Abstract
Objectives
The objective was to observe the effects of Astragalus polysaccharides on diabetes and on regulation of the TGF-β/Smad signaling pathway.
Methods
A type 2 diabetic rat model was established with a high-fat diet in combination with low-dose streptozotocin (35 mg/kg). Astragalus polysaccharides were applied as treatment intervention and changes in blood glucose and kidney morphology and function were assessed.
Results
Eight weeks after model establishment, kidney weight as a proportion of total weight (KW/TW) in the high-, medium-, and low-dose Astragalus polysaccharide groups was significantly lower than that in the model group, and the KW/TW value gradually decreased with increasing dose of polysaccharides in each treatment group. Fasting blood glucose in the low- and medium-dose Astragalus polysaccharide groups was numerically lower than that in the model group and fasting blood glucose in rats in the high-dose group was significantly lower than that in the model group. Levels of 24-hour urinary microalbumin, creatinine, blood urea nitrogen, collagens I, III, and IV, α-smooth muscle actin, transforming growth factor-β1, and Smad3 in Astragalus polysaccharide groups (all doses) were significantly lower than those in the model group.
Conclusions
Astragalus polysaccharide significantly improved blood glucose and protected kidney function in a rat diabetes model.
Keywords: Astragalus polysaccharides, diabetic nephropathy, transforming growth factor-β, Smad3, renal damage, fasting blood glucose
Introduction
Diabetic nephropathy (DN) is a principal microvascular complication in patients with type 2 diabetes.1,2 More than 30% of patients with type 2 diabetes will eventually suffer from end-stage renal disease, and patients at the end stage will require hemodialysis or kidney transplantation.3 The incidence of diabetes in China is as high as 11.6%,4 and the population developing DN expands annually. Therefore, there is an urgent need to find effective drugs to treat DN.
The Astragalus polysaccharide is a commonly used traditional Chinese herbal medicine; it is derived from a leguminous plant, Astragalus propinquus. The root of Astragalus propinquus has numerous effects, including increasing vitality, antiperspirant, and treatment of diuretic swelling and pus discharge. Modern pharmacological studies have shown that Astragalus propinquus enhances immune function, protects the liver, is a diuretic, and has anti-aging, antistress, antihypertensive, and antibacterial effects. Polysaccharides are a main active constituent of Astragalus propinquus. Astragalus polysaccharides have a hypoglycemic effect in experimental diabetic mice2 and can alleviate myocardial oxidative stress and fibrosis in diabetic rats. The mechanism involved in alleviating myocardial damage caused by diabetes may be related to inhibition of the expression of transforming growth factor (TGF)-β1 and tumor necrosis factor-α (TNF-α).5 However, there are few reports on the effects of Astragalus polysaccharides on DN.
Studies have shown that the TGF-β/Smad signaling pathway is one of the classical signaling pathways inducing fibrosis.6 Multiple studies report that the content of Smad2/3 is significantly higher in patients with end-stage renal disease than in healthy people.7,8 When the Smad3 gene of renal tubular epithelial cells is destroyed, matrix formation induced by angiotensin II (Ang II) is significantly reduced, which suggests that the TGF-β/Smad signaling pathway is involved in renal fibrosis. Therefore, in this study, we successfully established a rat model of type 2 diabetes, demonstrated the protective role of Astragalus polysaccharides on renal function of diabetic rats, and investigated the effect of Astragalus polysaccharides in regulating the TGF-β1/Smad signal transduction pathway.
Materials and methods
Reagents
Astragalus polysaccharides (purity >98%) were from Lanzhou Wotelaisi Biological Co. Ltd. (Lanzhou, China); streptozotocin (STZ) was from Sigma (St. Louis, MO, USA); rabbit anti-mouse TGF-β antibody, Smad3 polyclonal antibody, and mouse anti-β-actin polyclonal antibody were from Santa Cruz Biotechnology Inc. (Dallas, TX, USA); prestained protein marker was from New England Biolabs (Ipswich, MA, USA); bicinchoninic acid (BCA) quantitative protein assay kit was from Beyotime Biotechnology (Beijing, China); and the polyvinyl difluoride (PVDF) membrane was from Millipore (Hong Kong, China).
