Here, Räsch et al. investigated the underlying mechanisms that regulate the decay of mRNAs destabilized by AU-rich elements and miRNAs. They show that upon mRNA binding 4E-T, an eIF4E-binding protein (4E-BP) represses translation and promotes deadenylation via the recruitment of the CCR4–NOT deadenylase complex, and their findings provide insight into the mechanism of mRNA storage that controls localized translation and mRNA stability in P-bodies.
Keywords: deadenylation, decapping, eIF4E-binding proteins, P-bodies
Abstract
Human 4E-T is an eIF4E-binding protein (4E-BP) present in processing (P)-bodies that represses translation and regulates decay of mRNAs destabilized by AU-rich elements and microRNAs (miRNAs). However, the underlying regulatory mechanisms are still unclear. Here, we show that upon mRNA binding 4E-T represses translation and promotes deadenylation via the recruitment of the CCR4–NOT deadenylase complex. The interaction with CCR4–NOT is mediated by previously uncharacterized sites in the middle region of 4E-T. Importantly, mRNA decapping and decay are inhibited by 4E-T and the deadenylated target is stored in a repressed form. Inhibition of mRNA decapping requires the interaction of 4E-T with the cap-binding proteins eIF4E/4EHP. We further show that regulation of decapping by 4E-T participates in mRNA repression by the miRNA effector protein TNRC6B and that 4E-T overexpression interferes with tristetraprolin (TTP)- and NOT1-mediated mRNA decay. Thus, we postulate that 4E-T modulates 5′-to-3′ decay by swapping the fate of a deadenylated mRNA from complete degradation to storage. Our results provide insight into the mechanism of mRNA storage that controls localized translation and mRNA stability in P-bodies.
Ribosome recruitment in eukaryotes requires the assembly of the eukaryotic initiation factor (eIF)4F complex at the 5′ cap structure of the messenger (m)RNA (Topisirovic et al. 2011). This heterotrimeric complex is formed through the interaction of the scaffold eIF4G with the cap-binding protein eIF4E and the RNA helicase eIF4A. Together, these proteins trigger a series of events that result in the recruitment of the preinitiation complex, composed of the 40S ribosomal subunit and associated factors, and in the initiation of translation (Hashem and Frank 2018; Merrick and Pavitt 2018).
The function of eIF4F in translation initiation is tightly regulated by the eIF4E-binding proteins (4E-BPs). This group of translational repressors share with eIF4G canonical and noncanonical binding motifs that recognize a common surface on eIF4E (Peter et al. 2015; Grüner et al. 2016, 2018). Consequently, 4E-BPs compete with eIF4G for eIF4E binding, disrupting eIF4F assembly and blocking translation (Haghighat et al. 1995; Mader et al. 1995).
The eIF4E-transporter protein (4E-T), or eukaryotic translation initiation factor 4E nuclear import factor 1 (EIF4ENIF1), is a nucleocytoplasmic shuttling 4E-BP required for the localization of eIF4E to the nucleus (Dostie et al. 2000). However, in cells, 4E-T is predominantly located to processing (P)-bodies (Andrei et al. 2005; Ferraiuolo et al. 2005). P-bodies are dynamic cytoplasmic granules that form by the phase separation of RNA decay-associated proteins bound to translationally inactive transcripts (Standart and Weil 2018; Ivanov et al. 2019). These granules are thought to buffer the proteome through translational control and storage of mRNAs coding for regulatory proteins (Hubstenberger et al. 2017; Standart and Weil 2018).
In P-bodies, 4E-T establishes multiple interactions with proteins involved in mRNA turnover. In addition to the cap-binding proteins eIF4E and eIF4E homologous protein (4EHP), known 4E-T-binding partners include the cold-shock domain protein upstream of N-Ras (UNR), the RNA-dependent ATPase DDX6, the decapping factors LSM14A and PatL1, and the CCR4–NOT deadenylase complex (Kubacka et al. 2013; Kamenska et al. 2014; Ozgur et al. 2015; Brandmann et al. 2018). Several of these interactions are thought to be essential for P-body formation and to contribute to the control of translation and turnover of adenine and uracil (AU)-rich mRNAs destabilized by tristetraprolin (TTP) or transcripts repressed by micro (mi)RNAs (Ferraiuolo et al. 2005; Kamenska et al. 2014, 2016; Nishimura et al. 2015; Chapat et al. 2017; Jafarnejad et al. 2018).
In mammals, 4E-T is an important component of repressor complexes that regulate the expression of proneurogenic factors during neurogenesis (Yang et al. 2014; Amadei et al. 2015; Zahr et al. 2018). In addition, 4E-T is essential for meiosis in oocytes (Pfender et al. 2015), and mutations in the gene have been associated with female infertility (Kasippillai et al. 2013; Zhao et al. 2019). However, the mechanism by which 4E-T affects these developmental processes is unclear.
In this study, we examined the molecular effects of 4E-T in gene expression. Our work demonstrates that 4E-T coordinates deadenylation with the suppression of decapping to store mRNAs targeted by the CCR4–NOT complex in silenced messenger ribonucleoprotein particles (mRNPs).
Results
4E-T represses translation and promotes mRNA deadenylation
To study the mechanism by which 4E-T represses mRNA expression, we used a reporter assay in human cells. 4E-T fused to the bacteriophage MS2 coat protein and an HA (hemagglutinin) tag (MS2-HA-4E-T) was tethered to a Renilla (R-Luc) luciferase reporter containing six MS2-binding sites in the 3′ untranslated region (UTR; R-Luc-6xMS2bs) (Supplemental Fig. S1A). A plasmid encoding firefly luciferase (F-Luc-GFP) served as a transfection and normalization control. In HEK293T cells, MS2-HA-4E-T strongly reduced R-Luc activity compared with MS2-HA-GFP (Supplemental Fig. S1B, protein, black bars), as observed previously (Ferraiuolo et al. 2005; Kubacka et al. 2013; Kamenska et al. 2014). The abundance of the R-Luc mRNA did not significantly vary in the presence of 4E-T, as determined by Northern blotting (Supplemental Fig. S1B, mRNA, blue bars and S1C and D), indicating that 4E-T represses translation in the absence of mRNA decay. Furthermore, in cells expressing MS2-HA-4E-T the R-Luc mRNA migrated faster, resembling the transcript lacking the poly(A) tail (Supplemental Fig. S1C, lane 2, A0). Deadenylation, or removal of the poly(A) tail, by the multisubunit CCR4–NOT complex (acting often in combination with PAN2/3) is the first step in cytoplasmic mRNA turnover (Wahle and Winkler 2013). Importantly, 4E-T had no effect on the F-Luc-GFP control or an R-Luc reporter lacking the MS2 binding sites (Supplemental Fig. S1E–G).
We also tethered 4E-T to reporter mRNAs containing distinct coding sequences, F-Luc and β-GLOBIN (Fig. 1A; Supplemental Fig. S1H), or five BoxB elements in the 3′ UTR (R-Luc-5xBoxB) (Supplemental Fig. S1K; Lykke-Andersen et al. 2000; Pillai et al. 2004). We observed that independently of the reporter mRNA 4E-T induced translational repression and deadenylation without major changes in transcript abundance (Fig. 1B–D; Supplemental Fig. S1I,J,L–N).
Figure 1.

4E-T promotes mRNA deadenylation and blocks decapping of a bound mRNA. (A) β-GLOBIN reporters used in this study. (BGG) β-GLOBIN. The BGG-GAP reporter contains a truncated version of the GAPDH (GAP) gene to distinguish it from the BGG-6xMS2bs reporter by size (Lykke-Andersen et al. 2000). The BGG-6xMS2bs reporter contains six MS2 binding sites in the 3′ UTR. (B) Northern blot analysis of a tethering assay using the BGG-6xMS2bs reporter and MS2-HA-4E-T in HEK293T cells. A plasmid expressing BGG-GAP served as a transfection control and lacks the MS2-binding sites. The position of the deadenylated BGG-6xMS2bs reporter mRNA is marked with A0, whereas the position of the reporter mRNA with an intact poly(A) is indicated as An. (C) BGG-6xMS2bs mRNA levels determined by Northern blotting were normalized to those of BGG-GAP and set to 1 in cells expressing MS2-HA-GFP. Mean values ± standard deviation (SD) are shown (n = 3). (*) P < 0.05, paired t-test. (D) Western blot showing the expression levels of the tethered proteins. (E) RNA samples isolated from cells expressing MS2-HA-GFP or MS2-HA-4E-T, BGG-6xMS2bs, and BGG-GAP were treated with oligo(dT)15 in the presence (+) or absence (−) of RNase H and analyzed by Northern blot. Note that upon RNase H treatment the poly(A) tails of the BGG reporters are removed in the presence of oligo(dT). A0, deadenylated and An, polyadenylated reporter mRNAs. (F–H) Tethering assay with the BGG-6xMS2bs, BGG-GAP and MS2-HA-GFP, or MS2-HA-4E-T performed in cells expressing HA-MBP or the catalytic inactive mutant of NOT8 (HA-NOT8*). In the Northern blot depicted in F, A0 indicates the position of the deadenylated BGG-6xMS2bs mRNA while An indicates the position of the adenylated reporter mRNA. The graph in G depicts the relative quantification of the BGG-6xMS2bs mRNA levels, as described in B (n = 3). (*) P < 0.05; (ns) not significant, paired t-test. A representative Western blot showing the expression of all the proteins used in the assay is present in H. (I) RNA samples isolated from cells expressing MS2-HA-GFP or MS2-HA-4E-T, the BGG-6xMS2bs and the BGG-GAP reporters were incubated with Terminator 5′-phosphate-dependent exonuclease and analyzed by Northern blotting. 18S ribosomal RNA (rRNA) served as uncapped RNA control. (A0) Deadenylated reporter mRNAs;(An) polyadenylated reporter mRNAs.
