Abstract
Objective.
Although neck impairment has been described following surgical resection, limited studies have investigated its prevalence in nonsurgical treatment. The purpose of this study is to determine the prevalence and predictors of neck disability following head and neck cancer (HNC) treatment and to explore its association with quality of life (QOL).
Study Design.
Cross-sectional study.
Setting.
HNC survivorship clinic.
Subjects and Methods.
We identified 214 survivors who completed treatment ≥1 year prior to evaluation in the clinic. Self-reported neck impairment was measured using the Neck Disability Index. QOL was measured using the University of Washington QOL Questionnaire, with physical and social subscale scores calculated. Regression analysis and trend tests were employed to explore associations.
Results.
Over half of survivors (54.2%) reported neck disability. The odds of neck disability in survivors who received nonsurgical treatment and those who received surgery plus adjuvant treatment were 3.46 and 4.98 times higher compared to surgery alone (P = .008, P = .004). Survivors who underwent surgery only had higher physical and social QOL than those who received nonsurgical treatment (physical QOL: P < .001, social QOL: P = .023) and those who received surgery plus adjuvant treatment (physical QOL: P <.001, social QOL: P = .039).
Conclusion.
This study revealed a high prevalence of neck disability following nonsurgical treatment. While neck disability is an established sequela of surgical resection, the impact of nonsurgical treatment has gone unrecognized. Early identification and intervention to prevent progression of neck disability are crucial to optimize QOL.
Keywords: head and neck cancer, survivorship, neck disability, quality of life
The treatment of tumors in the head and neck has substantial consequences on critical physiologic functions, physical appearance, social interactions, and psychological well-being. Recent advances in head and neck cancer (HNC) therapies such as modified and selective neck dissections, organ-preserving laser surgery, microvascular flap reconstruction, and intensity-modulated radiation therapy (IMRT) reflect an increasing emphasis on improving functional outcomes while maintaining survival rates.1–5 As a result, assessments of functional outcomes and quality of life (QOL) have become important measures in HNC clinical trials. Since patients’ perspectives may vary widely from those of their treatment providers, patient-reported outcomes (PROs) are recognized as meaningful measures of functional impairment and QOL.6 These subjective measures have been shown to correlate with objective measures of dysfunction and provide advantages in clinical practice given their relatively low costs and ease of implementation.7
There has been a dramatic evolution in the demographics of HNC survivors attributable to the growing incidence of human papillomavirus (HPV)–associated oropharyngeal HNC, which tends to present in younger and healthier individuals.8 An increase in the utilization of chemoradiation (CRT) as a primary and adjuvant treatment has paralleled this trend and demonstrated advantages in attaining disease-free survival and locoregional control.9 Such patients demonstrate extended survivorship, presenting unique challenges to patients, caregivers, and health care providers. Consequently, there is a burgeoning population of long-term survivors who are at risk for unmet physical and psychosocial needs.10–12 Most patients with HNC, including HPV positive, present with locally advanced disease necessitating intense multimodal management.13–16 As the intensity of standard therapy has amplified, survivors experience substantial increases in acute toxicities.17 Data on late toxicities are limited and primarily focused on dysphagia, trismus, and pain.18,19
In the posttreatment setting, a physical impairment may worsen over time secondary to maladaptive postures and movement patterns with progressive fibrosis of tissues in suboptimal positions. Physical therapy, lymphedema therapy, pharmacotherapy, and integrative medicine approaches may be employed to improve musculoskeletal dysfunction.6 Thus, early detection of functional impairments in HNC survivors is critical to developing targeted interventional strategies for long-term amelioration of late treatment-related toxicities. Cervical and upper limb dysfunction are established sequelae of surgical resection and reconstruction.7,20–22 The impact of CRT, however, on musculoskeletal impairment is poorly understood. Although less frequently described, shoulder morbidity is a reported consequence of radiation therapy (RT) in the absence of surgery.23 Overall, there is a paucity of literature examining physical impairments after nonsurgical management.18,23,24
The purpose of this study was to determine the prevalence and predictors of neck disability following HNC treatment and to explore the association between neck disability and QOL. Through the systematic collection of patient-reported outcomes and comprehensive screening of functional impairments in HNC survivors, this investigation provides a unique insight into the scope and trajectory of acute and late toxicities related to HNC therapy.
