Abstract
Objective:
The aim of this study is to evaluate a novel composite measure of active range of motion (XA) and determine whether this measure correlates with active function.
Design:
Post hoc analysis of two randomized, placebo-controlled, double-blind studies with open-label extensions exploring changes in active function with abobotulinumtoxinA.
Setting:
Tertiary rehabilitation centers in Australia, Europe, and the United States.
Subjects:
Adults with upper (n = 254) or lower (n = 345) limb spastic paresis following stroke or brain trauma.
Interventions:
AbobotulinumtoxinA (⩽5 treatment cycles) in the upper or lower limb.
Main measures:
XA was used to calculate a novel composite measure (CXA), defined as the sum of XA against elbow, wrist, and extrinsic finger flexors (upper limb) or soleus and gastrocnemius muscles (lower limb). Active function was assessed by the Modified Frenchay Scale and 10-m comfortable barefoot walking speed in the upper limb and lower limb, respectively. Correlations between CXA and active function at Weeks 4 and 12 of open-label cycles were explored.
Results:
CXA and active function were moderately correlated in the upper limb (P < 0.0001–0.0004, r = 0.476–0.636) and weakly correlated in the lower limb (P < 0.0001–0.0284, r = 0.186–0.285) at Weeks 4 and 12 of each open-label cycle. Changes in CXA and active function were weakly correlated only in the upper limb (Cycle 2 Week 12, P = 0.0160, r = 0.213; Cycle 3 Week 4, P = 0.0031, r = 0.296). Across cycles, CXA improvements peaked at Week 4, while functional improvements peaked at Week 12.
Conclusion:
CXA is a valid measure for functional impairments in spastic paresis. CXA improvements following abobotulinumtoxinA injection correlated with and preceded active functional improvements.
Keywords: Active function, active range of motion, composite measure, passive range of motion, spastic paresis
Introduction
Deforming spastic paresis involves both muscular and neural mechanisms that lead to hypo-extensibility and muscle overactivity in antagonist muscles and to reduced motor command on agonist muscles.1,2 Consequently, paretic patients experience impairments in active range of movement, which are often associated with limitations in functional activities of daily life and restricted mobility.3
Much of the existing literature on focal interventions for the improvement of spastic paresis has focused, and often continues to focus, on ordinal measurements of resistance to passive movement: the most popular tools being Ashworth-derived scales.4,5 However, these subjective, unquantified, passive resistance measures lack clinical relevance when it comes to understanding whether substantial changes in active capacities and functioning have occurred after treatment.6–8
Motor impairment in patients with spastic paresis is governed primarily by the degree of antagonist resistance around not just a single joint but multiple joints in the affected limb;1 therefore, attempts at active movement are hindered in particular by antagonistic spastic cocontraction from several muscles.9 This symptom is overlooked in assessments of resistance to passive movements and in measures of active movements at a single joint.9 A composite measure of active range of motion may therefore be a more functionally relevant tool to estimate active movement capacities. To the best of our knowledge, there are no published data describing a composite active range of motion measurement, nor data to show correlations between this measure and improvements in active function.
Results of two recent Phase 3, randomized, double-blind studies and their open-label extensions conducted in adults with chronic spastic paresis of the upper or lower limb demonstrated improvements in range of active motion and gains in active function following repeat injections of botulinum toxin type A compared with placebo.10–12 Using data from these two studies, we evaluated a novel composite measure of active range of motion and determined whether changes in this measure correlated with changes in active function following repeated treatment with botulinum toxin type A.
Methods
We performed a post hoc analysis of two randomized, placebo-controlled, Phase 3, double-blind studies (upper limb: NCT01313299;10 lower limb: NCT0124940412) and their open-label extensions (NCT01313312 and NCT01251367,11,12 respectively) exploring the effects of abobotulinumtoxinA (Dysport®, Ipsen Biopharm Ltd, Wrexham, UK) in subjects with chronic hemiparesis. The objectives of this study were to evaluate the sensitivity to change and the correlation with active function of a novel composite measure of active range of motion (XA), for assessing change in global active range of motion in patients with upper or lower limb spastic paresis over repeated treatment cycles.
