Abstract
Tumor lymphatics play a key role in cancer progression as they are solely responsible for transporting malignant cells to regional lymph nodes (LNs), a process that precedes and promotes systemic lethal spread. It is broadly accepted that tumor lymphatic sprouting is induced mainly by soluble factors derived from tumor-associated macrophages (TAMs) and malignant cells. However, emerging evidence strongly suggests that a subset of TAMs, myeloid-lymphatic endothelial cell progenitors (M-LECP), also contribute to the expansion of lymphatics through both secretion of paracrine factors and a self-autonomous mode. M-LECP are derived from bone marrow (BM) precursors of the monocyte-macrophage lineage and characterized by unique co-expression of markers identifying lymphatic endothelial cells (LEC), stem cells, M2-type macrophages, and myeloid-derived immunosuppressive cells. This review describes current evidence for the origin of M-LECP in the bone marrow, their recruitment tumors and intratumoral trafficking, similarities to other TAM subsets, and mechanisms promoting tumor lymphatics. We also describe M-LECP integration into preexisting lymphatic vessels and discuss potential mechanisms and significance of this event. We conclude that improved mechanistic understanding of M-LECP functions within the tumor environment may lead to new therapeutic approaches to suppress tumor lymphangiogenesis and metastasis to lymph nodes.
Keywords: Bone marrow, Breast cancer, Endothelial cell lineage development, Hematopoietic stem cell differentiation, Inflammation, Lymphangiogenesis, Lymphatic metastasis, Lymphatic endothelial progenitors, M2-type macrophages, Myeloid-derived pro-vascular progenitors, Myeloid-derived suppressor cells, Tumor macrophages, Toll-like receptor 4, Tumor microenvironment, Vessel formation
7.1. Introduction
The lymphatic system consisting of lymph nodes (LNs) and the highly organized hierarchal network of lymphatic vessels is unique in the sense that it is an integral part of both the body’s immune defense and circulatory networks. As part of the immune defense, the lymphatic system is primarily responsible for transporting macrophages and dendritic cells (DC) from the tissues to regional lymph nodes where they present newly harvested antigens to regulatory and effector cells to help mount an adaptive immune response [4]. Lymphatic vessels also play important roles in the leukocyte trafficking and regulation of local immune responses [7, 89, 104]. As part of the circulatory system, lymphatic vessels are responsible for absorbing excessive protein and fluid from the interstitium and returning them to blood circulation [95]. This is particularly important during inflammation that is characterized by elevated vascular permeability [24] and, hence, a significant increase in water and blood proteins in the affected tissues. Specialized lymphatic vessels perform a variety of critical physiological functions in the skin, guts, and other organs [81].
The functions of the normal lymphatic system are beneficial for homeostasis, immune defense, and tissue restoration post-injury. Whereas induction of tumor lymphatics follows the same incentives as physiological lymphangiogenesis, tumor-induced lymphatics play a largely negative role. This is because tumor lymphatics are sole contributors to transporting malignant cells to local lymph nodes, a process that greatly increases systemic metastasis [12, 87]. An additional factor is that in the cancer environment, demands for generation of new vasculature are aggravated by high concentrations and imbalance of endothelium-promoting proteins over-expressed by malignant cells.
The two main factors that induce tumor and inflammatory lymphangiogenesis are vascular endothelial growth factor C (VEGF-C) and a related protein VEGF-D [55]. Both ligands bind the high-affinity tyrosine kinase receptor VEGFR-3 that is primarily expressed in lymphatic endothelial cells (LEC) [68]. VEGFR-3 activation increases proliferation, migration, and morphogenesis of LEC culminating in formation of new sprouts derived from the “mother” vessel. This canonical understanding of lymphatic vessel (LV) formation [27, 72] is now rapidly expanding by the emerging evidence indicating the critical contribution of lymphatic endothelial cell progenitors (LECP) [86, 88].
Although the existence and functional significance of LECP for lymphatic formation were debated in early studies [40, 48], it is now broadly accepted in the field [52, 77, 88]. Addition of exogenous LECP has been shown to increase lymphatic vessel density (LVD) in multiple in vivo models of inflammation [43, 64] and tumors [113], whereas ablation of bone marrow (BM)-derived mononuclear cells inhibits formation of new lymphatics [28]. Myeloid cell-derived LECP (i.e., M-LECP) appear to be the predominant type of lymphatic progenitors that contribute to inflammatory [77] and tumor [88] lymphangiogenesis in both human pathologies [110] and mouse experimental models [113]. Blood-circulating LECP are present at substantially higher levels in cancer patients compared with healthy subjects [9, 85, 113]. As we recently reported, the density of tumor-infiltrating M-LECP in clinical breast cancers significantly correlates with tumor-induced lymphatics and patient lymph node (LN) status [112]. This collective evidence strongly suggests an important role of BM-derived lymphatic progenitors in generation of tumor lymphatics and subsequent metastasis. This review summarizes the current knowledge in the LECP and M-LECP field with particular focus on their recruitment to tumors and interactions with the cells of the tumor microenvironment (TME).
7.1.1. Bone Marrow (BM) Origin of M-LECP
Adult LECP reportedly originate from various sources including the adipose tissue [118], cord blood [107, 110], mesenchymal stem cells [25], and hematopoietic stem cells [53]. However, most studies identified BM-derived immature CD11b-positive myeloid cells as an M-LECP primary source [28, 45, 63, 71, 90]. Supporting the myeloid origin, human blood-circulating mononuclear cells expressing lymphatic markers often co-express CD14, a specific marker of monocytes [19, 60, 110]. BM as the main source of M-LECP is also indicated by studies that showed reduction of myeloid-lymphatic cells upon depletion of BM cells by gamma irradiation and enhanced lymphangiogenesis upon administration of exogenous BM precursors [90]. Additional support is provided by the studies that showed detection of green fluorescent protein (GFP) in newly formed lymphatic vessels in mice following adoptive transfer of BM cells with constitutive GFP expression [88, 90]. It is also consistent with the known immature status of myeloid-lymphatic hybrid cells indicated by the absence of CD80 [45], a marker of mature macrophages, and high expression of a monocytic progenitor marker Ly6C [113]. Human LECP also express stem/progenitor markers such as CD133 as shown in VEGFR-3+ blood-circulating progenitors in both healthy subjects [19, 94] and cancer patients [9, 110]. Collectively, these reports strongly suggest that M-LECP are derived from BM myeloid progenitors rather than local tissue-differentiated macrophages.
7.1.2. Identification of M-LECP in Clinical Cancers and Experimental Tumor Models
M-LECP circulating in the blood or infiltrating tumors can be identified by combined immunostaining for three types of markers typically segregated to distinct lineages or different stages of maturation:
Specific markers of the myeloid lineage (e.g., CD11b in mouse and CD68 in human) indicating their origin
Specific markers of lymphatic endothelial lineage (e.g., VEGFR-3, LYVE-1, and podoplanin (PDPN)) indicating the destination of their cell fate
Stem/progenitor markers indicating their early differentiation status.
Mouse stem/progenitor markers associated with M-LECP include Sca-1 [63] and Ly6C [111], whereas human lymphatic progenitors were reported to express PU.1 [112], CD133, and CD34 [85, 94]. Co-expression of Ly6C, PU.1, and other stem cell markers in LEC-positive hematopoietic cells suggests that M-LECP are derived from the early precursors of the monocytic lineage because these markers are largely absent in mature myeloid cells [73, 114].
The presence of M-LECP in experimental tumor models has been shown in numerous studies by co-staining for CD11b, a specific marker of monocytes and macrophages, and one or more lymphatic markers. The most consistent lymphatic markers identifying mouse M-LECP are LYVE-1 [51, 96, 123] and podoplanin (PDPN) [63], whereas VEGFR-3 and PROX1 are less reliable due to their low or absent expression. This might be due to differential stages of maturity of tumor-recruited M-LECP. As we previously showed, VEGFR-3 signaling is required only for induction of pro-lymphatic differentiation characterized by upregulated LYVE-1 and PDPN but not for maintaining this lymphatic phenotype [43]. This is in contrast with mature LEC that express VEGFR-3, LYVE-1, and PDPN constitutively. Therefore, it stands to reason that LYVE-1+ and PDPN+ tumor-associated macrophages (TAMs) representing more mature LECP are detected at greater quantities than VEGFR-3+ or PROX1+ M-LECP, owing to the transient expression pattern of these markers during differentiation. Some examples of intratumoral mouse and human M-LECP identified by double staining using myeloid, stem, and lymphatic cell markers are shown in Figs. 7.1 and 7.2.
Fig. 7.1.