Materials and Methods
We conducted a retrospective review of patient-reported outcomes from a prospectively maintained database from the University of Pittsburgh Medical Center Head and Neck Cancer Survivorship Clinic. This retrospective review was approved by the University of Pittsburgh Institutional Review Board. All adult (≥18 years) survivors who completed primary treatment for head and neck squamous cell carcinoma of the oral cavity, oropharynx, or larynx/hypopharynx who were seen in the survivorship clinic between March 2017 and January 2018 were evaluated. Patients who underwent treatment for recurrence, distant metastasis, or second primaries were excluded. To be included in the analysis, survivors had to have completed both the University of Washington Quality of Life and Neck Disability Index questionnaires and be at least 1 year posttreatment. Figure 1 is a Consolidated Standards of Reporting Trials (CONSORT) flow diagram that details the screening process for this study based on these inclusion/exclusion criteria.
Figure 1.
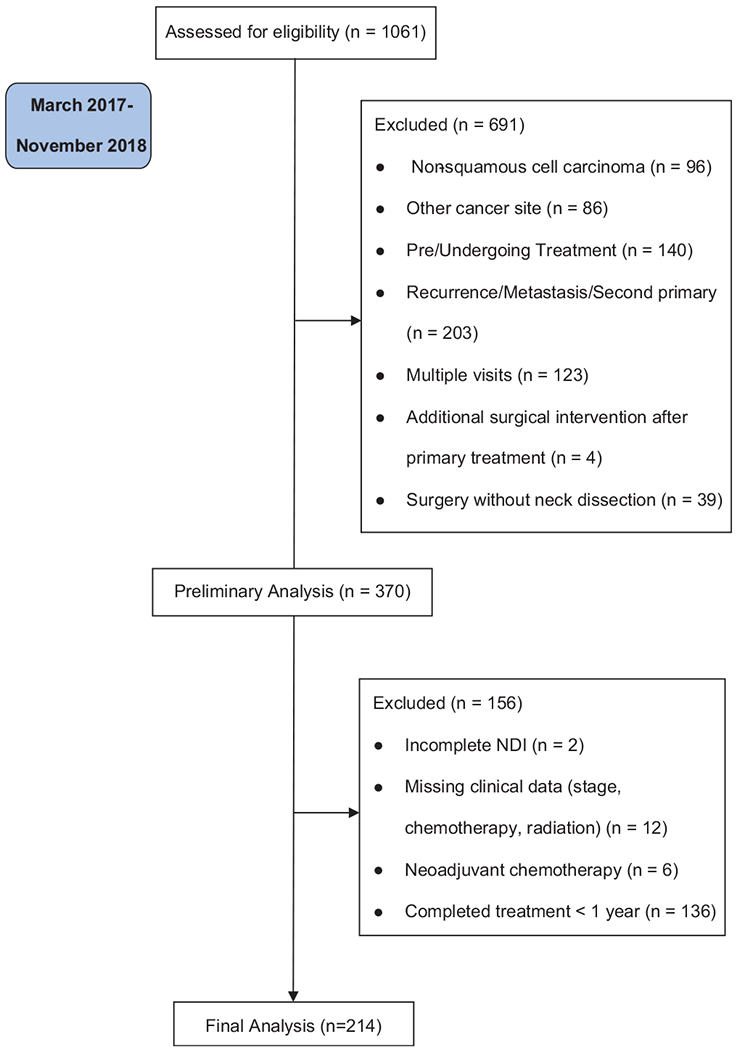
Consolidated Standards of Reporting Trials (CONSORT) flow diagram. NDI, Neck Disability Index.
Demographics and Clinical Characteristics
Demographics and clinical characteristics were abstracted from the medical record and included age, sex, race, marital status, tumor site, American Joint Committee on Cancer (AJCC) staging, HPV status for oropharyngeal tumors, and time since treatment completion. Treatment groups were categorized as (1) surgery alone, (2) nonsurgical (RT or CRT), or (3) surgery and adjuvant therapy (RT or CRT). All patients had to have undergone neck dissection to be included in one of the surgery treatment groups.