We then performed an oligo(dT)-targeted ribonuclease H (RNase H) cleavage assay to verify that the 4E-T-bound mRNAs are in fact deadenylated. In cells expressing MS2-HA-GFP, the BGG-6xMS2bs and BGG-GAP (control lacking the MS2bs) transcripts migrated faster after poly(A) tail cleavage (Fig. 1E, lanes 2 vs. 1, A0). In contrast, the 4E-T-bound BGG-6xMS2bs mRNA migrated like the deadenylated transcript before and after cleavage by RNase H (Fig. 1E, lanes 3,4). Moreover, in cells expressing MS2-HA-4E-T, the poly(A) tail of the control BGG-GAP mRNA was only removed after RNase H and oligo(dT) addition (Fig. 1E, lanes 4 vs. 3). Thus, we conclude that human 4E-T induces deadenylation of a bound mRNA.
4E-T-mediated mRNA deadenylation requires the CCR4–NOT complex
To determine whether the CCR4–NOT complex is involved in 4E-T-mediated mRNA deadenylation, we inhibited the deadenylase activity of the complex by overexpressing a catalytically inactive form of the NOT8 enzyme (NOT8*; D40A, E42A) in human cells. The mutant enzyme impedes CCR4–NOT-dependent mRNA deadenylation in a dominant-negative manner (Piao et al. 2010). The inactive NOT8, but not HA-MBP, blocked mRNA deadenylation targeted by 4E-T as the BGG-6xMS2bs reporter accumulated as a polyadenylated (An) mRNA (Fig. 1F [lanes 4 vs. 2], G,H). Our results show that mRNA deadenylation induced by 4E-T requires the CCR4–NOT complex.
4E-T blocks decapping of bound mRNA
In the 5′–3′ mRNA degradation pathway, removal of the poly(A) tail is followed by decapping and ultimately 5′–3′ exonucleolytic degradation by XRN1 (Franks and Lykke-Andersen 2008). The unusual accumulation of deadenylated mRNA in the presence of 4E-T could result from inhibition of decapping or, alternatively, inhibition of XRN1 activity after decapping. To investigate whether the deadenylated BGG-6xMS2bs mRNA was capped, we used the Terminator nuclease, a 5′–3′ exonuclease that degrades uncapped monophosphorylated RNA (Braun et al. 2012). The Terminator nuclease did not degrade the BGG-6xMS2bs reporter bound to MS2-HA-GFP or MS2-HA-4E-T (Fig. 1I), nor the BGG-GAP mRNA, indicating that these are capped transcripts. In contrast, the uncapped 18S rRNA was fully degraded upon addition of the Terminator nuclease (Fig. 1I, lanes 2,4). Our data suggest that 4E-T protects the deadenylated target mRNA from degradation by blocking decapping.
4E-T-dependent mRNA repression is independent of UNR, DDX6, PatL1, and LSM14A
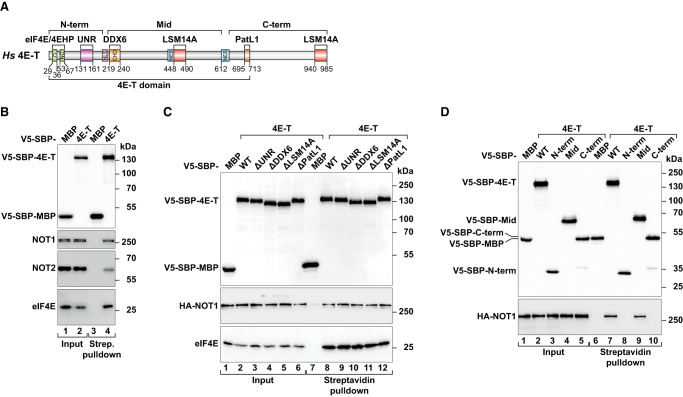
4E-T is a largely disordered protein with well-characterized short linear motifs (SLiMs) that mediate binding to DDX6, UNR, LSM14A, and PatL1 (Fig. 2A). To understand whether the interactions of 4E-T with these proteins are important to deadenylate and prevent decapping of a bound transcript, we made use of mutant proteins lacking each of the binding sites (ΔDDX6, ΔUNR, and ΔLSM14A) (Supplemental Table S1; Kamenska et al. 2014, 2016; Nishimura et al. 2015; Brandmann et al. 2018). The interaction of 4E-T with DDX6, UNR, and LSM14A was specifically abolished upon the deletion of the corresponding SLiMs, as assessed in pull-down assays following transient expression of the mutant proteins in human cells (Supplemental Fig. S2). In detail, deletion of the UNR binding site (ΔUNR, residues 131–161) prevented the interaction with UNR without affecting binding of 4E-T to eIF4E, DDX6, PatL1, and LSM14A (Supplemental Fig. S2A,B). Removal of the CUP homology domain (CHD, ΔDDX6, residues 219–240) only abrogated the association of 4E-T with DDX6 (Supplemental Fig. S2A,B). On the other hand, the interaction with LSM14A was strongly reduced upon the simultaneous deletion of two LSM14A binding sites present in 4E-T (residues 448–490 and 940–985, ΔLSM14A), whereas single deletion mutants (Δ448–490 or 1–939) had decreased binding to LSM14A (Supplemental Fig. S2B,C). The ΔLSM14A 4E-T protein still copurified with UNR, DDX6, PatL1, and eIF4E (Supplemental Fig. S2A,B).
Figure 2.
4E-T interacts with the CCR4–NOT complex via its Mid region. (A) Schematic overview of the domain architecture and binding regions of 4E-T. The N-terminal (N-term) region of 4E-T contains two eIF4E-binding motifs ([C] canonical eIF4E-binding motif; [NC] noncanonical eIF4E-binding motif) and the UNR-interacting region. The middle (Mid) region of 4E-T includes the CUP homology domain (CHD) that mediates interaction with DDX6, and one of the LSM14A-interacting regions. The C-term of 4E-T interacts with PatL1 and LSM14A. (NLS) Nuclear localization signal; (NES) nuclear export signal. The amino acid positions at the domain/motif boundaries are indicated below the protein. (B–D) Analysis of the interaction of V5-SBP-4E-T wild type (WT), deletion mutants (Δ), or fragments (N-, Mid, and C-) with endogenous NOT1 and NOT2 (B) or HA-NOT1 (C,D). SBP-tagged proteins were pulled down using streptavidin-coated beads. V5-SBP-MBP served as a negative control. eIF4E was used as a positive binding control. The inputs were 20% for V5-SBP-proteins, 2% for NOT1 and NOT2, 20% for eIF4E, or 2% for HA-NOT1, whereas bound fractions corresponded to 10% for V5-SBP-proteins, 40% for NOT1 and NOT2, 10% for eIF4E or 40% for HA-NOT1. Samples were analyzed by Western blotting using anti-NOT1, anti-NOT2, anti-V5, anti-eIF4E, and anti-HA antibodies.
PatL1 has been shown to interact with the C-terminal region of 4E-T (Fig. 2A; Kamenska et al. 2014, 2016). To define more precisely the binding site of PatL1, we tested whether a region of 4E-T spanning amino acids 695–713 was required for the interaction. This region is conserved in 4E-T proteins and contributes to P-body localization (Supplemental Fig. S2D; Kamenska et al. 2014). Indeed, its deletion (ΔPatL1) abolished 4E-T binding to PatL1 without affecting the interaction with other protein partners (Supplemental Fig. S2A,B).
We then examined the subcellular localization of the 4E-T mutant proteins in HeLa cells. GFP-4E-T colocalized with the P-body marker and decapping factor EDC4, as judged by antibody staining (Supplemental Fig. S3A; Kedersha and Anderson 2007). Since none of the amino acid deletions altered the nuclear localization (NLS) or the nuclear export signals (NES) in 4E-T, the mutant proteins were mainly cytoplasmic (Fig. 2A; Supplemental Fig. S3B–H). However, while the ΔUNR, ΔDDX6 and ΔPatL1 4E-T proteins also localized to P-bodies, the ΔLSM14A mutant (and thus the 4E-T 4xΔ protein that lacks the binding sites for UNR, DDX6, PatL1, and LSM14A) was dispersed throughout the cytoplasm and the nucleus (Supplemental Fig. S3B–H). These results indicate that LSM14A binding regulates 4E-T P-body localization.
We also observed that in the absence of single or combined (4xΔ mutant) interactions with UNR, DDX6, LSM14A, or PatL1, 4E-T still retained the ability to induce deadenylation and protect mRNA from decay upon tethering to the BGG-6xMS2bs reporter (Supplemental Fig. S4A–F). Moreover, all mutants still repressed the translation of the R-Luc-6xMS2bs reporter (Supplemental Fig. S4G,H).