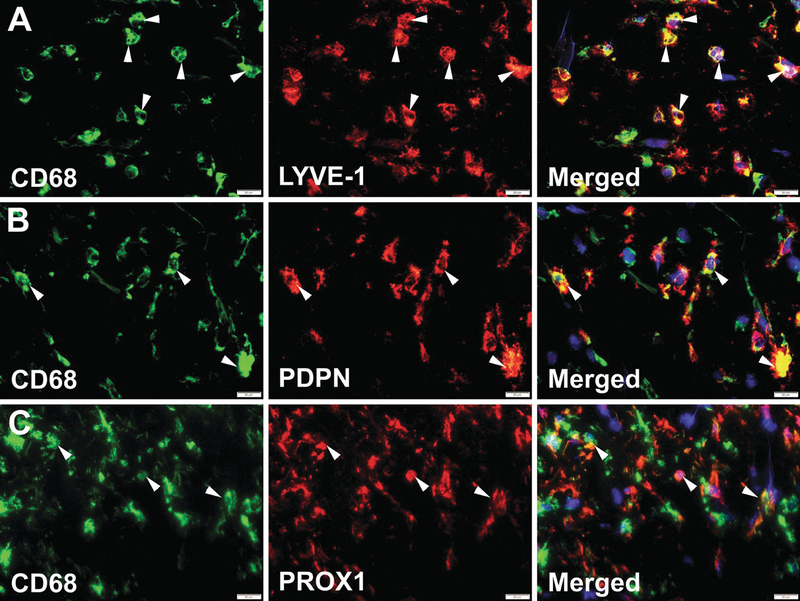
Human clinical breast cancers massively recruit M-LECP. Human BC specimens were co-stained for CD68 (green) and antibodies against markers of lymphatic vessels (red) including (a) LYVE-1, (b) PDPN, and (c) PROX1. Nuclei in merged images were identified by Hoechst stain. White arrowheads indicate cells that co-express CD68 and lymphatic markers. All images were acquired at 600× magnification
Fig. 7.2.
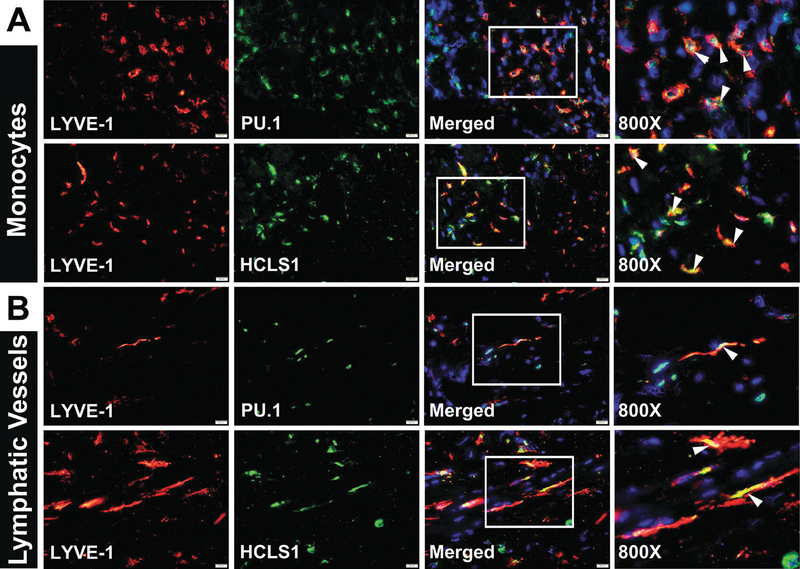
Both tumor M-LECP and lymphatic vessels in clinical breast cancers express stem/progenitor markers. BC specimens were co-stained with anti-LYVE-1, a marker of lymphatic vasculature, and hematopoietic stem markers PU.1 or HCLS1. Both markers were observed in (a) LYVE-1+ monocytes and (b) tumor lymphatic vasculature. All images were acquired at 400× magnification, with Hoechst stained nuclei present in merged images and 800× magnification panels. White boxes indicate areas highlighted in images taken at 800× magnification. White arrowheads point to cells and vessels expressing both LYVE-1 and stem cell markers
In human clinical tumors, M-LECP have been similarly identified by co-staining for LEC markers and CD68 that is broadly expressed in most myeloid cells [41], or CD14, a specific monocytic marker [121]. For instance, VEGFR-3-positive cells co-expressing CD14 and CD68 were shown in clinical cervical cancers [97], and LYVE-1+/CD68+ macrophages were detected in human melanoma [33]. We recently showed [112] that 100% of LYVE-1+ and PDPN+ cells infiltrating clinical breast cancers co-expressed classic monocyte-macrophage markers CD14, CD11b, CD18, MD2, MyD88, and Toll-like receptor 4 (TLR4) (Table 7.1). It is important to note that the first four markers are essential components of the TLR4 membrane complex, whereas the fifth marker (MyD88) is a major intracellular adapter of the activated TLR4. We previously showed that the TLR4 pathway plays a critical role in M-LECP differentiation [43, 113]. Therefore, this profile not only confirms the myeloid-macrophage identity of lymphatic progenitors but also demonstrates a direct link between the TLR4 pathway and lymphatic progenitors recruited to human cancers.
Table 7.1.
Protein expression profile of LYVE-1+ progenitors in clinical breast cancer
| Protein expressed in LYVE-1+ cells | Marker description or alias | Marker lineage expression | % marker positive of total LYVE-1+ cells | Comments |
|---|---|---|---|---|
| TLR4a | Toll-like receptor 4 | Myeloid, monocytes, macrophages | 100% | TLR4 regulates differentiation of M-LECP [88] |
| CD11ba | CD11b | Myeloid, monocytes, macrophages | 100% | CD11b is an essential co-receptor for TLR4 [79] and a marker of myeloid lineages [1] |
| CD14a | CD14 | Myeloid, monocytes, macrophages | 100% | CD14 is an essential co-receptor for TLR4 [39] and a specific marker of monocytes [121] |
| MD2b | Ly96 | Myeloid, monocytes, macrophages | 100% | MD2 is an essential co-receptor of TLR4 [13] |
| MyD88a | Myeloid differentiation factor 88 | Myeloid, monocytes, macrophages | 100% | MyD88 is a key intracellular mediator of the activated TLR4 pathway [22] |
| CXCR3b | CXCR3 | Monocytes, macrophages, stem cells | 100% | CXCR3 is a chemotactic receptor for stem cells [42], monocytes [15], and other immune cells [67] |
| STAB1b | Stabilin-1 | M2-type macrophages LEC | 100% | A marker of M2-type macrophages and lymphatic endothelial cells [57, 92] |
| CD38a | CD38 | Early progenitors | 80% | A specific marker of early BM progenitors [2] |
| HCLS1a | Hematopoietic cell-specific Lyn substrate-1 | Early progenitors | 50% | A specific marker of early BM progenitors [100] |
| PU.1a | Spi-1-protooncogene | Early myeloid progenitors | 50% | A key determinant of myelomonocytic differentiation [75] |
| CD146b | CD146 | Blood vascular endothelial cells (BEC) | 0% | A marker of blood vessels [35] and endothelial progenitors [30]; its absence suggests divergence from BEC lineage |
| CD3, CD4, CD8a | CD3, CD4, CD8 | T-cells | 0% | Absence of T-cell markers suggests lack of involvement of this lymphoid lineage |
| CD19a | CD19 | B-cells | 0% | Absence of B-cell markers suggests lack of involvement of this lymphoid lineage |
| FPR-1b | Formyl peptide receptor 1 | Mainly neutrophils | 0% | A specific marker of neutrophils [82]; the absence suggests divergence from granulocyte lineage |
| EMAa | Cytokeratins | Epithelial cells | 0% | Absence of this marker suggests lack of involvement of the epithelial lineages |
Data are taken from the reference [112]
Unpublished data
7.1.3. M-LECP Recruitment to Tumors and Their Intratumoral Trafficking
Because M-LECP are hybrid cells with dual myeloid-lymphatic phenotype, they express many chemokine receptors typical of macrophages [113]. It is therefore likely that tumor recruitment of M-LECP is mediated by similar chemoattraction pathways that mobilize other macrophage subsets. For instance, CSF1, one of the most potent monocyte attractants [31, 65], has been shown to recruit LYVE-1+ macrophages in a mouse osteosarcoma model [62]. Interference with CSF1 signaling using a CSF1R inhibitor, PLX3397, reduced TAM infiltration and lymphatic vessel density in a mouse breast cancer model MMTV-PyMT [112]. This suggests that LYVE-1+ macrophages follow the same tumor recruitment pathway as other BM-derived monocytes. A separate study showed that PLX3397 treatment of MMTV-PyMT-bearing mice not only reduced tumor infiltration by BM monocytes but also reduced metastasis [31]. Taken together, these studies suggest a direct link between recruitment of LYVE-1+ macrophages and tumor spread.
Another possible recruiter of M-LECP is VEGF-A, a common tumor-derived factor that promotes both angiogenesis and lymphangiogenesis [116]. VEGF-A plays a major role in the recruitment of BM monocytes via activation of one of its receptors, VEGFR-1 [74]. Consistent with the notion that M-LECP are recruited along with other BM-derived myeloid cells, VEGF-A has been shown to significantly increase the density of lymphatic progenitors in mouse models of human gastric, colorectal, and breast cancers [108]. In line with this report, VEGF-A neutralizing treatment of mice with MDA-MB-231 breast tumors reduced TAM infiltration concomitant with inhibition of lymphangiogenesis [116]. Consistently, treatment of patients with lung, breast, and colorectal cancers using anti-human VEGF-A antibody, bevacizumab, significantly reduced blood-circulating levels of immature myeloid cells [76] that represent a major source of M-LECP [88]. This suggests that VEGF-A targeting might be useful for inhibiting tumor infiltration of M-LECP and subsequent lymphangiogenesis in clinical settings.
