Abstract
Chronic obstructive pulmonary disease (COPD) increases postoperative morbidity and is associated with diminished long-term survival after lung cancer resection. Whether this is also true for mild-to-moderate COPD is uncertain. We conducted a retrospective analysis of all the patients who underwent lung cancer surgery between 2002 and 2012 in a university-affiliated hospital. The severity of airflow limitation was stratified according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) from stage 1 to 4. Data from 1456 cases of lung cancer surgery were reviewed and 1126 patients were included in the study: 672 (59.7%) patients had COPD (GOLD 1, n = 340; GOLD 2, n = 282; GOLD 3, n = 50) and 454 patients had a normal spirometry (controls). Following lung cancer resection, patients with COPD had a higher rate of postoperative morbidities of any kind (p < 0.0001), in particular, pneumonia (7.0% vs. 3.7%; p = 0.0251) and prolonged air leak (17.0% vs. 8.2%; p < 0.0001) than controls. In-hospital mortality was increased in GOLD 3 COPD but the incidence of other postoperative complications was not influenced by COPD severity. Neither COPD nor its severity influenced long-term survival in this population. To conclude, patients with COPD undergoing lung cancer surgery were at higher risk of postoperative complications than patients with normal respiratory function but the procedure was considered safe. The presence of COPD itself did not influence long-term survival. The results of our study apply mainly to patients with a GOLD 1 and 2 COPD since only a small number of patients with GOLD 3 COPD were involved.
Keywords: COPD, lung cancer, lung cancer treatment, thoracic surgery
Introduction
Lung cancer was estimated to cause over 1.7 million deaths worldwide in 2018, which made it the most frequent cause of death by cancer according to the Global Cancer Observatory estimates.1 Chronic obstructive pulmonary disease (COPD) coexists in 50–70% of lung cancer patients.2 The strong association between these conditions is partly the reflection of their shared risk factor, but COPD is also an independent risk factor for the occurrence of lung cancer even after controlling for tobacco exposure.3 This suggests that the relationship between lung cancer and COPD goes beyond smoking, involving common inflammatory and genetics pathways.4
The best treatment for early-stage non-small cell lung cancer remains curative-intent surgery.5 However, surgical resection may be problematic in patients with impaired lung function. Prior studies of surgical outcomes in patients with COPD have not always relied on objective spirometric criteria.6 A few studies described the postoperative outcome of patients with moderate-to-severe COPD; collectively, they highlighted a higher prevalence of complications.7,8 Patients investigated for lung cancer with concomitant COPD have predominantly mild and moderate airflow limitation.9 Recent studies explored the effect of the severity of COPD on surgical outcome but comprised only few patients with mild COPD.10–12 Hence, the difference in postoperative outcome of patients suffering from varying severity of COPD is uncertain.
The objectives of this study were (1) to determine if the rate of postoperative complications is higher in patients with COPD compared to individuals with normal lung function; (2) to evaluate the influence of the severity of COPD on postoperative complications; and (3) to determine if the long-term survival is affected by the presence of COPD.
Methods
Study population
The study population consisted of all lung cancer patients who underwent lung resection at the Institut Universitaire de Cardiologie et de Pneumologie de Québec (IUCPQ) between 2002 and 2012 and who provided written consent for their clinical data to be prospectively collected and archived for future research use in the institutional biobank clinical database. During this period, we estimate that more than 90% of lung resections for cancer in our institution were recorded in the biobank. The study was approved by the Institutional Ethics Committee (project CER 21184).
We included patients undergoing a first surgery for lung cancer with either normal pulmonary function or airflow limitation caused by COPD. COPD was defined as a forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC) ratio <0.70 and a smoking history of at least 10 pack-years. Airflow limitation was stratified in severity according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria13 with FEV1 ≥ 80% predicted for mild COPD (GOLD 1), 50 ≤ FEV1 < 80% for moderate COPD (GOLD 2), and 30 ≤ FEV1 < 50% for severe COPD (GOLD 3). Post-bronchodilator FEV1 was used when available (42.3% of patients); otherwise pre-bronchodilator value was used. Patients were excluded if they previously had lung cancer or undergone lung resection, if they had asthma, abnormal lung function not caused by COPD, no spirometry available, or if they received at surgery a diagnosis of pulmonary metastasis, small cell lung cancer, carcinoid tumor, or benign tumor.
