Abstract
To observe the mechanism of myocardial injury in diabetic rats after irbesartan intervention and analyze the role of nucleotide binding oligomerization domain-like receptor protein 3 (NLRP3) inflammatory pathway. The experiment was divided into four groups: normal control group (CON), high glucose and high caloric diet group (HC), diabetes group (DM) and diabetes+irbesartan group (DM+Ir). Compared with CON group, in HC group, triglyceride, total cholesterol and fasting blood glucose levels were increased; however, there was no significant difference of the cardiac function, the degree of myocardial fibrosis, NLRP3, ASC, Caspase-1 mRNA and protein expressions and the releasing of inflammatory factors interleukin (IL)-1β and IL-18. Compared with HC group, in DM group, triglyceride, total cholesterol, fasting blood glucose, IL-1β and IL-18 levels, NLRP3, ASC, Caspase-1 mRNA and protein expressions and the degree of myocardial fibrosis were increased, but the cardiac function was decreased. Compared with DM group, there were no changes in total cholesterol and fasting blood glucose, the degree of myocardial fibrosis cardiac function was attenuated, NLRP3, ASC, Caspase-1 expressions, IL-1β and IL-18 levels were reduced in DM+Ir group. The results suggested that irbesartan may exert myocardial protection by inhibiting the expression of the NLRP3/ASC/Caspase-1 pathway in diabetic rats.
Keywords: Cardiomyocyte, high glucose, inflammatory, NLRP3/ASC/Caspase-1 pathway, irbesartan
Introduction
Since Ruble first proposed the concept of diabetic cardiomyopathy (DCM) in 1974, people’s cognition of it has gradually deepened. DCM can cause cardiac myocyte hypertrophy and myocardial collagen fiber deposition, which accelerate the progression of diabetic cardiomyopathy.1,2 As an independent and specific cardiomyopathy, DCM is closely related to microvascular disease, oxidative damage, activation of the renin–angiotensin system (RAS), cardiac inflammation, fibrosis and apoptosis,3–7 and eventually leads to increased cardiac dysfunction and mortality. Studies have shown the specific mechanism by which irbesartan can improve myocardial injury in diabetic rats, but how it occurs and develops is unclear.
Angiotensin II is one of the important members of the RAS, which can contract blood vessels, and regulate water and salt metabolism, and thus affect the cardiovascular system function, and lead to deterioration of cardiac function. However, its main role is to induce cell hypertrophy, vascular proliferation and deposition of extracellular matrix proteins such as collagen, which causes ventricular remodeling. Angiotensin II plays a non-negligible regulatory role in the formation and development of myocardial hypertrophy and myocardial interstitial fibrosis. Studies have shown that irbesartan as an angiotensin II receptor antagonist plays an important role in myocardial protection in diabetic cardiomyopathy. As a commonly used angiotensin II receptor blocker (ARB), in addition to lowering blood pressure, irbesartan also has the function of regulating oxidative stress and protecting vital organs.8 In the myocardial fibrosis model, studies have shown that irbesartan can reduce myocardial fibrotic damage by inhibiting the activation of the extracellular signal-regulated kinase pathway.9 In addition, irbesartan can improve cardiac remodeling and dysfunction in patients with type 2 diabetes by inhibiting protein kinase D and endoplasmic reticulum stress activation.10 Therefore, irbesartan is a widely used ARB in clinical practice, especially in patients with diabetes and cardiac dysfunction.
The development of sterile inflammation after cell death is a ubiquitous response which occurs in all organs. The nucleotide binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome serves as an intracellular inflammatory machinery, which can be activated by a wide range of danger signals and lead to various chronic degenerative diseases. The novel cytosolic multiprotein complex is composed of NLRP3, apoptosis-associated speck-like protein containing CARD (ASC) and cysteinyl aspartate specific proteinase-1 (Caspase-1).11–13 During formation and activation of the NLRP3 inflammasome, cleaved Caspase-1 causes the maturation of pro-inflammatory cytokines, such as interleukin-1β (IL-1β) and interleukin-18 (IL-18). When the inflammatory reaction is induced, meanwhile, IL-1β and IL-18 are released, and more inflammatory cells are recruited to expand the inflammatory response. As a key link in cell death, NLRP3 inflammasome may provide a new target for the treatment of various inflammatory diseases, which plays an important role in many non-infectious inflammatory diseases such as gout and atherosclerosis.14,15 Furthermore, NLRP3 inflammasome activation may also induce cell metabolic disturbance, cell transformation and tissue damage as its uncanonical effects, which may be another important triggering mechanism for organ disease beyond inflammation. Based on the above background, we hypothesized that irbesartan may reduce myocardial injury in diabetic rats, and that this is associated with the NLRP3/ASC/Caspase-1 pathway signal. To test this hypothesis, we were prompted to investigate the effect of irbesartan in a diabetic model and to determine its underlying mechanisms.