Additional candidates for tumor recruitment of M-LECP are CXCL12 (SDF-1), a chemokine shown to recruit LYVE-1+ macrophages to adipose tissue via activation of its receptor CXCR4 [23], and CXCR3, a receptor for chemotactic factors CXCL9, CXCL10, and CXCL11 [91]. The potential for the latter receptor to control M-LECP migration is suggested by similar effects on various immune cells including monocytes [15] and mesenchymal stem cells [42]. Both CXCR3 and CXCR4 have been shown to promote lymphangiogenesis [59, 120] and metastasis [59, 122], which is consistent with their potential role in the recruitment of M-LECP. CXCR3 and, to a lesser degree, CXCR4 were detected in all analyzed M-LECP in our study of clinical breast cancers (Table 7.1). However, the direct chemotactic role of either CXCR3 or CXCR4 in tumor M-LECP mobilization has not been determined.
Upon arrival to tumors, M-LECP tend to accumulate near tumor lymphatic vessels [26], implying the existence of an intratumoral chemotactic gradient generated by LEC. This is not surprising because macrophages and DC commonly use lymphatic vessels to exit inflamed tissues on their journey to regional LNs [6, 17]. M-LECP retain the myeloid phenotype along with expression of lymphatic markers and therefore may use LV-generated chemotactic gradients of CCL19/CCL21 known to attract CCR7+ monocytes and dendritic cells (DC) [93, 105]. Monocyte-attracting chemokines CCL2, CCL3, and CCL5 might also be involved in M-LECP recruitment to tumors in general and to lymphatic vessels, specifically. This is supported by detection of the corresponding receptors of CCL2, CCL3, and CCL5 in M-LECP differentiated in vitro [113]. These cytokines have also been shown to attract blood vascular endothelial progenitors to intratumoral vessels [102], suggesting a similar role in recruitment of LECP. However, their promigratory functions in the context of lymphatic progenitors and vasculature have not been directly analyzed.
7.1.4. Relationships Between M-LECP and M2-TAMs
Tumor-associated macrophages (TAMs) are customarily divided into M1 (immunostimulatory) and M2 (immunosuppressive) types with the latter dominating the TME [99]. Some consider this an oversimplified categorization since many TAMs express both M1 and M2 markers [21, 66, 106] and display functional behavior associated with both types. However, it has been widely confirmed that TAMs express various scavenger receptors such as CD163, CD204, and CD206 that are regarded as specific M2-type markers. Scavenger receptors are a heterogeneous class of proteins with broad ligand specificity whose main function is to remove foreign elements from the inflamed or wounded tissue. Such proteins are highly upregulated in the type of macrophages responsible for cleansing and remodeling an injured site. Not surprisingly, accumulation of toxic material in the pathological TME attracts and retains macrophages expressing scavenger receptors. In relation to M-LECP, many TAMs expressing scavenger receptors also express the lymphatic marker LYVE-1 [36, 96]. TAMs with dual expression of M2 and LEC markers were identified in human clinical melanoma and a mouse B16 melanoma model [33]. TAMs expressing CD206 and another LEC marker, VEGFR-3, were found in syngeneic 4T1 breast tumors [36] as well as in other tumor models [96, 123]. We recently demonstrated in clinical breast cancers that a large fraction of LYVE-1+ TAMs co-express CD163 and CD204 [112]. The over-lapping expression of scavenger receptors in TAMs and tumor M-LECP not only confirms the myeloid-macrophage identity of lymphatic progenitors but also suggests a common immunosuppressive nature of both cell types.
While co-localization of LEC markers in M2-TAMs is fairly well established, the underlying reason remains obscure. However, the new understanding that co-signature of M2 macrophages and LEC markers identifies these cells as M-LECP supports a different perspective. As mentioned above, TAM gene expression suggests that their main function is not necessarily to stimulate or inhibit the immune system (they do a little bit of both) but to restore homeostasis disturbed by the TME. A similar macrophage type is found at the resolution phase of wound healing geared toward restoration of the tissue’s function after eliminating pathogens and re-creating lost structural components [69]. In such capacity, the M2-macrophages must contain a subset that restores blood vasculature for the obvious reason that no tissue expansion or remodeling can occur in the absence of adequate oxygen and nutrient supply. Angiogenesis is customarily followed by lymphangiogenesis to coordinate fluid and protein balance between the two circulatory systems. Therefore, it stands to reason that M2-type macrophages, the builders of the new site, would contain a subset of pro-vascular cells designated to regenerate both blood and lymphatic vessels. Indeed, TAMs have been repeatedly linked to tumor angiogenesis [20, 70]. Analogously, M2-TAMs expressing LEC markers (i.e., M-LECP) represent a subset of pro-vascular myeloid cells with a specific mission to create new lymphatics.
7.1.5. Relationships Between M-LECP and Myeloid-Derived Suppressive Cells (MDSC)
MDSC are defined as cells that express myeloid progenitor markers and have abilities to suppress functions of T-cells, B-cells, and NK cells [11]. In mouse models, MDSC are identified by CD11b+/Ly6Clow/Ly6G+ (defined as granulocytic PMN-MDSC), CD11b+/Ly6Chigh/Ly6G− (defined as monocytic M-MDSC), or Gr-1+/CD11b+ cells representing a mixed type [11]. Human markers for MDSC include CD14−/CD11b+/CD15+ (PMN-MDSC) and CD14+/CD11b+/HLA-DRlow (M-MDSC) [11]. In both species, MDSC are regarded as BM-derived immature myeloid cells accumulating in tumors due to high turnover of the existing TAMs [103].
Despite their significance, the exact definition of the MDSC phenotype is still evolving due, in part, to selected study methodology. For instance, many studies did not measure presumed MDSC immunosuppressive activity but rather identified tumor MDSC based solely on the surface markers shared with other myeloid subtypes. Additional confusion is caused by extensive use of RB6–8C5 antibody that recognizes the granulocyte differentiation 1 (Gr-1) epitope shared by two isoforms of Ly6 protein, Ly6G and Ly6C [38, 56]. Although Ly6G and Ly6C are co-expressed in early BM precursors, they are later aligned with either a granulocytic or monocytic lineage but not both [49]. The broad use of RB6–8C5 antibody that binds to the mixed Ly6G/Ly6C epitope adds another layer of uncertainty over specific markers that define MDSC.
With that being said, a number of studies did detect a significant overlap between M-LECP markers and those ascribed to MDSC. For instance, VEGFR-3 was detected in MDSC in lymphoid organs and TAMs infiltrating 4T1 tumors [36]. SAR131675, a specific inhibitor of VEGFR-3, was shown to suppress proliferation of TAMs in vitro and reduce their tumor density in vivo [18]. Analysis of clinical breast cancers showed that TIE-2+ macrophages expressing LEC markers LYVE-1, VEGFR-3, PDPN, and PROX1 exhibited not only pro-lymphangiogenic but also immunosuppressive activity [10]. These cells also co-expressed a monocytic marker CD14 considered as one of defining components of the MDSC signature. PDPN-positive myeloid cells in a mouse glioma model were also shown to possess immunosuppressive activity, and deletion of PDPN from these myeloid cells increased tumor influx of CD8+ cytotoxic T-cells [34]. This evidence collectively suggests that M-LECP, like many other tumor-infiltrating immune cells, suppress the anti-tumor activities of the host.
The potential ability of M-LECP to suppress immune responses might be important for their main function to induce new vasculature. Tumor vascular formation requires complex spatiotemporal coordination for differentiation and recruitment of endothelial and perivascular progenitors as well as intricate interactions with matrix and other cells in the TME. These complex processes might be prohibited in an environment generated by ongoing cytotoxic activities of immune cells, which likely exert bystander effects. It is possible that M-LECP and other pro-vascular progenitors have to be immunosuppressive to execute their functions in order to avoid structural disruption of newly created fragile vessels. Albeit currently speculative, this hypothesis is supported by documented immunosuppression of other sites associated with generation of new vessels such as late stages of wound healing and pregnancy [99].
7.1.6. Interactions of M-LECP with Tumor-Associated Lymphatic Endothelium
One cell type that LECP clearly interact with in the tumor environment is LEC lining preexisting lymphatic vessels. This conclusion is based on two main lines of evidence. First, tumor-infiltrating M-LECP are often found in proximity or close association with preexisting lymphatic vessels [90, 123]. Second, they structurally integrate specifically into lymphatic vessels even if blood vessels are present in the same field [113, 123]. It is also significant that LYVE-1+ progenitors integrate only into tumor-associated vessels but not those in nearby nonneoplastic tissues [10]. This suggests coordinated expression of complementary receptors on M-LECP and activated or inflamed lymphatic vessels that control their specific interaction.