Neck Disability Index
Neck pain and disability were assessed using the Neck Disability Index (NDI). The NDI is a 10-item measure of disability resulting from neck pain; the scale ranges from 0 to 50, with higher scores denoting more severe disability.25 NDI scores were tabulated by the degree of disability (none: 0-4, mild to moderate: 5-24, and severe to complete: 25-50). For this analysis, NDI was categorized into 2 groups: absence (0-4) or presence (5-50) of disability. Although initially developed to measure neck pain in patients with cervical spine injury due to whiplash, the NDI has been used to measure neck dysfunction in HNC survivors,7,22 demonstrates high reliability and internal consistency,25,26 and has an excellent ability to distinguish patients with different levels of perceived disability.27
University of Washington Quality of Life
QOL was measured using the University of Washington Quality of Life Scale (UWQOL) Version 4. The UWQOL consists of 12 single questions with response options ranging from 3 to 6 choices. The domains are pain, appearance, activity, recreation, swallowing, chewing, speech, shoulder, taste, saliva, mood, and anxiety. The physical subscale score is computed as an average of 6 domain scores, which include chewing, swallowing, speech, taste, saliva, and appearance.28 The social-emotional subscale score is an average of anxiety, mood, pain, activity, recreation, and shoulder function. Subscale scores range from 0 to 100, with higher scores indicating better functioning.29
Statistical Analysis
All statistical analysis was performed using SAS (9.4; SAS Institute, Cary, North Carolina) and RStudio (1.1.419; RStudio, Inc, Boston, Massachusetts). In the descriptive analysis, mean and standard deviation were calculated for continuous variables, while frequency and percentage were computed for categorical variables. For final analyses, we used the dichotomized version of NDI (presence/absence of neck disability). Logistic regression was performed, after adjusting for age, marital status, tumor site, AJCC stage, and time since treatment completion, to analyze the association between neck disability and HNC treatment groups (surgery alone, nonsurgical, or surgery and adjuvant therapy).
We performed a trend analysis to test the hypothesis that the probability of neck disability is ordered among 3 treatment groups: P (surgery alone) ≤ P (nonsurgical) ≤ P (surgery and adjuvant treatment), with at least 1 strict inequality. In addition to the trend test, we also performed a 1-sided pairwise test to evaluate differences between the surgery-alone and nonsurgical groups, as well as similarly between the surgery-alone and surgery plus adjuvant treatment groups. A nonparametric bootstrap methodology was used to perform these analyses.30 Ten thousand bootstrap samples were generated to obtain the P value.
To explore the impact of neck disability treatment modality on QOL (physical and social-emotional subscales), multiple linear regression was conducted after adjusting for age, marital status, tumor site, AJCC stage, and time since treatment completion. We performed 2 trend analyses to test 2 hypotheses: neck disability absence group has better QOL than the presence group, and QOL is ordered among 3 treatment groups: QOL (neck disability absence) ≥ QOL (neck disability presence), QOL (surgery alone) ≥ QOL (nonsurgical) ≥ QOL (surgery and adjuvant treatment). In addition to the trend test, we also performed a 1-sided pairwise test to evaluate differences between the surgery-alone and nonsurgical groups, as well as similarly between the surgery-alone and surgery and adjuvant treatment groups. An R package “CLME” was used to perform these analyses.31 Ten thousand bootstrap simulations were performed to obtain the bootstrap test.
Results
Demographics and Clinical Characteristics
In this study, 214 patients were included. Table 1 displays the demographics and clinical characteristics for the whole group and by neck disability group as well as the univariate analysis. The patients’ mean (SD) age was 64.5 (10.1) years. Locally advanced stage at diagnosis (AJCC III-IV) was found in 173 (80.8%) patients, while 41 (19.2%) were early stage. Oropharynx (n = 105, 49.1%) was the most common primary site of tumor followed by oral cavity (n = 63, 29.4%) and larynx/hypopharynx (n = 46, 21.5%). Eighty percent (n = 84 of 105) of the cases of oropharyngeal cancer were HPV positive.
Table 1.
Demographics and Clinical Characteristics for the Whole Group and by Neck Disability Status (N =214).