Experimental instruments
The One Touch blood glucose monitor was from Johnson & Johnson (New Brunswick, NJ, USA); the inverted microscope was from Olympus (Tokyo, Japan); the protein electrophoresis instrument was from Bio-Rad Laboratories (Hercules, CA, USA); and the gel imager was from the Shanghai Fudan Four-star High-tech Technology Company (Shanghai, China). The urinary microalbumin (MAU) ELISA kit (number 170822) was from Shanghai Fusheng Industrial Co. Ltd. (Shanghai, China). The Hitachi 7170A automatic biochemical analyzer was from Hitachi Ltd. (Tokyo, Japan).
Experimental animals
Sixty healthy male adult Sprague Dawley rats (specific-pathogen-free) with a body weight of 252 ± 2.7 g were provided by the Shanghai Animal Laboratory Center (Shanghai, China). All rats were fed in cages, with four rats in each cage. During 7 days of adaptive feeding, rats were allowed to eat and drink freely. The environment was well ventilated with relative humidity of 40% to 60%, an indoor temperature of 22°C to 24°C; and a 12-hour light/dark cycle.
Grouping and treatment of animals
Ten rats were randomly selected and assigned to the control group, fed a standard diet, and allowed to drink water freely. Fifty rats were fed a high-fat diet containing 70% basal feed, 20% fat, 5% egg yolk powder, and 5% milk powder. After 4 weeks, the rats were fasted for 12 hours and given streptozotocin (STZ; 35 mg/kg) by intraperitoneal injection. One week after model establishment, fasting blood glucose (FBG) of rats was tested. An FBG ≥11.1 mmol/L indicated successful establishment of the diabetes model. The rats remained on the high-fat diet. Of the 50 rats, 8 died and 6 failed to become diabetic, so 36 rats were successfully induced in the model. These 36 rats were randomly divided into four groups: the model group and high-, medium-, and low-dose Astragalus polysaccharides groups, with nine rats in each group. Rats in the high-, medium-, and low-dose Astragalus groups were given 25, 50, and 100 mg/kg of Astragalus polysaccharides, respectively, by intragastric administration once daily. The rats in the control and model groups were given an equal volume of normal saline by intragastric administration for 8 consecutive weeks. Animal housing, handling, and all procedures were approved by the ethical committees of Jining Medical University (IACUC: 1804021).
Measurement outcomes
General morphology observation
During the experiment, the general condition of the rats, including hair, activity, and mental status, was observed. The rats were weighed every 2 weeks (total weight, TW), and a metabolic cage was used to record feed and water intake. After the experiment, the rats were euthanized and the right kidney was isolated to determine kidney weight (KW); the kidney index was calculated as KW/TW.
Blood glucose measurement
On day 1 and at the end of weeks 2, 4, and 8 of the experiment, venous blood of fasting rats was collected from the tail, and the FBG of each group was detected using the One Touch blood glucose monitor.
24-hour urine microalbumin measurement
On day 1 and at the end of weeks 2, 4, and 8 of the experiment, urine samples were collected from rats using the metabolic cage, and 24-hour urine volumes of the rats were recorded. After centrifuging the urine at 4000 × g for 20 minutes, the supernatant was used to measure the level of urinary microalbumin using an ELISA method.
Measurement of serum creatinine and blood urea nitrogen
On the eighth weekend of the experiment, serum was obtained from all rats, and levels of creatinine (Cr) and blood urea nitrogen (BUN) were measured using the Hitachi 7170A automatic biochemical analyzer and averaged by group.
