Abstract
Background
Current measurements of multiple myeloma disease burden are suboptimal. Daratumumab is a monoclonal antibody that targets CD38, an antigen expressed on nearly all myeloma cells.
Purpose
To demonstrate preclinical and first-in-human application of an antibody composed of the native daratumumab labeled with the positron-emitting radionuclide zirconium 89 (89Zr) through the chelator deferoxamine (DFO), or 89Zr-DFO-daratumumab, for immunologic PET imaging of multiple myeloma.
Materials and Methods
89Zr-DFO-daratumumab was synthesized by conjugating 89Zr to daratumumab with DFO. A murine xenograft model using CD38-positive OPM2 multiple myeloma cells was used to evaluate CD38-specificity of 89Zr-DFO-daratumumab. Following successful preclinical imaging, a prospective phase I study of 10 patients with multiple myeloma was performed. Study participants received 74 MBq (2 mCi) of intravenous 89Zr-DFO-daratumumab. Each participant underwent four PET/CT scans over the next 8 days, as well as blood chemistry and whole-body counts, to determine safety, tracer biodistribution, pharmacokinetics, and radiation dosimetry. Because 89Zr has a half-life of 78 hours, only a single administration of tracer was needed to obtain all four PET/CT scans.
Results
89Zr-DFO-daratumumab was synthesized with radiochemical purity greater than 99%. In the murine model, substantial bone marrow uptake was seen in OPM2 mice but not in healthy mice, consistent with CD38-targeted imaging of OPM2 multiple myeloma cells. In humans, 89Zr-DFO-daratumumab was safe and demonstrated acceptable dosimetry. 89Zr-DFO-daratumumab uptake was visualized at PET in sites of osseous myeloma.
Conclusion
These data demonstrate successful CD38-targeted immunologic PET imaging of multiple myeloma in a murine model and in humans.
© RSNA, 2020
Summary
An immuno-PET antibody composed of the native daratumumab labeled with the positron-emitting radionuclide zirconium 89 through the chelator deferoxamine, or 89Zr-DFO-daratumumab, demonstrated successful uptake in osseous deposits in patients with multiple myeloma.
Key Results
■ CD38-targeted immuno-PET was safe and could successfully demonstrate and localize myeloma in animal models and patients.
■ CD38-targeted immuno-PET could demonstrate myeloma, which was occult at current standard-of-care imaging.
Introduction
Multiple myeloma is a plasma-cell neoplasm and the second most common hematologic malignancy in adults (1). Current measurements of multiple myeloma disease burden, whether by using blood analysis, imaging, or blind bone marrow biopsy, are suboptimal. Not all myeloma cells secrete abnormal immunoglobulins, limiting detection by using blood analysis (2–4). Standard imaging analyses with radiographic skeletal surveys are insensitive for disease detection, and more modern imaging methods with MRI and fluorodeoxyglucose PET/CT have limited sensitivity (5,6). Indeed, fluorodeoxyglucose PET/CT is only positive for multiple myeloma in 70% of patients with established multiple myeloma bone disease, and there is a high rate of false positivity due to inflammation and degeneration (7). The possibility of sampling error limits even invasive biopsies of the bone marrow, where multiple myeloma can grow in patches, as is well known (8).
This suboptimal assessment of disease burden impedes clinical care. For example, inaccurate assessment of disease burden confounds accurate staging along the spectrum of myeloma precursors and active disease. There is evidence that the disease responds better to treatment in its early stages, and a recent randomized trial targeting high-risk precursor myeloma demonstrated that early intervention was associated with better progression-free and overall survival (9)—hence the attractiveness of sensitive early detection and early initiation of therapy for patients on the pathway of developing multiple myeloma, before they develop complications and severe symptoms. In addition, advances in multiple myeloma therapy over the last decade have greatly improved response rates and survival, and the detection of minimal residual disease following therapy has become critical to treatment of patients with multiple myeloma (5,10–12). Thus, a sensitive method of detecting, localizing, and measuring tumor burden is greatly needed to optimize treatment of patients with multiple myeloma.
Here, we took advantage of daratumumab, a U.S. Food and Drug Administration–approved monoclonal antibody therapy for multiple myeloma that targets CD38. CD38 is a transmembrane glycoprotein that is expressed on nearly all myeloma cells, as well as expressed at lower levels on normal lymphoid and myeloid cells (13). Zirconium 89 (89Zr) is a radiometal whose half-life of 78 hours allows for imaging up to 5 or 6 days after administration, the time needed to generate sufficient target-to-background uptake of antibody-based PET radiotracers (14). The ability to perform imaging after 5 or 6 days offers a substantial advantage for imaging antibodies in humans (15,16) over the shorter half-life radionuclides currently available (17). Composed of the native daratumumab labeled with the positron-emitting radionuclide 89Zr through the chelator deferoxamine (DFO), 89Zr-DFO-daratumumab was designed to allow targeted noninvasive imaging of myeloma cells. In this article, we detail the synthesis, preclinical imaging, and phase I first-in-human imaging of 89Zr-DFO-daratumumab for successful noninvasive CD38-targeted immuno-PET of multiple myeloma.
Materials and Methods
Cell Culture
OPM2 cells used in preclinical studies are stably transfected through gammaretrovirus with firefly luciferase, allowing for tracking of these multiple myeloma cells with bioluminescent imaging. This cell line is derived from a 56-year-old woman with secondary plasma-cell leukemia and harbors high-risk cytogenetic features including t(4;14) translocation and homozygosity for TP53 mutations (18).
Animals and Tumor Models
All animal experiments were performed according to protocols approved by our institutional animal care and use committee and followed National Institutes of Health directives for animal welfare.
Preparation of DFO-Daratumumab
The chelator DFO was conjugated to daratumumab by adding 5 μL of a 10-mmol/L DFO-NCS (Macrocyclics, Plano, Tex) solution in dimethyl sulfoxide to a 2-mg/mL solution of antibody in 1 mL of phosphate-buffered saline. The pH of the solution was adjusted to 9 with 30 μL of 1 mol/L sodium carbonate. The reaction was then placed on a thermomixer at 37°C for 1 hour. Purification was achieved with a prepacked disposable PD-10 desalting column (GE Life Sciences, Chicago, Ill). After purification, the resulting DFO-daratumumab conjugate was concentrated with an Amicon centrifugal filter (molecular weight cutoff, 50 000; MilliporeSigma, Burlington, Mass). The final concentration was determined by using a NanoDrop 2000 spectrometer (Thermo Fisher Scientific, Waltham, Mass).
