Abstract
Abdominal wall masses, masslike lesions, and diffuse processes are common and often incidental findings at cross-sectional imaging. Distinguishing among these types of masses on the basis of imaging features alone can be challenging. The authors present a diagnostic algorithm that may help in distinguishing different types of abdominal wall masses accurately. Hernias may mimic discrete masses at clinical examination, and imaging is often ordered for evaluation of a possible abdominal wall mass. Once a discrete mass is confirmed to be present, the next step is to determine if it is a fat-containing, cystic, or solid mass. The most common fat-containing masses are lipomas. Fluid or cystic masses include postoperative abscesses, seromas, and rectus sheath hematomas. Solid masses are the most common abdominal wall masses and include desmoid tumors, sarcomas, endometriomas, and metastases. Multiple masses and other diffuse abdominal wall processes are often manifestations of an underlying condition or insult. The most frequently found diffuse processes are multiple injection granulomas from administration of subcutaneous medication. This article offers an algorithmic approach to characterizing abdominal wall masses on the basis of their composition and reviews abdominal wall diffuse processes.
Online supplemental material is available for this article.
©RSNA, 2020
SA-CME LEARNING OBJECTIVES
After completing this journal-based SA-CME activity, participants will be able to:
■ Discuss the most common abdominal wall masses, including their typical manifestations and any syndromic associations.
■ Describe typical imaging characteristics of common abdominal wall masses.
■ Identify select diffuse abdominal wall processes.
Introduction
Imaging is frequently performed for evaluation of palpable abdominal wall masses and masslike lesions. In addition, such masses, masslike processes, and diffuse abdominal wall masses can be encountered incidentally at cross-sectional imaging. Thus the abdominal wall and subcutaneous tissues should be included in the radiologist’s search pattern. In either scenario, knowledge of common abdominal wall abnormalities and the patient’s history guides the interpreting radiologist to make an accurate diagnosis or differential diagnosis.
Discrete abdominal wall masses can be evaluated on the basis of their composition. Evaluation of the mass first includes confirmation that the mass is not a masslike process, such as a hernia. Next, the internal composition of the mass should be determined (eg, fat, fluid, solid, or fibrous composition). In a radiologic study (1) of 365 patients who were referred to a sarcoma clinic for evaluation of an abdominal wall mass, the five most common masses in order of decreasing frequency were desmoid tumors (30% of the cohort), sarcomas of any type (20%), metastases (18%), lipomas (6%), and endometriomas (4%). Overall, a slight majority of abdominal wall lesions were benign (211 [58%] benign, 154 [42%] malignant). It was unclear if the lesions in this cohort were clinically palpable masses, masses incidentally discovered at imaging, or a mix of both. One surgical study (2) showed that abdominal wall masses in patients who were referred for resection showed a similar distribution of the three most common histologic diagnoses at resection, in descending order of frequency: desmoid tumor, soft-tissue sarcoma, and dermatofibrosarcoma protuberans.
Abdominal wall masses for which patients undergo imaging are more common in women than in men. In part, this is reflective of desmoid tumors being much more common in women and abdominal wall endometriosis being exclusive to women (1). Desmoid tumors, metastases, endometriomas, and sarcomas typically manifest with some degree of enhancement, whereas lipomas have fat composition. Although lipomas have characteristic homogeneous or near-homogeneous fat composition, differentiating liposarcoma from atypical lipomas can be challenging. Finally, certain patient histories, symptoms, or syndromic conditions can be helpful to know in the evaluation of abdominal wall masses. Examples include cyclic pain in an incision from a prior cesarean delivery in menstruating women as a characteristic of an abdominal wall endometrioma, use of anticoagulation medication with abdominal fullness or pain as a manifestation of a rectus sheath hematoma, multiple desmoid tumors associated with Gardner syndrome, and neurofibromas and malignant peripheral nerve sheath tumors associated with neurofibromatosis type I (3–5).
A different diagnostic approach is used for multifocal or diffuse processes in the abdominal wall. In comparison to the use of lesion composition and the knowledge of the common processes, a key factor in interpreting diffuse abdominal wall processes is knowledge of the patient history. Examples of commonly encountered diffuse processes include injection granulomas from subcutaneous administration of anticoagulation agents or insulin and diffuse soft-tissue calcifications in hypercalcemic states (6,7). Uncommon abdominal wall diffuse processes include lipohypertrophy related to insulin injections in diabetic patients, posttransplant lymphoproliferative disorder after solid organ transplants, and pancreatic panniculitis after pancreatitis (8–10).
The purpose of this article is to offer an algorithmic approach to characterization of abdominal wall masses and to review abdominal wall diffuse processes.
Anatomy
The abdominal wall is composed of anterior, anterolateral or lateral, and posterior sections. The anterior and anterolateral or lateral portions are sometimes grouped together or reported separately (11–13). From superficial to deep, the layers of the anterolateral abdominal wall include the skin, Camper superficial fascia (subcutaneous fat), Scarpa deep fascia (membranous fascia), deep fat, abdominal wall muscles, transversalis fascia, extraperitoneal fat, and parietal peritoneum (11–14). The fascial delineation is readily apparent on most CT images of the abdomen and pelvis (15,16). Figure 1 details the anatomy of the abdominal wall.
Figure 1.
Anatomy of the abdominal wall shown on an axial CT image (right) and a virtual cinematic rendering (left) in a 35-year-old man with abdominal pain and a normal CT examination.
The anterolateral abdominal wall muscles are composed of the three-layered lateral muscles including the external oblique, internal oblique, and transversus abdominis and the anterior and central rectus abdominis muscles. The arcuate line of Douglas is located approximately halfway between the umbilicus and the superior aspect of the pubic bone and is the site where the rectus sheath transitions from encircling the rectus abdominis to coursing anterior to it (11–14). This anatomic transition can be important when pathologic processes occupy the rectus sheath. Above the arcuate line, masses or fluid collections in the rectus sheath tend to be unilateral, whereas processes below the arcuate line can be bilateral. An example of this is shown with a rectus sheath hematoma in Figure 2. The linea alba is a vertically oriented fibrous thickening of the abdominal wall muscle aponeuroses at the midline that transects the middle of the rectus abdominis muscles, whereas the lineae semilunaris, or spigelian lines, are paired vertically oriented lines of aponeurosal thickening of the anterolateral abdominal wall muscles that form the lateral borders of the rectus sheath (11–14).
Figure 2a.
Compartmentalization of the rectus sheath relative to the arcuate line in a 58-year-old man. (a) Axial nonenhanced CT image shows a right rectus sheath hematoma (arrow) just below the level of the umbilicus, but above the arcuate line. (b) Axial nonenhanced CT image shows the rectus sheath hematoma as it travels caudally and past the level of the arcuate line, gains access to the posterior fascial layer, and transitions from unilateral to bilateral (white arrows). Spreading of the hematoma into the extraperitoneal space of the pelvis (black arrow) is also visible.
Figure 2b.
Compartmentalization of the rectus sheath relative to the arcuate line in a 58-year-old man. (a) Axial nonenhanced CT image shows a right rectus sheath hematoma (arrow) just below the level of the umbilicus, but above the arcuate line. (b) Axial nonenhanced CT image shows the rectus sheath hematoma as it travels caudally and past the level of the arcuate line, gains access to the posterior fascial layer, and transitions from unilateral to bilateral (white arrows). Spreading of the hematoma into the extraperitoneal space of the pelvis (black arrow) is also visible.
The arterial blood supply of the abdominal wall is primarily from the superior and inferior epigastric arteries and the lower intercostal arterial branches, lumbar arterial branches, and deep circumflex arteries. Both the superior and inferior epigastric arteries join at approximately the level of the umbilicus (11,12). The superficial branches of the superior and inferior epigastric arteries are important landmarks to avoid during US-guided biopsy, paracentesis, and CT-guided biopsy and for surgical port site placement.
Venous and lymphatic tributaries draining the abdominal wall can be divided into supraumbilical, paraumbilical, and infraumbilical distributions. Supraumbilical veins and lymphatic vessels drain into tributaries of the superior vena cava and axillary lymph nodes, whereas infraumbilical veins and lymph nodes drain into the inferior vena cava and inguinal lymph nodes. The paraumbilical vein is a tributary of and communicates with the left portal vein. In patients with portal hypertension, dilated paraumbilical veins are visible at the skin surface (ie, caput medusae). Paraumbilical lymphatic drainage communicates with lymphatic vessels of the liver, which may manifest as a site of umbilical lymph node metastasis (ie, Sister Mary Joseph node) (11,12).
A detailed description of named nerves of the abdominal wall is beyond the scope of this review. Although abdominal wall schwannomas and peripheral nerve sheath tumors may occur, they rarely occur in the abdominal wall and do not have a predilection for specific nerve distributions. For the disease processes in this article, we exclude conditions of the peritoneum and limit the boundary of the abdominal wall at its deepest muscular layer.
Imaging Approach
The American College of Radiology (ACR) Appropriateness Criteria for palpable abdominal masses includes two variants: one set of criteria for palpable abdominal masses suspected to be intra-abdominal neoplasms, and a separate set of criteria for masses in the abdominal wall. (17). The ACR categorizes imaging modalities used for each variant as “usually appropriate,” “may be appropriate,” and “usually not appropriate.” For the abdominal wall masses, US, contrast material–enhanced CT, and MRI without and with contrast material are usually appropriate; nonenhanced CT and MRI may be appropriate; and all other modalities, including radiography, PET, and fluoroscopy, are usually not appropriate.
US is the ideal first-choice modality for imaging and confident diagnosis of palpable soft-tissue masses in the abdominal wall if the patient’s history suggests that it is appropriate (18,19). US with the use of high-frequency linear transducers often provides excellent detail and resolution and allows dynamic palpation and manipulation of the mass in real time. The ACR Appropriateness Criteria for abdominal wall masses lists MRI as the second-choice modality, given the superior soft-tissue resolution of MRI. CT is listed as the third choice. However, the Appropriateness Criteria state that there are no recently published studies that compare the diagnostic yield for the different modalities that are used for abdominal wall masses. MRI can be tailored to a body wall mass protocol in which an MRI-visible marker is placed over the mass and the field of view is focused on imaging the mass.
CT is occasionally used as the initial modality to evaluate an abdominal wall mass, particularly when the patient has a history of malignancy, and metastasis is considered in the differential diagnosis. An imaging approach to initial workup of abdominal wall masses is presented in Figure 3. Image-guided percutaneous biopsy of abdominal wall masses is almost always performed with US, which allows real-time visualization, particularly of the superficial inferior epigastric arterial branches, and delineation of the deep border of the abdominal wall for deep or larger abdominal wall masses.
Figure 3.
Flowchart shows the imaging algorithm for evaluation of palpable abdominal wall masses. This algorithm is predominantly based on the ACR Appropriateness Criteria for palpable abdominal masses, abdominal wall variant (17).
The ACR Appropriateness Criteria for soft-tissue masses (18) have a predominantly musculoskeletal focus. However, superficial soft-tissue lesions of the abdomen are listed in their scope. The Appropriateness Criteria suggest an initial radiographic evaluation, or US can be used for lesions that are difficult to evaluate with radiography. Radiography is listed as usually appropriate in all categories (18). However, radiography of abdominal wall lesions is typically not helpful unless the soft-tissue lesion of interest closely approximates a bony landmark. For instance, a definitive diagnosis of a palpable mass in the anterior abdominal wall above the umbilicus generally is not made on the basis of radiography. However, for lesions that closely approximate bony landmarks such as the lower ribs, the lumbar spine, or the pelvis, radiography may provide diagnostic information about its relationship with bone. Overall, we recommend following the algorithm and diagnostic considerations of the ACR Appropriateness Criteria for palpable abdominal wall masses, which recommend initial evaluation with US. In addition, contrast-enhanced CT or MRI without and with contrast material enhancement are considered usually appropriate.
Diagnostic algorithms to guide the approach of discrete abdominal wall masses and diffuse processes are presented in Figures 4 and 5. The first step in the evaluation of an abdominal wall mass is to confirm that it actually is a discrete mass, rather than an abdominal wall hernia. Such findings are common in clinical practice, particularly with US (19). Once a discrete mass is determined to be present, its composition of fat, fluid, or soft tissue and imaging characteristics can further help to guide the algorithmic approach (Fig 4). Diffuse processes of the abdominal wall, with multiple small masses or calcifications in an anatomic region or diffusely scattered throughout the soft tissues of the abdominal wall, may be difficult to distinguish from one another on the basis of their imaging appearance alone. Rather, knowledge of the patient’s history or presenting symptoms can be more helpful in diagnosis of diffuse abdominal wall processes (Fig 5). In the following sections, we discuss specific abdominal wall masses and diffuse processes and the diagnostic algorithms shown in Figures 4 and 5.
Figure 4a.
Flowchart shows the diagnostic algorithm for abdominal wall masses, which is based primarily on mass characteristics for fat-containing masses (a), fluid or cystic masses (b), and solid masses (c).
Figure 5.
Flowchart shows the diagnostic algorithm for diffuse abdominal wall processes, which is based primarily on patient history.
Figure 4b.
Flowchart shows the diagnostic algorithm for abdominal wall masses, which is based primarily on mass characteristics for fat-containing masses (a), fluid or cystic masses (b), and solid masses (c).
