Abstract
Objective
Previous studies reported the effect of dexmedetomidine on intrathecal anesthesia. In this review, we explored the impact of dexmedetomidine as an adjunct for lumbar anesthesia in patients undergoing cesarean section.
Methods
Two authors searched eligible random controlled trials in electronic databases, including PubMed, Embase, Cochrane Library, Web of Science, China National Knowledge Infrastructure, the Chinese BioMedical database, Chinese Scientific Journal Database, and the Wanfang database.
Results
Ten trials comprising 970 patients were included in this review. Intrathecal dexmedetomidine significantly reduced the onset time of sensory block (standardized mean difference (SMD), −1.50, 95% confidence interval (CI) −2.15, −0.85, I2 = 92%) and motor block (SMD −0.77, 95% CI −1.50, −0.49, I2 = 60%) and prolonged the block duration time (sensory block: SMD 2.02, 95% CI 1.29, 2.74, I2 = 93%; motor block: SMD 1.90, 95% CI 1.07, 2.74, I2 = 94%). Patients who received dexmedetomidine showed a lower incidence of shivering. No significant difference was reported for the neonatal Apgar score and other complications.
Conclusion
The use of intrathecal dexmedetomidine during cesarean section can shorten the onset time of spinal anesthesia and enhance the effect of local anesthetic. It has no significant impact on neonates and there were no other adverse events.
Keywords: Dexmedetomidine, spinal anesthesia, cesarean section, meta-analysis, local anesthetic, neonate, adverse events
Background
Among pregnant women who undergo cesarean section, subarachnoid block has been a common and safe anesthesia method.1–3 To decrease maternal discomfort, sensory block has been required up to the level of T4.4 However, this level for single spinal anesthesia requires a high dose of local anesthetics such as bupivacaine, which might be closely related to hypotension, shivering, pruritus, nausea, and vomiting.5 Various studies demonstrated that different drugs could enhance the effect of local anesthetics,6–8 but no definitive conclusion has been reached. Therefore, it is necessary to find an auxiliary drug that enhances anesthesia and has fewer side effects.
Dexmedetomidine is a highly selective α2-adrenergic receptor agonist that produces sedative and analgesic effects9, and it has been widely used in different types of nerve blockade.10–12 Previous studies confirmed that dexmedetomidine might play a role in improving the effectiveness of spinal anesthesia while administered as an adjunct.13,14 A meta-analysis indicated that dexmedetomidine could shorten the spinal anesthesia onset time in cesarean section.15 However, the inclusion criteria are flawed, and neonate safety was not assessed. Thus, we performed this meta-analysis to explore the function of dexmedetomidine as an adjunct for spinal anesthesia in cesarean section.
Materials and methods
Reporting for this systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.16 All data in this study were from published studies and did not involve patients directly. Therefore, ethics committee approval and informed consent were not required.
Systematic literature search
Two independent investigators (Lu and Yuan) searched PubMed, the Cochrane library, Web of Science, Embase, China National Knowledge Infrastructure (CNKI), the Chinese BioMedical database (CBM), Chinese Scientific Journal Database (VIP), and the Wanfang database from database establishment to 30 September 2019, to find available randomized controlled trials (RCTs) without language restrictions. The search strategy for PubMed was as follows: ((“caesarean section”[All Fields] OR “cesarean section”[MeSH Terms] OR (“cesarean”[All Fields] AND “section”[All Fields]) OR “cesarean section”[All Fields]) OR (“cesarean section”[MeSH Terms] OR (“cesarean”[All Fields] AND “section”[All Fields]) OR “cesarean section”[All Fields] OR “c section”[All Fields])) OR (“cesarean section”[MeSH Terms] OR (“cesarean”[All Fields] AND “section”[All Fields]) OR “cesarean section”[All Fields] OR (“abdominal”[All Fields] AND “deliveries”[All Fields]) OR “abdominal deliveries”[All Fields])) AND ((((“dexmedetomidine”[MeSH Terms] OR “dexmedetomidine”[All Fields] OR “mpv 1440”[All Fields]) OR (“dexmedetomidine”[MeSH Terms] OR “dexmedetomidine”[All Fields] OR “precedex”[All Fields])) OR (“dexmedetomidine”[MeSH Terms] OR “dexmedetomidine”[All Fields] OR (“dexmedetomidine”[All Fields] AND “hydrochloride”[All Fields]) OR “dexmedetomidine hydrochloride”[All Fields])) OR (“dexmedetomidine”[MeSH Terms] OR “dexmedetomidine”[All Fields])). We also manually retrieved relevant studies and references from the included studies.
Selection criteria and data extraction
The inclusion criteria were as follows: (1) Patients (P): patients undergoing caesarean section under lumbar anesthesia; (2) Interventions (I): dexmedetomidine administered as an adjunct in spinal anesthesia; (3) Comparisons (C): local anesthetic plus dexmedetomidine vs. local anesthetic plus placebo; (4) Outcomes (O): the effect on the mother and neonate is provided; and (5) Study design (S): an RCT. The exclusion criteria included the following: (1) other types of anesthesia and surgery; (2) intravenous injection of dexmedetomidine; and (3) duplicate publications.
Two reviewers (Li and Yuan) independently extracted the following items: author, year of publication, sample size, anesthetic techniques, and outcomes. A conflict of opinion was resolved by a third reviewer (Zhou).
