIA-AA criteria for preclinical AD (Stage 3) [1], few studies have operationally-defined SCD [2,21-23]. Instead, subjective cognitive decline is often substituted for SCD; however, the literature on the utility of subjective cognitive decline as a predictor of progression is mixed and may be impacted by reduced self-awareness or anosognosia on subjective evaluation of cognitive status (e.g., can lead to underreporting of cognitive difficulties) [24] as well as how emotional factors such as anxiety and mood can impact one’s evaluation of their objective cognitive ability (e.g., can lead to over-reporting of cognitive difficulty) [25-27]. The majority of studies that have used objective cognitive measures for defining SCD have used a global or memory composite score with a set cutoff percentile for SCD, an approach that ensures that a specified proportion of the sample will meet SCD criteria [21-23]. Our criteria for SCD [2], and the current modification that includes process scores, use an approach similar to the well-validated Jak/Bondi criteria for MCI [14,15]. Rather than using a composite score that can be disproportionally impacted by poor performance on only one test, our criteria require reliability (two impaired scores as opposed to a single impaired score) that is balanced with sensitivity (impairment defined as performance >1 SD below normative mean) [14,15].
Limitations to the current study should be noted. The neuropsychological tests available in the ADNI database are relatively limited, particularly with regard to specific process and error scores. Future work should implement these SCD criteria in a dataset that includes a broader range of neuropsychological test process scores across multiple cognitive domains. Another limitation is that the neuropsychological test total scores used to identify SCD were also used to detect MCI, one of the outcome variables, in those who progressed. To address this potential limitation, we also examined progression to dementia since ADNI’s dementia criteria are independent of the neuropsychological test total scores and process scores used to identify SCD. The time-to-dementia analysis showed that both the E-SCD and L-SCD groups were more likely than the NC group to progress to dementia within 5- and 10-years. This finding supports the idea that the E-SCD criteria are clinically meaningful rather than a reflection of variability in cognitively normal participants.
Future studies should examine the specificity of SCD criteria for capturing early cognitive changes associated with AD versus other etiologies that are difficult to differentiate from AD in the preclinical stage (e.g., vascular, hippocampal sclerosis) [28,29]. Neuropsychological test process scores can help differentiate between Huntington’s disease and AD [4], and between various subcortical dementias [30], so it is possible that they may help detect and guide differential diagnoses of AD and other emerging pathologies early in the course of disease.
The results of our study show that memory test process scores integrated into an operational definition of SCD are useful for improving the sensitivity and prognostic value of SCD criteria. As shown in our prior work [13], cognitive inefficiencies and various types of errors that reflect processing deficits can be objectively measured by expanding the scope of the data we obtain from standard neuropsychological tests that are already administered in large-scale aging studies. Analysis and integration of these process scores into neuropsychological test-based operational definitions of SCD is a non-invasive and cost-effective way of identifying those at risk for cognitive decline prior to the emergence of the frank cognitive impairment associated with MCI and dementia.
Acknowledgements
This work was supported by NIH grants R01 AG049810 (M.W.B.) and K24 AG026431 (M.W.B.), the Alzheimer’s Association (AARF-17-528918 to K.R.T. and AARG-17-500358 to E.C.E.), and the U.S. Department of Veterans Affairs Clinical Sciences Research and Development Service (Career Development Award-2 1IK2 CX001415-01A1 to E.C.E.). Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
Conflict of Interest/Disclosure Statement
M.W.B. is paid royalties from Oxford University Press and serves as a consultant for Eisai and Novartis. D.P.S. serves as a consultant for CHDI Foundation, Novartis, and Bristol-Meyers Squibb. The other authors report no disclosures.
References
- [1].Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster M V, Phelps CH (2011) Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 7, 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, Bondi MW (2015) Subtle Cognitive Decline and Biomarker Staging in Preclinical Alzheimer’s Disease. J. Alzheimer’s Dis 47, 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Braak H, Zetterberg H, Del Tredici K, Blennow K (2013) Intraneuronal tau aggregation precedes diffuse plaque deposition, but amyloid-β changes occur before increases of tau in cerebrospinal fluid. Acta Neuropathol. 126, 631–641. [DOI] [PubMed] [Google Scholar]
- [4].Delis DC, Massman PJ, Butters N, Salmon DP, et al. (1991) Profiles of demented and amnesic patients on the California Verbal Learning Test: Implications for the assessment of memory disorders. Psychol. Assess 3, 19–26. [Google Scholar]
- [5].Welsh K, Butters N, Hughes J, Mohs R, Heyman A (1991) Detection of Abnormal Memory Decline in Mild Cases of Alzheimer’s Disease Using CERAD Neuropsychological Measures. Arch. Neurol 48, 278–281. [DOI] [PubMed] [Google Scholar]
- [6].Richard E, Schmand BA, Eikelenboom P, Van Gool WA, Alzheimer’s Disease Neuroimaging Initiative (2013) MRI and cerebrospinal fluid biomarkers for predicting progression to Alzheimer’s disease in patients with mild cognitive impairment: a diagnostic accuracy study. BMJ Open 3, e002541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bondi MW, Salmon DP, Galasko D, Thomas RG, Thal LJ (1999) Neuropsychological function and apolipoprotein E genotype in the preclinical detection of Alzheimer’s disease. Psychol. Aging 14, 295–303. [DOI] [PubMed] [Google Scholar]