Vascular integration of lymphatic progenitors has been tracked and quantified using various approaches. One approach is detection of exogenously introduced markers such as GFP [113, 123] or a fluorescent dye Dil [63] combined with immunostaining for lymphatic-specific (e.g., LYVE-1) and myeloid-macrophage markers such as CD11b and F4/80. An alternative method employed chimera mice reconstituted with the BM from GFP-expressing mice [90, 109, 113] which allows cell fate and lineage tracking of BM-derived cells. Detection of “green” lymphatic vessels that co-express LYVE-1 indicates insertion of the GFP mRNA or protein into new sprouts, which can occur only through physical interaction with GFP-positive BM-derived cells. This event was shown in multiple experimental models including fibrosarcoma [90], Rip1Tag2 insulinoma [123], melanoma [63], MMTV-PyMT breast [113], and TRAMPC-1 prostate [123] cancers. LYVE-1+ cells derived from transplanted GFP+ BM-derived hematopoietic stem cells were identified in intestinal tumors spontaneously developed in Apc (Min/+) mice [53]. BM-derived LYVE-1+ cells co-expressing a stem cell marker CD34 and a LEC marker VEGFR-3 were shown to integrate into peritumoral lymphatic vessels of mouse T241 fibrosarcoma [90]. CD11b+/PDPN+ tumor macrophages were detected in melanoma-associated lymphatic vessels [96]. In line with these reports, we found widespread lymphatic integration of adoptively transferred GFP+ M-LECP differentiated in vitro in a variety of syngeneic breast tumors EMT6 and MMTV-PyMT and xenografts of human breast carcinoma lines MDA-MB-231 and ZR-75 [112, 113]. Integration of LECP and M-LECP into tumor lymphatics in human cancers was shown by demonstrating highly expressed myeloid markers CD14 and CD68 [10, 112]. By contrast, lymphatic vessels in corresponding normal organs express low-level or no myeloid markers [112].
An example of complete M-LECP integration into tumor-associated lymphatic vessels in transgenic mouse MMTV-PyMT model is shown in Fig. 7.3. Confocal analysis showed that LYVE-1 and a macrophage marker F4/80 were co-expressed in the entire thickness of the vessel (Fig. 7.3, b1–b5 images). The same images show co-expression of lymphatic junctional protein VE-cadherin dispersed along the analyzed vessel (Fig. 7.3b). Co-expression of all three markers in the same vascular structure strongly favors coalescence of M-LECP with preexisting LEC rather than insertion of individual progenitors into the vascular wall. We detected in average 50% and up to 90% of tumor lymphatic vessels with myeloid-macrophage markers in both syngeneic and xenograft breast cancer models [112, 113]. Independent studies showed integration in ~60% of lymphatic vessels in LS174T colorectal and SK-BR-2 breast tumors [108]. Similar approaches detected LECP integration into lymphatic vessels in multiple inflammatory models [71, 96] as well as human tissues undergoing inflammatory lymphangiogenesis [60].
Fig. 7.3.

Confocal microscopy analysis shows evidence for M-LECP integration into tumor lymphatic vessels. MMTV-PyMT tumors were triple-stained for LYVE-1 and (a) CD11b and VEGFR-3 or (b) F4/80 and VE-cadherin. The region highlighted by a white box in b indicates the area analyzed by confocal Z-stack represented below in panels 1–5. Each image was captured 2 μm apart. All images were acquired at 1000× magnification
These observations are highly reminiscent of integration of blood vascular endothelial progenitors into tumor blood vessels [44] indicating that both blood vascular and lymphatic progenitors might follow the same process during inflammatory or tumor vascular formation. Further support for this conclusion is shown in studies with patients who received gender-mismatched BM transfusion years before tumor development [80]. Intriguingly, analysis of blood vessels in their cancers detected chromosomes from the opposite sex identified by in situ hybridization using specific probes to X and Y chromosomes [80]. Detection of the entire chromosome in the nuclei of tumor endothelial cells (EC) strongly suggests transfer of the whole cellular content of progenitors to existing EC rather than lineage infidelity, transcriptional aberration, or random upregulation of an isolated marker.
Another line of evidence that supports the donation of the entire progenitors’ contents is expression of protein tags experimentally introduced in LECP. We showed in both inflammatory [43] and tumor mouse models [113], as well as in human breast cancers [112], that endogenous myeloid markers and ectopic GFP are dispersed throughout lymphatic vessels after integration of M-LECP. An independent study using a pancreatic RT2 tumor model showed a similar pattern of GFP expression in tumor lymphatic vessels in mice that received a transfer of BM-derived GFP+ cells [123]. Using confocal microscopy and Z-stack analyses, the authors of this study distinguished among GFP+ cells closely associated with lymphatic vessels, GFP+ macrophages transmigrating through the vascular wall, and those truly integrated into the endothelial layer [123]. While all three events have been identified in expanding vasculature, only full integration of lymphatic progenitors into vessels can account for the broad GFP expression pattern in recipient lymphatic vessels [123] and longevity (>1 year) of GFP expression in these structures [53]. Taken together with the evidence described above, this suggests that pro-vascular progenitors might promote sprouting by transferring their cellular contents to the existing endothelium. Currently, however, the mechanisms of vascular integration of progenitors as well as the physiological impetus driving this process remain unknown.
7.1.7. Role of M-LECP in Generation of New Tumor Lymphatic Vessels
Although many aspects of M-LECP-mediated lymphangiogenesis are still poorly understood, three main mechanisms have been proposed in current literature. The most widely accepted concept suggests that myeloid-lymphatic cells promote lymphatic formation by virtue of over-expression of lymphangiogenic factors VEGF-A [108] and VEGF-C [32, 58, 61]. These factors stimulate, respectively, VEGFR-2 and VEGFR-3 expressed on LEC, and therefore their binding to these receptors is expected to induce the formation of new vasculature [50, 68]. This concept is supported by multiple lines of evidence from both experimental models and clinical studies. For instance, tumor M2-type macrophages [115, 117, 119] and myeloid cells with LEC markers [97] were shown to express much higher levels of lymphangiogenic factors than CD11b-negative cells [117]. Moreover, tumor expression of VEGF-A and VEGF-C is known to correlate with tumor LVD and lymphatic metastasis [8, 78, 98]. This mechanism is also supported by studies demonstrating suppression of tumor lymphangiogenesis by anti-VEGF-A antibody [116] or agents targeting the VEGFR-3 pathway [14, 46, 47, 84]. Suppression of tumor lymphangiogenesis and lymphatic metastasis by global elimination of macrophages also favors this concept [117].
While this evidence is generally consistent with the important role of TAM-produced paracrine factors in vascular formation, this mechanism does not effectively explain several findings, particularly those emerging in the M-LECP field. First, the majority of studies that supported a paracrine effect of VEGF-C did not compare the total amount of VEGF-C produced by TAMs with the amount derived from tumor cells. A single study that did compare the levels of VEGF-C transcripts showed a substantially higher expression in malignant cells compared with macrophages from the same tumor [123]. As shown in this study, for each 100 molecules of VEGF-C transcript expressed by tumor cells, macrophages produced only one to two molecules [123]. We recently confirmed this observation in a human breast cancer xenograft model, MDA-MB-231, by comparing the exact number of mouse and human VEGF-C transcript copies in the same tumor samples. We found that for each molecule of mouse VEGF-C produced by the entire tumor stroma, nearly 1000 transcript copies were produced by human malignant cells [112]. Based on the combined evidence from these two studies, it appears that the minuscule contribution of stroma including TAMs is unlikely to be significant for induction of new lymphatic vessels.
Another argument for the TAM pro-lymphangiogenic role mediated by paracrine factors is based on studies demonstrating inhibition of tumor lymphatics by anti-VEGF-C or anti-VEGFR-3 agents [47, 117]. However, the problem with this argument is that systemic inhibition of VEGFR-3 does not distinguish between local effects inhibiting VEGFR-3 on sprouting vessels and suppression of M-LECP generation in the BM that heavily relies on this pathway [43, 88]. Targeting macrophages in general also does not provide a clear mechanism since such treatment does not discriminate between elimination of soluble factors produced by M-LECP and alternative mechanisms relying on cell-cell interactions. Additional problem to explain the M-LECP role in lymphangiogenesis based only on production of soluble factors is the acquisition of the lymphatic phenotype by differentiated M-LECP [43, 113]. Arguably, VEGF-C transcription that can be induced in fibroblasts, epithelial cells, and other cell types requires no coincident expression of LEC-specific proteins in the producing cells. It is therefore unclear why M-LECP should express LYVE-1 and many other LEC markers if their sole function is to produce VEGF-C. Lastly, this mechanism does not address integration of M-LECP into preexisting LEC, an event that defies a logical explanation if the induction of lymphatics depends only on the paracrine support. This collective evidence argues that a cell-autonomous role of M-LECP might be more important for induction of lymphatic sprouting than their contribution to lymphangiogenic factors, particularly in the context of cancers secreting voluminous amounts of such proteins.
Another suggested mechanism of M-LECP-dependent lymphatic expansion is lympho-vasculogenesis, a process similar to generation of primitive lymphatic vasculature during embryonic development. Embryonic vascular formation is fundamentally different from that in the adults by virtue of the absence of preexisting vessels. Vasculogenesis is common during embryogenesis but extremely rare in adulthood. However, two independent studies in cornea injury models showed de novo lymphatic vessels arisen within the avascular limbus stroma at a considerable distance from preexisting lymphatic vessels [71, 110]. Moreover, the new vessels expressed GFP that could be derived only from GFP+ BM cells transplanted prior to injury [71]. The same study showed that isolated BM-derived CD11b+ cells created LYVE-1+/PDPN+ tubes in vitro [71], demonstrating their ability to replicate lympho-vascular morphology. Similar but rare instances of lympho-vasculogenesis were also observed in a model of peritonitis induced by a TLR4 ligand, LPS [43], and in MDA-MB-231 tumors activated by another TLR4 ligand, a chemotherapeutic drug paclitaxel [111]. The latter observation is potentially significant from a clinical perspective because paclitaxel was able to induce vessels in the center of the tumor normally devoid of lymphatics [111]. Intratumoral lymphatics are highly efficient in mediating metastasis due to proximity to tumor cells [5]. The enhanced LN metastatic burden was, indeed, demonstrated in paclitaxel-treated tumor-bearing mice [111]. Whether lympho-vasculogenesis commonly occurs in clinical cancers is currently unknown.