The Mid region of 4E-T interacts with CCR4–NOT
As our results highlighted mRNA deadenylation as a key event in the control of gene expression by 4E-T, we studied its interaction with the CCR4–NOT complex. In human cells, streptavidin-binding protein (SBP)-V5-4E-T copurified with the NOT1 and NOT2 subunits of CCR4–NOT, suggesting an association with the fully assembled complex (Fig. 2B). This interaction was not mediated by UNR, DDX6, LSM14A, and PatL1, as the corresponding 4E-T deletion mutants still associated with HA-NOT1 in pull-down assays (Fig. 2C).
Experimental evidence reported in the literature suggests that DDX6 bridges the interaction of 4E-T with NOT1 (Ozgur et al. 2015; Waghray et al. 2015). Our results indicate that 4E-T can also bind to the CCR4–NOT complex in the absence of an interaction with DDX6 (Fig. 2C). To confirm that 4E-T has additional interactions with the CCR4–NOT complex, we performed binding assays in DDX6-null HEK293T cells (Hanet et al. 2019). In cells depleted of DDX6, SBP-V5-4E-T still interacted with HA-NOT1 (Supplemental Fig. S5A). Thus, 4E-T establishes multiple and possibly redundant interactions with CCR4–NOT.
To delineate the region of 4E-T critical for the interaction with CCR4–NOT, we divided the protein into an N-terminal (N-term) fragment comprising the eIF4E and the UNR binding sites (residues 1–194), a middle fragment (Mid) containing the DDX6 and the first LSM14A-binding sites (residues 212–612), and a C-term fragment encompassing the PatL1 and the second LSM14A-binding sites (residues 639–985) (Fig. 2A; Supplemental Table S1). These 4E-T fragments were then tested for the ability to bind to HA-NOT1. We observed that the interaction of 4E-T with HA-NOT1 is mediated by its Mid fragment (Fig. 2D, lane 9).
To obtain additional insight into this interaction, we investigated the region of NOT1 responsible for binding to 4E-T. Using a similar approach, we tested in coimmunoprecipitation assays the binding of 4E-T to N-term (residues 1–1089), Central (residues 1085–1605), and C-term (residues 1595–2376) (Supplemental Table S1; Supplemental Fig. S5B) fragments of NOT1 known to assemble in discrete CCR4–NOT subcomplexes (Raisch et al. 2019). The NOT1 C-term, which associates with the NOT2 and NOT3 subunits of the deadenylase complex (Bhaskar et al. 2013; Boland et al. 2013), was sufficient to bind to 4E-T. The NOT1 N-term and Central fragments did not or only weakly interacted with 4E-T (Supplemental Fig. S5C, lanes 6–8).
The Mid region of 4E-T represses the expression of target mRNAs
We then separately used each 4E-T fragment in tethering assays. Remarkably, binding of 4E-T Mid to the BGG-6xMS2bs transcript triggered efficient mRNA degradation (Fig. 3A [lane 4], B,C). In contrast, the N-term had no effect on the reporter while the C-term partially reduced mRNA levels (Fig. 3A [lanes 2,3,5], B,C). All 4E-T fragments were dispersed in the cytoplasm and in the nucleus and compromised P-body integrity (Supplemental Fig. S3I–K). Consistent with the ability to bind CCR4–NOT, we observed that inhibition of decapping in cells overexpressing a catalytically inactive form of DCP2 (DCP2*, E148Q) (Wang et al. 2002; Chang et al. 2014) blocked decay induced by 4E-T Mid and resulted in the accumulation of the deadenylated reporter mRNA (Fig. 3A [lanes 7,9], B,C). Thus, the Mid region alone is able to trigger the decay of 4E-T-bound mRNAs through recruitment of CCR4–NOT. The reduction in reporter mRNA levels caused by the C-term was blocked in the presence of DCP2*. However, the C-term-bound mRNA was not deadenylated (Fig. 3A [lane 10], B).
Figure 3.
The Mid region of 4E-T promotes mRNA deadenylation. (A,B) HEK293T cells were transfected with plasmids coding for BGG-6xMS2bs, BGG-GAP, MS2-HA-GFP or MS2-HA-4E-T (WT or fragments) and F-Luc-GFP as a control or the catalytic inactive mutant of the decapping enzyme DCP2 (GFP-DCP2*). Northern blot analysis of representative RNA samples is shown in A. A0, deadenylated and An, polyadenylated reporter mRNAs. Quantification of reporter mRNA levels was performed as described in Figure 1C and is depicted in B (n = 3). (*) P < 0.05; (ns) not significant, paired t-test. (C) Expression levels of the proteins used in the tethering assay as analyzed by Western blotting.
Binding of 4E-T to eIF4E/4EHP inhibits decapping of deadenylated mRNA targets
In the context of full-length 4E-T, mRNA decay is blocked so that bound mRNAs are only deadenylated and not decapped and degraded by XRN1. In contrast, when in isolation, the 4E-T Mid region elicits the decay of a bound transcript. These observations indicate that the ability of 4E-T to protect an mRNA from decapping resides outside of its Mid region. As binding of cap-binding proteins to the cap protects the mRNA from 5′–3′ decay (Schwartz and Parker 2000), we addressed whether 4E-T interaction with eIF4E/4EHP regulates the stability of its target mRNAs. To this end, we generated 4E-T mutants carrying alanine substitutions in the canonical eIF4E-binding motif (C; Y30A, L35A) (Supplemental Table S1; Supplemental Fig. S6A; Dostie et al. 2000). These amino acid substitutions disrupted binding of 4E-T to eIF4E and 4EHP but not to NOT1, DDX6, PatL1, HA-UNR, or LSM14A (Fig. 4A; Supplemental Fig. S6B–D). 4E-T P-body localization was also independent of eIF4E and 4EHP binding (Supplemental Fig. S3L; Ferraiuolo et al. 2005; Kamenska et al. 2014).
Figure 4.

The 4E-T-eIF4E/4EHP interaction protects deadenylated mRNAs from degradation. (A) Streptavidin-based pull-down assays showing the association of V5-SBP-4E-T WT or canonical mutant (C) with eIF4E and NOT1. V5-SBP-MBP served as a negative control. The input (20% for the V5-SBP-tagged proteins and eIF4E, 2% for NOT1) and bound fractions (10% for the V5-SBP-tagged proteins and eIF4E, 40% for NOT1) were analyzed by Western blotting using the indicated antibodies. (B–D) Tethering assay using the plasmids coding for BGG-6xMS2bs, BGG-GAP, MS2-HA-GFP, or MS2-HA-4E-T (WT or the canonical eIF4E-binding motif mutant, C) in cells expressing F-Luc-GFP or mutant GFP-DCP2*. Northern blot analysis of representative RNA samples is shown in B. A0, deadenylated and An, polyadenylated reporter mRNAs. Quantification of mRNA levels was performed as described in Figure 1C and is represented in the graph depicted in C (n = 3). (*) P < 0.05, paired t-test. (D) Western blot analysis demonstrating the expression of the proteins used in the tethering assay.
We next examined whether 4E-T was still able to promote deadenylation and protect the BGG-6xMS2bs mRNA from decapping when impaired in eIF4E/4EHP binding. In contrast to wild-type protein, tethering of the 4E-T C mutant severely reduced the abundance of the BGG-6xMS2bs reporter (Fig. 4B [lane 3 vs. 2], C,D). The 4E-T C mutant induced 5′–3′ decay as indicated by the accumulation of deadenylated mRNA upon inhibition of decapping in cells coexpressing catalytically inactive DCP2 (DCP2*) (Fig. 4B [lanes 5,6], C,D). Thus, binding of 4E-T to eIF4E/4EHP blocks decapping of deadenylated mRNA. These observations also indicate that 4E-T can promote deadenylation and degradation of the reporter mRNA in the absence of an interaction with eIF4E/4EHP. This function is then mediated by 4E-T's Mid region.
CUP is a Drosophila-specific 4E-BP that promotes deadenylation and inhibits decapping of its target mRNAs. The mRNA protective function of CUP requires its noncanonical eIF4E-binding motif (Igreja and Izaurralde 2011). In contrast to CUP, the canonical motif of 4E-T is indispensable to protect the deadenylated mRNA from decay (Fig. 4B,C). To determine whether the noncanonical motif of 4E-T is also necessary to protect associated mRNAs from decapping, we introduced aspartate substitutions in two conserved tryptophans located C-terminal to the canonical motif (NC; W61D, W66D) (Supplemental Table S1; Supplemental Fig. S6A). The NC mutant had reduced binding to eIF4E (Supplemental Fig. S6E), indicating that human 4E-T also uses a bipartite binding mode to interact with the cap-binding protein. Moreover, the BGG-6xMS2 mRNA was degraded upon binding to the NC mutant of 4E-T (Supplemental Fig. S6F–H). We conclude that both the canonical and noncanonical eIF4E-binding motifs of human 4E-T are required to protect the deadenylated mRNA from degradation.
