In this prospective cohort study of children with suspected CAP, we evaluate the association between conventional host biomarkers and disease severity.
Abstract
Video Abstract
BACKGROUND:
Host biomarkers predict disease severity in adults with community-acquired pneumonia (CAP). We evaluated the association of the white blood cell (WBC) count, absolute neutrophil count (ANC), C-reactive protein (CRP), and procalcitonin with the development of severe outcomes in children with CAP.
METHODS:
We performed a prospective cohort study of children 3 months to 18 years of age with CAP in the emergency department. The primary outcome was disease severity: mild (discharged from the hospital), mild-moderate (hospitalized but not moderate-severe or severe), moderate-severe (eg, hospitalized with receipt of intravenous fluids, supplemental oxygen, complicated pneumonia), and severe (eg, intensive care, vasoactive infusions, chest drainage, severe sepsis). Outcomes were examined within the cohort with suspected CAP and in a subset with radiographic CAP.
RESULTS:
Of 477 children, there were no statistical differences in the median WBC count, ANC, CRP, or procalcitonin across severity categories. No biomarker had adequate discriminatory ability between severe and nonsevere disease (area under the curve [AUC]: 0.53–0.6 for suspected CAP and 0.59–0.64 for radiographic CAP). In analyses adjusted for age, antibiotic use, fever duration, and viral pathogen detection, CRP was associated with moderate-severe disease (odds ratio 1.12; 95% confidence interval, 1.0–1.25). CRP and procalcitonin revealed good discrimination of children with empyema requiring chest drainage (AUC: 0.83) and sepsis with vasoactive infusions (CRP AUC: 0.74; procalcitonin AUC: 0.78), although prevalence of these outcomes was low.
CONCLUSIONS:
WBC count, ANC, CRP, and procalcitonin are generally not useful to discriminate nonsevere from severe disease in children with CAP, although CRP and procalcitonin may have some utility in predicting the most severe outcomes.
What’s Known on This Subject:
Prognostic tools are limited for children with community-acquired pneumonia (CAP). Host biomarkers, including C-reactive protein (CRP) and procalcitonin, have been shown to be associated with severe clinical outcomes in adults with CAP. Data in children are limited.
What This Study Adds:
White blood cell count, CRP, and procalcitonin are generally not useful to discriminate overall disease severity in children with CAP. CRP and procalcitonin may have utility in predicting the most severe outcomes, but research is necessary to validate these findings.
Community-acquired pneumonia (CAP) is one of the most common serious infections in children, resulting in a substantial number of emergency department (ED) visits and hospitalizations.1 Accurate assessment of disease severity is essential to clinical decision-making. Although the site-of-care decision is one of the most important in the management of CAP, there are no validated prognostic tools to assist with this determination.
Biomarkers reflecting the host’s response to an infection offer an objective measure of disease severity that may improve prognostication of children with CAP. The white blood cell (WBC) count and absolute neutrophil count (ANC) are often evaluated in children with CAP; however, WBC count was not associated with disease severity in 1 study of children who were hospitalized.2,3 C-reactive protein (CRP) is an acute-phase reactant produced in response to interleukin-6, an inflammatory cytokine.4 Procalcitonin, a 116–amino acid precursor of calcitonin, is barely detectable in the blood of healthy individuals but increases in response to severe bacterial infection, sepsis, and multiple-organ dysfunction.5 Both CRP and procalcitonin have been studied as a means of differentiating viral from bacterial etiology; less work has been done to understand their prognostic abilities. Studies in adults with CAP have revealed that these markers are associated with disease severity6–10 but data in children remain limited.11–15
Our objective for this study was to evaluate the association of these host biomarkers with the development of severe outcomes in a prospective cohort of children with CAP.
Methods
Study Design, Setting, and Participants
The Catalyzing Ambulatory Research in Pneumonia Etiology and Diagnostic Innovations in Emergency Medicine (CARPE DIEM) study was a prospective cohort study of children aged 3 months to 18 years who presented to the ED with signs and symptoms of lower respiratory tract infection (LRTI) and received a chest radiograph (CXR) for clinical suspicion of CAP. The study was approved by the Cincinnati Children’s Hospital Medical Center (CCHMC) Institutional Review Board. Written informed consent was obtained from all legal guardians, and assent was obtained from children ≥11 years of age.
For this analysis, we included children aged 3 months to 18 years with signs and symptoms of LRTI who presented to the CCHMC ED from July 2013 to December 2017, had focal findings on a CXR indicating suspected CAP (defined as CXRs with focal opacities documented by the radiologist during the ED visit), and had blood drawn. LRTI was defined as ≥1 of the following: new or different cough or sputum production, chest pain, dyspnea, tachypnea, or abnormal auscultatory findings.16 We excluded children hospitalized for ≤14 days before the index ED visit, those with a history of aspiration or aspiration pneumonia, and those with immunocompromising or chronic medical conditions that predispose to severe or recurrent pneumonia (eg, immunodeficiency, chronic corticosteroid use, chronic lung disease, malignancy, sickle cell disease, congenital heart disease, patients dependent on tracheostomy, and neuromuscular disorders impacting respiration). Patients enrolled within 30 days before the ED visit were excluded to ensure a distinct infection episode.