Materials and methods
Materials
Male Sprague–Dawley rats (130–150 g) were provided by the Animal Center of Anhui Bengbu Medical College. All rats were subjected to a 12 h light/dark cycle at 22 ±2°C and 50–70% humidity and allowed free access to food and water. The procedure was performed in accordance with the guidelines for laboratory animal use and care developed by the College of Health Sciences and following the guidelines of the National Institutes of Health on laboratory animal care and welfare. Irbesartan was obtained from Sanofi (SA, Paris, France) and streptozotocin (STZ) was purchased from Sigma-Aldrich Inc. (St. Louis, Missouri, USA). The appropriate pretreatment dose of irbesartan was determined in preliminary experiments.
Induction of diabetes and experimental protocol
Forty male Sprague–Dawley rats were randomly divided into three groups: normal control group (CON group, n=10), high glucose and high fat group (HC group, n=10) and diabetes experimental group (DM group, n=20). The rats in CON group were given regular diet. The rats in the other groups were given a high-glucose and high-fat diet. After four weeks, the rats in the diabetes experimental group were given a single intraperitoneal injection of STZ 30 mg/kg to establish the diabetes model. Successful models were randomly divided into diabetic group (DM group, n=10) and diabetes+irbesartan group (DM+Ir group, n=10). Rats in the DM+Ir group were treated with 50 mg/kg per day irbesartan through intragastric administration for eight weeks. In this experiment, the typical symptoms of ‘three more and one less’ appeared in the DM group. The rats with fasting blood glucose (FBG) level of ⩾16.7 mmol/L after the injection were considered diabetic. The plasma triglyceride (TG), cholesterol (total cholesterol; TC) and FBG levels in the HC, DM and DM+Ir groups were maintained at a high level, suggesting that the diabetes rats showed abnormal lipid metabolism, and the diabetes model was replicated and successfully maintained on the basis of hyperlipidemia.
Detection of triglyceride, TC and FBG levels in plasma
The rats in each group were fasted for 12h and anesthetized by injection of 10% chloral hydrat,16 2 mL of blood was collected from the abdominal aorta and centrifuged for 5 min at 3000 rev/min, the plasma was collected and the plasma TG, TC and FBG levels were measured through an automatic biochemical analyzer (7180, Hitachi, Japan).
Echocardiographic image acquisition
Animals were anesthetized with 3% isoflurane (Baxter International, Deerfield, Illinois, USA) and fixed in supine position on a heatpad at 37°C (FUJIFILM VisualSonics, Toronto, Ontario, Canada). Isoflurane concentration was further reduced to a minimum of 1–2% to achieve constant and comparable heart rates during image acquisition.17,18 Ultrasound and two-dimensional ultrasound were used to measure the interventricular septum diameter (IVS(d)), using a Philips 7500 digital echocardiograph, left ventricular internal diameter at end-diastole (LVID(d)), left ventricular posterior wall diameter (LVPW(d)) and left ventricular ejection fraction (EF%).
Detection of cardiac pathological changes in rats by Masson staining
Following dewaxing and dehydration, myocardial tissue sections were incubated with 1% hydrochloric acid solution for 3–5 s at room temperature. After rinsing, the sections were stained with a mild alkali fuchsine solution (Beijing Solarbio Science and Technology Co., Ltd, Beijing, China) for 3 min at room temperature. After further rinsing with deionized water, samples were treated with 1% solution of phosphomolybdic acid for 1 min. Finally, sections were stained with 2% aniline blue solution (Beijing Solarbio Science and Technology Co., Ltd) for 2 min at room temperature, followed by dehydration with 95% ethanol and embedment. Following staining, myocardial collagen fibers were stained a bluish-green and the myocardium appeared red under light microscope at a magnification of ×400.
Detection of cardiac NLRP3, ASC and Caspase-1 at mRNA level by real-time quantitative polymerase chain reaction
Total RNA was extracted from myocardial cells using the RNAprep Pure Kit (Tiangen Biotech, Beijing, China) according to the manufacturer’s instructions. The first-strand cDNA was reverse-transcribed from the total RNA using the Superscript III Frist-Strand Synthesis System (Invitrogen, Carlsbad, California, USA). Real-time polymerase chain reaction (PCR) was performed with an Mx 3000 P Real-Time PCR Detection System (Agilent, Santa Clara, California, USA). Data were analyzed using 2–∆∆CT method with β-actin serving as an internal control. The sequences of the forward and reverse primers used are shown in Table 1.
Table 1.
List of primer sequences for reverse transcription-quantitative polymerase chain reaction.
| Gene | Primer | Sequence (5′–3′) | Product size (bp) |
|---|---|---|---|
| NLRP3 | Forward | CCATGAGCTCCCTTAAGCTG | 283 |
| Reverse | TTGCACAGGATCTTGCAGAC | ||
| ASC | Forward | CCCATAGACCTCACTGATAAAC | 260 |
| Reverse | AGAGCATCCAGCAA ACCA | ||
| Caspase-1 | Forward | TATGGAAAAGGCACGAGACC | 137 |
| Reverse | CAGCTGATGGACCTGACTGA |
NLRP3: nucleotide binding oligomerization domain-like receptor protein 3; ASC: apoptosis-associated speck-like protein containing CARD; Caspase-1: cysteinyl aspartate specific proteinase-1.
