Abstract
Background:
Alpine ski racing is known as a sport with unfavorable spinal loads and high rates of back overuse injuries at the elite level. However, little is known about overuse-related structural abnormalities occurring in the spine of youth athletes.
Purpose:
To describe the prevalence of abnormal magnetic resonance imaging (MRI) findings in the lumbar spine of youth competitive alpine skiers within the U16 category (under 16 years) with respect to sex, height growth, multifidus size, increasing age, and clinical relevance.
Study Design:
Cross-sectional study; Level of evidence, 3.
Methods:
A total of 108 youth competitive alpine skiers aged 13 to 15 years underwent MRI examination of the lumbar spine and measurement of the multifidus cross-sectional area on a 3-T Magnetom Prisma scanner. Complementary assessments included the determination of anthropometrics and biological maturation. Athletes were classified as symptomatic when, pursuant to the Oslo Sports Trauma Research Center questionnaire on health problems, at least 1 substantial back overuse–related health problem episode had been registered during the 12 months before the MRI examination.
Results:
Of the analyzed youth skiers, 37.0% presented with ≥1 abnormal MRI finding in the lumbar spine. The most prevalent findings in both sexes were disc degeneration (23.1%), Schmorl nodes (19.4%), end plate changes (10.2%), and pars interarticularis anomalies (10.2%); the pars interarticularis anomalies occurred exclusively in males. A smaller relative lumbar multifidus cross-sectional area was related to more frequent occurrence of disc protrusions (P = .018; R2 = 0.116) and end plate changes (P = .024; R2 = 0.096). Overall, the occurrence of abnormal MRI findings in the lumbar spine increased with age (P = .034; R2 = 0.054). Disc degeneration (particularly disc dehydration and disc protrusion) were significantly more prevalent in symptomatic versus asymptomatic athletes (P < .05 for all).
Conclusion:
As early as age 15 years or younger, competitive alpine skiers demonstrated distinct overuse-related structural abnormalities in the lumbar spine, with some of them being clinically relevant and restrictive of sports participation. As sex, height growth, multifidus size, and increasing age seem to play an important role for the occurrence of such abnormalities, considering these factors might be essential for prevention.
Keywords: low back pain, overuse injuries, musculoskeletal imaging, youth athletes, alpine skiing
To become a world-class competitive alpine skier, intensive physical conditioning and large amounts of on-snow training are required.14 Besides an accumulation of heavy mechanical loads over time, these training regimes expose athletes to unfavorable loading patterns, such as a combined occurrence of frontal bending, lateral bending, and torsion in the loaded trunk, with high vibration loads acting on the spine while skiing.34,35 Since such loading conditions are known to be related to high spinal disc loading, they have been suggested to be characteristic components of mechanisms leading to overuse injuries of the back in alpine ski racing.36 Indeed, the back has been reported to be the most frequently affected body part for overuse injuries among youth and elite competitive alpine skiers.4,19
In contrast to traumatic injuries, overuse injuries are characterized by gradual onset, recurrent symptoms, progression over time, and lack of an identifiable inciting event.12 Overuse injuries often develop undetected in the early stages. If not recognized at this time, they may increase in severity and consequently lead to an absence from training and competition in the follow-up. In an attempt to resolve this issue, a novel prospective injury registration method, called the Oslo Trauma Research Centre (OSTRC) questionnaire on health problems, was recently introduced and validated.9 However, this registration method is based on the presence of clinical symptoms or restrictions to sports participation, 2 signs often absent in the early stages of overuse injuries.
The accumulation of unphysiological loads, the development of structural abnormalities, and the presence of symptoms may occur at different points in time, making early recognition and adequate prevention challenging. Accordingly, in recent years, complementary information obtained by wearable sensors (load monitoring)13,34 or musculoskeletal imaging (early recognition of back overuse–related structural abnormalities)24,38,42 has gained in importance. With respect to the latter, the current gold standard is magnetic resonance imaging (MRI). It offers a sensitive and noninvasive way to identify early subclinical stages of spinal degeneration.24
Spinal abnormalities and back pain are known to increase with age2,3 and to be associated with atrophic changes in the multifidus muscles as a concomitant condition.15,22,37 Previous studies have highlighted the presence of higher rates of radiographic- and MRI-detected spinal abnormalities in 16- to 20-year-old alpine and mogul skiers than in age-matched controls,38,39,42 with a documented greater risk of developing back pain later during the career.20,21,28 However, little is known on the overuse-related structural abnormalities in the spine and multifidus muscles of youth athletes (<16 years old)24,32 and youth competitive alpine skiers in particular.29 Such information is essential for the development of effective prevention strategies.
Accordingly, the aims of the present study were (1) to describe the prevalence of overuse-related MRI findings in the lumbar spine of youth competitive alpine skiers of the under-16-years (U16) category with respect to sex- and sex-specific differences in height growth, (2) to assess the associations between MRI findings and biological maturation-dependent multifidus size, (3) to investigate the relationship of MRI findings with age, and (4) to compare the MRI findings of asymptomatic and symptomatic athletes and to explore their clinical relevance.
Methods
Participants and Study Design
A total of 108 youth competitive alpine skiers aged 13 to 15 years volunteered for this cross-sectional MRI study. Voluntary study participation was promoted within Swiss-Ski and related regional ski federations by official invitation letters/advertisements and a series of local information events directed to all interested athletes and parents. Participants were included if they were part of a certificated regional performance center of Swiss-Ski, which unites the approximately 220 best skiers of that age group in Switzerland. This is typically associated with competitive sports participation of 8 to 10 years and a training load of 5 to 9 units per week within the past 3 years. In the 12 months directly preceding the MRI examinations, our cohort was exposed to 8.46 ± 1.52 (mean ± SD) sport-specific training units of at least 30 minutes per week. There were no study dropouts. None of the interested participants met the exclusion criteria of former traumatic spine injuries or surgery or systemic pathologies such as inflammatory arthritis or diabetes mellitus. All athletes or their legal representatives completed an MRI safety questionnaire and signed an informed consent form. This study was approved by an institutional review board and the local ethics committee.