Figure 4c.
Flowchart shows the diagnostic algorithm for abdominal wall masses, which is based primarily on mass characteristics for fat-containing masses (a), fluid or cystic masses (b), and solid masses (c).
Hernias that Clinically Mimic Masses
Hernias are common, with 350 000 ventral or incisional abdominal wall hernia repairs performed annually in the United States (20). Hernias in the abdomen and pelvis can be divided into abdominal wall, pelvic, and groin hernias. Abdominal wall hernias usually are extraperitoneal protrusions of intra-abdominal contents through defects in the abdominal wall. Abdominal wall hernias can be further subdivided into ventral or incisional hernias (13). Spigelian hernias extend laterally to the rectus abdominis muscle and are contained by the external oblique muscle. Lumbar hernias of the posterior abdominal wall are frequently the result of trauma. Lumbar hernias occur through defects in either the superior or inferior lumbar triangle. A hernia through the superior lumbar triangle is termed a Grynfeltt hernia or Grynfeltt-Lesshaft hernia, whereas those through the inferior lumbar triangle are termed Petit hernias (13). There are numerous groin and pelvic hernias, with indirect and direct inguinal hernias representing the prototypical groin hernia. Abdominal wall hernias and their subtypes are discussed in the literature (13), and more in-depth discussion of these hernias is outside the scope of this article.
Although abdominal wall hernias are often accurately diagnosed clinically, they may mimic abdominal wall masses and prompt referral for diagnostic imaging. US offers real-time evaluation to help distinguish hernias from masses. Induction or exaggeration of hernias during real-time US can be facilitated by using the Valsalva maneuver. The US appearance varies, depending on the predominant content (ie, fat or bowel) of the hernia sac. CT and MRI are more sensitive for the diagnosis of hernias than is US (13) (Movies 1, 2). The diagnosis, the content of the sac, and the presence or absence of complications are often readily apparent at CT and MRI of uncomplicated abdominal wall or inguinal hernias. CT is the modality of choice for assessment of complications of irreducible hernias when there is clinical suspicion for strangulation. The CT findings of strangulation include fat stranding, fluid in the hernia sac, hypoenhancement of the bowel in the hernia, and the hernia serving as the transition point of an associated small bowel obstruction (21).
Movie 1.
Ventra hernia. Transverse sonographic cine demonstrates a supraumbilical ventral hernia containing a loop of small bowel. Note the subtle variations in the small bowel loop through the fascial defect as the transducer is moved and manipulated.
Movie 2.
Ventra hernia during Valsalva. Longitudinal sonographic cine demonstrates a supraumbilical ventral hernia containing omentum. As the patient performs the Valsalva maneuver, the omental fat freely herniates through the large fascial defect.
Mass Composition
As shown in the diagnostic algorithms (Figs 4, 5), the first step in evaluation of a potential abdominal wall mass is to determine whether it is a true mass or a hernia simulating a mass. If it is a discrete mass, the next step is to determine whether it predominantly contains fat, fluid, or soft tissue. US, CT, and MRI are all useful for differentiation of the tissue composition. At US, pure fat is usually hypoechoic, with an appearance similar to that of subcutaneous fat. Fat that is mixed with soft tissue, inflamed, or edematous is hyperechoic and attenuates sound. At CT, fat shows low attenuation, with a range of −10 to −100 HU, but it may be difficult to see in small masses (22). MRI best shows macroscopic fat as a loss of signal intensity in a mass on fat-saturated MR images. In- and opposed-phase MRI may show chemical shift, or “india ink” artifacts when lesions contain gross fat (23).
Fluid-containing lesions at US are typically anechoic and have posterior through transmission. Because US permits real-time imaging, debris in a mass can often be distinguished from solid material by demonstrating that it is freely mobile. The mobility of nonsolid material in a mass may be facilitated by changing the patient’s position or applying manual compression with the US transducer. At CT, simple fluid is approximately 0 HU and the fluid component of the lesion does not show enhancement. The attenuation value of fluid may be higher than that of simple fluid if there is hemorrhage, infection, or a microcystic component. Fluid has high signal intensity at T2-weighted MRI and has variable signal intensity at T1-weighted MRI, depending on the hemorrhage or protein content. Solid lesions can have variable echogenicity, attenuation, or signal intensity at US, CT, or MRI, respectively, and are predominantly composed of vascularized tissue that shows contrast material enhancement.
Fat-containing Masses
Fat-containing masses in the abdominal wall are primarily benign lipomas (1). Liposarcoma is the primary consideration in the differential diagnosis of fatty masses with an atypical appearance. Other fat-containing masses in the abdominal wall include hemangiomas, arteriovenous malformations, and hibernomas. Abdominal wall lipohypertrophy related to insulin injections is described in diffuse processes and is often bilateral (8).
Lipomas
Lipomas are common benign tumors of mature adipose tissue and comprise nearly half of all soft-tissue tumors. One large study (1) showed that lipomas are the second most common benign mass and fourth most common abdominal wall mass. Lipomas are often painless and asymptomatic. They usually come to clinical attention when the patient or clinician palpates the lesion during a physical examination (19,24,25).
The imaging findings of a lipoma at US, CT, and MRI are often diagnostic and obviate the need for further tests, biopsy, or treatment. At US, lipomas are oblong echogenic well-defined masses, without posterior acoustic shadowing or enhancement but with little internal vascularity, and sometimes with thin linear echogenic striations that parallel the skin (19,22). At CT, lipomas are encapsulated homogeneous fatty masses without a soft-tissue component (Fig 6). At MRI, lipomas are isointense compared with subcutaneous fat on images from all sequences. Thin septa (<2 mm) are frequently seen in lipomas. These septa typically demonstrate minimal or no enhancement (19,24,25). However, enhancing thickened or nodular septa have been reported in approximately one-third of benign lipomas and have an appearance that is indistinguishable from liposarcoma. Lipomas may interdigitate in the skeletal muscle and create a striated appearance that is not seen in liposarcomas (26).
Figure 6a.
Lipoma in a 29-year-old woman with a palpable right upper quadrant abdominal wall mass. (a) Color Doppler US image shows a hypovascular hypoechoic mass mirroring the subcutaneous fat with a thin hyperechoic capsule (arrow), which is consistent with a lipoma. (b) Axial CT image for evaluation of abdominal pain shows a fat-attenuation encapsulated mass (arrow), which is consistent with a body wall lipoma. Note how poorly discernible the capsule is at its lateral aspect.
Figure 6b.

Lipoma in a 29-year-old woman with a palpable right upper quadrant abdominal wall mass. (a) Color Doppler US image shows a hypovascular hypoechoic mass mirroring the subcutaneous fat with a thin hyperechoic capsule (arrow), which is consistent with a lipoma. (b) Axial CT image for evaluation of abdominal pain shows a fat-attenuation encapsulated mass (arrow), which is consistent with a body wall lipoma. Note how poorly discernible the capsule is at its lateral aspect.
Liposarcomas
Liposarcoma is the main differential diagnostic consideration for a fat-containing mass in the abdominal wall. Although liposarcomas are most commonly located in the deep soft tissues of the extremities and the retroperitoneum, they may also develop in the abdominal wall (1,23,26). Liposarcomas are the second most common soft-tissue sarcomas overall after undifferentiated pleomorphic sarcomas, and the World Health Organization (WHO) has subcategorized them as well-differentiated, myxoid, pleomorphic, mixed, and dedifferentiated types (27). In general, the presence of thickened or nodular enhancing septa or enhancing soft-tissue components in a mass, with fat attenuation at CT or fat signal intensity at MRI, should raise concern for liposarcoma (Fig 7). Myxoid liposarcoma may appear hyperintense at T2-weighted MRI and can mimic a complex cystic mass. Pleomorphic liposarcomas may appear as heterogeneous masses with hemorrhage or necrosis and may be devoid of any fatty components, which makes confident diagnosis on the basis of imaging a challenge (28). If percutaneous biopsy of a suspected fatty mass is performed, the enhancing soft-tissue component rather than the fatty components should be targeted.
Figure 7a.
Liposarcoma in a 77-year-old man who presented with a palpable mass in the right abdominal wall, which was initially seen at CT (not shown). Axial contrast-enhanced T1-weighted MR image (a) and axial FDG PET image (b) show the hypermetabolic enhancing soft-tissue component (black arrows) of this macroscopic fat–containing mass (white arrow). Biopsy results confirmed the diagnosis of liposarcoma.
Figure 7b.
Liposarcoma in a 77-year-old man who presented with a palpable mass in the right abdominal wall, which was initially seen at CT (not shown). Axial contrast-enhanced T1-weighted MR image (a) and axial FDG PET image (b) show the hypermetabolic enhancing soft-tissue component (black arrows) of this macroscopic fat–containing mass (white arrow). Biopsy results confirmed the diagnosis of liposarcoma.
Hibernomas
Hibernomas are rare tumors composed of brown fat. The abdominal wall is an uncommon location for hibernomas, which more typically occur in the proximal upper and lower extremities. Hibernomas are commonly encountered incidentally at fluorine 18 fluorodeoxyglucose (FDG) PET/CT, where they are typically hypermetabolic. The attenuation at CT or the signal intensity at MRI is only slightly different from that of the adjacent subcutaneous fat. Compared with lipomas, hibernomas may have more prominent septa and feeding vessels. As with lipomas, hibernomas may grow over time (29).
Hemangiomas and Arteriovenous Malformations
Hemangiomas of the abdominal wall are encapsulated fatty lesions that contain small capillaries, and are most frequently seen in young adult men (24,25). Abdominal wall hemangiomas in adults are often intramuscular or congenital (30). The patient’s family history is important to diagnosis, because some cases are autosomal dominant, and a history of trauma may also be a contributing factor. Hemangiomas are usually small slow-growing lesions that are often painful on palpation. Phleboliths in a fatty mass are suggestive of the diagnosis (Fig 8). High signal intensity at T2-weighted MRI and low signal intensity at T1-weighted MRI represent the vascular components, which enhance after administration of contrast material. Hemangiomas have no malignant potential and do not have a propensity for local recurrence after excision (24,25).
Figure 8.
Hemangioma in a 45-year-old man. Axial nonenhanced CT image shows an elongated fat-containing mass (white arrow) with several calcified phleboliths (black arrow) between the left external and internal oblique muscles. These findings are characteristic of an abdominal wall intramuscular hemangioma.
Arteriovenous or venous malformations are often interdigitated with fat, are unilateral in the abdominal wall, and are often located adjacent to or within the anterolateral muscle groups. Fast-flow malformations show brisk arterial enhancement, with early drainage veins at CT or MR angiography. Slow-flow malformations (often venous malformations) typically do not show arterial enhancement but instead show venous or delayed enhancement (Fig E1) (24,25). These malformations may be treated surgically or with sclerotherapy and other percutaneous techniques (30).
Fluid or Cystic Masses and Collections
Abdominal wall fluid-filled masses or collections often have characteristic imaging appearances and are suggestive of certain clinical scenarios. These fluid collections can be characterized broadly as hematomas, fluid collections in postoperative patients, or cystic masses in patients without a history of surgery.
Hematomas
Blunt and penetrating trauma to the abdomen, surgery, or exercise-related injury may result in contusions or hematomas in the abdominal wall. Abdominal wall hematomas may also develop spontaneously, particularly in patients with thrombocytopenia or coagulopathy or those who are administered systemic anticoagulation medication. Abdominal wall hematomas most commonly occur in the rectus abdominis muscle (ie, rectus sheath hematomas) (31). Less commonly, hematomas may involve the lateral and posterolateral abdominal wall. The rectus sheath envelops the rectus abdominis both anteriorly and posteriorly above the arcuate line. Below the arcuate line, the rectus sheath is only present anteriorly. Therefore, a rectus sheath hematoma located above the arcuate line is usually spindle shaped, because it is confined by the circumferential sheath, whereas a rectus sheath hematoma below the arcuate line is not confined posteriorly, tends to appear less circumscribed, and can extend posteriorly into the peritoneum and prevesical space (4,14) (Fig 2).
At US, an acute rectus sheath hematoma appears as a heterogeneously hyperechoic mass or a diffuse enlargement of the rectus muscle without internal Doppler flow. With time, a hematoma may liquefy and become hypoechoic or anechoic, with scattered echogenic components. At CT, a hyperattenuating mass or enlargement of the rectus muscle is the typical appearance of an acute rectus sheath hematomas. A fluid-fluid level may be apparent because of the layering of blood products of different densities, which is indicative of recent or active bleeding. Administration of intravenous contrast material permits assessment for active extravasation, usually from branches of the superior or inferior epigastric arteries (Fig 9). Over time, as the blood products in the hematoma break down, the CT attenuation of the hematoma decreases (4,14). Although MRI is not frequently performed for diagnosis, acute abdominal wall hematomas are usually T1 hyperintense and T2 hypointense relative to skeletal muscle. Over time, the T1 and T2 signal intensity varies, depending on the concentration of deoxyhemoglobin and hemosiderin (14).
Figure 9a.
Rectus sheath hematoma in an 81-year-old woman who was undergoing systemic anticoagulation therapy. US (a) and axial CT (b) images show a hematocrit layer (dashed arrow) within a right rectus sheath hematoma and active extravasation (solid arrow in b).