Quality and risk assessment
The risk of bias for the included studies was assessed based on the Cochrane guidelines (RevMan version 5.3, Copenhagen: The Nordic Cochrane Centre, 2014). The criteria were as follows: random sequence generation, allocation concealment, double-blinding, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Each trial was classified into a high risk, unclear, or low risk category. The assessment was reviewed independently by two team members (Lu and Yuan), and disagreement was resolved by a third reviewer (Zhou).
The quality of evidence was evaluated using Grading of Recommendations Assessment, Development, and Evaluation (GRADE)17 for the outcomes based on the following criteria: study design, risk of bias (for the included study), rating inconsistency in results (for the heterogeneity, I2 ≥50% without satisfactory explanation was considered suspect), rating indirectness of evidence (for the data converted from figures or different scales), and others. Each outcome was evaluated as high, moderate, low, or very low levels.
Statistical analysis
We performed this review using RevMan 5.3 (RevMan, version 5.3, Copenhagen). For dichotomous outcomes, we calculated a pooled risk ratio (RR) and 95% confidence intervals (CIs). For continuous data that were described as the median (range) in the studies, we converted it to the mean and standard deviation, based on the protocol.18,19 The mean difference (MD) and 95% CI were calculated for continuous data with the same measure-evaluation methods and units. Otherwise, the standardized mean difference (SMD) was applied. A P-value of less than 0.05 was considered to be statistically significant. The heterogeneity of trials was assessed using I2. High heterogeneity most likely existed because of the clinical and methodological factors, so the random effect model was applied in this meta-analysis even if I2 was small. Funnel plots were performed to examine the publication bias.
Our primary outcomes were onset time and duration of sensory and motor block. Apgar score, occurrence of hypotension, bradycardia, shivering, nausea, and vomiting were secondary outcomes.
Results
Search results
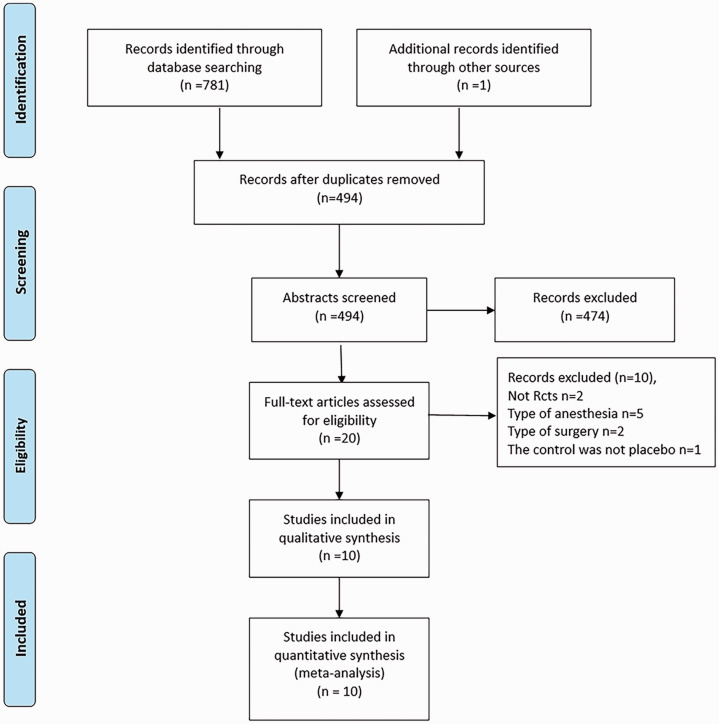
Initially, 782 relevant trials were identified after the database search. We excluded 288 duplicate studies, and another 474 trials were excluded based on their irrelevant titles and abstracts. Then, we carefully evaluated the full-text of 20 studies. Five trials were excluded because of epidural–spinal combined anesthesia.13,14,20–22 Two articles were excluded because they were not RCTs,23,24 two articles were excluded because of the type of surgery,25,26 and one trial was excluded because the control was not placebo.27 Finally, ten RCTs were included in our meta-analysis.4,28–36 The literature screening process is shown in Figure 1.
Figure 1.
Flow chart of study retrieval.