Detection of NLRP3, ASC and Caspase-1 protein expression by Western blot
After anesthesia in rats, about 100 mg of left ventricular myocardium was taken and stored in a refrigerator at –80°C. At the beginning of the experiment, 100 mg of myocardial tissue was lysed to obtain tissue homogenate, centrifuged at 4°C (12,000 rev/min per 10 min) and the supernatant was taken. After separation by 15% SDS PAGE, the membrane was electroporated to PVDP membrane, and NLRP3 (1:500, Abcam, UK), ASC (1:500, Abcam, UK), Caspase-1 (1:500, Abcam, UK) and β-actin (1:2000, Absin, China) primary antibody were added in sequence, and kept overnight at 4°C .The next day, the secondary antibody was added and incubated with HRP secondary IgG, and the ECL kit was subjected to exposure imaging. The gel imaging system measures the gray value of the bands and calculates the relative amounts of NLRP3, ASC and NLRP3/β-actin, ASC/β-actin and Caspase-1/β-actin protein expression.
Detection of IL-1β and IL-18 levels in heart tissue by enzyme-linked immunosorbent assay method
Heart tissue was boiled in 0.1 mol/L acetic acid at 100°C for 20 min and then homogenized in an ice bath. The homogenate was centrifuged at 13,000 × g for 4 min at 4°C to collect the supernatant. The peptide was then extracted from the supernatant of plasma or heart tissue using a kit comprising SEP-COLUMN and buffers A and B (Pack Phoenix Pharmaceuticals, Inc., Burlingame, California, USA) based on the manufacturer’s instructions. IL-1β and IL-18 levels from myocardial samples were measured using an enzyme immunoassay kit (Cayman Chemical, Ann Arbor, Michigan, USA) according to the manufacturer’s instructions.
Statistical analysis
All values are expressed as mean±SD. Statistical significance between the two groups was compared by one-way analysis of variance. p< 0.01 was considered statistically significant.
Results
General characteristics at the time of termination
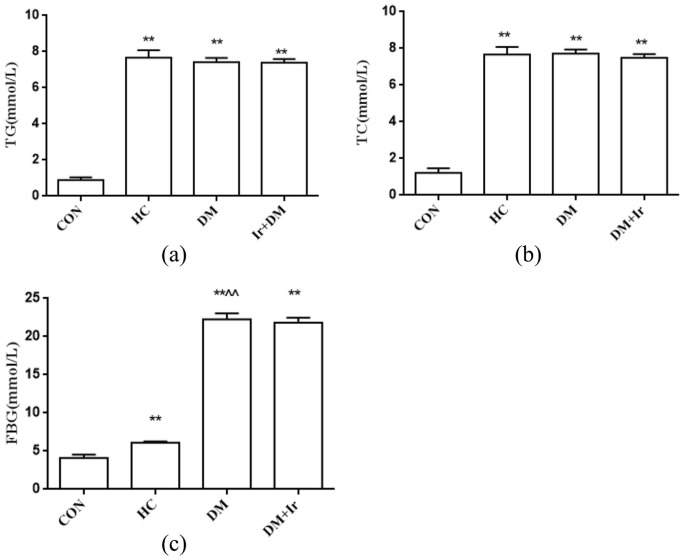
There were no significant differences of TG, TC and FBG levels among all groups at the beginning of the experiment. After eight weeks of artificial intervention, the plasma TG, TC and FBG levels in the HC group, DM group and DM+Ir group were maintained at a high level. In addition, an increase in water intake and food consumption was observed in diabetic rats. Compared with the CON group, the plasma levels of TG, TC and FBG in the HC group, DM group and DM+Ir group were significantly increased (p<0.01). Compared with the HC group, the TG and TC levels in the DM group and the DM+Ir group were not significantly increased (p>0.05), while FBS levels were significantly increased (p<0.01); compared with the DM group, there were no significant changes of TG, TC and FBG levels in the DM+Ir group (p>0.05) (Figure 1).
Figure 1.
The changes of triglyceride (TG), total cholesterol (TC) and fasting blood glucose (FBG) levels in different groups (mean±SD, n=6). The plasma (a) TG, (b) TC and (c) FBG levels in different groups.
**p<0.01 vs. CON
^^p<0.01 vs. HC.
CON: control group; HC: high glucose and high fat group; DM: diabetes group; DM+Ir: diabetes+irbesartan group.
