Abstract
The present pandemic of SARS-CoV-2 has been a tough task for the whole world to deal with. With the absence of specific drugs or vaccines against SARS-CoV-2, the situation is very difficult to control. Apart from the absence of specific therapies, the lack of knowledge about potential therapeutic targets and individual perception is adding to the complications. The present review describes the novel SARS-CoV-2 structure, surface proteins, asymptomatic and symptomatic transmission in addition to the genotype and phenotype of SARS-CoV-2 along with genetic strains and similarity between SARS, MERS and SARS-CoV-2. Therapeutic strategies such as inhibition of the endocytic pathway and suppressing RNA polymerase activity by metal ions, which could be quite beneficial for controlling COVID-19, are outlined. The drug repurposing for SARS-CoV-2 is discussed in detail along with therapeutic classes such as antivirals, antibiotics, and amino quinolones and their probable role in suppressing SARS-CoV-2 with reference to case studies. The ongoing clinical trials both with respect to drug repurposing and vaccines are summarized along with a brief description. The recent advancements and future perspective of ongoing research for therapy and detection of SARS-CoV-2 are provided. The review, in brief, summarizes epidemiology, therapy and the current scenario for combating SARS-CoV-2.
Graphical abstract
1. Introduction
Coronavirus (CoV) belonging to the Coronaviridae family has spikes on the outer surface, making it look like a crown, thus deriving its name (Corona in Latin is Crown). These enveloped viruses are made up of a single-stranded RNA genomic material along with a helical nucleocapsid bound to the RNA in a bead and string type continuous conformation. They have a diameter size range of 65–125 nm and length varying from 26–32 kbs. This virus family has subgroups based on their genomic structure namely α, β, ϒ and δ CoV [1]. Till now, four CoVs were identified in human circulation which have low pathogenicity and caused mild respiratory symptoms viz NL63 and 229E which are α CoVs; OC43 and HKU1 which are β-CoVs. In the 21st century, two severe respiratory tract infection (RTI) viz. severe acute respiratory syndrome (SARS) caused by SARS-CoV (β-CoV) emerged in Guangdong province of China in 2002–2003, and Middle East respiratory syndrome (MERS) caused by MERS-CoV (β-CoV) emerged in Saudi Arabia in 2012. Both these CoVs were of bat origin and had a fatality rate of 11% and 34%, respectively. The intermediary hosts between bats to humans in SARS were palm civet cats and in MERS were dromedary camels. SARS and MERS caused respiratory distress and lung injury leading to pulmonary failure and fatality [[2], [3], [4], [5], [6]].
1.1. COVID-19 origin and transmission
In Wuhan, capital of Hubei province, China, in late December 2019, there were clusters of cases with severe pneumonia due to unknown causes. Most of the initial cases were identified to have common exposure to the Huanan seafood market which was involved in selling dead seafood animals and trading of live animals. As China had a quick surveillance system after the SARS outbreak, the patient's respiratory samples were sent to reference labs for etiological examinations. Assessment of the patients for viral pneumonia was done by testing the broncho-alveolar lavage fluid using polymerase chain reaction, whole-genome sequencing and cell culturing. Chinese government notified the World Health Organization (WHO) and meanwhile closed the Huanan seafood market on the 1st of January, 2020. The number of cases started increasing drastically since then, even to those with no exposure to the seafood market, thus indicating human to human transmission [7]. The first fatality was reported on 11th January. This turned out to be an epidemic, initially spreading to other countries like Thailand, South Korea, and Japan as there was massive Chinese migration due to Chinese New Year's Eve.
This virus was identified as β-CoV on 7th January. It had 96.2% homology to bat coronavirus namely RaTG13 genome whereas 79.5% homology to SARS coronavirus. The samples taken from the surroundings of the Huanan market showed positive results for this virus, confirming its origin. This CoV used the same receptor as of SARS-CoV i.e. angiotensin-converting enzyme 2 (ACE 2) receptor, to infect the humans [8]. On 12th January, WHO officially named this CoV as 2019-novel coronavirus (2019-nCoV). Later on, the 11th February, WHO termed the disease as coronavirus disease 2019 (COVID-19) and CSG (Coronavirus Study Group) of the International Committee on Taxonomy of Viruses changed the virus name officially from 2019-nCoV to SARS-CoV-2, due to a lot of similarities with SARS-CoV [[9], [10], [11]]. Preliminary finding projected the R0 value (basic reproduction number) for SARS-CoV 2 in a range from 1.4 to 6.5 [12]. R0 value (represents an average of new infections produced by an infectious person in a total population) gives the warning for virus transmission with respect to an epidemic i.e if R0 > 1, the infected number could escalate and if R0 < 1, the transmission will die out soon.
The transmission from patients to healthcare workers was seen on 20th January. After this Wuhan and other cities of Hubei province were placed under complete lockdown. There were cases of COVID-19 in individuals who did not travel to China, which further suggested transmission between humans. All the domestic and international airports had a screening mechanism in place in order to detect any symptomatic travelers who were kept under quarantine and were allowed to go if they tested negative for the COVID-19 test. It soon became apparent that the disease could transmit from an asymptomatic person (before the commencement of any related symptom). All the countries evacuated their respective citizens from Wuhan and travelers from China and other affected countries were kept under quarantine/isolation for 14 days and went through the COVID-19 test. On 1st February, WHO declared COVID-19 as a Public Health Emergency of International Concern. Cases have continued to exponentially increase since then. On 11th March, WHO finally acknowledged COVID-19 as a Pandemic. As of April 16th, the total COID-19 cases are almost 2 million worldwide. It is to be noted that the number of cases has reduced in China, with a drastic increase in countries like the USA with 6.44 lacs cases, Spain and Italy with almost 1.8 and 1.6 lacs cases each. Most of the affected countries have declared a lockdown, with travel restrictions of any kind and border control measure taken to control the pandemic and reduce the transmission.
2. The similarity between COVID-19, SARS, and MERS
The novel human CoVs (SARS-CoV-2) was recognized as the source of an unidentified form of viral pneumonia called COVID-19 that originated in Wuhan, China and was avowed a pandemic by WHO on 11th March 2020 [13,14]. Prior to this, six forms of human CoVs were known which involve HCV-NL63 and HCoV229E, which belong to the genus α-coronavirus; and HCV-OC43, HCoVHKU1, Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) and the Middle East Respiratory Syndrome coronavirus (MERS-CoV) that belong to the genus β-coronavirus. CoVs were not widely known globally until the SARS pandemic in 2003, followed by MERS in 2012. An overview of the similarities and differences in pathogenesis, transmissibility and clinical condition between COVID-19, SARS and MERS is mentioned (Table 1 ) utilizing the most recent works concerning COVID-19 and compared with scientific data from previous findings of SARS and MERS.
Table 1.
Pathogenic, epidemiology and phylogenic features of SARS-CoV-2, SARS-CoV, MERS-CoV.
| Virus | Location of origin | Phylogenetic origin | Source/intermediate source | Receptor | Mortality rate | Ro |
|---|---|---|---|---|---|---|
| SARS-CoV-2 | Wuhan, China | Class I, Cluster IIa | Bats/unknown | ACE-2 | 2.3% | 2–2.5 |
| SARS-CoV | Guangdong, China, 2002–2003 | Class I, Cluster IIb | Bats/ palm civets or dromedary camels | ACE-2 | 9.5% | 1.7–1.9 |
| MERS-CoV | Saudi Arabia | Class II | Bats/ palm civets or dromedary camels | DDP4 | 34.4% | 0.7 |
2.1. Pathogenesis
MERS-CoV and SARS-CoV are considered extremely pathogenic and were transmitted by palm civets or dromedary camels to humans by means of bats. The characterization and genomic sequencing of the SARS-CoV-2 showed that the novel CoVs was 88% identical to the two earlier reported bat-derived SARS-like CoVs but were different from SARS-CoV (by 79%) and MERS-CoV ( by 50%), indicating that it evolved from bats but the intermediate source is not yet known. The phylogenetic analysis revealed that SARS-CoV-2 belonged to the genus Betacoronavirus and was genetically dissimilar from MERS-CoV and SARS-CoV [15]. The size of the coronavirus genome varies from approx. 26,000 to 32,000 bases and contains a 6 to 11 number of ORFs. The first ORF that encodes 16 non-structural proteins comprises of approx. 67% of the total genome while remaining ORF encodes the structural protein and accessory proteins. Among the four major structural glycoproteins (as shown in Fig. 1 [16]), spike glycoprotein(S) plays a vital part in binding to the host receptor. Homology modeling showed that SARS-CoV-2 and SARS-CoV enter the lung and utilize the same human cell receptor i.e. ACE-2 despite amino acid variation at key residues, while MERS-CoV enters the host cell via DPP4 [17]. Six mutations arose in the variable sections of the receptor-binding domain (RBD) of SARS-CoV-2, but no amino acid replacements were observed in RBD that directly interacts with ACE-2 receptors of the host cell [14]. Receptor affinity analysis claims that the SARS-CoV strain binds less efficiently than the novel SARS-CoV-2 strain, and further mutation in the nucleotide sequence of RBD may increase its pathogenicity [17]. It is uncertain whether higher SARS-CoV-2 affinity to ACE-2 receptor than SARS-CoV could lead to more severe lung connection. This should be further investigated since in vitro studies by inoculating SARS-CoV-2 in human airway epithelial cells have shown a cytopathic effect with the cessation of cilia movements [18]. Other than the lung, the ACE-2 receptors are highly expressed in the small intestine, kidney, testis, and liver, hence, these organs may also get affected by SARS-CoV-2 [19].
Fig. 1.
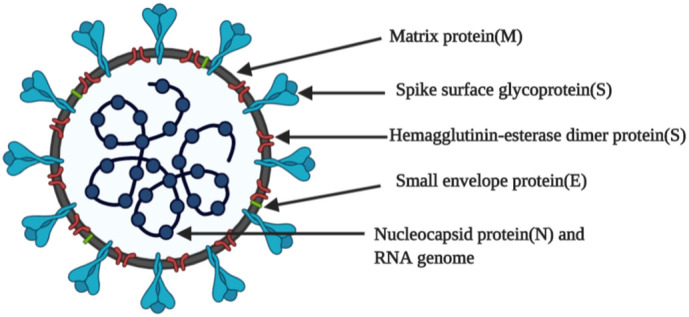
Structure of SARS-CoV-2 (COVID-19).
2.2. Clinical features
With comparison to the current clinical characteristics of COVID-19, it does not appear to be different from SARS and far less lethal than MERS, even though it spreads in the community at a rapid pace. Common symptoms include cough, sore throat, fever, and dyspnoea, with at least one symptom in all the infected patients. However, the Chinese Centre for Disease Control reports 81% of cases to have mild indications while 1.2% was asymptomatic [20]. Research laboratory results do not vary significantly in patients infected with COVID-19 than those diagnosed with other CoVs infections, the most common being lymphopenia together with low platelet amount and lower levels of albumin while the levels of creatine kinase, aminotransferases, lactic dehydrogenases, and C-protein increased [21]. Patients diagnosed with SARS-CoV-2 and SARS-CoV had an adverse clinical path with the onset of dyspnoea within 5 days followed by Acute Respiratory Distress Syndrome within 30% of the cases within a span of 5 days and need of mechanical ventilation in 17% of the cases. Due to the abundance of DDP4 receptors in tubules and glomeruli, a serious complication for MERS appears to be acute kidney injury, however, it rarely occurs in COVID-19 and SARS [22]. As symptoms commence, the viral loads in COVID-19 were found to be higher in the nose than in the throat and gradually decrease within days, contrary to SARS where the maximum shedding is observed after 10 days from onset of indications [23,24]. Through the experiences of the MERS and SARS epidemics, the lessons the world has learned are the finest cultural tools to tackle this novel worldwide danger.
3. Structure of SARS-CoV-2
SARS-CoV-2, the causative agent for the current pandemic situation is similar to SARS-CoV, which caused an epidemic in early 2003. Both viral strains belong to the B lineage of β-coronaviruses genera. These viruses are the largest RNA viruses reported to date. SARS coronaviruses have a single-stranded positive-sense RNA that complexes with capsid protein into a bead-on-the-string arrangement which forms the protein-ribonucleic helical core of the virus particle. The genetic core is enveloped in a lipid bilayer where structural proteins of the virus particle are anchored. Membrane (M) protein, the most abundant triple-spanning membrane protein that is crucial for virus assembly. Envelope (E) protein, the smallest among the other foremost structural proteins, predominantly localizes in vesicle trafficking organelles such as Golgi and ER/Golgi interface thus play a central role in maturation and budding. Spike (S) protein forms the trimeric spike which extends from the lipid membrane that gives a crown-like appearance; hence this family of the virus is named ‘corona’ viruses. Spike protein is crucial for the infectivity of the virus, tissue tropism, and virus internalization into the host cell. Structural proteins work in synchrony with each other to bring about virus replication in the host cell [[25], [26], [27]].
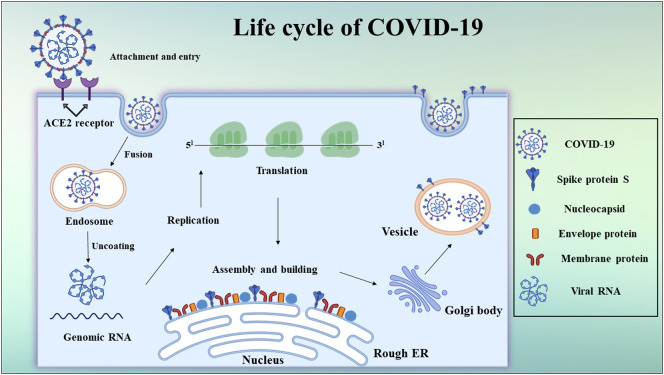
Recent studies have demonstrated that similar to SARS-CoV, SARS-CoV-2 also utilizes ACE-2 on the host cell surface for cellular entry. Upon binding to the receptor, virus is thought to be internalized by receptor mediated endocytosis and RNA (viral genomic material) is then released into host cell cytoplasm. Viral genome is translated to form non-structural proteins, which constitute replication transcription complex (RTC). The RTC leads to the formation of sub-genomic mRNAs which encode structural and accessory proteins. The new viral genomic RNA bound to nucleocapsid protein and other accessory proteins of virus present as virions (viral buds) in endoplasmic reticulum and golgi are transported via vesicle cargoes to fuse with the plasma membrane and finally are released out of cell as depicted in Fig. 2 [[28], [29], [30], [31], [32], [33], [34]].
Fig. 2.
Life cycle of SARS-CoV-2 (COVID-19).
3.1. RNA genome
SARS-CoV-2 is a positive-sense RNA virus, with a genome scope of 30 kb consisting of flanking untranslated regions (UTRs) at both 5′ and 3′ ends. The open reading frames ORF1a (open reading frame) and ORF1b present at 5′ proximal RNA genome encodes for a polyprotein, which upon proteolytic cleavage results in 16 putative nonstructural proteins (nsps) such as viral proteases (nsp3, cysteine protease, and nsp5), RNA-dependent RNA polymerase ([RdRp], nsp12), helicase (nsp13), and other nsps which are expected to be tangled in the viral transcription and replication. The distal portion of the viral genome towards 3′ end consists of 12 nested orfs which code for the structural proteins namely spike (S), Envelope (E), Membrane (M) and Nucleocapsid (N) and other accessory proteins. Genome analysis has tremendously helped in gaining more insights about the SARS CoV-2. Sequence analysis revealed a major distinction in spike, orf3b and orf 8 regions which were previously reported as recombination hotspots [35]. Several transcriptional regulatory elements such as stem-loop structures present in 5′ and 3′ UTRs and frameshift elements contribute to the complex transcription and translational properties to the viral RNA [[36], [37], [38], [39]].
Besides understanding about the genetic annotation, understanding the sequences in the sub-genomic mRNAs, and more insight into the secondary structures of the genomic RNA would enable in development of genome targeted therapeutics.
3.2. Spike (S) protein and COVID-19
Spike protein is a densely glycosylated class I fusion protein on the surface of virus particles. It comprises of a large ectodomain, a single-pass transmembrane domain that anchors the protein to lipid bilayer and a small intracellular segment. The ectodomain has two subunits S1 and S2 both forming homotrimers. The C-terminal functional domain of S1 subunit is involved in receptor binding and S2 subunit includes a fusion peptide, HR1 and HR2 (hepted repeat 1 and 2), a transmembrane domain and cytoplasmic domain that aids in viral envelope fusion with host cell membrane via the endosomal pathway.
The S protein on the surface of a virus particle is present in a pre-fusion form [40]. Upon contact with host cell, S protein undergo a priming by host cell membrane proteases such as TMPRSS2 (serine protease) to induce membrane wrapping and efficient internalization [41,42]. Structural analysis demonstrated that the RBD of SARS-CoV-2 bound with ~10 times advanced affinity to ACE-2 than that of SARS-CoV and in receptor unbound state, the S2 domain was more flexible [43]. Another notable change in the spike protein of SARS-CoV-2 is the presence of the S1/S2 furin-like rift spot that is absent in SARS-CoV. Several reports suggest that the presence of this proteolytic site increases the pathogenicity of the virus. Furin like rift spot is also reported to increase the tissue tropism of viruses [44,45]. So far, SARS-CoV-2 was thought to infect upper respiratory tissue, surprisingly a recent report shows the ability of the SARS-CoV-2 to infect T-lymphocytes also through membrane fusion process [46].