Distinct roles for the cap-binding proteins in the regulation of deadenylation and decapping by 4E-T
To understand which of the cap-binding proteins is used by 4E-T to inhibit mRNA decapping, we tethered 4E-T to the BGG-6xMS2 reporter in the absence of eIF4E or 4EHP. eIF4E partial depletion using short RNA (shRNA)-mediated knockdown (Supplemental Fig. S7A) increased the degradation of the 4E-T-bound reporter; however, relative to cells treated with a scramble (Scr) shRNA, the reduction in mRNA levels was not significant (P = 0.054) (Supplemental Fig. S7B,C). As complete depletion of the cap-binding protein results in decreased cellular viability, these results suggest that eIF4E binding most likely contributes to the protection of deadenylated transcripts associated with 4E-T. Moreover, since 4E-T may still associate with 4EHP in the absence of eIF4E, destabilization of the 4E-T-bound mRNA is less prominent than upon disruption of its interaction with both cap-binding proteins (eIF4E-binding mutants of 4E-T) (Fig. 4; Supplemental Fig. S6).
To address the importance of 4EHP binding, we generated a 4EHP-null HEK293T cell line using CRISPR-Cas9 gene editing (Supplemental Fig. S7D). 4EHP-null cells proliferated slower compared with control (Ctrl) cells but had no obvious changes in general translation, as assessed by polysome profiling analysis (Supplemental Fig. S7E). In the absence of 4EHP, tethered 4E-T was still able to deadenylate and protect the reporter mRNA from further degradation (Supplemental Fig. S7F,G). In contrast to eIF4E depletion, the 4E-T-bound mRNA had a heterogeneous poly(A) tail in 4EHP-null cells, with a large fraction of the mRNA remaining polyadenylated (Supplemental Fig. S7F, lane 4 vs. 2). This observation suggests that deadenylation of the 4E-T-bound mRNA is lessened in the absence of 4EHP.
Overall, these results indicate that 4E-T protects a bound and deadenylated mRNA from degradation when in the presence of eIF4E or 4EHP.
Involvement of 4E-T in TNRC6B-mediated mRNA repression
Our results indicate that 4E-T protects deadenylated and repressed mRNAs from degradation. As 4E-T contributes to miRNA-mediated gene silencing (Kamenska et al. 2014, 2016; Nishimura et al. 2015; Jafarnejad et al. 2018), we explored the possibility that 4E-T could influence the fate of deadenylated miRNA targets from decay to storage. Interestingly, the miRISC-associated TNRC6B protein regulates gene expression using a combination of translation repression, deadenylation, and mRNA degradation (Lazzaretti et al. 2009). In fact, upon tethering of TNRC6B to an R-Luc reporter, about 40% of the bound transcripts are not degraded and remain silenced in the deadenylated form (Lazzaretti et al. 2009). To investigate whether stabilization of the TNRC6B-bound reporter requires 4E-T, we tethered MS2-HA-TNRC6B to the BGG-6xMS2bs reporter in the presence or absence of 4E-T. Relative to MS2-HA-GFP, 50% of the BGG-6xMS2bs reporter was degraded upon TNRC6B binding (Fig. 5A,B). As observed before, a fraction of the transcripts also accumulated in the deadenylated form in cells expressing TNRC6B (Fig. 5A [lanes 2 vs. 1], B). shRNA-mediated knockdown (KD) led to a pronounced decrease of 4E-T protein levels without affecting MS2-HA-TNRC6B expression (Fig. 5C). 4E-T depletion compromised the accumulation of the deadenylated TNRC6B-bound reporter, which was then mostly degraded (Fig. 5A [lane 4], B). The levels of the deadenylated TNRC6B-bound reporter were restored upon coexpression of a V5-SBP-tagged and shRNA-resistant version of 4E-T (Fig. 5A [lane 6], B,C).
Figure 5.
Inhibition of decapping by 4E-T participates in TNRC6B-mediated mRNA repression. (A,B) Tethering of MS2-HA-TNRC6B or MS2-HA-GFP to the BGG-6xMS2bs reporter in control, scramble (Scr) shRNA, and 4E-T-depleted (4E-T shRNA) HEK293T cells expressing V5-SBP-MBP or shRNA resistant V5-SBP-4E-T. BGG-GAP was used as a transfection control. A representative Northern blot analysis is shown in A. (A0) Deadenylated reporter mRNAs; (An) polyadenylated reporter mRNAs. (B) BGG-6xMS2bs mRNA levels were normalized to that of BGG-GAP and set to 1 in the experimental conditions using MS2-HA-GFP. Mean values ± SD are shown (n = 3). (*) P < 0.05, paired t-test. (C) Western blot analysis showing the expression of V5-SBP-MBP, V5-SBP-4E-T, MS2-HA-GFP, and MS2-HA-TNRC6B proteins used in the assay described in A. (Top panel) The samples were also analyzed with anti-4E-T antibodies to show the decrease in endogenous 4E-T expression upon shRNA-mediated depletion. (Bottom panel) TUBULIN was used as a loading control. (D,E) Scramble shRNA and 4E-T shRNA-treated cells were treated with actinomycin D (ActD) and harvested at the indicated time points. (D) RNA samples were analyzed by Northern blotting. The same membrane was incubated with 32P-labeled probes specific for the BGG mRNA and the 18S rRNA. Band intensities were quantified by PhosphorImager. (E) BGG-6xMS2bs mRNA levels were normalized to that of 18S rRNA and set to 1 for time point zero. The values at the remaining time points were calculated relative to time point zero and plotted as a function of time after ActD addition. Error bars represent the SD from three independent experiments. The half-lives of the BGG-6xMS2bs mRNA in the different experimental conditions are shown below the Northern blot panels and are represented as the mean ± SD.
To determine the decay rate of the reporter bound to TNRC6B in the presence and absence of 4E-T, we blocked transcription with actinomycin D. Reporter mRNA levels were determined in Scr shRNA and 4E-T shRNA-treated cells at different time points upon actinomycin D addition. We observed that BGG-6xMS2bs mRNA was destabilized in the absence of 4E-T. The half-life of the reporter mRNA decreased to 1.8 h ± 0.18 h in 4E-T-depleted cells compared with 5.1 h ± 1.5 h in Scr shRNA-treated cells (Fig. 5D,E). Moreover, the stability of the BGG-6xMS2bs reporter bound to TNRC6B was restored to 4.9 h ± 1.7 h upon re-expression of V5-SBP-4E-T (Fig. 5D,E).
Collectively, these data support the role of 4E-T in protecting TNRC6B-targeted mRNAs from decapping and further decay.
4E-T overexpression blocks decay of transcripts destabilized by TTP and NOT1
To broaden its role as a decapping inhibitory factor, we addressed the consequences of 4E-T overexpression in human cells, a condition that could mimic the localized and enriched presence of the protein in P-bodies (Hubstenberger et al. 2017) or the high expression levels observed in oocytes (Villaescusa et al. 2006; Minshall et al. 2007). In this context, we investigated steady-state levels of a β-GLOBIN (BGG) reporter mRNA targeted to degradation by TTP due to the presence of the FOS AU-rich element (ARE) in the 3′ UTR (BGG-ARE) (Fig. 6A; Ferraiuolo et al. 2005). Overexpression of TTP in HEK293T cells resulted in a reduction of the BBG-ARE mRNA levels to 50% relative to cells expressing MBP (Fig. 6B [lane 3 vs. 1], C). Coexpression of 4E-T inhibited TTP-mediated decay of the BGG-ARE reporter, which accumulated as a deadenylated decay intermediate (Fig. 6B [lane 4], C,D). These results are consistent with a role of 4E-T in blocking deadenylation-dependent mRNA decapping. In control cells, the abundance of the polyadenylated BGG-ARE reporter increased in the presence of 4E-T (Fig. 6B [lanes 2 vs. 1], C), indicating that 4E-T also inhibits TTP-independent degradation of an mRNA destabilized by the FOS ARE.
Figure 6.

Overexpression of 4E-T blocks deadenylation-dependent decapping. (A) Schematic representation of the BGG-ARE reporter used in this study. A copy of the ARE sequence present in the 3′ UTR of the FOS mRNA was cloned downstream from the β-GLOBIN ORF. The FOS ARE is recognized by tristetraprolin (TTP) to promote target mRNA decay upon recruitment of the CCR4–NOT complex (Fabian et al. 2013; Bulbrook et al. 2018). (B,C) HEK293T cells were transfected with the BGG-ARE reporter and plasmids expressing λN-HA-MBP or λN-HA-TTP, V5-SBP-MBP, or V5-SBP-4E-T. The BGG-GAP reporter served as a transfection and normalization control. A representative Northern blot is shown in B. (A0) Deadenylated reporter mRNAs; (An) polyadenylated reporter mRNAs. (C) BGG-ARE mRNA levels were normalized to those of the BGG-GAP and set to1 in cells expressing λN-HA-MBP. Mean values ± SD are shown (n = 3). (*) P < 0.05, paired t-test. (D) Expression levels of the proteins used in B and C analyzed by Western blotting. TUBULIN served as a loading control. (E,F) Tethering of MS2-HA-NOT1 or MS2-HA-GFP to the BGG-6xMS2bs reporter in cells overexpressing V5-SBP-4E-T or V5-SBP-MBP. BGG-GAP was used as a transfection control. A representative Northern blot analysis is shown in E. A0, deadenylated and An, polyadenylated reporter mRNAs. (F) BGG-6xMS2bs mRNA levels were normalized to those of BGG-GAP and set to 1 in the experimental condition using MS2-HA-GFP and V5-SBP-MBP. Mean values ± SD are shown (n = 3). (*) P < 0.05; (ns) not significant, paired t-test. (G) The expression levels of the proteins used in E and F were verified using Western blotting. TUBULIN served as a loading control. Proteins were detected using anti-HA, anti-4E-T, anti-V5, and anti-TUBULIN antibodies.