Changes of cardiac function in heart
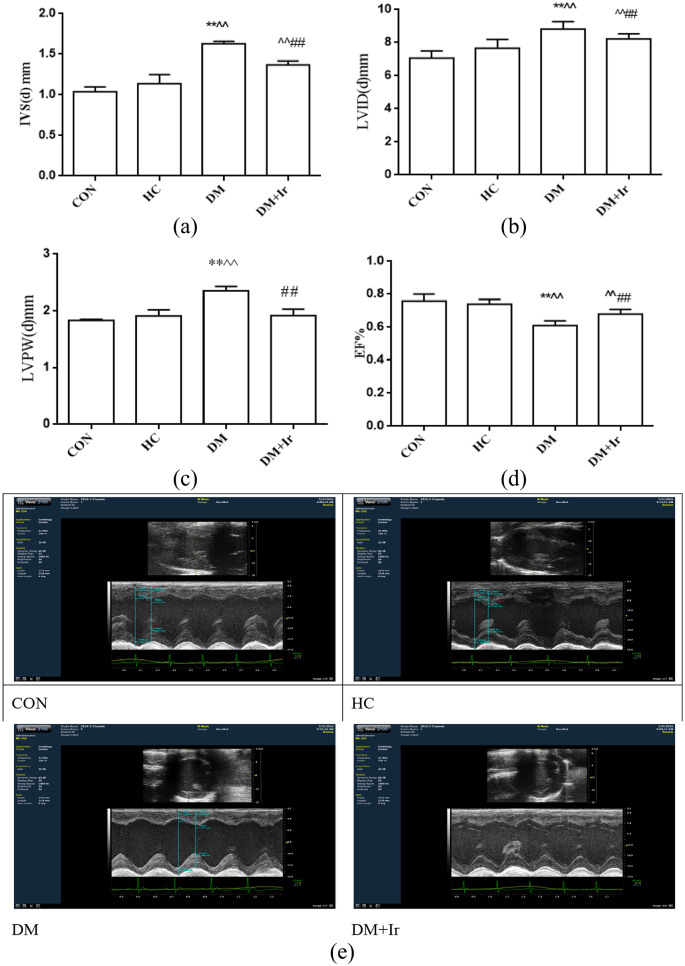
Compared with the CON group, there was no significant effect on cardiac function in the HC group. Compared with the HC group, IVS(d) and LVID(d) were increased and EF% was decreased in the DM and DM+Ir groups (p<0.01); however, LVPW(d) was increased in the DM group, with no significant change in the DM+Ir group. Compared with the DM group, in the DM+Ir group, IVS(d), LVID(d) and LVPW(d) were decreased, while EF% was increased (Figure 2).
Figure 2.
The heart functions in the different groups (mean±SD, n=6). The (a) IVS(d), (b) LVID(d), (c) LVPW(d) and EF% in different groups. (e) Typical echocardiographic images in the different groups.
**p<0.01 vs. CON
^^p<0.01 vs. HC
##p<0.01 vs. DM.
CON: control group; HC: high glucose and high fat group; DM: diabetes group; DM+Ir: diabetes+irbesartan group; IVS(d): interventricular septum diameter; LVID(d): left ventricular internal diameter at end-diastole; LVPW(d): left ventricular posterior wall diameter; EF%: ejection fraction percentage.
Masson staining results to assess myocardial damage
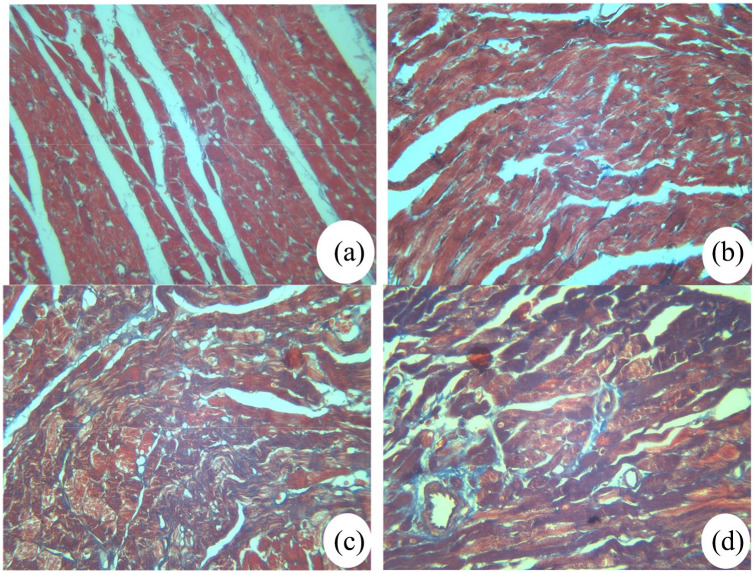
The Masson staining was performed to assess the effect of irbesartan on cardiac fibrosis. As presented in Figure 3, the red myocardial tissues in CON and HC groups were regular and clear. Blood vessels were clear with a small quantity of blue collagen surrounding them. However, the red myocardial tissue was markedly decreased and appeared disorganized, while, the blue collagen fiber was increased in the DM group. Compared with the DM group, the number of blue collagen fibers in the DM+Ir group was lower while the amount of red myocardial tissue was higher. These results indicated that irbesartan reduced the myocardial fibrosis of cardiac tissue.
Figure 3.
Masson staining of rat myocardial tissues in different groups (original magnification: 100×, n=4 ). (a) Control group; (b) high glucose and high fat group; (c) diabetes group; (d) diabetes+irbesartan group.