Study Procedures
Potential participants were identified by trained research coordinators. After informed consent, demographic and historical information were obtained from the patient and/or parent by using a structured questionnaire. Clinicians completed a case report form assessing clinical signs and severity impressions. Blood, urine, and/or nasopharyngeal swabs were collected at enrollment from those who consented to collection of each specimen type. Complete blood cell count, CRP and procalcitonin assays were performed within 1 hour after blood collection. Clinicians caring for enrolled patients were blinded to all study laboratory test results, with the exception of values from the complete blood cell count that warranted immediate action (eg, low hemoglobin level). Clinicians did not have access to CRP or procalcitonin test results in all cases. Clinical data, including vital signs, medications, and interventions, were extracted from the electronic medical record. Patients received a follow-up phone call ∼7 to 15 days after they were discharged from the ED or hospital to assess disease course.
Given the variation in CXR interpretation, a subset of the cohort with suspected CAP was defined as having radiographic CAP, which was defined on the basis of the evaluations of 2 independent study radiologists blinded to clinical information after the study visit. Radiographs were classified as normal, definite/probable atelectasis, atelectasis versus pneumonia, or definite/probable pneumonia. Radiographic CAP was defined as both radiologists classifying CXR findings as atelectasis versus pneumonia or definite/probable pneumonia.17 In cases of disagreement, the attending radiologist’s reading from the ED visit was used as a tiebreaker. If there was persistent disagreement, consensus was reached during an in-person meeting with the study radiologists.
Outcome Measurements
The primary outcome, assessed after the ED visit, was disease severity. Mild disease was defined as discharge from the ED and not returning for hospitalization within 7 days. Mild-moderate disease was defined as those hospitalized (including those initially discharged who were subsequently hospitalized within 7 days) but not meeting moderate-severe or severe criteria. Moderate-severe disease was classified as hospitalization with at least 1 of the following occurring as an inpatient: receipt of at least 1 intravenous (IV) fluid bolus, continuous IV fluids for >12 hours, supplemental oxygen, broadening of antibiotics from aminopenicillin to any other antibiotic class, complicated pneumonia (moderate-large pleural effusion, metastatic infection associated with pneumonia, lung abscess, or lung necrosis), or presumed sepsis (systemic inflammatory response syndrome with receipt of antibiotics and ≥40 mL/kg of IV bolus fluid). Severe disease required at least 1 of the following: treatment in the ICU, positive-pressure ventilation (including continuous positive-pressure ventilation, bilevel positive-pressure ventilation, and intubation with mechanical ventilation), vasoactive infusions, chest drainage, extracorporeal membrane oxygenation, severe sepsis or septic shock (by using validated International Classification of Diseases, Ninth Revision diagnosis codes), or death.18
Biomarker Measurements
WBC count and ANC assays were performed on the CELL-DYN Sapphire (Abbott Diagnostics, Lake Forest, IL). CRP assays were performed on the Dimension Vista 1500 (Siemens Medical Solutions USA, Inc, Malvern, PA) with a functional sensitivity of 0.29 mg/dL. Procalcitonin was measured by using the VIDAS B.R.A.H.M.S procalcitonin assay performed on the MINI VIDAS instrument (BioMerieux, Marcy-l'Étoile, France) with a functional sensitivity of 0.1 ng/mL. Biomarker measurements less than the limit of detection were replaced with estimates equal to the limit of detection divided by the square root of 2.19
Statistical Analysis
The median biomarker concentrations with 25th and 75th percentiles were reported. Differences across outcome categories were tested by using a Wilcoxon rank test. Statistical tests were not conducted if any outcome category had a prevalence of <5%.20
Biomarker concentrations were log-base-2 transformed for modeling because they were right-skewed. Odds ratios (ORs) were thus interpreted as the multiplicative change in odds for a doubling of each biomarker concentration. Logistic regression models were used to calculate unadjusted and adjusted ORs to compare 3 groups: (1) mild versus mild-moderate, moderate-severe, and severe, (2) mild and mild-moderate versus moderate-severe and severe, and (3) nonsevere (mild, mild-moderate, and moderate-severe) versus severe disease in children with CAP and radiographic CAP. Proportional odds logistic regression models were used by considering disease severity an ordered factor, with proportionally similar differences in multiplicative risks between mild, mild-moderate, moderate-severe, and severe disease states. Multivariate models were adjusted for age (as a natural cubic spline with 3 degrees of freedom), receipt of antibiotics before arrival, and duration of fever because these variables were hypothesized a priori to affect biomarker concentrations and disease severity outcomes. In addition, although receipt of IV fluids as an inpatient (bolus or continuous for >12 hours) is often in response to lack of oral intake or dehydration, the receipt of IV fluids may also be due to other, sometimes arbitrary, factors. Therefore, we performed sensitivity analyses with no IV fluid variables in the composite outcome.
We characterized the discriminatory ability of each biomarker by creating receiver operating characteristic (ROC) curves and calculating the area under the curve (AUC). Using an empirical cut point estimation method for maximizing classification accuracy, we calculated the optimal threshold for classification and calculated the resulting test characteristics with 95% confidence intervals (CIs) at the nearest point to the upper left corner of the ROC curve. All analyses were repeated for the individual outcomes included in the composite disease severity outcome.