Radiolabeling of DFO-Daratumumab
89Zr was supplied by the Radiochemistry and Molecular Imaging Probe Core Facility at Memorial Sloan Kettering Cancer Center (New York, NY) in 0.1 mol/L oxalic acid. After adjusting the pH of the solution to 6.8 with 1 mol/L sodium carbonate, approximately 1 mg of DFO-daratumumab was added to 3 mCi (111 MBq) of neutralized 89Zr. Radiochemical purity was assessed by using radio–thin-layer chromatography and was always greater than 99% before injection.
Mouse Imaging and Biodistribution Studies
Bioluminescent imaging was used to monitor the progression of disease after intravenous injection of OPM2 cells into female NSG mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ; 6–8 weeks old; Jackson Laboratories, Bar Harbor, Maine). Mice were injected with 100 μL of 15 mg/mL luciferin approximately 10 minutes before imaging with an IVIS SpectrumCT (PerkinElmer, Melville, NY). Image acquisition times were between 10 and 30 seconds. Mice were imaged with bioluminescent imaging within 24 hours before the radiotracer was administered.
For the imaging and biodistribution studies, mice were intravenously injected with 200 μCi (7400 kBq) of activity in 70 μg of 89Zr-DFO-daratumumab. For the initial evaluation of 89Zr-DFO-daratumumab, a single group of five animals was used.
Because of the NSG background of the mice used in these studies, 500 µg of nonspecific human immunoglobulin G was either coinjected with 89Zr-DFO-daratumumab or, in the case of the blocking study, injected 3 days prior to the tracer to avoid anomalous biodistribution caused by the lack of endogenous immunoglobulin G in NSG mice (19). Mice were anesthetized with 1%–2% isoflurane and PET/CT images were acquired with an Inveon micro PET/CT instrument (Siemens, Erlangen, Germany).
Biodistribution studies were conducted by killing five mice at discrete time points after injection of 89Zr-DFO-daratumumab. Biodistribution values are presented as the percent of the injected dose, determined by counting relevant standards along with organ samples.
For blocking studies (n = 3 mice per group), blocked mice were coinjected with a 25-fold excess of unlabeled DFO-daratumumab to block relevant receptors, demonstrating specific binding of the tracer to CD38.
For all preclinical studies, the statistical test run was the Student t test. Any P value < .05 was considered to indicate statistical significance.
First-in-Human Imaging with 89Zr-DFO-Daratumumab
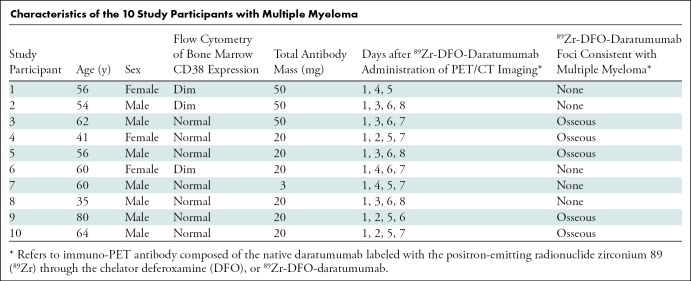
Following successful preclinical imaging of 89Zr-DFO-daratumumab, per institutional review board protocol, an Investigational New Drug Application from the Food and Drug Administration was obtained for first-in-human phase I imaging (ClinicalTrials.gov identifier, NCT03665155). Our institutional review board approved the study protocol. Inclusion criteria were as follows: CD38-positive multiple myeloma; at least one tumor lesion at CT, MRI, or fluorodeoxyglucose PET/CT within 60 days of protocol enrollment; and Eastern Cooperative Oncology Group performance status of 0 to 2. CD38-positivity of multiple myeloma was determined with pathologic analysis by using flow cytometry of bone marrow, and CD38 expression was graded as absent, dim, or within normal range (Table). Exclusion criteria were as follows: life expectancy less than 3 months, pregnancy or lactation, ineligibility for PET/CT scanning because of weight greater than 450 pounds, and/or history of anaphylactic reaction to humanized or human antibodies or a grade 3 or 4 administration reaction during administration of daratumumab. All study participants provided written informed consent.
Characteristics of the 10 Study Participants with Multiple Myeloma
Because of known potential reactions to daratumumab exposure (20), participants were premedicated with acetaminophen 650 mg orally, diphenhydramine 25 mg intravenously, and dexamethasone 20 mg intravenously. Antibody administration was performed intravenously, through a catheter flushed with 5% human serum albumin to prevent antibody retention to the catheter tubing. An amount of 0 mg, 17 mg, or 47 mg of nonradiolabeled (“cold”) daratumumab was administered over 30 minutes. Then 74 MBq (2 mCi) ± 10% of 89Zr-DFO-daratumumab was intravenously administered in a mass of approximately 3 mg, to bring the total daratumumab antibody mass to 3 mg, 20 mg, or 50 mg for each study participant. The first three study participants received 50 mg total antibody mass, the next three study participants received 20 mg, and the subsequent study participant received 3 mg. After visual inspection of the images for these first seven study participants, the 20-mg and 50-mg antibody mass biodistributions were similarly effective for imaging, while the 3-mg antibody mass was not. Coupled with the intention to keep the antibody mass as low as possible, the principle investigator decided that the final three study participants would receive 20-mg total antibody mass. Study participants were monitored for adverse effects on the day of and the day after 89Zr-DFO-daratumumab administration.
Up to four whole-body PET/CT scans were obtained for each study participant on days 1, 2–4, 5–6, and 7–8 following administration (day 0) of 89Zr-DFO-daratumumab. Ranges for days of imaging were preselected before opening the protocol to allow both comprehensive multiday imaging and scheduling flexibility, particularly over the weekend. Study participants were imaged from skull apex to midthigh with a dedicated research PET/CT scanner (Discovery 710 PET/CT; GE Healthcare, Chicago, Ill) in three-dimensional mode with emission time per bed position extending from 4 minutes (day 1) to 8 minutes (days 7–8). Low-dose CT scans were acquired with an x-ray tube current of 80 mA. PET/CT images were reconstructed with attenuation, scatter, and other standard corrections applied and using iterative reconstruction. 89Zr-DFO-daratumumab PET/CT scans were interpreted by a nuclear radiologist (G.A.U., with 10 years of experience in imaging with novel radiotracers) with knowledge of the study participants' medical history and prior imaging. Expected sites of physiologic uptake were determined from the initial study participants and included blood pool, liver, and spleen. Nonphysiologic radiotracer uptake was considered suspicious for CD38-positive malignancy. Three-dimensional volumes of interest were drawn for target lesions, blood pool background, and liver background on PET/CT images, and tracer uptake was quantified by using maximum standardized uptake value (SUV), calculated as follows: SUV = decay-corrected mean region of interest activity (μCi/mL)/(injected dose [μCi]/body weight [g]).