Changes of NLRP3, ASC and Caspase-1 at mRNA expression
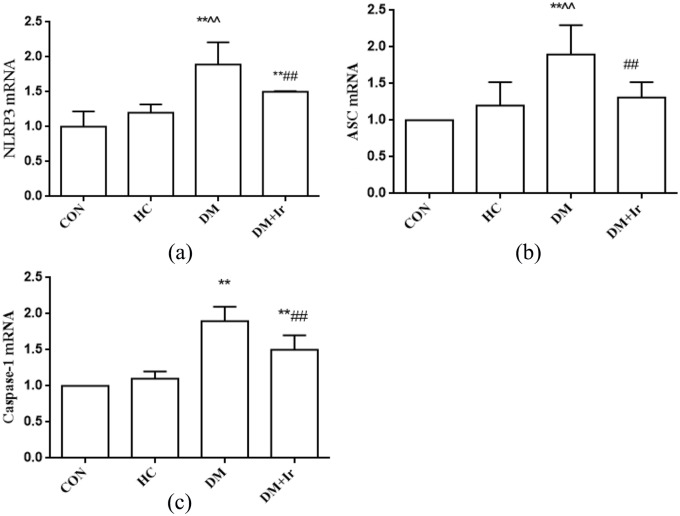
Compared with the CON group, the NLRP3, ASC and Caspase-1 mRNA levels in the HC group were not significantly increased (p>0.05), but the NLRP3 and Caspase-1 mRNA levels were increased in the DM and DM+Ir groups, the ASC mRNA level was increased in the DM group and there were no significant changes in the DM+Ir group. Compared with the HC group, NLRP3, ASC and Caspase-1 mRNA levels were further increased in the DM group. Compared with the DM group, the mRNA expression of NLRP3, ASC and Caspase-1 were inhibited in the DM+Ir group (p<0.01) (Figure 4).
Figure 4.
Changes of NLRP3, ASC and Caspase-1 levels in the myocardial tissues (mean±SD, n=6).
(a) NLRP3, (b) ASC and (c) Caspase-1 mRNA levels in myocardial tissues.
**p<0.01 vs. CON
^^p<0.01 vs. HC
##p<0.01 vs. DM.
NLRP3: nucleotide binding oligomerization domain-like receptor protein 3; ASC: apoptosis-associated speck-like protein containing CARD; Caspase-1: cysteinyl aspartate specific proteinase-1; CON: control group; HC: high glucose and high fat group; DM: diabetes group; DM+Ir: diabetes +irbesartan group.
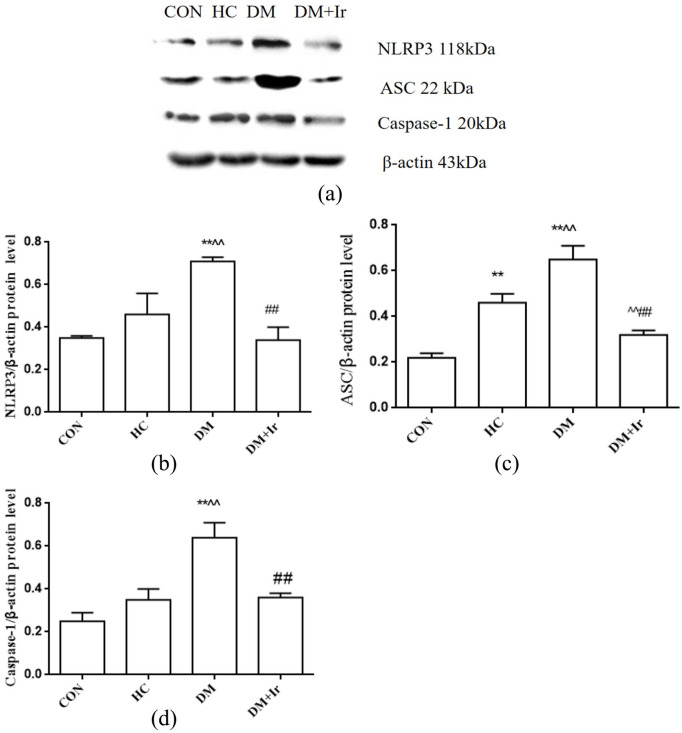
Changes of NLRP3, ASC and Caspase-1 protein expressions in heart
Compared with the CON group, the ASC protein expression in the HC group was significantly increased (p<0.01), but the protein expressions of NLRP3 and Caspase-1 were not changed. Compared with the CON group, the NLRP3, ASC and Caspase-1 protein expressions in the DM group were increased. The expressions of NLRP3, ASC and Caspase-1 protein in the DM+Ir group were significantly lower than those in the DM group (p<0.01) (Figure 5).
Figure 5.
Changes of NLRP3, ASC and Caspase-1 at protein expressions in different groups (mean+SD, n=6).
(a) Typical Western blot bands of NLRP3, ASC and Caspase-1 protein expressions; B, C, D: Statistical analysis of (b) NLRP3, (c) ASC and (d) Caspase-1 protein expression.
**p<0.01 vs. CON
^^p<0.01 vs. HC
##p<0.01 vs. DM.
NLRP3: nucleotide binding oligomerization domain-like receptor protein 3; ASC: apoptosis-associated speck-like protein containing CARD; Caspase-1: cysteinyl aspartate specific proteinase-1; CON: control group; HC: high glucose and high fat group; DM: diabetes group; DM+Ir: diabetes +irbesartan group.