Statistical computing was conducted in R (version 3.5.3; R Foundation for Statistical Computing, Vienna, Austria), SAS (version 9.4; SAS Institute, Inc, Cary, NC), and Stata (version 14.2; Stata Corp, College Station, TX).
Results
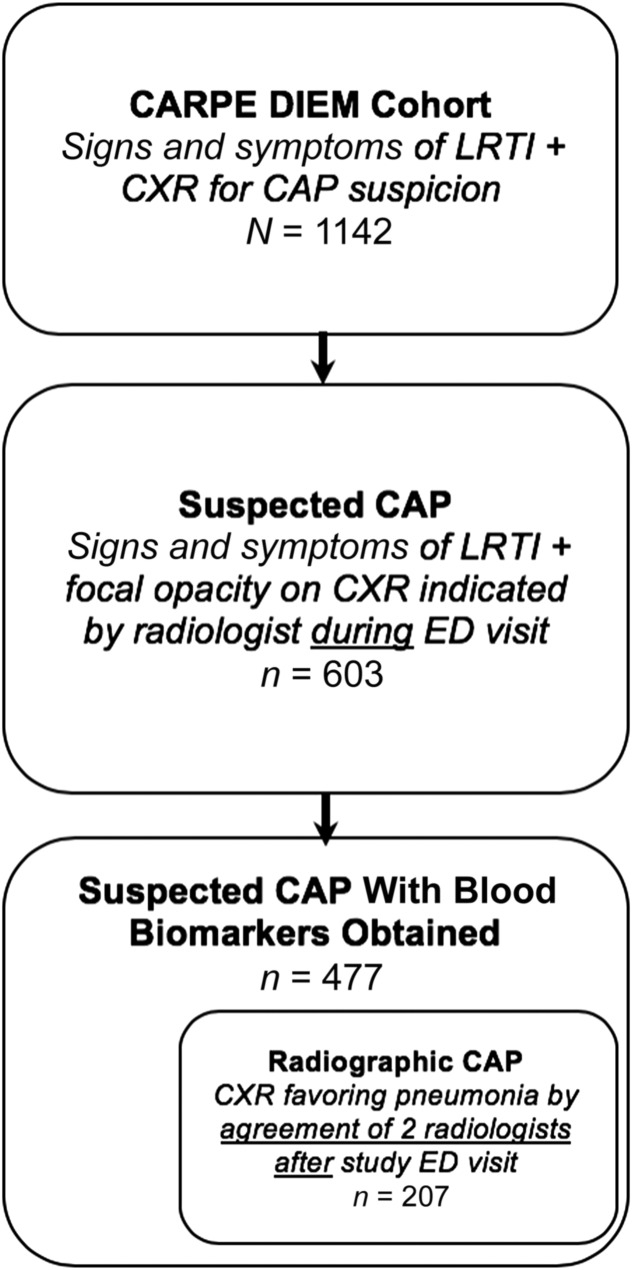
A total of 1142 children were enrolled in CARPE DIEM. Of these, 603 children had a focal CXR opacity and 477 had blood samples taken and were included in this analysis. All 477 children had a WBC count and ANC performed; 456 children had CRP and procalcitonin measurements performed. A total of 207 children had radiographic CAP and blood drawn (Fig 1). The mean age of the cohort was 5.6 years, and 52.6% (n = 251) of children were boys (Table 1).
FIGURE 1.
Study flow diagram.
TABLE 1.
Cohort Characteristics
| N = 477 | |
|---|---|
| Age, y, mean (SD) | 5.6 (4.6) |
| Male sex, n (%) | 251 (52.6) |
| Race (n = 476), n (%) | |
| White | 319 (67) |
| African American | 128 (26.9) |
| Other | 29 (6.1) |
| Insurance (n = 475), n (%) | |
| Private | 236 (49.7) |
| Public | 231 (48.6) |
| Self-pay | 8 (1.7) |
| Home antibiotics (n = 470), n (%) | 189 (40.2) |
| Home antibiotic class, n (%)a | |
| Aminopenicillin | 106 (56.1) |
| Cephalosporin | 32 (16.9) |
| Macrolide | 27 (14.3) |
| Other | 48 (25.4) |
| Fever | 418 (87.6) |
| Duration of fever, d, mean (SD) | 4.56 (4.85) |
| CXR results, n (%)b | |
| Normal | 34 (7.1) |
| Definite or probable atelectasis | 236 (49.5) |
| Atelectasis versus pneumonia | 39 (8.2) |
| Definite or probable pneumonia | 168 (35.2) |
| Pathogen detected, n (%) | 276 (57.9) |
| Virus | 248 (52.0) |
| Rhinovirus or enterovirus | 109 (22.9) |
| Respiratory syncytial virus | 83 (17.4) |
| Influenza | 29 (6.1) |
| Parainfluenza | 14 (2.9) |
| Human metapneumovirus | 23 (4.8) |
| Adenovirus | 4 (0.8) |
| Bocavirus | 8 (1.7) |
| Coronavirus | 10 (2.1) |
| Mycoplasma pneumoniae | 35 (7.3) |
| Typical bacteria | 3 (0.6) |
| Staphylococcus aureus | 3 (0.6) |
| Streptococcus pneumoniae | 0 (0.0) |
| Disease severity, n (%) | |
| Mild | 121 (25.4) |
| Mild-moderate | 126 (26.4) |
| Moderate-severe | 179 (37.5) |
| Severe | 51 (10.7) |
Patients may have been taking >1 class of antibiotic.