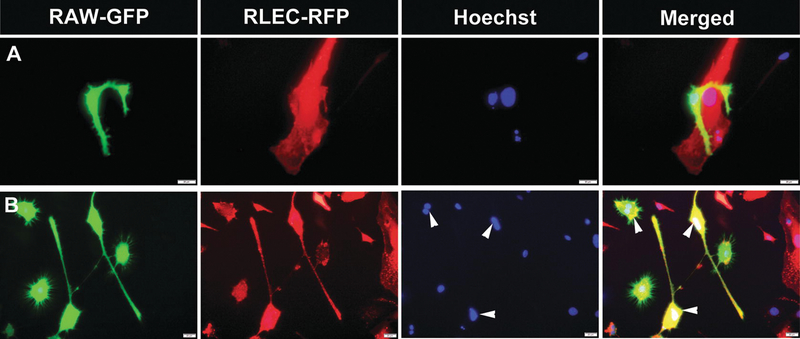
The third proposed mechanism for M-LECP induction of tumor lymphangiogenesis involves integration of M-LECP into preexisting lymphatic vessels observed during both inflammatory [64] and tumor lymphatic formation [90, 108, 113, 123]. This event was previously described as “incorporation” [53, 90, 109], “integration” [16, 63, 123], or “insertion” [10] of myeloid-lymphatic cells into tumor vasculature. However, a more accurate description might be “fusion.” This is because histological and immunohistochemical analyses of tumors in vivo show a complete overlap between myeloid and lymphatic markers in vessels rather than insertion of individual myeloid cells between two adjacent LEC. Several additional lines of evidence also support the theory of M-LECP fusion with LEC. Confocal microscopy analyses showed that myeloid markers derived from M-LECP are detected throughout the length and depth of the lymphatic vascular structures and are not restricted to “inserted” myeloid cells (Fig. 7.3). Chimera mice reconstituted with GFP+ BM generated “green” LV in which GFP was evenly distributed through the entire thickness of the vessels identified by LYVE-1 and VE-cadherin markers [112]. Independent studies showed coalescence of lymphatic progenitors with LEC during inflammatory lymphangiogenesis by detecting Y chromosome in lymphatic vessels in female patients undergoing rejection of gender-mismatched kidney transplants [60]. This is reminiscent of detection of XX and Y chromosomes in the nuclei of tumor (but not normal) blood vascular endothelial cells (BEC) in patients who received gender-mismatched BM transplants years before tumor development [44]. Clearly, the long-term presence of one or more chromosomes in remodeled vasculature indicates not just cell-cell interaction but donation of the entire genomic material, which is difficult to explain by any other mechanism but fusion. In support of this concept, we recently demonstrated that conditions mimicking TME promote fusion of GFP+ mouse macrophage line co-cultured with red fluorescent protein (RFP)-tagged LEC [112]. Fusion was detected by both color overlap (i.e., presence of yellow cells) and shared nuclei [112]. An example of fusion of LEC and inflamed macrophages induced by TME-mimicking conditions in vitro is shown in Fig. 7.4. This assay also detected a substantially increased nuclear multiplication in the fused cells, suggesting that transfer of the M-LECP genomic material to LEC might be necessary for cell division, a key prerequisite for generation of new sprouts.
Fig. 7.4.
Fusion is a possible mechanism of M-LECP integration into tumor lymphatics. Rat lymphatic endothelial cells expressing RFP (RLEC-RFP) and macrophage cell line RAW264.7 expressing GFP (RAW-GFP) were co-cultured for 4–6 days in serum-free medium containing 3 nM of LPS. (a) RAW-GFP migrated to RLEC-RFP displayed intimate cell-cell interactions. (b) After 24–48 hours, many cells underwent fusion indicated by yellow color and multi-nucleation highlighted by white arrowheads. Homogenous color throughout fused cells indicates complete donation of the RAW-GFP cell contains to RFP-tagged lymphatic endothelial cells. All images were acquired at 600× magnification
It should be noted that stem and progenitor cells routinely use fusion for direct transfer of biological material to cells requiring recovery or functional reprogramming [3]. This is particularly noted under injury [29], tumor [83], and inflammatory conditions [54] reminiscent of TME. Fusion and other means of transferring cellular contents are the common mechanisms of stem/progenitor cells recruited to damaged and injured sites that have been programmed to restore the lost components of these tissues [37, 101]. Conceptually, tumor M-LECP are similar to other progenitors attempting to restore functions of the wounded organs. Fusion used by other BM progenitors is the most effective way to provide injured cells in peripheral organs with the survival factors, promitotic signals, and nuclear transcription factors needed to direct structural expansion and to enforce reprogramming. Fusion asserts the breadth, the speed, and the exquisite specificity of delivered factors to the target cells. Such assertion cannot be matched by diffusion of paracrine soluble factors limited in vivo only to a few hundred microns by anatomic barriers. This is particularly relevant to generation of new adult vasculature known for resistance to endothelial cell division under normal circumstances. It is well established that major expansion of either blood or lymphatic vasculature during adulthood is strictly reserved to drastic and unresolved changes in homeostasis such as chronic inflammatory diseases and cancer. It is therefore tempting to suggest that while soluble paracrine TAM-derived factors can aid in new vessel formation, the key mechanism forcing the adult endothelium to undergo highly complex changes required for sprouting must be induced by more drastic cell-transforming mechanism such as fusion. Only fusion can directly deliver genome-remodeling regulatory proteins imposing a fundamentally new behavior on the needed cells. If this theory is proven to be correct in future studies, this might explain how a relatively small number of BM progenitors can produce an extensive network of new vessels.
In summary, currently proposed mechanisms of progenitor-mediated lymphangiogenesis include the following:
Production of soluble pro-lymphatic factors directly acting on existing endothelium
Embryonic-like lympho-vasculogenesis that does not require preexisting vessels
Full donation of the progenitors’ contents to LEC mediated by fusion or other means of protein and gene transfer
The latter is suggested to enable the existing LEC to undergo complex processes required for sprouting that are typically prohibited under normal or transient inflammatory conditions to prevent promiscuous vessel formation. A better understanding of the proportional contribution of these mechanisms to, and their collective impact on, the formation of tumor lymphatics is likely to emerge in future studies.
7.2. Future Directions
The M-LECP field is now entering an exciting new phase. In the past decade, inflammation-dependent induction of M-LECP in humans and mice was firmly established. Myeloid-lymphatic progenitors have been shown to mobilize from the bone marrow to sites of inflammation where they significantly contribute to structural expansion and function of new lymphatics, in part, by integration into preexisting vasculature. In the absence of preexisting lymphatics, M-LECP undergo an embryonic-like lympho-vasculogenesis. Throughout these processes, M-LECP retain their myeloid and stem-like identities while adding LEC features without becoming mature endothelial cells. Five out-standing questions that need to be addressed in future studies are as follows:
What are the differentiation mechanisms in the BM diverting the myeloid-macrophage precursors toward acquisition of the lymphatic phenotype?
Which chemokines are responsible for M-LECP mobilization to the blood, to tumor recruitment, and specifically toward tumor lymphatic vessels?
What mechanisms regulate de novo formation of lymphatics via adult lympho-vasculogenesis?
What mechanisms control M-LECP integration into the vasculature? What is the nature of this event? What happens after integration?
What are the differences and similarities between LECP generated in the BM and those produced by peripheral tissues?
Regarding the first question, the current evidence suggests that M-LECP differentiation requires continuous presence of inflammatory cytokines that promote generation of the macrophage lineage (e.g., CSF1) as well as potent immunomodulators such as TLR4 ligands. However, identification of the specific transcription factors that control myeloid-lymphatic transition still awaits future studies. With regard to the second question, the screening of individual chemokines needs to be conducted to determine whether M-LECP take advantage of classic trafficking pathways of inflammatory monocytes or express their own receptors to direct migration to inflamed tissues. Analysis of the third question will require side-by-side comparison of specific transcription factors and cellular events as well as interaction with the cells in the local environment during embryonic and adult lymphatic formation.
The fourth question is arguably the most intriguing of all because of the paucity of current evidence illuminating the mechanisms of vascular integration of either blood or lymphatic progenitors and the lack of any information regarding the molecular consequences of this event. The physiological impetus for M-LECP to undergo such a process also remains undefined. The only direct clue to this question is the recent evidence suggesting that integration indicated by co-localization of myeloid and lymphatic markers in new vessels might reflect fusion of M-LECP with preexisting LEC [112]. Future studies will need to validate this hypothesis, and if confirmed, tease out specific steps and molecular regulation of this process.