Spike protein being the first contact site between viruses and cells is subjected to tremendous evolutionary pressure. Any changes in spike protein would have a profound effect on the infectivity and transmission of viruses. In the spike protein of the present pandemic SARS-CoV-2, changes acquired such as furin-like cleavage site, structural changes at the receptor binding sites are being correlated for the species jumping and efficient human-to-human transmission. The virus is also found to form syncytium which enables virus spreading by cell-cell fusion which might also leads to the rapid infectivity of the SARS-CoV-2 virus [47].
3.3. SARS-CoV-2 main protease
The main protease (Mpro) also recognized as 3C-like protease is encoded by nsp5. It is the first protein that is auto-cleaved and it further cleaves the polyprotein into individual members of non-structural proteins at the cleavage site LeuGln↓ (Ser, Ala, Gly). It attains a stable active form as an octamer while it's monomer and dimer exist in equilibrium. [48]. SARS-CoV-2 shares 96% arrangement resemblance with SARS-CoV. The crystal structure of SARS-CoV-2 Mpro showed that polar amino acids in the dimer interface have been replaced with non-polar ones. This mutation has resulted in the increased catalytic activity of the dimer while dimer dissociation constants have remained the same [49]. Protease is essential for virus proteome production and replication. Therefore, Mpro is considered as one of the potential targets to treat COVID-19.
3.4. SARS-CoV-2 RNA dependent RNA polymerase (RdRp)
Viral replication and transcription of SARS CoV-2 is carried out by complex protein machinery. Proteins of this complex are translated from ORF1a and ORF1b and are the part of large polypeptide chain. Overall architecture of RdRp complex of SARS-CoV-2 is found to be analogous to that of SARS CoV. The key constituent of this enzyme complex is RNA-dependent RNA polymerase (nsp12). It forms the complex with other co-factors such as nsp7 and nsp8 and catalyses the development of viral genomic RNA. The complex has a central groove where template-directed RNA synthesis id catalysed. The nascent RNA strands exit path is positively charged which bring about electrostatic interaction between nascent RNA strand and enzyme complex. This exit path is also accessible to the solvents [10,50]
RNA polymerase unit (nsp12) is the promising target to develop viral inhibitors and the promising drug remdesivir, which has entered into clinical trials now for the treatment of COVID-19, also inhibits the activity of RdRp.
4. Enzymes and pathways as potential therapeutic targets
Upon infection from any microbes, the proteins exposed on the surface of these intruders are at first recognized by our immune system. A systematic and specific immune response is then elicited to fight back these infectious agents. However, in certain infections, the immune system might be taken over by the infectivity of the microbes which results in pathological conditions. Thus, in this condition supporting the immune system with synthetic drugs would not only help in combating the infection but might also enable in the recovery of defence of our body. Similar to the approach of the immune system, surface structures are always the first class of probable candidates to be addressed by the therapeutics development. Drugs to combat SARS-CoV-2 infection are in search. In particular, proteins of SARS-CoV-2 such as spike protein (S), RNA dependent RNA polymerase and Main protease are being studied to develop potential inhibitors. However, a detailed study of the other non-structural proteins is the need of the hour to increase the spectrum of proteins which can be targeted for control of COVID-19 infection.
4.1. Role of metal ions in suppressing RNA polymerase activity
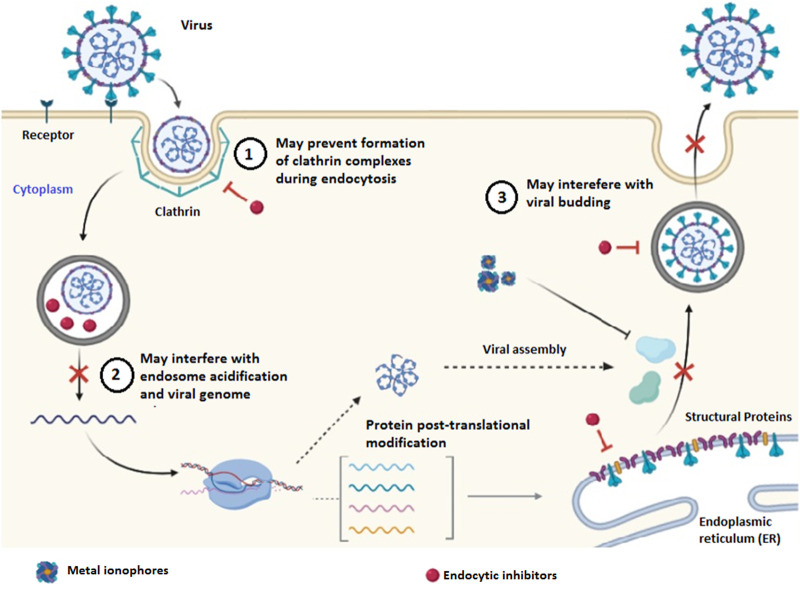
Metal ions are an essential part of some viral proteins and play a vital part in their pathogenesis and existence. Binding of metal ions might activate or inactivate the protein. Because metal ions can bind to viral proteins, different metal ion-conjugates were tested for their inhibitory properties of 3CL protease of SARS-CoV. Hg2+, Zn2+, and Cu2+ exhibited inhibitory properties with Zn2+ being more effective. These metals exhibited non-competitive inhibition [51]. Many of the putative enzymes coded from the nsp cluster of the SARS-CoV genome have a Zn2+ binding domain [52]. A detailed molecular interaction study demonstrated that Zn2+ ions and Zn2+ ionophores effectively inhibited replicase and also RdRp at the elongation phase [53]. Helicase is one of the enzymes from the nsp group of proteins in SARS-CoV. It is involved in unwinding the dsRNA or dsDNA in 5′ to 3′ polarity. The activity of helicase is vital for replication and also the transcription of the viral genome. Bismuth compounds efficiently inhibited the helicase activity [54]. Metals being versatile in their utility can be used to minimize the survival of viruses (Fig. 3 ). In the current pandemic, metal ion based coating to the hospital surfaces and personal protection equipment (PPE) are being extensively explored. However, molecular interactions and mechanism of the inhibitory properties of metal ions need to be explored in detail.
Fig. 3.
Alternate pathways to control virus replication.
4.2. Clathrin-mediated endocytosis as potential targets?
Besides targeting viral proteins, the cellular mechanisms involved in virus internalization can also be a viable therapeutic strategy (Fig. 3). The entry mechanism and internalization process for SARS-CoV-2 into cells have not been clarified yet. Since SARS-CoV-2 binds to the similar receptor as SARS-CoV, and is also vulnerable to chloroquine, a lysosomotropic agent; hence there is an increased probability that the SARS CoV-2 also utilize the similar endocytic mechanism for the entry into the host cells. Clathrin-mediated endocytosis is the most prevalent process of internalization including that of SARS-CoV. Therefore, drugs such as chlorpromazine inhibiting clathrin-mediated endocytosis are tested for their potential in inhibiting SARS.CoV-2 [55].
5. Drug repurposing and COVID-19
Currently, there is no particular antiviral therapy suggested for COVID-19 and no vaccine is presently available as its pathogenesis and proliferation pathways still remain unknown. The treatment is based on symptoms, and oxygen therapy is the main treatment method for severely infected patients. In cases of respiratory failure refractory to oxygen therapy and artificial ventilation may be required while hemodynamic assistance is important for septic shock management [56]. However, therapeutic treatment is a must for the future and some of available drugs can be repurposed for the treatment of COVID-19. There are many choices for containing or avoiding emerging COVID-19 infections, involving vaccines, oligonucleotide-based drugs, monoclonal antibodies, interferon treatments, peptides, and small-molecule medicines [57]. However, it's likely to take months to years to develop new drug/vaccine associated interventions. Given the severity of the COVID-19 pandemic, we here focused on the drug repurposing techniques and the ability to repurpose current therapeutic agents for COVID-19 treatment. Drug repurposing (reprofiling, drug repositioning or re-tasking) is a process to redevelop a compound /drug for the use in a different disease other than that of its original use. It is a strategy of identifying the approved, discontinued, shelved and investigational drugs for new uses that are authorized for the treatment of other diseases [58]. About one-third of the approvals involve repurposed drugs [59]. The basic principle involved in drug repurposing is that a common molecular pathway is responsible for many diseases and a host of detailed information is available on the formulation, dose, toxicity, pharmacology and clinical trial data of the approved/ shelved or discontinued drugs. The advantages of drug repurposing are in most of the cases safety assessment, preclinical testing, and in some cases formulation development won't take much time and thus reducing the time required for the drug development. For repurposed drugs the chances of failure will be less as the preliminary stages of evaluating drug efficacy and safety have been already completed and the drug has already been found to be adequately safe in a preclinical and human model. Hence, repurposed drugs open a new avenue for the research of new pathways and targets [60] and finally, investment for repurposing the drug is less as it often bypasses the Phase-1 trial in comparison to the development of a new drug entity [61].
5.1. Potential candidates for repurposing for SARS-CoV-2
5.1.1. Antimalarial agents
5.1.1.1. Chloroquine
The SARS-CoV-2 (COVID-19) host interactions can be modulated by small-molecule agents authorized for other human diseases. Chloroquine (CQ), a commonly used anti-malarial drug and authorized immune modulator, has been recently identified as a possible broad-spectrum antiviral drug [62,63]. CQ is reported to prevent virus infection by elevating the endosomal pH needed for virus/cell fusion and by interacting with the glycosylation of SARS-CoV cell receptors [64]. In one of the recent findings, CQ demonstrates inhibitory activity towards COVID-19 (EC50 = 1.13 μM in Vero E6 cells) [65] and is examined in an open-label trial (ChiCTR2000029609) [66]. Subsequently, a number of clinical trials (ChiCTR2000029542, ChiCTR2000029559, ChiCTR2000029609, ChiCTR2000029740, ChiCTR2000029760, ChiCTR2000029761, ChiCTR2000029762, ChiCTR2000029803, ChiCTR2000029826, ChiCTR2000029837, ChiCTR2000029868, ChiCTR2000029898, ChiCTR2000029899, ChiCTR2000029935 and ChiCTR2000029939) were immediately carried out in China for the safety and efficacy of CQ and hydroxychloroquine for treating COVID-19 linked pneumonia [66]. Reports have so far shown that chloroquine phosphate is preferred in managing the treatment by constraining the worsening of pneumonia, increasing lung imaging tests, encouraging virus-negative transformation, and reducing the duration of illness [67]. CQ is an inexpensive and effective drug and has been in use for over 70 years, making it potential and clinically beneficial towards COVID-19.
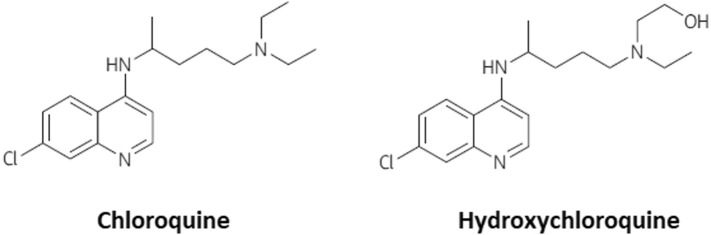
5.1.1.2. Hydroxychloroquine
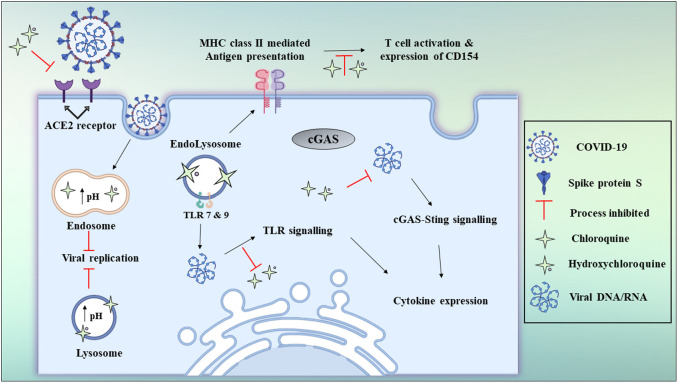
Hydroxychloroquine (HCQ), which demonstrates a highly related structure (as shown in Fig. 4 ) and antiviral activity to that of CQ, may function as a safer treatment method for COVID 19, as it demonstrates anti-SARS-CoV activity in vitro [68] with better clinical safety profile as that of CQ (during long-term use) which will allow high daily dose [69]. Both compounds can interact with ACE-2 glycosylation and decrease the binding capacity between ACE-2 on the host cells and the COVID-19 surface spike protein. They may also raise the pH of lysosomes and endosomes, by preventing the virus fusion mechanism with host cells and corresponding replication. When HCQ enters antigen-presenting cells (APCs) it inhibits the development of antigen and the autoantigen exposure to T cells controlled by major histocompatibility complex (MHC) class II. It represses subsequent stimulation of T cells and CD154 expression and other cytokines. Furthermore, HCQ disrupts DNA / RNA's interaction with toll-like receptors (TLRs) and the cGAS nucleic acid detector, and thus the expression of pro-inflammatory genes cannot be triggered. Consequently, CQ and HCQ administration is not only expected to alleviate COVID-19's extreme development by preventing its replication and invasion but it also reduces the risk of cytokine storm by blocking T cell stimulation as depicted in Fig. 5 [70]. In a more recent study, an open-label non-randomized study investigating the impact of HCQ (EU Clinical Trial Number: 2020-000890-25) reported on a group of 36 patients. It stated a substantial decrease in nasopharyngeal swab viral positivity, relative to control, 6 days following inclusion in the HCQ group. However, 16 patients were assigned as controls in a deviation from their chart defined protocol, and 6 patients obtained simultaneous azithromycin (AZM) treatment to avoid bacterial superinfection. Patients who received AZM were chosen based on clinical assessment. After 6 days, the subgroup getting AZM had negative virus swabs relative to 57% (8/14) with HCQ alone and 12.5% (2/16) with control [71]. It shows that AZM added to HCQ has been significantly more effective in eliminating the virus. However, this study is constrained by its low sample size and lack of randomization which gives a lot of room for study with a larger sample size.
Fig. 4.
The chemical composition of chloroquine and hydroxychloroquine.
Fig. 5.
Schematic representation of chloroquine and hydroxychloroquine antiviral mechanisms.
5.1.2. Antiviral agents
5.1.2.1. Lopinavir and ritonavir
Several kinds of research have concentrated on repurposing existing antiviral therapies, in particular those that have shown previous effectiveness towards MERS-CoV and SARS-CoV. Authorized protease inhibitors were confirmed to be effective towards SARS-CoV and MERS-CoV such as lopinavir (LPV) and ritonavir (RTV). Clinical trials (e.g., ChiCTR2000029539) are conducted to test HIV protease inhibitors like LPV and RTV in patients with COVID-19 infection [66]. Initially, LPV and RTV were suspected to suppress SARS and MERS 3-chymotrypsin-like protease and proved to be linked with better clinical results of SARS patients in a non-randomized open-label study [72]. However, it is up for debate that LPV and RTV could efficiently block the COVID-19 3-chymotrypsin-like and papain-like proteases. HIV protease is from the family of aspartic proteases while the two coronavirus proteases belong to the family of cysteine proteases. In fact, HIV protease inhibitors have been precisely designed to match the C2 structure in the HIV protease dimer catalytic site, however, this C2-symmetric structure is missing in coronavirus proteases [57]. Assuming that HIV protease inhibitors modify host pathways to interact with coronavirus infection indirectly, their potency remains a matter of concern. In the recent study, 51 COVID-19 patients with conventional Chinese medicine, interferon, LPV, RTV and brief-term (3 to 5 days) corticosteroids were successfully treated and 50 patients were recovered and discharged [73]. A 54-year-old man having COVID-19 was successfully treated with LPV and RTV from day 10 of infection, 2 tablets (LPV 200 mg/RTV 50 mg) every 12 h was mentioned in a study by Lim et al. The viral β-coronavirus load was begun to decrease after the first day of administration and since then, few to no significant coronavirus titers were identified [74]. The observation suggest that although there are no drugs for COVID-19 at present, the repurposing of antiretroviral drug which have been previously approved by United States Food and Drug Administration (FDA) is really helpful in supressing the virus as well as treating already infected individual. Similar combination of other retroviral agents with appropriate dose and dosing frequency will be quite helpful in facing this situation.