As determined by 2 radiologists independently assessing CXRs.
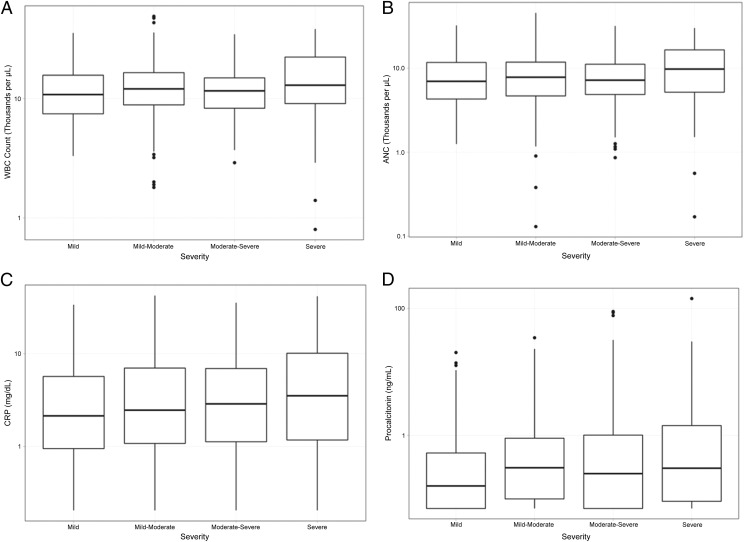
Of the 477 children included, 121 (25.4%) had mild disease, 126 (26.4%) had mild-moderate disease, 179 (37.5%) had moderate-severe disease, and 51 (10.7%) had severe disease. There were no statistical differences in the median WBC count, ANC, CRP level, or procalcitonin level across the 4 severity categories (Fig 2). In sensitivity analyses used to compare those with mild disease with the rest of the cohort, those with mild and mild-moderate disease with those with moderate-severe and severe disease, and those with nonsevere (ie, mild, mild-moderate, and moderate-severe) disease with those with severe disease, results did not change. When IV fluids were removed from the composite outcome, results also did not change.
FIGURE 2.
Blood biomarkers and disease severity. The median biomarker concentration is represented by the middle line in each box. The lower and upper boarders of the box represent the 25th and 75th percentiles, respectively. A, WBC count (P = .24). B, ANC (P = .26). C, CRP (P = .6). D, Procalcitonin (P = .21).
Use of composite outcomes necessitates the examination of its individual components. WBC count and ANC were largely not associated with the presence of most individual outcomes (Supplemental Table 4). CRP and procalcitonin did reveal statistical association with several of the components of the composite severity outcome. Small sample sizes precluded statistical testing on several individual outcomes; however, higher median values of all biomarkers were found in children who developed complicated pneumonia or received chest-drainage procedures or positive-pressure ventilation. Median CRP and procalcitonin values were higher in children who had sepsis and required vasoactive infusions.
In analyses adjusted for age, antibiotic receipt before arrival, fever duration, and viral pathogen detection, WBC count, ANC, and procalcitonin were not associated with disease severity in suspected or radiographic CAP (Table 2). CRP was modestly associated with disease severity in suspected CAP (mild severity versus more severe: OR 1.19 [95% CI, 1.04–1.35]; mild + mild-moderate versus moderate-severe + severe: OR 1.12 [95% CI, 1.00–1.25]). Similarly, CRP was associated with disease severity of radiographic CAP only when comparing mild severity with more severe groups (OR 1.23 [95% CI, 1.01–1.51]). In adjusted proportional odds regression models of the ordinal 4-tiered severity outcome, WBC count and ANC were not associated with disease severity, whereas CRP and procalcitonin were modestly associated (Supplemental Table 5). In radiographic CAP, none of the biomarkers were associated with increasing severity in adjusted analyses.
TABLE 2.