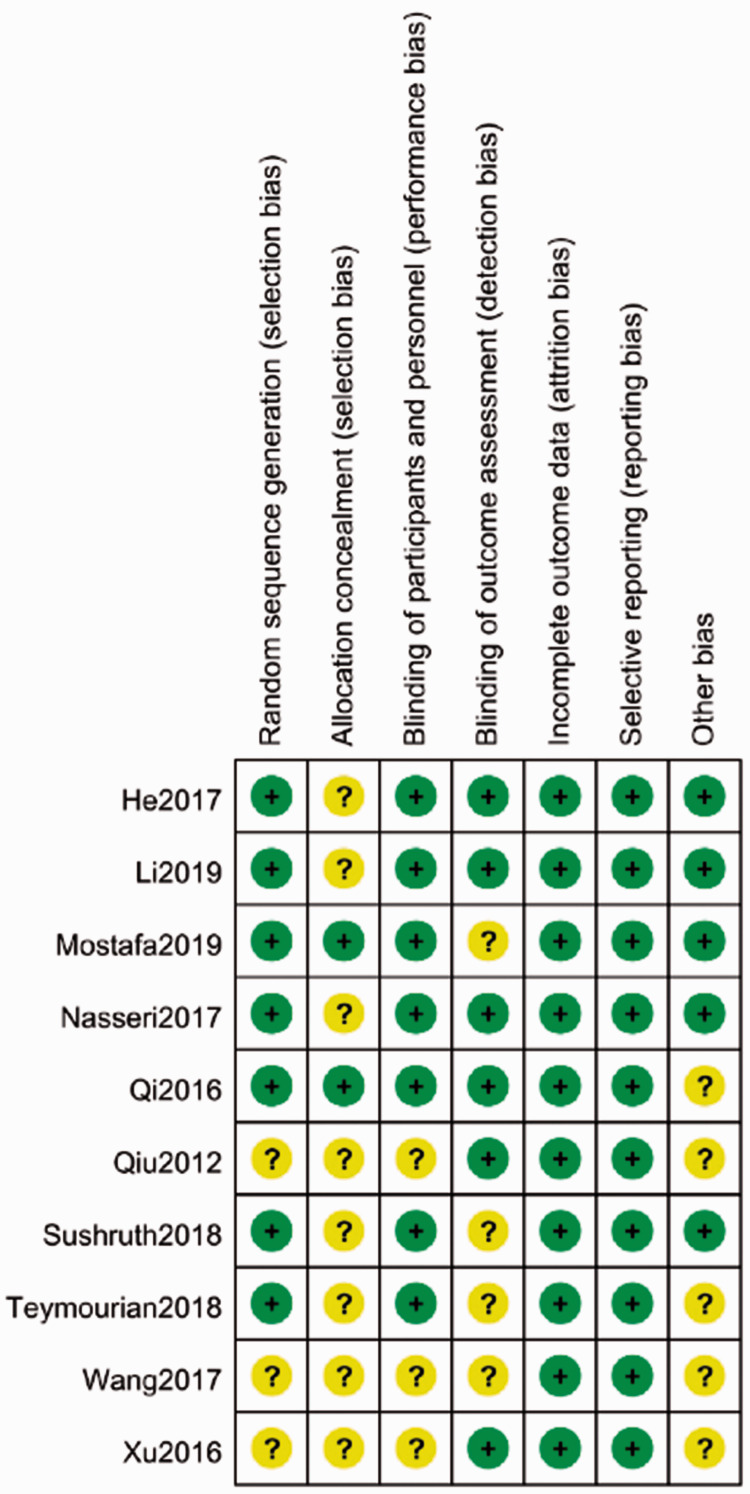
Assessment of bias
Seven studies4,28–33 explicitly reported the method of random sequence generation and two trials29,31 described allocation concealment. Double-blinding was used in seven trials.4,28–33 Six studies28,30,31,33,34,36 mentioned that the assessors were blinded, and they evaluated attrition bias. No selective reporting was reported. Five trials28–30,32,33 did not have sample size calculations before interventions. The summary of the risk of bias is shown in Figure 2.
Figure 2.
Risk bias in the included studies.
Study characteristics
Table 1 shows detailed information about the included studies. Dexmedetomidine was administrated as an adjunct for spinal anesthesia in all trials. The American Society of Anesthesiology (ASA) physical status ranged from I–III. The publication years were 2016 to 2019.
Table 1.
Detailed information about the included studies.
| Study | Samplesize | ASA Grade | Anesthesia position | Local anesthetic | Intervention | Comparison | Outcome |
|---|---|---|---|---|---|---|---|
| Teymourian 20184 | 152 | I–II | Sitting | Bupivacaine | 7.5 µg DEX | DEX vs. placebo | (5) |
| Sushruth 201832 | 60 | II | Right lateral | Bupivacaine | 5 µg DEX | DEX vs. placebo | (1) (2) (3) (4) (6) (7) (9) |
| Qi 201631 | 80 | I–II | Lateral decubitus | Bupivacaine | 5 µg DEX | DEX vs. placebo | (1) (2) (3) (4) (5) (6) (7) (8) (9) |
| Nasseri 201730 | 50 | I–II | Sitting | Bupivacaine | 5 µg DEX | DEX vs. placebo | (6) (7) (8) (9) |
| Mostafa 201929 | 60 | I–II | Sitting | Bupivacaine | 5 µg DEX | DEX vs. placebo | (1) (3) (5) |
| He 201728 | 90 | I–II | Left lateral | Bupivacaine | Group 1: 2.5 µg DEX Group 2: 5 µg DEX |
DEX vs. placebo | (5) (7) (8) |
| Li 201933 | 100 | I–II | Left lateral | Bupivacaine | 5 µg DEX | DEX vs. placebo | (1) (2) (3) (4) (6) (8) (9) |
| Qiu 201234 | 80 | II–III | Left lateral | Bupivacaine | 5 µg DEX | DEX vs. placebo | (1) (2) (3) (4) (6) (9) |
| Wang 201735 | 100 | II–III | Left lateral | Bupivacaine | 5 µg DEX | DEX vs. placebo | (1) (2) (3) (4) (5) (6) (8) (9) |
| Xu 201636 | 120 | I–II | Left lateral | Bupivacaine | 6 µg DEX | DEX vs. placebo | (1) (2) (3) (4) (6) (9) |
Abbreviations: ASA, American Society of Anesthesiology; DEX, dexmedetomidine.