Figure 9b.
Rectus sheath hematoma in an 81-year-old woman who was undergoing systemic anticoagulation therapy. US (a) and axial CT (b) images show a hematocrit layer (dashed arrow) within a right rectus sheath hematoma and active extravasation (solid arrow in b).
Morel-Lavallee Lesions
Morel-Lavallee lesions are shearing injuries that result from a traumatic force that separates the overlying skin and subcutaneous fat from the underlying fascia. Although it is traumatically sheared and separated internally, the skin is not breached in these closed degloving injuries. In this injury, arterial branches, venous tributaries, and lymphatic channels are disrupted. Acutely, this will result in a hematoma in the new posttraumatic space between the subcutaneous fat and the underlying fascial layer. More subacute or chronic fluid may be seromatous, serosanguineous, or lymphatic (14). Although Morel-Lavallee lesions are more common around the hips, pelvis, and thighs, they do occasionally arise in the abdominal wall, particularly over the lumbar spine. In subtle cases, they may manifest as thickening with a small volume of fluid around fascial planes. In more pronounced or acute manifestations, the volume of fluid may be larger, and air or active extravasation may be present (Fig 10). The appearances at US and MRI vary considerably and are reflections of the volume, acuity, and composition of the fluid accumulation around the fascial planes. The superior contrast resolution of MRI compared with that of CT may help in delineation of the extent of fascial involvement (14).
Figure 10a.
Morel-Lavallee lesion in a 50-year-old male pedestrian who was struck by a motor vehicle. Axial (a) and coronal reformatted (b) CT images show a closed degloving injury extending from the lower right chest wall to the most caudal profiled extent at the right hip, with predominant subcutaneous collection of gas (black arrow) and fluid (white arrow in a) centered in the right lateral abdominal wall. The coronal reformatted image shows the craniocaudal extent of this Morel-Lavallee lesion and two comminuted displaced anterolateral rib fractures (square outline in b) and the pneumomediastinum (* in b), which was secondary to a displaced sternal body fracture (not shown). Air-filled small bowel loops in a large right inguinal hernia are partially shown (white arrow in b). No intra-abdominal visceral injury is visible.
Figure 10b.

Morel-Lavallee lesion in a 50-year-old male pedestrian who was struck by a motor vehicle. Axial (a) and coronal reformatted (b) CT images show a closed degloving injury extending from the lower right chest wall to the most caudal profiled extent at the right hip, with predominant subcutaneous collection of gas (black arrow) and fluid (white arrow in a) centered in the right lateral abdominal wall. The coronal reformatted image shows the craniocaudal extent of this Morel-Lavallee lesion and two comminuted displaced anterolateral rib fractures (square outline in b) and the pneumomediastinum (* in b), which was secondary to a displaced sternal body fracture (not shown). Air-filled small bowel loops in a large right inguinal hernia are partially shown (white arrow in b). No intra-abdominal visceral injury is visible.
Seromas
Seromas are defined as collections of sterile serous fluid that contain plasma and relatively few red blood cells. They are commonly seen after abdominal surgery but can be difficult to distinguish from hematomas and hernias on the basis of the physical examination alone. At US, seromas are usually anechoic unilocular thin-walled circumscribed fluid collections with posterior through transmission. At CT or MRI, seromas are circumscribed low-attenuation or low–T1 signal intensity fluid collections, respectively, without enhancement. Unless recent surgery has been performed or there is a superimposed infection, an uncomplicated seroma is devoid of gas and adjacent inflammatory changes (6,19).
Abscesses
An abscess is defined as an infected fluid collection. Clinical symptoms are often suggestive of the diagnosis, including the presence of leukocytosis, fever, purulent drainage, and tenderness. At US, abscesses are typically multilocular complex hypoechoic (not anechnoic) collections with thickened septa and hyperemia. Gas in an abscess at US appears as echogenic reflectors with ring-down artifacts. At CT and MRI, a thick-walled peripherally enhancing fluid collection that often contains gas with adjacent fat stranding is characteristic of an abscess (Fig E2) (6,19,24). Diffusion restriction may be present at MRI. Image-guided aspiration or drainage is both diagnostic and therapeutic.
Cystic Masses in Patients with No History of Surgery
Epidermal inclusion cysts, or sebaceous cysts, are cystic lesions filled with keratin debris and are bounded by a wall of stratified squamous epithelium. They may form during embryogenesis as the result of occlusion of the pilosebaceous unit or after epidermal elements are implanted into deeper tissue during a surgical procedure. They may be centered superficially in the soft tissues or deeper. When they are set deeper, they mimic other processes (24,32,33). At US, epidermal inclusion cysts are usually circumscribed round or ovoid mildly hyperechoic lesions with posterior through transmission and no internal Doppler flow (Fig E3). Occasionally, internal linear echogenic reflectors and a thin hypoechoic rim are present (32) (Movie 3). They are usually circumscribed nodules of soft-tissue attenuation at CT and show high signal intensity at T2-weighted MRI (Fig E3). Internal components may demonstrate signal hyperintensity at T1-weighted MRI and signal hypointensity at T2-weighted MRI that reflect keratin debris. Unruptured epidermal inclusion cysts typically show a thin rim of enhancement, with central nonenhancement. In comparison, ruptured epidermal inclusion cysts show thick and irregular rim enhancement and surrounding soft-tissue edema and/or inflammation, which may be difficult to distinguish from an abscess or a neoplastic process (33).
Movie 3.
Epidermal inclusion cyst. Longitudinal sonographic cine of a palpable right upper quadrant abdominal wall mass demonstrates a mixed echogenicity oval mass centered in the subcutaneous fat with a hypoechoic rim and bright internal reflectors. This mass was avascular on Doppler imaging (not shown) and most consistent with an epidermal inclusion cyst.
Myxomas are benign hypercellular spindle cell neoplasms with an excess mucopolysaccharide or myxoid stroma. Most commonly, they develop in the heart, with subcutaneous tissue the second most common site. Most soft-tissue myxomas are intramuscular and predominantly manifest in the extremities, particularly in the thigh (approximately 50%). In one imaging study (30) of 45 soft-tissue myxomas in 44 patients, only two (4%) myxomas were in the abdominal wall, none were in the anterior or anterolateral abdominal wall, and four (9%) were in the buttocks or groin. At CT and MRI, myxomas tend to mimic the appearance of cysts. CT shows a hypoattenuating oval or round mass (Fig E4), and MRI typically shows T1 hypointensity and marked T2 hyperintensity. Internal enhancement, which is best shown at MRI, may be present. US can suggest the soft-tissue component of myxomas, which typically manifest as hypoechoic masses, a feature that helps distinguish a myxoma from an anechoic cyst (Fig E4) (34).
Solid Masses in Patients with a Relevant Medical History
Patients with abdominal wall masses that arise in areas of a prior surgical incision or biopsy can have a history that is suggestive of cancer, which may help to establish an accurate diagnosis. The primary consideration is abdominal wall metastasis in a patient who underwent prior abdominal surgery for cancer resection. Benign entities can also seed surgical incisions, and most abdominal wall endometriomas arise in women who have undergone cesarean delivery. Women with abdominal wall endometriosis are typically of menstruating age and may have cyclical pain associated with the abdominal wall implant (3,6,24). Finally, abdominal or body wall masses are commonly found in patients with syndromic conditions. Some examples include desmoid tumors in Gardner syndrome and neurofibromas and peripheral nerve sheath tumors in neurofibromatosis type I (5,6,24). Desmoid tumors associated with Gardner syndrome are discussed in the “Desmoid Tumor” section.
Abdominal Wall Metastasis
The soft tissues of the abdominal wall are a relatively rare site of metastatic disease (8,24). Nevertheless, a soft-tissue nodule in the abdominal wall in a patient with a history of malignancy should be viewed with suspicion and investigated further. Primary tumors that may metastasize to the abdominal wall include melanoma; cancers of the breast, pancreas, and lung; gastrointestinal and genitourinary cancers; and lymphoma. In rare cases, the abdominal wall may be a primary site of cutaneous lymphoma, which is discussed in the subsequent dedicated section. The Sister Mary Joseph nodule is a metastatic nodule at the umbilicus that is identified at physical examination of a patient with advanced abdominal and pelvic malignancy (35) (Fig 11).
Figure 11.
Sister Mary Joseph nodule in a 60-year-old woman with metastatic ovarian serous adenocarcinoma. Axial CT image shows umbilical lymph node metastasis (square outline) from an ovarian serous cancer. Note the calcifications (arrows), which mimic metastatic disease elsewhere in this patient (not shown).
Imaging findings of metastatic disease to the abdominal wall are nonspecific and include a single or multiple enhancing soft-tissue nodules. The presence of widespread metastatic disease elsewhere in the body should further raise suspicion for this diagnosis. Melanoma metastases have a propensity for hemorrhage. Hypermetabolism at FDG PET is usually present, but definitive diagnosis often requires tissue sampling (1,21). Occasionally, omental metastases in a ventral or incisional hernia may be a blind spot for developing metastases, or omental metastases in an abdominal wall hernia may mimic an abdominal wall soft-tissue mass.
Seeding of Prior Surgery or Biopsy Track
Metastases at the site of prior surgery or biopsy should be suspected in a patient with known malignancy and growing skin nodules or plaques at the site of prior intervention. Needle track seeding in the abdominal wall is associated with poorly differentiated tumor histologic results and a subcapsular tumor location (24) (Fig 12). Laparoscopic port site metastasis (also termed port site recurrence) at sites of prior laparoscopic colectomy for colon cancer is a well-described phenomenon (36).
Figure 12a.
Metastatic seeding of a prior biopsy track in a 68-year-old man with cirrhosis and hepatocellular carcinoma. (a) Axial contrast-enhanced T1-weighted MR image shows a rim-enhancing mass in the left lateral section (arrow), which shows washout and features of hepatocellular carcinoma. The patient underwent percutaneous biopsy at an outside institution, the results of which confirmed the imaging diagnosis. (b) Axial contrast-enhanced CT image acquired after the patient underwent liver transplant shows metastatic seeding from the prior percutaneous biopsy track (arrow).
Figure 12b.
Metastatic seeding of a prior biopsy track in a 68-year-old man with cirrhosis and hepatocellular carcinoma. (a) Axial contrast-enhanced T1-weighted MR image shows a rim-enhancing mass in the left lateral section (arrow), which shows washout and features of hepatocellular carcinoma. The patient underwent percutaneous biopsy at an outside institution, the results of which confirmed the imaging diagnosis. (b) Axial contrast-enhanced CT image acquired after the patient underwent liver transplant shows metastatic seeding from the prior percutaneous biopsy track (arrow).
Surgical incision or biopsy track seeding is not limited to malignant processes. Abdominal wall leiomyomas have been described in patients who have undergone previous surgery for uterine fibroids, presumably because of microscopic seeding of tissue in the abdominal wall (37) (Fig E5). Splenosis refers to automatic transplantation of ectopic splenic tissue into another anatomic compartment after splenic rupture. In the subcutaneous tissue, splenosis is associated with the scar at the site of previous surgery or traumatic injury (38). Foci of splenosis resemble the attenuation at CT, the signal intensity at MRI, and the enhancement of normal splenic tissue (39) (Fig E6).
Suture material used for closure of incisions or stray surgical material from port sites, such as a gallstone dropped during laparoscopic cholecystectomy, can cause a localized inflammatory granulomatous response and can manifest as a mass or masslike process (40). Suture granulomas often have a typical US appearance. One imaging cohort (41) of patients with suture granulomas showed a hypoechoic mass in all of the patients, and the suture material manifested as linear hyperechogenicity in the hypoechoic mass in 91% of the patients (Movie 4). CT shows nonspecific soft-tissue attenuation (6).
Movie 4.
Suture granuloma. 68-year-old woman who had prior resection of primary peritoneal adenocarcinoma with a 2 cm nodule in the left rectus sheath that had progressively enlarged over the course of two preceding surveillance CT examinations (not shown). She was referred on the basis of these CT findings for ultrasound-guided biopsy. Longitudinal sonographic cine demonstrates a hypoechoic oval nodule just to the left of the patient’s midline laparotomy incision. Multiple hyperechoic linear foci are present within this hypoechoic nodule, suggestive of suture material. Ultrasound-guided biopsy was performed and confirmed the diagnosis of benign inflammatory change about the sonographically profile suture material, consistent with a diagnosis of suture granuloma.
Endometriosis
Endometriosis is defined as ectopic functional endometrial tissue located outside of the endometrial cavity, and it is usually seen in young adult women. Although endometriosis typically involves the ovaries and pelvic peritoneum, unusual extrapelvic sites of endometriosis include the thorax and abdominal wall. The latter is often associated with cesarean delivery or hysterectomy scars (Fig 13). However, in rare cases, abdominal wall endometriosis can develop in patients with no history of surgery (Fig 14). The classic clinical history of a patient with abdominal wall endometriosis is cyclical abdominal pain associated with menses and a painful palpable nodule in the abdominal wall close to a cesarean delivery scar (3,6). Endosalpingiosis is ectopic fallopian tube epithelial tissue of müllerian origin that can manifest in the abdominal wall either in isolation or concurrently with endometriosis (42) (Fig 14).