Data collection
The following information was extracted from the biobank’s database: gender, age, body mass index (BMI), smoking history (in pack-years), pulmonary function tests, extent of lung resection, histopathologic diagnosis, and pathologic TNM staging. TNM staging established before 2010 was revised according to the AJCC seventh edition criteria.14 Medical charts were reviewed to collect information about comorbidities, including coronary artery disease, peripheral artery disease, hypertension, dyslipidemia, diabetes, and chronic kidney disease. We also documented from the medical chart the surgical approach (thoracoscopy vs. thoracotomy), length of hospital stay, intensive care unit length of stay, pleural space drainage duration, and the occurrence of postoperative complications, including mechanical ventilation, pneumonia, pulmonary embolism, myocardial infarction, prolonged air leak (air leak > 7 days), respiratory failure (defined as hypoxemic or hypercapnic respiratory failure), death, and any other complications not listed above. Postoperative complications were deemed to occur when mentioned in the discharge summary or in the medical record. Survival status until May 1, 2017, was obtained from the Régie de l’assurance maladie du Québec (RAMQ), a provincial organization to which all deaths occurring in the province of Québec are reported.
Statistical analysis
Continuous descriptive and nominal variables are reported as means ± standard deviations (SD) and proportions, respectively. Proportions were analyzed using χ 2 or Fisher’s exact test, whereas continuous variables were analyzed with one-way analysis of variance. Survival curves were constructed using Kaplan–Meier estimates and compared with the log-rank test. Cox proportional hazard regression analysis was performed to model event-free (death) at follow-up. Patient’s characteristics were investigated to identify the prognostic factors that may explain the time until death event occur. Variables with a probability value <0.20 were candidates for multivariate regression using a forward approach. Statistical significance was present with the two-tailed p value <0.05.
Results
Between 2002 and 2012, 1456 lung cancer resections were performed at the IUCPQ and were recorded in the biobank; 1126 patients met our inclusion criteria. The COPD group comprised 672 patients (59.7%), distributed in 340 GOLD 1, 282 GOLD 2, and 50 GOLD 3 patients. The remaining 454 patients, who had a normal respiratory function, formed the control group.
Patients’ characteristics are presented in Table 1. In the COPD group, we observed a lower proportion of women and patients were on average older than in the control group. Patients with COPD were heavier smokers and tended to exhibit more often peripheral artery disease, but this did not reach statistical significance. On the other hand, controls were more likely to undergo video-assisted thoracoscopic surgery than patients with COPD.
Table 1.
Patients’ characteristics.
| COPD (N = 672) | Controls (N = 454) | p Value | |
|---|---|---|---|
| Women, n (%) | 277 (41.2) | 259 (57.1) | <0.0001 |
| Age (years) | 65.6 ± 8.3 | 62.3 ± 9.6 | <0.0001 |
| BMI (kg/m2) | 25.5 ± 4.7 | 26.7 ± 5.4 | 0.0002 |
| Current/former/never smoker (%) | 31.3/68.8/0 | 22.3/68.1/9.7 | <0.0001 |
| Smoking history (pack-years) | 50.2 ± 27.0 | 35.6 ± 26.2 | <0.0001 |