MRI Examination
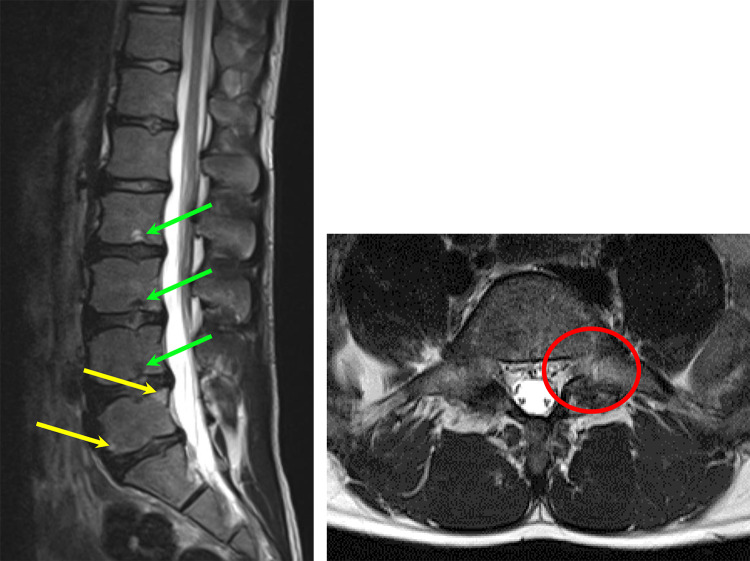
All participants underwent an MRI examination of the lumbar spine from T10 to S1. For that purpose, a 3-T Magnetom Prisma MRI scanner (Siemens Healthcare) with a dedicated spine coil (32-channel receiver) was used. The scan protocol included an axial T2-weighted turbo spin echo sequence (repetition time [TR], 5430 ms; echo time [TE], 96 ms; slice thickness, 4 mm; field of view [FOV], 320 mm × 20 mm; matrix, 384 × 384), a sagittal T2-weighted turbo spin echo sequence in Dixon technique (TR, 3500 ms; TE, 86 ms; slice thickness, 4 mm; FOV, 220 mm × 220 mm; matrix, 384 × 384), and an axial 3D T1-weighted gradient echo sequence (volumetric interpolated breath-hold examination [VIBE]) of the whole body (TR, 3.23 ms; TE, 1.23 ms; flip angle, 15°; FOV, 450 mm × 338 mm × 288 mm; matrix, 264 × 352 × 96). All examinations were conducted by a radiographer. A sample image is illustrated in Figure 1.
Figure 1.
Left: sagittal T2-weighted Dixon magnetic resonance image (MRI) of an athlete presenting multiple Schmorl nodes (green arrows) and disc dehydration at the L2-S1 levels, as well as end plate changes (yellow arrows) at the superior and inferior end plate of L5. Right: axial T2-weighted MRI of an athlete presenting left-sided spondylolysis (red circle) at the L5 level.
Anthropometric Assessments and Biological Maturation
In addition to the MRI examinations, data on the athletes’ anthropometrics were collected. Biological maturation was described as the age at peak height velocity (APHV) and the maturity offset, determined according to the noninvasive estimation method of Mirwald et al.27 This method includes the athlete’s chronological age and the anthropometric factors of body height, sitting height, and subischial leg length into a sex-specific formula for determining the maturity offset (ie, the estimated time before or after reaching APHV). Body mass index (kg/m2) was calculated, and, where available from previous assessments, Δheight was computed as the absolute growth in centimeters in the past 12 months. In 2 missing cases, the last body height assessment was performed 18 months before the MRI examinations. Therefore, a linear interpolation was used for approximation.
Classification of Asymptomatic Versus Symptomatic Skiers
Over a span of 12 months preceding the MRI examinations, all athletes were prospectively assessed by use of the OSTRC questionnaire on health problems. This questionnaire was demonstrated to be a sensitive and valid methodology to document the pattern of traumatic injuries, overuse injuries, and illnesses in a large heterogeneous group of athletes in a previous study.9 Every second week, the participating athletes received an email with a personalized link to a online questionnaire implemented by REDCap (powered by Vanderbilt University). Participants who failed to respond within 2 days were automatically reminded by email. After 3 days without any response, a manual text message was sent daily to the athletes and their parents. The deadline of submitting any valid response was defined as 7 days after the initial email contact. To verify the correctness and completeness of all OSTRC questionnaire–based data entries recorded, at the end of the prospective observation period, personal retrospective interviews and physical examinations were performed by a sports physician (S.F.). Special focus was laid on (1) the correct differentiation of overuse complaints from traumatic back injuries, (2) the precise injury localization, and (3) the suitable allocation of a subsequent event being a new injury. Prospective OSTRC questionnaire entries were amended only in the rare cases for which there were, based on the supplemental interviews, clear indications for accidentally incorrect or incomplete information.
In the course of data analysis, all back overuse–related health problems were subdivided into “substantial” or “nonsubstantial” depending on their OSTRC severity score, as done previously.9 Substantial problems were defined as those resulting in “moderate or severe reductions in training volume,” “moderate or severe reductions in sports performance,” or “complete inability to participate in sport” (ie, skiers having selected option 3, 4, or 5 in question 2 or 3 of the OSTRC questionnaire).9 Finally, athletes were classified as “symptomatic” when at least 1 substantial back overuse–related health problem episode had been registered during the 12 months before the MRI examination.