Synthesized results
Primary outcomes
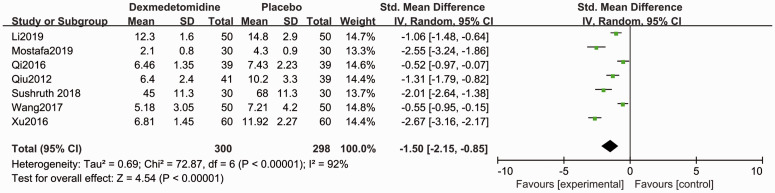
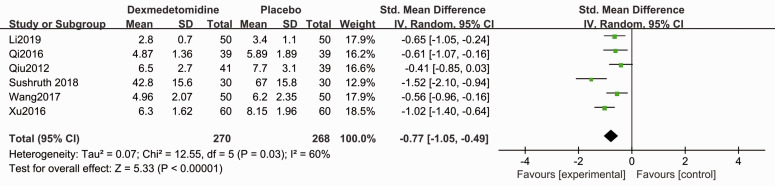
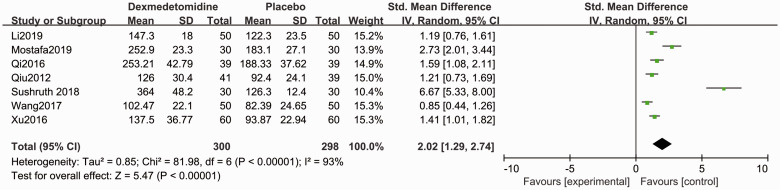
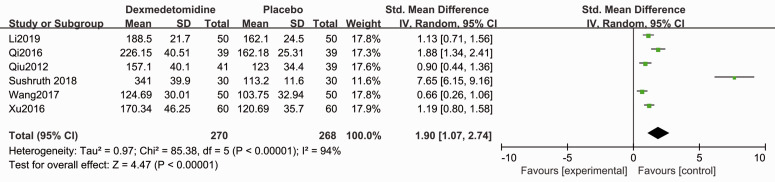
Compared with placebo, patients in the dexmedetomidine group showed shorter sensory block onset time (SMD −1.50, 95% CI −2.15, −0.85, P < 0.05, I2 = 92%, Figure 3) and motor block onset time (SMD −0.77, 95% CI −1.50, −0.49, P < 0.05, I2 = 60%, Figure 4). Forest plots revealed that dexmedetomidine significantly prolonged the sensory block duration (SMD 2.02, 95% CI 1.29, 2.74, P < 0.05, I2 = 93%, Figure 5) and motor block duration (SMD 1.90, 95% CI 1.07, 2.74, P < 0.05, I2 = 94%, Figure 6).
Figure 3.
Forest plot of the pooled analysis showing the onset time of sensory block.
Figure 4.
Forest plot of the pooled analysis showing the onset time of motor block.
Figure 5.
Forest plot of the pooled analysis showing the duration of sensory block.
Figure 6.
Forest plot of the pooled analysis showing the duration of motor block.
Second outcomes
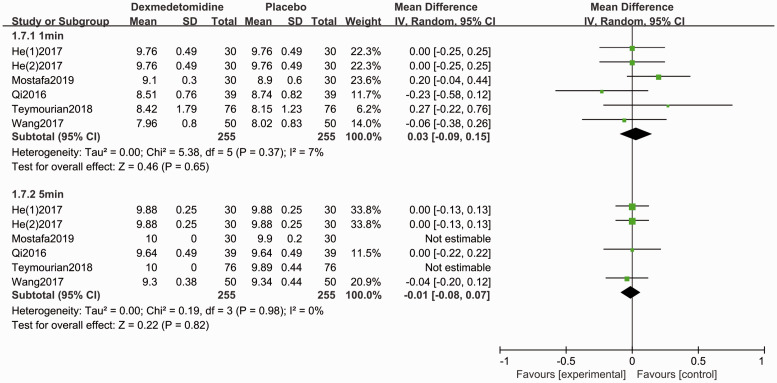
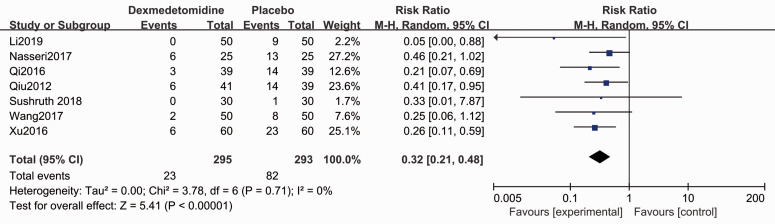
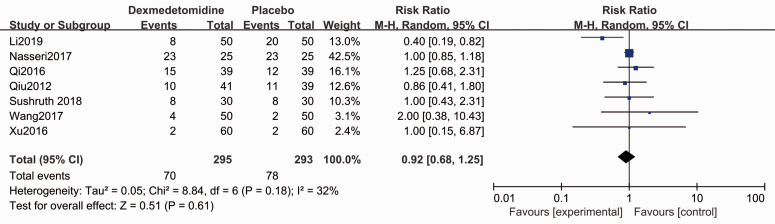
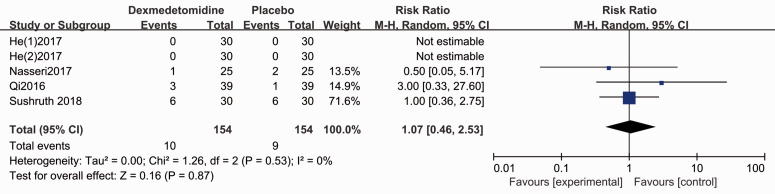
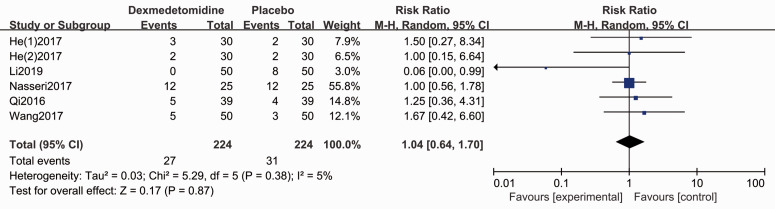
The Apgar score for the neonate was evaluated in five studies.4,28,29,31,35 Forest plots show no difference for the Apgar scores (1-minute: MD 0.03, 95% CI −0.09, 0.15, P > 0.05, I2 = 7%; 5-minute: MD −0.01, 95% CI −0.08, 0.07, P < 0.05, I2 = 0%, Figure 7). Patients who administrated dexmedetomidine had a lower incidence of shivering (RR 0.32, 95% CI 0.21, 0.48, P < 0.05, I2 = 0%, Figure 8), while no significant differences were reported for other complications (Figures 9–11).
Figure 7.
Forest plot of the pooled analysis showing the Apgar scores.
Figure 8.
Forest plot of the pooled analysis showing the incidence of shivering.
Figure 9.
Forest plot of the pooled analysis showing the incidence of hypotension.
Figure 10.
Forest plot of the pooled analysis showing the incidence of bradycardia.
Figure 11.
Forest plot of the pooled analysis showing the incidence of nausea and vomiting.
GRADE evaluation
The GRADE levels of evidence for onset time of sensory and motor block and the duration of sensory and motor block were moderate, while the other results (Apgar scores at 1 and 5 minutes, shivering, hypotension, bradycardia, and nausea and vomiting) had high GRADE levels (Table 2). The overall results are shown in Table 2.