| Pulmonary function tests | |||
| FEV1 (L) | 2.0 ± 0.6 | 2.5 ± 0.6 | <0.0001 |
| FEV1 (% predicted) | 78.1 ± 17.3 | 99.9 ± 14.7 | <0.0001 |
| FVC (L) | 3.2 ± 0.8 | 3.3 ± 0.8 | 0.0476 |
| FVC (% predicted) | 98.3 ± 18.8 | 104.2 ± 15.6 | <0.0001 |
| FEV1/FVC | 60.8 ± 8.0 | 75.5 ± 4.8 | <0.0001 |
| TLC (L) | 6.2 ± 1.2 | 5.7 ± 1.2 | <0.0001 |
| TLC (% predicted) | 110.4 ± 15.3 | 106.2 ± 13.7 | <0.0001 |
| D LCO (ml/min/mmHg) | 17.1 ± 5.1 | 19.6 ± 6.0 | <0.0001 |
| D LCO (% predicted) | 81.7 ± 21.5 | 94.5 ± 22.4 | <0.0001 |
| RV (L) | 3.1 ± 3.8 | 2.4 ± 2.5 | 0.0003 |
| RV (% predicted) | 134.4 ± 38.3 | 114.2 ± 29.7 | <0.0001 |
| Coronary artery disease, n (%) | 142 (21.1) | 85 (18.7) | 0.36 |
| Peripheral artery disease, n (%) | 114 (17.0) | 57 (12.6) | 0.05 |
| Hypertension, n (%) | 331 (49.3) | 198 (43.6) | 0.07 |
| Dyslipidemia, n (%) | 283 (42.1) | 190 (41.9) | 0.95 |
| Diabetes, n (%) | 83 (12.4) | 51 (11.2) | 0.64 |
| Chronic kidney disease, n (%) | 22 (3.3) | 10 (2.2) | 0.36 |
| VATS, n (%) | 247 (36.8) | 203 (44.7) | 0.0056 |
| Extent of resection, n (%) | |||
| Pneumonectomy | 74 (11.0) | 43 (9.5) | 0.3428 |
| Bilobectomy | 67 (10.0) | 47 (10.4) | |
| Lobectomy | 456 (67.9) | 328 (72.3) | |
| Sublobar resection | 75 (11.1) | 36 (7.9) | |
| Histologic diagnosis, n (%) | |||
| Adenocarcinoma | 408 (60.7) | 316 (69.6) | 0.0012 |
| Squamous cell carcinoma | 229 (34.1) | 106 (23.4) | |
| Large-cell carcinoma | 15 (2.2) | 11 (2.4) | |
| Other | 20 (3.0) | 21 (4.6) | |
| Pathologic staging, n (%) | |||
| I | 387 (57.6) | 267 (58.8) | 0.80 |
| II | 168 (25.0) | 109 (24.0) | |
| III | 108 (16.1) | 69 (15.2) | |
| IV | 9 (1.3) | 9 (2.0) |
FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; TLC: total lung capacity; D LCO: diffusion capacity for carbon monoxide; RV: residual volume; VATS: video-assisted thoracoscopic surgery; BMI: body mass index.
The hospital (p = 0.0012) and intensive care unit length of stay (p = 0.0054) were significantly longer in COPD in comparison to controls (Table 2). Pleural space drainage duration (p = 0.0029) was also significantly longer in COPD in comparison to controls (Table 2). However, when comparing COPD GOLD 1, 2, and 3 groups, length of hospital stay, intensive care unit length of stay, and pleural space drainage duration were not statistically significantly different (Table 3).
Table 2.
Postoperative outcomes in COPD and controls.
| Complications, n (%) | COPD (N = 672) | Controls (N = 454) | p Value |
|---|---|---|---|
| Mechanical ventilation | 30 (4.5) | 12 (2.6) | 0.15 |
| Pneumonia | 47 (7.0) | 17 (3.7) | 0.0251 |
| Pulmonary embolism | 6 (0.9) | 2 (0.4) | 0.49 |
| Myocardial infarction | 3 (0.5) | 0 (0.0) | 0.28 |
| Prolonged air leak | 114 (17.0) | 37 (8.2) | <0.0001 |
| Respiratory failure | 26 (3.9) | 12 (2.6) | 0.31 |
| Other morbidity | 254 (37.8) | 136 (30.0) | 0.0073 |
| Any complication | 329 (49.0) | 160 (35.2) | <0.0001 |
| 30-Day mortality | 9 (1.3) | 1 (0.2) | 0.06 |
| Mortality during hospitalization | 10 (1.5) | 1 (0.2) | 0.06 |
| Hospital length of stay (days) | 9.6 ± 10.4 | 7.7 ± 8.8 | 0.0012 |
| ICU length of stay (days)a | 3.0 ± 8.0 | 1.7 ± 3.2 | 0.0054 |
| Chest tube duration (days)a | 5.9 ± 6.6 | 4.5 ± 5.5 | 0.0029 |
ICU: intensive care unit; COPD: chronic obstructive pulmonary disease.
a Data obtained in 673/1126 and 706/1126 subjects, respectively.