We also tested the effect of 4E-T overexpression on the decay of mRNAs directly targeted by the CCR4–NOT complex. Therefore, we tethered MS2-HA-NOT1 to the BGG-6xMS2bs reporter in the presence or absence of V5-SBP-4E-T. Relative to MS2-HA-GFP, this reporter is degraded to 30% when bound by NOT1 (Fig. 6E [lanes 3 vs. 1], F,G). Overexpression of 4E-T blocked NOT1-dependent decapping and the deadenylated reporter accumulated in cells (Fig. 6F,G, lanes 4 vs. 3).
In conclusion, our data supports the notion that 4E-T, in complex with eIF4E or 4EHP, stabilizes deadenylated mRNAs by interfering with decapping.
Discussion
Just as germ cell granules, neuronal granules or stress granules, P-bodies coordinate the storage of translationally inactive mRNAs in the cell cytoplasm (Bhattacharyya et al. 2006; Voronina et al. 2011; Hutten et al. 2014; Hubstenberger et al. 2017; Schütz et al. 2017; Ivanov et al. 2019). In this study, we describe 4E-T, an essential P-body component and a 4E-BP, as a regulator of mRNA storage. 4E-T-bound mRNAs are translationally repressed, deadenylated, and protected from decapping-dependent decay. We show that regulation of deadenylation and decapping by 4E-T relies on specific protein partners. mRNA deadenylation is a consequence of the interaction of 4E-T's Mid region with the CCR4–NOT complex, whereas inhibition of decapping and subsequent degradation requires interaction with the cap-binding proteins eIF4E/4EHP. Our data also highlights that posttranscriptional regulation by 4E-T is of significance in the context of mRNAs targeted by the miRNA effector TNRC6B.
4E-T is a binding platform for multiple RNA-associated factors
The human 4E-T protein is a large disordered protein with multiple low-complexity regions that confer binding to translation, deadenylation, and decapping factors (Kamenska et al. 2016). Here, we reveal that in addition to the binding sites identified for eIF4E, UNR, DDX6, and LSM14A (Dostie et al. 2000; Ozgur et al. 2015; Kamenska et al. 2016; Brandmann et al. 2018), other short linear motifs (SLIMs) present in 4E-T convene independent binding to PatL1 and possibly to the CCR4–NOT complex as well. A conserved sequence motif in the C-term and previously known to be important but not essential for the localization of 4E-T to P-bodies (Kamenska et al. 2014) mediates the interaction with PatL1 (Fig. 2A; Supplemental Fig. S2). On the other hand, the interaction of 4E-T with the CCR4–NOT is confined to the Mid region of the protein (Fig. 2). Attempts to narrow down and identify the SLIMs involved in CCR4–NOT interaction within this region were unsuccessful, as multiple sequences seemed to be required (data not shown). Further work on the architecture of 4E-T complexes will be necessary to determine whether these protein interactions occur simultaneously or consecutively, and their role in cells.
One important finding in our studies is that the interaction with the CCR4–NOT complex induces deadenylation of the bound mRNA and accounts for one of the eIF4E-independent mechanisms involved in 4E-T mediated mRNA repression (Kamenska et al. 2014). As 4E-T participates in posttranscriptional events regulated by miRNAs and AU-rich element binding proteins (Ferraiuolo et al. 2005; Kamenska et al. 2014; Nishimura et al. 2015; Chapat et al. 2017), its interaction with the deadenylase complex most likely contributes to and/or sustains the repressed state of the targeted transcript.
4E-T blocks decapping by binding eIF4E/4EHP
Another important observation in this work is that, similar to the fly-specific 4E-BP CUP (Igreja and Izaurralde 2011), interaction of 4E-T with eIF4E/4EHP protects the deadenylated target mRNA from decapping-dependent decay. In the absence of eIF4E and 4EHP-binding, mRNA deadenylation promoted by the Mid region causes the decay of the 4E-T-bound mRNA (Fig. 4). Although the mechanism is still unclear, inhibition of decapping by 4E-T could be achieved by increasing eIF4E or 4EHP affinity for the cap structure. The direct interaction between 4E-T and 4EHP enhances binding to the cap structure and is a requisite for competition with the eIF4F complex during repression of translation initiation by miRNAs (Chapat et al. 2017). Similarly, binding of CUP to eIF4E increases the affinity of the latter to the cap (Kinkelin et al. 2012) and contributes to the translational regulation of localized mRNAs during early Drosophila development (Wilhelm et al. 2003; Nakamura et al. 2004; Zappavigna et al. 2004).
Additional mechanisms used to block decapping could involve the competition of 4E-T with unknown proteins that facilitate eIF4E or 4EHP dissociation from the cap structure. Similar to eIF4G, direct or indirect RNA-binding activity of 4E-T could also play a role in anchoring eIF4E or 4EHP to the mRNA and increase their association with the cap structure (Yanagiya et al. 2009).
Independent of the mechanism that prevents decapping, the interaction of 4E-T with eIF4E or 4EHP could be subject to regulation by posttranslational modifications or binding partners, so that 4E-T-bound mRNAs can be either stored in a repressed and deadenylated form in P-bodies or fully degraded depending on their sequence and binding proteins. For example, 4E-T-associated transcripts targeted by miRNAs and 4EHP (Chapat et al. 2017) or TNRC6B (this work) undergo translational repression while mRNAs targeted by 4E-T and the CCR4–NOT are degraded (Nishimura et al. 2015).
We also observed that cellular depletion of each cap-binding protein has distinct effects on the 4E-T-bound mRNA. Reduction of eIF4E expression appeared to sensitize the mRNA to further degradation. In contrast, 4EHP loss affected the initiation of deadenylation by 4E-T. The molecular details associated with these differences remain unclear but may be associated with distinct composition of the 4E-T mRNA–protein complexes.
4E-T as a coordinator of P-body-associated mRNA repression
The molecular mechanisms underlying selective translational regulation in P-bodies remain largely unknown. 4E-T is an essential P-body component (Andrei et al. 2005; Ferraiuolo et al. 2005) and thus emerges as an important regulator of the expression of transcripts present in these RNA granules. As a binding platform for various proteins, 4E-T coordinates the repression and protection of P-body-associated mRNAs. The interaction of 4E-T with eIF4E or 4EHP brings the cap binding proteins into P-bodies (Ferraiuolo et al. 2005; Kubacka et al. 2013), promotes translational repression (Ferraiuolo et al. 2005; Kamenska et al. 2014) and prevents decapping (this study). Moreover, 4E-T association with CCR4–NOT likely sustains the deadenylated and repressed state of the mRNA (this work and Waghray et al. 2015). Interestingly, P-bodies contain deadenylases, lack PABP, and associated mRNAs have been suggested to contain either a heterogeneous or no poly(A) tail (Cougot et al. 2004; Andrei et al. 2005; Kedersha et al. 2005; Aizer et al. 2014; Hubstenberger et al. 2017). Additionally, the interaction of 4E-T with DDX6 is also required for P-body assembly and translational repression (Kamenska et al. 2016).
4E-T localization to P-bodies is also subject to regulation. Our study highlights that binding to LSM14A is important for the recruitment of 4E-T, and consequently of eIF4E, 4EHP, and associated mRNAs, to P-bodies (Supplemental Fig. S3). Arginine methylation controls LSM14A recruitment to P-bodies (Matsumoto et al. 2012) and thus it may regulate the presence of 4E-T in these cytoplasmic RNA granules. 4E-T itself is posttranslationally modified by phosphorylation under arsenite-induced oxidative stress. In these conditions, P-body size increases (Cargnello et al. 2012). Interestingly, the majority of the phospho-regulated sites are in the Mid region of the protein that is responsible for the interaction with CCR4–NOT. The significance of these regulatory events to the function of 4E-T-containing mRNPs or to the dynamic nature of P-bodies remains uncharacterized.
4E-T driven mRNA storage in germinal and neuronal granules
The role of 4E-T in the specification of alternative fates for bound mRNAs (i.e., storage in a deadenylated, repressed form for later use, or complete degradation) has important biological implications. Spatial and temporal control of mRNA translation is a common posttranscriptional mechanism operating in oocytes, eggs, and early embryos of many organisms and in neuronal cells (Martin and Ephrussi 2009; Jung et al. 2014; Formicola et al. 2019). Interestingly, 4E-T is a component of germinal and neuronal granules and regulates oocyte and neuronal development via poorly understood mechanisms (Villaescusa et al. 2006; Minshall et al. 2007; Kasippillai et al. 2013; Yang et al. 2014; Amadei et al. 2015; Pfender et al. 2015; Zahr et al. 2018; Zhao et al. 2019). In somatic cells, 4E-T has also documented roles in miRNA-mediated gene silencing and in the control of the expression of AU-rich mRNAs (Kamenska et al. 2014; Nishimura et al. 2015; Chapat et al. 2017). Thus, the identification of the target mRNAs and mechanisms used and governing 4E-T function in cells will advance our knowledge on the control of gene expression in fundamental developmental processes.