Table 2.
Summary of the results.
| Outcomes | RR/SMD/MD (95%CI) | P | I2 | GRADE |
|---|---|---|---|---|
| Onset time of sensory block | −1.50 (−2.15, −0.85) | <0.05 | 92% | ⨁⨁⨁◯ MODERATE |
| Onset time of motor block | −0.77 (−1.05, −0.49) | <0.05 | 60% | ⨁⨁⨁◯ MODERATE |
| Duration of sensory block | 2.02 (1.29, 2.74) | <0.05 | 93% | ⨁⨁⨁◯ MODERATE |
| Duration of motor block | 1.90 (1.07, 2.74) | <0.05 | 94% | ⨁⨁⨁◯ MODERATE |
| Apgar scores at 1 minute | 0.03 (−0.19, 0.15) | N.S. | 7% | ⨁⨁⨁⨁ HIGH |
| Apgar scores at 5 minute | −0.01 (−0.08, 0.07) | N.S. | 0% | ⨁⨁⨁⨁ HIGH |
| Shivering | 0.32 (0.21, 0.48) | <0.05 | 0% | ⨁⨁⨁⨁ HIGH |
| Hypotension | 0.92 (0.68, 1.25) | N.S. | 32% | ⨁⨁⨁⨁ HIGH |
| Bradycardia | 1.07 (0.46, 2.52) | N.S. | 0% | ⨁⨁⨁⨁ HIGH |
| Nausea and vomiting | 1.04 (0.64, 1.70) | N.S. | 5% | ⨁⨁⨁⨁ HIGH |
Outcome: (1) sensory block duration; (2) motor block duration; (3) sensory block onset time; (4) motor block onset time; (5) Apgar score; (6) hypotension; (7) bradycardia; (8) nausea and vomiting; (9) shivering
N.S., not significant, CI, confidence interval; RR, relative risk; MD, mean difference; SMD, standardized mean difference.
Publication bias
We performed funnel plots for the onset time of sensory and motor block. The funnel plots showed a symmetric distribution, which indicates that there was no obvious publication bias.
Discussion
The current meta-analysis was performed to investigate the impact of dexmedetomidine as an adjuvant for single spinal anesthesia. The synthesized results showed that dexmedetomidine shortened the onset time of local anesthetic, prolonged the duration of sensory and motor block, and reduced the occurrence of shivering, while having no impact on the neonate. The drug-related side effects also did not increase.
Recently, dexmedetomidine has been commonly applied as an assistant drug for a subarachnoid block during the perioperative period.37–39 A previous meta-analysis by Liu et al.15 considered that the addition of dexmedetomidine could significantly reduce the onset time of spinal anesthesia. However, two trials14,20 in that meta-analysis did not meet the inclusion criteria because of the combined spinal and epidural anesthesia. In addition, only studies published in English were included and neonate safety was not demonstrated. Furthermore, the effect of dexmedetomidine on the duration of local anesthetic has not been evaluated. Thus, it was necessary for us to conduct this review.