| Variables | Total (N = 214) | No Neck Disability (n = 98) | Neck Disability (n = 116) | P Value |
|---|---|---|---|---|
| Age, ya | 64.5 ± 10.1 | 66.2 ± 9.1 | 63.1 ± 10.7 | .023b |
| Sex | .052 | |||
| Male | 159 (74.3) | 79 (80.6) | 80 (69.0) | |
| Female | 55 (25.7) | 19 (19.4) | 36 (31.0) | |
| Marital status | .004 | |||
| Married | 149 (69.6) | 78 (79.6) | 71 (61.2) | |
| Divorced/LWP/widowed/single | 65 (30.4) | 20 (20.4) | 45 (38.8) | |
| Race | .013b | |||
| White | 199 (93.0) | 96 (98.0) | 103 (88.8) | |
| Other | 15 (7.0) | 2 (2.0) | 13 (11.2) | |
| Tumor site | .563 | |||
| Oral cavity | 63 (29.4) | 31 (31.6) | 32 (27.6) | |
| Oropharynx | 105 (49.1) | 49 (50.0) | 56 (48.3) | |
| Larynx/hypopharynx | 46 (21.5) | 18 (18.4) | 28 (24.1) | |
| AJCC stage | .069 | |||
| Tis-IIc | 41 (19.2) | 24 (24.5) | 17 (14.7) | |
| III-IV | 173 (80.8) | 74 (75.5) | 99 (85.3) | |
| Treatment modality | .007 | |||
| Surgery alone | 40 (18.7) | 27 (27.5) | 13 (11.2) | |
| Nonsurgical | 96 (44.9) | 42 (42.9) | 54 (46.6) | |
| Surgery + adjuvant treatment | 78 (36.4) | 29 (29.6) | 49 (42.2) | |
| Neck dissection | .588 | |||
| No | 96 (44.9) | 42 (42.9) | 54 (46.6) | |
| Yes | 118 (55.1) | 56 (57.1) | 62 (53.5) | .385 |
| Unilateral | 71 (60.2) | 36 (64.3) | 35 (56.5) | |
| Bilateral | 47 (39.8) | 20 (35.7) | 27 (43.5) | |
| Chemotherapy (n = 213) | .036 | |||
| None | 78 (36.6) | 44 (45.4) | 34 (29.3) | |
| Platinum based | 118 (55.4) | 48 (49.5) | 70 (60.3) | |
| Nonplatinum based | 17 (8.0) | 5 (5.1) | 12 (10.4) | |
| Radiation | .012b | |||
| None | 40 (18.7) | 27 (27.6) | 13 (11.2) | |
| IMRT | 153 (71.5) | 60 (61.2) | 93 (80.2) | |
| Three-dimensional conformal | 9 (4.2) | 5 (5.1) | 4 (3.5) | |
| XRT NOS | 12 (5.6) | 6 (6.1) | 6 (5.1) | |
| Time since treatment completion, ya,d | 5.8 ± 6.0 | 6.7 ± 7.4 | 5.0 ± 4.3 | .345b |
Abbreviations: AJCC, American Joint Committee on Cancer; IMRT, intensity-modulated radiation therapy; LWP, living with partner; XRT NOS, radiation type not specified.
Mean ± SD. All other data are reported as n (%).
P value is for Fisher exact test for categorical variables or t test for continuous variables. Otherwise, reported P values are for χ2 test.
Tis = 1.
Median is 2.0 years.
Of 214 patients, 40 (18.7%) underwent surgical intervention alone, 96 (44.9%) received nonsurgical therapy, and 78 (36.4%) received both surgery and adjuvant treatment. Of the 96 patients who underwent nonsurgical treatment, only 10 (10.4%) received radiation alone. For those patients who underwent surgery and adjuvant treatment, 34.6% (n = 27 of 78) received radiation alone. For 1 patient who underwent surgery plus adjuvant treatment, we were unable to determine the type of chemotherapy given. Most of the radiation was intensity-modulated radiotherapy (n = 153 of 174, 87.9%). Patients in both the nonsurgical and surgery with adjuvant treatment groups, who received chemotherapy as part of their treatment, most often received platinum-based chemotherapy (n = 71 of 85, 83.5% and n = 47 of 50, 94.0%, respectively). The mean (SD) time since treatment completion was 5.8 (6.0) years, with a median of 4.0 years (range, 1-40 years).
Overall, 116 (54.2%) patients reported the presence of neck disability. Among the survivors who reported neck disability, the majority (n = 77, 66.4%) had mild or moderate disability, and 33.6% (n = 39) reported severe to complete disability. Within the nonsurgical treatment group, the presence of neck disability was not significantly associated with treatment modality (RT alone or CRT, P = .744), with similar results noted in the surgery plus adjuvant treatment group (surgery plus RT vs surgery plus CRT, P = .461). Therefore, 3 treatment groups were used: surgery, nonsurgical treatment, and surgery plus adjuvant treatment.
The mean (SD) score of the physical subscale was 74.1 (18.7), and the social-emotional subscale was 78.1 (18.4). Among those with no neck disability, the mean (SD) physical subscale score was 84.7 (10.6), and for those with disability, the mean (SD) physical subscale score was 64.9 (19.2). Among those with no neck disability, the mean (SD) social subscale score was 91.5 (7.4), and for those with disability, the mean (SD) social subscale score was 66.3 (17.1).