MRI Interpretation
The MRI assessments and measurements were performed with Merlin PACS software (Phönix-PACS). The diagnostic analysis of the scans was performed by an experienced radiologist in musculoskeletal imaging (C.S.) who was blinded to the results of the clinical assessment. Image interpretation focused on the presence of the specific MRI findings of disc degeneration (subdivided in disc dehydration, annulus tear, protrusion, and extrusion), Schmorl node, end plate change, and anomaly of the pars interarticularis and facet joints, as well as on interspinal bursitis, as defined in Table 1.
Table 1.
Definition of the Specific MRI Findings of the Lumbar Spine (T10 to S1)a
| MRI Finding | Definition |
|---|---|
| Disc dehydration | Reduced signal intensity of intervertebral disc on T2-weighted MR image |
| Disc annulus tear | Linear hyperintense signal within the hypointense annulus fibrosus on T2-weighted MR image |
| Disc protrusion | Displacement of disc material beyond the margins of intervertebral disc space; the diameter of the hernia is widest at its base in the axial and sagittal plane |
| Disc extrusion | Displacement of disc material beyond the margins of intervertebral disc space; the diameter of the hernia is wider at its periphery than at its base in the axial or sagittal plane |
| Schmorl node | Herniation of disc material through the vertebral end plate into the adjacent vertebra |
| End plate changes | Morphologic changes of vertebral end plate (eg, edema, fatty/sclerotic changes, spondylophyte) |
| Anomaly of pars interarticularis | Morphologic or signal intensity change of the pars interarticularis of the vertebral arch (eg, edema, fatty changes, spondylolysis) |
| Anomaly of facet joints | Atypical configuration of facet joints, degenerative or inflammatory changes of facet joints with or without adjacent soft tissue inflammation |
| Interspinal bursitis | Focal edema or accumulation of fluid between spinal processes on fat-suppressed T2-weighted MR image |
aMR, magnetic resonance; MRI, magnetic resonance imaging.
Additionally, based on the axial T2-weighted MRI, an experienced rater (L.P.) determined the cross-sectional area (CSA) of the lumbar multifidus muscles using the software ImageJ (Version 1.48; National Institutes of Health). Such an approach has been demonstrated to be highly reliable.5,10 First, the center of the first lumbar vertebral body was defined in the sagittal image plane, and multifidus CSA was determined in the corresponding transversal plane. Subsequently, starting from the midvertebral level, the adjacent cranial and caudal images were analyzed. For each lumbar vertebral body and both sides, the CSA of the multifidus muscles was then computed as the mean of these 3 CSA measurements. Because muscle CSA is dependent not only on the degree of muscle conditioning but also on the athlete’s body dimensions in general, left and right CSAs were summed and were divided by the total CSA of the associated vertebral body, as done previously.8,26 By such a procedure, a relative multifidus CSA was calculated that can be compared among athletes of different anthropometrics.31 Finally, this relative CSA of the multifidus muscles was determined for each of the 5 lumbar vertebral bodies, and the L1-L5 mean of the relative multifidus CSA was computed.
Statistical Analysis
All data were assessed for normality with the Shapiro-Wilk test, graphical techniques, and shape parameters (skewness and kurtosis coefficients).30 Since the variables of age and Δheight revealed significant results at the Shapiro-Wilk test (P < .05) but corresponding skewness and kurtosis values were ≤0.3 and ≤1.4 (ie, markedly below common reference boundaries of substantial departure from a normal distribution: skewness >2 and kurtosis >7 according to West et al40), all statistical tests including age or Δheight were backed up by bias-corrected accelerated bootstrapping with 10,000 samples; in all other cases, standard parametric tests were applied. In all analyses, statistical significance was set at P < .05.
First, the participants’ mean baseline characteristics and multifidus size were calculated. Differences between subgroups were analyzed with unpaired sample t tests. Second, the prevalence of skiers with specific MRI findings was expressed as the percentage proportion of the overall group, as well as the percentage proportions within the female and male subgroups (number of participants affected / total number of participants per group × 100). Corresponding sex differences were assessed by Pearson chi-square tests. Potential associations of specific MRI findings with sex-specific differences in Δheight were analyzed by univariate binary logistic regression. Third, to investigate the associations between (1) MRI findings and the L1-L5 mean of the relative multifidus CSA and (2) between the L1-L5 mean of the relative multifidus CSA and maturity offset, univariate binary logistic and linear regression analyses were performed, respectively. Fourth, univariate binary logistic regression models were used to describe the relationship between MRI findings and participants’ age. Finally, to explore the clinical relevance of specific MRI findings, their overall percentage proportion was reported, as well as that within the asymptomatic and symptomatic athletes. Corresponding group differences were tested for significance by Pearson chi-square tests. Owing to missing OSTRC data in 11 athletes of the entire cohort of 108 athletes, this clinical symptom–related analysis of the MRI findings was conducted within a subgroup of 97 athletes. All calculations were performed in Microsoft Excel and/or SPSS (v 23; IBM).
Results
Participant Characteristics and Multifidus Size
An overview of the participants’ baseline characteristics and multifidus size is given in Table 2. Despite comparable chronological age, the group of males reached their estimated APHV significantly later than females. Accordingly, at the time of the MRI examination, males were a mean 1.62 years closer to their APHV than females, and their growth rate within the past 12 months was significantly greater. Moreover, as on average male and female athletes had already passed their APHV, males were significantly taller than females.
Table 2.