We found that dexmedetomidine can enhance the effect of local anesthetic and prolong the duration of analgesia. Several studies had a similar result.40,41 Gautam et al.42 suggested that dexmedetomidine is better than fentanyl as an intrathecal adjuvant to reduce visceral pain and in prolonged post-operative analgesia. Some studies considered that dexmedetomidine induces vasoconstriction by acting on the α2-adrenergic receptor to help prolong the period of analgesia,43,44 while Yoshitomi et al.45 suggested that dexmedetomidine directly affects its ability via the α2-adrenergic receptor.
Perioperative shivering is a common complication after spinal anesthesia. In our study, dexmedetomidine prevented the occurrence of shivering. The mechanism is complex. Several studies have demonstrated that dexmedetomidine alleviated shivering effects via α2-adrenergic receptors, which are widely distributed in the hypothalamus to mediate thermoregulatory inhibition.46 Other studies confirmed that dexmedetomidine directly increased the temperature range without affecting thermoregulatory defenses, thereby decreasing the occurrence of shivering.47,48
The Apgar score is widely used for evaluating neonates.49 In our study, 1- and 5-minute Apgar scores and the umbilical blood pH were not significantly different between the two groups. Therefore, we considered that intrathecal dexmedetomidine was safe for neonates. Other complications, including hypotension, bradycardia, pruritus, nausea and vomiting, showed an occurrence rate that was not significantly different between the groups. In addition, no spinal anesthesia-related neurological complications were reported in the included studies. However, the dexmedetomidine dose in our study was small, ranging from 2.5 to 7.5 µg. More high-quality studies are required to ensure the dose safety of dexmedetomidine.
Heterogeneity was high in most of the outcomes, which likely has several explanations. First, most of the outcomes were continuous data, and there was high heterogeneity. Second, the units were inconsistent in the included studies. Third, there might be high clinical heterogeneity.
There were some limitations in this meta-analysis. There was a very small number of eligible RCTs and patients, which may be subject to a small-study effect bias. Various dosages of dexmedetomidine, different anesthesia techniques, and the surgeon’s experience all lead to high clinical heterogeneity. Therefore, a random effects model was used in this meta-analysis. This meta-analysis does not have a registered protocol, which might cause some bias.
Conclusion
Intrathecal dexmedetomidine was shown to be safe for the mother and neonate. In addition, it can shorten the onset time of local anesthesia, prolong the block duration time, and decrease the occurrence of shivering without increasing the drug-related side effects. However, this result should be interpreted with caution because of the high heterogeneity. Further well-designed studies with a larger sample size are required to verify the efficacy and safety.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by grants from Jiaxing Key Discipline of Medicine –Anesthesiology (2019-zc-06), Jiaxing Science and Technology Bureau (2018AD32080).
ORCID iD
Qi-hong Shen https://orcid.org/0000-0003-3365-779X
References
- 1.Loubert C, Gagnon PO, Fernando R. Minimum effective fluid volume of colloid to prevent hypotension during caesarean section under spinal anesthesia using a prophylactic phenylephrine infusion: an up-down sequential allocation study. J Clin Anesth 2017; 36: 194–200. DOI: 10.1016/j.jclinane.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Hirose N, Kondo Y, Maeda T, et al. Prophylactic infusion of phenylephrine is effective in attenuating the decrease in regional cerebral blood volume and oxygenation during spinal anesthesia for cesarean section. Int J Obstet Anesth 2019; 37: 36–44. DOI: 10.1016/j.ijoa.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Sakata K, Yoshimura N, Tanabe K, et al. Prediction of hypotension during spinal anesthesia for elective cesarean section by altered heart rate variability induced by postural change. Int J Obstet Anesth 2017; 29: 34–38. DOI: 10.1016/j.ijoa.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Teymourian H, Khorasanizadeh S, Ansar P, et al. Comparison of the effect of bupivacaine in combination with dexmedetomidine with bupivacaine plus placebo on neonatal Apgar score, bispectral index, and sedation level of parturient women. Anesth Pain Med 2018; 8: e81947. DOI: 10.5812/aapm.81947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal A, Kishore K. Complications and controversies of regional anaesthesia: A review. Indian J Anaesth 2009; 53: 543–553. [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang D, Zhou C, Wei D, et al. Dexamethasone added to local anesthetics in ultrasound-guided transversus abdominis plain (TAP) block for analgesia after abdominal surgery: A systematic review and meta-analysis of randomized controlled trials. PLoS One 2019; 14: e0209646. DOI: 10.1371/journal.pone.0209646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guay J, Nishimori M, Kopp SL. Epidural local anesthetics versus opioid-based analgesic regimens for postoperative gastrointestinal paralysis, vomiting, and pain after abdominal surgery: A Cochrane review. Anesth Analg 2016; 123: 1591–1602. DOI: 10.1213/ane.0000000000001628. [DOI] [PubMed] [Google Scholar]