Table 3.
Comparison of postoperative outcomes according to GOLD stage.
| Complications, n (%) | GOLD 1 (N = 340) | GOLD 2 (N = 282) | GOLD 3 (N = 50) | p Value |
|---|---|---|---|---|
| Mechanical ventilation | 15 (4.4) | 12 (4.3) | 3 (6.0) | 0.77 |
| Pneumonia | 20 (5.9) | 21 (7.5) | 6 (12.0) | 0.22 |
| Pulmonary embolism | 2 (0.6) | 3 (1.1) | 1 (2.0) | 0.41 |
| Myocardial infarction | 1 (0.3) | 1 (0.4) | 1 (2.0) | 0.28 |
| Prolonged air leak | 54 (15.9) | 49 (17.4) | 11 (22.0) | 0.51 |
| Respiratory failure | 12 (3.5) | 11 (3.9) | 3 (6.0) | 0.60 |
| Other morbidity | 117 (34.4) | 117 (41.5) | 20 (40.0) | 0.18 |
| Any complications | 152 (44.7) | 149 (52.8) | 28 (56.0) | 0.07 |
| 30-Day mortality | 7 (2.1) | 1 (0.4) | 1 (2.0) | 0.10 |
| Mortality during hospitalization | 7 (2.1) | 1 (0.4) | 2 (4.0) | 0.0425 |
| Hospital length of stay (days) | 9.0 ± 10.6 | 10.2 ± 10.4 | 10.2 ± 8.5 | 0.45 |
| ICU length of stay (days) | 2.8 ± 8.6 | 3.0 ± 7.5 | 3.7 ± 6.3 | 0.59 |
| Chest tube duration (days) | 5.6 ± 5.8 | 6.0 ± 6.8 | 7.2 ± 10.0 | 0.21 |
ICU: intensive care unit.
The frequency of occurrence of any postoperative complications was significantly higher in patients with COPD than in controls (p < 0.0001; Table 2). In particular, pneumonia and prolonged air leak occurred significantly more frequently in COPD patients than in controls. There was a tendency for in-hospital mortality and 30-day mortality to be higher in patients with COPD, but this did not reach statistical significance.
Postoperative morbidity according to the GOLD airflow limitation grade is provided in Table 3. Most patients were in the GOLD 1 and 2 categories, with only 50 patients in the GOLD 3 category. Even though the prevalence of most postoperative morbidity and mortality was numerically greater in the GOLD 3 category, none of the differences were statistically significant. We observed a higher in-hospital mortality in GOLD 3 patients (GOLD 1: 2.1% vs. GOLD 2: 0.4% vs. GOLD 3: 4.0%; p = 0.0425) but the 30-day mortality was not statistically significantly different across the different GOLD categories.