Association of Biomarkers With Disease Severity in Children With CAP, Adjusted for Age, Receipt of Antibiotics Before Arrival, Fever Duration, and Viral Pathogen Detected
| Biomarker | Suspected CAP, OR (95% CI) | Radiographic CAP, OR (95% CI) | ||||
|---|---|---|---|---|---|---|
| Mild Versus Mild-Moderate + Moderate-Severe + Severe | Mild + Mild-Moderate Versus Moderate-Severe + Severe | Mild + Mild-Moderate + Moderate-Severe Versus Severe | Mild Versus Mild-Moderate + Moderate-Severe + Severe | Mild + Mild-Moderate Versus Moderate-Severe + Severe | Mild + Mild-Moderate + Moderate-Severe Versus Severe | |
| WBC count | 1.06 (0.81–1.38) | 0.95 (0.75–1.2) | 1.3 (0.87–1.97) | 1.11 (0.75–1.63) | 1.05 (0.94–1.18) | 1.13 (0.69–1.96) |
| ANC | 1.06 (0.87–1.29) | 1.01 (0.85–1.2) | 1.31 (0.97–1.82) | 1.11 (0.82–1.48) | 1.11 (0.85–1.46) | 1.14 (0.77–1.76) |
| CRP | 1.19 (1.04–1.35) | 1.12 (1.00–1.25) | 1.22 (0.99–1.51) | 1.23 (1.01–1.51) | 1.08 (0.91–1.3) | 1.28 (0.93–1.82) |
| Procalcitonin | 1.11 (1.00–1.23) | 1.06 (0.98–1.16) | 1.13 (0.98–1.29) | 1.11 (0.97–1.28) | 1.05 (0.94–1.18) | 1.15 (0.96–1.37) |
Biomarkers were analyzed by using log2 transformation. OR is interpreted as the odds of developing the more severe outcome for every doubling of the biomarker value.
None of the 4 biomarkers examined had adequate discriminatory ability (ie, AUC) for severe versus nonsevere disease, with only fair sensitivity and specificity (Table 3). Discriminatory ability improved slightly in children with radiographic CAP, particularly for WBC count, ANC, and procalcitonin. When the mild group was compared with all others and when the mild and mild-moderate groups were compared with the moderate-severe and severe groups, test characteristics of all 4 biomarkers examined remained poor to fair (Supplemental Tables 6 and 7).
TABLE 3.
Performance Characteristics of Biomarkers To Predict Severe Versus Nonsevere Disease in Children With CAP
| Biomarker | Thresholda | AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | LR+ (95% CI) | LR− (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Suspected CAP | ||||||||
| WBC count | 16.8 | 0.6 (0.54–0.67) | 0.4 (0.28–0.54) | 0.8 (0.77–0.84) | 0.2 (0.13–0.28) | 0.92 (0.89–0.94) | 2.03 (1.42–2.91) | 0.75 (0.6–0.92) |
| ANC | 9.6 | 0.58 (0.51–0.64) | 0.52 (0.38–0.65) | 0.64 (0.6–0.68) | 0.15 (0.1–0.2) | 0.92 (0.88–0.94) | 1.43 (1.09–1.87) | 0.76 (0.58–0.99) |
| CRP | 3.3 | 0.56 (0.48–0.63) | 0.54 (0.39–0.68) | 0.57 (0.48–0.63) | 0.13 (0.09–0.19) | 0.91 (0.87–0.94) | 1.26 (0.96–1.67) | 0.8 (0.59–1.1) |
| Procalcitonin | 0.35 | 0.53 (0.46–0.61) | 0.48 (0.34–0.62) | 0.59 (0.54–0.64) | 0.13 (0.09–0.19) | 0.9 (0.86–0.93) | 1.17 (0.86–1.58) | 0.88 (0.67–1.16) |
| Radiographic CAP | ||||||||
| WBC count (n = 206) | 15 | 0.62 (0.51–0.73) | 0.52 (0.31–0.73) | 0.71 (0.64–0.78) | 0.19 (0.1–0.3) | 0.92 (0.87–0.96) | 1.8 (1.15–2.83) | 0.67 (0.43–1.04) |
| ANC (n = 206) | 11.2 | 0.63 (0.52–0.74) | 0.57 (0.35–0.77) | 0.69 (0.62–0.76) | 0.19 (0.1–0.3) | 0.93 (0.87–0.96) | 1.81 (1.19–2.76) | 0.63 (0.39–1.02) |
| CRP (n = 200) | 6.23 | 0.59 (0.47–0.7) | 0.57 (0.34–0.78) | 0.60 (0.53–0.68) | 0.15 (0.08–0.24) | 0.92 (0.86–0.96) | 1.4 (0.95–2.2) | 0.71 (0.43–1.18) |
| Procalcitonin (n = 205) | 0.52 | 0.64 (0.53–0.76) | 0.62 (0.38–0.82) | 0.67 (0.6–0.74) | 0.18 (0.1–0.28) | 0.94 (0.88–0.97) | 1.87 (1.26–2.77) | 0.57 (0.33–0.99) |
LR+, positive likelihood ratio; LR−, negative likelihood ratio.
Empirical cut point estimation at the point closest to perfect sensitivity and specificity (ie, upper left-hand corner of the ROC curve).
When test characteristics of biomarkers for individual outcomes were examined, WBC count and ANC generally did not discriminate from those who did and did not develop individual severe outcomes. CRP and procalcitonin had moderately good ability to discriminate those who developed complicated pneumonia, required chest drainage, or received vasoactive infusions (Supplemental Table 8).