Neck Disability
Considering both clinical and statistical significance identified during the univariate analysis (Table 1), the logistic regression model included the following factors: treatment type (surgery alone, nonsurgical, surgery plus adjuvant treatment), age (years), marital status (dichotomous), tumor site, AJCC stage, and time since treatment completion (years). After controlling for covariates in the model, marital status and treatment type were significantly associated with neck disability. Married patients were less likely to report neck disability (P = .004). Compared to surgery alone, neck disability was significantly higher in patients who underwent surgery and adjuvant treatment (P = .016) (Table 2). The trend test (Figure 2) revealed that the probabilities of neck disability presence were ordered among 3 treatment types (P = .005) in the following sequence: P (surgery alone) ≤P (nonsurgical) ≤P (surgery and adjuvant treatment). The probability of reporting neck dysfunction was lowest among patients who received surgery alone and highest among those who received surgery and adjuvant treatment. In addition, the logistic regression analysis and the trend tests demonstrated that the odds of having neck disability for patients receiving nonsurgical therapy were 3.46 times higher than those undergoing surgery alone (P = .008). The odds of reporting neck dysfunction were even more pronounced for those receiving surgery and adjuvant treatment compared to surgery alone (odds ratio [OR], 4.98; P = .004). However, the presence of neck disability was not significantly different between the nonsurgical and surgery plus adjuvant treatment groups (OR, 1.44; P = .217).
Table 2.
Logistic Regression of Demographics and Clinical Characteristics Associated with Neck Disability.
| Variables | OR (95% CI) | P Value |
|---|---|---|
| Age, y | 0.977 (0.947-1.008) | .152 |
| Time since treatment completion, y | 0.970 (0.918-1.024) | .263 |
| AJCC stage | ||
| Tis-IIa | 1.195 (0.451-3.166) | .720 |
| III-IV | (Base) | |
| Tumor site | ||
| Oral cavity | 1.116 (0.413-3.014) | .476 |
| Oropharynx | 0.691 (0.311-1.533) | |
| Larynx/hypopharynx | (Base) | |
| Treatment modality | ||
| Surgery alone | 0.201 (0.067-0.602) | .016 |
| Nonsurgical | 0.695 (0.333-1.448) | |
| Surgery + adjuvant treatment | (Base) | |
| Marital status | ||
| Married | (Base) | .004 |
| Divorced/LWP/widowed/single | 2.698 (1.371-5.308) |
Abbreviations: AJCC, American Joint Committee on Cancer; CI, confidence interval; LWP, living with partner; OR, odds ratio.
Tis = 1.
Figure 2.
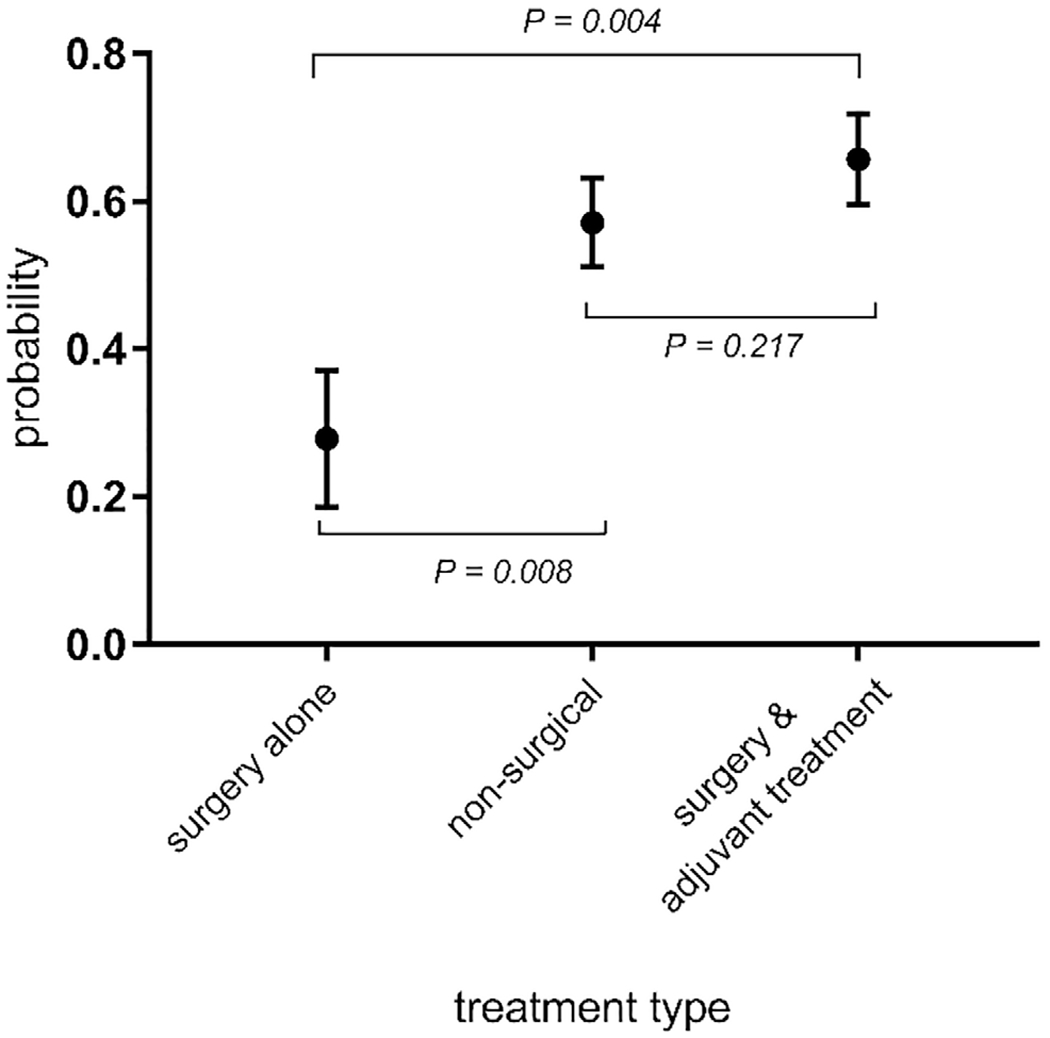
Probability of having neck disability for each treatment type. Probabilities were obtained after adjusting for age, marital status, tumor site, American Joint Committee on Cancer stage, and time since treatment completion at their average values.