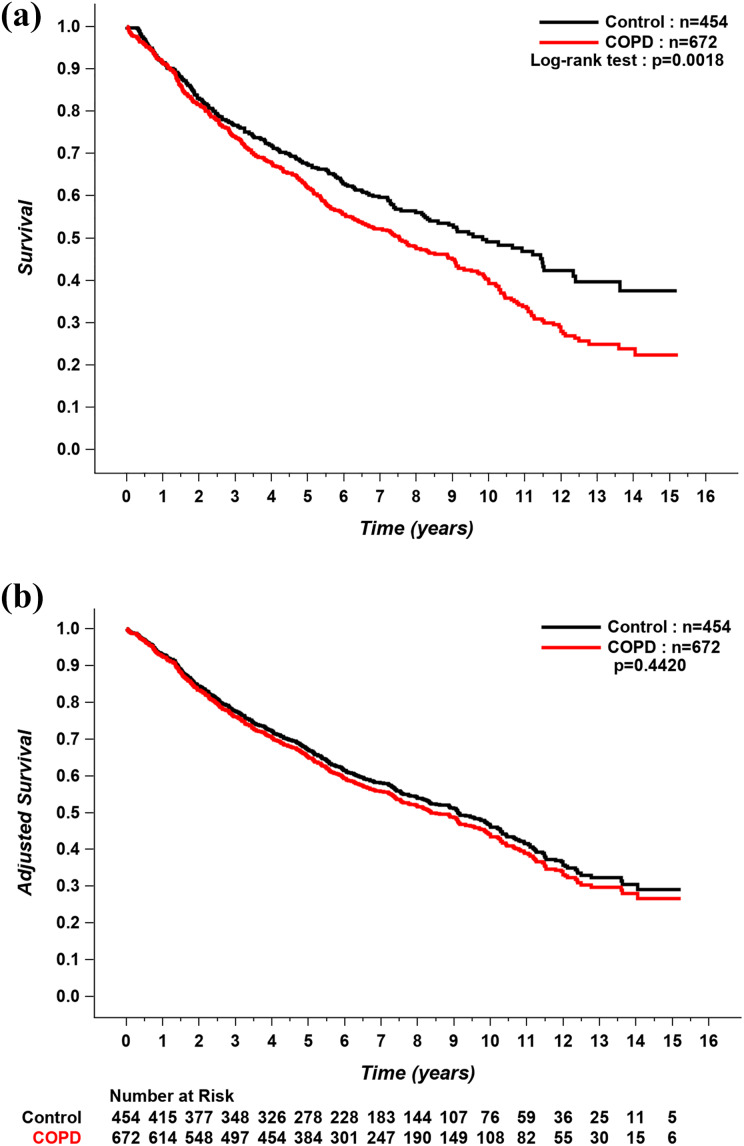
Current or previous smoking history, cumulative tobacco exposure, age, a diagnosis of COPD, the presence of coronary or peripheral artery disease, diabetes, the need for thoracotomy, the need for pneumonectomy or bilobectomy versus lobectomy, pathological TNM stage II, III, or IV, and GOLD stage 1 or 2 were all associated with long-term mortality in the univariate analyses (Table 4). However, in the multivariate analysis, the effect of COPD on mortality was lost, with age, cumulative tobacco exposure, the presence of coronary artery disease, and pathological TNM stage II, III, or IV remaining as the only predictors of mortality (Table 5). There was no significant impact of GOLD category on mortality. A Kaplan–Meier analysis of long-term survival in patients with COPD and controls is shown in Figure 1. During the follow-up period, 389 patients with COPD and 213 controls died. Patients with COPD had lower survival compared to controls; however, when the curves were adjusted for mortality predictors in the multivariate analysis, long-term survival was similar between the two groups.
Table 4.
Univariate predictors of mortality.
| Covariate | Hazard ratio | 95% Confidence intervals | p Value |
|---|---|---|---|
| Women | 0.72 | 0.61–0.84 | <0.0001 |
| Former smoker | 1.98 | 1.14–3.45 | 0.0150 |
| Current smoker | 2.35 | 1.34–4.13 | 0.0029 |
| Pack-years | 1.01 | 1.01–1.01 | <0.0001 |
| Age (years) | 1.03 | 1.02–1.03 | <0.0001 |
| BMI (kg/m2) | 1.00 | 0.98–1.01 | 0.75 |
| COPD | 1.30 | 1.10–1.54 | 0.0019 |
| Coronary artery disease | 1.48 | 1.23–1.78 | <0.0001 |
| Peripheral artery disease | 1.31 | 1.06–1.61 | 0.0111 |
| Hypertension | 1.04 | 0.89–1.22 | 0.62 |
| Dyslipidemia | 1.06 | 0.90–1.25 | 0.49 |
| Diabetes | 1.43 | 1.14–1.80 | 0.0020 |
| Chronic kidney disease | 1.30 | 0.83–2.03 | 0.25 |
| Thoracotomy | 1.45 | 1.21–1.73 | <0.0001 |
| Extent of resection | |||
| Pneumonectomy versus lobectomy | 1.86 | 1.46–2.36 | <0.0001 |
| Bilobectomy versus lobectomy | 1.49 | 1.16–1.91 | 0.0017 |
| Pathological TNM stage | |||
| II | 1.71 | 1.41–2.06 | < 0.0001 |
| III | 2.92 | 2.37–3.59 | < 0.0001 |
| IV | 1.89 | 1.08–3.30 | 0.0248 |
| Histologic diagnosis | |||
| Adenocarcinoma | 0.55 | 0.37–0.81 | 0.0022 |
| Squamous cell carcinoma | 0.78 | 0.52–1.15 | 0.21 |
| Large cell carcinoma | 0.75 | 0.41–1.39 | 0.36 |
| GOLD stage | |||
| GOLD 1 | 1.23 | 1.01–1.49 | 0.0395 |
| GOLD 2 | 1.42 | 1.16–1.74 | 0.0006 |
| GOLD 3 | 1.19 | 0.79–1.79 | 0.40 |
GOLD: Global Initiative for Chronic Obstructive Lung Disease; COPD: chronic obstructive pulmonary disease; BMI: body mass index.