Discussion
We evaluated the association with and predictive ability for disease severity of 4 widely available conventional host biomarkers (WBC count, ANC, CRP, and procalcitonin) in a prospective cohort of children with ED encounters for suspected or radiographic CAP. Biomarker levels did not differ by disease severity overall, nor did any biomarker adequately discriminate among various degrees of severity. A modest association was observed for CRP and disease severity in models adjusted for age, previous antibiotic use, fever duration, and viral pathogen detection. In individual clinical outcomes, host biomarker levels, particularly CRP and procalcitonin, were higher in those who developed rare, but serious, outcomes, including empyema requiring chest-drainage procedures and sepsis requiring vasoactive infusions; however, we cannot reach definitive conclusions.
Consistent with previous literature, we found that WBC count has limited ability to predict severity in children with suspected CAP.3,21,22 Studies of CRP and disease severity in adults with CAP yield conflicting results.6,7 Some studies have revealed that CRP is associated with severe outcomes, such as mortality, complicated pneumonia, positive-pressure ventilation, and inotropic support, whereas others have revealed no association with severity or no value in adding CRP to existing clinical severity scores.6,7,22–24Procalcitonin has been shown to be superior to CRP in predicting mortality and severe outcomes in adults.9,21,25–28 Recently, a large prospective cohort study of 1770 adults revealed that procalcitonin was strongly associated with the risk of requiring invasive respiratory or vasopressor support.9
Data regarding the association of host biomarkers and severe disease in children are limited and inconsistent. Studies revealed that both CRP and procalcitonin levels are elevated in children with complicated compared with uncomplicated CAP.29,30 Agnello et al15 found that CRP, but not procalcitonin, was a predictor of pleural effusion in 119 hospitalized children with CAP 1 to 14 years old in Italy. In contrast, Stockmann et al12 found that increasing procalcitonin levels were associated with ICU admission, empyema requiring chest drainage, and longer hospital length of stay in 532 hospitalized children with CAP in the United States. In 100 children with CAP, Don et al11 found that procalcitonin levels were higher in children who were hospitalized and those with alveolar, as opposed to interstitial, CAP. A study of 265 children 4 months to 14 years old hospitalized for suspected CAP revealed that both CRP and procalcitonin were associated with severe disease, as defined by the British Thoracic Society pneumonia guideline criteria.14 However, at cutoffs of 1.04 mg/L for CRP and 0.093 ng/mL for procalcitonin, the sensitivity (77.2% for CRP and 76.8% for procalcitonin), specificity (41.2% for CRP and 57.7% for procalcitonin), and discriminatory performance (AUC: 0.58 for CRP and 0.65 for procalcitonin) for both markers were only fair. These previous studies were limited by smaller sample sizes, a focus on children who were hospitalized, and use of outcomes that were either focused on specific entities (eg, pleural effusion) or were insensitive for predicting need for hospitalization (eg, the British Thoracic Society criteria).31
In our study, we examined not only complications of CAP, such as empyema or sepsis, but also more common indicators of disease severity and requirements for hospitalization, such as use of IV fluids, supplemental oxygen, positive-pressure ventilation, and broadening of antibiotics. We therefore looked at CAP severity more broadly than most previous studies by focusing on both interventions and diagnoses that required hospitalization. We found that CRP and procalcitonin were statistically associated with use of IV fluids and that CRP was associated with broadening of antibiotics. Interquartile ranges (IQRs) were overlapping, making the clinical significance of these differences unclear. Similar to previous results, we also found elevated CRP and procalcitonin levels in children with complicated pneumonia, empyema requiring chest drainage, positive-pressure ventilation, sepsis, and receipt of vasoactive infusions; however, smaller numbers of children with these outcomes precludes definitive conclusions.
Clinicians making site-of-care and initial treatment decisions often do not make choices with a single individual outcome in mind, but rather by considering a group of outcomes indicating the need for hospitalization or focused therapies. Therefore, we believe it more clinically relevant to examine disease severity as a composite outcome encompassing many facets of severity. There were no differences in biomarker levels for increasing degrees of severity. These biomarkers were not able to adequately discriminate children who would go on to develop more severe outcomes from those who would not. Our data suggest that CRP and procalcitonin may be useful in predicting the development of specific severe outcomes, such as complicated pneumonia and sepsis. Given the higher negative predictive values (NPVs) of these markers in discriminating severe from nonsevere disease, it may be these markers have a role in ruling out the most severe outcomes.
Our study has several important strengths. Our study is larger than most previous studies, and it began in the ED (the setting of most site-of-care decisions) and included children regardless of their disposition. We were also able to examine the role of these biomarkers in patients with clinically suspected CAP and the subset with radiographic CAP, which is important because CAP is often diagnosed without a radiograph, yet radiographs are considered the current reference standard for diagnosis. Our results are not substantively different between those with suspected CAP and those with radiographic CAP confirmed by independent radiologist review. Temporal associations may be inferred because these biomarkers were measured before occurrence of the severity outcomes. Finally, detailed clinical outcomes data allowed for examination of a breadth of severity outcomes.