- [8].Kaplan E (1988) The process approach to neuropsychological assessment. Aphasiology. [DOI] [PubMed] [Google Scholar]
- [9].Loewenstein DA, Acevedo A, Luis C, Crum T, Barker WW, Duara R (2004) Semantic interference deficits and the detection of mild Alzheimer’s disease and mild cognitive impairment without dementia. J. Int. Neuropsychol. Soc 10, 91–100. [DOI] [PubMed] [Google Scholar]
- [10].Libon DJ, Bondi MW, Price CC, Lamar M, Eppig J, Wambach DM, Nieves C, Delano-Wood L, Giovannetti T, Lippa C, Kabasakalian A, Cosentino S, Swenson R, Penney DL (2011) Verbal Serial List Learning in Mild Cognitive Impairment: A Profile Analysis of Interference, Forgetting, and Errors. J. Int. Neuropsychol. Soc 17, 905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Salmon DP, Bondi MW (2009) Neuropsychological Assessment of Dementia. Annu. Rev. Psychol 60, 257–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Woodard JL, Dunlosky J, Salthouse TA (1999) Task Decomposition Analysis of Intertrial Free Recall Performance on the Rey Auditory Verbal Learning Test in Normal Aging and Alzheimer’s Disease. J. Clin. Exp. Neuropsychol. (Neuropsychology, Dev. Cogn. Sect. A) 21, 666–676. [DOI] [PubMed] [Google Scholar]
- [13].Thomas KR, Eppig J, Edmonds EC, Jacobs DM, Libon DJ, Au R, Salmon DP, Bondi MW, for ADNI. Word-list Intrusion Errors Predict Progression to Mild Cognitive Impairment. Neuropsychology, 32, 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, Delis DC (2009) Quantification of Five Neuropsychological Approaches to Defining Mild Cognitive Impairment. Am. J. Geriatr. Psychiatry 17, 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, Nation DA, Libon DJ, Au R, Galasko D, Salmon DP (2014) Neuropsychological Criteria for Mild Cognitive Impairment Improves Diagnostic Precision, Biomarker Associations, and Progression Rates. J. Alzheimer’s Dis 42, 275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–44. [DOI] [PubMed] [Google Scholar]
- [17].Rey A (1964) L’examen Clinique en Psychologie, Paris, France. [Google Scholar]
- [18].Edmonds EC, Delano-Wood L, Clark LR, Jak AJ, Nation DA, McDonald CR, Libon DJ, Au R, Galasko D, Salmon DP, Bondi MW (2015) Susceptibility of the conventional criteria for mild cognitive impairment to false-positive diagnostic errors. Alzheimer’s Dement. 11, 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hansson O, Seibyl J, Stomrud E, Zetterberg H, Trojanowski JQ, Bittner T, Lifke V, Corradini V, Eichenlaub U, Batrla R, Buck K, Zink K, Rabe C, Blennow K, Shaw LM, Swedish BioFINDER study group, Alzheimer’s Disease Neuroimaging Initiative (2018) CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers. Dement epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bittner T, Zetterberg H, Teunissen CE, Ostlund RE, Militello M, Andreasson U, Hubeek I, Gibson D, Chu DC, Eichenlaub U, Heiss P, Kobold U, Leinenbach A, Madin K, Manuilova E, Rabe C, Blennow K (2016) Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of β-amyloid (1-42) in human cerebrospinal fluid. Alzheimer’s Dement. 12, 517–526. [DOI] [PubMed] [Google Scholar]
- [21].Jack CR, Knopman DS, Weigand SD, Wiste HJ, Vemuri P, Lowe V, Kantarci K, Gunter JL, Senjem ML, Ivnik RJ, Roberts RO, Rocca WA, Boeve BF, Petersen RC (2012) An operational approach to National Institute on Aging-Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann. Neurol 71, 765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Knopman DS, Jack CR, Wiste HJ, Weigand SD, Vemuri P, Lowe V, Kantarci K, Gunter JL, Senjem ML, Ivnik RJ, Roberts RO, Boeve BF, Petersen RC (2012) Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology 78, 1576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, Cairns NJ, Morris JC, Holtzman DM, Fagan AM (2013) Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 12, 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hill NL, Mogle J, Wion R, Munoz E, DePasquale N, Yevchak AM, Parisi JM (2016) Subjective Cognitive Impairment and Affective Symptoms: A Systematic Review. Gerontologist 56, e109–e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ryu SY, Lee SB, Kim TW, Lee TJ (2016) Subjective memory complaints, depressive symptoms and instrumental activities of daily living in mild cognitive impairment. Int. Psychogeriatrics 28, 487–494. [DOI] [PubMed] [Google Scholar]
- [26].Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, Bondi MW (2014) Subjective Cognitive Complaints Contribute to Misdiagnosis of Mild Cognitive Impairment. J. Int. Neuropsychol. Soc 20, 836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yates JA, Clare L, Woods RT, CFAS M (2017) Subjective memory complaints, mood and MCI: a follow-up study. Aging Ment. Health 21, 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dubois B, Feldman HH, Jacova C, DeKosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P (2007) Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS–ADRDA criteria. Lancet Neurol. 6, 734–746. [DOI] [PubMed] [Google Scholar]
- [29].Brenowitz WD, Monsell SE, Schmitt FA, Kukull WA, Nelson PT (2014) Hippocampal Sclerosis of Aging is a Key Alzheimer’s Disease Mimic: Clinical-Pathologic Correlations and Comparisons with both Alzheimer’s Disease and Non-Tauopathic Frontotemporal Lobar Degeneration. J. Alzheimer’s Dis 39, 691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Massman PJ, Delis DC, Butters N, Levin BE, Salmon DP (1990) Are all subcortical dementias Alike?: Verbal learning and memory in Parkinson’s and huntington’s disease patients. J. Clin. Exp. Neuropsychol 12, 729–744. [DOI] [PubMed] [Google Scholar]