Figure 13.
Typical abdominal wall endometriosis in a Pfannenstiel incision in a 40-year-old woman. Axial CT image shows a mass (white arrow) to the left of midline with rim and nodular peripheral enhancement arising from an incision from a prior low transverse cesarean delivery (black arrow). The diagnosis of endometriosis was confirmed at resection.
Figure 14.
Atypical abdominal wall endometriosis in a 26-year-old woman who had undergone no prior surgery and who presented for evaluation of a palpable periumbilical abnormality. Axial contrast-enhanced CT image shows a poorly defined enhancing periumbilical lesion. This lesion was targeted for biopsy with US guidance (not shown), and the percutaneous core biopsy results were interpreted as endosalpingiosis (ectopic fallopian tube epithelium). However, at surgical excision, the interpreting pathologist diagnosed it as endometriosis.
At US, abdominal wall endometriosis usually appears as a solid hypoechoic nodule with internal vascularity near the site of a scar on the skin. The margins may be smooth or irregular, and small cystic areas may be present, presumably from repeated episodes of menstrual bleeding (6,24). CT and MRI findings are nonspecific and show an enhancing nodule in the anterior abdominal wall (Fig 13). The nodule is usually isointense to mildly hyperintense compared with skeletal muscle at T1- and T2-weighted MRI (3). The imaging findings must be correlated with patient history and clinical findings to arrive at the correct diagnosis.
A pitfall for diagnosis of abdominal wall endometriosis is that desmoid tumors manifest similarly in menstruating women, who typically have a history of pregnancy and birth (1,2). Furthermore, desmoid tumors may be associated with a cesarean delivery or hysterectomy incision site, although it is uncommon. The clinical history of cyclical pain with menses may help in the differential diagnoses if there is overlap in imaging findings. However, this classic history is only present in approximately 50% of patients with abdominal wall endometriosis (3,6,24). If MRI of the body wall is used to characterize an abdominal wall mass in the differential diagnosis of abdominal wall endometriosis and desmoid tumors, pelvic sequences may be helpful to look for pelvic endometrial implants.
Neurofibromas
Neurofibromas are benign nerve sheath tumors that may occur in the abdominal wall and elsewhere. Multiple neurofibromas are pathognomonic for neurofibromatosis type 1. Neurofibromas may be small round lobulated or tubular masses. They may also be large and may be found between the tissue planes in a patient with plexiform neurofibroma. At US, neurofibromas are circumscribed masses with smooth margins (43). Neurofibromas are homogeneously hypoattenuating at CT, show hypointensity at T1-weighted MRI and heterogeneous hyperintensity at T2-weighted MRI, and demonstrate enhancement after the administration of intravenous contrast material. Small cystic spaces are sometimes present owing to myxoid degeneration, and a “target” appearance has been described (ie, a high-signal-intensity periphery with a low-signal-intensity center on T2-weighted MR images) (24,44).
Malignant Peripheral Nerve Sheath Tumors
A malignant peripheral nerve sheath tumor is an aggressive malignant neoplasm that may originate from a peripheral nerve sheath or a plexiform neurofibroma. The lifetime risk of a patient with neurofibromatosis type 1 developing a malignant peripheral nerve sheath tumor is approximately 5%. Nerve sheath tumors tend to manifest at a younger age in patients with neurofibromatosis type 1 than they do in patients without the disease, and neurofibromatosis type 1 is associated with a worse prognosis (45). The imaging appearance is usually heterogeneous attenuation at CT or heterogeneous signal intensity at MRI, with heterogeneous enhancement due to regions of necrosis (Fig E7). The margins are usually poorly defined and infiltrative, and destructive changes in adjacent osseous structures may be seen, although peripheral nerve sheath tumors may also have an indolent appearance (46). FDG PET/CT is useful in profiling the metabolic activity and differentiating between malignant peripheral nerve sheath tumors and neurofibromas.
Solid Mass in Patients with No Relevant Medical History
Enhancing abdominal wall solid masses that manifest without a relevant patient history or related syndrome can be challenging to diagnose accurately. Desmoid tumors are the most common abdominal wall mass overall (1,2). Although imaging patterns of desmoid tumors have been described, there is substantial overlap in the imaging appearance of desmoid tumors and other abdominal wall masses, and tissue sampling is often required to secure the diagnosis. Abdominal wall sarcomas are rare and have a spectrum of different appearances, sizes, and degrees of local invasion. Abdominal wall schwannomas and primary cutaneous lymphoma are two rare entities that are also discussed in this section.
Desmoid Tumors
Desmoid tumors are fibroblastic tumors that most often occur sporadically but can be associated with Gardner syndrome (familial adenomatous polyposis) (5). They are commonly located in the mesentery, retroperitoneum, and abdominal wall. Although a desmoid tumor is a benign neoplasm with no metastatic potential, it can be locally invasive and has a high rate of local recurrence after resection (2). Desmoid tumors are the most common abdominal wall masses that are confirmed at histologic examination. Abdominal wall desmoid tumors are much more common in women than in men (1,2). In the previously mentioned retrospective imaging study (1) of 365 patients with abdominal wall masses, desmoid tumors were the most common mass (30% in that cohort) and showed a female-to-male ratio of 11 to 1. Traditionally, surgical resection is the treatment of choice; however, asymptomatic patients may be simply observed with serial imaging and clinical follow-up. Radiation therapy, chemotherapy, nonsteroidal anti-inflammatory drugs, and antiestrogen pharmacotherapy are additional noninvasive options for patients with unresectable disease, recurrence, or progression on observation (47).
At US, a desmoid tumor appears as a homogeneously hypoechoic solid mass with internal Doppler US flow (1). CT and MRI permit assessment of the location of the tumor with respect to the abdominal wall musculature, peritoneum, bowel, and vasculature. At CT, an abdominal wall desmoid tumor appears as a hyperattenuating enhancing mass that may have circumscribed or ill-defined margins (6,48) (Fig 15). Patients with Gardner syndrome are more likely to have multiple and large desmoid tumors than are patients with nonsyndromic desmoid tumors (Fig 16).
Figure 15a.
Desmoid tumor in the abdominal wall of a 35-year-old woman, which was confirmed on the basis of percutaneous biopsy. (a) Axial CT image shows a hyperenhancing abdominal wall mass centered in the left rectus sheath (arrow). (b–d) Axial nonenhanced T1-weighted MR images show a hypointense mass. After administration of a gadolinium contrast agent, there is progressive homogeneous enhancement of the mass (arrow in b–d) on sequential arterial phase (c), portal venous phase (not shown), and delayed phase (d) images. (e) US image shows a hypovascular hypoechoic mass (arrow).
Figure 16a.
Desmoid tumors in a 34-year-old woman with Gardner syndrome and multiple palpable abdominal masses. Axial contrast-enhanced CT images show that the masses (black arrow) are centered in the underlying rectus abdominis muscles and have an appearance most consistent with that of desmoid tumors. A gastric mass (white arrow in a) was later characterized as adenocarcinoma, and changes from prior colectomy are also noted (* in b).
Figure 15b.
Desmoid tumor in the abdominal wall of a 35-year-old woman, which was confirmed on the basis of percutaneous biopsy. (a) Axial CT image shows a hyperenhancing abdominal wall mass centered in the left rectus sheath (arrow). (b–d) Axial nonenhanced T1-weighted MR images show a hypointense mass. After administration of a gadolinium contrast agent, there is progressive homogeneous enhancement of the mass (arrow in b–d) on sequential arterial phase (c), portal venous phase (not shown), and delayed phase (d) images. (e) US image shows a hypovascular hypoechoic mass (arrow).
Figure 15c.
Desmoid tumor in the abdominal wall of a 35-year-old woman, which was confirmed on the basis of percutaneous biopsy. (a) Axial CT image shows a hyperenhancing abdominal wall mass centered in the left rectus sheath (arrow). (b–d) Axial nonenhanced T1-weighted MR images show a hypointense mass. After administration of a gadolinium contrast agent, there is progressive homogeneous enhancement of the mass (arrow in b–d) on sequential arterial phase (c), portal venous phase (not shown), and delayed phase (d) images. (e) US image shows a hypovascular hypoechoic mass (arrow).
Figure 15d.
Desmoid tumor in the abdominal wall of a 35-year-old woman, which was confirmed on the basis of percutaneous biopsy. (a) Axial CT image shows a hyperenhancing abdominal wall mass centered in the left rectus sheath (arrow). (b–d) Axial nonenhanced T1-weighted MR images show a hypointense mass. After administration of a gadolinium contrast agent, there is progressive homogeneous enhancement of the mass (arrow in b–d) on sequential arterial phase (c), portal venous phase (not shown), and delayed phase (d) images. (e) US image shows a hypovascular hypoechoic mass (arrow).
Figure 15e.
Desmoid tumor in the abdominal wall of a 35-year-old woman, which was confirmed on the basis of percutaneous biopsy. (a) Axial CT image shows a hyperenhancing abdominal wall mass centered in the left rectus sheath (arrow). (b–d) Axial nonenhanced T1-weighted MR images show a hypointense mass. After administration of a gadolinium contrast agent, there is progressive homogeneous enhancement of the mass (arrow in b–d) on sequential arterial phase (c), portal venous phase (not shown), and delayed phase (d) images. (e) US image shows a hypovascular hypoechoic mass (arrow).
Figure 16b.
Desmoid tumors in a 34-year-old woman with Gardner syndrome and multiple palpable abdominal masses. Axial contrast-enhanced CT images show that the masses (black arrow) are centered in the underlying rectus abdominis muscles and have an appearance most consistent with that of desmoid tumors. A gastric mass (white arrow in a) was later characterized as adenocarcinoma, and changes from prior colectomy are also noted (* in b).
The MRI appearance of desmoid tumors varies depending on the dominant element in the mass (Fig 15). Some authors (1,48–49) have adopted stages of desmoid tumors, with the underlying concept that desmoid tumors in the earlier stages have a predominantly myxoid composition and in the later stages, they progress to a predominantly fibrous element. Some evidence (1) suggests that desmoid tumors in the earlier stages have high rates of local recurrence after resection.
These tumors enhance briskly and show signal intensity that mirrors that of fluid, with hypo- to isointensity at T1-weighted MRI and hyperintensity at T2-weighted MRI. In the intermediate stages, the fibrous or collagen element starts to develop in the myxoid background, and this fibrous deposition manifests as T1- and T2-hypointense bandlike structures. As the myxoid element is replaced in the intermediate stages, T2-weighted MRI may show isointensity or mixed areas of hyper- and hypointensity that reflect the myxoid and fibrous elements. In later stages, the dominant element is fibrous tissue, which may manifest as hypointensity at T1- and T2-weighted MRI, akin to the bandlike structures comprising most of the matrix. As the matrix shifts from myxoid to fibrous elements, the degree of initial arterial or venous enhancement decreases. However, the fibrous element may demonstrate delayed-sequence enhancement (1,6,48,49).
Sarcomas
Soft-tissue sarcomas can arise in the abdominal wall and may be located in the superficial subcutaneous tissue or the abdominal wall musculature. With few exceptions, the various subtypes of sarcoma are not distinguishable with imaging alone. Abdominal wall sarcomas are the most worrisome entity in the differential diagnosis for enhancing solid masses of the abdominal wall. In one study (1) of patients with abdominal wall masses who were referred to a sarcoma clinic, sarcomas (all subtypes) were the second most common abdominal wall mass after desmoid tumors. Sarcomas can have a spectrum of differing imaging appearances (Fig 17). This section highlights some sarcomas that involve the abdominal wall, including leiomyosarcoma, dermatofibrosarcoma protuberans, rhabdomyosarcoma, epithelioid angiosarcoma, and synovial sarcoma.
Figure 17a.
Variable appearances of abdominal wall sarcomas in six different patients. Compare the appearances of discrete palpable abdominal wall masses (a–c) to those of superficial ulcerative masses (d–f). (a) Axial CT image in a 38-year-old man shows a well-circumscribed small epithelioid sarcoma (arrow) in the subcutaneous fat (thought to be an inguinal hernia on examination). (b) Axial CT image in a 33-year-old man shows a leiomyosarcoma (arrows) deep in the underlying muscle of the abdomen (the patient also had a tumor in the right thigh [not shown]). (c) Axial CT image in a 21-year-old man shows a large rhabdomyosarcoma (arrow). The patient also had a tumor in the left thigh (not shown). (d) Axial CT image in a 24-year-old man shows a superficial dermatofibrosarcoma protuberans (arrow) that is not grossly ulcerative at imaging, but physical examination showed edematous skin and bleeding. (e) Axial CT image in a 71-year-old woman shows a superficial epithelioid angiosarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. (f) Axial CT image in a 62-year-old woman shows a deep and infiltrating synovial sarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. All diagnoses were confirmed by means of surgical resection or biopsy.
Figure 17d.