5.1.2.2. Remdesivir
Remdesivir (RDV) is a broad-spectrum anti-viral and an adenosine triphosphate nucleoside analog. It is an investigational small molecule developed by Gilad Sciences. Inc in 2015 primarily for the treatment of Ebola and it is in Phase -II trials for its activity against the Ebola virus-induced hemorrhagic fever [75]. In vivo and in vitro animal study data shows that RDV is active against a host of virus-like: filoviridae, parmyxoviridae and the coronaviridae (Middle East Respiratory Syndrome-Corona Virus [MERS CoV], Severe Acute Respiratory Syndrome-Corona Virus 2 [SARS-CoV-2]) [7,76]. The prodrug of RDV (GS-443902) constrains the viral RNA dependent RNA polymerase at the early stage of the viral infectious cycle. Chain termination and lethal mutagenesis are the other possible mechanisms of action of RDV [77]. RDV is an antiviral that is known to reduce the viral load by decreasing viral replication. The animal studies showed that the RDV efficiently decrease the viral load in the lung tissue of MERS-CoV infected mice and further it improved the pathological damage done to the lung tissue and lung function [78]. Another study showed that the metabolite of RDV GS-5734 was operative against an array of CoVs like pre-pandemic bat CoVs, bat CoVs and circulating contemporary human CoV in primary human lung cells [79]. They also showed that GS-5734 halts the replication of MERS-CoV and SARS-CoV and thereby reduces the viral load in primary human epithelial cell cultures. RDV blocks SARS-CoV infection at a micromolecular and at a half cytotoxic concentration [65]. There is no sufficient data available on the pharmacokinetics of RDV, however, the data is extrapolated from in vivo mouse and non-human primate data. RDV is metabolized into its prodrug by anabolic intracellular kinase [80]. Once-daily dosing was found to reach a maximum concentration with a half-life of approximately 20 h in vivo non-human primate model and an in vitro human lung cell model [81]. The data relating to protein binding, hepatic clearance, adverse effects, and dosing information is not yet available. As per the clinical study data currently, a loading dose of 200 mg intravenous RDV is given followed by 100 mg intravenously for 5 to 10 days [82]. In order to evaluate the safety and efficacy of RDV in COVID-19 patients, several clinical trials have been started. A randomized, double-blind, controlled trial to evaluate the safety and efficacy of RDV in hospitalized patients with moderate to mild COVID-19 respiratory disease has been initiated by China-Japan friendship hospital, China. The trial consists of 08 patients divided into 2 groups with one group receiving active RDV and the other control group receiving RDV placebo. The results of the trials are expected to be obtained by the end of April 2020 [83]. Gilead Lifesciences has initiated a phase-3 randomized study to evaluate the efficacy of RDV regimens with respect to clinical status by a 7-point ordinal scale on day 14 with 2400 participants The study completion date is expected to be May 2020 [84]. National Institute of Allergy and Infectious Diseases (NIAID) has initiated a multicenter, double-blind, randomized, adaptive, placebo-controlled trial to assess the safety and efficacy of RDV in hospitalized adults diagnosed with COVID-19 [85].
5.1.2.3. Ribavirin
Ribavirin (RBV) is an antiviral purine nucleoside analog approved for chronic hepatitis C virus (HCV). It is a broad-spectrum anti-viral drug having activity against hepatitis B and respiratory syncytial virus (MERS-CoV, SARS-CoV-2) [86]. RBV is metabolized by adenosine kinase to its prodrug mono, di and triphosphate metabolites. The synthesis of viral mRNA polymerase is inhibited by the direct binding of RBV triphosphate (RTP) to the nucleotide-binding site of the enzyme which results in the reduction in the production of defective virions and decreases the viral load [87]. RBV also competitively inhibits host inosine monophosphate dehydrogenase (IMPDH) which further inhibits the guanine nucleotides synthesis. The decrease in guanosine triphosphate leads to the depletion of viral protein synthesis and halts the process of viral genome replication [88]. RBV also has an immunomodulatory effect on the host by promoting the production of cytokines which stimulates the humoral response and enhances immunity towards the virus. The mechanism of action of RBV that includes the inhibition of mRNA capping which results in induction of mutation in the viral replication is the main reason for considering RBV as a potential treatment approach to treat SARS-CoV-2 [89]. High doses of RBV are required for treatment. RBV, when given as a monotherapy, shows resistance against SARS and MERS and therefore it's given in combination with other antiviral drugs like lopinavir, chloroquine analogs and with interferon-α [90]. A Study on Vero and LLC-MK2 cells showed that the combination of RBV with interferon-α inhibits the replication of novel coronavirus (nCoV) isolate hCoV-EMC/2012 in the cell lines with a lower concentration of both RBV and interferon-α, thus making RBV as a promising treatment for COVID-19 [90]. The pharmacokinetic profile of RBV is well established. It is rapidly and extensively absorbed orally with an oral bioavailability of 64%. It is metabolized in the liver by adenosine kinase to its mono, di and tri phosphate metabolites. The half-life of RBV is about 120–170 h. For the treatment of COVID-19, intravenous RBV is given in a dose of 500 mg, 2–3 times daily in combination with lopinavir/ritonavir or interferon-α for not more than 10 days [91]. The main adverse effects of RBV are hypocalcemia, hemolytic anemia and hypomagnesemia [92]. RBV should be avoided in patients with preexisting cardiac disease and in patients with poor renal function. RBV is known to cause teratogenic effects and therefore it should be avoided in pregnant women [93]. Several clinical trials to assess the potency of RBV in treating COVID-19 are underway. A randomized, open labeled, controlled phase-2 trial to assess the efficacy of combination therapy of lopinavir/ritonavir, RBV and interferon beta-1b has been initiated by The University of Honkong, China. The aim of the study is to assess the effect of combination therapy in suppressing the viral load, shorten the hospitalization time, recovery and reduce the mortality in patients with COVID-19 infection compared with lopinavir/ ritonavir [94].
5.1.2.4. Arbidol
Arbidol (ARB) or Umifenovir is an antiviral drug currently approved in China and Russia for the prophylaxis treatment of respiratory viral infections and influenza [95]. It is a broad-spectrum antiviral drug that has demonstrated activity against many other viruses like the Lassa virus, zika virus, Ebola virus, flavivirus, herpes simplex and in food – mouth disease [96]. Furthermore, it has shown activity in vitro against a host of other viruses like hantaan virus, chikungunya virus, hepatitis B and C virus, reovirus and coxsackievirus [97]. ARB has shown activity against SARS-CoV-2 virus and thus it's been currently investigated for its potential to treat COVID-19 prophylactically [95]. The broad-spectrum activity of ARB is mainly because of its dual action as a host targeting agent (HTA) that acts on one or multiple stages of the viral life cycle and as a direct-acting antiviral (DAA) which induces direct virucidal effects on virus [95]. The DAA action is mainly because of the ability of the ARB to form interactions with amino acid residues. ARB interferes with intracellular trafficking, clathrin-mediated endocytosis and directly with viral lipid envelope [98]. The interaction of ARB with viral glycoprotein aromatic residues that are involved in fusion and cellular recognition is the main reason for ARB's antiviral activity [99]. Because of its ability to interact with both viral proteins and lipids ARB can interfere with the later stages of the viral life cycle [100]. The antiviral activity of ARB against SARS-CoV-2 has gained the attention of researchers in the use of ARB as a potential treatment therapy alone or in combination therapy to treat COVID-19. Currently, China is conducting various studies to evaluate the potential of ARB in treating COVID-19 and has recommended the use of ARB as a treatment option. A study conducted in China reveals that ARB effectively inhibits SARS-CoV-2 infection at low concentration of 10–30 μM in vitro. The National Health Commission (NHC) of the People's Republic of China has included ARB in the latest version of the Guidelines for the Prevention, Diagnosis and Treatment of Novel Coronavirus-induced Pneumonia for the tentative treatment of COVID-19. ARB is rapidly absorbed following its oral administration. The maximum concentration of the drug in plasma is estimated to be 415–467 ng/ml and the time taken to reach maximum plasma concentration is between 0.65–1.8 h [101]. It undergoes cytochrome P450 dependent hepatic metabolism and it is also metabolized by intestinal microsomes [102]. Phase-1 metabolic pathways include hydroxylation, sulfoxidation, and N-demethylation, whereas the Phase-2 pathway is mainly due to glucuronide and sulfate conjugation reactions [103]. The plasma half-life of ARB is 17–21 h and it is eliminated majorly through feces. Currently to treat COVD-19 a dose of 200 mg of ARB 3 times a day is given orally for not more than 10 days [104]. A phase-4 for pneumonia caused by SARS-CoV-2, randomized, multicenter, open study has been initiated by Jieming QU, Rujin Hospital, China to observe the safety and efficacy of ARB in the treatment of pneumonia induced by SARS-CoV-2 [105]. Another randomized, open study to assess the safety and efficacy of Bromhexine Hydrochloride tablets given in combination with ARB and recombinant human interferon-α 2b in the treatment of mild to moderate COVID-19 pneumonia has also been initiated [106].
5.1.2.5. Favipiravir
Favipiravir (FPV), a derivative of pyrazine carboxamide, is a promising broad-spectrum antiviral drug initially developed for influenza several years ago. It acts by preventing the influenza viral RNA-dependent polymerase RNA, thus blocking the replication process by acting negatively on genetic copying. It has been screened against many RNA viruses including H1N1, Ebola, Arena virus, and Bunyavirus because of its action against RNA viruses. It was however approved in Japan and France and not in other countries for a few indications [107].
In order to determine the viability of FPV for COVID-19 repurposing, we carried out a literature survey of clinical trials using FPV as an investigational drug for different indications. Results of the research published for a multi-centric Phase II study (NCT01068912) showed clinical effectiveness of FPV in influenza in low-dose and high-dose treatment modalities with no mortality and no substantial possibility of severe adverse reactions [108]. Results of the multi-centric Phase III study (NCT02026349) released on EudraCT showed that FPV could reduce symptoms and overcome influenza fever without any mortality or significant adverse effect [109]. Evidently, FPV in Influenza could contribute to positive treatment interventions. FPV may also have possible antiviral activity on SARS-CoV-2 which is an RNA virus. A clinical study on FPV for the treatment of COVID-19 conducted by the Shenzhen Third People's Hospital and the Clinical Medical Research Centre of the National Infectious Diseases obtained positive results on 14 February. Preliminary outcomes from a total of 80 patients (along with the experimental group and the control group) showed that FPV had a more potent antiviral action than LPV / RTV. There were no major adverse reactions in the treatment group FPV, and there were slightly less adverse effects than the LPV / RTV group. In terms of disease development and viral clearance FPV demonstrated stronger therapeutic responses to COVID-19 [110].
5.1.2.6. Darunavir
Darunavir (DRV) is a second-generation protease inhibitor against HIV-1. Scientists in China declared on 4 February 2020 that DRV prevented in vitro SARS-CoV-2 infection. Cell research demonstrated that DRV reported to inhibit viral replication at a concentration of 300 μM in vitro, and its inhibition efficacy was 280 times that of the untreated group [111]. In patients with COVID-19 pneumonia, DRV is used in trial number NCT04252274 in combination with cobicistat [112]. Such a combination is currently approved by the FDA in treating AIDS. DRV is a HIV protease inhibitor, and cobicistat is a supplement to enhance DRV's pharmacodynamics and pharmacokinetics by inhibiting cytochrome P450 (CYP3A) [113,114].
5.1.2.7. Oseltamivir
The other antiviral drugs are also widely used in COVID-19 treatment around the world. Oseltamivir is another medication licensed for the treatment of influenza A and B; it prevents the viral neuraminidase and thereby prevents the releasing of viral particles from host cells, thus minimising the spread throughout the respiratory tract [115]. Usage of Oseltamivir with or without antibiotics and corticosteroids is reported in China [116]. Oseltamivir is also used in clinical trials with chloroquine and FPV in multiple combinations [117].
5.1.2.8. Interferons
Interferons (INF) are a cluster of cytokines that are secreted by dendritic, plasmacytoid and other types of cells [118]. These are the body's natural defense against viral infection and also play an important role in combating tumors and regulating immunity. FDA has approved INFs for the treatment of various conditions like multiple sclerosis, hepatitis B and C viral infection, hairy cell leukemia and Acquired Immuno Deficiency Syndrome (AIDS) [119]. INFs are the first cytokines released during a viral infection. The interferon stimulating genes (ISG) which are mainly involved in immunomodulation, signaling and inflammation are activated by the INF fixation on Interferon α/β Receptor (IFNAR) receptors present on the plasma membrane of most of the cells [120]. The ISGs activates the adaptive immunity by decreasing the secretion or metabolism of cytokines by interfering with viral replication. ISGs further prevents membrane fusion by decreasing the membrane fluidity and sensitize the cells to pathogens thereby inhibiting the virus-cell cycle steps [121]. INF, when given as a monotherapy, showed that higher serum concentration of INF is required to halt viral replication. Whereas the in vitro studies of INF given in combination with ribavirin showed a potent synergistic effect in treating SARS [122]. The combination of INF and ribavirin at a lower dose showed an inhibitory action on the replication of MERS-CoV [90]. Among the subtypes of INFs, INFβ1b and INFβ1a are the most potent inhibitors of SARS-CoV [123] and MERS-CoV [124]. With respect to coronavirus infection INFβ1 plays an important role because of its protective role in the lungs. It maintains the endothelial barrier function of the lungs by up-regulating Cluster of Differentiation 73 (CD73) in the endothelial cells of lungs and results in the secretion of anti-inflammatory adenosine [125]. These studies showed that INF can be used in the prophylactic treatment of COVID-19. INF is an investigational drug and hence its complete pharmacokinetic profile is not yet established. However, the half-life of the pegylated form of INF is 4.6–22 h. As of now, there is no established dosage regimen for INF in treating COVID-19. Several clinical trials with INF alone and in combination with other drugs have been initiated. An early phase randomized open blank control study has been planned to evaluate the safety and efficacy of recombinant INFα1b as monotherapy in treating corona virus infection [126]. In another case, an randomized open-label controlled trial on the combination therapy of lopinavir/ ritonavir, ribavirin and INFβ1b in comparison with monotherapy of lopinavir/ ritonavir as a treatment for novel coronavirus infection has been planned [94]. Peginterferon alfa-2a marketed as Pegasys has been used in the treartment of chronic Hepatitis C in combination with Ribavirin. With repect to COVID-19 several clinical trials with using Pegylated interferon alfa have been initiated. Open label, controlled, randomised Phase II clinical trial to estimate the antiviral efficacy and safety of Pegylated INF Lamda (NCT04343976) and Pegylated INF Lamda-1A (NCT04388709) in patients with COVID-19 infection have been initiated [127,128].
5.1.3. Antibiotic
5.1.3.1. Azithromycin
Azithromycin (AZ) is a semi synthetic broad spectrum macrolide antibiotic approved by FDA for the treatment of genitourinary tract infections, enteric and respiratory tract related infections [129]. Along with its anti-bacterial activity, AZ has also shown antiviral activity by inducing the production of interferon-stimulated genes in a rhino virus infected bronchial epithelial cells [130]. Studies showed that AZ is also effective against zika and Ebola virus. AZ acts against the virus by a host of multiple mechanism. AZ inhibits viral genetic shedding from lysosome and blocks endocytosis by getting accumulated intracellularly in the lysosomes and endosomal vesicles leading to the increase in pH levels and thereby limits the replication of viral genetic material [131]. The anti-viral activity of AZ is also attributed to its ability to induce interferon mediated antiviral response which reduces the replication of the virus [132]. AZ also decreases the mucus secretion and enables lung function by directly acting on bronchial epithelial cells [130]. AZ has shown a potential action against SARS-CoV-2. It interacts among SARS-CoV-2 spike protein and host angiotensin converting enzyme-2 (ACE-2) protein and thereby inhibits the viral entry into the host cell [133]. The bioavailability of AZ following oral administration is 37%. It has a high tissue penetration and a longer half life (68 h). It is primarily metabolized in the liver and excreted through biliary excretion. The volume of distribution of AZ is 31 L/kg. The side effects of AZ include macrolide antibiotic resistances, hearing loss and cardiac arrythmias (QT prolongation) [129]. There are several reports suggesting that a combination of chloroquine and AZ is effective in reducing the viral load in the treatment of COVID-19. Usually a dose of 1–2 g of azithromycin in combination with hydroxychloroquine or chloroquine is used in the treatment of COVID-19.
There are around 81 clinical trial registered for testing the efficacy of AZ in combination with several other drugs for treating COVID-19. An open label, randomized interventional study has been initiated to compare the efficiency of the two drugs hydroxychloroquine and AZ to see which one is better in the treatment of confirmed or suspected cases of COVID-19 [134]. A multicentre, randomized, open labelled clinical trial has been started to assess the safety and efficacy of tocilizumab given in combination with azithromycin and hydroxychloroquine in the treatment of SARS-CoV-2 [135]. A phase II B, double blind, randomized, placebo-controlled trial has been initiated to evaluate the safety and effectiveness of hydroxychloroquine and AZ to prevent the hospitalization or death in symptomatic adult outpatients with COVID-19 [136]. Gautret et al., conducted a non-randomized, observational, open-label, external control trial on COVID-19 patients of >12 yrs age to deduce the efficacy of combination therapy of hydroxychloroquine and AZ [137]. They concluded that hydroxychloroquine (500 mg/day) and AZ (250 mg/ day for 5 days) successfully reduced the viral load. The activity of hydroxychloroquine in reducing the viral load was further reinforced by AZ.