Lastly, future research will need to compare the molecular profiles and mechanisms of differentiation of LECP that originate from other sources than hematopoietic stem cells or myeloid precursors. Such studies should provide critical information for understanding the diversity of M-LECP population and the role of local tissue sources for lymphatic regeneration and remodeling.
In summary, future studies of M-LECP-dependent promotion of lymphatics are expected not only to clarify the mechanisms of tumor lymphangiogenesis and associated metastasis but also to illuminate the consequences of chronic inflammation associated with many human disorders. Such studies should also advance the understanding of the fundamental mechanisms of tissue regeneration during adulthood.
Acknowledgments
The authors are grateful to Susan Ryherd for critical review and editing. This manuscript was supported by a grant # R01CA199649 awarded to Sophia Ran by the National Institutes of Health and a Team Science Grant from Simmons Cancer Institute funded by proceeds of the Denim and Diamonds charity event.
Contributor Information
Sophia Ran, Department of Medical Microbiology, Immunology, and Cell Biology, Southern Illinois University School of Medicine, Springfield, IL, USA; Simmons Cancer Institute, Springfield, IL, USA.
Lisa Volk-Draper, Department of Medical Microbiology, Immunology, and Cell Biology, Southern Illinois University School of Medicine, Springfield, IL, USA.
References
- 1.Ahn GO, Tseng D, Liao CH, Dorie MJ, Czechowicz A, Brown JM (2010) Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc Natl Acad Sci USA 107:8363–8368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albeniz I, Turker-Sener L, Bas A, Kalelioglu I, Nurten R (2012) Isolation of hematopoietic stem cells and the effect of CD38 expression during the early erythroid progenitor cell development process. Oncol Lett 3:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambrosi DJ, Rasmussen TP (2005) Reprogramming mediated by stem cell fusion. J Cell Mol Med 9:320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angeli V, Randolph GJ (2006) Inflammation, lymphatic function, and dendritic cell migration. Lymphat Res Biol 4:217–228 [DOI] [PubMed] [Google Scholar]
- 5.Beasley NJ, Prevo R, Banerji S, Leek RD, Moore J, van Trappen P et al. (2002) Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res 62:1315–1320 [PubMed] [Google Scholar]
- 6.Bellingan GJ, Xu P, Cooksley H, Cauldwell H, Shock A, Bottoms S et al. (2002) Adhesion molecule-dependent mechanisms regulate the rate of macrophage clearance during the resolution of peritoneal inflammation. J Exp Med 196:1515–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Betterman KL, Harvey NL (2016) The lymphatic vasculature: development and role in shaping immunity. Immunol Rev 271:276–292 [DOI] [PubMed] [Google Scholar]
- 8.Bjorndahl MA, Cao R, Burton JB, Brakenhielm E, Religa P, Galter D et al. (2005) Vascular endothelial growth factor-a promotes peritumoral lymphangiogenesis and lymphatic metastasis. Cancer Res 65:9261–9268 [DOI] [PubMed] [Google Scholar]
- 9.Bogos K, Renyi-Vamos F, Dobos J, Kenessey I, Tovari J, Timar J et al. (2009) High VEGFR-3-positive circulating lymphatic/vascular endothelial progenitor cell level is associated with poor prognosis in human small cell lung cancer. Clin Cancer Res 15:1741–1746 [DOI] [PubMed] [Google Scholar]
- 10.Bron S, Henry L, Faes-Van’t Hull E, Turrini R, Vanhecke D, Guex N et al. (2016) TIE-2-expressing monocytes are lymphangiogenic and associate specifically with lymphatics of human breast cancer. Oncoimmunology 5:e1073882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF et al. (2016) Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 7:12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown M, Assen FP, Leithner A, Abe J, Schachner H, Asfour G et al. (2018) Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science 359:1408–1411 [DOI] [PubMed] [Google Scholar]
- 13.Bryant CE, Spring DR, Gangloff M, Gay NJ (2010) The molecular basis of the host response to lipopolysaccharide. Nat Rev Microbiol 8:8–14 [DOI] [PubMed] [Google Scholar]
- 14.Burton JB, Priceman SJ, Sung JL, Brakenhielm E, An DS, Pytowski B et al. (2008) Suppression of prostate cancer nodal and systemic metastasis by blockade of the lymphangiogenic axis. Cancer Res 68:7828–7837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butler KL, Clancy-Thompson E, Mullins DW (2017) CXCR3(+) monocytes/macrophages are required for establishment of pulmonary metastases. Sci Rep 7:45593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buttler K, Lohrberg M, Gross G, Weich HA, Wilting J (2016) Integration of CD45-positive leukocytes into newly forming lymphatics of adult mice. Histochem Cell Biol 145:629–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao C, Lawrence DA, Strickland DK, Zhang L (2005) A specific role of integrin Mac-1 in accelerated macrophage efflux to the lymphatics. Blood 106:3234–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cecco S, Aliberti M, Baldo P, Giacomin E, Leone R (2014) Safety and efficacy evaluation of albumin-bound paclitaxel. Expert Opin Drug Saf 13:511–520 [DOI] [PubMed] [Google Scholar]
- 19.Changming W, Xin L, Hua T, Shikun W, Qiong X, Zhigeng Z et al. (2011) Monocytes can be induced to express lymphatic phenotypes. Lymphology 44:48–53 [PubMed] [Google Scholar]
- 20.Chen P, Huang Y, Bong R, Ding Y, Song N, Wang X et al. (2011) Tumor-associated macrophages promote angiogenesis and melanoma growth via adrenomedullin in a paracrine and autocrine manner. Clin Cancer Res 17:7230–7239 [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Tan W, Wang C (2018) Tumor-associated macrophage-derived cytokines enhance cancer stem-like characteristics through epithelial-mesenchymal transition. Onco Targets Ther 11:3817–3826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng Z, Taylor B, Ourthiague DR, Hoffmann A (2015) Distinct single-cell signaling characteristics are conferred by the MyD88 and TRIF pathways during TLR4 activation. Sci Signal 8:ra69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho CH, Koh YJ, Han J, Sung HK, Jong LH, Morisada T et al. (2007) Angiogenic role of LYVE-1-positive macrophages in adipose tissue. Circ Res 100:e47–e57 [DOI] [PubMed] [Google Scholar]
- 24.Claesson-Welsh L (2015) Vascular permeability--the essentials. Ups J Med Sci 120:135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conrad C, Niess H, Huss R, Huber S, von Luettichau I, Nelson PJ et al. (2009) Multipotent mesenchymal stem cells acquire a lymphendothelial phenotype and enhance lymphatic regeneration in vivo. Circulation 119:281–289 [DOI] [PubMed] [Google Scholar]
- 26.Corliss BA, Azimi MS, Munson JM, Peirce SM, Murfee WL (2016) Macrophages: an inflammatory link between angiogenesis and lymphangiogenesis. Microcirculation 23:95–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cueni LN, Detmar M (2008) The lymphatic system in health and disease. Lymphat Res Biol 6:109–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C et al. (2004) VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest 113:1040–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies PS, Powell AE, Swain JR, Wong MH (2009) Inflammation and proliferation act together to mediate intestinal cell fusion. PLoS One 4:e6530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delorme B, Basire A, Gentile C, Sabatier F, Monsonis F, Desouches C et al. (2005) Presence of endothelial progenitor cells, distinct from mature endothelial cells, within human CD146+ blood cells. Thromb Haemost 94:1270–1279 [DOI] [PubMed] [Google Scholar]
- 31.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF et al. (2011) Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov 1:54–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding M, Fu X, Tan H, Wang R, Chen Z, Ding S (2012) The effect of vascular endothelial growth factor C expression in tumor-associated macrophages on lymphangiogenesis and lymphatic metastasis in breast cancer. Mol Med Rep 6:1023–1029 [DOI] [PubMed] [Google Scholar]
- 33.Dollt C, Becker K, Michel J, Melchers S, Weis CA, Schledzewski K et al. (2017) The shedded ectodomain of Lyve-1 expressed on M2-like tumor-associated macrophages inhibits melanoma cell proliferation. Oncotarget 8:103682–103692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisemann T, Costa B, Peterziel H, Angel P (2019) Podoplanin positive myeloid cells promote glioma development by immune suppression. Front Oncol 9:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elshal MF, Khan SS, Takahashi Y, Solomon MA, McCoy JP Jr (2005) CD146 (Mel-CAM), an adhesion marker of endothelial cells, is a novel marker of lymphocyte subset activation in normal peripheral blood. Blood 106:2923–2924 [DOI] [PubMed] [Google Scholar]