- 8.Bhardwaj S, Devgan S, Sood D, et al. Comparison of local wound infiltration with ropivacaine alone or ropivacaine plus dexmedetomidine for postoperative pain relief after lower segment cesarean section. Anesth Essays Res 2017; 11: 940–945. DOI: 10.4103/aer.AER_14_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: A review of clinical applications. Curr Opin Anaesthesiol 2008; 21: 457–461. [DOI] [PubMed] [Google Scholar]
- 10.Fu Q, Evangelista MC, Doodnaught GM, et al. Sciatic and femoral nerve blockade using bupivacaine alone, or in combination with dexmedetomidine or buprenorphine in cats. Acta Anaesthesiol Scand 2017; 180: 592. DOI: 10.1136/vr.104152. [DOI] [PubMed] [Google Scholar]
- 11.Keplinger M, Marhofer P, Kettner SC, et al. A pharmacodynamic evaluation of dexmedetomidine as an additive drug to ropivacaine for peripheral nerve blockade: A randomised, triple-blind, controlled study in volunteers. Vet Rec 2015; 32: 790–796. DOI: 10.1097/EJA.0000000000000246 [DOI] [PubMed] [Google Scholar]
- 12.Tu Z, Tan X, Li S, et al. The efficacy and safety of dexmedetomidine combined with bupivacaine on caudal epidural block in children: A meta-analysis. Med Sci Monit 2019; 25: 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi WT, Zhang P. Effect of dexmedetomidine combined with lumbar anesthesia on Th1/Th2 in maternal patients and neonates undergoing caesarean section. Exp Ther Med 2019; 18: 1426–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Y, Xu Y, Wang GN. Comparative evaluation of intrathecal bupivacaine alone, bupivacaine-fentanyl, and bupivacaine-dexmedetomidine in caesarean section. Drug Res (Stuttg) 2014; 65: 468–472. DOI: 10.1055/s-0034-1387740. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Zhang X, Wang X, et al. Comparative evaluation of intrathecal bupivacaine alone and bupivacaine combined with dexmedetomidine in cesarean section using spinal anesthesia: A meta-analysis. J Int Med Res 2019; 47: 2785–2799. DOI: 10.1177/0300060518797000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 2009; 6: e1000097. DOI: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao H, Liang F, Fang Y, et al. Application of Grading of Recommendations Assessment, Development, and Evaluation (GRADE) to the guideline development for clinical practice with acupuncture and moxibustion. Front Med 2017; 11: 590–594. DOI: 10.1007/s11684-017-0537-4. [DOI] [PubMed] [Google Scholar]
- 18.Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018; 27: 1785–1805. DOI: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 19.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. DOI: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bi YH, Cui XG, Zhang RQ, et al. Low dose of dexmedetomidine as an adjuvant to bupivacaine in cesarean surgery provides better intraoperative somato-visceral sensory block characteristics and postoperative analgesia. Oncotarget 2017; 8: 63587–63595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng Y, Li ZY, Wu JH, et al. Effect of dexmedetomidine combined with ropivacaine in subarachnoid block on cesarean section block. Modern Med J China 2018; 20: 55–57. [Google Scholar]
- 22.Zhang JH, Zhang L, Yang CX. . The application of bupivacaine combined with sufentanil and dexmedetomidine in subarachnoid block anesthesia in hysterotomy. Jilin Med J 2015; 36: 1350–1353. [Google Scholar]
- 23.Liu L, Qian J, Shen B, et al. Intrathecal dexmedetomidine can decrease the 95% effective dose of bupivacaine in spinal anesthesia for cesarean section: A prospective, double-blinded, randomized study. Medicine (Baltimore) 2019; 98: e14666. DOI: 10.1097/md.0000000000014666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia F, Chang XY, Zhang YF, et al. The effect of intrathecal dexmedetomidine on the dose requirement of hyperbaric bupivacaine in spinal anaesthesia for caesarean section: A prospective, double-blinded, randomized study. BMC Anesthesiol 2018; 18: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imani F, Rahimzadeh P, Faiz HR, et al. Comparison of the post-caesarean analgesic effect of adding dexmedetomidine to paracetamol and ketorolac: A randomized clinical trial. Anesth Pain Med 2018; 8: e85311. DOI: 10.5812/aapm.85311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y, Xin Y, Liu YB, et al. Effect of epidural dexmedetomidine combined with ropivacaine in labor analgesia. Clin J Pain 2017; 33: 319–324. [DOI] [PubMed] [Google Scholar]
- 27.Kamali A, Azadfar R, Pazuki S, et al. Comparison of dexmedetomidine and fentanyl as an adjuvant to lidocaine 5% for spinal anesthesia in women candidate for elective caesarean. Open Access Maced J Med Sci 2018; 6: 1862–1867. DOI: 10.3889/oamjms.2018.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He L, Xu JM, Liu SM, et al. Intrathecal dexmedetomidine alleviates shivering during cesarean delivery under spinal anesthesia. Biol Pharm Bull 2017; 40: 169–173. DOI: 10.1248/bpb.b16-00651. [DOI] [PubMed] [Google Scholar]