5.1.3.2. Tetracycline
Tetracyclines (doxycycline, tetracyclines, minocycline) are a class of polyketide antibiotics having a broad-spectrum bacteriostatic activity against various bacterial infections. The bactericidal activity of tetracycline is mainly due to its ability to reversibly bind to the bacterial ribosomes and there by leading to the leakage of the intracellular components from the bacterial cell by altering the cytoplasmic membrane [138]. Several studies have shown that along with anti-bacterial activity tetracyclines also possess a potential antiviral activity against a host of virus including SARS-CoV-2. Tetracycline act by three possible mechanism against SARS-Cov-2 virus. Coronavirus depends on matrix metalloproteinases (MMPs) for replication, survival, cell to cell adhesion and cell infiltration having zinc as a part of MMPs complex [139]. Tetracyclines are known to chelate with the zinc compounds present on the MMPs complex and thereby helps in decreasing the severity of COVID-19 infection. Secondly, tetracyclines decreases the levels of cytokines and other inflammatory agents by down regulation of NFKB pathways [140] and thereby aiding in the treatment of COVID-19 as corona virus causes an elevated release of cytokines (IL-6, TNF-α and IL-1β) and triggers the release of inflammatory agents like protease and histamine along with cytokines by inducing proliferation of the respiratory submucosa mast cells. The third possible mechanism of tetracycline in treating COVID-19 is by inhibiting the replication of viral DNA in the lungs which is mainly attributed to the high tissue penetrating ability and lipophilic nature of tetracycline. The bioavailability of tetracycline upon intramuscular injection is 40% and the oral bioavailability is around 60–80%. It is a highly protein bound drug with a half-life of 6–12 h. Vomiting, diarrhoea, loss of appetite and oral sores are the possible adverse effects of tetracyclines. Nantes University Hospital, France has been given approval to start a double blinded, randomized, placebo controlled, multi centred clinical trial. The aim of this study is to assess the efficacy of doxycycline in reducing/ abolishing the cytokine storm which is induced by the SARS-CoV-2 virus in the COVID-19 positive patients upon hospitalization [141].
5.1.3.3. Teicoplanin
Teicoplanin is a glycopeptide antibiotic produced by the actinomycete Actinoplanes teichomyceticus [142]. It is currently approved to treat Gram-positive bacterial infections like Staphylococcal infections [143]. Teicoplanin has also shown its activity against an array of viruses like the influenza virus, hepatitis C virus, Ebola, HIV, flavivirus and on corona virus-like SARS-CoV and MERS-CoV [144]. Teicoplanin inhibits the transpeptidase activities and transglycosylation reaction by nonspecifically binding to the outside layers of the peptidoglycan structure followed by binding to the terminal amino acids which constitute the building blocks of the peptidoglycan cell wall [145]. Thus, teicoplanin specifically stops bacterial growth by inhibiting the biosynthesis of the bacterial cell wall [146]. Teicoplanin binds to peptidoglycan and inhibits the cell wall lytic enzymes resulting in cell freezing [145]. Teicoplanin acts against coronavirus, by inhibiting the genome viral RNA release and thereby preventing the replication of the virus-cell cycle. This inhibition activity is because of teicoplanin acting on the early stage of the viral life cycle by inhibiting the low pH cleavage of viral spike protein by cathepsin L in the late endosome [144]. In the case of SARS-CoV-2 teicoplanin acts in the same way by targeting to the cleavage site for cathepsin L present in the spike protein. A study to test the efficacy of teicoplanin on 2019-nCoV showed that teicoplanin efficiently inhibits the entry of the 2019-nCoV spike pseudovirus into the cytoplasm in a dose-dependent manner [147]. Teicoplanin is poorly absorbed after oral administration. It has 90% bioavailability upon administering intramuscularly. The protein binding of the drug is 90–95% and it is metabolized in the liver by hydroxylation reaction to give metabolites 1 and 2 [148]. The mean volume of distribution at steady state for teicoplanin was found to be 40–70 L. It is eliminated by the renal pathway [149]. Hypersensitivity at the site of injection is the major side effect associated with teicoplanin. Other than this it may cause bronchospasm and anaphylactic shock. Teicoplanin can also cause nephrotoxicity and ototoxicity. Tachycardia, fatigue, headache, tremors, diarrhea, and elevation of liver enzymes are the other reported side effects [149].
5.1.4. Immunosuppresents
5.1.4.1. Sirolimus
Sirolimus is a potent immunosuppressive agent obtained from Streptomyces hygroscopicus. It is commercially available as Rapamune (Pfizer) and is mainly used in treating lymphangioleiomymatosis and as a prophylactic agent to prevent organ transplant rejection. The immunosuppressive activity of sirolimus is mainly due to its ability to inhibit T-lymphocyte proliferation/ activation and cytokine production by selectively blocking the transcriptional activation of cytokines. Along with possessing antineoplastic and anti-fungal properties, it also has anti-viral activity. Sirolimus binds to immunophilin in the cell to form a immunosuppressive complex and inhibits the mammalian target of rapamycin (mTOR) kinase which in turn halts the formation of mTORC1 protein complex which is said to play an important role in viral replication. Studies showed that sirolimus successfully inhibited mTOR signalling pathway and there by inhibited MERS-CoV [150], thus making sirolimus a suitable candidate to further test and use in treating COVID-19. The University of Cincinnati has been granted approval for performing a double blind, randomized, placebo controlled clinical trial on the use of sirolimus in treating COVD-19. The aim of the study is to determine the efficacy of sirolimus in improving the clinical outcomes among the patients hospitalized for the treatment of COVD-19 pneumonia [151]. A placebo controlled, two-arm, double blinded, randomized, phase-I clinical trial has been initiated to assess the virological efficacy of sirolimus. The main of this trial is to evaluate the pharmacokinetics, pharmacodynamics, virological efficacy, tolerability and safety of sirolimus as an adjuvant therapy in treating patients with COVID-19 [152].
5.1.4.2. Baricitinib
Baricitinib is an immunosuppressant approved by the FDA for the treatment of moderate to severe rheumatoid arthritis. It is a janus kinase (JAK) inhibitor which acts by selectively and reversibly binding to JAK receptors there by inhibiting JAK1 and JAK2. This inhibition leads in halting the signal transduction from growth receptors or cytokines leading to reduction in immune cell function and haematopoiesis. Baricitinib also prevents the formation of viral particles and intracellular passage of viral cells by binding to associated protein kinase 1 (AAK1) [15]. Baricitinib is considered as one of the drug candidates to test against COVID-19. The patients with COVID-19 are prone to secondary hemophagocytic lymphohistocytosis (SHL) [153], which leads to an elevated inflammatory and ferritin marker levels in the COVID-19 patients resulting in hyperinflammation. The use of JAK inhibitors like baricitinib will aid in reducing the inflammation and it also prevents the viral infection and endocytosis by inducing a inhibitory effect o AAK1 receptors [77]. The pharmacokinetic profile of the baricitinib is well established. The bioavailability of baricitinib upon oral administration is 80% and it reaches a peak plasma concentration within 1 h. the half life of baricitinib is 12.5 h with a volume of distribution of 76 L and its 50% protein binding. It is metabolised in liver by oxidation mediated through CYP3A4 and it is eliminated unchanged from the urine [154]. The main side effects associated with baricitinib are thrombosis, upper respiratory tract infections and malignancy. There are several ongoing clinical trials for determining the exact role and the efficacy of baricitinib in the treatment of COVID-19. A phase-II, non-randomized, open labelled clinical trial has been initiated to investigate the safety and efficacy of combination therapy of lopinavir/ritonavir, hydroxychloroquine sulphate and baricitinib in the treatment of moderate to severe COVD-19 in hospitalized patients [155]. Another randomized, phase-II, open labelled clinical trial to evaluate the safety and efficacy of the combination of drugs in treating SARS-CoV-2 associated pneumonia has been initiated. The aim of this current study is to design a study design with drugs of different classes and investigate their efficacy in treating COVID-19. A combination of hydroxychloroquine (200 mg), ritonavir (50 mg), baricitinib (4 mg) and imatinib (400 mg) will be administered to the patients with SARS-CoV-2 pneumonia for 7 days and the efficacy and safety of this combination therapy will be evaluated [156].
5.1.4.3. Cyclosporine
Cyclosporine is a immunosuppressive agent isolated form the fungus Beaueria nivea. It is widely approved for the treatment of lupus nephritis vasculitis psoriatic arthritis and as a immunosuppressant agent in organ transplantation [157]. Cyclosporine acts as a calcineurin inhibitor. It binds to cyclophilin receptor in the cells and forms a cyclosporine-cyclophilin complex. This complex will further block the calcium dependent interleukin (IL-2) production pathway and suppresses the gene transcription of IL-2 thereby reducing the inflammatory responses [158]. In the case of COVID-19, cyclosporine blocks RNA-dependent-RNA polymerase and peptidyl-prolyl isomerase activity of SARS-CoV-2 virus there by inhibiting the cyclophilin function of the virus [159]. To enter into the cell corona virus bids to the angiotensin converting enzyme-2 (ACE-2) at a low cytosolic Ph, cyclosporine s known to maintain the cytosolic pH at normal levels and there by aids in decreasing the viral load [160]. Cytokine storm in SARS-CoV-2 infection is mainly caused due to SHL and cyclosporine is the choice of drug to treat SHL as cyclosporine blocks the production of IL-2 and inhibits the proliferation and survival of T-cells [161]. Cyclosporine is absorbed variably with a volume of distribution 0f 4–8 L/kg. The half-life of cyclosporine is 19 h and its metabolized in intestine and liver by CYP450 enzymes [158]. Gingival hyperplasia, nephrotoxicity and hyperlipidaemia are the possible side effects of cyclosporine. Despite of its activity against SARS-CoV-2 virus it is not yet recommended for the treatment of COVID-19 as there is no proper pre-clinical and clinical data proving the safety and efficacy of cyclosporine in the treatment of COVID-19 available.
5.1.5. Monoclonal antibodies (MAb)
5.1.5.1. Tocilizumab (TCZ)
Tocilizumab is the first humanized recombinant MAb which is used to treat autoimmune and inflammatory conditions like cytokine release syndrome, giant cell arthritis and rheumatoid arthritis. TCZ targets and binds to the membrane bound IL-6 receptors thus preventing the interaction of IL-6 with IL-6 receptors. This leads to the reduction of the cytokine's pro inflammatory activity resulting in the blockade of antibody production and induction of T-cell proliferation by IL-6 [162]. In the course of COVID-19 disease progression, the patients develop conditions like acute interstitial pneumonia, fever, lung injury, arthralgia, leukopenia, thrombocytopenia, myocarditis and biological inflammatory changes which is caused due to the hyper production of cytokines resulting in a cytokine storm [163]. This cytokine storm is induced by the replication of the virus which activates the innate immune system to produce ILs. TCZ binds to these IL receptors and blunts the hyper secretion of ILs thereby reducing the inflammation and other related conditions [164]. The half-life of TCZ is concentration dependent and unlike other MAbs, TCZ is also metabolized to smaller proteins by the action of proteolytic enzymes [165]. A dose of 400 mg of intravenous TCZ once daily is administered in patients with COVID-19 [163]. Skin reactions, headaches, hypertension and gastrointestinal perforation are the major adverse effects associated with TCZ. A host of clinical trials have been initiated to investigate the use of TCZ in the treatment of COVD-19. A multicentred, open-labelled, phase-II, single arm clinical trial study has been initiated by the National Cancer Institute, Naples to evaluate the tolerability and efficacy of TCZ in treating COVID-19 pneumonia [166]. Another open-label, multi-centre clinical trial has been initiated with the main aim to evaluate the use of TCZ when given in combination with azithromycin and hydroxychloroquine in treating the patients hospitalized with COVID-19 [135]. A clinical study design to investigate the use of TCZ in manging COVID-19 patients with suspected pulmonary hyperinflammation has been initiated. It is an open-label, randomized multi centred, two arm study with the aim to test the hypothesis that TCZ can effectively reduce the inflammation caused as a result of virus induced cytokine storm and resulting in the rapid improvement of clinical conditions [167].
5.1.6. Anti-helmintics
5.1.6.1. Ivermectin
Ivermectin is a broad-spectrum anti-parasite drug which is approved for the treatment of onchocerciasis and intestinal strongyloidiasis. Ivermectin acts by selectively binding to the glutamate-gated chloride on channels of the parasite and leads to hyperpolarization of the cell which results in the paralysis and death of the parasite. The anti-viral activity of ivermectin was first discovered with its ability to block the interaction between the nuclear transport receptor importin α/β(IMP) and integrase molecule of HIV [168]. It is also known to block the viral replication of host of viruses including influenza, flavivirus and dengue virus [169]. The in vitro study of ivermectin anti-viral activity against SARS-CoV-2 virus showed an inhibition of the viral replication up to 5000-fold within 48 h which is mediated by inhibiting the IMP α/β mediated nuclear import of viral proteins [170]. A phase-III, double blind, randomized clinical trial has been started with a aim to determine the safety and efficacy profile of the combination therapy of hydroxychloroquine and ivermectin I the treatment of hospitalized COVD-19 patients [171].
5.1.7. Angiotensin converting enzyme inhibitors (ACE-2 inhibitors)
ACE-2 inhibitors (captopril, ramipril) are widely used in treating hypertension. ACE-2 receptors are overexpressed on the epithelial cells of the oral mucosa and they are also present immune reactive cells like lungs blood vessels macrophages and intestine [172]. In the case of SARS-CoV and SARS-CoV-2 ACE-2 receptors serves as a door way for the virus to enter into the host cell [173]. SARS-CoV has a transmembrane spike glycoprotein (S-protein) on its outer shell which binds to the ACE-2 receptors. Once the virus is attached to the ACE-2 receptors of the host cell the cellular proteases starts priming the S-protein and allows the virus to get fused into the cellular membrane of the host cell which results in the entry of the virus and replication in the host target cell [41]. The amino acid configuration of SARS-CoV-S protein is almost 80% similar to that of SARS-Co-2-S-protein and thus shares the same binding affinity to ACE-2 receptors as like SARS-CoV. Hence, SARS-CoV-2 also utilizes ACE-2 receptors as an entry point into the host cell [174]. Thus, treating with ACE-2 inhibitors and blocking the entry of SARS-CoV-2 virus into the cell has been considered as a potential therapeutic strategy to treat COVID-19 and in this regard many clinical trials have been initiated.
5.1.8. Corticosteroids
In particular, corticosteroids are not approved for treating COVID-19 or any viral pneumonia [175]. In septic shock, corticosteroids benefit from blunting the host's immune reaction to the release of bacterial toxins. The frequency of shock is remarkably low in patients with COVID-19 (5% of cases). Having to deliver oxygenated blood flow and thoracic pressure from breathing results in increased heart activity leading to cardiogenic shock. Corticosteroids can cause damage via immunosuppressant activity during the infection treatment and have failed to offer an advantage in many viral epidemics, including influenza infection, respiratory syncytial virus (RSV) infection, MERS and SARS [176]. With COVID-19, early recommendations for treating chronically ill people indicate when to use minimal-dose corticosteroids and when to stop using corticosteroids. Recommendations rely on the particular clinical condition (e.g. refractory trauma, artificially ventilated Acute respiratory distress syndrome (ARDS) patients); however, they are based on data identified as weak [177]. Indeed, a study was performed in Wuhan China identifying clinical outcomes of patients infected with COVID-19 (N = 201). Eighty-four (41.8%) patients acquired ARDS and 44 (52.4%) of those died. Methylprednisolone therapy reduced the risk of mortality in ARDS patients [178]. A retrospective open-label trial (ChiCTR2000029386) is planned to examine clinical progress in patients administered with methylprednisolone IV [66,179]. In another study, Zhou et al. indicated that, in 10 COVID-19 patients, the brief-term optimum-dose corticosteroid (160 mg/day) and immunoglobulin (20 g / day) substantially decreased lung damage, stabilized body temperature, C-reactive protein, lymphocyte, and oxygenation levels [180]. However, when examining 416 COVID-19 patients, Shang et al. observed that corticosteroid treatment and the administration of gamma globulin raised fatalities and considered only effective in patients with low lymphocyte levels [181]. The delivery of corticosteroids for COVID-19 patients is also uncertain according to the above scientific findings.