Quality of Life
The linear regression models for both physical and social QOL included the following factors: neck disability status (absence, presence), treatment type (surgery alone, nonsurgical, surgery and adjuvant treatment), age (years), marital status (dichotomous), tumor site, AJCC stage, and time since treatment completion (years). Table 3 shows that after adjusting for other covariates in the model, AJCC stage, tumor site, treatment modality, and neck disability were significantly associated with physical QOL. The linear regression analysis for social QOL (Table 4) shows that neck disability and time since treatment completion were significantly associated with social QOL while adjusting for other variables in the model. To further evaluate the association between neck disability and QOL and the association between treatment modality and QOL, a nonparametric bootstrap test (CLME)31 was performed. The 1-sided tests showed that both physical life and social QOL were significantly better for patients with no reported neck disability compared to those with neck disability (P < .001) (Figure 3a,b). As for the influence of treatment type on QOL, the trend test (Figure 3c) revealed that the physical QOL is ordered among 3 treatment types (P < .001) in the following sequence: physical QOL (surgery alone) ≥ physical QOL (nonsurgical) ≥ physical QOL (surgery and adjuvant treatment). One-sided hypothesis tests revealed that the surgery-alone group had significantly better physical QOL than both the nonsurgical group (P < .001) and surgery and adjuvant treatment group (P < .001) (Figure 3c). However, physical QOL was not significantly different between the nonsurgical and surgery and adjuvant treatment groups (P = .409) (Figure 3c).
Table 3.
Multiple Linear Regression of Variables Associated with the University of Washington Physical Quality of Life Subscale.
| Variables | Coefficient (95% CI) | P Value |
|---|---|---|
| Age, y | −0.095 (−0.306, 0.115) | .373 |
| Time since treatment completion, y | 0.326 (−0.029 to −.680) | .072 |
| AJCC stage | ||
| Tis-IIa | 8.235 (1.612 to 14.858) | .015 |
| III-IV | (Base) | |
| Tumor site | ||
| Oral cavity | −11.350 (−17.855 to −4.844) | .003 |
| Oropharynx | −4.910 (−10.189 to 0.368) | |
| Larynx/hypopharynx | (Base) | |
| Treatment modality | ||
| Surgery alone | 13.654 (6.362 to 20.945) | .0008 |
| Nonsurgical | 0.099 (−4.840 to 5.038) | |
| Surgery + adjuvant treatment | (Base) | |
| Neck disability | ||
| No | 16.640 (12.520 to 20.760) | <.0001 |
| Yes | (Base) | |
| Marital status | ||
| Married | 2.001 (−2.519 to 6.521) | .384 |
| Divorced/LWP/widowed/single | (Base) |
Abbreviations: AJCC, American Joint Committee on Cancer; CI, confidence interval; LWP, living with partner.
Tis = 1.
Table 4.