Full details of the protocols for the original upper limb10,11 and lower limb12 studies, including eligibility criteria, study interventions, and assessments, have been previously published. In brief, eligible patients were aged between 18 and 80 years and had spastic hemiparesis following one clinically defined stroke episode or brain trauma ⩾6 months prior to enrollment. Key exclusion criteria included major limitations in passive range of motion in the affected limb, physiotherapy initiated less than four weeks before expected enrollment, or treatment with botulinum toxin type A of any type in the previous four months. Patients could receive up to five treatment cycles with abobotulinumtoxinA, inclusive of the initial double-blind, randomized, placebo-controlled treatment cycle. Dosing during the open-label treatment cycles was at the investigator’s discretion, up to a maximum of 1500 U. In all studies, patients attended mandatory follow-up visits at Weeks 4 and 12 of each treatment cycle, with discretionary visits at Weeks 16, 20, and 24. Retreatment was possible on or after Week 12 of each cycle. Maximum study duration for patients completing both double-blind and open-label studies was 18 months.
The key assessment used in this post hoc analysis was the composite active range of motion, or CXA, which was defined as follows: upper limb CXA = XA for elbow flexors + XA for wrist flexors + XA for extrinsic finger flexors; lower limb CXA = XA for soleus + XA for gastrocnemius muscles. Each of these muscle groups was included in the calculation regardless of whether the muscle groups were injected or not. For a given antagonist, XA is the maximal active range of motion against the resistance of this antagonist, reflecting the net result, in terms of joint angles, of the force developed by the command reaching the agonist, minus the passive (stiffness) and active (spastic cocontraction) antagonist resistances over a single movement.1,13 Assessment of XA followed the measurement procedures described by Gracies and colleagues.1,13 For each antagonist tested, XA was measured by goniometry, using zero angle as the theoretical position of minimal stretch of the antagonist assessed.1,13 Measurements were taken in a seated position for the upper limb and in supine position for the lower limb. The XA measures have shown intra- and inter-rater reliability in the muscles evaluated in the present study.14
Active upper limb function was assessed using the Modified Frenchay Scale,13,15,16 a modified version of the original Frenchay Arm Test.17 The Modified Frenchay Scale measures active upper limb function in hemiparesis based on a video review of 10 everyday living tasks.15 Six tasks are bimanual and four are unimanual with the paretic hand. Each task is rated on a 10 point visual analog scale by independent assessors (0 = no movement to 10 = normal movement; 5 = task barely accomplished). Active lower limb function was measured using the 10-m walking speed test at comfortable and maximal walking speeds without walking aids, while wearing shoes and while barefoot. In the present analysis, comfortable barefoot walking speed data were used.
Study populations included in this analysis were the intention-to-treat populations for each study, defined as all patients who received at least one injection of study medication. Efficacy analyses were recorded as the mean ± standard deviation and 95% confidence interval of change from baseline to Week 4 (post-injection) for double-blind cycles for CXA, and to Weeks 4 and 12 (post-injection) of open-label treatment cycles for CXA and measures of function. All open-label analyses for CXA and measures of function included only subgroups of patients with data available for both Week 4 and Week 12 of each treatment cycle (Cycles 1–3 only, as n = 0 at Cycle 4 Week 12). Baseline values were considered to be Day 1 (prior to injection) of the double-blind phase. Analyses were performed by treatment group in the double-blind phase (placebo vs. abobotulinumtoxinA) and for all patients in the open-label extension (all doses combined). Linear relationships between CXA and measures of function were explored using Pearson’s correlation coefficients on changes from baseline to Weeks 4 and 12 of open-label treatment cycles for all patients with data available (Cycles 1–4).