Furthermore, the current view that cytoplasmic granules such as P-bodies are reservoirs of repressed transcripts opens the possibility that, according to the cellular needs or developmental stage, specific mRNAs can be mobilized into the translating pool. Reactivation of silenced transcripts has been described during oocyte maturation, early embryonic development, mitotic cell cycle progression, in neurons, following stress relief or associated to the rhythmic expression of clock-controlled genes (Vassalli et al. 1989; Simon et al. 1992; Gebauer et al. 1994; Wu et al. 1998; Oh et al. 2000; Bhattacharyya et al. 2006; Novoa et al. 2010; Carbonaro et al. 2011; Kojima et al. 2012, Udagawa et al. 2012; Aizer et al. 2014). The mechanisms involved in translational activation following P-body storage most likely require remodeling and processing (e.g., polyadenylation) of the repressed mRNA. The repressor machinery must be replaced by factors that promote translation and polyadenylation of the stored transcripts. 4E-T, again, can play a crucial role in this mechanism, as regulation of the interactions with its protein partners might control the assembly and disassembly of the repressor complex, such as evidenced by the decay of the 4E-T-bound mRNA in the absence of interaction with eIF4E and 4EHP. This topic merits further investigation as multiple and redundant interactions (RNA–protein and protein–protein) operate in the control of gene expression in P-bodies.
Overall, our findings highlight 4E-T as a coordinator of mRNA turnover. As a binding platform for cap-binding proteins, the CCR4–NOT deadenylase complex and decapping factors, 4E-T guarantees that silenced mRNAs are protected from degradation in cytoplasmic granules associated with germline and neuronal development.
Materials and methods
DNA constructs
The DNA constructs used in this study are described in the Supplemental Material and listed in Supplemental Table S1. All constructs and mutations were confirmed by sequencing.
Tethering assays in human cells
All the conditions used in the tethering assays are described in the Supplemental Material.
Knockdown and complementation assays
HEK293T cells (0.8 × 106 cells per well) were transfected 24 h after seeding in six-well plates with 3 μg of plasmid expressing scramble (control), 4E-T, or eIF4E shRNA using Lipofectamine 2000 (Invitrogen). Forty-eight hours after transfection, the cells were transfected a second time. In the TNRC6B tethering assay, the transfection mixtures contained 0.5 μg of BGG-6xMS2bs, 0.5 μg of BGG-GAP, 0.4 μg of MS2-HA-TNRC6B, 0.15 μg of V5-SBP-MBP or 0.3 μg of V5-SBP-4E-T (shRNA resistant), and 1 μg of vector expressing 4E-T shRNA. Following eIF4E depletion with 1 μg of the respective shRNA, cells were transfected with 0.5 μg of BGG-6xMS2bs, 0.5 μg of BGG-GAP, 0.4 μg of MS2-HA-4E-T or MS2-HA-GFP, and 1 μg of eIF4E shRNA. Total RNA was isolated with TRIzol (Thermo Scientific) and analyzed as described above.
Half-life experiments
To determine reporter mRNA decay rates, cells were treated with actinomycin D (10 µg/mL final concentration) 24 h after transfection and collected at the indicated time points. mRNA levels determined by Northern blotting were normalized to the levels of 18S rRNA. To determine the half-lives (t1/2 = 50% of remaining mRNA) indicated below the panels in the figures, the normalized BGG-6xMS2bs mRNA levels were set to 1 at time point zero and plotted against time.
RNase H digestion
Ten micrograms of RNA were incubated with 3 μL of 5 U/µL RNase H (New England Biolabs) and 6 μM oligo(dT) 15-mer in 100 μL of H2O for 1 h at 37°C. The RNase H-treated RNA was purified by phenol-chloroform extraction and analyzed by Northern blotting.
Terminator assay
Ten micrograms of RNA treated with 1 μL of Terminator 5′-phosphate-dependent exonuclease 1 U/μL (Epicentre) in 20 μL of H2O for 1 h at 37°C was purified by phenol-chloroform extraction and analyzed via Northern blotting.
Coimmunoprecipitation (co-IP) and pull-down assays
The conditions used in the co-IP and pull-down assays are described in the Supplemental Material. All antibodies used in the co-IP and pull-down assays are listed in Supplemental Table S2.
Immunofluorescence
Immunofluorescence was performed as described in Lazzaretti et al. (2009). Details are included in the Supplemental Material.
Generation of the 4EHP-null cell line
Clonal cell lines were obtained and confirmed for gene editing as described previously (Peter et al. 2017). Details are described in the Supplemental Material.
Polysome profiling
Polysome profiles for HEK293T wild-type and 4EHP-null cells were performed as described before (Kuzuoğlu-Öztürk et al. 2016).
Statistics
Experiments were done in triplicates and all data is reported as the mean ± the standard deviation (SD) represented as error bars. Statistical analyses were performed with a two-tailed paired Student's t-test. P-values < 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We dedicate this manuscript to the memory of Elisa Izaurralde, who passed away before the conclusion of this work. All experiments were conceived and performed in her laboratory. We are thankful to Praveen Bawankar, Heike Budde, and Lara Wohlbold for cloning. We also thank Daniel Peter and Eugene Valkov for helpful suggestions on the manuscript. This work was supported by the Max Planck Society.
Author contributions: F.R. performed most of the experiments and generated several of the constructs. R.W. generated the 4EHP-null cell line and performed the polysome profiling experiment. E.I. was the principal investigator. C.I. coordinated the project. F.R. and C.I. wrote the manuscript.
Footnotes
Supplemental material is available for this article.
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.336073.119.
Freely available online through the Genes & Development Open Access option.
References
- Aizer A, Kalo A, Kafri P, Shraga A, Ben-Yishay R, Jacob A, Kinor N, Shav-Tal Y. 2014. Quantifying mRNA targeting to P-bodies in living human cells reveals their dual role in mRNA decay and storage. J Cell Sci 127: 4443–4456. 10.1242/jcs.152975 [DOI] [PubMed] [Google Scholar]
- Amadei G, Zander MA, Yang G, Dumelie JG, Vessey JP, Lipshitz HD, Smibert CA, Kaplan DR, Miller FD. 2015. A Smaug2-Based translational repression complex determines the balance between precursor maintenance versus differentiation during mammalian neurogenesis. J Neurosci 35: 15666–15681. 10.1523/JNEUROSCI.2172-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrei MA, Ingelfinger D, Heintzmann R, Achsel T, Rivera-Pomar R, Lührmann R. 2005. A role for eIF4E and eIF4E-transporter in targeting mRNPs to mammalian processing bodies. RNA 11: 717–727. 10.1261/rna.2340405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar V, Roudko V, Basquin J, Sharma K, Urlaub H, Séraphin B, Conti E. 2013. Structure and RNA-binding properties of the Not1-Not2-Not5 module of the yeast CCR4–NOT complex. Nat Struct Mol Biol 20: 1281–1288. 10.1038/nsmb.2686 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. 2006. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell 125: 1111–1124. 10.1016/j.cell.2006.04.031 [DOI] [PubMed] [Google Scholar]
- Boland A, Chen Y, Raisch T, Jonas S, Kuzuoğlu-Öztürk D, Wohlbold L, Weichenrieder O, Izaurralde E. 2013. Structure and assembly of the NOT module of the human CCR4–NOT complex. Nat Struct Mol Biol 20: 1289–1297. 10.1038/nsmb.2681 [DOI] [PubMed] [Google Scholar]
- Brandmann T, Fakim H, Padamsi Z, Youn JY, Gingras AC, Fabian MR, Jinek M. 2018. Molecular architecture of LSM14 interactions involved in the assembly of mRNA silencing complexes. EMBO J 37: e97869 10.15252/embj.201797869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JE, Truffault V, Boland A, Huntzinger E, Chang CT, Haas G, Weichenrieder O, Coles M, Izaurralde E. 2012. A direct interaction between DCP1 and XRN1 couples mRNA decapping to 5′ exonucleolytic degradation. Nat Struct Mol Biol 19: 1324–1331. 10.1038/nsmb.2413 [DOI] [PubMed] [Google Scholar]
- Bulbrook D, Brazier H, Mahajan P, Kliszczak M, Fedorov O, Marchese FP, Aubareda A, Chalk R, Picaud S, Strain-Damerell C, et al. 2018. Tryptophan-mediated interactions between tristetraprolin and the CNOT9 subunit are required for CCR4–NOT deadenylase complex recruitment. J Mol Biol 430: 722–736. 10.1016/j.jmb.2017.12.018 [DOI] [PubMed] [Google Scholar]