Our study also has several limitations. First, composite outcomes can be challenging to interpret because of potential heterogeneity and the impact of individual outcomes on the whole. We performed several sensitivity analyses, including proportional odds logistic regression and different groupings of the severity outcomes, and found similar results. We also analyzed each outcome individually, although sample sizes were small for some. We cannot draw definitive conclusions for these outcomes (eg, severe sepsis). This was a single-center study, and results may not be generalizable; validation across centers is important. Finally, we did not examine serial biomarker measurements, making us unable to draw conclusions on temporal changes or the impact of repeat studies during hospitalization on outcomes. Only 30 children had repeat WBC count, CRP measurement, or procalcitonin measurement performed, and many of the components of the composite outcome are not dependent on these values (eg, development of complicated pneumonia and use of positive predictive value [PPV]), therefore it is unlikely that these repeated studies played a substantial role in the results. To account for illness duration and the effect of antibiotic use on biomarker measurement, we adjusted for these in our multivariable analyses, with similar results.
Conclusions
Our results suggest that conventionally measured biomarkers, including CRP and procalcitonin, are not generally useful for predicting illness severity in children evaluated for CAP in the ED. Given their high NPV, these markers may be helpful in ruling out the most severe outcomes. Further research is required to understand if there are specific clinical situations (eg, severe outcomes) in which these biomarkers improve current predictive ability, allowing for targeted interventions in high-risk children.
Acknowledgments
We acknowledge Judd Jacobs and Jessi Lipscomb for their role in data management for the CARPE DIEM study. Elizabeth Moneka provided administrative assistance in manuscript preparation. Stacey Tobin provided editorial support. We are grateful to the entire research team and patient services staff in the Divisions of Emergency Medicine and Hospital Medicine at CCHMC for their assistance with study procedures. Finally, we are especially grateful to the patients and families who enrolled in the CARPE DIEM study.
Glossary
- ANC
absolute neutrophil count
- AUC
area under the curve
- CAP
community-acquired pneumonia
- CARPE DIEM
Catalyzing Ambulatory Research in Pneumonia Etiology and Diagnostic Innovations in Emergency Medicine
- CCHMC
Cincinnati Children’s Hospital Medical Center
- CI
confidence interval
- CRP
C-reactive protein
- CXR
chest radiograph
- ED
emergency department
- IQR
interquartile range
- IV
intravenous
- LRTI
lower respiratory tract infection
- NPV
negative predictive value
- PPV
positive predictive value
- OR
odds ratio
- ROC
receiver operating characteristic
- WBC
white blood cell
Footnotes
Dr Florin conceptualized and designed the study, supervised participant enrollment and data acquisition, performed the statistical analysis and interpreted the data, and drafted the initial manuscript; Dr Ambroggio conceptualized and designed the study, supervised participant enrollment and data acquisition, and participated in data interpretation; Dr Brokamp and Mr Zhang performed the statistical analysis and interpreted the data; Drs Rattan and Crotty interpreted all chest radiographs and participated in data interpretation; Mr Belsky, Ms Krueger, and Mr Epperson participated in data acquisition and interpretation; Ms Kachelmeyer supervised participant enrollment, coordinated the study activities, and interpreted the data; Drs Ruddy and Shah conceptualized and designed the study and participated in data interpretation; and all authors reviewed and revised the manuscript, approved the final manuscript as submitted, and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the National Institutes of Health National Institute of Allergy and Infectious Diseases (K23AI121325 to Dr Florin and K01AI125413 to Dr Ambroggio), the Gerber Foundation (to Dr Florin), the National Institutes of Health National Center for Research Resources, and the Cincinnati Center for Clinical and Translational Science and Training (5KL2TR000078 to Dr Florin). The funders did not have any role in the study design, data collection, statistical analysis, or manuscript preparation. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Keren R, Luan X, Localio R, et al. ; Pediatric Research in Inpatient Settings (PRIS) Network . Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155–1164 [DOI] [PubMed] [Google Scholar]
- 2.Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237–244 [DOI] [PubMed] [Google Scholar]
- 3.Williams DJ, Zhu Y, Grijalva CG, et al. Predicting severe pneumonia outcomes in children. Pediatrics. 2016;138(4):e20161019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luna CM. C-reactive protein in pneumonia: let me try again. Chest. 2004;125(4):1192–1195 [DOI] [PubMed] [Google Scholar]
- 5.Becker KL, Nylén ES, White JC, Müller B, Snider RH Jr.. Clinical review 167: procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab. 2004;89(4):1512–1525 [DOI] [PubMed] [Google Scholar]
- 6.Zhydkov A, Christ-Crain M, Thomann R, et al. ; ProHOSP Study Group . Utility of procalcitonin, C-reactive protein and white blood cells alone and in combination for the prediction of clinical outcomes in community-acquired pneumonia. Clin Chem Lab Med. 2015;53(4):559–566 [DOI] [PubMed] [Google Scholar]