Baseline Characteristics and Multifidus Sizea
| Overall (N = 108) | Females (n = 42) | Males (n = 66) | P Value | |
|---|---|---|---|---|
| Age, y | 14.83 ± 0.58 | 14.74 ± 0.66 | 14.88 ± 0.52 | .245 |
| Maturity offset, y | 1.27 ± 1.09 | 2.26 ± 0.59 | 0.64 ± 0.83b | <.001 |
| APHV, y | 13.56 ± 1.05 | 12.48 ± 0.46 | 14.24 ± 0.68b | <.001 |
| Body height, cm | 166.4 ± 7.7 | 163.1 ± 5.9 | 168.5 ± 8.0b | <.001 |
| Δheight, cm | 4.95 ± 3.06 | 2.70 ± 2.54 | 6.39 ± 2.44b | <.001 |
| Body weight, kg | 56.6 ± 9.2 | 55.3 ± 7.5 | 57.4 ± 10.2 | .237 |
| BMI, kg/m2 | 20.33 ± 2.34 | 20.78 ± 2.50 | 20.04 ± 2.20 | .118 |
| L1-L5 mean of the relative multifidus CSA, cm2/cm2 | 1.04 ± 0.19 | 1.05 ± 0.17 | 1.02 ± 0.20 | .449 |
aAll data are expressed as mean ± SD. Level of significance based on unpaired sample t tests and backed up by bias-corrected accelerated bootstrapping with 10,000 samples. APHV, age at peak height velocity; BMI, body mass index; CSA, cross-sectional area; Δheight, growth in body height during the past year.
bP < .001.
MRI Findings With Respect to Sex and Sex-Specific Differences in Height Growth
A total of 108 participants underwent the MRI examination. The major results are summarized in Table 3. Of the analyzed youth athletes, 37.0% presented at least ≥1 MRI finding. The most prevalent specific MRI findings were disc degeneration (23.1%), Schmorl node (19.4%), end plate changes (10.2%), and anomalies of the pars interarticularis (10.2%). Pars interarticularis anomalies were exclusively found in males (16.7% of all male participants), while in females such anomalies were absent. Moreover, in this connection, an additional binary logistic regression analysis revealed a direct association of pars interarticularis anomalies and Δheight (R 2 Nagelkerke = 0.065; B = 0.204 [95% CI, 0.011-0.459]; SEB = 0.289; P = .024).
Table 3.
MRI Findings in the Lumbar Spine (T10 to S1) of Overall, Female, and Male Youth Competitive Alpine Skiersa
| MRI Finding | Overall (N = 108) | Females (n = 42) | Males (n = 66) | Chi-square | P Value |
|---|---|---|---|---|---|
| ≥1 MRI finding | 37.0 | 33.3 | 39.4 | 0.255 | .614 |
| Disc degeneration | 23.1 | 21.4 | 24.2 | 0.088 | .767 |
| Disc dehydration | 16.7 | 16.7 | 16.7 | 0.000 | ≥.999 |
| Disc annulus tear | 5.6 | 7.1 | 4.5 | 0.312 | .577 |
| Disc protrusion | 8.3 | 9.5 | 7.6 | 0.117 | .732 |
| Disc extrusion | 3.7 | 2.4 | 4.5 | 0.325 | .569 |
| Schmorl node | 19.4 | 16.7 | 21.2 | 0.273 | .602 |
| End plate changes | 10.2 | 7.1 | 12.1 | 0.625 | .429 |
| Anomaly of pars interarticularis | 10.2 | 0.0 | 16.7 b | 7.000 | .008 |
| Anomaly of facet joints | 2.8 | 2.4 | 3.0 | 0.039 | .844 |
| Interspinal bursitis | 5.6 | 9.5 | 3.0 | 1.948 | .163 |
aPrevalence data are expressed as the percentage proportion of specific MRI findings on the overall group as well as within the subgroups (number affected / number per group × 100). Levels of significance for sex differences are based on Pearson chi-square tests. MRI, magnetic resonance imaging.
bP < .01.
Association of MRI Findings and Biological Maturation-Dependent Multifidus Size
The univariate binary logistic regression-based association of the L1-L5 mean of the relative multifidus CSA with each MRI finding is presented in Table 4. A lower L1-L5 mean of the relative multifidus CSA was significantly associated with a higher occurrence of disc protrusion (P = .018) and end plate changes (P = .024), while for all other MRI findings no such relation was found. Moreover, in an additional linear regression analysis, a direct relationship was observed between the L1-L5 mean of the relative multifidus CSA and the athletes’ maturity offset (R 2 = 0.062; B = 0.043; SEB = 0.016; P = .009).
Table 4.
Univariate Binary Logistic Regression Models Describing the Association Between the Predictor L1-L5 Mean of the Relative Multifidus CSA (cm2/cm2) and MRI Findings of the Lumbar Spine (T10 to S1)a
| Model (N = 108) | |||||||
|---|---|---|---|---|---|---|---|
| χ2 | P Value | R 2 Nagelkerke | Cohen f | Dependent Variable (yes, no) | B | SEB | P Value |
| 0.137 | .711 | 0.002 | 0.04 | ≥1 MRI finding | –0.384 | 1.039 | .712 |
| 0.495 | .482 | 0.007 | 0.08 | Disc degeneration | –0.866 | 1.234 | .483 |
| 0.206 | .650 | 0.003 | 0.05 | Disc dehydration | 0.634 | 1.400 | .651 |
| 2.480 | .115 | 0.065 | 0.065 | Disc annulus tear | –3.624 | 2.361 | .125 |
| 5.628 | .018 | 0.116 | 0.36 | Disc protrusion | –4.639b | 2.061 | .024 |
| 0.000 | .985 | 0.000 | 0.00 | Disc extrusion | 0.051 | 2.747 | .985 |
| 2.253 | .133 | 0.033 | 0.18 | Schmorl node | –1.987 | 1.341 | .138 |
| 5.113 | .024 | 0.096 | 0.33 | End plate changes | –4.007b | 1.848 | .030 |
| 0.004 | .952 | 0.000 | 0.00 | Anomaly of pars interarticularis | –0.102 | 1.714 | .952 |
| 0.052 | .819 | 0.002 | 0.04 | Anomaly of facet joints | 0.724 | 3.179 | .820 |
| 0.252 | .615 | 0.007 | 0.08 | Interspinal bursitis | –1.135 | 2.261 | .616 |
aCSA, cross-sectional area; MRI, magnetic resonance imaging.
bP < .05.