Results
Patients
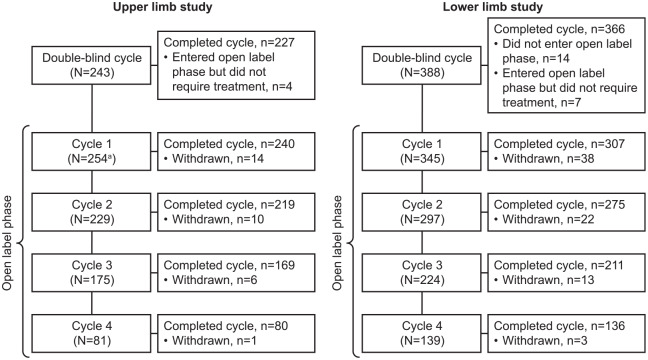
In the upper and lower limb open-label studies, 254 and 345 patients, respectively, received repeated injections.10–12 The number of patients who received injection in, and completed each cycle is shown in Figure 1.10–12 Full details of patient flow and of patient demographics, baseline characteristics, and mean dose of abobotulinumtoxinA injected into each of the muscle groups can be found in the original study publications.10–12,18 In general, patient demographics and baseline characteristics were similar between studies; across double-blind treatment groups of both studies, the mean age was 51 to 53 years, 62% to 70% of patients were male, and in 83% to 92% of patients, the cause of spastic paresis was stroke. CXA values at baseline of the double-blind study are shown in Table 1. Baseline values for active function at Cycle 1 were a mean [95% confidence interval] score of 3.9 [3.7; 4.1] out of 10 on the Modified Frenchay Scale and a 10-m comfortable barefoot walking speed of 0.447 [0.424–0.469] m/s.
Figure 1.
Patients in the upper and lower limb studies.
Further details on patient flow and reasons for withdrawal are reported in the primary publications for the upper and lower limb studies.10–12
aIncludes 223 patients from the double-blind study and 31 newly recruited patients.
Table 1.
Change from baseline in composite active range of motion during the upper limb and lower limb double-blind studies.
| Time point | Upper limb |
Lower limb |
||
|---|---|---|---|---|
| Placebo (n = 79) | AbobotulinumtoxinA (n = 159) | Placebo (n = 128) | AbobotulinumtoxinA (n = 253) | |
| Baseline (n) Mean [95% CI] |
58 302.1 [30.0; 540.0] |
133 294.4 [70.0; 540.0] |
128 150.7 [95.0; 210.0] |
253 149.9 [80.0; 220.0] |
| Change from baseline to Week 4 (n) Mean [95% CI] |
55 5.5 [−5.0; 15.9] |
128 36.6 [27.7; 45.4] |
127 4.4 [1.5; 7.2] |
253 6.3 [4.3; 8.3] |
CI: confidence interval; n: number of patients with data available.
Data are for double-blind intention-to-treat populations. Data are presented in degrees (°).
Change in composite range of active motion (CXA)
In the upper limb double-blind trial, mean change in CXA from baseline to Week 4 was markedly greater following abobotulinumtoxinA injection (doses pooled) than following placebo injection, in contrast with results from the lower limb double-blind trial (Table 1).
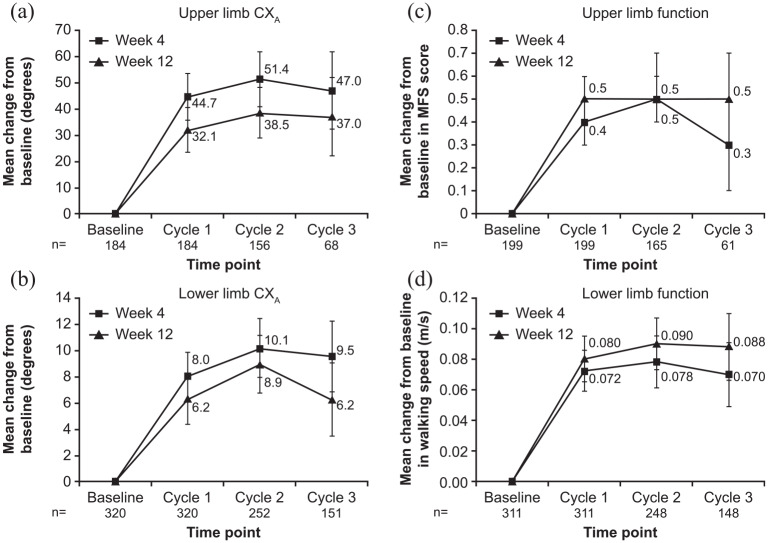
In the open-label extension studies, there were sustained improvements in CXA across Cycles 1 to 3 at Weeks 4 and 12 in both the upper and lower limb trials (doses pooled; Figure 2(a) and (b)), except from Cycle 2 to Cycle 3 where the number of remaining patients in the trial was markedly reduced. Results were not analyzed for Cycle 4 as patient numbers were too low. During both the upper and lower limb studies, mean change in CXA was numerically higher at Week 4 of each cycle compared with Week 12.