- 7.Hohenthal U, Hurme S, Helenius H, et al. Utility of C-reactive protein in assessing the disease severity and complications of community-acquired pneumonia. Clinical Microbiol Infect. 2009;15(11):1026–1032 [DOI] [PubMed] [Google Scholar]
- 8.Andrijevic I, Matijasevic J, Andrijevic L, Kovacevic T, Zaric B. Interleukin-6 and procalcitonin as biomarkers in mortality prediction of hospitalized patients with community acquired pneumonia. Ann Thorac Med. 2014;9(3):162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Self WH, Grijalva CG, Williams DJ, et al. Procalcitonin as an early marker of the need for invasive respiratory or vasopressor support in adults with community-acquired pneumonia. Chest. 2016;150(4):819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bello S, Lasierra AB, Minchole E, et al. Prognostic power of proadrenomedullin in community-acquired pneumonia is independent of aetiology. Eur Respir J. 2012;39(5):1144–1155 [DOI] [PubMed] [Google Scholar]
- 11.Don M, Valent F, Korppi M, et al. Efficacy of serum procalcitonin in evaluating severity of community-acquired pneumonia in childhood. Scand J Infect Dis. 2007;39(2):129–137 [DOI] [PubMed] [Google Scholar]
- 12.Stockmann C, Ampofo K, Killpack J, et al. Procalcitonin accurately identifies hospitalized children with low risk of bacterial community-acquired pneumonia. J Pediatric Infect Dis Soc. 2018;7(1):46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallihan RG, Suárez NM, Cohen DM, et al. Molecular distance to health transcriptional score and disease severity in children hospitalized with community-acquired pneumonia. Front Cell Infect Microbiol. 2018;8:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esposito S, Di Gangi M, Cardinale F, et al. ; Ita-CAP Study Group . Sensitivity and specificity of soluble triggering receptor expressed on myeloid cells-1, midregional proatrial natriuretic peptide and midregional proadrenomedullin for distinguishing etiology and to assess severity in community-acquired pneumonia. PLoS One. 2016;11(11):e0163262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agnello L, Bellia C, Di Gangi M, et al. Utility of serum procalcitonin and C-reactive protein in severity assessment of community-acquired pneumonia in children. Clin Biochem. 2016;49(1–2):47–50 [DOI] [PubMed] [Google Scholar]
- 16.Jain S, Williams DJ, Arnold SR, et al. ; CDC EPIC Study Team . Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neuman MI, Monuteaux MC, Scully KJ, Bachur RG. Prediction of pneumonia in a pediatric emergency department. Pediatrics. 2011;128(2):246–253 [DOI] [PubMed] [Google Scholar]
- 18.Balamuth F, Weiss SL, Hall M, et al. Identifying pediatric severe sepsis and septic shock: accuracy of diagnosis codes. J Pediatr. 2015;167(6):1295–1300.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46–51 [Google Scholar]
- 20.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–1379 [DOI] [PubMed] [Google Scholar]
- 21.Park JH, Wee JH, Choi SP, Oh SH. The value of procalcitonin level in community-acquired pneumonia in the ED. Am J Emerg Med. 2012;30(7):1248–1254 [DOI] [PubMed] [Google Scholar]
- 22.Müller B, Harbarth S, Stolz D, et al. Diagnostic and prognostic accuracy of clinical and laboratory parameters in community-acquired pneumonia. BMC Infect Dis. 2007;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalmers JD, Singanayagam A, Hill AT. C-reactive protein is an independent predictor of severity in community-acquired pneumonia. Am J Med. 2008;121(3):219–225 [DOI] [PubMed] [Google Scholar]
- 24.Krüger S, Ewig S, Papassotiriou J, et al. ; CAPNETZ Study Group . Inflammatory parameters predict etiologic patterns but do not allow for individual prediction of etiology in patients with CAP: results from the German competence network CAPNETZ. Respir Res. 2009;10:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krüger S, Ewig S, Marre R, et al. ; CAPNETZ Study Group . Procalcitonin predicts patients at low risk of death from community-acquired pneumonia across all CRB-65 classes. Eur Respir J. 2008;31(2):349–355 [DOI] [PubMed] [Google Scholar]
- 26.Masiá M, Gutiérrez F, Shum C, et al. Usefulness of procalcitonin levels in community-acquired pneumonia according to the patients outcome research team pneumonia severity index. Chest. 2005;128(4):2223–2229 [DOI] [PubMed] [Google Scholar]
- 27.Lacoma A, Rodríguez N, Prat C, et al. Usefulness of consecutive biomarkers measurement in the management of community-acquired pneumonia. Eur J Clin Microbiol Infect Dis. 2012;31(5):825–833 [DOI] [PubMed] [Google Scholar]
- 28.Huang DT, Weissfeld LA, Kellum JA, et al. ; GenIMS Investigators . Risk prediction with procalcitonin and clinical rules in community-acquired pneumonia. Ann Emerg Med. 2008;52(1):48–58.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fonseca TS, Gendrel D, Ruuskanen O, Nascimento-Carvalho CM. Pleural effusion increases serum procalcitonin values in children with community-acquired pneumonia. Pediatr Infect Dis J. 2015;34(8):914–915 [DOI] [PubMed] [Google Scholar]
- 30.Lahti E, Peltola V, Virkki R, Alanen M, Ruuskanen O. Development of parapneumonic empyema in children. Acta Paediatr. 2007;96(11):1686–1692 [DOI] [PubMed] [Google Scholar]
- 31.Ambroggio L, Brokamp C, Mantyla R, et al. Validation of the British Thoracic Society severity criteria for pediatric community-acquired pneumonia. Pediatr Infect Dis J. 2019;38(9):894–899 [DOI] [PMC free article] [PubMed] [Google Scholar]