Relationship of MRI Findings With Age
The results of the univariate binary logistic regression models assessing the association of age with MRI findings are illustrated in Table 5. The occurrence of ≥1 MRI finding was found to be significantly related to an increase in age (P = .034).
Table 5.
Univariate Binary Logistic Regression Model Describing the Association Between the Predictor Increasing Age and MRI Findings of the Lumbar Spine (T10 to S1)a
| Model (N = 108) | |||||||
|---|---|---|---|---|---|---|---|
| χ2 | P Value | R 2 Nagelkerke | Cohen f | Dependent Variable (yes, no) | B (95% CI)b | SEB | P Value |
| 4.479 | .034 | 0.054 | 0.24 | ≥1 MRI finding | 0.714c (0.023 to 1.590) | 0.359 | .037 |
| 3.110 | .078 | 0.043 | 0.21 | Disc degeneration | 0.708 (–0.064 to 1.591) | 0.390 | .056 |
| 0.603 | .437 | 0.009 | 0.10 | Disc dehydration | 0.347 (–0.457 to 1.230) | 0.422 | .385 |
| 0.926 | .336 | 0.024 | 0.16 | Disc annulus tear | 0.715 (–0.638 to 2.966) | 0.885 | .188 |
| 2.456 | .117 | 0.052 | 0.23 | Disc protrusion | 0.985 (–0.217 to 3.031) | 0.882 | .071 |
| 0.000 | .999 | 0.000 | 0.00 | Disc extrusion | 0.001 (–1.779 to 1.896) | 0.873 | .998 |
| 0.045 | .831 | 0.001 | 0.03 | Schmorl node | –0.089 (–0.789 to 0.604) | 0.363 | .803 |
| 0.077 | .782 | 0.001 | 0.03 | End plate change | –0.152 (–1.287 to 0.955) | 0.552 | .760 |
| 0.072 | .788 | 0.001 | 0.03 | Anomaly of pars interarticularis | –0.147 (–1.224 to 0.898) | 0.543 | .761 |
| 0.025 | .874 | 0.001 | 0.03 | Anomaly of facet joints | –0.159 (–2.722 to 7.205) | 11.427 | .912 |
| 0.717 | .397 | 0.019 | 0.14 | Interspinal bursitis | 0.626 (–1.429 to 3.507) | 1.015 | .370 |
aMRI, magnetic resonance imaging.
bData are expressed as regression coefficient B and the lower and upper bias-corrected accelerated bootstrapping-based 95% CIs with 10,000 samples.
cP < .05.
Differences in MRI Findings Between Asymptomatic and Symptomatic Athletes
Table 6 shows the prevalence data of the MRI findings present in the subgroup of 97 asymptomatic and symptomatic athletes. Disc degeneration (particularly disc dehydration and disc protrusion) was significantly more prevalent in symptomatic versus asymptomatic athletes (P = .037, .010, and .024, respectively).
Table 6.
MRI Findings in the Lumbar Spine (T10 to S1) of Asymptomatic and Symptomatic Youth Competitive Alpine Skiersa
| MRI Finding | Overall (n = 97) | Asymptomatic (n = 81) | Symptomatic (n = 16) | Chi-square | P Value |
|---|---|---|---|---|---|
| ≥1 MRI finding | 39.2 | 34.6 | 62.5 | 2.661 | .103 |
| Disc degeneration | 25.8 | 21.0 | 50.0b | 4.363 | .037 |
| Disc dehydration | 18.6 | 13.6 | 43.8b | 6.554 | .010 |
| Disc annulus tear | 6.2 | 4.9 | 12.5 | 1.235 | .266 |
| Disc protrusion | 9.3 | 6.2 | 25.0b | 5.104 | .024 |
| Disc extrusion | 4.1 | 3.7 | 6.3 | 0.210 | .647 |
| Schmorl node | 20.6 | 17.3 | 37.5 | 2.648 | .104 |
| End plate change | 11.3 | 8.6 | 25.0 | 3.153 | .076 |
| Anomaly of pars interarticularis | 10.3 | 11.1 | 6.3 | 0.306 | .580 |
| Anomaly of facet joints | 3.1 | 3.7 | 0.0 | 0.593 | .441 |
| Interspinal bursitis | 6.2 | 6.2 | 6.3 | 0.000 | .991 |
aPrevalence data are expressed as the percentage proportion of specific MRI findings on the overall group as well as within the subgroups (number affected / number per group × 100). Clinical symptoms–related analysis of the MRI findings was conducted with a subgroup of 97 athletes. Level of significance for sex differences was based on Pearson chi-square tests. MRI, magnetic resonance imaging.
bP < .05.
Discussion
The major findings of the study were as follows: (1) 37.0% of all analyzed and 62.5% of all symptomatic youth competitive alpine skiers presented at least 1 abnormal MRI finding of the lumbar spine. The most prevalent findings in our study population were disc degenerations, Schmorl nodes, end plate changes, and pars interarticularis anomalies. Anomalies of the pars interarticularis occurred only in males, with all other findings equally distributed between the sexes. (2) Smaller relative lumbar multifidus CSA was related to a higher prevalence of disc protrusions or end plate changes. (3) Overall, the presence of abnormal MRI findings in the lumbar spine increased with age. (4) Disc degenerations—in particular, disc dehydrations and disc protrusions—were significantly more prevalent in symptomatic than asymptomatic athletes.