Table 5.
Multivariate predictors of mortality.
| Covariate | Hazard ratio | 95% Confidence intervals | p Value |
|---|---|---|---|
| Age (years) | 1.02 | 1.01–1.03 | <0.0001 |
| Pack-years | 1.01 | 1.00–1.01 | 0.0003 |
| Coronary artery disease | 1.43 | 1.17–1.74 | 0.0004 |
| Thoracotomy | 1.08 | 0.89–1.31 | 0.42 |
| Pathological TNM stage | |||
| Stage II | 1.65 | 1.35–2.02 | <0.0001 |
| Stage III | 3.08 | 2.47–3.85 | <0.0001 |
| Stage IV | 2.32 | 1.29–4.16 | 0.0048 |
| GOLD stage | 0.4710 | ||
| GOLD 1 | 1.02 | 0.83–1.25 | 0.88 |
| GOLD 2 | 1.11 | 0.90–1.38 | 0.33 |
| GOLD 3 | 1.33 | 0.88–2.03 | 0.18 |
GOLD: Global Initiative for Chronic Obstructive Lung Disease.
Figure 1.
(a) Unadjusted and (b) adjusted Kaplan–Meier curves for long-term survival in COPD patients and controls. Covariates include age, smoking history (pack-years), coronary artery disease, and TNM stages. COPD: chronic obstructive pulmonary disease.
Discussion
In this study, we found that COPD was highly prevalent among patients with lung cancer undergoing thoracic surgery. Patients with COPD were more likely to suffer from postoperative morbidities, especially from pneumonia and prolonged air leak, than those having a normal respiratory function. COPD severity did not influence the occurrence of postoperative morbidity. While long-term survival appeared inferior in patients with COPD, this survival disadvantage disappeared once mortality predictors in the multivariate analysis, with age, pack-years, coronary artery disease, and TNM pathological stage were taken into account.
Our study adds to the literature because we have a large and a well-characterized study population, including an objective spirometric assessment of lung function in all study participants. Another positive feature of our study is the considerable size of the GOLD 1 and GOLD 2 groups. The high COPD prevalence (60%) we found in this population with lung cancer undergoing surgical resection is in agreement with the one in patients with newly diagnosed lung cancer reported by Loganathan et al.2 Our data showing a higher prevalence of postoperative complications in patients with COPD compared to individuals with normal lung function including pneumonia and prolonged air leak is also consistent with previous reports.7,8,10,12,15,16 A number of factors may explain why patients with COPD may be at increased risk of complications following lung resection, including advanced age, active smoking, limited ventilatory reserve, enhanced susceptibility to respiratory tract infection, and presence of comorbid conditions, such as coronary artery disease.5
The overall 30-day and in-hospital mortality rates in control subjects (0.2%) and patients with COPD (1.5%) were low and within the expected mortality rates for lung cancer resection.17 The small mortality signal in patients with GOLD 3 COPD is difficult to interpret given the small number of patients in this category and the low number of events (n = 2). An interesting finding of this study was that COPD was not independently associated with reduced long-term survival once confounders, such as age, smoking history, coronary artery disease, and TNM pathological stage, were considered. This highlights the importance of not considering the presence of COPD in isolation in the preoperative assessment of potential lung resection candidates but rather that all comorbidities should be weighted in the decisional process. In one study, the presence of self-reported physician diagnosed COPD was associated with reduced survival in patients undergoing lung cancer resection even when important covariates such as age, sex, and smoking history were taken into account.6 However, this study was based on self-reported diagnosis of COPD. This is problematic because