Figure 2.
Change from baseline to Week 4 and Week 12 in (a) upper limb CXA, (b) lower limb CXA, (c) upper limb active function, and (d) lower limb active function, during the open-label extension studies.
CXA: composite active range of motion; MFS: Modified Frenchay Scale; n: number of patients with data at both Week 4 and Week 12 of each cycle.
Data are presented as mean [95% confidence interval]. Active function was assessed by the MFS (upper limb) and unaided comfortable barefoot walking speed (lower limb).
Change in active function
Patients receiving repeated abobotulinumtoxinA injections during the open-label extension studies showed improvements in active function following treatment. At Week 4, mean improvements from baseline in upper limb active function (Modified Frenchay Scale score) increased between Cycles 1 and 2, then decreased slightly during Cycle 3 (Figure 2(c)). At Week 12, improvements in active function were numerically greater than they were at Week 4, and this effect was consistent across cycles (Figure 2(c)). Similarly, in the lower limb study, improvements in comfortable barefoot walking speed were maintained across open-label treatment cycles at Weeks 4 and 12, with consistently higher improvements observed at Week 12 than at Week 4 (Figure 2(d)).
Correlations between CXA and functional outcomes
As shown in Figure 3 and Table 2, there were correlations between absolute values for CXA and measures of active function in the upper limb (P < 0.0001–0.0004) and lower limb (P < 0.0001–0.0284) at Week 4 and Week 12 of each open-label cycle. Although significant, these correlations were weak to moderate (r = 0.186–0.636).
Figure 3.
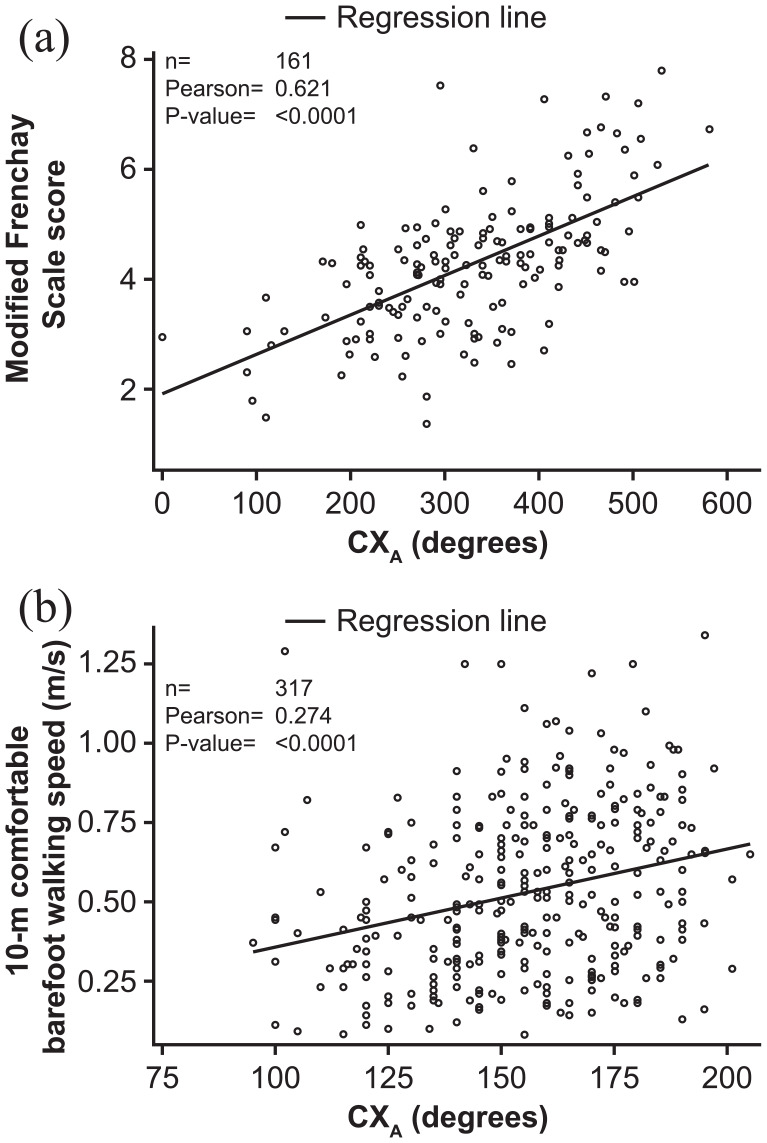
Scatterplot and correlation analysis for CXA and active function at Week 12 of Cycle 1 in (a) upper limb and (b) lower limb.