- Carbonaro M, O'Brate A, Giannakakou P. 2011. Microtubule disruption targets HIF-1α mRNA to cytoplasmic P-bodies for translational repression. J Cell Biol 192: 83–99. 10.1083/jcb.201004145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargnello M, Tcherkezian J, Dorn JF, Huttlin EL, Maddox PS, Gygi SP, Roux PP. 2012. Phosphorylation of the eukaryotic translation initiation factor 4E-transporter (4E-T) by c-Jun N-terminal kinase promotes stress-dependent P-body assembly. Mol Cell Biol 32: 4572–4584. 10.1128/MCB.00544-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CT, Bercovich N, Loh B, Jonas S, Izaurralde E. 2014. The activation of the decapping enzyme DCP2 by DCP1 occurs on the EDC4 scaffold and involves a conserved loop in DCP1. Nucleic Acids Res 42: 5217–5233. 10.1093/nar/gku129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapat C, Jafarnejad SM, Matta-Camacho E, Hesketh GG, Gelbart IA, Attig J, Gkogkas CG, Alain T, Stern-Ginossar N, Fabian MR, et al. 2017. Cap-binding protein 4EHP effects translation silencing by microRNAs. Proc Natl Acad Sci 114: 5425–5430. 10.1073/pnas.1701488114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougot N, Babajko S, Séraphin B. 2004. Cytoplasmic foci are sites of mRNA decay in human cells. J Cell Biol 165: 31–40. 10.1083/jcb.200309008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostie J, Ferraiuolo M, Pause A, Adam SA, Sonenberg N. 2000. A novel shuttling protein, 4E-T, mediates the nuclear import of the mRNA 5′ cap-binding protein, eIF4E. EMBO J 19: 3142–3156. 10.1093/emboj/19.12.3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian MR, Frank F, Rouya C, Siddiqui N, Lai WS, Karetnikov A, Blackshear PJ, Nagar B, Sonenberg N. 2013. Structural basis for the recruitment of the human CCR4–NOT deadenylase complex by tristetraprolin. Nat Struct Mol Biol 20: 735–739. 10.1038/nsmb.2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraiuolo MA, Basak S, Dostie J, Murray EL, Schoenberg DR, Sonenberg N. 2005. A role for the eIF4E-binding protein 4E-T in P-body formation and mRNA decay. J Cell Biol 170: 913–924. 10.1083/jcb.200504039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formicola N, Vijayakumar J, Besse F. 2019. Neuronal ribonucleoprotein granules: dynamic sensors of localized signals. Traffic 20: 639–649. [DOI] [PubMed] [Google Scholar]
- Franks TM, Lykke-Andersen J. 2008. The control of mRNA decapping and P-body formation. Mol Cell 32: 605–615. 10.1016/j.molcel.2008.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer F, Xu W, Cooper GM, Richter JD. 1994. Translational control by cytoplasmic polyadenylation of c-mos mRNA is necessary for oocyte maturation in the mouse. EMBO J 13: 5712–5720. 10.1002/j.1460-2075.1994.tb06909.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüner S, Peter D, Weber R, Wohlbold L, Chung MY, Weichenrieder O, Valkov E, Igreja C, Izaurralde E. 2016. The structures of eIF4E-eIF4G complexes reveal an extended interface to regulate translation initiation. Mol Cell 64: 467–479. 10.1016/j.molcel.2016.09.020 [DOI] [PubMed] [Google Scholar]
- Grüner S, Weber R, Peter D, Chung MY, Igreja C, Valkov E, Izaurralde E. 2018. Structural motifs in eIF4G and 4E-BPs modulate their binding to eIF4E to regulate translation initiation in yeast. Nucleic Acids Res 46: 6893–6908. 10.1093/nar/gky542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighat A, Mader S, Pause A, Sonenberg N. 1995. Repression of cap-dependent translation by 4E-binding protein 1: competition with p220 for binding to eukaryotic initiation factor-4E. EMBO J 14: 5701–5709. 10.1002/j.1460-2075.1995.tb00257.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanet A, Räsch F, Weber R, Ruscica V, Fauser M, Raisch T, Kuzuoğlu-Öztürk D, Chang CT, Bhandari D, Igreja C, et al. 2019. HELZ directly interacts with CCR4–NOT and causes decay of bound mRNAs. Life Sci Alliance 2: e201900405 10.26508/lsa.201900405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem Y, Frank J. 2018. The jigsaw puzzle of mRNA translation initiation in eukaryotes: a decade of structures unraveling the mechanics of the process. Annu Rev Biophyss 47: 125–151. 10.1146/annurev-biophys-070816-034034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubstenberger A, Courel M, Bénard M, Souquere S, Ernoult-Lange M, Chouaib R, Yi Z, Morlot JB, Munier A, Fradet M, et al. 2017. P-Body purification reveals the condensation of repressed mRNA regulons. Mol Cell 68: 144–157.e5. 10.1016/j.molcel.2017.09.003 [DOI] [PubMed] [Google Scholar]
- Hutten S, Sharangdhar T, Kiebler M. 2014. Unmasking the messenger. RNA Biol 11: 992–997. 10.4161/rna.32091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igreja C, Izaurralde E. 2011. CUP promotes deadenylation and inhibits decapping of mRNA targets. Genes Dev 25: 1955–1967. 10.1101/gad.17136311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov P, Kedersha N, Anderson P. 2019. Stress granules and processing bodies in translational control. Cold Spring Harb Perspect Biol 11: a032813 10.1101/cshperspect.a032813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafarnejad SM, Chapat C, Matta-Camacho E, Gelbart IA, Hesketh GG, Arguello M, Garzia A, Kim SH, Attig J, Shapiro M, et al. 2018. Translational control of ERK signaling through miRNA/4EHP-directed silencing. Elife 7: e35034 10.7554/eLife.35034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Gkogkas CG, Sonenberg N, Holt CE. 2014. Remote control of gene function by local translation. Cell 157: 26–40. 10.1016/j.cell.2014.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenska A, Lu WT, Kubacka D, Broomhead H, Minshall N, Bushell M, Standart N. 2014. Human 4E-T represses translation of bound mRNAs and enhances microRNA-mediated silencing. Nucleic Acids Res 42: 3298–3313. 10.1093/nar/gkt1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenska A, Simpson C, Vindry C, Broomhead H, Bénard M, Ernoult-Lange M, Lee BP, Harries LW, Weil D, Standart N. 2016. The DDX6-4E-T interaction mediates translational repression and P-body assembly. Nucleic Acids Res 44: 6318–6334. 10.1093/nar/gkw565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasippillai T, MacArthur DG, Kirby A, Thomas B, Lambalk CB, Daly MJ, Welt CK. 2013. Mutations in eIF4ENIF1 are associated with primary ovarian insufficiency. J Clin Endocrinol Metab 98: E1534–E1539. 10.1210/jc.2013-1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Anderson P. 2007. Mammalian stress granules and processing bodies. Methods Enzymol 431: 61–81. 10.1016/S0076-6879(07)31005-7 [DOI] [PubMed] [Google Scholar]
- Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fritzler MJ, Scheuner D, Kaufman RJ, Golan DE, Anderson P. 2005. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol 169: 871–884. 10.1083/jcb.200502088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkelin K, Veith K, Grunwald M, Bono F. 2012. Crystal structure of a minimal eIF4E-Cup complex reveals a general mechanism of eIF4E regulation in translational repression. RNA 18: 1624–1634. 10.1261/rna.033639.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Sher-Chen EL, Green CB. 2012. Circadian control of mRNA polyadenylation dynamics regulates rhythmic protein expression. Genes Dev 26: 2724–2736. 10.1101/gad.208306.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubacka D, Kamenska A, Broomhead H, Minshall N, Darzynkiewicz E, Standart N. 2013. Investigating the consequences of eIF4E2 (4EHP) interaction with 4E-transporter on its cellular distribution in HeLa cells. PLoS One 8: e72761 10.1371/journal.pone.0072761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzuoğlu-Öztürk D, Bhandari D, Huntzinger E, Fauser M, Helms S, Izaurralde E. 2016. miRISC and the CCR4–NOT complex silence mRNA targets independently of 43S ribosomal scanning. EMBO J 35: 1186–1203. 10.15252/embj.201592901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaretti D, Tournier I, Izaurralde E. 2009. The C-terminal domains of human TNRC6A, TNRC6B, and TNRC6C silence bound transcripts independently of Argonaute proteins. RNA 15: 1059–1066. 10.1261/rna.1606309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykke-Andersen J, Shu MD, Steitz JA. 2000. Human Upf proteins target an mRNA for nonsense-mediated decay when bound downstream of a termination codon. Cell 103: 1121–1131. 10.1016/S0092-8674(00)00214-2 [DOI] [PubMed] [Google Scholar]