Multiple Linear Regression of Variables Associated with the University of Washington Social Quality of Life Subscale.
| Variables | Coefficient (95% CI) | P Value |
|---|---|---|
| Age, y | 0.077 (−0.114 to 0.268) | .429 |
| Time since treatment completion, y | 0.343 (0.018 to 0.668) | .039 |
| AJCC stage | ||
| Tis-IIa | 4.694 (−1.278 to 10.666) | .123 |
| III-IV | (Base) | |
| Tumor site | ||
| Oral cavity | −0.281 (−6.256 to 5.695) | .055 |
| Oropharynx | 5.034 (0.186 to 9.882) | |
| Larynx/hypopharynx | (Base) | |
| Treatment modality | ||
| Surgery alone | 5.556 (−1.094 to 12.207) | .215 |
| Nonsurgical | −0.267 (−4.806 to 4.272) | |
| Surgery + adjuvant treatment | (Base) | |
| Neck disability | ||
| No | 22.666 (18.864 to 26.468) | <.0001 |
| Yes | (Base) | |
| Marital status | ||
| Married | 0.804 (−3.398 to 5.005) | .706 |
| Divorced/LWP/widowed/single | (Base) |
Abbreviations: AJCC, American Joint Committee on Cancer; LWP, living with partner.
Tis = 1.
Figure 3.
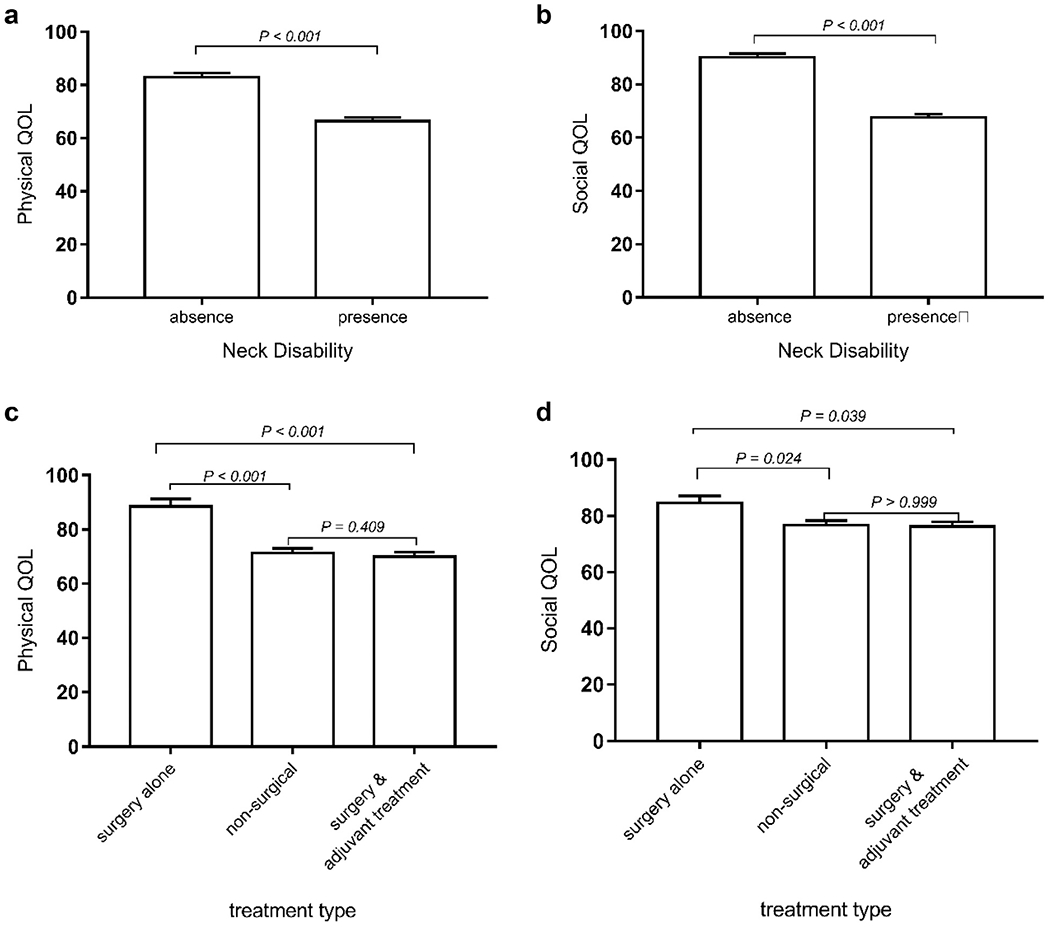
(a, b) Association between neck disability and quality of life (QOL). (c, d) Association between treatment type and QOL. (Both physical QOL and social QOL were obtained after adjusting for age, marital status, tumor site, AJCC stage, and time since treatment completion.