![Variable appearances of abdominal wall sarcomas in six different patients. Compare the appearances of discrete palpable abdominal wall masses (a–c) to those of superficial ulcerative masses (d–f). (a) Axial CT image in a 38-year-old man shows a well-circumscribed small epithelioid sarcoma (arrow) in the subcutaneous fat (thought to be an inguinal hernia on examination). (b) Axial CT image in a 33-year-old man shows a leiomyosarcoma (arrows) deep in the underlying muscle of the abdomen (the patient also had a tumor in the right thigh [not shown]). (c) Axial CT image in a 21-year-old man shows a large rhabdomyosarcoma (arrow). The patient also had a tumor in the left thigh (not shown). (d) Axial CT image in a 24-year-old man shows a superficial dermatofibrosarcoma protuberans (arrow) that is not grossly ulcerative at imaging, but physical examination showed edematous skin and bleeding. (e) Axial CT image in a 71-year-old woman shows a superficial epithelioid angiosarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. (f) Axial CT image in a 62-year-old woman shows a deep and infiltrating synovial sarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. All diagnoses were confirmed by means of surgical resection or biopsy.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/c4ec/7263290/518f74e7ab40/rg.2020190170.fig17d.jpg)
Variable appearances of abdominal wall sarcomas in six different patients. Compare the appearances of discrete palpable abdominal wall masses (a–c) to those of superficial ulcerative masses (d–f). (a) Axial CT image in a 38-year-old man shows a well-circumscribed small epithelioid sarcoma (arrow) in the subcutaneous fat (thought to be an inguinal hernia on examination). (b) Axial CT image in a 33-year-old man shows a leiomyosarcoma (arrows) deep in the underlying muscle of the abdomen (the patient also had a tumor in the right thigh [not shown]). (c) Axial CT image in a 21-year-old man shows a large rhabdomyosarcoma (arrow). The patient also had a tumor in the left thigh (not shown). (d) Axial CT image in a 24-year-old man shows a superficial dermatofibrosarcoma protuberans (arrow) that is not grossly ulcerative at imaging, but physical examination showed edematous skin and bleeding. (e) Axial CT image in a 71-year-old woman shows a superficial epithelioid angiosarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. (f) Axial CT image in a 62-year-old woman shows a deep and infiltrating synovial sarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. All diagnoses were confirmed by means of surgical resection or biopsy.
A leiomyosarcoma is composed of malignant tumor cells that resemble smooth muscle. Although leiomyosarcoma usually arises in the retroperitoneum and/or the inferior vena cava, it uncommonly originates in the soft tissues of the abdominal wall. Compared with retroperitoneal leiomyosarcoma, those that arise in the abdominal wall are usually small at the time of diagnosis, and thus often lack necrosis and hemorrhage, which are typical of large retroperitoneal leiomyosarcomas (50). However, larger leiomyosarcomas in the abdominal wall may demonstrate heterogeneous attenuation at CT, which reflects varying degrees of degeneration, necrosis, or hemorrhage, with enhancement of the solid portions (51) (Fig 17b). Leiomyosarcomas are heterogeneously hyperintense at T2-weighted MRI and isointense compared with skeletal muscle at T1-weighted MRI, with heterogeneous enhancement (52); however, these imaging features are not specific, and fatty components are characteristically absent.
Figure 17b.
Variable appearances of abdominal wall sarcomas in six different patients. Compare the appearances of discrete palpable abdominal wall masses (a–c) to those of superficial ulcerative masses (d–f). (a) Axial CT image in a 38-year-old man shows a well-circumscribed small epithelioid sarcoma (arrow) in the subcutaneous fat (thought to be an inguinal hernia on examination). (b) Axial CT image in a 33-year-old man shows a leiomyosarcoma (arrows) deep in the underlying muscle of the abdomen (the patient also had a tumor in the right thigh [not shown]). (c) Axial CT image in a 21-year-old man shows a large rhabdomyosarcoma (arrow). The patient also had a tumor in the left thigh (not shown). (d) Axial CT image in a 24-year-old man shows a superficial dermatofibrosarcoma protuberans (arrow) that is not grossly ulcerative at imaging, but physical examination showed edematous skin and bleeding. (e) Axial CT image in a 71-year-old woman shows a superficial epithelioid angiosarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. (f) Axial CT image in a 62-year-old woman shows a deep and infiltrating synovial sarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. All diagnoses were confirmed by means of surgical resection or biopsy.
Dermatofibrosarcoma protuberans is a locally aggressive neoplasm of the skin and superficial tissue that typically comes to clinical attention because of growth that results in a characteristic protuberance at physical examination. It is most frequently seen in young to middle-aged black women. Dermatofibrosarcoma protuberans has a high rate of local recurrence after simple resection (approximately 20%), while the use of Mohs microsurgery is associated with a less than 1% recurrence rate. Fibrosarcoma develops in 10%–15% of cases and can result in lung metastasis (53,54). At CT, dermatofibrosarcoma protuberans is a noncalcified soft-tissue–attenuation mass that involves the skin and subcutaneous fat and demonstrates variable degrees of contrast material enhancement (Fig 17e). Enhancement patterns of dermatofibrosarcoma protuberans tend to be homogeneous. One retrospective study (55) in which contrast-enhanced CT was used to image patients with histologically confirmed dermatofibrosarcoma protuberans found that most patients (80%) showed homogeneous enhancement, and the tumors were smaller than 5 cm. Heterogeneous enhancement in larger dermatofibrosarcoma protuberans is due to internal necrosis and cystic degeneration (55). Dermatofibrosarcoma protuberans is isointense or hyperintense at T1- and T2-weighted MRI and shows similar enhancement patterns to those found at CT (56).
Figure 17e.
Variable appearances of abdominal wall sarcomas in six different patients. Compare the appearances of discrete palpable abdominal wall masses (a–c) to those of superficial ulcerative masses (d–f). (a) Axial CT image in a 38-year-old man shows a well-circumscribed small epithelioid sarcoma (arrow) in the subcutaneous fat (thought to be an inguinal hernia on examination). (b) Axial CT image in a 33-year-old man shows a leiomyosarcoma (arrows) deep in the underlying muscle of the abdomen (the patient also had a tumor in the right thigh [not shown]). (c) Axial CT image in a 21-year-old man shows a large rhabdomyosarcoma (arrow). The patient also had a tumor in the left thigh (not shown). (d) Axial CT image in a 24-year-old man shows a superficial dermatofibrosarcoma protuberans (arrow) that is not grossly ulcerative at imaging, but physical examination showed edematous skin and bleeding. (e) Axial CT image in a 71-year-old woman shows a superficial epithelioid angiosarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. (f) Axial CT image in a 62-year-old woman shows a deep and infiltrating synovial sarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. All diagnoses were confirmed by means of surgical resection or biopsy.
Rhabdomyosarcoma is rarely seen in adults but is the most common soft-tissue sarcoma in children. It can occur in various sites throughout the body such as the head, neck, trunk, and extremities, including sites that lack normal striated muscle cells. MRI is the modality of choice for determining the site of origin and evaluating the extent of local disease and regional nodal involvement. The imaging appearance of rhabdomyosarcoma is similar to that of other soft-tissue sarcomas, which typically show low to intermediate signal intensity at T1-weighted MRI, high signal intensity at T2-weighted MRI, and heterogeneous contrast material enhancement (57). The CT appearance varies and is usually an enhancing soft-tissue–attenuation nodule or mass (Fig 17c).
Figure 17c.
Variable appearances of abdominal wall sarcomas in six different patients. Compare the appearances of discrete palpable abdominal wall masses (a–c) to those of superficial ulcerative masses (d–f). (a) Axial CT image in a 38-year-old man shows a well-circumscribed small epithelioid sarcoma (arrow) in the subcutaneous fat (thought to be an inguinal hernia on examination). (b) Axial CT image in a 33-year-old man shows a leiomyosarcoma (arrows) deep in the underlying muscle of the abdomen (the patient also had a tumor in the right thigh [not shown]). (c) Axial CT image in a 21-year-old man shows a large rhabdomyosarcoma (arrow). The patient also had a tumor in the left thigh (not shown). (d) Axial CT image in a 24-year-old man shows a superficial dermatofibrosarcoma protuberans (arrow) that is not grossly ulcerative at imaging, but physical examination showed edematous skin and bleeding. (e) Axial CT image in a 71-year-old woman shows a superficial epithelioid angiosarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. (f) Axial CT image in a 62-year-old woman shows a deep and infiltrating synovial sarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. All diagnoses were confirmed by means of surgical resection or biopsy.
Epithelioid angiosarcoma is a rare malignant tumor of endothelial origin. It most commonly manifests in the deep soft tissue of the extremities, but it may also be found in the skin, head, neck, lungs, breasts, adrenal glands, and bones (58). These tumors, similar to rhabdomyosarcomas, usually demonstrate early nodal and solid organ metastasis (59). CT and MRI are helpful for evaluation of the extent of disease and vascular involvement and differentiation of tumor recurrence from postoperative changes (Fig 17e).
Synovial sarcoma is a relatively common type of sarcoma that is usually seen in adolescents and young adults (60). Despite the name, the cell of origin is not clear, and this malignant neoplasm does not arise from the synovium. However, it is usually seen in close proximity to joint spaces. The most common CT appearance is a heterogeneous mass located in the deep soft tissues, with attenuation similar to or slightly lower than that of muscle (61). However, patients with advanced stages of synovial sarcoma may present with an ulcerative wound (Fig 17f). Calcifications may be present, and areas of lower attenuation representing necrosis or higher attenuation representing hemorrhage may be seen. Synovial sarcoma usually demonstrates heterogeneous enhancement, has signal intensity that is similar to or slightly higher than that of skeletal muscle at T1-weighted MRI, and has heterogeneous signal intensity at T2-weighted MRI with areas of hemorrhage, cystic regions, and septa (61).
Figure 17f.
Variable appearances of abdominal wall sarcomas in six different patients. Compare the appearances of discrete palpable abdominal wall masses (a–c) to those of superficial ulcerative masses (d–f). (a) Axial CT image in a 38-year-old man shows a well-circumscribed small epithelioid sarcoma (arrow) in the subcutaneous fat (thought to be an inguinal hernia on examination). (b) Axial CT image in a 33-year-old man shows a leiomyosarcoma (arrows) deep in the underlying muscle of the abdomen (the patient also had a tumor in the right thigh [not shown]). (c) Axial CT image in a 21-year-old man shows a large rhabdomyosarcoma (arrow). The patient also had a tumor in the left thigh (not shown). (d) Axial CT image in a 24-year-old man shows a superficial dermatofibrosarcoma protuberans (arrow) that is not grossly ulcerative at imaging, but physical examination showed edematous skin and bleeding. (e) Axial CT image in a 71-year-old woman shows a superficial epithelioid angiosarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. (f) Axial CT image in a 62-year-old woman shows a deep and infiltrating synovial sarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. All diagnoses were confirmed by means of surgical resection or biopsy.
Schwannomas
A schwannoma is a benign tumor of Schwann cells composed of Antoni A (cellular) and Antoni B (myxoid) components. The Antoni A portion of the schwannoma enhances after administration of intravenous contrast material. Ancient schwannomas are a subtype of classic schwannomas, with a predominance of degenerative changes including formation of cysts, calcification, hemosiderin deposition, interstitial fibrosis, and vascular hyaline degeneration (62). The anterior and anterolateral abdominal wall are uncommon locations for schwannomas. At CT, a schwannoma appears as a circumscribed mass with cystic spaces and a variable degree of enhancement that likely reflects the degree of cellularity (62). Schwannomas are typically hypointense at T1-weighted MRI and heterogeneously hyperintense at T2-weighted MRI, with enhancing solid components and nonenhancing cystic or necrotic components (Fig E8) (63).
Primary Cutaneous Lymphoma
Primary cutaneous lymphoma is extranodal non-Hodgkin lymphoma that is isolated to the skin at initial staging and accounts for up to 19% of primary extranodal forms of non-Hodgkin lymphoma. Both T-cell and B-cell lineages can manifest as primary cutaneous lymphoma. T-cell primary cutaneous lymphoma typically (ie, in up to 90% of patients) manifests as multiple skin lesions that may show diffuse skin thickening with or without ulceration and may be associated with mycosis fungoides or Sézary syndrome. Its clinical appearance can mimic cellulitis. In comparison, B-cell primary cutaneous lymphoma more commonly manifests as an isolated skin lesion (in approximately 50% of cases) and tends to develop in older individuals rather than in those with T-cell lineage. Both forms tend to be isolated to one area of the body. In one large single-center study (64) of 395 patients, B-cell primary cutaneous lymphoma most commonly manifested in the head and neck, whereas T-cell lineages affected the trunk.
One imaging study (65) of 41 patients was focused on the US appearance of primary cutaneous lymphoma, with PET/CT correlation, when available. In that study, the overall incidence of an abdominal or pelvic location was low (10%). B-cell primary cutaneous lymphoma more commonly manifests as a nodule or mass. US imaging shows dermal thickening, which primarily manifests as discrete nodules, organized plaques of matted tissue, or combinations of both. CT may allow delineation of the nodule or mass if it is large enough, especially nodules or masses of B-cell lineage (Fig 18). However, particularly in nodules or masses of T-cell lineage, CT may show nonspecific dermal thickening with subcentimeter granular or nodular opacities. FDG PET/CT almost always shows increased FDG avidity (66).
Figure 18a.