CXA: composite active range of motion; MFS: Modified Frenchay Scale; n: number of patients with data available.
Data are presented as absolute values.
Table 2.
Relationship between composite active range of motion and measures of active function in the upper and lower limb studies (absolute values and changes from baseline).
| Time point | Upper limb study |
Lower limb study |
||||||
|---|---|---|---|---|---|---|---|---|
| Week 4 |
Week 12 |
Week 4 |
Week 12 |
|||||
| Absolute values | Change from baseline | Absolute values | Change from baseline | Absolute values | Change from baseline | Absolute values | Change from baseline | |
| Cycle 1 (n) | 179 | 170 | 161 | 152 | 335 | 334 | 317 | 316 |
| Correlation coefficient | 0.575 | 0.052 | 0.621 | 0.122 | 0.221 | −0.036 | 0.274 | −0.021 |
| P value | <0.0001 | 0.5007 | <0.0001 | 0.1331 | <0.0001 | 0.5158 | <0.0001 | 0.7052 |
| Cycle 2 (n) | 158 | 148 | 137 | 128 | 286 | 285 | 252 | 252 |
| Correlation coefficient | 0.601 | 0.155 | 0.608 | 0.213 | 0.222 | −0.052 | 0.211 | −0.065 |
| P value | <0.0001 | 0.0601 | <0.0001 | 0.0160 | 0.0001 | 0.3854 | 0.0008 | 0.3059 |
| Cycle 3 (n) | 104 | 98 | 53 | 50 | 215 | 215 | 150 | 150 |
| Correlation coefficient | 0.636 | 0.296 | 0.476 | 0.059 | 0.186 | −0.086 | 0.285 | 0.021 |
| P value | <0.0001 | 0.0031 | 0.0003 | 0.6819 | 0.0064 | 0.2076 | 0.0004 | 0.8029 |
| Cycle 4 (n) | 46 | 43 | 0 | 0 | 134 | 134 | 0 | 0 |
| Correlation coefficient | 0.503 | 0.150 | – | – | 0.189 | −0.025 | – | – |
| P value | 0.0004 | 0.3367 | – | – | 0.0284 | 0.7785 | – | – |
n: number of patients with data available.
Linear relationships analyzed using the Pearson correlation coefficient on absolute values and changes from baseline.
Correlations between changes from baseline in CXA and in active function were significant only at open-label Cycle 2 Week 12 (P = 0.0160) and Cycle 3 Week 4 (P = 0.0031) of the upper limb study.