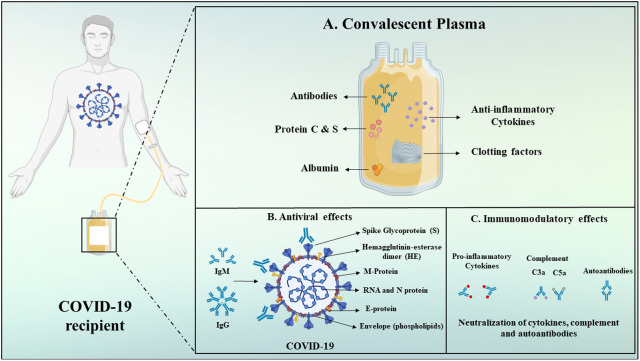
6. Convalescent plasma
The FDA provides access to convalescent plasma (CP), materials abundant in antibodies obtained from suitable donors who are healed from COVID-19. The FDA indicates that evaluating its safety and effectiveness by clinical trials is necessary prior to clinically prescribing CP for patients with COVID-19, and for the same FDA has issued guidelines to investigators and health care providers. For investigators seeking to review CP for use in patients with extreme or imminent life-threatening COVID-19, the FDA has posted details through the use of the single-patient emergency Investigational New Drug (IND) application process for particular patients. Also, the FDA is closely interacting with investigators to explore the prospect of cooperation on creating a master protocol for the use of CP, with the aim of minimizing duplicative attempts [182]. CP composition is complex and involve a wide range of components deriving from blood. Plasma includes a mixture of inorganic salts, organic compounds, water, and over 1000 proteins. The others factors identified are albumin, immunoglobulins, complements, coagulation and antithrombotic factors. Main convalescent plasma components are depicted in Fig. 6A [183,184]. Additional protective antibodies, including immunoglobulin G (IgG) and immunoglobulin M (IgM), are found in plasma in addition to neutralizing antibodies (NAbs) which might show antiviral effect. IgG and IgM are the key isotypes, although immunoglobulin A (IgA) can also be important, especially for viral mucosal infections. Non-NAbs which bind to the virus but do not affect its ability to replicate could contribute to prophylaxis and/or improvement in recovery. The humoral immune response targets mainly spike (S) protein. (Fig. 6B) [184,185]. CP's anti-inflammatory effects include the autoantibodies network and the regulation of an overactive immune system. Additionally, some antibodies prevent complement cascade (i.e., C3a and C5a), and limit immune complex formation (Fig. 6C) [184,186,187]. Researchers have investigated the effectiveness of CP therapy on 10 severe COVID-19 adult patients, aged 34–78 years. The pilot study findings indicate CP treatment could be a safe and effective therapeutic alternative for severe COVID-19 infections. A group from China administered a single 200 ml dose of CP to 10 patients with severe COVID-19 with elevated levels of anti-SARS-CoV-2 antibodies. Clinical symptoms, such as cough, fever, chest pain and shortness of breath, substantially improved three days after the injection, as per the investigators. The patients have had elevated numbers of lymphocytes, better liver function and decreased inflammation. The researchers also reported that the levels of neutralizing antibodies in the patients either increased or stayed elevated following CP transfusion [188].
Fig. 6.
Schematic depiction of convalescent plasma components and its mechanisms of action.
7. Ongoing clinical trials
After the COVID-19 outbreak was stated pandemic by the WHO Director-General Dr. Tedros on the 11th of March, the world started noticing the alarming levels of the upswing in the COVID-19 cases. Hitherto, the patients suffering from COVID-19, are receiving symptomatic treatment due to the unavailability of a specific therapy. In severe cases, the treatment includes support to the functioning of the vital organs [189]. The understanding of the whole genome sequence of COVID-19 allows the refinement of current technologies in developing the potent and targeted therapeutics [190]. Though, several clinical trials are presently in progress in various parts of the world to meet the vital requirements in developing the effective therapeutics and vaccines, WHO recently launched a global mega-trial called ‘Solidarity’ which emphasizes on the four most assuring therapies: the antimalarial medications chloroquine and hydroxychloroquine, an antiviral compound remdesivir, a combination of two anti-HIV drugs, ritonavir, and lopinavir, and lastly the same combination along with an immune system messenger, interferon-beta that can aid in paralyzing the virus [191]. Chloroquine and hydroxychloroquine prevent the entry and transport of the virus by altering the pH of endosomes. Remdesivir a nucleotide analog, likely terminates the RNA synthesis leading to the induction of mutagenesis. Both ritonavir and lopinavir being protease inhibitors block the viral cellular entry where ritonavir inhibits Cyt-P450 and prolongs the half-life of lopinavir. Interferon-beta inhibits viral replication [192]. In quest of an effective therapeutic moiety, various research institutes and pharma companies are repurposing several antivirals, antibiotics, and their combinations. If the efficacy of these repurposed drugs reveals beneficial outcomes against COVID-19, it would be a case of good fortune rather than good preparedness. The current strategy for pandemic drugs is to develop or repurpose a drug right after the discovery of a novel outbreak. Several aspects of the pandemic seem to have shown good progress using this strategy. Within weeks, viral genome sequences were understood and published which helped in identifying the suitable drug candidates. Clinical trials of interferons, ACE inhibitors, remdesivir, hydroxychloroquine, and monoclonal antibodies are under progress [193] . Though the window of the usefulness of steroids for COVID-19 patients is narrower, they are still preferred because of their capability of preventing lung fibrosis and stopping cytokine storm [194]. Testing of the proposed drugs takes more time particularly when it is performed on humans as a part of clinical trials.
On the other hand, the development of vaccines is hard and takes relatively more time than repurposing an existing drug especially when the novel technologies available have not been tested for safety. In the pipeline of vaccines, an mRNA-based vaccine developed by Modera and Vaccine Research Center at NIH expresses target antigen after being injected in the form of lipid nanoparticle encapsulated mRNA. It has reached a phase I clinical trial (NCT04283461). Curevac is currently in the preclinical phase working on a similar vaccine. Additional strategies which are still in the preclinical phase include S protein-focused vaccines such as viral-vector based vaccines (Geovax, CanSino Biologics, Vaxart and the University of Oxford), recombinant-protein based vaccines (Novavax, University of Queensland, and Biopharmaceuticals), DNA vaccines (Applied DNA sciences and Inovio), inactivated virus vaccines and live attenuated vaccines (Serum Institute of India with Codagenix). Johnson & Johnson is working on an experimental adenovirus vector that is not yet a licensed vaccine [195]. Biontech SE in collaboration with Pfizer very recently (April 29, 2020), started recruiting humans to evaluate the safety and efficacy of RNA vaccine candidates through phase I and II of clinical trials (NCT04368728). To combat this pandemic COVID-19, vaccines might enter the market lately but will be useful in the ultimate eradication of the virus. In Table 2 , we have exemplarily summarized the various therapeutics for COVID-19, which are under clinical trials.
Table 2.
Clinical trials of the various therapeutics for COVID-19.
| Sr. no. | Therapeutic moiety/combinations | Phase of clinical trial | Route of administration | Category and purpose in COVID-19 | Sponsor | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|---|
| 1 | Chloroquine | 2 & 3, 4, 2 | Oral route | Antimalarial, to treat mild Symptomatic and Asymptomatic cases of COVID-19 | HaEmek Medical Center, Israel, Wroclaw Medical University, Poland, Oxford University Clinical Research Unit, Vietnam | NCT04333628, NCT04331600, NCT04328493 |
| 2 | Hydroxychloroquine | 3, 3, 2, 2, 2 | Oral route | Antimalarial, to prevent severe COVID-19 disease | Dr. Michael Hill, University of Calgary, Canada, Germany, United States, Baylor University Medical Center, United States, California, Rambam Health Care Campus | NCT04329611, NCT04340544, NCT04329923, NCT04333225, NCT04335084, NCT04323631 |
| 3 | Remdesivir | 3 | I.V infusion | Antiviral in treatment of COVID-19 | Gilead Sciences, | NCT04292899, NCT04292730 |
| 4 | Azithromycin | 3 | Oral route | Antibiotic, to prevent COVD-19 disease progression | University of California, San Francisco | NCT04332107 |
| 5 | Atovaquone/Azithromycin | NA | Oral route | Anti-Malarial/Anti-Infective Combination to treat COVID-19 patients | HonorHealth Research Institute, United States | NCT04339426 |
| 6 | Lopinavir/ritonavir | 2 | Oral route | Antiretroviral, to treat COVID-19 patients | Sunnybrook Health Sciences Centre, Canada | NCT04330690 |
| 7 | Lopinavir/ritonavir vs Hydroxychloroquine sulfate | 2 | Oral route | To treat Mild COVID-19 | Asan Medical Center, Korea | NCT04307693 |
| 8 |
|
2 | 1, 2, & 3 Oral route, 4. S.C injection | To treat moderate to severe COVID-19 | Lisa Barrett, Canada | NCT04321993 |
| 9 | Remdesivir, Lopinavir/ritonavir, interferon β-1A, and hydroxychloroquine | 3 | I.V infusion, oral route, S.C injection, and oral route respectively | To treat COVID-19 patients | National Institute of Health and Medical Research, France | NCT04315948 |
| 10 | Hydroxychloroquine, Oseltamivir, Azithromycin | 3 | Oral route | To treat COVID-19 patients | Shehnoor Azhar, Pakistan | NCT04338698 |
| 11 | Hydroxychloroquine and Nitazoxanide | 2, 3 | Oral route | To evaluate safety and efficacy in COVID-19 patients | Tanta University | NCT04361318 |
| 12 | Hydroxychloroquine vs. Azithromycin | 2, 3 | Oral route | To treat COVID-19 patients | Intermountain Health Care, Inc., United States, Intermountain Medical Center, United States | NCT04329832, NCT04334382 |
| 13 | 1. Lopinavir/ritonavir, 2. Ribavirin and 3. Interferon beta-1b |
2 | 1 & 2 oral route, 3 S.C injection | To treat COVID-19 patients | The University of Hong Kong, Hong Kong | NCT04276688 |
| 14 | Clevudine | 2 | Oral route | Antiviral, to evaluate safety and efficacy in COVID-19 patients | Bukwang Pharmaceutical, Korea | NCT04347915 |
| 15 | Arbidol | 4 | Oral route | Antiviral, to treat patients with COVID-19 pneumonia | Jieming QU, Ruijin Hospital, China | NCT04260594 |
| 16 | Isotretinoin | 3 | Oral route | Retinoid, to evaluate the safety and efficacy in COVID-19 | Tanta University, Egypt | NCT04361422 |
| 17 | Ivermectin and Nitazoxanide | 2, 3 | Oral route | To evaluate the safety and efficacy of the combination in COVID-19 | Tanta University, Egypt | NCT04360356 |
| 18 | Recombinant human Interferon α1β | 1 | Nebulization | To treat COVID-19 patients | Tongji Hospital, China | NCT04293887 |
| 19 |
|
NA | 1 & 2 oral route, 3 spray | To treat patients with COVID-19 pneumonia | Second Affiliated Hospital of Wenzhou Medical University, China | NCT04273763 |
| 20 | Deferoxamine | 1 & 2 | Intravenous infusion | Iron chelator, to reduce the Severity of COVID-19 Manifestations | Kermanshah University of Medical Sciences, Iran | NCT04333550 |
| 21 | Dexamethasone | 4 | I.V | Steroid, to treat patients with ARDS caused by COVID-19 | Dr. Negrin University Hospital, Spain | NCT04325061 |
| 22 | Piclidenoson | 2 | Oral route | A3 adenosine receptor agonist, to treat COVID-19 patients | Can-Fite BioPharma, Israel | NCT04333472 |
| 23 | Favipiravir | 3 | Oral route | Antiviral, to evaluate safety and efficacy in COVID-19 patients | Giuliano Rizzardini, Italy | NCT04336904 |
| 24 | Huaier Granule | 2 & 3 | Oral route | Adjuvant treatment of COVID-19 | Tongji Hospital, China | NCT04291053 |
| 25 | Tranexamic acid | 2 | Oral route | Antifibrinolytics, to study its effect on COVID-19 | The University of Alabama at Birmingham, United States | NCT04338126 |
| 26 | BLD-2660 | 2 | Oral route | Antiviral small molecule, to treat COVID-19 | Blade Therapeutics, United States | NCT04334460 |
| 27 | Sildenafil citrate | 3 | Oral route | Phosphodiesterase inhibitor, to treat COVID-19 patients | Tongji Hospital, China | NCT04304313 |
| 28 | Losartan | 1 | Oral route | Angiotensin receptor blocker, to treat respiratory failure due to COVID-19 | University of Kansas Medical Center, United States | NCT04335123 |
| 29 | Telmisartan | 2 | Oral route | Angiotensin receptor blocker, to evaluate the efficacy in COVID-19 patients | Laboratorio Elea Phoenix S.A., Argentina | NCT04355936 |
| 30 | Atorvastatin | 2 | Oral route | Statin, to assess the efficacy in reducing the deteroration of COVID-19 patients | Mount Auburn Hospital | NCT04380402 |
| 31 | Prazosin | 2 | Oral route | Alpha-blockers, to evaluate the safety and efficacy of drug to prevent cytokine storm | Johns Hopkins University, United States | NCT04365257 |
| 32 | Chlorpromazine | 3 | Oral route | Antipsychotics, to repurpose the drug for COVID-19 treatment | Centre Hospitalier St Anne, France | NCT04366739 |
| 33 | Lenalidomide | 4 | Oral route | Antiangiogenic agent, to treat mild to moderate COVID-19 patients | Getafe University Hospital, Spain | NCT04361643 |
| 34 | FT516 - induced pluripotent stem cell | 1 | – | To identify the maximum tolerated dose for the treatment of COVID-19 | Masonic Cancer Center, University of Minnesota | NCT04363346 |
| 35 | Allogeneic Mesenchymal Stromal Cells | 2 | I.V injection | To evaluate safety and efficacy of cells in patients with COVID-19 Pneumonia | Cell Therapy Network, Spain | NCT04361942 |
| 36 | Human Mesenchymal Stem Cells | 1 & 2 | I.V | Improve immune factor and to treat patients with COVID-19 pneumonia | Puren Hospital, China | NCT04339660 |
| 37 | Anti-SARS-CoV-2 convalescent plasma | 2 | I.V infusion | To assess the safety and efficacy | Medical College of Wisconsin, Hackensack Meridian Health, United States, Joakim Dillner, Sweden | NCT04354831, NCT04343755, NCT04390178, NCT04384497 |
| 38 | Anti-SARS-CoV-2 convalescent plasma | 3 | I.V infusion | To evaluate the efficacy | Central Directorate of the Army Health Service, France, National Institute of Medical Sciences and Nutrition Salvador Zubiran, United Mexican States |
NCT04372979, NCT04388410 |
| 39 | Hyperimmune plasma with standard therapy | 2 & 3 | I.V | To evaluate safety and efficacy in COVID-19 patients | University of Catanzaro, Italy | NCT04385043 |
| 40 | bac-TRL-Spike | 1 | Oral route | To prevent COVID-19 in Healthy adults | Symvivo Corporation, Canada | NCT04334980 |
| 41 | IFX-1 with Best supportive care | 2 & 3 | I.V | C5a antibody, to treat pneumonia. | InflaRx GmbH, Netherlands | NCT04333420 |
| 42 | Acalabrutinib with Best supportive care | 2 | Oral route | Bruton's tyrosine kinase inhibitor, To evaluate safety, efficacy and pharmacokinetics | AstraZeneca, United States | NCT04380688, NCT04346199 |
| 43 | Baricitinib | 2 & 3 | I.V | Janus Kinase inhibitor, to treat COVID-19 | University of Colorado, United States | NCT04340232 |
| 44 | Tocilizumab | 2 | I.V injection | Immunosuppressive, to treat patients with COVID-19 pneumonia | National Cancer Institute, Naples, University Hospital Inselspital, Berne, Switzerland | NCT04317092, NCT04335071 |
| 45 | Canakinumab | 3 | I.V infusion | Anti-human-IL-1β monoclonal antibody, To study the safety and efficacy in COVID-19 patients with cytokine release syndrome | Novartis Pharmaceuticals, United States | NCT04362813 |
| 46 | Ruxolitinib | 2 | – | Kinase inhibitor, to treat hyper inflammation in patients with stage II/III COVID-19 | Dr. Andreas Hochhaus, Germany | NCT04338958 |
| 47 | Ruxolitinib | 3 | Oral route | To evaluate safety and efficacy in COVID-19 patients with Cytokine storm | Novartis Pharmaceuticals, United Kingdom | NCT04362137 |
| 48 | BCG vaccine | 3 | Intracutaneous injection | To reduce Health Care Workers Absenteeism in COVID-19 | UMC Utrecht, Netherlands, Murdoch Children Research Institute, Australia | NCT04328441, NCT04327206 |
| 49 | Artificial Antigen Presenting Cell (aAPC) Vaccine | 1 | S.C injection | To treat COVID-19 | Shenzhen Geno-Immune Medical Institute, China | NCT04299724 |
| 50 | ChAdOx1 nCoV-19 Vaccine | 1 & 2 | I.M | To treat COVID-19 | University of Oxford, United Kingdom | NCT04324606 |
8. Recent advancement and future perspective
Considerable progress was made in the in vitro diagnostic test (IVD) for COVID-19. Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) is the basic ideology for IVD which takes a few hours for the results. Few industries have managed to reduce the test time from 3 h (Cepheid) to 5 min (Abbot). A wide array of serology immunoassays (IAs) that primarily detect IgM and IgG were also developed (automated chemiluminescence IA, manual ELISA, rapid lateral flow IA), complementing the COVID-19 diagnostic molecular assays [196]. The production of SARS-CoV-2 antiviral drugs remains problematic as there are no officially approved drugs and vaccines for the treatment of COVID-19. Hence, we must rely solely on implementing preventive and stringent control measures to reduce the possibility of potential transmission of diseases. Furthermore, the currently limited knowledge for therapeutic drugs must be used thoroughly to establish strategies for drug application to inhibit and manage SARS-CoV-2 transmission. While research is underway to improve COVID-19 prevention, treatment, and control, the reported clinical data on various therapeutic approaches are limited. Several preclinical trials show that S protein is a key viral antigen for vaccine development. Detailed clinical trials for already licensed products are being performed on COVID-19 affected patients since they have a well-established safety profile. Studies performed in vitro for an antiviral and antimalarial drug namely remdesivir and chloroquine have shown promising results in suppressing the virus. In a recent study dated 9th April 2020, 1061 patients were treated for COVID-19 using a combination of Hydroxychloroquine-Azithromycin (HZQ-AZ) for 3 days and a follow up of 9 days. The results depict that 91.7% of the patients were cured while 4.3% required intensive care units and 0.47% of patients died. The authors reported that HZQ-AZ combination is a safe and effective treatment if begun immediately after diagnosis, and in most cases clears up the virus persistence and contiguity [197]. In the future, research must be carried out using an effective animal model that analyses the virus's replication, transmission and pathogenesis also integrating the imaging and molecular diagnosis will help to efficiently track and treat COVID-19 [19,198,199].