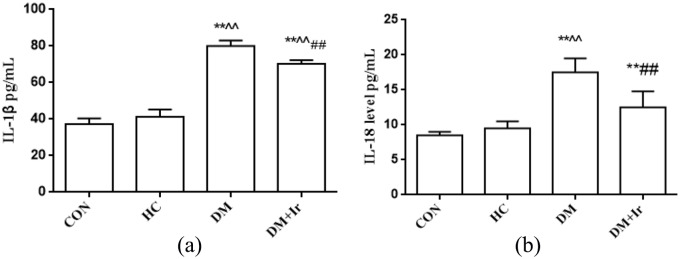
Changes of plasma IL-1β and IL-18 levels in heart
Compared with the CON group, there was no significant change of plasma IL-18 and IL-1β levels in the HC group. The levels of IL-1β and IL-18 in the myocardial tissue of the DM group and the DM+Ir group were significantly increased (p<0.01). Compared with the DM group, the levels of IL-1β and IL-18 in myocardial tissue of the DM+Ir group were lower than those in the DM group (p< 0.01) (Figure 6).
Figure 6.
Enzyme immunoassay for detecting the levels of interleukin (IL)-18 and IL-1β in myocardial tissue (mean±SD, n=6). (a) The IL-1β level in different groups; (b) the IL-18 level in different groups.
**p<0.01 vs. CON
^^p<0.01 vs. HC
##p<0.01 vs. DM
CON: control group; HC: high glucose and high fat group; DM: diabetes group; DM+Ir: diabetes+ irbesartan group.
Discussion
In the study, we aimed to explore the effect and potential mechanisms of NLRP3/ASC/Caspase-1 signal in type 2 diabetes-induced cardiac injury. The present study demonstrated that administered irbesartan may alleviate diabetic rats’ induced myocardial injury through inhibiting inflammation and myocardial fibrosis.
In the study, we successfully established a rat model of type 2 diabetes. In the irbesartan intervention group, the myocardial morphology and function were significantly improved compared with in the diabetic group; the likely mechanism was related to inhibiting the activity of the NLRP3/ASC/Caspase-1 pathway. Thus, NLRP3 inflammasome has a pivotal role in DCM and may be an attractive target for the treatment of type 2 diabetes.
The pathogenesis of DCM is complex, and it is currently recognized that it encompasses metabolic disorders, myocardial fibrosis and activation of the renin–angiotensin–aldosterone system (RAAS). Metabolic disorders include hyperglycemia, disorders of lipid metabolism, and insulin resistance. Hyperglycemia is the triggering factor of DCM. Abnormal lipid metabolism runs through the entire course of diabetes and promotes the development of diabetes and its complications.[19,20] In this experiment, the type 2 diabetes model was induced by a high-calorie diet and chemical reagent STZ. The plasma TC, triglyceride and FBG levels were maintained at a high level in the HC, DM and DM+Ir groups. These results suggest that the diabetes model has a disorder of lipid metabolism, and the diabetes model replicates and maintains success on the basis of hyperlipidemia.
RAAS has been recognized as an important signal relay station in the process of myocardial fibrosis, and angiotensin II (Ang II) is one of the most important effectors of RAAS, which can stimulate and mediate myocardial fibroblast synthesis and collagen secretion by binding to angiotensin receptors, eventually leading to the occurrence of myocardial tissue fibrosis. Previous studies have shown that Ang II could induce the secretion of transforming growth factor-β1 by direct induction of fibroblasts, which in turn leads to cascade fibrosis.21–23 As an Ang II receptor inhibitor, irbesartan can reduce the oxidative stress of the blood vessel wall, reduce the formation of atherosclerotic plaque and reduce ischemia–reperfusion injury.24 It has been found that irbesartan may attenuate protein kinase D and endoplasmic reticulum stress to improve diabetes induced ventricular remodeling.10 Tang et al. observed that in diabetic rats the degree of damage and myocardial fibrosis of DCM can be reduced after irbesartan intervention.25,26 In our study, after administration of irbesartan for seven weeks, the IVS(d), LVID(d) and LVPW(d) were increased, the EF% was decreased significantly, and the Masson staining results showed that the degree of myocardial fibrosis was significantly improved compared with the DM group, which indicated that irbesartan can reduce the occurrence of myocardial fibrosis by inhibiting the binding of Ang II to its receptor.
Inflammation plays a key role in inflammatory diseases, including diabetes, heart disease and Parkinson’s disease. Inflammation is the activation of the immune system in response to infection, injury or irritation. It has been reported that chronic inflammation is a possible trigger for diabetes; even with optimal treatment, patients with diabetes still have a high chance of dying from chronic inflammation.27 IL-1β and IL-18 are the common pro-inflammatory factors in the body, and the release of pro-inflammatory factors induced by hyperglycemia can cause persistent myocardial damage and lead to cardiac dysfunction.28 Caspase-1 is a component of the NLRP3 inflammasome that is involved in the maturation of pro-inflammatory factors (IL-1β and IL-18, etc.).29 The previous studies have shown that NLRP3 inflammasome-related pyroptosis plays a vital role in the development of diabetic cardiomyopathy.30,31 Ang II has an important relationship with NLRP3. Ang II can cause NLRP3 inflammasome activation by promoting endoplasmic reticulum stress and release of cathepsin B.32,33 Activated NLRP3 inflammasome causes cell damage, mitochondrial dysfunction, smooth muscle phenotypic transformation and vascular remodeling, causing abnormalities in tissues and organs.34,35 Some studies have shown that the effects of angiotensin-converting enzyme inhibitors or ARBs on diabetes may be achieved through the Caspase pathway.36,37
However, the mechanism of NLRP3 inflammasome activation in diabetes, which is the basis of the hyperlipidemia condition, remains to be explored. Whether irbesartan can alleviate the occurrence of diabetes has not been reported. Our results showed that after high-calorie diet and chemical reagent STZ intervention, NLRP3, ASC, Caspase-1 expressions, IL-1β and IL-18 levels were all increased and cardiac function was reduced; after administration of irbesartan in the diabetic rat model, NLRP3, ASC, Caspase-1 expressions were reduced, IL-1β and IL-18 levels were also reduced, and the cardiac function was improved, indicating that irbesartan could reduce inflammation and improve ventricular remodeling.