Human Whole-Body and Serum Clearance Measurements
Whole-body clearance was determined with serial measurements of count rate by using a 12.7-cm-thick sodium iodide scintillation detector at a fixed 3 m from the study participant. Background-corrected geometric mean counts were obtained after infusion, before and after first voiding, and subsequently at the times of the PET/CT scans. Count rates were normalized to the value immediately after infusion (taken as 100%) to yield relative retained activities (in percentage). Multiple blood samples were obtained at approximately 15 minutes, 30 minutes, and 1–2 hours after injection, and subsequently at the times of each PET scan. Aliquots of serum were counted by using a gamma well-type detector (Wallac Wizard 1480 Gamma Counter; PerkinElmer) and measured activity concentrations converted to percent of injected activity per liter (% IA/L). A monoexponential function was fitted to the whole-body probe data and a biexponential function fitted to the serum activity concentration data by using SAAM software (21). Areas under the curve and corresponding residence times were derived by using analytic integration.
Human Normal Tissue Dosimetry
Normal tissue absorbed radiation dose estimates were derived as described previously (22,23). Corresponding values for heart contents and red marrow were estimated from the serum area under the curve (24). The residence time for the remainder of the body was derived by subtracting all individually estimated residence times from the whole-body residence time. Thereafter, absorbed radiation doses to individual organs were calculated by using the OLINDA/EXM software application (25).
Statistics
Kinetic parameters and absorbed dose estimates were calculated for each study participant on an individual basis. Subsequently, these were summarized by using descriptive statistics.
Results
Synthesis of 89Zr-DFO-Daratumumab and in Vitro Evaluation
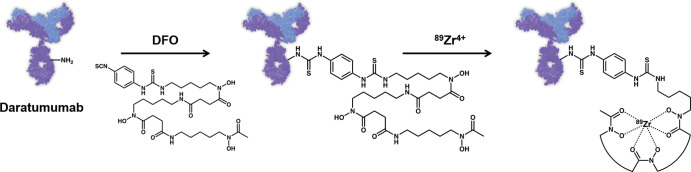
For use as an immuno-PET agent, the antibody daratumumab was chemically modified to contain a chelating moiety for the incorporation of a PET radioisotope (Fig 1a). To that end, the isothiocyanate derivative of the chelator DFO was conjugated to the antibody via free amines found on lysine residues. Once modified with DFO, the antibody could be labeled with 89Zr. Radiochemical purity of 89Zr-DFO-daratumumab was greater than 99%, demonstrating that little, if any, free 89Zr was present. For in vitro studies, human multiple myeloma OPM2 cells were used and their expression of CD38 was validated with flow cytometry (Fig E1 [online]). The immunoreactivity of the DFO-labeled antibody was determined by using modified Lindmo assay (26) and was consistently greater than 90%.
Figure 1a:
Images show synthesis of immuno-PET antibody composed of native daratumumab labeled with positron-emitting radionuclide zirconium 89 (89Zr) through chelator deferoxamine (DFO), or 89Zr-DFO-daratumumab, and evaluation in murine model of multiple myeloma. (a) Schematic representation illustrates conjugation of DFO chelator to daratumumab with subsequent labeling with 89Zr (not to scale). (b) Bioluminescent imaging of OPM2-injected (top) and healthy (bottom) mice demonstrates location of cancer cells in disease model (left, prone; right, supine). Bioluminescence images were taken no more than 24 hours before administration of radiotracer. (c) PET/CT images of OPM2-injected and healthy mice 144 hours after injection of 89Zr-DFO-daratumumab. OPM2 mice exhibit higher level of tracer uptake than do healthy mice. Uptake pattern of 89Zr-DFO-daratumumab matches pattern of cancer localization as seen in bioluminescence images. MIP = maximum intensity projection. White arrows indicate knees, green arrows indicate lumbar spine, and yellow arrows indicate pelvis. Dotted line on coronal images defines plane of trans-axial images. (d) Biodistribution of 89Zr-DFO-daratumumab at 144 hours after injection. %ID/g = percent injected dose per gram. LI = large intestine, SI = small intestine.
In Vivo Evaluation of 89Zr-DFO-Daratumumab in a Murine Model of Multiple Myeloma
Once the target and PET tracer had been validated in vitro, we aimed to study the biodistribution and imaging potential of 89Zr-DFO-daratumumab in a systemic murine model of human multiple myeloma. The model was initiated by intravenously injecting 1 × 106 OPM2 cells, stably transfected with firefly luciferase, into mice with an NSG background (27). This allowed the myeloma tumor burden in the mice to be monitored by using bioluminescent imaging (Fig 1b). Myeloma cells primarily accumulated in the bone marrow of the femurs, sternum, and spine. Approximately 3–4 weeks after OPM2 cell injection, the mice were injected with 89Zr-DFO-daratumumab and imaged by using PET/CT.
Figure 1b:

Images show synthesis of immuno-PET antibody composed of native daratumumab labeled with positron-emitting radionuclide zirconium 89 (89Zr) through chelator deferoxamine (DFO), or 89Zr-DFO-daratumumab, and evaluation in murine model of multiple myeloma. (a) Schematic representation illustrates conjugation of DFO chelator to daratumumab with subsequent labeling with 89Zr (not to scale). (b) Bioluminescent imaging of OPM2-injected (top) and healthy (bottom) mice demonstrates location of cancer cells in disease model (left, prone; right, supine). Bioluminescence images were taken no more than 24 hours before administration of radiotracer. (c) PET/CT images of OPM2-injected and healthy mice 144 hours after injection of 89Zr-DFO-daratumumab. OPM2 mice exhibit higher level of tracer uptake than do healthy mice. Uptake pattern of 89Zr-DFO-daratumumab matches pattern of cancer localization as seen in bioluminescence images. MIP = maximum intensity projection. White arrows indicate knees, green arrows indicate lumbar spine, and yellow arrows indicate pelvis. Dotted line on coronal images defines plane of trans-axial images. (d) Biodistribution of 89Zr-DFO-daratumumab at 144 hours after injection. %ID/g = percent injected dose per gram. LI = large intestine, SI = small intestine.
For in vivo imaging, mice were separated into three groups (five mice per group). Two groups comprised diseased mice injected with OPM2 cells, while the third group contained healthy mice. One of the groups of diseased mice received a coinjection of a blocking dose of unlabeled DFO-daratumumab with 89Zr-DFO-daratumumab to confirm the specificity of the uptake.