MRI Findings in the Lumbar Spine of Youth Competitive Alpine Skiers and Sex-Specific Differences in Height Growth
In the present study, the prevalence of having at least 1 MRI finding in the lumbar spine was as high as 37% (see Table 3). On first view, this appears to be markedly lower than previously reported rates for other competitive sports17,25; however, given the substantially younger age of the current cohort of youth competitive alpine skiers (14.83 ± 0.58 years) as compared with elite athletes investigated in the aforementioned studies, this prevalence magnitude must be seen from a different perspective. The same must be considered when comparing the MRI findings of the current study with those observed for alpine and mogul skiers in earlier studies (eg, >80% were reported to show degenerative disc changes): the analyzed athletes were on average 3 years older.38,42 Thus, on second view, one could argue that despite their very young age, youth competitive alpine skiers already demonstrate high rates of overuse-related structural abnormalities in the lumbar spine. Moreover, although a direct comparison is difficult, the magnitudes of the mean prevalence for specific MRI findings in youth competitive alpine skiers were clearly above those previously reported for nonathletic children of comparable age.23 A similar tendency was demonstrated in recent studies directly comparing the abnormal MRI findings of 16- to 20-year-old skiers with age-matched controls.38,39,42
The 3 most prevalent findings in our cohort of youth competitive alpine skiers were disc degenerations, Schmorl nodes, and end plate changes. This is in line with the results of published competitive alpine skiing–related studies29,42 and might be attributable to the typical loading patterns of the spine while skiing, including a combined occurrence of frontal bending, lateral bending and torsion in the trunk, high ground-reaction forces, and excessive exposure to low-frequency whole-body vibrations (∼4-10 Hz).34,35
Interestingly, there was a direct association between the occurrence of pars interarticularis anomalies and an increase in Δheight. This may lead to the entirely plausible presumption that during phases of accelerated growth, bony structures are more prone to overuse-related structural changes. In a similar context, muscular imbalances after accelerated growth have been reported to predispose young athletes to overuse physeal injuries.1 Moreover, such a line of argumentation is supported by our finding of anomalies of the pars interarticularis being observed only in male athletes (see Table 3). Despite comparable chronological age, the group of males was on average 1.62 years closer to their APHV and consequently exposed to a 2-times-higher growth rate within the past 12 months than the group of females (see Table 2). A previous study in the general nonathletic public also reported adult males to have a 2-times-higher occurrence of spondylolysis: a finding that may open an, at least partially, other line of argumentation.6
Important Role of the Multifidus Muscle Size and Its Dependency on Biological Maturation
The multifidus muscles, belonging to the paraspinal back muscles, are an important contributor to the stabilization of the lumbar spine.11 Because competitive alpine skiing is a sport with unfavorable spinal loading patterns, including high axial loads in excessively forward-bent postures,34,35 their stabilizing function is essential for preventing back overuse injuries.18 In such forward-bent postures, it is plausible that spinal torques may be higher than in the upright stance, as the occurring ground-reaction forces are acting with a longer lever arm.16 Moreover, as a direct result of the very short lever arms of the paraspinal muscles, the forces required to stabilize the trunk in such postures must be relatively high,16 implying a logical link to multifidus size.
In fact, in this study, a lower L1-L5 mean of the relative multifidus CSA was significantly associated with a higher occurrence of disc protrusion and end plate changes (Table 4). Disc protrusions and end plate changes might be attributable to excessive loading of the immature spine in an extensively forward-bent posture, as is typical for competitive alpine skiing.33,35 In previous studies, frontal bending in the spine was demonstrated to result in higher spinal disc loading41 and was suggested to be responsible for a significantly higher rate of anterior end plate lesions in elite alpine skiers than in controls.29
Another interesting finding of the current study is the direct linear relationship between the L1-L5 mean of the relative multifidus CSA and skiers’ maturity offset, indicating that skiers closer to their APHV have less developed paraspinal muscles. As it is thoroughly plausible that undersized multifidus muscles may have limited capability to stabilize the spine under adverse loading conditions, this study highlights the importance of focusing prevention efforts on the vulnerable phases of rapid musculoskeletal growth during athletes’ puberty.
MRI Findings Increase With Age
With regard to increasing age, our results support the widespread stakeholder belief that degenerative processes of the spine may arise from the growth spurt on. In the analyzed cohort of competitive alpine skiers of the U16 category, chronological age was found to be decisive for the frequency at which structural abnormalities in the spine occur (Table 5). This is convincing, as the nature of back overuse injuries implies an accumulation of adverse spinal loading and a reinforcement of MRI findings over time. The latter has been particularly well documented by previous studies.2,3
More MRI Findings—More Pain?
In the current study, lumbar disc degenerations were significantly more prevalent in symptomatic than asymptomatic skiers (Table 6). This is in line with previous findings in the general, nonathletic public for which a meta-analysis of epidemiologic studies demonstrated that MRI evidence of disc bulge, disc degeneration, disc extrusions, and protrusions is significantly associated with low back pain in adult patients.7 However, because longitudinal data are widely lacking, such association between degenerative findings and pain should not be interpreted as causation.7 At the same time, subclinical MRI findings (ie, findings with no difference between symptomatic and asymptomatic athletes) should be considered potentially meaningful, as corresponding structural abnormalities may not cause substantial back pain now but can progress into aggravated, more severe grades in the future.2
Methodological Considerations
By presenting new data on a relatively rarely explored cohort of youth competitive alpine skiers (athletes of the U16 category), this study provides novel information about the prevalence of overuse-related back MRI findings with respect to sex, height growth, multifidus size, increasing age, and clinical relevance. Nevertheless, when interpreting the study findings, one should be aware of some limitations.
First, the chosen cross-sectional design cannot conclusively answer questions of causal relationship, as it can only depict current parameter associations, not predict future ones. Thus, for conclusively clarifying the clinical relevance of early signs of spinal degeneration and the causative relation between MRI findings and multifidus size, further longitudinal studies are needed.