- 36.Espagnolle N, Barron P, Mandron M, Blanc I, Bonnin J, Agnel M et al. (2014) Specific inhibition of the VEGFR-3 tyrosine kinase by SAR131675 reduces peripheral and tumor associated immunosuppressive myeloid cells. Cancers (Basel) 6:472–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrand J, Noel D, Lehours P, Prochazkova-Carlotti M, Chambonnier L, Menard A et al. (2011) Human bone marrow-derived stem cells acquire epithelial characteristics through fusion with gastrointestinal epithelial cells. PLoS One 6:e19569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fleming TJ, Fleming ML, Malek TR (1993) Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6–8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol 151:2399–2408 [PubMed] [Google Scholar]
- 39.Gangloff M, Weber AN, Gay NJ (2005) Conserved mechanisms of signal transduction by toll and toll-like receptors. J Endotoxin Res 11:294–298 [DOI] [PubMed] [Google Scholar]
- 40.Gordon EJ, Rao S, Pollard JW, Nutt SL, Lang RA, Harvey NL (2010) Macrophages define dermal lymphatic vessel calibre during development by regulating lymphatic endothelial cell proliferation. Development 137:3899–3910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gough PJ, Gordon S, Greaves DR (2001) The use of human CD68 transcriptional regulatory sequences to direct high-level expression of class a scavenger receptor in macrophages in vitro and in vivo. Immunology 103:351–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo YC, Chiu YH, Chen CP, Wang HS (2018) Interleukin-1beta induces CXCR3-mediated chemo-taxis to promote umbilical cord mesenchymal stem cell transendothelial migration. Stem Cell Res Ther 9:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall KL, Volk-Draper LD, Flister MJ, Ran S (2012) New model of macrophage acquisition of the lymphatic endothelial phenotype. PLoS One 7:e31794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hammerling GJ, Ganss R (2006) Vascular integration of endothelial progenitors during multistep tumor progression. Cell Cycle 5:509–511 [DOI] [PubMed] [Google Scholar]
- 45.Hamrah P, Chen L, Cursiefen C, Zhang Q, Joyce NC, Dana MR (2004) Expression of vascular endothelial growth factor receptor-3 (VEGFR-3) on monocytic bone marrow-derived cells in the conjunctiva. Exp Eye Res 79:553–561 [DOI] [PubMed] [Google Scholar]
- 46.Harris AR, Perez MJ, Munson JM (2018) Docetaxel facilitates lymphatic-tumor crosstalk to promote lymphangiogenesis and cancer progression. BMC Cancer 18:718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T et al. (2002) Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst 94:819–825 [DOI] [PubMed] [Google Scholar]
- 48.He Y, Rajantie I, Ilmonen M, Makinen T, Karkkainen MJ, Haiko P et al. (2004) Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer Res 64:3737–3740 [DOI] [PubMed] [Google Scholar]
- 49.Hestdal K, Ruscetti FW, Ihle JN, Jacobsen SE, Dubois CM, Kopp WC et al. (1991) Characterization and regulation of RB6–8C5 antigen expression on murine bone marrow cells. J Immunol 147:22–28 [PubMed] [Google Scholar]
- 50.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H et al. (1997) Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 276:1423–1425 [DOI] [PubMed] [Google Scholar]
- 51.Jeon BH, Jang C, Han J, Kataru RP, Piao L, Jung K et al. (2008) Profound but dysfunctional lymphangiogenesis via vascular endothelial growth factor ligands from CD11b+ macrophages in advanced ovarian cancer. Cancer Res 68:1100–1109 [DOI] [PubMed] [Google Scholar]
- 52.Ji RC (2012) Macrophages are important mediators of either tumor-or inflammation-induced lymphangiogenesis. Cell Mol Life Sci 69:897–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang S, Bailey AS, Goldman DC, Swain JR, Wong MH, Streeter PR et al. (2008) Hematopoietic stem cells contribute to lymphatic endothelium. PLoS One 3:e3812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johansson CB, Youssef S, Koleckar K, Holbrook C, Doyonnas R, Corbel SY et al. (2008) Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nat Cell Biol 10:575–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jussila L, Alitalo K (2002) Vascular growth factors and lymphangiogenesis. Physiol Rev 82:673–700 [DOI] [PubMed] [Google Scholar]
- 56.Jutila MA, Kroese FG, Jutila KL, Stall AM, Fiering S, Herzenberg LA et al. (1988) Ly-6C is a monocyte/macrophage and endothelial cell differentiation antigen regulated by interferon-gamma. Eur J Immunol 18:1819–1826 [DOI] [PubMed] [Google Scholar]
- 57.Karikoski M, Marttila-Ichihara F, Elima K, Rantakari P, Hollmen M, Kelkka T et al. (2014) Clever-1/stabilin-1 controls cancer growth and metastasis. Clin Cancer Res 20:6452–6464 [DOI] [PubMed] [Google Scholar]
- 58.Kataru RP, Jung K, Jang C, Yang H, Schwendener RA, Baik JE et al. (2009) Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood 113:5650–5659 [DOI] [PubMed] [Google Scholar]
- 59.Kawada K, Taketo MM (2011) Significance and mechanism of lymph node metastasis in cancer progression. Cancer Res 71:1214–1218 [DOI] [PubMed] [Google Scholar]
- 60.Kerjaschki D, Huttary N, Raab I, Regele H, Bojarski-Nagy K, Bartel G et al. (2006) Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med 12:230–234 [DOI] [PubMed] [Google Scholar]
- 61.Kim KE, Koh YJ, Jeon BH, Jang C, Han J, Kataru RP et al. (2009) Role of CD11b+ macrophages in intraperitoneal lipopolysaccharide-induced aberrant lymphangiogenesis and lymphatic function in the diaphragm. Am J Pathol 175:1733–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M et al. (2009) M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med 206:1089–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee JY, Park C, Cho YP, Lee E, Kim H, Kim P et al. (2010) Podoplanin-expressing cells derived from bone marrow play a crucial role in postnatal lymphatic neovascularization. Circulation 122:1413–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee SJ, Park C, Lee JY, Kim S, Kwon PJ, Kim W et al. (2015) Generation of pure lymphatic endothelial cells from human pluripotent stem cells and their therapeutic effects on wound repair. Sci Rep 5:11019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin EY, Nguyen AV, Russell RG, Pollard JW (2001) Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 193:727–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin X, Zheng W, Liu J, Zhang Y, Qin H, Wu H et al. (2013) Oxidative stress in malignant melanoma enhances tumor necrosis factor-alpha secretion of tumor-associated macrophages that promote cancer cell invasion. Antioxid Redox Signal 19:1337–1355 [DOI] [PubMed] [Google Scholar]
- 67.Liu Y, Poon RT, Hughes J, Feng X, Yu WC, Fan ST (2005) Chemokine receptors support infiltration of lymphocyte subpopulations in human hepatocellular carcinoma. Clin Immunol 114:174–182 [DOI] [PubMed] [Google Scholar]
- 68.Lohela M, Saaristo A, Veikkola T, Alitalo K (2003) Lymphangiogenic growth factors, receptors and therapies. Thromb Haemost 90:167–184 [DOI] [PubMed] [Google Scholar]
- 69.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M (2013) Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 229:176–185 [DOI] [PubMed] [Google Scholar]
- 70.Mantovani A, Marchesi F, Porta C, Sica A, Allavena P (2007) Inflammation and cancer: breast cancer as a prototype. Breast 16(Suppl 2):S27–S33 [DOI] [PubMed] [Google Scholar]
- 71.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M et al. (2005) Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest 115:2363–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McColl BK, Loughran SJ, Davydova N, Stacker SA, Achen MG (2005) Mechanisms of lymphangiogenesis: targets for blocking the metastatic spread of cancer. Curr Cancer Drug Targets 5:561–571 [DOI] [PubMed] [Google Scholar]
- 73.Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J et al. (2010) Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res 70:5728–5739 [DOI] [PubMed] [Google Scholar]
- 74.Murakami M, Zheng Y, Hirashima M, Suda T, Morita Y, Ooehara J et al. (2008) VEGFR1 tyrosine kinase signaling promotes lymphangiogenesis as well as angiogenesis indirectly via macrophage recruitment. Arterioscler Thromb Vasc Biol 28:658–664 [DOI] [PubMed] [Google Scholar]
- 75.Nerlov C, Graf T (1998) PU.1 induces myeloid lineage commitment in multipotent hematopoietic progenitors. Genes Dev 12:2403–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Osada T, Chong G, Tansik R, Hong T, Spector N, Kumar R et al. (2008) The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother 57:1115–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park C, Lee JY, Yoon YS (2011) Role of bone marrow-derived lymphatic endothelial progenitor cells for lymphatic neovascularization. Trends Cardiovasc Med 21:135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pepper MS, Skobe M (2003) Lymphatic endothelium: morphological, molecular and functional properties. J Cell Biol 163:209–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perera PY, Mayadas TN, Takeuchi O, Akira S, Zaks-Zilberman M, Goyert SM et al. (2001) CD11b/CD18 acts in concert with CD14 and toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J Immunol 166:574–581 [DOI] [PubMed] [Google Scholar]
- 80.Peters BA, Diaz LA, Polyak K, Meszler L, Romans K, Guinan EC et al. (2005) Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med 11:261–262 [DOI] [PubMed] [Google Scholar]