Histopathological observation
After the experiment, the kidneys of each rat were isolated and the right kidney was weighed and cut along the longitudinal axis after removal of the capsule. Part of the tissue of the right kidney was fixed in 10% neutral formalin, sectioned using conventional paraffin embedding, and stained with hematoxylin and eosin (HE) to observe the morphological changes in kidney tissue under the microscope.
Expression of renal tissue-related proteins by western blot
One milligram of renal tissue was isolated, protein was extracted with 1 mL of radioimmunoprecipitation assay (RIPA) lysate, the supernatant was obtained after 15 min of centrifugation at 1200 × g at 4°C, and protein content was measured by the BCA method. Twenty micrograms of protein was sampled and isolated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis; 5% bovine serum albumin was used to seal, and the corresponding primary antibody was added for incubation overnight at 4°C. The membrane was flushed three times with Tris buffer saline with Tween 20 (TBST) and the secondary antibody was added for incubation for 1 hour. The membrane was flushed 3 times with TBST and the images were developed with enhanced chemiluminescence developer. The gray value of the target band was analyzed with ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data were analyzed using Stata 10.0 statistical software (StataCorp., College Station, TX, USA). Measurement data were expressed as mean ± standard deviation and compared with one-way analysis of variance (ANOVA) and q test. P < 0.05 implied a significant difference.
Results
General condition of rats
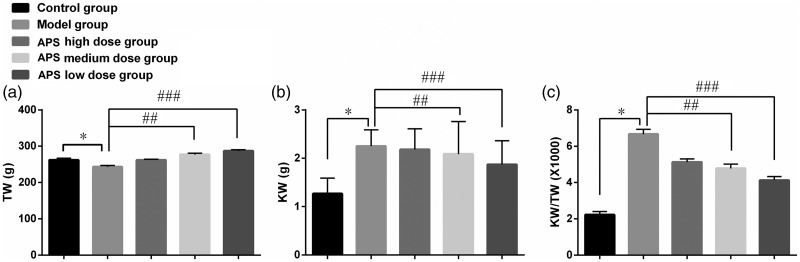
Rats in the control group were in good general condition. They drank normally, were active, reacted swiftly, had shiny hair, and their body weight increased steadily in the 8-week experimental period. Rats in the diabetes model group were in poor general condition. They ate less, drank less, and acted slowly; they had dry, weathered hair and were slow to gain weight. The general condition of rats in the Astragalus polysaccharide groups (all three doses) was superior to that of rats in the model group. Eight weeks after model establishment, the condition of rats in Astragalus polysaccharide groups was intermediate between that of the model group and the control group. Rats treated with the higher dose of polysaccharides were better conditioned than rats treated with the lower doses. Eight weeks after model establishment, the KW/TW value of rats in the model group was significantly higher than that of control rats, whereas KW/TW values in the high-, medium-, and low-dose Astragalus polysaccharide groups were significantly lower (P < 0.05) than that in the model group. KW/TW decreased gradually with increasing dose of Astragalus polysaccharides (P < 0.05) (Figure 1).
Figure 1.
Effect of Astragalus polysaccharides on KW/TW value of DN rats (n=10) 8 weeks after model establishment. (a) Value of TW among all groups; (b) value of KW among all groups; (c) value of KW/TW among all groups. *P < 0.05 compared with the control group; #P < 0.05 compared with the model group. Error bars represent standard deviations. APS, Astragalus polysaccharides; TW, total weight; KW, kidney weight; DN, diabetic neuropathy.
Comparison of FBG in rats
Two weeks after model establishment, FBG in the model group and the Astragalus polysaccharide groups (all doses) were significantly higher (P < 0.05) than that in the control group. Eight weeks after model establishment, FBG in the low- and medium-dose Astragalus polysaccharide groups were not lower than that in the model group, but FBG in the high-dose group was significantly lower (P < 0.05) (Figure 2a–2d).
Figure 2.