- Mader S, Lee H, Pause A, Sonenberg N. 1995. The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-4 gamma and the translational repressors 4E-binding proteins. Mol Cell Biol 15: 4990–4997. 10.1128/MCB.15.9.4990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KC, Ephrussi A. 2009. mRNA localization: gene expression in the spatial dimension. Cell 136: 719–730. 10.1016/j.cell.2009.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Nakayama H, Yoshimura M, Masuda A, Dohmae N, Matsumoto S, Tsujimoto M. 2012. PRMT1 is required for RAP55 to localize to processing bodies. RNA Biol 9: 610–623. 10.4161/rna.19527 [DOI] [PubMed] [Google Scholar]
- Merrick WC, Pavitt GD. 2018. Protein synthesis initiation in eukaryotic cells. Cold Spring Harb Perspect Biol 10: a033092 10.1101/cshperspect.a033092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshall N, Reiter MH, Weil D, Standart N. 2007. CPEB interacts with an ovary-specific eIF4E and 4E-T in early Xenopus oocytes. J Biol Chem 282: 37389–37401. 10.1074/jbc.M704629200 [DOI] [PubMed] [Google Scholar]
- Nakamura A, Sato K, Hanyu-Nakamura K. 2004. Drosophila cup is an eIF4E binding protein that associates with Bruno and regulates oskar mRNA translation in oogenesis. Dev Cell 6: 69–78. 10.1016/S1534-5807(03)00400-3 [DOI] [PubMed] [Google Scholar]
- Nishimura T, Padamsi Z, Fakim H, Milette S, Dunham WH, Gingras AC, Fabian MR. 2015. The eIF4E-Binding protein 4E-T is a component of the mRNA decay machinery that bridges the 5′ and 3′ Termini of Target mRNAs. Cell Rep 11: 1425–1436. 10.1016/j.celrep.2015.04.065 [DOI] [PubMed] [Google Scholar]
- Novoa I, Gallego J, Ferreira PG, Mendez R. 2010. Mitotic cell-cycle progression is regulated by CPEB1 and CPEB4-dependent translational control. Nat Cell Biol 12: 447–456. 10.1038/ncb2046 [DOI] [PubMed] [Google Scholar]
- Oh B, Hwang S, McLaughlin J, Solter D, Knowles BB. 2000. Timely translation during the mouse oocyte-to-embryo transition. Development 127: 3795–3803. [DOI] [PubMed] [Google Scholar]
- Ozgur S, Basquin J, Kamenska A, Filipowicz W, Standart N, Conti E. 2015. Structure of a human 4E-T/DDX6/CNOT1 complex reveals the different interplay of DDX6-binding proteins with the CCR4–NOT complex. Cell Rep 13: 703–711. 10.1016/j.celrep.2015.09.033 [DOI] [PubMed] [Google Scholar]
- Peter D, Igreja C, Weber R, Wohlbold L, Weiler C, Ebertsch L, Weichenrieder O, Izaurralde E. 2015. Molecular architecture of 4E-BP translational inhibitors bound to eIF4E. Mol Cell 57: 1074–1087. 10.1016/j.molcel.2015.01.017 [DOI] [PubMed] [Google Scholar]
- Peter D, Weber R, Sandmeir F, Wohlbold L, Helms S, Bawankar P, Valkov E, Igreja C, Izaurralde E. 2017. GIGYF1/2 proteins use auxiliary sequences to selectively bind to 4EHP and repress target mRNA expression. Genes Dev 31: 1147–1161. 10.1101/gad.299420.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfender S, Kuznetsov V, Pasternak M, Tischer T, Santhanam B, Schuh M. 2015. Live imaging RNAi screen reveals genes essential for meiosis in mammalian oocytes. Nature 524: 239–242. 10.1038/nature14568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao X, Zhang X, Wu L, Belasco JG. 2010. CCR4–NOT deadenylates mRNA associated with RNA-induced silencing complexes in human cells. Mol Cell Biol 30: 1486–1494. 10.1128/MCB.01481-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai RS, Artus CG, Filipowicz W. 2004. Tethering of human Ago proteins to mRNA mimics the miRNA-mediated repression of protein synthesis. RNA 10: 1518–1525. 10.1261/rna.7131604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisch T, Chang CT, Levdansky Y, Muthukumar S, Raunser S, Valkov E. 2019. Reconstitution of recombinant human CCR4–NOT reveals molecular insights into regulated deadenylation. Nat Commun 10: 3173 10.1038/s41467-019-11094-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütz S, Nöldeke ER, Sprangers R. 2017. A synergistic network of interactions promotes the formation of in vitro processing bodies and protects mRNA against decapping. Nucleic Acids Res 45: 6911–6922. 10.1093/nar/gkx353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DC, Parker R. 2000. mRNA decapping in yeast requires dissociation of the cap binding protein, eukaryotic translation initiation factor 4E. Mol Cell Biol 20: 7933–7942. 10.1128/MCB.20.21.7933-7942.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R, Tassan JP, Richter JD. 1992. Translational control by poly(A) elongation during Xenopus development: differential repression and enhancement by a novel cytoplasmic polyadenylation element. Genes Dev 6: 2580–2591. 10.1101/gad.6.12b.2580 [DOI] [PubMed] [Google Scholar]
- Standart N, Weil D. 2018. P-bodies: cytosolic droplets for coordinated mRNA storage. Trends Genet 34: 612–626. 10.1016/j.tig.2018.05.005 [DOI] [PubMed] [Google Scholar]
- Topisirovic I, Svitkin YV, Sonenberg N, Shatkin AJ. 2011. Cap and cap-binding proteins in the control of gene expression. Wiley Interdiscip Rev RNA 2: 277–298. 10.1002/wrna.52 [DOI] [PubMed] [Google Scholar]
- Udagawa T, Swanger SA, Takeuchi K, Kim JH, Nalavadi V, Shin J, Lorenz LJ, Zukin RS, Bassell GJ, Richter JD. 2012. Bidirectional control of mRNA translation and synaptic plasticity by the cytoplasmic polyadenylation complex. Mol Cell 47: 253–266. 10.1016/j.molcel.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli JD, Huarte J, Belin D, Gubler P, Vassalli A, O'Connell ML, Parton LA, Rickles RJ, Strickland S. 1989. Regulated polyadenylation controls mRNA translation during meiotic maturation of mouse oocytes. Genes Dev 3: 2163–2171. 10.1101/gad.3.12b.2163 [DOI] [PubMed] [Google Scholar]
- Villaescusa JC, Allard P, Carminati E, Kontogiannea M, Talarico D, Blasi F, Farookhi R, Verrotti AC. 2006. Clast4, the murine homologue of human eIF4E-Transporter, is highly expressed in developing oocytes and post-translationally modified at meiotic maturation. Gene 367: 101–109. 10.1016/j.gene.2005.09.026 [DOI] [PubMed] [Google Scholar]
- Voronina E, Seydoux G, Sassone-Corsi P, Nagamori I. 2011. RNA granules in germ cells. Cold Spring Harb Perspect Biol 3: a002774 10.1101/cshperspect.a002774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghray S, Williams C, Coon JJ, Wickens M. 2015. Xenopus CAF1 requires NOT1-mediated interaction with 4E-T to repress translation in vivo. RNA 21: 1335–1345. 10.1261/rna.051565.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahle E, Winkler GS. 2013. RNA decay machines: deadenylation by the CCR4–NOT and Pan2-Pan3 complexes. Biochim Biophys Acta 1829: 561–570. 10.1016/j.bbagrm.2013.01.003 [DOI] [PubMed] [Google Scholar]
- Wang Z, Jiao X, Carr-Schmid A, Kiledjian M. 2002. The hDcp2 protein is a mammalian mRNA decapping enzyme. Proc Natl Acad Sci 99: 12663–12668. 10.1073/pnas.192445599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm JE, Hilton M, Amos Q, Henzel WJ. 2003. Cup is an eIF4E binding protein required for both the translational repression of oskar and the recruitment of Barentsz. J Cell Biol 163: 1197–1204. 10.1083/jcb.200309088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Wells D, Tay J, Mendis D, Abbott MA, Barnitt A, Quinlan E, Heynen A, Fallon JR, Richter JD. 1998. CPEB-mediated cytoplasmic polyadenylation and the regulation of experience-dependent translation of α-CaMKII mRNA at synapses. Neuron 21: 1129–1139. 10.1016/S0896-6273(00)80630-3 [DOI] [PubMed] [Google Scholar]
- Yanagiya A, Svitkin YV, Shibata S, Mikami S, Imataka H, Sonenberg N. 2009. Requirement of RNA binding of mammalian eukaryotic translation initiation factor 4GI (eIF4GI) for efficient interaction of eIF4E with the mRNA cap. Mol Cell Biol 29: 1661–1669. 10.1128/MCB.01187-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Smibert CA, Kaplan DR, Miller FD. 2014. An eIF4E1/4E-T complex determines the genesis of neurons from precursors by translationally repressing a proneurogenic transcription program. Neuron 84: 723–739. 10.1016/j.neuron.2014.10.022 [DOI] [PubMed] [Google Scholar]
- Zahr SK, Yang G, Kazan H, Borrett MJ, Yuzwa SA, Voronova A, Kaplan DR, Miller FD. 2018. A Translational repression complex in developing mammalian neural stem cells that regulates neuronal specification. Neuron 97: 520–537.e6. 10.1016/j.neuron.2017.12.045 [DOI] [PubMed] [Google Scholar]
- Zappavigna V, Piccioni F, Villaescusa JC, Verrotti AC. 2004. Cup is a nucleocytoplasmic shuttling protein that interacts with the eukaryotic translation initiation factor 4E to modulate Drosophila ovary development. Proc Natl Acad Sci 101: 14800–14805. 10.1073/pnas.0406451101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Feng F, Chu C, Yue W, Li L. 2019. A novel EIF4ENIF1 mutation associated with a diminished ovarian reserve and premature ovarian insufficiency identified by whole-exome sequencing. J Ovarian Res 12: 119 10.1186/s13048-019-0595-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.