- 81.Petrova TV, Koh GY (2018) Organ-specific lymphatic vasculature: from development to pathophysiology. J Exp Med 215:35–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pittman K, Kubes P (2013) Damage-associated molecular patterns control neutrophil recruitment. J Innate Immun 5:315–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Powell AE, Anderson EC, Davies PS, Silk AD, Pelz C, Impey S et al. (2011) Fusion between intestinal epithelial cells and macrophages in a cancer context results in nuclear reprogramming. Cancer Res 71:1497–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pytowski B, Goldman J, Persaud K, Wu Y, Witte L, Hicklin DJ et al. (2005) Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst 97:14–21 [DOI] [PubMed] [Google Scholar]
- 85.Qiu H, Cao L, Wang D, Xu H, Liang Z (2013) High levels of circulating CD34+/VEGFR3+ lymphatic/vascular endothelial progenitor cells is correlated with lymph node metastasis in patients with epithelial ovarian cancer. J Obstet Gynaecol Res 39:1268–1275 [DOI] [PubMed] [Google Scholar]
- 86.Ran S, Montgomery KE (2012) Macrophage-mediated lymphangiogenesis: the emerging role of macrophages as lymphatic endothelial progenitors. Cancers 4:618–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ran S, Volk L, Hall K, Flister MJ (2009) Lymphangiogenesis and lymphatic metastasis in breast cancer. Pathophysiology 17:229–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ran S, Wilber A (2017) Novel role of immature myeloid cells in formation of new lymphatic vessels associated with inflammation and tumors. J Leukoc Biol 102:253–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Randolph GJ, Angeli V, Swartz MA (2005) Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol 5:617–628 [DOI] [PubMed] [Google Scholar]
- 90.Religa P, Cao R, Bjorndahl M, Zhou Z, Zhu Z, Cao Y (2005) Presence of bone marrow-derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood 106:4184–4190 [DOI] [PubMed] [Google Scholar]
- 91.Reynders N, Abboud D, Baragli A, Noman MZ, Rogister B, Niclou SP et al. (2019) The distinct roles of CXCR3 variants and their ligands in the tumor microenvironment. Cell 8:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Riabov V, Yin S, Song B, Avdic A, Schledzewski K, Ovsiy I et al. (2016) Stabilin-1 is expressed in human breast cancer and supports tumor growth in mammary adenocarcinoma mouse model. Oncotarget 7:31097–31110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Russo E, Teijeira A, Vaahtomeri K, Willrodt AH, Bloch JS, Nitschke M et al. (2016) Intralymphatic CCL21 promotes tissue egress of dendritic cells through afferent lymphatic vessels. Cell Rep 14:1723–1734 [DOI] [PubMed] [Google Scholar]
- 94.Salven P, Mustjoki S, Alitalo R, Alitalo K, Rafii S (2003) VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood 101:168–172 [DOI] [PubMed] [Google Scholar]
- 95.Scallan JP, Zawieja SD, Castorena-Gonzalez JA, Davis MJ (2016) Lymphatic pumping: mechanics, mechanisms and malfunction. J Physiol 594:5749–5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schledzewski K, Falkowski M, Moldenhauer G, Metharom P, Kzhyshkowska J, Ganss R et al. (2006) Lymphatic endothelium-specific hyaluronan receptor LYVE-1 is expressed by stabilin-1+, F4/80+, CD11b+ macrophages in malignant tumours and wound healing tissue in vivo and in bone marrow cultures in vitro: implications for the assessment of lymphangiogenesis. J Pathol 209:67–77 [DOI] [PubMed] [Google Scholar]
- 97.Schoppmann SF, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C et al. (2002) Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol 161:947–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schoppmann SF, Fenzl A, Nagy K, Unger S, Bayer G, Geleff S et al. (2006) VEGF-C expressing tumor-associated macrophages in lymph node positive breast cancer: impact on lymphangiogenesis and survival. Surgery 139:839–846 [DOI] [PubMed] [Google Scholar]
- 99.Sica A, Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122:787–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Skokowa J, Klimiankou M, Klimenkova O, Lan D, Gupta K, Hussein K et al. (2012) Interactions among HCLS1, HAX1 and LEF-1 proteins are essential for G-CSF-triggered granulopoiesis. Nat Med 18:1550–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Spees JL, Whitney MJ, Sullivan DE, Lasky JA, Laboy M, Ylostalo J et al. (2008) Bone marrow progenitor cells contribute to repair and remodeling of the lung and heart in a rat model of progressive pulmonary hypertension. FASEB J 22:1226–1236 [DOI] [PubMed] [Google Scholar]
- 102.Spring H, Schuler T, Arnold B, Hammerling GJ, Ganss R (2005) Chemokines direct endothelial progenitors into tumor neovessels. Proc Natl Acad Sci USA 102:18111–18116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Strachan DC, Ruffell B, Oei Y, Bissell MJ, Coussens LM, Pryer N et al. (2013) CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8+ T cells. Oncoimmunology 2:e26968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Swartz MA (2014) Immunomodulatory roles of lymphatic vessels in cancer progression. Cancer Immunol Res 2:701–707 [DOI] [PubMed] [Google Scholar]
- 105.Tal O, Lim HY, Gurevich I, Milo I, Shipony Z, Ng LG et al. (2011) DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J Exp Med 208:2141–2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Talmadge JE, Donkor M, Scholar E (2007) Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev 26:373–400 [DOI] [PubMed] [Google Scholar]
- 107.Tan YZ, Wang HJ, Zhang MH, Quan Z, Li T, He QZ (2014) CD34+ VEGFR-3+ progenitor cells have a potential to differentiate towards lymphatic endothelial cells. J Cell Mol Med 18:422–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tawada M, Hayashi S, Ikegame Y, Nakashima S, Yoshida K (2014) Possible involvement of tumor-producing VEGF-A in the recruitment of lymphatic endothelial progenitor cells from bone marrow. Oncol Rep 32:2359–2364 [DOI] [PubMed] [Google Scholar]
- 109.Tawada M, Hayashi S, Osada S, Nakashima S, Yoshida K (2012) Human gastric cancer organizes neighboring lymphatic vessels via recruitment of bone marrow-derived lymphatic endothelial progenitor cells. J Gastroenterol 47:1057–1060 [DOI] [PubMed] [Google Scholar]
- 110.Van’t Hull EF, Bron S, Henry L, Ifticene-Treboux A, Turrini R, Coukos G et al. (2014) Bone marrow-derived cells are implicated as a source of lymphatic endothelial progenitors in human breast cancer. Oncoimmunology 3:e29080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Volk-Draper L, Hall K, Griggs C, Rajput S, Kohio P, DeNardo D et al. (2014) Paclitaxel therapy promotes breast cancer metastasis in a TLR4-dependent manner. Cancer Res 74:5421–5434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Volk-Draper L, Patel R, Bhattarai N, Yang J, Wilber A, DeNardo D et al. (2019) Myeloid-derived lymphatic endothelial cell progenitors significantly contribute to lymphatic metastasis in clinical breast Cancer. Am J Pathol 189(11):2269–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Volk-Draper LD, Hall KL, Wilber AC, Ran S (2017) Lymphatic endothelial progenitors originate from plastic myeloid cells activated by toll-like receptor-4. PLoS One 12:e0179257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang D, D’Costa J, Civin CI, Friedman AD (2006) C/EBPalpha directs monocytic commitment of primary myeloid progenitors. Blood 108:1223–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Watari K, Shibata T, Kawahara A, Sata K, Nabeshima H, Shinoda A et al. (2014) Tumor-derived interleukin-1 promotes lymphangiogenesis and lymph node metastasis through M2-type macrophages. PLoS One 9:e99568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Whitehurst B, Flister MJ, Bagaitkar J, Volk L, Bivens CM, Pickett B et al. (2007) Anti-VEGF-A therapy reduces lymphatic vessel density and expression of VEGFR-3 in an orthotopic breast tumor model. Int J Cancer 121:2181–2191 [DOI] [PubMed] [Google Scholar]
- 117.Yang H, Kim C, Kim MJ, Schwendener RA, Alitalo K, Heston W et al. (2011) Soluble vascular endothelial growth factor receptor-3 suppresses lymphangiogenesis and lymphatic metastasis in bladder cancer. Mol Cancer 10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang Y, Chen XH, Li FG, Chen YX, Gu LQ, Zhu JK et al. (2015) In vitro induction of human adipose-derived stem cells into lymphatic endothelial-like cells. Cell Reprogram 17:69–76 [DOI] [PubMed] [Google Scholar]
- 119.Zhang B, Zhang Y, Yao G, Gao J, Yang B, Zhao Y et al. (2012) M2-polarized macrophages promote metastatic behavior of Lewis lung carcinoma cells by inducing vascular endothelial growth factor-C expression. Clinics (Sao Paulo) 67:901–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhuo W, Jia L, Song N, Lu XA, Ding Y, Wang X et al. (2012) The CXCL12-CXCR4 chemokine path-way: a novel axis regulates lymphangiogenesis. Clin Cancer Res 18:5387–5398 [DOI] [PubMed] [Google Scholar]
- 121.Ziegler-Heitbrock HW, Ulevitch RJ (1993) CD14: cell surface receptor and differentiation marker. Immunol Today 14:121–125 [DOI] [PubMed] [Google Scholar]
- 122.Zlotnik A (2006) Involvement of chemokine receptors in organ-specific metastasis. Contrib Microbiol 13:191–199 [DOI] [PubMed] [Google Scholar]
- 123.Zumsteg A, Baeriswyl V, Imaizumi N, Schwendener R, Ruegg C, Christofori G (2009) Myeloid cells contribute to tumor lymphangiogenesis. PLoS One 4:e7067 [DOI] [PMC free article] [PubMed] [Google Scholar]