Primary cutaneous lymphoma in a 55-year-old man with no relevant medical history who presented with a palpable mass in the anterior abdominal wall. (a) Axial CT image shows an enhancing mass at the area of palpable concern (arrow). (b) Axial fused PET/CT image shows high FDG avidity (arrow). Pathologic results showed B-cell lymphoma.
Figure 18b.
Primary cutaneous lymphoma in a 55-year-old man with no relevant medical history who presented with a palpable mass in the anterior abdominal wall. (a) Axial CT image shows an enhancing mass at the area of palpable concern (arrow). (b) Axial fused PET/CT image shows high FDG avidity (arrow). Pathologic results showed B-cell lymphoma.
Diffuse Processes
Abdominal wall processes may manifest as multiple small nodules, masses, or calcifications in an anatomic region or diffusely throughout the soft tissues of the abdominal wall. In comparison with the algorithm-based approach to diagnosis of discrete abdominal wall masses, these diffuse processes have fewer distinguishing features. However, knowledge of the patient’s history may help to secure an accurate diagnosis (Fig 5).
Injection Granulomas
Injection granulomas represent localized fat necrosis, scar formation, and dystrophic calcification in response to injection of medication into the subcutaneous fat. Small calcified nodules in the ventral abdominal wall or gluteal region in a patient with a history of injection of medication in these locations is highly suggestive of the diagnosis. At CT, injection granulomas often appear as small nodules with soft-tissue attenuation in the subcutaneous fat of the anterior abdominal wall (Fig 19). Occasionally, small arterial branches or venous tributaries in the abdominal wall may be violated, resulting in a hematoma, or in rare cases, an active hemorrhage. Recently administered medications appear as regions of fluid attenuation or signal intensity with poorly defined margins, and small foci of gas may be present (8). The nodules are hypointense at T1-weighted MRI and variable at T2-weighted MRI (hyperintense if an inflammatory reaction predominates, hypointense if a fibrous reaction predominates).
Figure 19.
Injection granulomas in a 46-year-old bedridden woman who was administered daily subcutaneous injections of enoxaparin. CT image shows multiple injection granulomas (outlined area).
Focal areas of subcutaneous fat lipohypertrophy in the abdominal wall may occur at sites of subcutaneous insulin injection. This focal hypertrophic proliferation of fat is secondary to the localized anabolic effect of insulin. At imaging, abdominal wall lipohypertrophy manifests as local asymmetric distribution of the subcutaneous fat, which may be masslike. The typical location is adjacent to the umbilicus in a bilateral distribution (Fig 20). Infiltrative fat necrosis may be present in the fat proliferation (8).
Figure 20a.
Lipohypertrophy at insulin injection sites in a 42-year-old man with insulin-dependent diabetes mellitus after a pancreas transplant. Axial CT image below the umbilicus (a) and oblique sagittal maximum intensity projection CT image (b) show bilateral symmetric infraumbilical masslike protuberances (arrows in a; outlined square in b). These insulin injection sites show bilateral subcutaneous fat hypertrophy with central infiltrative soft-tissue attenuation. These are manifestations of abdominal wall lipohypertrophy at insulin injection sites.
Figure 20b.

Lipohypertrophy at insulin injection sites in a 42-year-old man with insulin-dependent diabetes mellitus after a pancreas transplant. Axial CT image below the umbilicus (a) and oblique sagittal maximum intensity projection CT image (b) show bilateral symmetric infraumbilical masslike protuberances (arrows in a; outlined square in b). These insulin injection sites show bilateral subcutaneous fat hypertrophy with central infiltrative soft-tissue attenuation. These are manifestations of abdominal wall lipohypertrophy at insulin injection sites.
Silicone injection for cosmetic purposes is typically performed for breast or gluteal augmentation. These injections are often performed in nonsterile environments and are frequently complicated by infection, including abscesses, cellulitis, and mycobacterial infection. Illegal silicone injections are highly associated with HIV-positive status (67). At imaging, they appear as fine to large granular nodules or masses (Fig 21). Often they are encountered incidentally and mistaken for other diffuse processes, if the appropriate history is not known by the ordering clinician or interpreting radiologist (68).
Figure 21.
Gluteal augmentation with silicone injections in a 41-year-old woman. Axial contrast-enhanced CT image shows diffuse soft-tissue attenuation and subcentimeter nodules around the subcutaneous fat overlying the buttocks bilaterally.
Soft-Tissue Calcifications
Diffuse soft-tissue calcifications are often due to increased circulating calcium-phosphorous products or systemic processes that lead to deposition of calcium products in soft tissue with a normal serum calcium level. When diffuse or bizarre calcifications are encountered at imaging in the abdomen and pelvis (they are most apparent at CT), the cause can often be ascertained on the basis of patients’ comorbidities and laboratory values. Metastatic calcifications refer to soft-tissue calcifications with increased circulating calcium and phosphorous products, and dystrophic calcifications refer to soft-tissue calcifications in patients with normal circulating calcium and phosphorous products (7). At imaging, soft-tissue calcifications manifest predominantly in the periarticular soft tissue around large joints such as the hips (69,70).
Collagen vascular diseases fall under the classification of dystrophic calcifications and occur in areas of subcutaneous necrosis. Calcinosis universalis is the main subtype of dystrophic calcification that is seen in collagen vascular diseases and manifests at imaging as sheetlike deposits of calcification in subcutaneous tissue and fascial planes and with intramuscular distribution (Fig E9). Although this pattern, which is most associated with dermatomyositis, is more common in the extremities, it is the most likely type of diffuse calcification in the abdomen and pelvis (7).
Posttransplant Lymphoproliferative Disorder
Posttransplant lymphoproliferative disorder is a complication after solid organ transplantation and most commonly manifests as abnormalities in intra-abdominal extranodal sites but may rarely manifest as subcutaneous or intramuscular nodes or masses. The mechanism of posttransplant lymphoproliferative disorder may be due to either Epstein-Barr virus–mediated polyclonal proliferation that resembles infectious mononucleosis or aggressive monomorphic proliferation that resembles lymphoma (71). Lung and small bowel transplants are the two most common precipitating surgical procedures, with an incidence of posttransplant lymphoproliferative disorder of 5% and 20%, respectively. At imaging, posttransplant lymphoproliferative disorder appears as multiple soft-tissue masses (Fig 22). In one cohort of 51 patients with histologically confirmed posttransplant lymphoproliferative disorder with 36 abnormalities at CT of the abdomen and pelvis, 8% of cases showed disease manifestation in the abdominal wall (9).
Figure 22.
Posttransplant lymphoproliferative disorder in a 54-year-old man after kidney transplant. Axial CT image shows multiple supraumbilical abdominal wall nodules (arrows). Atrophic native kidneys are also noted. Biopsy confirmed the diagnosis of posttransplant lymphoproliferative disorder.
Pancreatic Panniculitis
Pancreatic panniculitis is a rare manifestation of subcutaneous fat necrosis that is associated with underlying pancreatic adenocarcinoma or pancreatitis, which affects less than 1% of patients with underlying pancreatic disease. These subcutaneous nodules may be painful or tender on palpation. If they are associated with acute pancreatitis, they may precede, develop simultaneous to, or follow the episode of pancreatitis. The underlying mechanism of pancreatic panniculitis is related to an individual’s reaction to systemically elevated serum lipase levels. Skin biopsy is required for diagnosis and is characterized by ghostlike fat cells with a thick wall and no nucleus. Even rarer is pancreatic panniculitis paired with polyarthritis (10,72).
To our knowledge, the imaging findings of pancreatic panniculitis have not been well characterized in large studies, but scattered case reports appear in the radiology literature (10,73). They manifest as hyperattenuating small subcutaneous fat nodules at CT and may manifest as hyperechoic small nodules at US (Fig 23). Subcutaneous fat necrosis may manifest in the extremities or the abdominal wall, either in isolation or in tandem. When pancreatic panniculitis is associated with polyarthritis, MRI of the joint shows intraosseous fat necrosis, which may not necessarily be periarticular. Nonspecific findings in the joint may include synovitis and joint effusions (10,72).
Figure 23.
Pancreatic panniculitis in a 46-year-old man with prior episodes of pancreatitis. Axial CT image shows multiple areas of small subcutaneous nodules (boxes) in the abdominal wall and an area of fat necrosis (arrow). A conglomerate of these nodules was sampled with US-guided biopsy (not shown), which yielded a diagnosis of pancreatic panniculitis.
Kaposi Sarcoma
Kaposi sarcoma is a low-grade vascular neoplasm that most commonly affects the skin, oral mucosa, and thoracic and abdominal viscera. There are four recognized subtypes, with the most common being related to HIV/AIDS, where it has up to a 50% estimated lifetime incidence. Additional subtypes are related to the classic sporadic cases, endemic cases in Eastern and Central Africa, and organ transplant–related disease. Its pathogenesis is not completely understood but has a clear relationship to human herpesvirus type 8 (73). At imaging, Kaposi sarcoma manifests cutaneously as nodules or diffuse infiltration of the skin and subcutaneous fat. If it manifests as skin nodules, they are clinically apparent and may be large (Fig E10). Chest wall manifestations are much more common than is abdominal wall involvement (73).
Melanoma
Melanoma is the fifth most common new cancer diagnosis in men and women in the United States (74). Imaging has a limited role in diagnosis of cutaneous melanoma, although imaging with PET/CT, PET/MRI, or CT has a role in staging or surveillance of patients at follow-up. Although a solitary abdominal wall primary cutaneous melanoma or isolated metastases do occur, the most frequent manifestation is multiple subcentimeter to small (approximately 2.5 cm) subcutaneous nodules. At CT, these multiple subcutaneous nodules tend to be isoattenuating in comparison to skeletal muscle, with homogeneous enhancement (75) (Fig E11). At US, subcutaneous melanoma tends to be homogeneously hypoechoic, with increased through transmission, although this appearance varies (76). In larger nodules or masses, the increased through transmission at US may cause the interpreting radiologist to confuse the finding with a cystic mass or fluid collection. The MRI appearance manifests as intrinsically T1-hyperintense nodules. Occasionally, these subcutaneous nodules undergo internal necrosis, which varies their typical appearance (75,76).
Conclusion
Abdominal wall masses are categorized on the basis of their composition, and imaging is supplemented with patient history to arrive at the correct diagnosis. The most common abdominal wall masses include desmoid tumors, lipomas, metastases, endometriomas, and sarcomas. Lipomas have fat composition and usually are not a diagnostic challenge, whereas diagnosis of metastases and endometriomas often relies on patient history. Desmoid tumors and sarcomas have similar imaging features. Although desmoid tumors may have locally infiltrative borders, sarcomas may demonstrate local invasion that violates fascial planes and borders. The imaging appearances of the four most common solid abdominal wall masses are summarized in the Table.
US, CT, and MRI Features of the Four Most Common Solid Abdominal Wall Masses
In comparison to the algorithm-based approach that includes the lesional composition of abdominal wall masses and masslike processes, diagnosis of diffuse abdominal wall processes often requires knowledge of and correlation with pertinent patient history and presentation. However, some diffuse processes have characteristic imaging appearances, such as diffuse soft-tissue calcifications. Knowledge of these common and uncommon abdominal wall manifestations helps radiologists to provide accurate interpretations when these processes are profiled with imaging or are incidentally encountered.
SUPPLEMENTAL FIGURES
Supported by the National Institutes of Health top-tier grant T32-EB021955. D.H.B. supported by the National Institutes of Health top-tier grant T32-EB021955.
Recipient of a Magna Cum Laude award for an education exhibit at the 2018 RSNA Annual Meeting.
For this journal-based SA-CME activity, the authors D.C.O., M.L., and P.J.P have provided disclosures; all other authors, the editor, and the reviewers have disclosed no relevant relationships.
Disclosures of Conflicts of Interest.—: D.C.O. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: consultancy for VisualDx. Other activities: disclosed no relevant relationships. M.L. Activities related to the present article: grants from Philips and Ethicon. Activities not related to the present article: disclosed no relevant relationships. Other activities: disclosed no relevant relationships. P.J.P. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: consultancy for Bracco and stock/stock options for Cellectar, Elucent, and Shine. Other activities: disclosed no relevant relationships.