For PET/CT imaging, mice were intravenously injected with 7.4 MBq (200 μCi) in 70 ug of 89Zr-DFO-daratumumab. Mice were imaged by using PET/CT at 24, 72, 96, 120, and 144 hours after injection (Fig 1c). A complete biodistribution study was performed after the mice were killed at 144 hours after injection (Fig 1d). Because the OPM2 cells localized to the bone marrow, it was necessary to extract the bone marrow from the hind limbs of the mice by using centrifugation (to count separately from the bone) for accurate biodistribution (28).
Figure 1c:
Images show synthesis of immuno-PET antibody composed of native daratumumab labeled with positron-emitting radionuclide zirconium 89 (89Zr) through chelator deferoxamine (DFO), or 89Zr-DFO-daratumumab, and evaluation in murine model of multiple myeloma. (a) Schematic representation illustrates conjugation of DFO chelator to daratumumab with subsequent labeling with 89Zr (not to scale). (b) Bioluminescent imaging of OPM2-injected (top) and healthy (bottom) mice demonstrates location of cancer cells in disease model (left, prone; right, supine). Bioluminescence images were taken no more than 24 hours before administration of radiotracer. (c) PET/CT images of OPM2-injected and healthy mice 144 hours after injection of 89Zr-DFO-daratumumab. OPM2 mice exhibit higher level of tracer uptake than do healthy mice. Uptake pattern of 89Zr-DFO-daratumumab matches pattern of cancer localization as seen in bioluminescence images. MIP = maximum intensity projection. White arrows indicate knees, green arrows indicate lumbar spine, and yellow arrows indicate pelvis. Dotted line on coronal images defines plane of trans-axial images. (d) Biodistribution of 89Zr-DFO-daratumumab at 144 hours after injection. %ID/g = percent injected dose per gram. LI = large intestine, SI = small intestine.
Figure 1d:

Images show synthesis of immuno-PET antibody composed of native daratumumab labeled with positron-emitting radionuclide zirconium 89 (89Zr) through chelator deferoxamine (DFO), or 89Zr-DFO-daratumumab, and evaluation in murine model of multiple myeloma. (a) Schematic representation illustrates conjugation of DFO chelator to daratumumab with subsequent labeling with 89Zr (not to scale). (b) Bioluminescent imaging of OPM2-injected (top) and healthy (bottom) mice demonstrates location of cancer cells in disease model (left, prone; right, supine). Bioluminescence images were taken no more than 24 hours before administration of radiotracer. (c) PET/CT images of OPM2-injected and healthy mice 144 hours after injection of 89Zr-DFO-daratumumab. OPM2 mice exhibit higher level of tracer uptake than do healthy mice. Uptake pattern of 89Zr-DFO-daratumumab matches pattern of cancer localization as seen in bioluminescence images. MIP = maximum intensity projection. White arrows indicate knees, green arrows indicate lumbar spine, and yellow arrows indicate pelvis. Dotted line on coronal images defines plane of trans-axial images. (d) Biodistribution of 89Zr-DFO-daratumumab at 144 hours after injection. %ID/g = percent injected dose per gram. LI = large intestine, SI = small intestine.
At 24 hours after injection, PET imaging showed a significant amount of 89Zr-DFO-daratumumab in the blood circulation (Fig E2 [online]). In diseased mice at later time points, an increasing accumulation of the tracer was observed in the hind limbs, pelvic region, and sternum. This uptake was consistent with the pathology of the cancer model and confirmed with the bioluminescence data. In the group of mice that received a coinjected blocking dose of unlabeled antibody, uptake in the bone marrow was significantly less at 72 hours (16.2% ID/g vs 5.4% ID/g) as seen at PET/CT imaging, confirming the specificity of the tracer to the CD38 target (Fig 2).
Figure 2:
Blocking study demonstrates specific target accumulation of immuno-PET antibody composed of native daratumumab labeled with positron-emitting radionuclide zirconium 89 (89Zr) through chelator deferoxamine (DFO), or 89Zr-DFO-daratumumab. A, PET/CT images at 72 hours after injection demonstrate effect of blocking dose of unlabeled DFO-daratumumab on uptake of 89Zr-DFO-daratumumab in OPM2 mice. Blocking dose of 25× (1.75 mg) occupies receptors and prevents 89Zr-DFO-daratumumab from binding, reducing bone marrow uptake at this time point, as seen in PET images (arrows). B, Decrease in binding is also evident in biodistribution data (** indicate P < .005). %ID/g = percent injected dose per gram.
89Zr-DFO-Daratumumab Biodistribution in Normal Mice
Figure E3 (online) shows the time course of the biodistribution of 89Zr-DFO-daratumumab in healthy BALB/cAnN mice, assessed with ex vivo biodistribution. At 24 hours, the highest activity concentrations were seen in blood pool and blood pool–dominated lung, slowly (approximately 70% retention at 144 hours) decreasing over the observation period. In a group of tissues including liver, spleen, kidneys, and bone marrow, activity concentrations remained relatively constant. However, there was a steady increase in bone uptake from approximately 4% ID/g to approximately 10% ID/g over the observation period, possibly reflecting free 89Zr.
First-in-Human Imaging with 89Zr-DFO-Daratumumab
Between September 2018 and January 2019, 10 study participants with pathologically proven CD38-positive multiple myeloma completed the study protocol (Table). Three study participants were women and seven were men. Ages of study participants ranged from 35 to 80 years (mean age ± standard deviation, 57 years ± 12). Study participants were administered a nominal activity of 74 MBq (2 mCi) of 89Zr-DFO-daratumumab on day 0 and underwent imaging on days 1, 2–4, 5–6, and/or 7–8, as prescribed prospectively in the protocol. The second study participant demonstrated tachycardia and tachypnea after 89Zr-DFO-daratumumab administration, a known potential reaction to daratumumab exposure (20) which resolved within 2 hours during observation. No other study participants had adverse reactions to the tracer.
Time of imaging affected physiologic and pathologic uptake of 89Zr-DFO-daratumumab (Fig 3). At earlier time points (1–4 days after tracer administration), blood pool and liver activity were high. At later time points (5–8 days after tracer administration), blood pool and, to a lesser extent, liver activity decreased. With increased time following tracer administration, uptake in focal skeletal lesions consistent with multiple myeloma increased. For example, a distal left femoral lesion in the study participant in Figure 3 increased from standard uptake value of 5.4 at day 1 to standard uptake value of 9.5 on day 7. The combination of decreased background uptake and increased lesion uptake at the later time points resulted in better visualization of lesions at days 5–8.