Discussion
This post hoc analysis of recent double-blind and open-label extension studies of abobotulinumtoxinA in chronic spastic paresis showed significant, albeit moderate, correlations between CXA (composite active range of motion) and active function in the upper and lower limbs. This analysis also showed that changes in CXA and active function after abobotulinumtoxinA therapy followed a different time course, such that improvements in CXA peaked at Week 4 post-injection, whereas improvements in active function peaked at Week 12. Finally, we showed that CXA was sensitive to change following treatment for upper and lower limb spastic paresis. The pattern of changes in CXA, which showed an overall trend toward greater improvements with repeated treatment cycles followed by a plateau at later cycles, reflects the results observed for other outcome measures of the original studies.10–12
Historically, composite outcome measures used in rehabilitation studies have tended to be generated from passive resistance measures, for example, by summing modified Ashworth Scale scores of multiple joints to assess muscle tone changes in the affected limb.18–30 Although helpful, these approaches have overlooked assessment of active motion or active function. A “composite functional index” has been developed, which assesses perceived function involving mostly passive movements.31 This index combined subjective assessments (ease of cleaning palm, cutting fingernails, and putting arm through sleeve) with Barthel Index items (grooming, feeding, and dressing), but true active function, such as that assessed via the Modified Frenchay Scale, was not explored.31
In the present study, a trend was observed toward consistently greater improvements in active function at Week 12 compared with Week 4 following injection, although 95% confidence intervals were overlapping. In the lower limb study in particular, change from baseline in comfortable barefoot walking speed was increased at Week 12 of Cycles 1 to 3 compared with Week 4, while this was the opposite for CXA in both the upper and lower limbs. This discrepancy may be due to time required for motor learning processes and improved inter-segment coordination to develop, that is, time needed to acquire new motor control to adjust to the new situation of a weaker and looser antagonist between Weeks 4 and 12 following injection, during which time direct blocking effects of the toxin on antagonist cocontractions start declining.10–12 An additional reason could be the window of opportunity to retrain, that is, patients may have walked more and/or used their arm in a more intensive way between Week 4 and Week 12, and therefore increased their activity or their intensity of rehabilitation. This increase in activity may have been sufficient to enhance functional effects of treatment, even when direct effects of the toxin on actual range of active motion diminished. Overall, this observation may indicate that the optimal assessment time of active range of motion following injection may be around Week 4, whereas changes in active function post-injection may be more efficiently assessed around Week 12.
Although this analysis demonstrated a significant correlation between absolute values for CXA and active function in the upper and lower limbs at Weeks 4 and 12 across all treatment cycles, there was no clear correlation between changes from baseline in CXA and active function when explored between both values at the same time point. Again, this may be due to the time lag between maximal change in CXA (Week 4) and maximal change in function (Week 12), which would contribute to lesser correlation between the two than between absolute values of each at a given time.
So far as limitations for the present analysis, CXA, particularly when measured on a small number of limb muscles as done here, may not capture all clinically relevant aspects of motor function along the whole limb. In particular, at Week 4, additional limitations of movements may not have been captured (e.g. limitations due to pronator or shoulder extensor overactivity in the upper limb, or due to overactivity in proximal muscles of the lower limb). A further limitation was the relatively short duration of the assessment period evaluated here, as motor commands may require more than 12 weeks to adapt to the effects of a muscle-weakening therapy.
Nevertheless, use of CXA as a marker of active function may provide several benefits over existing measures. Furthermore, XA is easy to learn and use in a clinical setting. With these points in mind, CXA could be a measure of choice to approach overall level of active function in patients with upper or lower limb spasticity. Because the effects of botulinum toxin type A injections have been well established on tone and spasticity reduction, we suggest that either CXA (early) or measures of active function (late) are now used with higher functional relevance as primary or secondary outcome measures for assessing effects of treatment in the paretic upper and lower limbs.
Clinical messages.
A novel composite score of active range of motion correlates with active function in patients with upper or lower limb spastic paresis.
Changes in the composite score of active range of motion are associated with active functional improvements observed eight weeks later.
Acknowledgments
The authors thank all patients involved in these studies, as well as their caregivers, care team, investigators, and research staff in participating institutions.
Footnotes
Author contributions: All authors made a substantial contribution to the concept/design of the analyses presented in this manuscript, acquisition of data, critical revision of the draft for important intellectual content, and interpretation of data, and provided approval of the version to be published. P.M. and R.R. were involved in the analysis of data.
Data sharing statement: Where patient data can be anonymized, Ipsen will share all individual participant data that underlie the results reported in this article with qualified researchers who provide a valid research question. Study documents, such as the study protocol and clinical study report, are not always available. Proposals should be submitted to DataSharing@Ipsen.com and will be assessed by a scientific review board. Data are available beginning six months and ending five years after publication of the original trials; after this time, only raw data may be available.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: N.B. has nothing to disclose. P.M. is employed by Ipsen. R.R. is employed by Ividata, subcontracted to Ipsen at the time of manuscript development. J.B. was employed by Ipsen at the time the analyses were run. J.-M.G. received research grants from Allergan, Ipsen, and Merz; consultancy fees from Allergan, Ipsen, and Merz; and was an investigator on an Ipsen trial.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by Ipsen.