Effect of Astragalus polysaccharides on fasting blood glucose level in diabetic rats (a) 0 weeks, (b) 2 weeks, (c) 4 weeks, and (d) 8 weeks after model establishment in all groups. *P < 0.05 compared with the control group; #P < 0.05 compared with the model group. APS, Astragalus polysaccharides; CB, concentration.
Comparison of 24-hour urine microalbumin levels
At the end of week 2 of model establishment, 24-hour urine microalbumin levels between the model group and the Astragalus polysaccharide groups were not significantly different. However, 4 and 8 weeks after model establishment, the level of 24-hour urinary microalbumin in the model group was significantly higher (P < 0.05) than that in the control group. The levels of 24-hour urinary microalbumin in the Astragalus polysaccharide groups (all doses) were significantly lower (P < 0.05) than that in the model group (Figure 3a–3d).
Figure 3.
Effect of Astragalus polysaccharides on levels of 24-hour urine microalbumin in diabetic rats (ρB/mg·L−1) at the end of week 0 (a), week 2 (b), week 4 (c), and week 8 (d) of model establishment. Error bars represent standard deviations. *P < 0.05 compared with the control group; #P < 0.05 compared with the model group. APS, Astragalus polysaccharides; ρB, mass concentration of microalbumin.
Comparison of serum Cr and BUN levels
In week 8 of model establishment, levels of serum Cr and BUN in the model group were significantly higher (P < 0.05) than those in the blank control group. The levels of Cr and BUN in the high-dose Astragalus polysaccharide group were significantly lower (P < 0.05) than that in the model group (Figure 4a and 4b).
Figure 4.
Effect of Astragalus polysaccharides on levels of Cr (a) and BUN (b) in diabetic rats in week 8 of model establishment among all groups. Error bars represent standard deviations. Cr, creatinine; BUN, blood urea nitrogen. APS, Astragalus polysaccharides; CB, concentration.
Observation of kidney pathological tissues
In week 8 of model establishment, HE staining showed that the renal tissue structure of rats in the control group was clear and distributed normally, the glomerular capsule wall was smooth and complete, the mesangial cells were arranged regularly without hyperplasia, the capillaries were normally distributed in the renal tissues, and the renal tubule lumen was smooth and round without degeneration, necrosis, or interstitial inflammatory cell infiltration. In the model group, the renal tissue structure of the rats was disorderly, the glomerular capsule was enlarged, the cystic cavity was arranged irregularly, massive mesangial cells proliferated, the basement membrane was thickened and widened, the renal tubules were narrower, the epithelial cells underwent vacuolar degeneration and atrophy, and a large amount of interstitial inflammatory cell infiltration was observed. Renal damage in the Astragalus polysaccharide groups (all doses) was less severe compared that in the model group. Partial glomerular enlargement, mesangial cell proliferation, and mild tubular stenosis were observed, although these signs were milder than in the model group and relieved to a greater degree in the higher dose group than in the lower dose groups (Figure 5a–5e).
Figure 5.
Effect of Astragalus polysaccharides on renal pathological tissues in diabetic rats (200× in all panels) in (a) control group; (b) model group; (c) Astragalus polysaccharide high-dose group; (d) Astragalus polysaccharide medium-dose group; and (e) Astragalus polysaccharide low-dose group. APS, Astragalus polysaccharides.
Expression of epithelial–mesenchymal transition-related proteins in rats
Eight weeks after model establishment, levels of proteins related to epithelial–mesenchymal transition, collagens I, III, and IV and α-smooth muscle actin (α-SMA), in renal tissue in the model group were significantly higher (P < 0.05) than those in the control group. Expression levels of collagens I, III, and IV, and α-SMA in the Astragalus polysaccharide groups (all doses) were significantly decreased (P < 0.05) in a dose-dependent manner (Figure 6a–6e).
Figure 6.