Figure 3a:
Images show effect of imaging time on biodistribution of immuno-PET antibody composed of native daratumumab labeled with positron-emitting radionuclide zirconium 89 (89Zr) through chelator deferoxamine (DFO), or 89Zr-DFO-daratumumab. (a) Maximum intensity projection images of study participant 4 following administration of 74 MBq (2 mCi) of 89Zr-DFO-daratumumab in 20-mg total antibody mass and PET/CT imaging 1, 2, 5, and 7 days after injection. At day 1, blood pool and liver uptake predominate. With later imaging, blood pool background decreases and osseous myeloma uptake increases. Dashed red lines demonstrate level of trans-axial images in b. (b) Transaxial CT and fused PET/CT images from level of dashed red lines in a demonstrate increasing 89Zr-DFO-daratumumab avidity with time in distal right femur osseous lesion (straight arrows) and decreasing blood pool in the popliteal vessels (curved arrows). (c) Time course of 89Zr-DFO-daratumumab uptake in index regions for study participant 4 in terms of standardized uptake value (SUV). Blood pool represents cardiac left ventricle; femoral lesion represents maximum SUV for distal right femur lesion demonstrated in b. Increasing lesion uptake coupled with decreasing blood pool illustrates improvement in lesion discrimination with time after administration.
Figure 3b:
Images show effect of imaging time on biodistribution of immuno-PET antibody composed of native daratumumab labeled with positron-emitting radionuclide zirconium 89 (89Zr) through chelator deferoxamine (DFO), or 89Zr-DFO-daratumumab. (a) Maximum intensity projection images of study participant 4 following administration of 74 MBq (2 mCi) of 89Zr-DFO-daratumumab in 20-mg total antibody mass and PET/CT imaging 1, 2, 5, and 7 days after injection. At day 1, blood pool and liver uptake predominate. With later imaging, blood pool background decreases and osseous myeloma uptake increases. Dashed red lines demonstrate level of trans-axial images in b. (b) Transaxial CT and fused PET/CT images from level of dashed red lines in a demonstrate increasing 89Zr-DFO-daratumumab avidity with time in distal right femur osseous lesion (straight arrows) and decreasing blood pool in the popliteal vessels (curved arrows). (c) Time course of 89Zr-DFO-daratumumab uptake in index regions for study participant 4 in terms of standardized uptake value (SUV). Blood pool represents cardiac left ventricle; femoral lesion represents maximum SUV for distal right femur lesion demonstrated in b. Increasing lesion uptake coupled with decreasing blood pool illustrates improvement in lesion discrimination with time after administration.
Figure 3c:

Images show effect of imaging time on biodistribution of immuno-PET antibody composed of native daratumumab labeled with positron-emitting radionuclide zirconium 89 (89Zr) through chelator deferoxamine (DFO), or 89Zr-DFO-daratumumab. (a) Maximum intensity projection images of study participant 4 following administration of 74 MBq (2 mCi) of 89Zr-DFO-daratumumab in 20-mg total antibody mass and PET/CT imaging 1, 2, 5, and 7 days after injection. At day 1, blood pool and liver uptake predominate. With later imaging, blood pool background decreases and osseous myeloma uptake increases. Dashed red lines demonstrate level of trans-axial images in b. (b) Transaxial CT and fused PET/CT images from level of dashed red lines in a demonstrate increasing 89Zr-DFO-daratumumab avidity with time in distal right femur osseous lesion (straight arrows) and decreasing blood pool in the popliteal vessels (curved arrows). (c) Time course of 89Zr-DFO-daratumumab uptake in index regions for study participant 4 in terms of standardized uptake value (SUV). Blood pool represents cardiac left ventricle; femoral lesion represents maximum SUV for distal right femur lesion demonstrated in b. Increasing lesion uptake coupled with decreasing blood pool illustrates improvement in lesion discrimination with time after administration.
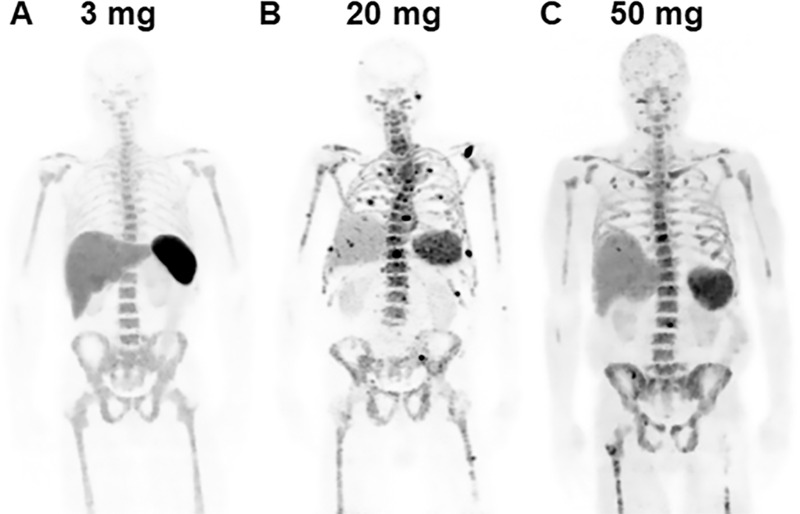
Total antibody mass may affect physiologic uptake of 89Zr-DFO-daratumumab (Fig 4). At 3 mg of total antibody mass, intense, probably physiologic, uptake was observed in the spleen, limiting available tracer for localization of multiple myeloma. At both 20-mg and 50-mg total antibody mass, splenic uptake was lower and uptake in focal skeletal lesions was visualized, consistent with multiple myeloma.
Figure 4:
Effect of total antibody mass on tracer distribution. Total of 74 MBq (2 mCi) of immuno-PET antibody composed of native daratumumab labeled with positron-emitting radionuclide zirconium 89 (89Zr) through chelator deferoxamine (DFO), or 89Zr-DFO-daratumumab, was administered to study participants with CD38-positive multiple myeloma at total antibody masses of, A, 3-mg total antibody mass (3-mg radiolabeled and 0-mg cold antibody) in study participant 7, a 60-year-old man; B, 20-mg total antibody mass (3-mg radiolabeled and 17-mg cold antibody) in study participant 9, an 80-year-old man; and, C, 50-mg total antibody mass (3-mg radiolabeled and 47-mg cold antibody) in study participant 3, a 62-year-old man. All maximum intensity projection images were obtained 5–6 days following tracer administration. Higher physiologic splenic uptake at 3-mg total antibody mass is relatively reduced at 20-mg and 50-mg total antibody doses. Foci consistent with 89Zr-DFO-daratumumab–avid osseous myeloma are visualized at 20-mg and 50-mg total antibody doses.
Five of 10 study participants demonstrated foci of osseous 89Zr-DFO-daratumumab–avidity, consistent with multiple myeloma (Fig 5, Table). These five participants were all suspected of having active multiple myeloma at the time of imaging. Foci often correlated with lytic osseous lesions at CT at sites of known multiple myeloma. However, additional foci without CT correlates were also visualized at previously unsuspected sites of disease.