Abbreviations:
- ACR
- American College of Radiology
- FDG
- fluorine 18 fluorodeoxyglucose
References
- 1.Bashir U, Moskovic E, Strauss D, et al. Soft-tissue masses in the abdominal wall. Clin Radiol 2014;69(10): e422–e431. [DOI] [PubMed] [Google Scholar]
- 2.Stojadinovic A, Hoos A, Karpoff HM, et al. Soft tissue tumors of the abdominal wall: analysis of disease patterns and treatment. Arch Surg 2001;136(1):70–79. [DOI] [PubMed] [Google Scholar]
- 3.Busard MPH, Mijatovic V, van Kuijk C, Hompes PGA, van Waesberghe JHTM. Appearance of abdominal wall endometriosis on MR imaging. Eur Radiol 2010;20(5): 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berná JD, Garcia-Medina V, Guirao J, Garcia-Medina J. Rectus sheath hematoma: diagnostic classification by CT. Abdom Imaging 1996;21(1):62–64. [DOI] [PubMed] [Google Scholar]
- 5.Clark SK, Phillips RK. Desmoids in familial adenomatous polyposis. Br J Surg 1996;83(11):1494–1504. [DOI] [PubMed] [Google Scholar]
- 6.Davis BS, Dunn DP, Hostetler VC. Beyond hernias: a multimodality review of abdominal wall pathology. Br J Radiol 2017;90(1069):20160719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalinka MK, Melchior EL. Soft tissue calcifications in systemic disease. Bull N Y Acad Med 1980;56(6):539–563. [PMC free article] [PubMed] [Google Scholar]
- 8.Gayer G, Park C. Abdominal Wall Masses: CT Findings and Clues to Differential Diagnosis. Semin Ultrasound CT MR 2018;39(2):230–246. [DOI] [PubMed] [Google Scholar]
- 9.Pickhardt PJ, Siegel MJ. Posttransplantation lymphoproliferative disorder of the abdomen: CT evaluation in 51 patients. Radiology 1999;213(1):73–78. [DOI] [PubMed] [Google Scholar]
- 10.Kang DJ, Lee SJ, Choo HJ, Her M, Yoon HK. Pancreatitis, panniculitis, and polyarthritis (PPP) syndrome: MRI features of intraosseous fat necrosis involving the feet and knees. Skeletal Radiol 2017;46(2):279–285. [DOI] [PubMed] [Google Scholar]
- 11.Turnage RH, Badgwell B. Abdominal Wall, Umbilicus, Peritoneum, Mesenteries, Omentum, and Retroperitoneum. In: Townsend C, Beauchamp R, Evers B, Mattox K, eds. Sabiston Textbook of Surgery. 19th ed. Philadelphia, Pa: Elsevier Saunders, 2012. [Google Scholar]
- 12.Moore K, Dalley A. Clinically oriented anatomy. 7th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 1980; 178–191. [Google Scholar]
- 13.Cabarrus MC, Yeh BM, Phelps AS, Ou JJ, Behr SC. From Inguinal Hernias to Spermatic Cord Lipomas: Pearls, Pitfalls, and Mimics of Abdominal and Pelvic Hernias. RadioGraphics 2017;37(7):2063–2082. [DOI] [PubMed] [Google Scholar]
- 14.Matalon SA, Askari R, Gates JD, Patel K, Sodickson AD, Khurana B. Don’t Forget the Abdominal Wall: Imaging Spectrum of Abdominal Wall Injuries after Nonpenetrating Trauma. RadioGraphics 2017;37(4):1218–1235. [DOI] [PubMed] [Google Scholar]
- 15.Abu-Hijleh MF, Roshier AL, Al-Shboul Q, Dharap AS, Harris PF. The membranous layer of superficial fascia: evidence for its widespread distribution in the body. Surg Radiol Anat 2006;28(6):606–619. [DOI] [PubMed] [Google Scholar]
- 16.Harley OJH, Pickford MA. CT analysis of fat distribution superficial and deep to the Scarpa’s fascial layer in the mid and lower abdomen. J Plast Reconstr Aesthet Surg 2013;66(4):525–530. [DOI] [PubMed] [Google Scholar]
- 17.Expert Panel on Gastrointestinal Imaging , Fowler KJ, Garcia EM, et al. ACR Appropriateness Criteria Palpable Abdominal Mass-Suspected Neoplasm. J Am Coll Radiol 2019;16(11S):S384–S391. [DOI] [PubMed] [Google Scholar]
- 18.Expert Panel on Musculoskeletal Imaging , Kransdorf MJ, Murphey MD, et al. ACR Appropriateness Criteria Soft-Tissue Masses. J Am Coll Radiol 2018;15(5S):S189–S197. [DOI] [PubMed] [Google Scholar]
- 19.Gokhale S. Sonography in identification of abdominal wall lesions presenting as palpable masses. J Ultrasound Med 2006;25(9):1199–1209. [DOI] [PubMed] [Google Scholar]
- 20.Poulose BK, Shelton J, Phillips S, et al. Epidemiology and cost of ventral hernia repair: making the case for hernia research. Hernia 2012;16(2):179–183. [DOI] [PubMed] [Google Scholar]
- 21.Loftus TJ, Go KL, Jordan JR, et al. Computed tomography evidence of fluid in the hernia sac predicts surgical site infection following mesh repair of acutely incarcerated ventral and groin hernias. J Trauma Acute Care Surg 2017;83(1):170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prasad SR, Wang H, Rosas H, et al. Fat-containing lesions of the liver: radiologic-pathologic correlation. RadioGraphics 2005;25(2):321–331. [DOI] [PubMed] [Google Scholar]
- 23.Pereira JM, Sirlin CB, Pinto PS, Casola G. CT and MR imaging of extrahepatic fatty masses of the abdomen and pelvis: techniques, diagnosis, differential diagnosis, and pitfalls. RadioGraphics 2005;25(1):69–85. [DOI] [PubMed] [Google Scholar]
- 24.Virmani V, Sethi V, Fasih N, Ryan J, Kielar A. The abdominal wall lumps and bumps: cross-sectional imaging spectrum. Can Assoc Radiol J 2014;65(1):9–18. [DOI] [PubMed] [Google Scholar]
- 25.Murphey MD, Carroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of the AFIP: benign musculoskeletal lipomatous lesions. RadioGraphics 2004;24(5):1433–1466. [DOI] [PubMed] [Google Scholar]
- 26.Kransdorf MJ, Bancroft LW, Peterson JJ, Murphey MD, Foster WC, Temple HT. Imaging of fatty tumors: distinction of lipoma and well-differentiated liposarcoma. Radiology 2002;224(1):99–104. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen GP, Mandahl N. Adipocytic tumors. In: Christopher D, Unni K, Mertens F, eds. World Health Organization classification of tumors. Pathology and genetics: tumors of soft tissue and bone. Lyon, France: IARC, 2002; 19–46. [Google Scholar]
- 28.Sung MS, Kang HS, Suh JS, et al. Myxoid liposarcoma: appearance at MR imaging with histologic correlation. RadioGraphics 2000;20(4):1007–1019. [DOI] [PubMed] [Google Scholar]
- 29.Gupta P, Potti TA, Wuertzer SD, Lenchik L, Pacholke DA. Spectrum of Fat-containing Soft-Tissue Masses at MR Imaging: The Common, the Uncommon, the Characteristic, and the Sometimes Confusing. RadioGraphics 2016;36(3):753–766. [DOI] [PubMed] [Google Scholar]
- 30.Merrow AC, Gupta A, Patel MN, Adams DM. 2014 Revised Classification of Vascular Lesions from the International Society for the Study of Vascular Anomalies: Radiologic-Pathologic Update. RadioGraphics 2016;36(5):1494–1516. [DOI] [PubMed] [Google Scholar]
- 31.Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore) 2006;85(2):105–110. [DOI] [PubMed] [Google Scholar]
- 32.Kim HK, Kim SM, Lee SH, Racadio JM, Shin MJ. Subcutaneous epidermal inclusion cysts: ultrasound (US) and MR imaging findings. Skeletal Radiol 2011;40(11):1415–1419. [DOI] [PubMed] [Google Scholar]
- 33.Hong SH, Chung HW, Choi JY, Koh YH, Choi JA, Kang HS. MRI findings of subcutaneous epidermal cysts: emphasis on the presence of rupture. AJR Am J Roentgenol 2006;186(4):961–966. [DOI] [PubMed] [Google Scholar]
- 34.Murphey MD, McRae GA, Fanburg-Smith JC, Temple HT, Levine AM, Aboulafia AJ. Imaging of soft-tissue myxoma with emphasis on CT and MR and comparison of radiologic and pathologic findings. Radiology 2002;225(1):215–224. [DOI] [PubMed] [Google Scholar]
- 35.Tso S, Brockley J, Recica H, Ilchyshyn A. Sister Mary Joseph’s nodule: an unusual but important physical finding characteristic of widespread internal malignancy. Br J Gen Pract 2013;63(615):551–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleshman J, Sargent DJ, Green E, et al. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg 2007;246(4):655–662; discussion 662–664. [DOI] [PubMed] [Google Scholar]
- 37.Moon HS, Koo JS, Park SH, Park GS, Choi JG, Kim SG. Parasitic leiomyoma in the abdominal wall after laparoscopic myomectomy. Fertil Steril 2008;90(4):1201.e1–1201.e2. [DOI] [PubMed] [Google Scholar]
- 38.Yeh CJ, Chuang WY, Kuo TT. Unusual subcutaneous splenosis occurring in a gunshot wound scar: pathology and immunohistochemical identification. Pathol Int 2006;56(6):336–339. [DOI] [PubMed] [Google Scholar]
- 39.Lake ST, Johnson PT, Kawamoto S, Hruban RH, Fishman EK. CT of splenosis: patterns and pitfalls. AJR Am J Roentgenol 2012;199(6):W686–W693. [DOI] [PubMed] [Google Scholar]
- 40.Nayak L, Menias CO, Gayer G. Dropped gallstones: spectrum of imaging findings, complications and diagnostic pitfalls. Br J Radiol 2013;86(1028):20120588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rettenbacher T, Macheiner P, Hollerweger A, Gritzmann N, Weismann C, Todoroff B. Suture granulomas: sonography enables a correct preoperative diagnosis. Ultrasound Med Biol 2001;27(3):343–350. [DOI] [PubMed] [Google Scholar]
- 42.Papavramidis TS, Sapalidis K, Michalopoulos N, Karayannopoulou G, Cheva A, Papavramidis ST. Umbilical endosalpingiosis: a case report. J Med Case Reports 2010;4(1):287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen W, Jia JW, Wang JR. Soft tissue diffuse neurofibromas: sonographic findings. J Ultrasound Med 2007;26(4):513–518. [DOI] [PubMed] [Google Scholar]
- 44.Bhargava R, Parham DM, Lasater OE, Chari RS, Chen G, Fletcher BD. MR imaging differentiation of benign and malignant peripheral nerve sheath tumors: use of the target sign. Pediatr Radiol 1997;27(2):124–129. [DOI] [PubMed] [Google Scholar]
- 45.King AA, Debaun MR, Riccardi VM, Gutmann DH. Malignant peripheral nerve sheath tumors in neurofibromatosis 1. Am J Med Genet 2000;93(5):388–392. [PubMed] [Google Scholar]
- 46.Levy AD, Patel N, Dow N, Abbott RM, Miettinen M, Sobin LH. From the archives of the AFIP: abdominal neoplasms in patients with neurofibromatosis type 1–radiologic-pathologic correlation. RadioGraphics 2005;25(2):455–480. [DOI] [PubMed] [Google Scholar]
- 47.Kasper B, Ströbel P, Hohenberger P. Desmoid tumors: clinical features and treatment options for advanced disease. Oncologist 2011;16(5):682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu H, Koo HJ, Lim S, et al. Desmoid-Type Fibromatosis of the Thorax: CT, MRI, and FDG PET Characteristics in a Large Series From a Tertiary Referral Center. Medicine (Baltimore) 2015;94(38):e1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JC, Thomas JM, Phillips S, Fisher C, Moskovic E. Aggressive fibromatosis: MRI features with pathologic correlation. AJR Am J Roentgenol 2006;186(1):247–254. [DOI] [PubMed] [Google Scholar]
- 50.Levy AD, Manning MA, Al-Refaie WB, Miettinen MM. Soft-Tissue Sarcomas of the Abdomen and Pelvis: Radiologic-Pathologic Features, Part 1: Common Sarcomas–From the Radiologic Pathology Archives. RadioGraphics 2017;37(2):462–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lane RH, Stephens DH, Reiman HM. Primary retroperitoneal neoplasms: CT findings in 90 cases with clinical and pathologic correlation. AJR Am J Roentgenol 1989;152(1):83–89. [DOI] [PubMed] [Google Scholar]
- 52.O’Sullivan PJ, Harris AC, Munk PL. Radiological imaging features of non-uterine leiomyosarcoma. Br J Radiol 2008;81(961):73–81. [DOI] [PubMed] [Google Scholar]
- 53.Kreicher KL, Kurlander DE, Gittleman HR, Barnholtz-Sloan JS, Bordeaux JS. Incidence and Survival of Primary Dermatofibrosarcoma Protuberans in the United States. Dermatol Surg 2016;42(Suppl 1):S24–S31. [DOI] [PubMed] [Google Scholar]
- 54.Llombart B, Serra-Guillén C, Monteagudo C, López Guerrero JA, Sanmartín O. Dermatofibrosarcoma protuberans: a comprehensive review and update on diagnosis and management. Semin Diagn Pathol 2013;30(1):13–28. [DOI] [PubMed] [Google Scholar]