Medical writing support: The authors thank Jacqueline Harte, BSc (Hons) and Germanicus Hansa-Wilkinson, MSc of Watermeadow Medical, an Ashfield Company, for providing medical writing and editorial support, which was funded by Ipsen in accordance with Good Publication Practice guidelines.
ORCID iD: Jean-Michel Gracies  https://orcid.org/0000-0003-4859-8390
https://orcid.org/0000-0003-4859-8390
References
- 1. Gracies JM. Coefficients of impairment in deforming spastic paresis. Ann Phys Rehabil Med 2015; 58(3): 173–178. [DOI] [PubMed] [Google Scholar]
- 2. Gracies JM. Pathophysiology of spastic paresis. II: emergence of muscle overactivity. Muscle Nerve 2005; 31: 552–571. [DOI] [PubMed] [Google Scholar]
- 3. Barnes M, Kocer S, Murie Fernandez M, et al. An international survey of patients living with spasticity. Disabil Rehabil 2017; 39(14): 1428–1434. [DOI] [PubMed] [Google Scholar]
- 4. Van Wijck FM, Pandyan AD, Johnson GR, et al. Assessing motor deficits in neurological rehabilitation: patterns of instrument usage. Neurorehabil Neural Repair 2001; 15(1): 23–30. [DOI] [PubMed] [Google Scholar]
- 5. Wein T, Esquenazi A, Jost WH, et al. OnabotulinumtoxinA for the treatment of poststroke distal lower limb spasticity: a randomized trial. PM R 2018; 10(7): 693–703. [DOI] [PubMed] [Google Scholar]
- 6. Norton BJ, Bomze HA, Sahrmann SA, et al. Correlation between gait speed and spasticity at the knee. Phys Ther 1975; 55: 355–359. [DOI] [PubMed] [Google Scholar]
- 7. Patrick E, Ada L. The Tardieu Scale differentiates contracture from spasticity whereas the Ashworth Scale is confounded by it. Clin Rehabil 2006; 20(2): 173–182. [DOI] [PubMed] [Google Scholar]
- 8. Fleuren JF, Voerman GE, Erren-Wolters CV, et al. Stop using the Ashworth Scale for the assessment of spasticity. J Neurol Neurosurg Psychiatry 2010; 81: 46–52. [DOI] [PubMed] [Google Scholar]
- 9. Vinti M, Couillandre A, Hausselle J, et al. Influence of effort intensity and gastrocnemius stretch on co-contraction and torque production in the healthy and paretic ankle. Clin Neurophysiol 2013; 124(3): 528–535. [DOI] [PubMed] [Google Scholar]
- 10. Gracies JM, Brashear A, Jech R, et al. Safety and efficacy of abobotulinumtoxinA for hemiparesis in adults with upper limb spasticity after stroke or traumatic brain injury: a double-blind randomised controlled trial. Lancet Neurol 2015; 14: 992–1001. [DOI] [PubMed] [Google Scholar]
- 11. Gracies JM, O’Dell M, Vecchio M, et al. Effects of repeated abobotulinumtoxinA injections in upper limb spasticity. Muscle Nerve 2018; 57(2): 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gracies JM, Esquenazi A, Brashear A, et al. Efficacy and safety of abobotulinumtoxinA in spastic lower limb: randomized trial and extension. Neurology 2017; 89(22): 2245–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gracies JM, Bayle N, Vinti M, et al. Five-step clinical assessment in spastic paresis. Eur J Phys Rehabil Med 2010; 46(3): 411–421. [PubMed] [Google Scholar]
- 14. Baude M, Loche CM, Gault-Colas C, et al. Intra- and inter-raters reliabilities of a stepped clinical assessment of chronic spastic paresis in adults. Ann Phys Rehabil Med 2015; 58: e4–e5. [Google Scholar]
- 15. Gracies JM, Hefter H, Simpson D, et al. Botulinum toxin in spasticity. In: Moore P, Naumann M. (eds) Handbook of botulinum toxin. 2nd Ed. New York: Blackwell Science, 2002, pp.221–274. [Google Scholar]
- 16. Baude M, Mardale V, Loche CM, et al. Intra- and inter-rater reliability of the Modified Frenchay Scale to measure active upper limb function in hemiparetic patients. Ann Phys Rehabil Med 2016; 59(suppl.): e59–e60. [Google Scholar]