Second, the clinical assessment with the OSTRC questionnaire is based on self-reported health problems and therefore relies on the correctness and quality of the answers provided. The risk of potential recall bias was addressed by a prospective data collection with regular 2-week intervals. The risk of reporting bias was faced by conducting retrospective interviews to verify and complete the self-reported data.
Third, the mean relative CSA of the lumbar multifidus muscles is only 1 of several measures and muscles relevant for core stability; however, the causation of structural abnormalities is expected to be multifactorial. Moreover, as mean relative multifidus CSA calculations were based on the manual segmentations of a single rater, despite an excellent intrarater reliability of the used approach,10 absolute values may suffer from a certain systematic bias attributed to subjectivity.
Conclusion
As illustrated in this study, youth competitive alpine skiers have high rates of overuse-related structural abnormalities in the lumbar spine, with some of them already being clinically relevant and restrictive for athletes’ sports participation or performance. Because sex, height growth, multifidus size, and increasing age seem to play an important role for the occurrence of such abnormalities, the factors should be considered for effective prevention.
Acknowledgment
The authors thank all athletes and coaches involved. This work is based on experiments performed at the Swiss Center for Musculoskeletal Imaging, Balgrist Campus AG, Zürich.
Footnotes
Final revision submitted March 18, 2020; accepted March 31, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was generously supported by the Balgrist Foundation, Swiss-Ski, the Stiftung Passion Schneesport, and the Stiftung zur Förderung des alpinen Skisportes in der Schweiz. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the regional ethics commission of Zürich Canton (KEK-ZH-NR: 2017-01395).
References
- 1. Arnold A, Thigpen CA, Beattie PF, Kissenberth MJ, Shanley E. Overuse physeal injuries in youth athletes. Sports Health. 2017;9(2):139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baranto A, Hellstrom M, Cederlund CG, Nyman R, Sward L. Back pain and MRI changes in the thoraco-lumbar spine of top athletes in four different sports: a 15-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1125–1134. [DOI] [PubMed] [Google Scholar]
- 3. Baranto A, Hellstrom M, Nyman R, Lundin O, Sward L. Back pain and degenerative abnormalities in the spine of young elite divers: a 5-year follow-up magnetic resonance imaging study. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):907–914. [DOI] [PubMed] [Google Scholar]
- 4. Bergstrom KA, Brandseth K, Fretheim S, Tvilde K, Ekeland A. Back injuries and pain in adolescents attending a ski high school. Knee Surg Sports Traumatol Arthrosc. 2004;12(1):80–85. [DOI] [PubMed] [Google Scholar]
- 5. Berry DB, Padwal J, Johnson S, Parra CL, Ward SR, Shahidi B. Methodological considerations in region of interest definitions for paraspinal muscles in axial MRIs of the lumbar spine. BMC Musculoskelet Disord. 2018;19(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beutler WJ, Fredrickson BE, Murtland A, Sweeney CA, Grant WD, Baker D. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine (Phila Pa 1976). 2003;28(10):1027–1035. [DOI] [PubMed] [Google Scholar]
- 7. Brinjikji W, Diehn FE, Jarvik JG, et al. MRI findings of disc degeneration are more prevalent in adults with low back pain than in asymptomatic controls: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2015;36(12):2394–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen YY, Pao JL, Liaw CK, Hsu WL, Yang RS. Image changes of paraspinal muscles and clinical correlations in patients with unilateral lumbar spinal stenosis. Eur Spine J. 2014;23(5):999–1006. [DOI] [PubMed] [Google Scholar]
- 9. Clarsen B, Ronsen O, Myklebust G, Florenes TW, Bahr R. The Oslo Sports Trauma Research Center questionnaire on health problems: a new approach to prospective monitoring of illness and injury in elite athletes. Br J Sports Med. 2014;48(9):754–760. [DOI] [PubMed] [Google Scholar]
- 10. Fortin M, Battie MC. Quantitative paraspinal muscle measurements: inter-software reliability and agreement using OsiriX and ImageJ. Phys Ther. 2012;92(6):853–864. [DOI] [PubMed] [Google Scholar]
- 11. Freeman MD, Woodham MA, Woodham AW. The role of the lumbar multifidus in chronic low back pain: a review. PM R. 2010;2(2):142–146. [DOI] [PubMed] [Google Scholar]
- 12. Fuller CW, Ekstrand J, Junge A, et al. Consensus statement on injury definitions and data collection procedures in studies of football (soccer) injuries. Br J Sports Med. 2006;40(3):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gilgien M, Kröll J, Spörri J, Crivelli P, Müller E. Application of dGNSS in alpine ski racing: basis for evaluating physical demands and safety. Front Physiol. 2018;9:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gilgien M, Reid R, Raschner C, Supej M, Holmberg HC. The training of Olympic alpine ski racers. Front Physiol. 2018;9:1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goubert D, Oosterwijck JV, Meeus M, Danneels L. Structural changes of lumbar muscles in non-specific low back pain: a systematic review. Pain Physician. 2016;19(7):e985–e1000. [PubMed] [Google Scholar]
- 16. Haid C, Fischler S. Biomechanische Belastungsaspekte der Wirbelsäule beim Golfschwung. Sports Orthopaedics and Traumatology. 2013;29(2):89–95. [Google Scholar]
- 17. Hangai M, Kaneoka K, Hinotsu S, et al. Lumbar intervertebral disk degeneration in athletes. Am J Sports Med. 2009;37(1):149–155. [DOI] [PubMed] [Google Scholar]