Effect of Astragalus polysaccharides on the expression of EMT-related proteins 8 weeks after model establishment: (a) collagen I, (b) collagen III, (c) collagen IV, and (d) α-SMA levels in renal tissues among all groups; (e) western blot of collagen I, III, and IV, α-SMA, and β-actin levels in renal tissues among all groups. Error bars represent standard deviations. *P < 0.05 compared with the control group; #P < 0.05 compared with the model group. APS, Astragalus polysaccharides; EMT, epithelial–mesenchymal transition; α-SMA, α-smooth muscle actin.
Expression of TGF-β1 and Smad3 proteins in kidney of rats detected by western blotting
Eight weeks after model establishment, expression of TGF-β1 and Smad3 in the model group were significantly increased (P < 0.05) compared with the control group. In addition, expression of TGF-β1 and Smad3 in the Astragalus polysaccharide groups were significantly decreased (P < 0.05) compared with the model group in a dose-dependent manner (Figure 7a–7c).
Figure 7.
Effect of Astragalus polysaccharides on expression of (a) TGF-β1 and (b) Smad3 proteins in renal tissues of diabetic rats 8 weeks after model establishment; (c) western blot of TGF-β1 and Smad3 protein levels of renal tissues among all groups. Error bars represent standard deviations. *P < 0.05 compared with the control group; #P < 0.05 compared with the model group. APS, Astragalus polysaccharides; TGF-β1, transforming growth factor-β1.
Discussion
DN is a serious complication of type 2 diabetes, next only to cardiovascular and cerebrovascular disease. In clinical practice, pathological changes including kidney enlargement, thickening and widening of glomerular capillary basement membrane, glomerulus sclerosis, tubular atrophy, and renal interstitial fibrosis9,10 are always observed, end-stage renal disease may develop, and hemodialysis or kidney transplantation is required to prolong life. Astragalus polysaccharide is a type of polysaccharide extracted from the Chinese herb Astragalus propinquus Schischkin. In this study, we successfully established a rat model of type 2 diabetes and administered Astragalus polysaccharides to model rats. The results showed that the Astragalus polysaccharides may play a significant role in preventing DN, and the mechanism of action may be associated with renal function improvement, regulation of blood lipids and blood glucose, and regulation of TGF-β/Smad signaling pathway in renal tissues.
Increases in indexes of renal function, including serum Cr, BUN, and urinary microalbumin, in patients with diabetes indicate damage to kidney tissue.11 With progressing DN, high glucose levels may induce an oxidative stress response, directly damage kidney tissue, and lead to an increase of transmembrane pressure of the glomerulus capillary, destruction of glomerular filtration function, an increase in renal interstitial fibrosis, and acceleration of DN. Patients may show symptoms of kidney enlargement, an increase in serum Cr and BUN levels, and very high levels of urine protein.12,13 In this study, we established a diabetic rat model by using a high-fat diet in combination with intraperitoneal injection of STZ. Two weeks after model establishment, FBG in the model group increased to >13.9 mmol/L, which satisfied the criterion for diabetes. At the same time, KW/TW, serum Cr, BUN, and 24-hour urine microalbumin in the model group were higher than those in the control group. Micropathological analysis showed evidence of renal damage in model rats. These findings indicated that rats in the model group had enlarged kidneys, renal function damage, and 24-hour microalbumin elevation, simulating symptoms of DN and confirming that the model was successfully established and applicable for studying DN in clinical practice.
After treatment with high, medium, and low doses of Astragalus polysaccharides, KW, KW/TW, serum Cr, BUN, and 24-hour urinary microalbumin were significantly decreased. In the Astragalus polysaccharide groups (all doses), renal damage was significantly milder compared with that in the model group. Partial glomerular enlargement, glomerular mesangial cell proliferation, and mild tubular stenosis were also observed, but were milder than those in the model group and dose-dependently decreased. Thus, Astragalus polysaccharides could improve the renal function of DN rats, delay the pathological process of kidney injury, and protect the kidney to some extent.