In conclusion, this study showed that in a diabetic rat model, with the occurrence of myocardial injury, the activation of NLRP3/ASC/Caspase-1 signal pathway was effected after the intervention of irbesartan, the cardiac structure and function have been improved to various degrees in diabetic rats, and this improvement may be related to inhibition of the NLRP3/ASC/Caspase-1 pathway. This study provides an experimental basis for the study of irbesartan in improving myocardial damage in diabetic patients, and explored the correlation between irbesartan and NLRP3/ASC/Caspase-1 pathway.
Footnotes
Author contribution: PK and HW contributed equally to this work.
Ethical approval: Ethical approval was given by the medical ethics committee of Bengbu Medical College with the following reference number: [2017] number 075.
Data availability: The data used to support the findings of this study are available from the corresponding author upon request.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Chinese National Natural Science Foundation (grant numbers 81770297, 81970313), Anhui Province Natural Foundation (grant number 1908085QH353) and Science and Technology Project of Anhui Province (grant number 1804h08020246).
ORCID iDs: Pinfang Kang  https://orcid.org/0000-0001-7084-7862
https://orcid.org/0000-0001-7084-7862
References
- 1. Masuda T, Muto S, Fujisawa G, et al. Heart angiotensin II-induced cardiomyocyte hypertrophy suppresses coronary angiogenesis and progresses diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol 2012; 302(9): H1871-883. [DOI] [PubMed] [Google Scholar]
- 2. Tan Y, Li X, Brittian K R, et al. Angiotensin II plays a critical role in alcohol-induced cardiac nitrative damage, cell death, remodeling, and cardiomyopathy in a protein kinase C/nicotinamide adenine dinucleotide phosphate oxidase-dependent manner. J Am Coll Cardiol 2012; 59(16): 1477-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rani N, Bharti S, Bhatia J, et al. Chrysin, a PPAR-γ agonist improves myocardial injury in diabetic rats through inhibiting AGE-RAGE mediated oxidative stress and inflammation. Chem Biol Interact 2016; 250: 59-67. [DOI] [PubMed] [Google Scholar]
- 4. Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006; 444: 860-867. [DOI] [PubMed] [Google Scholar]
- 5. Dong B, Yu QT, Dai HY, et al. Angiotensin-converting enzyme-2 overexpression improves left ventricular remodeling and function in a rat model of diabetic cardiomyopathy. J Am Coll Cardiol 2012; 59(8): 739-747. [DOI] [PubMed] [Google Scholar]
- 6. Thomas CM, Yong QC, Rosa RM, et al. Cardiac-specific suppression of NF-κB signaling prevents diabetic cardiomyopathy via inhibition of the renin angiotensin system. Am J Physiol Heart Circ Physiol 2014; 307: H1036-H1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wen HL, Liang ZS, Rui Z, et al. Anti-inflammatory effects of triptolide improve left ventricular function in a rat model of diabetic cardiomyopathy. Cardiovasc Diabetol 2013; 12: 50. DOI:10.1186/1475-2840-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ren X, Guan G, Liu G, et al. Irbesartan ameliorates diabetic nephropathy by reducing the expression of connective tissue growth factor and alpha-smooth-muscle actin in the tubulointerstitium of diabetic rats. Pharmacology 2009; 83(2): 80-87. [DOI] [PubMed] [Google Scholar]
- 9. Zhang GJ, Zhang WP, Wang KC, et al. The mechanism of irbesartan against diabetes induced myocardial fibrosis in rat model. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2016; 32(3): 221-224. [DOI] [PubMed] [Google Scholar]
- 10. Liu X, Xu Q, Wang X, et al. Irbesartan ameliorates diabetic cardiomyopathy by regulating protein kinase D and ER stress activation in a type 2 diabetes rat model. Pharmacol Res 2015; 93: 43-51. [DOI] [PubMed] [Google Scholar]
- 11. Bracey NA, Beck PL, Muruve DA, et al. The Nlrp3 Inflammasome promotes myocardial dysfunction in structural cardiomyopathy through IL-1β. Exper Physiol 2012; 98(2): 462-472. [DOI] [PubMed] [Google Scholar]
- 12. Mezzaroma E, Toldo S, Farkas D, et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci U S A 2011; 108: 19725-19730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kawaguchi M, Takahashi M, Hata T, et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 2011; 123(6): 594-604. [DOI] [PubMed] [Google Scholar]
- 14. Rheinheimer J, de Souza BM, Cardoso NS, et al. Current role of the NLRP3 inflammasome on obesity and insulin resistance: A systematic review. Metabolism 2017; 74: 1-9. [DOI] [PubMed] [Google Scholar]