Figure 5:
Images show visualization of skeletal myeloma by using immuno-PET antibody composed of native daratumumab labeled with positron-emitting radionuclide zirconium 89 (89Zr) through chelator deferoxamine (DFO), or 89Zr-DFO-daratumumab, in an 80-year-old man with osseous myeloma. A, Maximum intensity projection (MIP) image from 89Zr-DFO-daratumumab PET/CT demonstrates multiple foci of osseous avidity, including left scapular focus (arrow). B, Axial CT and, C, fused PET/CT images from 89Zr-DFO-daratumumab PET/CT demonstrate left scapular focus localizes to lytic osseous lesion at CT (arrows). D, MIP image from fluorine 18 fluorodeoxyglucose PET/CT 1 week prior fails to identify lesions seen at 89Zr-DFO-daratumumab PET/CT.
Pharmacokinetics
Whole-body and serum clearance generally conformed to mono- and biexponential kinetics, respectively. Summed biologic clearance curves are shown in Figure E4 (online). Summary statistics for the clearance parameters are provided in Table E1 (online). In vitro stability was determined in human serum and the radiopharmaceutical showed high stability out to 7 days.
Normal Tissue Radiation Dose Estimates
Participants demonstrated a pattern typical of antibody tracers (15,16,23,29–31), with blood pool and liver uptake in days 1–4 replaced by tumor uptake in later days. The urinary bladder was not visualized on any PET/CT scan. Bowel excretion was visualized in seven of 10 participants, for whom the maximum gut activity was, on average, 5% ± 2 of that in the total body.
Absorbed dose estimates for normal tissues are provided in Table E2 (online). The organs receiving the highest doses were spleen (1.8 mSv/MBq ± 1.1) and liver (1.6 mSv/MBq ± 0.3), with an average effective dose of 0.49 mSv/MBq ± 0.07.
Discussion
We used daratumumab, a U.S. Food and Drug Administration–approved CD38-targeting antibody for multiple myeloma, for the synthesis, preclinical validation, and first-in-human phase I imaging of 89Zr-DFO-daratumumab, an antibody composed of the native daratumumab labeled with the positron-emitting radionuclide zirconium 89 (89Zr) through the chelator deferoxamine (DFO). Using a systemic multiple myeloma model in NSG mice with ex vivo orthotopic bone marrow distribution, we demonstrated 89Zr-DFO-daratumumab uptake was specific for CD38-overexpressing OPM2 cells. The specificity of the uptake was confirmed with blocking tumor accumulation by coinjection of excess unlabeled tracer. First-in-human imaging demonstrated visualization of active multiple myeloma, including sites of previously unknown disease. In 10 study participants, one adverse reaction was observed (an infusion reaction typical for antibody-based radiopharmaceuticals that resolved within 2 hours). No other adverse events were reported. Absorbed dose estimates given in Table E2 (online) are comparable to other antibody PET tracers and well within limits of safety for a diagnostic imaging agent. These results provide proof-of-principle of CD38-targeted immuno-PET, including successful imaging in patients with multiple myeloma.
Noninvasive CD38-targeted immuno-PET introduces several potential applications of clinical value. First, 89Zr-DFO-daratumumab has promise for highly sensitive detection of multiple myeloma, allowing both disease localization before therapy and quantification of disease burden. Second, it may allow for detection of minimal residual disease positivity following therapy, which continues to grow in importance as a prognostic marker and end point in multiple myeloma clinical trials (12). Third, while proven effective, not all patients respond to daratumumab therapy (11). Nijhof et al (32) demonstrated a significant positive association between CD38 expression levels on myeloma cells and the efficacy of daratumumab to induce cell death. Therefore, 89Zr-DFO-daratumumab PET before therapy should be evaluated as a biomarker of tumor responsiveness to daratumumab therapy, just as immuno-PET biomarkers of ERBB2 (formerly HER2) expression have demonstrated success predicting responsiveness to HER2-targeted therapies in patients with breast cancer (33,34). Fourth, 89Zr-DFO-daratumumab may also be useful for monitoring response following daratumumab therapy. Fifth, successful CD38-targeted imaging can be leveraged to design CD38-targeted radioimmunotherapy, creating a theranostic partner for 89Zr-DFO-daratumumab. CD38-targeted radioimmunotherapy has been previously demonstrated in preclinical models utilizing anti-CD38 antibody-streptavidin and yttrium 90-DOTA-biotin (35), as well as with bismuth 213 anti-CD38 antibody (36). Finally, as CD38 expression has been demonstrated in other malignancies such as lung cancers (37) and B-cell lymphomas (38), as well as autoimmune disorders such as lupus (39) and rheumatoid arthritis (40), there may be several disease processes that can benefit from CD38-targeted immuno-PET.
Preclinical CD38-targeted immuno-PET has been achieved with CD38-positive MM1.S myeloma cells (17,41), A549 lung cancer cells, and Ramos lymphoma cells (38). As there is rising interest in CD38-targeted imaging, the question of the optimal agent will be debated. Full antibodies used for immuno-PET require long systemic circulation times, in the range of 5–8 days, for optimal target localization and background clearance in humans (15,16,23). The full antibody labeled with 89Zr in our article takes advantage of the relatively long radioactive half-life of 89Zr (78 hours) to allow imaging during this period. Immuno-PET with the shorter half-life copper 64 (13 hours) was successful in a mouse model (17) 1 day following tracer administration, but may not be optimal for the longer imaging times required for full antibody imaging in humans. The currently required multiple-day uptake times for immuno-PET imaging with full antibodies is a relative disadvantage for clinical use when compared with other agents that can be imaged on the same day as administration. Potential alternatives include smaller antibody fragments and nanobodies (42–46), which achieve target localization in shorter periods.
Not all study participants demonstrated disease visible at 89Zr-DFO-daratumumab. Of note, three study participants with dim CD38 expression by using flow cytometry of bone marrow samples were among the study participants without 89Zr-DFO-daratumumab–avid disease. Clinically standard CD38 flow cytometry is only semiquantitative, with “dim” reported when CD38 fluorescence is in the range of 3 to 100 times lower than normal intensity. This is clinically significant, as patients with lower CD38 fluorescence have lower response rates to CD38-targeted therapy (47). Thus, it seems likely that patients with dim CD38 by using flow cytometry will be more difficult to image with CD38-targeted imaging. In addition, study participant 7 received only 3 mg of total antibody mass, which may not have been sufficient to saturate nonspecific binding in the liver (15). The imaging results are promising but will need further validation.