self-reported diagnosis of COPD may lead to both under- and overdiagnosis of COPD,18,19 making it difficult to draw firm conclusions about the survival impact of COPD in a study, where this diagnosis was not confirmed by spirometry. In two studies in which objective assessment of COPD was obtained, survival following lung resection was not reduced in the presence of GOLD 1 and 2 COPD.10,20 In contrast to our findings, these two studies reported that GOLD 3 COPD was associated with poorer long-term survival compared to individuals with normal lung function. Similar to the present study, the number of patients with GOLD 3 COPD was small in these two previous studies (n = 36 and n = 51, respectively). The differences between their and our study could also be explained by differences in the patient selection process, leading to variations in the physical fitness of patients with GOLD 3 COPD across studies. Together, the present and previous studies support that patients with mild-to-moderate COPD should not be denied surgical lung resection solely on the presence of COPD. Whether lung resection surgery can be offered in a safe manner to patients with severe COPD cannot be currently ascertained because of the small number of patients involved. This decision should be carefully considered on an individual basis by medical and surgical teams.
Our retrospective study has limitations however. Only a small number of patients with GOLD 3 COPD were involved, a reflection of the preoperative patient selection process. Quality of life was not measured. This issue of quality of life has already been studied by Pompili et al.21 who reported, in a small group of patients, that postoperative quality of life (measured by a questionnaire) in patients with moderate and severe COPD was comparable to that of patients with normal respiratory function. Due to the retrospective design of our study, there were some missing information regarding the intensive care unit length of stay and chest tube duration that could not be retrieved from the medical chart in all patients. Also, postoperative complications were deemed to occur when mentioned in the discharge summary or medical record, without the use of standardized definition, leaving the possibility of misdiagnosis for some of them. Finally, whether selection bias in our cohort exists is uncertain. In our institution, standard preoperative evaluation protocols are followed, as recommended by official organizations such as the American College of Chest Physicians.5 Only patients who were considered fit for surgery underwent lung resection. This could explain the favorable postoperative outcome and low mortality rate in this cohort. Nevertheless, this information provides reassurance that these recommendations lead to safe thoracic surgical procedures in patients with mild-to-moderate COPD.
Conclusion
Patients with mild-to-moderate COPD were at higher risk of post lung resection complications, in particular, pneumonia and persistent air leak compared to patients with a normal respiratory function. These findings should help inform clinicians in their decision to offer surgical lung resection in individuals with COPD. The prevention and early detection of postoperative complications could be the next step to improve the postoperative outcome of this group of patients. Future studies should also explore if these results could be extended to patients with severe COPD.
Acknowledgments
The authors would like to thank Christine Racine and Sabrina Biardel from the Institutional Biobank for their help in data collection and the members of the thoracic surgery clinic.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the la Fondation de L’IUCPQ et le Fonds sur les maladies respiratoires J.-D.-Bégin–P.-H.-Lavoie.