9. Conclusion
With the rapid increase in COVID-19 cases through out the world, the research for development of drugs and vaccines have gained a momentum. During this prevailing condition of pandemic, the fast development of vaccines is required but it will take time as ongoing clinical trials are necessary for assessing the biocompatibility and toxicity of developed vaccines. Drug repurposing has proved to be the most efficient approach towards the treatment of COVID-19. Drugs from various therapeutic category alone or in combination have been explored and many combinations specially antivirals have proved to be effective against the disease although the dose and combination of repurposed drugs has been selected on trial basis and clinical trials on many repurposed drugs have been called off sighting toxicity and efficacy issues. The concept of drug repurposing is quite useful and may have demonstrated effectiveness but this might vary with the severity as well as with subjects on which treatment is being done. In case of vaccines, multiple vaccines have demonstrated positive results and will be goining to next phase soon giving hope for early commercial launch of vaccines against COVID-19. Amongst alternative therapeutic strategy, Indian council of Medical Research recently approved clinical trial for use of herbal formulation against COVID-19 while plasma therapy has demonstrated significant results and may be explored more in near future. With the ongoing pace of research being conducted in this area, the world will be able to tackle this pandemic and life could be normal like before very soon.
Declaration of competing interest
The authors report no conflict of interest.
Acknowledgement
The authors acknowledge European Molecular Biology Organization (EMBO), Germany for providing EMBO Short-term Fellowship to Dr. Abhjeet Pandey. The authors are thankful to i) Manipal Academy of Higher Education (MAHE), Manipal, India (for Postdoctoral Fellowship to Dr Abhijeet Pandey and Dr TMA Pai Doctoral Fellowship to Gasper Fernandes), ii) Themis Medicare Limited, Mumbai (for Junior Research Fellowship to Ajinkya N Nikam), iii) Science and Engineering Research Board (SERB), Government of India, New Delhi (for Research Fellowship to A B Shreya), Department of Science and Technology (DST), Government of India, New Delhi (for INSPIRE fellowship to Sadhana P Mutalik), Indian Council for Medical Education (ICMR), Government of India, New Delhi (for Senior Research Fellowship to Divya Gopalan) and All India Council for Technical Education (AICTE), Government of India, New Delhi (for National Doctoral Fellowship to Bharath S Padya). The authors are also thankful to Manipal and Manipal College of Pharmaceutical Sciences, MAHE, Manipal, India for providing necessary facilities.
Contributor Information
Srinivas Mutalik, Email: ss.mutalik@manipal.edu.
Ruth Prassl, Email: ruth.prassl@medunigraz.at.
References
- 1.Richman D.D., Whitley R.J., Hayden F.G. John Wiley & Sons; 2016. Clinical Virology. [Google Scholar]
- 2.Chan-Yeung M., Xu R.-H. SARS: epidemiology. Respirology. 2003;(8 Suppl):S9–14. doi: 10.1046/j.1440-1843.2003.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.W.H. Organization Middle East respiratory syndrome coronavirus (MERS-CoV) 2016. http://www.who.int/emergencies/mers-cov/en Erişim Tarihi. 1 (n.d.)
- 4.Wang N., Shi X., Jiang L., Zhang S., Wang D., Tong P., Guo D., Fu L., Cui Y., Liu X., Arledge K.C., Chen Y.-H., Zhang L., Wang X. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013;23:986–993. doi: 10.1038/cr.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23:130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhong N., Zheng B., Li Y., Poon L., Xie Z., Chan K., Li P., Tan S., Chang Q., Xie J., Liu X., Xu J., Li D., Yuen K., Peiris J., Guan Y. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet. 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.W.H. Organization . World Health Organization; 2020. Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases: Interim Guidance, 2 March 2020. [Google Scholar]
- 12.Liu Y., Gayle A.A., Wilder-Smith A., Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coronavirus (COVID-19) events as they happen, (n.d.). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen (accessed April 10, 2020).
- 14.Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J., Sheng J., Quan L., Xia Z., Tan W., Cheng G., Jiang T. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mousavizadeh L., Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94 doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H., Liu S.-M., Yu X.-H., Tang S.-L., Tang C.-K. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Severe outcomes among patients with coronavirus disease 2019 (COVID-19) — United States, February 12–March 16, 2020 | MMWR, (n.d.). https://www.cdc.gov/mmwr/volumes/69/wr/mm6912e2.htm (accessed April 10, 2020). [DOI] [PMC free article] [PubMed]
- 21.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cha R., Joh J.-S., Jeong I., Lee J.Y., Shin H.-S., Kim G., Kim Y., C.C.T. of N.M. Center Renal complications and their prognosis in korean patients with middle east respiratory syndrome-coronavirus from the central MERS-CoV designated hospital. J. Korean Med. Sci. 2015;30:1807–1814. doi: 10.3346/jkms.2015.30.12.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peiris J., Chu C., Cheng V., Chan K., Hung I., Poon L., Law K., Tang B., Hon T., Chan C., Chan K., Ng J., Zheng B., Ng W., Lai R., Guan Y., Yuen K. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J., Guo Q., Song T., He J., Yen H.-L., Peiris M., Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bárcena M., Oostergetel G.T., Bartelink W., Faas F.G.A., Verkleij A., Rottier P.J.M., Koster A.J., Bosch B.J. Cryo-electron tomography of mouse hepatitis virus: insights into the structure of the coronavirion. Proc. Natl. Acad. Sci. U. S. A. 2009;106:582–587. doi: 10.1073/pnas.0805270106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tseng Y.-T., Chang C.-H., Wang S.-M., Huang K.-J., Wang C.-T. Identifying SARS-CoV membrane protein amino acid residues linked to virus-like particle assembly. PLoS One. 2013;8 doi: 10.1371/journal.pone.0064013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Wilde A.H., Snijder E.J., Kikkert M., van Hemert M.J. Host factors in coronavirus replication. In: Tripp R.A., Tompkins S.M., editors. Roles of Host Gene and Non-coding RNA Expression in Virus Infection. Springer International Publishing; Cham: 2018. pp. 1–42. [DOI] [Google Scholar]
- 29.Hussain S., Pan J., Chen Y., Yang Y., Xu J., Peng Y., Wu Y., Li Z., Zhu Y., Tien P., Guo D. Identification of novel subgenomic RNAs and noncanonical transcription initiation signals of severe acute respiratory syndrome coronavirus. J. Virol. 2005;79:5288–5295. doi: 10.1128/JVI.79.9.5288-5295.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrier A., Bonnin A., Desmarets L., Danneels A., Goffard A., Rouillé Y., Dubuisson J., Belouzard S. The C-terminal domain of the MERS coronavirus M protein contains a trans-Golgi network localization signal. J. Biol. Chem. 2019;294:14406–14421. doi: 10.1074/jbc.RA119.008964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawicki S.G., Sawicki D.L. Coronavirus transcription: a perspective. In: Enjuanes L., editor. Coronavirus Replication and Reverse Genetics. Springer; Berlin, Heidelberg: 2005. pp. 31–55. [DOI] [Google Scholar]
- 32.Xia S., Zhu Y., Liu M., Lan Q., Xu W., Wu Y., Ying T., Liu S., Shi Z., Jiang S., Lu L. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cellular & Molecular Immunology. 2020:1–3. doi: 10.1038/s41423-020-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu F., Du L., Ojcius D.M., Pan C., Jiang S. Measures for diagnosing and treating infections by a novel coronavirus responsible for a pneumonia outbreak originating in Wuhan, China. Microbes Infect. 2020;22:74–79. doi: 10.1016/j.micinf.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang N., Jiang S., Du L. Current advancements and potential strategies in the development of MERS-CoV vaccines. Expert Review of Vaccines. 2014;13:761–774. doi: 10.1586/14760584.2014.912134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan J.F.-W., Kok K.-H., Zhu Z., Chu H., To K.K.-W., Yuan S., Yuen K.-Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerging Microbes & Infections. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sola I., Almazán F., Zúñiga S., Enjuanes L. Continuous and discontinuous RNA synthesis in coronaviruses. Annu Rev Virol. 2015;2:265–288. doi: 10.1146/annurev-virology-100114-055218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irigoyen N., Firth A.E., Jones J.D., Chung B.Y.-W., Siddell S.G., Brierley I. High-resolution analysis of coronavirus gene expression by RNA sequencing and ribosome profiling. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R. Rangan, I.N. Zheludev, R. Das, RNA Genome Conservation and Secondary Structure in SARS-CoV-2 and SARS-related Viruses, BioRxiv. (2020) 2020.03.27.012906. doi: 10.1101/2020.03.27.012906. [DOI] [PMC free article] [PubMed]
- 39.D.E. Gordon, G.M. Jang, M. Bouhaddou, J. Xu, K. Obernier, M.J. O'Meara, J.Z. Guo, D.L. Swaney, T.A. Tummino, R. Huettenhain, R.M. Kaake, A.L. Richards, B. Tutuncuoglu, H. Foussard, J. Batra, K. Haas, M. Modak, M. Kim, P. Haas, B.J. Polacco, H. Braberg, J.M. Fabius, M. Eckhardt, M. Soucheray, M.J. Bennett, M. Cakir, M.J. McGregor, Q. Li, Z.Z.C. Naing, Y. Zhou, S. Peng, I.T. Kirby, J.E. Melnyk, J.S. Chorba, K. Lou, S.A. Dai, W. Shen, Y. Shi, Z. Zhang, I. Barrio-Hernandez, D. Memon, C. Hernandez-Armenta, C.J.P. Mathy, T. Perica, K.B. Pilla, S.J. Ganesan, D.J. Saltzberg, R. Ramachandran, X. Liu, S.B. Rosenthal, L. Calviello, S. Venkataramanan, J. Liboy-Lugo, Y. Lin, S.A. Wankowicz, M. Bohn, P.P. Sharp, R. Trenker, J.M. Young, D.A. Cavero, J. Hiatt, T.L. Roth, U. Rathore, A. Subramanian, J. Noack, M. Hubert, F. Roesch, T. Vallet, B. Meyer, K.M. White, L. Miorin, O.S. Rosenberg, K.A. Verba, D. Agard, M. Ott, M. Emerman, D. Ruggero, A. García-Sastre, N. Jura, M. von Zastrow, J. Taunton, A. Ashworth, O. Schwartz, M. Vignuzzi, C. d'Enfert, S. Mukherjee, M. Jacobson, H.S. Malik, D.G. Fujimori, T. Ideker, C.S. Craik, S. Floor, J.S. Fraser, J. Gross, A. Sali, T. Kortemme, P. Beltrao, K. Shokat, B.K. Shoichet, N.J. Krogan, A SARS-CoV-2-Human Protein-Protein Interaction Map Reveals Drug Targets and Potential Drug-repurposing, BioRxiv. (2020) 2020.03.22.002386. doi: 10.1101/2020.03.22.002386. [DOI]
- 40.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020 doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng J., Zhao Y., Xu G., Zhang K., Jia W., Sun Y., Zhao J., Xue J., Hu Y., Zhang G. The S2 subunit of QX-type infectious bronchitis coronavirus spike protein is an essential determinant of neurotropism. Viruses. 2019;11 doi: 10.3390/v11100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir. Res. 2020;176 doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X., Xu W., Hu G., Xia S., Sun Z., Liu Z., Xie Y., Zhang R., Jiang S., Lu L. SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cellular & Molecular Immunology. 2020:1–3. doi: 10.1038/s41423-020-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S., Qi F., Bao L., Du L., Liu S., Qin C., Sun F., Shi Z., Zhu Y., Jiang S., Lu L. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia B., Kang X. Activation and maturation of SARS-CoV main protease. Protein Cell. 2011;2:282–290. doi: 10.1007/s13238-011-1034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020 doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Subissi L., Posthuma C.C., Collet A., Zevenhoven-Dobbe J.C., Gorbalenya A.E., Decroly E., Snijder E.J., Canard B., Imbert I. One severe acute respiratory syndrome coronavirus protein complex integrates processive RNA polymerase and exonuclease activities. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E3900–E3909. doi: 10.1073/pnas.1323705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu J.T.-A., Kuo C.-J., Hsieh H.-P., Wang Y.-C., Huang K.-K., Lin C.P.-C., Huang P.-F., Chen X., Liang P.-H. Evaluation of metal-conjugated compounds as inhibitors of 3CL protease of SARS-CoV. FEBS Lett. 2004;574:116–120. doi: 10.1016/j.febslet.2004.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chaturvedi U.C., Shrivastava R. Interaction of viral proteins with metal ions: role in maintaining the structure and functions of viruses. FEMS Immunol. Med. Microbiol. 2005;43:105–114. doi: 10.1016/j.femsim.2004.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.te Velthuis A.J.W., van den Worm S.H.E., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang N., Tanner J.A., Wang Z., Huang J.-D., Zheng B.-J., Zhu N., Sun H. Inhibition of SARS coronavirus helicase by bismuth complexes. Chem. Commun. 2007:4413–4415. doi: 10.1039/B709515E. [DOI] [PubMed] [Google Scholar]
- 55.Yang N., Shen H.-M. Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19. Int. J. Biol. Sci. 2020;16:1724–1731. doi: 10.7150/ijbs.45498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. StatPearls. StatPearls Publishing; Treasure Island (FL): 2020. Features, evaluation and treatment coronavirus (COVID-19)http://www.ncbi.nlm.nih.gov/books/NBK554776/ [PubMed] [Google Scholar]
- 57.Li G., Clercq E.D. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 58.Ashburn T.T., Thor K.B. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 59.Talevi A., Bellera C.L. Challenges and opportunities with drug repurposing: finding strategies to find alternative uses of therapeutics. Expert Opin. Drug Discovery. 2020;15:397–401. doi: 10.1080/17460441.2020.1704729. [DOI] [PubMed] [Google Scholar]
- 60.Pushpakom S., Iorio F., Eyers P.A., Escott K.J., Hopper S., Wells A., Doig A., Guilliams T., Latimer J., McNamee C., Norris A., Sanseau P., Cavalla D., Pirmohamed M. Drug repurposing: progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019;18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 61.Oprea T.I., Bauman J.E., Bologa C.G., Buranda T., Chigaev A., Edwards B.S., Jarvik J.W., Gresham H.D., Haynes M.K., Hjelle B., Hromas R., Hudson L., Mackenzie D.A., Muller C.Y., Reed J.C., Simons P.C., Smagley Y., Strouse J., Surviladze Z., Thompson T., Ursu O., Waller A., Wandinger-Ness A., Winter S.S., Wu Y., Young S.M., Larson R.S., Willman C., Sklar L.A. Drug repurposing from an academic perspective. Drug Discovery Today: Therapeutic Strategies. 2011;8:61–69. doi: 10.1016/j.ddstr.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Savarino A., Trani L.D., Donatelli I., Cauda R., Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect. Dis. 2006;6:67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan Y., Zou Z., Sun Y., Li X., Xu K.-F., Wei Y., Jin N., Jiang C. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res. 2013;23:300–302. doi: 10.1038/cr.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chinese Clinical Trial Register (ChiCTR) - The world health organization international clinical trials registered organization registered platform, (n.d.). http://www.chictr.org.cn/showprojen.aspx?proj=49145 (accessed April 8, 2020).