An inherent limitation of our first-in-human clinical trial was the small sample size. Because this was a phase I study, pathologic correlates were not obtained for individual foci seen on research PET/CT scans.
In summary, the successful first-in-human visualization and localization of CD38-positive multiple myeloma in patients is an exciting proof-of-principle for multiple myeloma immuno-PET and offers multiple opportunities for future clinical trials of an immuno-PET antibody composed of the native daratumumab labeled with the positron-emitting radionuclide zirconium 89, or 89Zr-DFO-daratumumab, for measurement of disease burden, evaluation of minimal residual disease, and prediction of daratumumab therapy response.
APPENDIX
SUPPLEMENTAL FIGURES
G.A.U., J.A.O., R.M., and C.O.L. supported by the Lymphoma and Leukemia Society. G.A.U. and C.O.L. supported by the Rising Tide Foundation. L.M.C. supported by National Institutes of Health (NIH) (EB-025050) J.S.L. supported by National Cancer Institute (NCI) (R35 CA232130).
Supported by the Memorial Sloan Kettering Cancer Center Radiochemistry and Molecular Imaging Probe Core, funded by NIH/NCI Cancer Center Support Grant (P30 CA008748).
G.A.U. and N.B.S. contributed equally to this work.
Disclosures of Conflicts of Interest: G.A.U. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Sanofi. Other relationships: disclosed no relevant relationships. N.B.S. disclosed no relevant relationships. J.A.O. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Janssen Research & Development. Other relationships: disclosed no relevant relationships. A.S.K. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is employed by Memorial Sloan Kettering Cancer Center. Other relationships: disclosed no relevant relationships. C.C.R. disclosed no relevant relationships. R.M. disclosed no relevant relationships. E.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Bristol Meyers Squibb, Precision Biosciences, and Fate Therapeutics; has grants/grants pending with Bristol Meyers Squibb and Fate Therapeutics; has patents (planned, pending, or issued) with and receives royalties from Bristol Meyers Squibb. Other relationships: disclosed no relevant relationships. L.M.C. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is employed by Memorial Sloan Kettering Cancer Center. Other relationships: disclosed no relevant relationships. S.K.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is employed by Memorial Sloan Kettering Cancer Center. Other relationships: disclosed no relevant relationships. J.S.L. disclosed no relevant relationships. C.O.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: has grants/grants pending with AMGEN, Janssen, Takeda, and Celgene; received payment for lectures including service on speakers bureaus from AMGEN, Janssen, and Bristol Meyers Squibb. Other relationships: disclosed no relevant relationships.
Abbreviations:
- DFO
- deferoxamine
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Chawla SS, Kumar SK, Dispenzieri A, et al. Clinical course and prognosis of non-secretory multiple myeloma. Eur J Haematol 2015;95(1):57–64. [DOI] [PubMed] [Google Scholar]
- 3.Dimopoulos MA, Kastritis E, Terpos E. Non-secretory myeloma: one, two, or more entities? Oncology (Williston Park) 2013;27(9):930–932. [PubMed] [Google Scholar]
- 4.Lopes da Silva R, Monteiro A, Veiga J. Non-secretory multiple myeloma relapsing as extramedullary liver plasmacytomas. J Gastrointestin Liver Dis 2011;20(1):81–83. [PubMed] [Google Scholar]
- 5.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 2016;17(8):e328–e346. [DOI] [PubMed] [Google Scholar]
- 6.Messiou C, Hillengass J, Delorme S, et al. Guidelines for Acquisition, Interpretation, and Reporting of Whole-Body MRI in Myeloma: Myeloma Response Assessment and Diagnosis System (MY-RADS). Radiology 2019;291(1):5–13. [DOI] [PubMed] [Google Scholar]
- 7.Hillengass J, Landgren O. Challenges and opportunities of novel imaging techniques in monoclonal plasma cell disorders: imaging “early myeloma”. Leuk Lymphoma 2013;54(7):1355–1363. [DOI] [PubMed] [Google Scholar]
- 8.Mailankody S, Korde N, Lesokhin AM, et al. Minimal residual disease in multiple myeloma: bringing the bench to the bedside. Nat Rev Clin Oncol 2015;12(5):286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mateos MV, Landgren O. MGUS and Smoldering Multiple Myeloma: Diagnosis and Epidemiology. Cancer Treat Res 2016;169:3–12. [DOI] [PubMed] [Google Scholar]
- 10.Anderson KC, Auclair D, Kelloff GJ, et al. The Role of Minimal Residual Disease Testing in Myeloma Treatment Selection and Drug Development: Current Value and Future Applications. Clin Cancer Res 2017;23(15):3980–3993. [DOI] [PubMed] [Google Scholar]
- 11.Landgren O, Rajkumar SV. New Developments in Diagnosis, Prognosis, and Assessment of Response in Multiple Myeloma. Clin Cancer Res 2016;22(22):5428–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landgren O, Lu SX, Hultcrantz M. MRD Testing in Multiple Myeloma: The Main Future Driver for Modern Tailored Treatment. Semin Hematol 2018;55(1):44–50. [DOI] [PubMed] [Google Scholar]
- 13.Nooka AK, Kaufman JL, Hofmeister CC, et al. Daratumumab in multiple myeloma. Cancer 2019;125(14):2364–2382. [DOI] [PubMed] [Google Scholar]
- 14.Brandt M, Cardinale J, Aulsebrook ML, Gasser G, Mindt TL. An Overview of PET Radiochemistry, Part 2: Radiometals. J Nucl Med 2018;59(10):1500–1506. [DOI] [PubMed] [Google Scholar]
- 15.Dijkers EC, Oude Munnink TH, Kosterink JG, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther 2010;87(5):586–592. [DOI] [PubMed] [Google Scholar]
- 16.Ulaner GA, Lyashchenko SK, Riedl C, et al. First-in-Human Human Epidermal Growth Factor Receptor 2-Targeted Imaging Using 89Zr-Pertuzumab PET/CT: Dosimetry and Clinical Application in Patients with Breast Cancer. J Nucl Med 2018;59(6):900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caserta E, Chea J, Minnix M, et al. Copper 64-labeled daratumumab as a PET/CT imaging tracer for multiple myeloma. Blood 2018;131(7):741–745 [Published correction appears in Blood 2018;131(25):2869.] 10.1182/blood-2017-09-807263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juge-Morineau N, François S, Puthier D, Godard A, Bataille R, Amiot M. The gp 130 family cytokines IL-6, LIF and OSM but not IL-11 can reverse the anti-proliferative effect of dexamethasone on human myeloma cells. Br J Haematol 1995;90(3):707–710. [DOI] [PubMed] [Google Scholar]
