To the Editor,
The place of extracorporeal membrane oxygenation (ECMO) therapy in the coronavirus disease 2019 (COVID-19) outbreak is undefined.1 Our tertiary hospital is situated in Picardy (northern France), one of the areas most affected by the outbreak in France. We report a prospective case-series that describes the clinical course of patients with COVID-19 with respiratory failure requiring veno-venous ECMO between March 2020 and April 2020.
After ethical approval, we prospectively collected data on consecutive COVID-19 patients (confirmed with reverse transcription polymerase chain reaction testing) admitted to our referral centre for ECMO therapy. Demographic, biological, and clinical data were collected during ECMO therapy. Data on outcomes were reported. Fourteen patients were eligible for ECMO during this period; two of them died in peripheral centres during ECMO cannulation (one patient was in refractory septic shock and one patient had a massive pulmonary embolism). Twelve patients were admitted to our centre; all had percutaneous femoro-jugular cannulation. Patients were mainly male with a medical history of hypertension and diabetes (eTable 1 in the Electronic Supplementary Material [ESM]). Prior to ECMO, patients were severely hypoxemic with a median [interquartile range (IQR)] arterial oxygen partial pressure/fractional inspired oxygen (PaO2/FiO2) ratio of 76 [66–83] mmHg, pH of 7.31 [7.22–7.36], and partial pressure of carbon dioxide of 55 [42–60] mmHg. In line with current data, we found a mildly impaired respiratory system compliance of 30 [27–32] mL·mmHg−12 All patients were treated with inhaled nitric oxide, neuromuscular blockade, and prone positioning prior to ECMO therapy. Median [IQR] intensive care unit (ICU) length of stay before ECMO initiation was 6 [4–8] days. Median [IQR] lymphocyte count was 600 [400–1000] mm−3, fibrinogen 7.5 [5.1–9] g·L−1, and C-reactive protein 257 [181–295] mg·L−1 (eTable 2 as ESM).
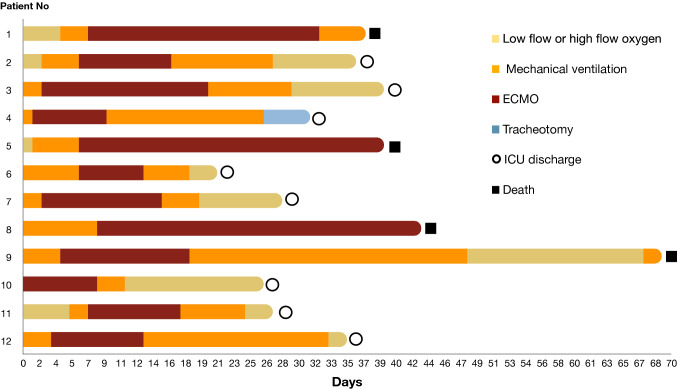
Ten (83%) patients were weaned from ECMO and two patients died under ECMO. Duration of ECMO therapy was 12 [9–22] days. Nine patients (75%) were weaned from mechanical ventilation. Overall, eight patients (67%) were discharged from the ICU and four (33%) died (Figure). Lung-protective ventilation was maintained during ECMO. Duration of mechanical ventilation was 25 [19–30] days and ten (83%) patients developed ventilator associated pneumonia (VAP). All patients received heparin treatment for an anti-Xa level target of 0.2–0.3 UI·mL−1. Thrombotic events occurred in 11 (92%) patients: deep vein thrombosis (four patients), renal replacement therapy (RRT) circuit clotting (two patients), complete clotting of the ECMO circuit (three patients), and pulmonary embolism (two patients). Eleven (92%) patients had Kidney Disease: Improvement of Global Outcomes 2 or 3 classification of acute kidney injury (AKI) and eight (67%) required RRT (eTable 3 as ESM).
Figure.
ECMO course for COVID-19 patients. COVID-19 = coronavirus disease 2019; ECMO = extracorporeal membrane oxygenation.
For patients weaned from ECMO, biological data showed an increase in lymphocyte count (from 560 [401–927] mm−3 to 1,280 [745–1,494] mm−3) and a decrease in fibrinogen (from 6.8 [5.1–8] g·L−1 to 3.6 [3.2–5.0] g·L−1). We observed an increase in PaO2/FiO2 ratio from 129 [104–210] mmHg to 268 [213–340] mmHg, and an initial decrease in respiratory compliance from 29.3 [26.7–30] to 19.3 [17.6–20] mL·mmHg−1, followed by an increase to 26.8 [25.4–27.1] mL·mmHg−1 (eFigure as ESM).
In this case-series of patients with COVID-19-related respiratory failure, we found a high rate of ECMO-weaning. Complications such as AKI, thrombosis, and VAP occurred frequently. A high risk of thrombosis for COVID-19 patients under ECMO has been suggested previously.3 At the initiation of ECMO, patients had low lymphocyte counts that increased progressively until weaning, in accordance with previous reports showing that most severe COVID-19 cases had persistently low lymphocyte counts.4 In our experience, a reduction in fibrinogen correlates with improvements in oxygenation. Decreasing fibrinogen levels may be a marker for improvement in the coagulopathy and a reduction in disease severity, with improvement in oxygenation.5 Studies with a larger sample size are needed to draw formal conclusions about the benefit of ECMO therapy for COVID-19-related respiratory failure.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Disclosures
None.
Funding statement
None.
Editorial responsibility
This submission was handled by Dr. Sangeeta Mehta, Associate Editor, Canadian Journal of Anesthesia.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ramanathan K, Antognini D, Combes A, et al. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. Covid-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beyls C, Huette P, Abou-Arab O, Berna P, Mahjoub Y. Extracorporeal membrane oxygenation for COVID-19-associated severe acute respiratory distress syndrome and risk of thrombosis. Br J Anaesth. 2020 doi: 10.1016/j.bja.2020.04.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Hu B, Hu C, Characteristics Clinical, et al. Clinical Characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients in severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.