- 67.Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 68.Biot C., Daher W., Chavain N., Fandeur T., Khalife J., Dive D., De Clercq E. Design and synthesis of hydroxyferroquine derivatives with antimalarial and antiviral activities. J. Med. Chem. 2006;49:2845–2849. doi: 10.1021/jm0601856. [DOI] [PubMed] [Google Scholar]
- 69.Marmor M.F., Kellner U., Lai T.Y.Y., Melles R.B., Mieler W.F. American academy of ophthalmology, recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision) Ophthalmology. 2016;123:1386–1394. doi: 10.1016/j.ophtha.2016.01.058. [DOI] [PubMed] [Google Scholar]
- 70.D. Zhou, S.-M. Dai, Q. Tong, COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression, J. Antimicrob. Chemother.. (n.d.). doi: 10.1093/jac/dkaa114. [DOI] [PMC free article] [PubMed]
- 71.Gautret P., Lagier J.C., Parola P., Hoang V.T., Medded L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honore S., Colson P., Chabriere E., Scola B.L., Rolain J.M., Brouqui P., Raoult D. 2020. Hydroxychloroquine and Azithromycin as a Treatment of COVID-19: Preliminary Results of an Open-label Non-randomized Clinical Trial. MedRxiv. 2020.03.16.20037135. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Zumla A., Chan J.F.W., Azhar E.I., Hui D.S.C., Yuen K.-Y. Coronaviruses — drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lei L., Jian-ya G. 2020. Clinical Characteristics of 51 Patients Discharged From Hospital With COVID-19 in Chongqing, China. MedRxiv. 2020.02.20.20025536. [DOI] [Google Scholar]
- 74.Lim J., Jeon S., Shin H.-Y., Kim M.J., Seong Y.M., Lee W.J., Choe K.-W., Kang Y.M., Lee B., Park S.-J. Case of the index patient who caused tertiary transmission of coronavirus disease 2019 in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 pneumonia monitored by quantitative RT-PCR. J. Korean Med. Sci. 2020;35 doi: 10.3346/jkms.2020.35.e79. [DOI] [PubMed] [Google Scholar]
- 75.Hoenen T., Groseth A., Feldmann H. Therapeutic strategies to target the Ebola virus life cycle. Nat Rev Microbiol. 2019;17:593–606. doi: 10.1038/s41579-019-0233-2. [DOI] [PubMed] [Google Scholar]
- 76.Lo M.K., Feldmann F., Gary J.M., Jordan R., Bannister R., Cronin J., Patel N.R., Klena J.D., Nichol S.T., Cihlar T., Zaki S.R., Feldmann H., Spiropoulou C.F., de Wit E. Remdesivir (GS-5734) protects African green monkeys from Nipah virus challenge. Sci. Transl. Med. 2019;11:eaau9242. doi: 10.1126/scitranslmed.aau9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barlow A., Landolf K.M., Barlow B., Yeung S.Y.A., Heavner J.J., Claassen C.W., Heavner M.S. Review of emerging pharmacotherapy for the treatment of coronavirus disease 2019. Pharmacotherapy. 2020 doi: 10.1002/phar.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O., Spahn J.E., Bauer L., Sellers S., Porter D., Feng J.Y., Cihlar T., Jordan R., Denison M.R., Baric R.S. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11:1–14. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M.O., Mackman R.L., Spahn J.E., Palmiotti C.A., Siegel D., Ray A.S., Cihlar T., Jordan R., Denison M.R., Baric R.S. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9:eaal3653. doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V., Siegel D., Perron M., Bannister R., Hui H.C., Larson N., Strickley R., Wells J., Stuthman K.S., Van Tongeren S.A., Garza N.L., Donnelly G., Shurtleff A.C., Retterer C.J., Gharaibeh D., Zamani R., Kenny T., Eaton B.P., Grimes E., Welch L.S., Gomba L., Wilhelmsen C.L., Nichols D.K., Nuss J.E., Nagle E.R., Kugelman J.R., Palacios G., Doerffler E., Neville S., Carra E., Clarke M.O., Zhang L., Lew W., Ross B., Wang Q., Chun K., Wolfe L., Babusis D., Park Y., Stray K.M., Trancheva I., Feng J.Y., Barauskas O., Xu Y., Wong P., Braun M.R., Flint M., McMullan L.K., Chen S.-S., Fearns R., Swaminathan S., Mayers D.L., Spiropoulou C.F., Lee W.A., Nichol S.T., Cihlar T., Bavari S. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Madelain V., Nguyen T.H.T., Olivo A., de Lamballerie X., Guedj J., Taburet A.-M., Mentré F. Ebola virus infection: review of the pharmacokinetic and pharmacodynamic properties of drugs considered for testing in human efficacy trials. Clin. Pharmacokinet. 2016;55:907–923. doi: 10.1007/s40262-015-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Severe 2019-nCoV Remdesivir RCT - full text view - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04257656 (accessed April 9, 2020).
- 83.Mild/moderate 2019-nCoV Remdesivir RCT - full text view - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04252664 (accessed April 9, 2020).
- 84.Study to evaluate the safety and antiviral activity of remdesivir (GS-5734™) in participants with severe coronavirus disease (COVID-19) - full text view - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04292899 (accessed April 9, 2020).
- 85.Adaptive COVID-19 treatment trial (ACTT) - full text view - ClinicalTrials.gov, (n.d.). Https://Clinicaltrials.Gov/Ct2/Show/Nct04280705 (accessed April 9, 2020).
- 86.Tan E.L.C., Ooi E.E., Lin C.-Y., Tan H.C., Ling A.E., Lim B., Stanton L.W. Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs. Emerg. Infect. Dis. 2004;10:581–586. doi: 10.3201/eid1004.030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Te H.S., Randall G., Jensen D.M. Mechanism of action of ribavirin in the treatment of chronic hepatitis C. Gastroenterol Hepatol (N Y) 2007;3:218–225. [PMC free article] [PubMed] [Google Scholar]
- 88.Graci J.D., Cameron C.E. Mechanisms of action of ribavirin against distinct viruses. Rev. Med. Virol. 2006;16:37–48. doi: 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ölschläger S., Neyts J., Günther S. Depletion of GTP pool is not the predominant mechanism by which ribavirin exerts its antiviral effect on Lassa virus. Antivir. Res. 2011;91:89–93. doi: 10.1016/j.antiviral.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 90.Falzarano D., de Wit E., Martellaro C., Callison J., Munster V.J., Feldmann H. Inhibition of novel β coronavirus replication by a combination of interferon-α2b and ribavirin. Sci. Rep. 2013;3:1686. doi: 10.1038/srep01686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discoveries & Therapeutics. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 92.Knowles S.R., Phillips E.J., Dresser L., Matukas L. Common adverse events associated with the use of ribavirin for severe acute respiratory syndrome in Canada. Clin. Infect. Dis. 2003;37:1139–1142. doi: 10.1086/378304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chu C.M., Cheng V.C.C., Hung I.F.N., Wong M.M.L., Chan K.H., Chan K.S., Kao R.Y.T., Poon L.L.M., Wong C.L.P., Guan Y., Peiris J.S.M., Yuen K.Y. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lopinavir/Ritonavir, Ribavirin and IFN-beta combination for nCoV treatment - full text view - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04276688 (accessed April 9, 2020).
- 95.Blaising J., Polyak S.J., Pécheur E.-I. Arbidol as a broad-spectrum antiviral: an update. Antivir. Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hulseberg C.E., Fénéant L., Szymańska-de Wijs K.M., Kessler N.P., Nelson E.A., Shoemaker C.J., Schmaljohn C.S., Polyak S.J., White J.M. Arbidol and other low-molecular-weight drugs that inhibit lassa and ebola viruses. J. Virol. 2019;93 doi: 10.1128/JVI.02185-18. e02185-18, /jvi/93/8/JVI.02185-18.atom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pécheur E.-I., Borisevich V., Halfmann P., Morrey J.D., Smee D.F., Prichard M., Mire C.E., Kawaoka Y., Geisbert T.W., Polyak S.J. The synthetic antiviral drug arbidol inhibits globally prevalent pathogenic viruses. J. Virol. 2016;90:3086–3092. doi: 10.1128/JVI.02077-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Teissier E., Zandomeneghi G., Loquet A., Lavillette D., Lavergne J.-P., Montserret R., Cosset F.-L., Böckmann A., Meier B.H., Penin F., Pécheur E.-I. Mechanism of inhibition of enveloped virus membrane fusion by the antiviral drug arbidol. PLoS One. 2011;6 doi: 10.1371/journal.pone.0015874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kadam R.U., Wilson I.A. Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proc. Natl. Acad. Sci. U. S. A. 2017;114:206–214. doi: 10.1073/pnas.1617020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zeng L.-Y., Yang J., Liu S. Investigational hemagglutinin-targeted influenza virus inhibitors. Expert Opin. Investig. Drugs. 2017;26:63–73. doi: 10.1080/13543784.2017.1269170. [DOI] [PubMed] [Google Scholar]
- 101.Liu M.-Y., Wang S., Yao W.-F., Wu H., Meng S.-N., Wei M.-J. Pharmacokinetic properties and bioequivalence of two formulations of arbidol: an open-label, single-dose, randomized-sequence, two-period crossover study in healthy chinese male volunteers. Clin. Ther. 2009;31:784–792. doi: 10.1016/j.clinthera.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Deng P., Zhong D., Yu K., Zhang Y., Wang T., Chen X. Pharmacokinetics, metabolism, and excretion of the antiviral drug arbidol in humans. Antimicrob. Agents Chemother. 2013;57:1743–1755. doi: 10.1128/AAC.02282-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Song J.-H., Fang Z.-Z., Zhu L.-L., Cao Y.-F., Hu C.-M., Ge G.-B., Zhao D.-W. Glucuronidation of the broad-spectrum antiviral drug arbidol by UGT isoforms: UGT isoforms for arbidol glucuronidation. J. Pharm. Pharmacol. 2013;65:521–527. doi: 10.1111/jphp.12014. [DOI] [PubMed] [Google Scholar]
- 104.Jin Y.-H., Cai L., Cheng Z.-S., Cheng H., Deng T., Fan Y.-P., Fang C., Huang D., Huang L.-Q., Huang Q., Han Y., Hu B., Hu F., Li B.-H., Li Y.-R., Liang K., Lin L.-K., Luo L.-S., Ma J., Ma L.-L., Peng Z.-Y., Pan Y.-B., Pan Z.-Y., Ren X.-Q., Sun H.-M., Wang Y., Wang Y.-Y., Weng H., Wei C.-J., Wu D.-F., Xia J., Xiong Y., Xu H.-B., Yao X.-M., Yuan Y.-F., Ye T.-S., Zhang X.-C., Zhang Y.-W., Zhang Y.-G., Zhang H.-M., Zhao Y., Zhao M.-J., Zi H., Zeng X.-T., Wang Y.-Y., Wang X.-H., for the Zhongnan Hospital of Wuhan University Novel Coronavirus Management and Research Team, Evidence-Based Medicine Chapter of China International Exchange and Promotive Association for Medical and Health Care (CPAM) A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Military Medical Research. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Clinical study of arbidol hydrochloride tablets in the treatment of pneumonia caused by novel coronavirus - full text view - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04260594 (accessed April 9, 2020).
- 106.Evaluating the efficacy and safety of bromhexine hydrochloride tablets combined with standard treatment/standard treatment in patients with suspected and mild novel coronavirus pneumonia (COVID-19) - full text view - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04273763 (accessed April 9, 2020).
- 107.K. K., A. D. Potential repurposing of Favipiravir in COVID-19 outbreak based on current evidence. Travel Med. Infect. Dis. 2020:101710. doi: 10.1016/j.tmaid.2020.101710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dose-finding study of favipiravir in the treatment of uncomplicated influenza, ClinicalTrials.Gov. (n.d.). https://clinicaltrials.gov/ct2/show/NCT01068912 (accessed May 16, 2020).
- 109.Phase 3 efficacy and safety study of favipiravir for treatment of uncomplicated influenza in adults - T705US316, ClinicalTrials.Gov. (n.d.). https://clinicaltrials.gov/ct2/show/NCT02026349 (accessed May 16, 2020).
- 110.Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., Liao X., Gu Y., Cai Q., Yang Y., Shen C., Li X., Peng L., Huang D., Zhang J., Zhang S., Wang F., Liu J., Chen L., Chen S., Wang Z., Zhang Z., Cao R., Zhong W., Liu Y., Liu L. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020 doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Researchers find two new drugs that can effectively inhibit coronavirus, (n.d.). https://news.cgtn.com/news/2020-02-04/Researchers-find-two-drugs-that-can-effectively-inhibit-coronavirus-NOFpci7NJK/index.html (accessed May 17, 2020).
- 112.Lu H. Efficacy and safety of darunavir and cobicistat for treatment of COVID-19, clinicaltrials.gov. 2020. https://clinicaltrials.gov/ct2/show/NCT04252274
- 113.Santos J.R., Curran A., Navarro-Mercade J., Ampuero M.F., Pelaez P., Pérez-Alvarez N., Clotet B., Paredes R., Moltó J. Simplification of antiretroviral treatment from darunavir/ritonavir monotherapy to darunavir/cobicistat monotherapy: effectiveness and safety in routine clinical practice. AIDS Res. Hum. Retrovir. 2019;35:513–518. doi: 10.1089/aid.2018.0178. [DOI] [PubMed] [Google Scholar]
- 114.Mathias A.A., German P., Murray B.P., Wei L., Jain A., West S., Warren D., Hui J., Kearney B.P. Pharmacokinetics and pharmacodynamics of GS-9350: a novel pharmacokinetic enhancer without anti-HIV activity. Clinical Pharmacology & Therapeutics. 2010;87:322–329. doi: 10.1038/clpt.2009.228. [DOI] [PubMed] [Google Scholar]
- 115.Uyeki T.M. Oseltamivir treatment of influenza in children. Clin. Infect. Dis. 2018;66:1501–1503. doi: 10.1093/cid/cix1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kongsaengdao D.S. A 6 week prospective, open label, randomized, in Multicenter Study of, Oseltamivir Plus Hydroxychloroquine Versus Lopipinavir/Ritonavir Plus Oseltamivir Versus Darunavir/Ritonavir Plus Oseltamivir Plus Hydroxychloroquine in Mild COVID-19 AND Lopipinavir/Ritonavir Plus Oseltamivir Versus Favipiravir Plus Lopipinavir/Ritonavir Versus Darunavir/Ritonavir Plus Oseltamivir Plus Hydroxychloroquine Versus Favipiravir Plus Darunavir and Ritonavir Plus Hydroxychloroquine in Moderate to Critically Ill COVID-19, clinicaltrials.gov. 2020. https://clinicaltrials.gov/ct2/show/NCT04303299
- 118.Liu Y.-J. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 119.Jakimovski D., Kolb C., Ramanathan M., Zivadinov R., Weinstock-Guttman B. Interferon β for multiple sclerosis. Cold Spring Harb Perspect Med. 2018;8:a032003. doi: 10.1101/cshperspect.a032003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Totura A.L., Baric R.S. SARS coronavirus pathogenesis: host innate immune responses and viral antagonism of interferon. Current Opinion in Virology. 2012;2:264–275. doi: 10.1016/j.coviro.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schneider W.M., Chevillotte M.D., Rice C.M. Interferon-stimulated genes: a complex web of host defenses. Annu. Rev. Immunol. 2014;32:513–545. doi: 10.1146/annurev-immunol-032713-120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Omrani A.S., Saad M.M., Baig K., Bahloul A., Abdul-Matin M., Alaidaroos A.Y., Almakhlafi G.A., Albarrak M.M., Memish Z.A., Albarrak A.M. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect. Dis. 2014;14:1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hensley L.E., Fritz E.A., Jahrling P.B., Karp C., Huggins J.W., Geisbert T.W. Interferon-β 1a and SARS coronavirus replication. Emerg. Infect. Dis. 2004;10:317–319. doi: 10.3201/eid1002.030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chan J.F.W., Chan K.-H., Kao R.Y.T., To K.K.W., Zheng B.-J., Li C.P.Y., Li P.T.W., Dai J., Mok F.K.Y., Chen H., Hayden F.G., Yuen K.-Y. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J. Infect. 2013;67:606–616. doi: 10.1016/j.jinf.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shen K.-L., Yang Y.-H. Diagnosis and treatment of 2019 novel coronavirus infection in children: a pressing issue. World J. Pediatr. 2020 doi: 10.1007/s12519-020-00344-6. s12519-020-00344–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Efficacy and safety of IFN-α2β in the treatment of novel coronavirus patients - full text view - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04293887 (accessed April 10, 2020).
- 127.Pegylated interferon lambda treatment for COVID-19 - full text view - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04343976 (accessed May 23, 2020).
- 128.Interferon lambda therapy for COVID-19 - full text view - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04388709 (accessed May 23, 2020).