- 19.Sharma SK, Chow A, Monette S, et al. Fc-Mediated Anomalous Biodistribution of Therapeutic Antibodies in Immunodeficient Mouse Models. Cancer Res 2018;78(7):1820–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCullough KB, Hobbs MA, Abeykoon JP, Kapoor P. Common Adverse Effects of Novel Therapies for Multiple Myeloma (MM) and Their Management Strategies. Curr Hematol Malig Rep 2018;13(2):114–124. [DOI] [PubMed] [Google Scholar]
- 21.Barrett PH, Bell BM, Cobelli C, et al. SAAM II: Simulation, Analysis, and Modeling Software for tracer and pharmacokinetic studies. Metabolism 1998;47(4):484–492. [DOI] [PubMed] [Google Scholar]
- 22.Pandit-Taskar N, O’Donoghue JA, Beylergil V, et al. ⁸⁹Zr-huJ591 immuno-PET imaging in patients with advanced metastatic prostate cancer. Eur J Nucl Med Mol Imaging 2014;41(11):2093–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Donoghue JA, Lewis JS, Pandit-Taskar N, et al. Pharmacokinetics, Biodistribution, and Radiation Dosimetry for 89Zr-Trastuzumab in Patients with Esophagogastric Cancer. J Nucl Med 2018;59(1):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sgouros G, Stabin M, Erdi Y, et al. Red marrow dosimetry for radiolabeled antibodies that bind to marrow, bone, or blood components. Med Phys 2000;27(9):2150–2164. [DOI] [PubMed] [Google Scholar]
- 25.Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med 2005;46(6):1023–1027. [PubMed] [Google Scholar]
- 26.Lindmo T, Boven E, Cuttitta F, Fedorko J, Bunn PA, Jr. Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods 1984;72(1):77–89. [DOI] [PubMed] [Google Scholar]
- 27.Lawson MA, Paton-Hough JM, Evans HR, et al. NOD/SCID-GAMMA mice are an ideal strain to assess the efficacy of therapeutic agents used in the treatment of myeloma bone disease. PLoS One 2015;10(3):e0119546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amend SR, Valkenburg KC, Pienta KJ. Murine Hind Limb Long Bone Dissection and Bone Marrow Isolation. J Vis Exp 2016 (110):. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ulaner GA, Hyman DM, Lyashchenko SK, Lewis JS, Carrasquillo JA. 89Zr-Trastuzumab PET/CT for Detection of Human Epidermal Growth Factor Receptor 2-Positive Metastases in Patients With Human Epidermal Growth Factor Receptor 2-Negative Primary Breast Cancer. Clin Nucl Med 2017;42(12):912–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laforest R, Lapi SE, Oyama R, et al. [89Zr]Trastuzumab: Evaluation of Radiation Dosimetry, Safety, and Optimal Imaging Parameters in Women with HER2-Positive Breast Cancer. Mol Imaging Biol 2016;18(6):952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bensch F, Smeenk MM, van Es SC, et al. Comparative biodistribution analysis across four different 89Zr-monoclonal antibody tracers-The first step towards an imaging warehouse. Theranostics 2018;8(16):4295–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nijhof IS, Groen RW, Lokhorst HM, et al. Upregulation of CD38 expression on multiple myeloma cells by all-trans retinoic acid improves the efficacy of daratumumab. Leukemia 2015;29(10):2039–2049. [DOI] [PubMed] [Google Scholar]
- 33.Gebhart G, Lamberts LE, Wimana Z, et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann Oncol 2016;27(4):619–624. [DOI] [PubMed] [Google Scholar]
- 34.Ulaner GA, Hyman DM, Ross DS, et al. Detection of HER2-Positive Metastases in Patients with HER2-Negative Primary Breast Cancer Using 89Zr-Trastuzumab PET/CT. J Nucl Med 2016;57(10):1523–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green DJ, Orgun NN, Jones JC, et al. A preclinical model of CD38-pretargeted radioimmunotherapy for plasma cell malignancies. Cancer Res 2014;74(4):1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teiluf K, Seidl C, Blechert B, et al. α-Radioimmunotherapy with 213Bi-anti-CD38 immunoconjugates is effective in a mouse model of human multiple myeloma. Oncotarget 2015;6(7):4692–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehlerding EB, England CG, Jiang D, et al. CD38 as a PET Imaging Target in Lung Cancer. Mol Pharm 2017;14(7):2400–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang L, Jiang D, England CG, et al. ImmunoPET imaging of CD38 in murine lymphoma models using 89Zr-labeled daratumumab. Eur J Nucl Med Mol Imaging 2018;45(8):1372–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pavón EJ, Zumaquero E, Rosal-Vela A, et al. Increased CD38 expression in T cells and circulating anti-CD38 IgG autoantibodies differentially correlate with distinct cytokine profiles and disease activity in systemic lupus erythematosus patients. Cytokine 2013;62(2):232–243. [DOI] [PubMed] [Google Scholar]
- 40.Chang X, Yue L, Liu W, et al. CD38 and E2F transcription factor 2 have uniquely increased expression in rheumatoid arthritis synovial tissues. Clin Exp Immunol 2014;176(2):222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghai A, Maji D, Cho N, et al. Preclinical Development of CD38-Targeted [89Zr]Zr-DFO-Daratumumab for Imaging Multiple Myeloma. J Nucl Med 2018;59(2):216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sörensen J, Sandberg D, Sandström M, et al. First-in-human molecular imaging of HER2 expression in breast cancer metastases using the 111In-ABY-025 affibody molecule. J Nucl Med 2014;55(5):730–735. [DOI] [PubMed] [Google Scholar]
- 43.Keyaerts M, Xavier C, Heemskerk J, et al. Phase I Study of 68Ga-HER2-Nanobody for PET/CT Assessment of HER2 Expression in Breast Carcinoma. J Nucl Med 2016;57(1):27–33. [DOI] [PubMed] [Google Scholar]
- 44.Bannas P, Hambach J, Koch-Nolte F. Nanobodies and Nanobody-Based Human Heavy Chain Antibodies As Antitumor Therapeutics. Front Immunol 2017;8:1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fumey W, Koenigsdorf J, Kunick V, et al. Nanobodies effectively modulate the enzymatic activity of CD38 and allow specific imaging of CD38+ tumors in mouse models in vivo. Sci Rep 2017;7(1):14289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oberle A, Brandt A, Alawi M, et al. Long-term CD38 saturation by daratumumab interferes with diagnostic myeloma cell detection. Haematologica 2017;102(9):e368–e370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nijhof IS, Casneuf T, van Velzen J, et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood 2016;128(7):959–970. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.