The TGF-β/Smads signaling pathway is important in the generation and development of DN.14,15 TGF-β1 is an important regulatory molecule for the synthesis of extracellular matrix in the kidney16 and an important initiating factor in the development of DN. When the kidney is damaged by high glucose and high lipid, the expression of TGF-β1 in the kidney may be upregulated, collagen synthesis in the kidney cells may be increased, and deposition of extracellular matrix is elevated and degradation decreases; as a result, the thickening of glomerular and tubular basement membrane, extracellular matrix deposition, and renal interstitial fibrosis process are promoted.17,18 Studies have shown that when the mesangial cells are stimulated by hyperglycemia, TGF-β1 synthesis is increased and extracellular matrix is deposited by induction.19 Smad protein is the only substrate of TGF-β1 signaling pathway; when expression of TGF-β1 is upregulated, expression of Smad protein is increased correspondingly, which may lead to transduction of the TGF-β1 signal from the receptor to the nucleus, accelerating the process of tissue fibrosis.20 Studies have shown that expression of Smad3 in fibrotic kidney tissue is significantly upregulated21 and that when the Smad3 gene is knocked out from renal tubular epithelial cells, Ang II-induced matrix is significantly reduced and renal fibrosis process may be stopped. We showed that 8 weeks after model establishment, expression of collagens I, III, and IV, α-SMA, TGF-β1, and Smad3 in renal tissue of the model rats was significantly increased compared with that in the control group. The expression levels of these proteins were significantly decreased in the Astragalus polysaccharide groups in a dose-dependent manner, suggesting that Astragalus polysaccharides may inhibit the activity of TGF-β/Smad signaling pathway, reduce the formation of extracellular matrix, and protect the kidney from renal interstitial fibrosis.
In summary, Astragalus polysaccharides can improve blood glucose levels and renal function of diabetic rats and protect the kidney. The mechanism may be associated with inhibition of the TGF-β/Smad signaling pathway and decreased formation of extracellular matrix.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_0300060520903612 for Astragalus polysaccharides protect renal function and affect the TGF-β/Smad signaling pathway in streptozotocin-induced diabetic rats by Xue Meng, Mingmin Wei, Dong Wang, Xiaohan Qu, Kun Zhang, Nan Zhang and Xinjian Li in Journal of International Medical Research
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Xinjian Li https://orcid.org/0000-0003-3698-1763
References
- 1.Gnudi L, Coward RJM, Long DA. Diabetic nephropathy: perspective on novel molecular mechanisms. Trends Endocrinol Metab 2016; 27: 820–830. [DOI] [PubMed] [Google Scholar]
- 2.Wada J, Makino H. Innate immunity in diabetes and diabetic nephropathy. Nat Rev Nephrol 2016; 12: 13–26. [DOI] [PubMed] [Google Scholar]
- 3.Gao J, Gu Z, Xu Y, et al. Peritoneal dialysis treatment of metformin-associated lactic acidosis in a diabetic nephropathy patient. Clin Nephrol 2016; 86: 279–282. [DOI] [PubMed] [Google Scholar]
- 4.Zheng T, Liu Y, Qin S, et al. Increased plasma dipeptidyl peptidase-4 activities are associated with high prevalence of diabetic nephropathy in Chinese patients with newly diagnosed type 2 diabetes: a cross-sectional study. Diab Vasc Dis Res 2016; 13: 127. [DOI] [PubMed] [Google Scholar]
- 5.Xiao B, Sun Z, Cao F, et al. Brain pharmacokinetics and the pharmacological effects on striatal neurotransmitter levels of Pueraria lobata isoflavonoids in rat. Front Pharmacol 2017; 8: 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu M, Cai J, Wei H, et al. Scoparone protects against pancreatic fibrosis via TGF-β/Smad signaling in rats. Cell Physiol Biochem 2016; 40: 277. [DOI] [PubMed] [Google Scholar]