- 129.Fohner A.E., Sparreboom A., Altman R.B., Klein T.E. PharmGKB summary: macrolide antibiotic pathway, pharmacokinetics/pharmacodynamics. Pharmacogenet. Genomics. 2017;27:164–167. doi: 10.1097/FPC.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gielen V., Johnston S.L., Edwards M.R. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur. Respir. J. 2010;36:646–654. doi: 10.1183/09031936.00095809. [DOI] [PubMed] [Google Scholar]
- 131.Tyteca D. Azithromycin, a lysosomotropic antibiotic, has distinct effects on fluid-phase and receptor-mediated endocytosis, but does not impair phagocytosis in J774 macrophages. Exp. Cell Res. 2002;281:86–100. doi: 10.1006/excr.2002.5613. [DOI] [PubMed] [Google Scholar]
- 132.Li C., Zu S., Deng Y.-Q., Li D., Parvatiyar K., Quanquin N., Shang J., Sun N., Su J., Liu Z., Wang M., Aliyari S.R., Li X.-F., Wu A., Ma F., Shi Y., Nielsen-Saines K., Jung J.U., Qin F.X.-F., Qin C.-F., Cheng G. Azithromycin protects against zika virus infection by upregulating virus-induced Type I and III interferon responses. Antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.00394-19. e00394-19, /aac/63/12/AAC.00394-19.atom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Damle B., Vourvahis M., Wang E., Leaney J., Corrigan B. Clinical pharmacology perspectives on the antiviral activity of azithromycin and use in COVID-19. Clin. Pharmacol. Ther. 2020 doi: 10.1002/cpt.1857. (cpt.1857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hydroxychloroquine vs. azithromycin for hospitalized patients with suspected or confirmed COVID-19 - full text view - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04329832 (accessed May 18, 2020).
- 135.Clinical trial of combined use of hydroxychloroquine, azithromycin, and tocilizumab for the treatment of COVID-19 - full text view - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04332094 (accessed May 18, 2020).
- 136.Evaluating the efficacy of hydroxychloroquine and azithromycin to prevent hospitalization or death in persons with COVID-19 - full text view - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04358068 (accessed May 18, 2020).
- 137.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.-M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 138.Griffin M.O., Fricovsky E., Ceballos G., Villarreal F. Tetracyclines: a pleitropic family of compounds with promising therapeutic properties. Review of the literature. Am. J. Phys. Cell Phys. 2010;299:C539–C548. doi: 10.1152/ajpcell.00047.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Phillips J.M., Gallagher T., Weiss S.R. Neurovirulent murine coronavirus JHM.SD uses cellular zinc metalloproteases for virus entry and cell-cell fusion. J. Virol. 2017;91 doi: 10.1128/JVI.01564-16. e01564-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Henehan M., Montuno M., De Benedetto A. Doxycycline as an anti-inflammatory agent: updates in dermatology. J. Eur. Acad. Dermatol. Venereol. 2017;31:1800–1808. doi: 10.1111/jdv.14345. [DOI] [PubMed] [Google Scholar]
- 141.Nantes University Hospital Doxycycline versus placebo in COVID-19 + patients without hospitalization criteria: prospective, multicenter, randomized, double-blind study, clinicaltrials.gov. 2020. https://clinicaltrials.gov/ct2/show/NCT04371952
- 142.Parenti F., Beretta G., Berti M., Arioli V. Teichomycins, new antibiotics from Actinoplanes teichomyceticus nov. sp. I. Description of the producer strain, fermentation studies and biological properties. J. Antibiot. 1978;31:276–283. doi: 10.7164/antibiotics.31.276. [DOI] [PubMed] [Google Scholar]
- 143.Colson P., Raoult D. Fighting viruses with antibiotics: an overlooked path. Int. J. Antimicrob. Agents. 2016;48:349–352. doi: 10.1016/j.ijantimicag.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhou N., Pan T., Zhang J., Li Q., Zhang X., Bai C., Huang F., Peng T., Zhang J., Liu C., Tao L., Zhang H. Glycopeptide antibiotics potently inhibit cathepsin l in the late endosome/lysosome and block the entry of ebola virus, middle east respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus (SARS-CoV) J. Biol. Chem. 2016;291:9218–9232. doi: 10.1074/jbc.M116.716100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Parenti F. Structure and mechanism of action of teicoplanin. J. Hosp. Infect. 1986;7:79–83. doi: 10.1016/0195-6701(86)90011-3. [DOI] [PubMed] [Google Scholar]
- 146.Somma S., Gastaldo L., Corti A. Teicoplanin, a new antibiotic from Actinoplanes teichomyceticus nov. sp. Antimicrob. Agents Chemother. 1984;26:917–923. doi: 10.1128/AAC.26.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang J., Ma X., Yu F., Liu J., Zou F., Pan T., Zhang H. Teicoplanin potently blocks the cell entry of 2019-nCoV. Microbiology. 2020 doi: 10.1101/2020.02.05.935387. [DOI] [Google Scholar]
- 148.Bernareggi A., Borghi A., Borgonovi M., Cavenaghi L., Ferrari P., Vekey K., Zanol M., Zerilli L.F. Teicoplanin metabolism in humans. Antimicrob. Agents Chemother. 1992;36:1744–1749. doi: 10.1128/AAC.36.8.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Campoli-Richards D.M., Brogden R.N., Faulds D. Teicoplanin. Drugs. 1990;40:449–486. doi: 10.2165/00003495-199040030-00007. [DOI] [PubMed] [Google Scholar]
- 150.Kindrachuk J., Ork B., Hart B.J., Mazur S., Holbrook M.R., Frieman M.B., Traynor D., Johnson R.F., Dyall J., Kuhn J.H., Olinger G.G., Hensley L.E., Jahrling P.B. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for middle east respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob. Agents Chemother. 2015;59:1088–1099. doi: 10.1128/AAC.03659-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gupta N. Sirolimus treatment in hospitalized patients with COVID-19 pneumonia (The SCOPE Trial), clinicaltrials.gov. 2020. https://clinicaltrials.gov/ct2/show/NCT04341675
- 152.Kraft W.K., Randomized A. Double-blinded, placebo-controlled trial evaluating the virological efficacy, safety, tolerability, pharmacokinetics, and pharmacodynamics of sirolimus adjuvant therapy in patients with coronavirus disease (COVID-19), clinicaltrials.gov. 2020. https://clinicaltrials.gov/ct2/show/NCT04371640
- 153.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kuriya B., Cohen M.D., Keystone E. Baricitinib in rheumatoid arthritis: evidence-to-date and clinical potential. Therapeutic Advances in Musculoskeletal. 2017;9:37–44. doi: 10.1177/1759720X16687481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Treatment of moderate to severe coronavirus disease (COVID-19) in hospitalized patients - full text view - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04321993 (accessed May 19, 2020).
- 156.Clinical trial to evaluate efficacy of 3 types of treatment in patients with pneumonia by COVID-19 - full text view - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04346147 (accessed May 19, 2020).
- 157.Chighizola C.B., Ong V.H., Meroni P.L. The use of cyclosporine a in rheumatology: a 2016 comprehensive review. Clinic Rev Allerg Immunol. 2017;52:401–423. doi: 10.1007/s12016-016-8582-3. [DOI] [PubMed] [Google Scholar]
- 158.Faulds D., Goa K.L., Benfield P. Cyclosporin: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in immunoregulatory disorders. Drugs. 1993;45:953–1040. doi: 10.2165/00003495-199345060-00007. [DOI] [PubMed] [Google Scholar]
- 159.de Wilde A.H., Zevenhoven-Dobbe J.C., van der Meer Y., Thiel V., Narayanan K., Makino S., Snijder E.J., van Hemert M.J. Cyclosporin A inhibits the replication of diverse coronaviruses. J. Gen. Virol. 2011;92:2542–2548. doi: 10.1099/vir.0.034983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cure E., Cumhur Cure M. Can dapagliflozin have a protective effect against COVID-19 infection? A hypothesis. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020;14:405–406. doi: 10.1016/j.dsx.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Behrens E.M., Koretzky G.A. Review: cytokine storm syndrome: looking toward the precision medicine era. Arthritis & Rheumatology. 2017;69:1135–1143. doi: 10.1002/art.40071. [DOI] [PubMed] [Google Scholar]
- 162.Abdallah H., Hsu J.C., Lu P., Fettner S., Zhang X., Douglass W., Bao M., Rowell L., Burmester G.R., Kivitz A. Pharmacokinetic and pharmacodynamic analysis of subcutaneous tocilizumab in patients with rheumatoid arthritis from 2 randomized, controlled trials: SUMMACTA and BREVACTA: pharmacokinetic and pharmacodynamic analysis of subcutaneous. J. Clin. Pharmacol. 2017;57:459–468. doi: 10.1002/jcph.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U. S. A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Keizer R.J., Huitema A.D.R., Schellens J.H.M., Beijnen J.H. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin. Pharmacokinet. 2010;49:493–507. doi: 10.2165/11531280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 166.National Cancer Institute, Naples Multicenter study on the efficacy and tolerability of tocilizumab in the treatment of patients with COVID-19 pneumonia, clinicaltrials.gov. 2020. https://clinicaltrials.gov/ct2/show/NCT04317092 [DOI] [PMC free article] [PubMed]
- 167.The use of tocilizumab in the management of patients who have severe COVID-19 with suspected pulmonary hyperinflammation - full text view - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04377750 (accessed May 20, 2020).
- 168.Wagstaff K.M., Sivakumaran H., Heaton S.M., Harrich D., Jans D.A. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem. J. 2012;443:851–856. doi: 10.1042/BJ20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yang S.N.Y., Atkinson S.C., Wang C., Lee A., Bogoyevitch M.A., Borg N.A., Jans D.A. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antivir. Res. 2020;177 doi: 10.1016/j.antiviral.2020.104760. [DOI] [PubMed] [Google Scholar]
- 170.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;178 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Centenario Hospital Miguel Hidalgo Efficacy and safety of hydroxychloroquine and ivermectin in hospitalized no critical patients secondary to COVID-19 infection: randomized controlled trial, clinicaltrials.gov. 2020. https://clinicaltrials.gov/ct2/show/NCT04391127
- 172.Anguiano L., Riera M., Pascual J., Valdivielso J.M., Barrios C., Betriu A., Mojal S., Fernández E., Soler M.J. Circulating angiotensin-converting enzyme 2 activity in patients with chronic kidney disease without previous history of cardiovascular disease. Nephrol. Dial. Transplant. 2015;30:1176–1185. doi: 10.1093/ndt/gfv025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Clinical management of severe acute respiratory infection when COVID-19 is suspected, (n.d.). https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected (accessed April 10, 2020).
- 176.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Alhazzani W., Møller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E., Oczkowski S., Levy M.M., Derde L., Dzierba A., Du B., Aboodi M., Wunsch H., Cecconi M., Koh Y., Chertow D.S., Maitland K., Alshamsi F., Belley-Cote E., Greco M., Laundy M., Morgan J.S., Kesecioglu J., McGeer A., Mermel L., Mammen M.J., Alexander P.E., Arrington A., Centofanti J.E., Citerio G., Baw B., Memish Z.A., Hammond N., Hayden F.G., Evans L., Rhodes A. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020 doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhou Y.-H., Qin Y.-Y., Lu Y.-Q., Sun F., Yang S., Harypursat V., Tang S.-Q., Huang Y.-Q., He X.-Q., Zeng Y.-M., Li Y., Xu X.-L., Zhao T., Chen Y.-K. Effectiveness of glucocorticoid therapy in patients with severe novel coronavirus pneumonia: protocol of a randomized controlled trial. Chin. Med. J. 2020 doi: 10.1097/CM9.0000000000000791. Publish Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhou Z.-G., Xie S.-M., Zhang J., Zheng F., Jiang D.-X., Li K.-Y., Zuo Q., Yan Y.-S., Liu J.-Y., Xie Y.-L., Peng H., Zhang L. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. 2020. https://www.preprints.org/manuscript/202003.0065/v2
- 181.Shang J., Du R., Lu Q., Wu J., Xu S., Ke Z., Cai Z., Gu Y., Huang Q., Zhan Y., Yang J., Liu Y., Hu Y., Zhang H., Huang H., Xie Z., Li X., Hu W., Gong J., Ke W., Shao Z., Liu Z., Xie J. 2020. The Treatment and Outcomes of Patients With COVID-19 in Hubei, China: A Multi-centered, Retrospective, Observational Study, Social Science Research Network, Rochester, NY. [DOI] [Google Scholar]
- 182.C. for B.E. and Research FDA; 2020. Recommendations for investigational COVID-19 convalescent plasma. https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma
- 183.Benjamin R.J., McLaughlin L.S. Plasma components: properties, differences, and uses. Transfusion. 2012;52:9S–19S. doi: 10.1111/j.1537-2995.2012.03622.x. [DOI] [PubMed] [Google Scholar]
- 184.Rojas M., Rodríguez Y., Monsalve D.M., Acosta-Ampudia Y., Camacho B., Gallo J.E., Rojas-Villarraga A., Ramírez-Santana C., Díaz-Coronado J.C., Manrique R., Mantilla R.D., Shoenfeld Y., Anaya J.-M. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun. Rev. 2020 doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bloch E.M., Shoham S., Casadevall A., Sachais B.S., Shaz B., Winters J.L., van Buskirk C., Grossman B.J., Joyner M., Henderson J.P., Pekosz A., Lau B., Wesolowski A., Katz L., Shan H., Auwaerter P.G., Thomas D., Sullivan D.J., Paneth N., Gehrie E., Spitalnik S., Hod E., Pollack L., Nicholson W.T., Pirofski L., Bailey J.A., Tobian A.A.R. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J. Clin. Invest. 2020 doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lutz H.U., Späth P.J. Anti-inflammatory effect of intravenous immunoglobulin mediated through modulation of complement activation. Clinic Rev Allerg Immunol. 2005;29:207–212. doi: 10.1385/CRIAI:29:3:207. [DOI] [PubMed] [Google Scholar]
- 187.Basta M., Van Goor F., Luccioli S., Billings E.M., Vortmeyer A.O., Baranyi L., Szebeni J., Alving C.R., Carroll M.C., Berkower I., Stojilkovic S.S., Metcalfe D.D. F(ab)′2-mediated neutralization of C3a and C5a anaphylatoxins: a novel effector function of immunoglobulins. Nat. Med. 2003;9:431–438. doi: 10.1038/nm836. [DOI] [PubMed] [Google Scholar]
- 188.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L., Meng S., Hu Y., Peng C., Yuan M., Huang J., Wang Z., Yu J., Gao X., Wang D., Yu X., Li L., Zhang J., Wu X., Li B., Xu Y., Chen W., Peng Y., Hu Y., Lin L., Liu X., Huang S., Zhou Z., Zhang L., Wang Y., Zhang Z., Deng K., Xia Z., Gong Q., Zhang W., Zheng X., Liu Y., Yang H., Zhou D., Yu D., Hou J., Shi Z., Chen S., Chen Z., Zhang X., Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. PNAS. 2020 doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gabutti G., d’Anchera E., Sandri F., Savio M., Stefanati A. Coronavirus: update related to the current outbreak of COVID-19. Infect. Dis. Ther. 2020 doi: 10.1007/s40121-020-00295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.The Lancet Infectious Diseases Challenges of coronavirus disease 2019. Lancet Infect. Dis. 2020;20:261. doi: 10.1016/S1473-3099(20)30072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kupferschmidt K., Cohen J. WHO launches global megatrial of the four most promising coronavirus treatments. Science. 2020;22 [Google Scholar]
- 192.Singh A.K., Singh A., Shaikh A., Singh R., Misra A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: a systematic search and a narrative review with a special reference to India and other developing countries. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020;14:241–246. doi: 10.1016/j.dsx.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pannu J. Running ahead of pandemics: achieving in-advance antiviral drugs. SSRN Journal. 2020 doi: 10.2139/ssrn.3570737. [DOI] [Google Scholar]
- 194.Yuen K.-S., Ye Z.-W., Fung S.-Y., Chan C.-P., Jin D.-Y. SARS-CoV-2 and COVID-19: the most important research questions. Cell Biosci. 2020;10:40. doi: 10.1186/s13578-020-00404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Amanat F., Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020 doi: 10.1016/j.immuni.2020.03.007. S1074761320301205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Vashist S.K. In vitro diagnostic assays for COVID-19: recent advances and emerging trends. Diagnostics. 2020;10:202. doi: 10.3390/diagnostics10040202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.covexit, Professor Didier Raoult releases the results of a New Hydroxychloroquine Treatment Study on 1061 patients – COVEXIT.com, (n.d.). http://covexit.com/professor-didier-raoult-releases-the-results-of-a-new-hydroxychloroquine-treatment-study-on-1061-patients/ (accessed April 10, 2020).
- 198.K. Dhama, K. Sharun, R. Tiwari, M. Dadar, Y.S. Malik, K.P. Singh, W. Chaicumpa, COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics, Human Vaccines & Immunotherapeutics. 0 (2020) 1–7. doi: 10.1080/21645515.2020.1735227. [DOI] [PMC free article] [PubMed]
- 199.Vellingiri B., Jayaramayya K., Iyer M., Narayanasamy A., Govindasamy V., Giridharan B., Ganesan S., Venugopal A., Venkatesan D., Ganesan H., Rajagopalan K., Rahman P.K.S.M., Cho S.-G., Kumar N.S., Subramaniam M.D. COVID-19: A promising cure for the global panic. Sci. Total Environ. 2020;725:138277. doi: 10.1016/j.scitotenv.2020.138277. [DOI] [PMC free article] [PubMed] [Google Scholar]