- 55.Li X, Zhang W, Xiao L, et al. Computed tomographic and pathological findings of dermatofibrosarcoma protuberans. J Comput Assist Tomogr 2012;36(4):462–468. [DOI] [PubMed] [Google Scholar]
- 56.Zhang L, Liu QY, Cao Y, Zhong JS, Zhang WD. Dermatofibrosarcoma Protuberans: Computed Tomography and Magnetic Resonance Imaging Findings. Medicine 2015;94(24):e1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allen SD, Moskovic EC, Fisher C, Thomas JM. Adult rhabdomyosarcoma: cross-sectional imaging findings including histopathologic correlation. AJR Am J Roentgenol 2007;189(2):371–377. [DOI] [PubMed] [Google Scholar]
- 58.Deshpande V, Rosenberg AE, O’Connell JX, Nielsen GP. Epithelioid angiosarcoma of the bone: a series of 10 cases. Am J Surg Pathol 2003;27(6):709–716. [DOI] [PubMed] [Google Scholar]
- 59.Hart J, Mandavilli S. Epithelioid angiosarcoma: a brief diagnostic review and differential diagnosis. Arch Pathol Lab Med 2011;135(2):268–272. [DOI] [PubMed] [Google Scholar]
- 60.Kransdorf MJ. Malignant soft-tissue tumors in a large referral population: distribution of diagnoses by age, sex, and location. AJR Am J Roentgenol 1995;164(1):129–134. [DOI] [PubMed] [Google Scholar]
- 61.Murphey MD, Gibson MS, Jennings BT, Crespo-Rodríguez AM, Fanburg-Smith J, Gajewski DA. From the archives of the AFIP: Imaging of synovial sarcoma with radiologic-pathologic correlation. RadioGraphics 2006;26(5):1543–1565. [DOI] [PubMed] [Google Scholar]
- 62.Cohen LM, Schwartz AM, Rockoff SD. Benign schwannomas: pathologic basis for CT inhomogeneities. AJR Am J Roentgenol 1986;147(1):141–143. [DOI] [PubMed] [Google Scholar]
- 63.Kim SH, Choi BI, Han MC, Kim YI. Retroperitoneal neurilemoma: CT and MR findings. AJR Am J Roentgenol 1992;159(5):1023–1026. [DOI] [PubMed] [Google Scholar]
- 64.Lee WJ, Lee SH, Moon IJ, et al. Relative Frequency, Clinical Features, and Survival Outcomes of 395 Patients with Cutaneous Lymphoma in Korea: A Subgroup Analysis per 10-year Period. Acta Derm Venereol 2016;96(7):888–893. [DOI] [PubMed] [Google Scholar]
- 65.Mandava A, Koppula V, Wortsman X, Catalano O, Alfageme F. The clinical value of imaging in primary cutaneous lymphomas: role of high resolution ultrasound and PET-CT. Br J Radiol 2019;92(1095):20180904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Otero HJ, Jagannathan JP, Prevedello LM, et al. CT and PET/CT findings of T-cell lymphoma. AJR Am J Roentgenol 2009;193(2):349–358. [DOI] [PubMed] [Google Scholar]
- 67.Bertin C, Abbas R, Andrieu V, et al. Illicit massive silicone injections always induce chronic and definitive silicone blood diffusion with dermatologic complications. Medicine 2019;98(4):e14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin DJ, Wong TT, Ciavarra GA, Kazam JK. Adventures and Misadventures in Plastic Surgery and Soft-Tissue Implants. RadioGraphics 2017;37(7):2145–2163. [DOI] [PubMed] [Google Scholar]
- 69.Steinbach LS, Johnston JO, Tepper EF, Honda GD, Martel W. Tumoral calcinosis: radiologic-pathologic correlation. Skeletal Radiol 1995;24(8):573–578. [DOI] [PubMed] [Google Scholar]
- 70.Stewart VL, Herling P, Dalinka MK. Calcification in soft tissues. JAMA 1983;250(1):78–81. [PubMed] [Google Scholar]
- 71.Bakker NA, van Imhoff GW, Verschuuren EAM, van Son WJ. Presentation and early detection of post-transplant lymphoproliferative disorder after solid organ transplantation. Transpl Int 2007;20(3):207–218. [DOI] [PubMed] [Google Scholar]
- 72.Narváez J, Bianchi MM, Santo P, et al. Pancreatitis, panniculitis, and polyarthritis. Semin Arthritis Rheum 2010;39(5):417–423. [DOI] [PubMed] [Google Scholar]
- 73.Restrepo CS, Martínez S, Lemos JA, et al. Imaging manifestations of Kaposi sarcoma. RadioGraphics 2006;26(4):1169–1185. [DOI] [PubMed] [Google Scholar]
- 74.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 75.Juan YH, Saboo SS, Tirumani SH, et al. Malignant skin and subcutaneous neoplasms in adults: multimodality imaging with CT, MRI, and 18F-FDG PET/CT. AJR Am J Roentgenol 2014;202(5):W422–W438. [DOI] [PubMed] [Google Scholar]
- 76.Nazarian LN, Alexander AA, Kurtz AB, et al. Superficial melanoma metastases: appearances on gray-scale and color Doppler sonography. AJR Am J Roentgenol 1998;170(2):459–463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.

















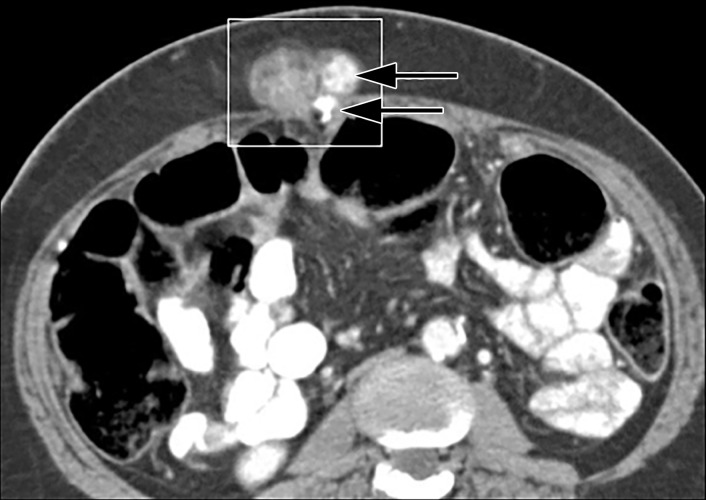








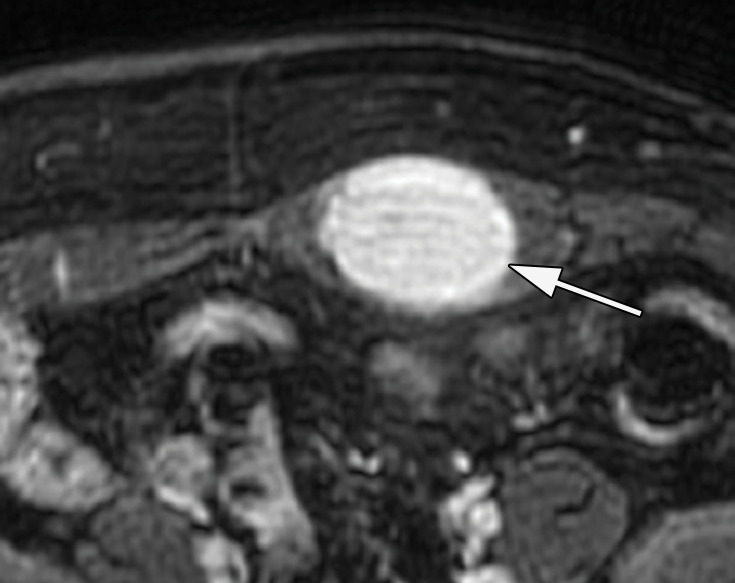


![Variable appearances of abdominal wall sarcomas in six different patients. Compare the appearances of discrete palpable abdominal wall masses (a–c) to those of superficial ulcerative masses (d–f). (a) Axial CT image in a 38-year-old man shows a well-circumscribed small epithelioid sarcoma (arrow) in the subcutaneous fat (thought to be an inguinal hernia on examination). (b) Axial CT image in a 33-year-old man shows a leiomyosarcoma (arrows) deep in the underlying muscle of the abdomen (the patient also had a tumor in the right thigh [not shown]). (c) Axial CT image in a 21-year-old man shows a large rhabdomyosarcoma (arrow). The patient also had a tumor in the left thigh (not shown). (d) Axial CT image in a 24-year-old man shows a superficial dermatofibrosarcoma protuberans (arrow) that is not grossly ulcerative at imaging, but physical examination showed edematous skin and bleeding. (e) Axial CT image in a 71-year-old woman shows a superficial epithelioid angiosarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. (f) Axial CT image in a 62-year-old woman shows a deep and infiltrating synovial sarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. All diagnoses were confirmed by means of surgical resection or biopsy.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/c4ec/7263290/72bc2c2ce8cb/rg.2020190170.fig17a.jpg)
![Variable appearances of abdominal wall sarcomas in six different patients. Compare the appearances of discrete palpable abdominal wall masses (a–c) to those of superficial ulcerative masses (d–f). (a) Axial CT image in a 38-year-old man shows a well-circumscribed small epithelioid sarcoma (arrow) in the subcutaneous fat (thought to be an inguinal hernia on examination). (b) Axial CT image in a 33-year-old man shows a leiomyosarcoma (arrows) deep in the underlying muscle of the abdomen (the patient also had a tumor in the right thigh [not shown]). (c) Axial CT image in a 21-year-old man shows a large rhabdomyosarcoma (arrow). The patient also had a tumor in the left thigh (not shown). (d) Axial CT image in a 24-year-old man shows a superficial dermatofibrosarcoma protuberans (arrow) that is not grossly ulcerative at imaging, but physical examination showed edematous skin and bleeding. (e) Axial CT image in a 71-year-old woman shows a superficial epithelioid angiosarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. (f) Axial CT image in a 62-year-old woman shows a deep and infiltrating synovial sarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. All diagnoses were confirmed by means of surgical resection or biopsy.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/c4ec/7263290/f4c5d0e6dea9/rg.2020190170.fig17b.jpg)
![Variable appearances of abdominal wall sarcomas in six different patients. Compare the appearances of discrete palpable abdominal wall masses (a–c) to those of superficial ulcerative masses (d–f). (a) Axial CT image in a 38-year-old man shows a well-circumscribed small epithelioid sarcoma (arrow) in the subcutaneous fat (thought to be an inguinal hernia on examination). (b) Axial CT image in a 33-year-old man shows a leiomyosarcoma (arrows) deep in the underlying muscle of the abdomen (the patient also had a tumor in the right thigh [not shown]). (c) Axial CT image in a 21-year-old man shows a large rhabdomyosarcoma (arrow). The patient also had a tumor in the left thigh (not shown). (d) Axial CT image in a 24-year-old man shows a superficial dermatofibrosarcoma protuberans (arrow) that is not grossly ulcerative at imaging, but physical examination showed edematous skin and bleeding. (e) Axial CT image in a 71-year-old woman shows a superficial epithelioid angiosarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. (f) Axial CT image in a 62-year-old woman shows a deep and infiltrating synovial sarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. All diagnoses were confirmed by means of surgical resection or biopsy.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/c4ec/7263290/bf38a7793228/rg.2020190170.fig17e.jpg)
![Variable appearances of abdominal wall sarcomas in six different patients. Compare the appearances of discrete palpable abdominal wall masses (a–c) to those of superficial ulcerative masses (d–f). (a) Axial CT image in a 38-year-old man shows a well-circumscribed small epithelioid sarcoma (arrow) in the subcutaneous fat (thought to be an inguinal hernia on examination). (b) Axial CT image in a 33-year-old man shows a leiomyosarcoma (arrows) deep in the underlying muscle of the abdomen (the patient also had a tumor in the right thigh [not shown]). (c) Axial CT image in a 21-year-old man shows a large rhabdomyosarcoma (arrow). The patient also had a tumor in the left thigh (not shown). (d) Axial CT image in a 24-year-old man shows a superficial dermatofibrosarcoma protuberans (arrow) that is not grossly ulcerative at imaging, but physical examination showed edematous skin and bleeding. (e) Axial CT image in a 71-year-old woman shows a superficial epithelioid angiosarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. (f) Axial CT image in a 62-year-old woman shows a deep and infiltrating synovial sarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. All diagnoses were confirmed by means of surgical resection or biopsy.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/c4ec/7263290/d1ff6988571d/rg.2020190170.fig17c.jpg)
![Variable appearances of abdominal wall sarcomas in six different patients. Compare the appearances of discrete palpable abdominal wall masses (a–c) to those of superficial ulcerative masses (d–f). (a) Axial CT image in a 38-year-old man shows a well-circumscribed small epithelioid sarcoma (arrow) in the subcutaneous fat (thought to be an inguinal hernia on examination). (b) Axial CT image in a 33-year-old man shows a leiomyosarcoma (arrows) deep in the underlying muscle of the abdomen (the patient also had a tumor in the right thigh [not shown]). (c) Axial CT image in a 21-year-old man shows a large rhabdomyosarcoma (arrow). The patient also had a tumor in the left thigh (not shown). (d) Axial CT image in a 24-year-old man shows a superficial dermatofibrosarcoma protuberans (arrow) that is not grossly ulcerative at imaging, but physical examination showed edematous skin and bleeding. (e) Axial CT image in a 71-year-old woman shows a superficial epithelioid angiosarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. (f) Axial CT image in a 62-year-old woman shows a deep and infiltrating synovial sarcoma (arrows) that manifested as a neglected ulcerative wound at presentation. All diagnoses were confirmed by means of surgical resection or biopsy.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/c4ec/7263290/0177a3b40224/rg.2020190170.fig17f.jpg)