- 29.Mostafa MF, Herdan R, Fathy GM, et al. Intrathecal dexmedetomidine versus magnesium sulphate for postoperative analgesia and stress response after caesarean delivery; randomized controlled double-blind study. Eur J Pain 2020; 24: 182–191. DOI: 10.1002/ejp.1476. [DOI] [PubMed] [Google Scholar]
- 30.Nasseri K, Ghadami N, Nouri B. Effects of intrathecal dexmedetomidine on shivering after spinal anesthesia for cesarean section: a double-blind randomized clinical trial. Drug Des Devel Ther 2017; 11: 1107–1113. DOI: 10.2147/dddt.s131866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi XF, Chen DL, Li GH, et al. Comparison of intrathecal dexmedetomidine with morphine as adjuvants in cesarean sections. Biol Pharm Bull 2016; 39: 1455–1460. [DOI] [PubMed] [Google Scholar]
- 32.Sushruth MR, Rao DG. Effect of adding intrathecal dexmedetomidine as an adjuvant to hyperbaric bupivacaine for elective cesarean section. Anaesthes Pain Intens Care 2018; 22: 348–354. [Google Scholar]
- 33.Li YM, Li XX, S L. Safety and efficacy of bupivacaine combined with dexmedetomidine in subarachnoid anesthesia for cesarean section. J Clin Anesthesiol 2019; 35: 885–888. [Google Scholar]
- 34.Qiu LC, Chen YQ. . Clinical observation of intrathecal dexmedetomidine in mild preeclampsia parturient undergoing cesarean section. J Clin Anesthesiol 2012; 28: 372–374. [Google Scholar]
- 35.Wang H, Chen JB. . Effect of intrathecal injection of dexmedetomidine on the anaesthesia of parturient and newborn in pre-eclampsia cesarean section. Maternal Child Health Care China 2017; 32: 4575–4577. [Google Scholar]
- 36.Xu P, Ran JH, Yang HD. . Anesthetic effect and safety of dexmedetomidine on patients with preeclampsia undergoing cesarean section. Chinese J Woman Child Health Res 2016; 27: 765–767. [Google Scholar]
- 37.Dolma L, Salhotra R, Rautela RS, et al. Isobaric ropivacaine with or without dexmedetomidine for surgery of neck femur fracture under subarachnoid block. J Anaesthesiol Clin Pharmacol 2018; 34: 518–523. DOI: 10.4103/joacp.JOACP_226_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh DR, Nag K, Nagella AB, et al. Efficacy of dexmedetomidine infusion for procedural comfort and intraoperative sedation in patients undergoing surgeries with subarachnoid block: A randomized double-blind clinical trial. Anesth Essays Res 2017; 11: 294–299. DOI: 10.4103/0259-1162.204209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vatsalya T, Waikar C, Singh M. Comparison of intravenous bolus and infusion of dexmedetomidine on characteristics of subarachnoid block. Anesth Essays Res 2018; 12: 190–193. DOI: 10.4103/aer.AER_111_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srinivas DB, Lakshminarasimhaiah G. Comparison of subcutaneous dexmedetomidine versus clonidine as an adjuvant to spinal anesthesia: a randomized double blind control trial. Local Reg Anesth 2019; 12: 29–36. DOI: 10.2147/lra.s197386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang MJ, Wang LY, Chen H, et al. Efficacy of dexmedetomidine as a neuraxial adjuvant for elective cesarean sections: A meta-analysis of randomized trials. Int J Clin Exp Med 2018; 11: 8855–8864. [Google Scholar]
- 42.Gautam B, Tabdar S, Shrestha U. Comparison of fentanyl and dexmedetomidine as intrathecal adjuvants to spinal anaesthesia for abdominal hysterectomy. JNMA J Nepal Med Assoc 2018; 56: 848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, et al. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth 2009; 103: 268–274. DOI: 10.1093/bja/aep159. [DOI] [PubMed] [Google Scholar]
- 44.Masuki S, Dinenno FA, Joyner MJ, et al. Selective alpha2-adrenergic properties of dexmedetomidine over clonidine in the human forearm. J Appl Physiol (1985) 2005; 99: 587–592. DOI: 10.1152/japplphysiol.00147.2005. [DOI] [PubMed] [Google Scholar]
- 45.Yoshitomi T, Kohjitani A, Maeda S, et al. Dexmedetomidine enhances the local anesthetic action of lidocaine via an alpha-2A adrenoceptor. Anesth Analg 2008; 107: 96–101. DOI: 10.1213/ane.0b013e318176be73. [DOI] [PubMed] [Google Scholar]
- 46.Lewis SR, Nicholson A, Smith AF, et al. Alpha-2 adrenergic agonists for the prevention of shivering following general anaesthesia. Cochrane Database Syst Rev 2015; 8: Cd011107. DOI: 10.1002/14651858.CD011107.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bicer C, Esmaoglu A, Akin A, et al. Dexmedetomidine and meperidine prevent postanaesthetic shivering. Eur J Anaesthesiol 2006; 23: 149–153. DOI: 10.1017/s0265021505002061. [DOI] [PubMed] [Google Scholar]
- 48.Elvan EG, Oc B, Uzun S, et al. Dexmedetomidine and postoperative shivering in patients undergoing elective abdominal hysterectomy. Eur J Anaesthesiol 2008; 25: 357–364. DOI: 10.1017/s0265021507003110. [DOI] [PubMed] [Google Scholar]
- 49.White LD, Hodsdon A, An GH, et al. Induction opioids for caesarean section under general anaesthesia: A systematic review and meta-analysis of randomised controlled trials. Int J Obstet Anesth 2019; 40: 4–13. DOI: 10.1016/j.ijoa.2019.04.007. [DOI] [PubMed] [Google Scholar]