- 17. Wade D, Langton-Hewer R, Wood VA, et al. The hemiplegic arm after stroke: measurement and recovery. J Neurol Neurosurg Psychiatry 1983; 46(6): 521–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O’Dell MW, Brashear A, Jech R, et al. Dose-dependent effects of abobotulinumtoxinA (Dysport) on spasticity and active movements in adults with upper limb spasticity: secondary analysis of a phase 3 study. PM R 2018; 10: 1–10. [DOI] [PubMed] [Google Scholar]
- 19. Bakheit AMO, Thilmann AF, Ward AB, et al. A randomized, double-blind, placebo-controlled, dose-ranging study to compare the efficacy and safety of three doses of botulinum toxin type A (Dysport) with placebo in upper limb spasticity after stroke. Stroke 2000; 31(10): 2402–2406. [DOI] [PubMed] [Google Scholar]
- 20. Platz T, Vuadens P, Eickhof C, et al. REPAS, a summary rating scale for resistance to passive movement: item selection, reliability and validity. Disabil Rehabil 2008; 30(1): 44–53. [DOI] [PubMed] [Google Scholar]
- 21. Lu X, Hu N, Deng S, et al. The reliability, validity and correlation of two observational gait scales assessed by video tape for Chinese subjects with hemiplegia. J Phys Ther Sci 2015; 27(12): 3717–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ng SS, Hui-Chan CW. Contribution of ankle dorsiflexor strength to walking endurance in people with spastic hemiplegia after stroke. Arch Phys Med Rehabil 2012; 93(6): 1046–1051. [DOI] [PubMed] [Google Scholar]
- 23. Ng SS, Hui-Chan CW. Transcutaneous electrical nerve stimulation combined with task-related training improves lower limb functions in subjects with chronic stroke. Stroke 2007; 38(11): 2953–2959. [DOI] [PubMed] [Google Scholar]
- 24. Zhu W, Zheng G, Gu Y, et al. Clinical efficacy and sEMG analysis of a new traditional Chinese medicine therapy in the treatment of spasticity following apoplectic hemiparalysis. Acta Neurol Belg 2014; 114(2): 125–129. [DOI] [PubMed] [Google Scholar]
- 25. Xu KS, Yan TB, Mai JN. [Effects of botulinum toxin guided by electric stimulation on spasticity in ankle plantar flexor of children with cerebral palsy: a randomized trial]. Zhonghua Er Ke Za Zhi 2006; 44(12): 913–917. [PubMed] [Google Scholar]
- 26. Carruthers A, Carruthers J. Botulinum toxin. 4th ed. Philadelphia, PA: Elsevier Saunders, 2017. [Google Scholar]
- 27. Snow BJ, Tsui JK, Bhatt MH, et al. Treatment of spasticity with botulinum toxin: a double-blind study. Ann Neurol 1990; 28: 512–515. [DOI] [PubMed] [Google Scholar]
- 28. Barden HL, Baguley IJ, Nott MT, et al. Quantifying patterns of upper limb motor change following BTX-A injection in adult spasticity management. Brain Inj 2015; 29(12): 1452–1459. [DOI] [PubMed] [Google Scholar]
- 29. Jung KS, In TS, Cho HY. Effects of sit-to-stand training combined with transcutaneous electrical stimulation on spasticity, muscle strength and balance ability in patients with stroke: a randomized controlled study. Gait Posture 2017; 54: 183–187. [DOI] [PubMed] [Google Scholar]
- 30. Oo WM. Efficacy of addition of transcutaneous electrical nerve stimulation to standardized physical therapy in subacute spinal spasticity: a randomized controlled trial. Arch Phys Med Rehabil 2014; 95(11): 2013–2020. [DOI] [PubMed] [Google Scholar]
- 31. Francis HP, Wade DT, Turner-Stokes L, et al. Does reducing spasticity translate into functional benefit? An exploratory meta-analysis. J Neurol Neurosurg Psychiatry 2004; 75(11): 1547–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]