- 18. Hides JA, Stanton WR, McMahon S, Sims K, Richardson CA. Effect of stabilization training on multifidus muscle cross-sectional area among young elite cricketers with low back pain. J Orthop Sports Phys Ther. 2008;38(3):101–108. [DOI] [PubMed] [Google Scholar]
- 19. Hildebrandt C, Raschner C. Traumatic and overuse injuries among elite adolescent alpine skiers: a two-year retrospective analysis. International Sportmed Journal. 2013;14(4):245–255. [Google Scholar]
- 20. Iwamoto J, Abe H, Tsukimura Y, Wakano K. Relationship between radiographic abnormalities of lumbar spine and incidence of low back pain in high school and college football players: a prospective study. Am J Sports Med. 2004;32(3):781–786. [DOI] [PubMed] [Google Scholar]
- 21. Iwamoto J, Abe H, Tsukimura Y, Wakano K. Relationship between radiographic abnormalities of lumbar spine and incidence of low back pain in high school rugby players: a prospective study. Scand J Med Sci Sports. 2005;15(3):163–168. [DOI] [PubMed] [Google Scholar]
- 22. Kalichman L, Carmeli E, Been E. The association between imaging parameters of the paraspinal muscles, spinal degeneration, and low back pain. Biomed Res Int. 2017;2017:2562957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kjaer P, Leboeuf-Yde C, Sorensen JS, Bendix T. An epidemiologic study of MRI and low back pain in 13-year-old children. Spine (Phila Pa 1976). 2005;30(7):798–806. [DOI] [PubMed] [Google Scholar]
- 24. Kobayashi A, Kobayashi T, Kato K, Higuchi H, Takagishi K. Diagnosis of radiographically occult lumbar spondylolysis in young athletes by magnetic resonance imaging. Am J Sports Med. 2013;41(1):169–176. [DOI] [PubMed] [Google Scholar]
- 25. Külling FA, Florianz H, Reepschläger B, Gasser J, Jost B, Lajtai G. High prevalence of disc degeneration and spondylolysis in the lumbar spine of professional beach volleyball players. Orthop J Sports Med. 2014;2(4):2325967114528862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee JC, Cha JG, Kim Y, Kim YI, Shin BJ. Quantitative analysis of back muscle degeneration in the patients with the degenerative lumbar flat back using a digital image analysis: comparison with the normal controls. Spine (Phila Pa 1976). 2008;33(3):318–325. [DOI] [PubMed] [Google Scholar]
- 27. Mirwald RL, Baxter-Jones AD, Bailey DA, Beunen GP. An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc. 2002;34(4):689–694. [DOI] [PubMed] [Google Scholar]
- 28. Ogon M, Riedl-Huter C, Sterzinger W, Krismer M, Spratt KF, Wimmer C. Radiologic abnormalities and low back pain in elite skiers. Clin Orthop Relat Res. 2001;390:151–162. [DOI] [PubMed] [Google Scholar]
- 29. Rachbauer F, Sterzinger W, Eibl G. Radiographic abnormalities in the thoracolumbar spine of young elite skiers. Am J Sports Med. 2001;29(4):446–449. [DOI] [PubMed] [Google Scholar]
- 30. Razali NM, Yap BW. Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling tests. Journal of Statistical Modeling and Analytics. 2011;2:21–33. [Google Scholar]
- 31. Rezazadeh F, Taheri N, Okhravi SM, Hosseini SM. The relationship between cross-sectional area of multifidus muscle and disability index in patients with chronic non-specific low back pain. Musculoskelet Sci Pract. 2019;42:1–5. [DOI] [PubMed] [Google Scholar]
- 32. Shimozaki K, Nakase J, Yoshioka K, et al. Incidence rates and characteristics of abnormal lumbar findings and low back pain in child and adolescent weightlifter: a prospective three-year cohort study. PLoS One. 2018;13(10):e0206125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Spörri J, Kröll J, Fasel B, Aminian K, Müller E. Course setting as a prevention measure for overuse injuries of the back in alpine ski racing: a kinematic and kinetic study of giant slalom and slalom. Orthop J Sports Med. 2016;4(2):2325967116630719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spörri J, Kröll J, Fasel B, Aminian K, Müller E. The use of body worn sensors for detecting the vibrations acting on the lower back in alpine ski racing. Front Physiol. 2017;8:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spörri J, Kröll J, Haid C, Fasel B, Müller E. Potential mechanisms leading to overuse injuries of the back in alpine ski racing: a descriptive biomechanical study. Am J Sports Med. 2015;43(8):2042–2048. [DOI] [PubMed] [Google Scholar]
- 36. Spörri J, Kröll J, Supej M, Müller E. Reducing the back overuse-related risks in alpine ski racing: let’s put research into sports practice. Br J Sports Med. 2019;53(1):2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun D, Liu P, Cheng J, Ma Z, Liu J, Qin T. Correlation between intervertebral disc degeneration, paraspinal muscle atrophy, and lumbar facet joints degeneration in patients with lumbar disc herniation. BMC Musculoskelet Disord. 2017;18(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thoreson O, Kovac P, Sward A, Agnvall C, Todd C, Baranto A. Back pain and MRI changes in the thoraco-lumbar spine of young elite Mogul skiers. Scand J Med Sci Sports. 2017;27(9):983–989. [DOI] [PubMed] [Google Scholar]
- 39. Todd C, Kovac P, Sward A, et al. Comparison of radiological spino-pelvic sagittal parameters in skiers and non-athletes. J Orthop Surg Res. 2015;10:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. West SG, Finch JF, Curran PJ. Structural equation models with nonnormal variables: problems and remedies In: Hoyle RH, ed. Structural Equation Modeling: Concepts, Issues, and Applications. Sage Publications Inc; 1995:56–75. [Google Scholar]
- 41. Wilke HJ, Neef P, Caimi M, Hoogland T, Claes LE. New in vivo measurements of pressures in the intervertebral disc in daily life. Spine (Phila Pa 1976). 1999;24(8):755–762. [DOI] [PubMed] [Google Scholar]
- 42. Witwit WA, Kovac P, Sward A, et al. Disc degeneration on MRI is more prevalent in young elite skiers compared to controls. Knee Surg Sports Traumatol Arthrosc. 2018;26(1):325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]