ORCID iD: François Maltais  https://orcid.org/0000-0002-6809-4651
https://orcid.org/0000-0002-6809-4651
References
- 1. Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer, https://gco.iarc.fr/today (2018, accessed 6 July 2019). [Google Scholar]
- 2. Loganathan RS, Stover DE, Shi W, et al. Prevalence of COPD in women compared to men around the time of diagnosis of primary lung cancer. Chest 2006; 129(5): 1305–1312. [DOI] [PubMed] [Google Scholar]
- 3. Young RP, Hopkins RJ, Christmas T, et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J 2009; 34(2): 380–386. [DOI] [PubMed] [Google Scholar]
- 4. Vermaelen K, Brusselle G. Exposing a deadly alliance: novel insights into the biological links between COPD and lung cancer. Pulm Pharmacol Ther 2013; 26(5): 544–554. [DOI] [PubMed] [Google Scholar]
- 5. Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143(5 Suppl): e166S–e190S. [DOI] [PubMed] [Google Scholar]
- 6. Zhai R, Yu X, Shafer A, et al. The impact of coexisting COPD on survival of patients with early-stage non-small cell lung cancer undergoing surgical resection. Chest 2014; 145(2): 346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sekine Y, Behnia M, Fujisawa T. Impact of COPD on pulmonary complications and on long-term survival of patients undergoing surgery for NSCLC. Lung Cancer 2002; 37(1): 95–101. [DOI] [PubMed] [Google Scholar]
- 8. Subotic DR, Mandaric DV, Eminovic TM, et al. Influence of chronic obstructive pulmonary disease on postoperative lung function and complications in patients undergoing operations for primary non-small cell lung cancer. J Thorac Cardiovasc Surg 2007; 134(5): 1292–1299. [DOI] [PubMed] [Google Scholar]
- 9. Hashimoto N, Matsuzaki A, Okada Y, et al. Clinical impact of prevalence and severity of COPD on the decision-making process for therapeutic management of lung cancer patients. BMC Pulm Med 2014; 14: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sekine Y, Suzuki H, Yamada Y, et al. Severity of chronic obstructive pulmonary disease and its relationship to lung cancer prognosis after surgical resection. Thorac Cardiovasc Surg 2013; 61(2): 124–130. [DOI] [PubMed] [Google Scholar]
- 11. Yoshida Y, Kage H, Murakawa T, et al. Worse prognosis for stage IA lung cancer patients with smoking history and more severe chronic obstructive pulmonary disease. Ann Thorac Cardiovasc Surg 2015; 21(3): 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim ES, Kim YT, Kang CH, et al. Prevalence of and risk factors for postoperative pulmonary complications after lung cancer surgery in patients with early-stage COPD. Int J Chron Obstruct Pulmon Dis 2016; 11: 1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187(4): 347–365. [DOI] [PubMed] [Google Scholar]
- 14. Compton CC, Byrd DR, Garcia-Aguilar J, et al. (eds). AJCC cancer staging atlas: a companion to the seventh editions of the AJCC cancer staging manual and handbook. 2nd ed New York: Springer-Verlag, 2012, pp. 311–328. [Google Scholar]
- 15. Petrella F, Rizzo S, Radice D, et al. Predicting prolonged air leak after standard pulmonary lobectomy: computed tomography assessment and risk factors stratification. Surgeon 2011; 9(2): 72–77. [DOI] [PubMed] [Google Scholar]
- 16. Elsayed H, McShane J, Shackcloth M. Air leaks following pulmonary resection for lung cancer: Is it a patient or surgeon related problem? Ann R Coll Surg Engl 2012; 94(6): 422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Green A, Hauge J, Iachina M, et al. The mortality after surgery in primary lung cancer: results from the Danish Lung Cancer Registry. Eur J Cardiothorac Surg 2016; 49(2): 589–594. [DOI] [PubMed] [Google Scholar]
- 18. Labonte LE, Tan WC, Li PZ, et al. Undiagnosed chronic obstructive pulmonary disease contributes to the burden of health care use. Data from the CanCOLD study. Am J Respir Crit Care Med 2016; 194(3): 285–298. [DOI] [PubMed] [Google Scholar]
- 19. Lacasse Y, Brooks D, Goldstein RS. Trends in the epidemiology of COPD in Canada, 1980 to 1995. COPD and Rehabilitation Committee of the Canadian Thoracic Society. Chest 1999; 116(2): 306–313. [DOI] [PubMed] [Google Scholar]
- 20. Bugge A, Lund MB, Brunborg C, et al. Survival after surgical resection for lung cancer in patients with chronic obstructive pulmonary disease. Ann Thorac Surg 2016; 101(6): 2125–2131. [DOI] [PubMed] [Google Scholar]
- 21. Pompili C, Brunelli A, Refai M, et al. Does chronic obstructive pulmonary disease affect postoperative quality of life in patients undergoing lobectomy for lung cancer? A case-matched study. Eur J Cardiothorac Surg 2010; 37(3): 525–530. [DOI] [PubMed] [Google Scholar]