- 15. Yang CS, Shin DM, Jo EK. The role of NLR-related protein 3 inflammasome in host defense and inflammatory diseases. Int Neurourol J 2012; 16: 2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li T, Yao W. Therapeutic effect of irbesartan combined with atorvastatin calcium in the treatment of rats with coronary heart disease. Exp Ther Med 2018; 16: 4119-4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grune J, Ritter D, Krater K, et al. Accurate assessment of LV function using the first automated 2D-border detection algorithm for small animals–evaluation to models of LV dysfunction. Cardiovasc Ultrasound 2019; 17(1): 7. DOI:10.1186/s 12947-01900156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilck N, Marko L, Blogh A, et al. Nitric oxide-sensitive guanylyl cyclase stimulation improves experimental heart failure with preserved ejection fraction. JCI Insight 2018; 3(4): e96006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Touyz RM, Berry C. Recent advances in angiotensin II signaling. Braz J Med Biol Res 2002; 35: 1001-1015. [DOI] [PubMed] [Google Scholar]
- 20. Guo DF, Sun YL, Hamet P, et al. The angiotensin II Type 1 receptor and receptor-associated proteins. Cell Res 2001; 11: 165-180. [DOI] [PubMed] [Google Scholar]
- 21. Ruiz-Ortega M, Rupercz M, Estcban V, et al. Angiotensin II: A key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol Dial Transplant 2006; 21(1): 16-20. [DOI] [PubMed] [Google Scholar]
- 22. Tunon J, Ruiz-Ortega M, Egido J. Regulation of matrix proteins and impact on vascular structure. Curr Hypertens Rep 2000; 2(1): 106-113. [DOI] [PubMed] [Google Scholar]
- 23. Aneja Tang WH, Bansilal S, et al. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med 2008; 121(9): 748-757. [DOI] [PubMed] [Google Scholar]
- 24. Yan Z, Ayahisa W, Songji Z, et al. Suppressive effects of irbesartan on inflammation and apoptosis in atherosclerotic plaques of apoE / mice: Molecular imaging with14 C / FDG and 99m Tc, Annexin A5. PLoS One 2014; 9(2): e89338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma GT, Xie XM, Wu XH, et al. Short- and long-term therapeutic effects of combination therapy with perindopril and irbesartan in a rat model of dilated cardiomyopathy. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2007; 32(4): 594-598. [PubMed] [Google Scholar]
- 26. Tang RN, Lv LL, Zhang JD, et al. Effects of angiotensin II receptor blocker on myocardial endothelial-to-mesenchymal transition in diabetic rats. Int J Cardiol 2013; 162(2): 92-99. [DOI] [PubMed] [Google Scholar]
- 27. Wei X, Song H, Yin L, et al. Fatty acid synthesis configures the plasma membrane for inflammation in diabetes. Nature 2016; 539(7628): 294-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bell DS. Diabetes: A cardiac condition manifesting as hyperglycemia. Endocr Pract 2008; 14(7): 924-932. [DOI] [PubMed] [Google Scholar]
- 29. Sharma BR, Karki R, Kanneganti TD. Role of AIM2 inflammasome in inflammatory diseases, cancer and infection. Eur J Immunol 2019; 49(11): 1998-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luo B, Li B, Wang W, et al. NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model. PLoS One 2014; 9: e104771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giordano A, Murano I, Mondini E, et al. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J Lipid Res 2013; 54: 2423-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang J, Wen Y, Lv LL, et al. Involvement of endoplasmic reticulum stress in angiotensin II-induced NLRP3 inflammasome activation in human renal proximal tubular cells in vitro. Acta Pharmacol Sin 2015; 36(7): 821-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lian D, Lai J, Wu Y, et al. Cathepsin B-mediated NLRP3 inflammasome formation and activation in angiotensin II-induced hypertensive mice: Role of macrophage digestion dysfunction. Cell Physiol Biochem 2018; 50: 1585-1600. [DOI] [PubMed] [Google Scholar]
- 34. Min Z, Mi B, Guixia D, et al. Angiotensin II stimulates the NLRP3 inflammasome to induce podocyte injury and mitochondrial dysfunction. Kidney Dis 2018; 4(2): 83-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ren XS, Tong Y, Ling L, et al. NLRP3 gene deletion attenuates angiotensin II-induced phenotypic transformation of vascular smooth muscle cells and vascular remodeling. Cell Physiol Biochem 2017; 44(6): 2269-2280. [DOI] [PubMed] [Google Scholar]
- 36. Ding LH, Liu D, Xu M, et al. Enalapril inhibits tubulointerstitial inflammation and NLRP3 inflammasome expression in BSA-overload nephropathy of rats. Acta Pharmacol Sin 2014; 35(010): 1293-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou X, Liang L, Zhao Y, et al. Epigallocatechin-3-gallate ameliorates angiotensin II-induced oxidative stress and apoptosis in human umbilical vein endothelial cells through the activation of Nrf2/Caspase-3 signaling. J Vasc Res 2017; 54(5): 299-308. [DOI] [PubMed] [Google Scholar]