- 7.Meng XM, Chung AC, Lan HY. Role of the TGF-β/BMP-7/Smad pathways in renal diseases. Clin Sci (Lond) 2013; 124: 243–254. [DOI] [PubMed] [Google Scholar]
- 8.Raina P, Sikka R, Kaur R, et al. Association of transforming growth factor beta-1 (TGF-β1) genetic variation with type 2 diabetes and end stage renal disease in two large population samples from North India. OMICS 2015; 19: 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Najafian B, Alpers CE, Fogo AB. Pathology of human diabetic nephropathy In: Lai KN and Tang SCW (eds) Diabetes and the kidney. Basel: Karger Publishers, 2011, pp.36–47. [DOI] [PubMed] [Google Scholar]
- 10.Alpers CE, Hudkins KL. Pathology identifies glomerular treatment targets in diabetic nephropathy. Kidney Res Clin Pract 2018; 37: 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin S, Jian T, Li J, et al. Association of chemerin and vascular endothelial growth factor (VEGF) with diabetic nephropathy. Med Sci Monit 2016; 22: 3209–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y, Liu J, Ten S, et al. Plasma heparanase is associated with blood glucose levels but not urinary microalbumin excretion in type 2 diabetic nephropathy at the early stage. Ren Fail 2017; 39: 698–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varma V, Varma M, Varma A, et al. Serum total sialic acid and highly sensitive C-reactive protein: prognostic markers for the diabetic nephropathy. J Lab Physicians 2016; 8: 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang F, Hao Y, Zhang X, et al. Effect of echinacoside on kidney fibrosis by inhibition of TGF-β1/Smads signaling pathway in the db/db mice model of diabetic nephropathy. Drug Des Devel Ther 2017; 11: 2813–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu ZJ, Shu S, Li ZJ, et al. Liuwei Dihuang pill treats diabetic nephropathy in rats by inhibiting of TGF-β/SMADS, MAPK, and NF-kB and upregulating expression of cytoglobin in renal tissues. Medicine (Baltimore) 2017; 96: e5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moritz RJ, Lebaron RG, Phelix CF, et al. Macrophage TGF-β1 and the proapoptotic extracellular matrix protein BIGH3 induce renal cell apoptosis in prediabetic and diabetic conditions. Int J Clin Med 2016; 7: 496–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou SX, Huo DM, He XY, et al. High glucose/lysophosphatidylcholine levels stimulate extracellular matrix deposition in diabetic nephropathy via platelet-activating factor receptor. Mol Med Rep 2018; 17: 2366–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, Zhang MH, Li Z, et al. Protective effects and mechanism of glycosides/phenol component of Moutan Cortex on renal injury of diabetic nephropathy rats. Zhongguo Zhong Yao Za Zhi 2016; 41: 1990. [DOI] [PubMed] [Google Scholar]
- 19.Sharma SR, Mishra SR, Wagle K, et al. Social determinants of common metabolic risk factors (high blood pressure, high blood sugar, high body mass index and high waist-hip ratio) of major non-communicable diseases in South Asia region: a systematic review protocol. Syst Rev 2017; 6: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong C, Gongora R, Sosulski ML, et al. Regulation of transforming growth factor-beta1 (TGF-β1)-induced pro-fibrotic activities by circadian clock gene BMAL1. Respir Res 2016; 17: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delić D, Eisele C, Schmid R, et al. Urinary exosomal miRNA signature in type II diabetic nephropathy patients. PLoS One 2016; 11: e0150154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_0300060520903612 for Astragalus polysaccharides protect renal function and affect the TGF-β/Smad signaling pathway in streptozotocin-induced diabetic rats by Xue Meng, Mingmin Wei, Dong Wang, Xiaohan Qu, Kun Zhang, Nan Zhang and Xinjian Li in Journal of International Medical Research