The trend test revealed that social QOL is ordered among the 3 treatment types (P = .022) in the following sequence: social QOL (surgery alone) ≥ social QOL (nonsurgical) ≥ social QOL (surgery and adjuvant treatment). One-sided hypothesis tests revealed that the surgery-alone group had significantly better social QOL than both the nonsurgical group (P = .024) and surgery and adjuvant treatment group (P = .039). However, social QOL was not significantly different between the nonsurgical and surgery and adjuvant treatment groups (P > .999).
Discussion
The current trend toward increased intensity of standard treatment for HPV-negative HNC cancer will result in an increasing number of survivors who will live with the resulting acute and late toxicities of treatment. These cumulative effects of cancer and treatment contribute to a progressive decline in QOL, which encompasses diminished functionality among these survivors. While neck disability has been associated with surgical intervention,7,20–22 studies evaluating neck dysfunction across treatment modalities are limited. A recent small descriptive study suggested that patients receiving nonsurgical measures may also experience mild neck disability.24 This study aims to address the critical knowledge gap that exists in understanding physical impairment in the setting of multimodal and nonsurgical HNC management.
Our study demonstrated that 54.2% of survivors reported some neck disability, with most of those survivors (66.4%, n = 77 of 116) reporting mild to moderate disability. In prior studies that evaluated neck disability through clinical assessment and evaluation, the prevalence ranged from 11% to 77%.24,32,33 Marital status and treatment type were significantly associated with neck disability. Married patients were less likely to report neck disability (P = .004). Previous studies have shown that patients with head and neck cancer who are married have less metastatic disease, are more likely to receive adequate treatment, and may experience a survival benefit.34,35 The researchers postulate that spouses may support patients in visual and symptom surveillance. While further analysis is needed, spouses may also support and aid patients in symptom management, which may contribute to less reported neck disability.
In a previous study, the highest reported neck disability occurred in patients who underwent nonsurgical treatment.24 In our study, treatment modality was a predictor of neck disability (Table 2). Patients who received nonsurgical treatment or surgery with adjuvant treatment were more likely to experience neck disability than those who received surgery alone, although there was no difference in the prevalence of neck disability between nonsurgical treatment and surgery plus adjuvant treatment groups. While neck impairment has been attributed to surgical insult in previous studies,7,20–22 our findings suggest that RT, perhaps partly through fibrotic changes, may also play a significant role in the development and progression of neck impairment. It also should be noted that many of our patients who received nonsurgical therapy received chemoradiation, which brings up the question as to the risk of neck disability with adding chemotherapy concurrent with RT. Because of the limited variability in chemotherapeutic agents, we were unable to explore the potential influence of concurrent chemotherapy in this study. However, this investigation highlights that a better understanding of the biological mechanisms related to fibrotic changes and the impact of functional outcomes is needed.
The cumulative impact of neck impairment on daily activities may contribute to lower physical and social/emotional QOL reported by patients in this study. In a previous study of 167 patients, myofascial pain syndrome, which is characterized by intense, deep pain and limited range of motion, was associated with lower overall QOL.33 Studies have linked neck dissection with lower QOL,36,37 but most of these studies focus on impairment of the shoulder.37 Further work needs to be done to explore the phenomenon of neck impairment, including impairments in range of motion, posture, and presence of pain in nonsurgical patients and the relationship with QOL.
By using patient-reported outcomes measures, such as the Neck Disability Index, health care providers may screen for symptoms and treatment-related toxicities in the clinical setting. Integration of such metrics into clinical practice may help providers identify acute and late treatment-related effects. More important, through systematic identification of patients’ symptom burden and impact on QOL, care teams may intervene earlier in the survivorship trajectory and monitor for changes over time. Early intervention via rehabilitation and exercise programs38,39 may help mitigate the neck impairment that these survivors, like the ones examined in our study, experience. There are several limitations to this study. Most of the survivors evaluated in our multidisciplinary HNC survivorship clinic were treated at a single institution, and our sample had limited variability in race and marital status. A larger, more diverse sample from several institutions would allow for further exploration on the influence of these variables on neck disability. Furthermore, the survivorship clinic is designed to meet the multifaceted needs of survivors with an emphasis on the multidisciplinary management of late and long-term effects of treatment. The purpose of this clinic may lead to a selection bias of patients with a high burden of symptom and treatment-related effects. In addition, both the UWQOL social-emotional subscale and NDI contain items assessing pain and recreation, thereby potentially confounding the association between social QOL and NDI noted in this analysis. Future studies should employ objective measures of neck function, such as cervical range of motion, and physical assessment to corroborate and elaborate patient-reported outcome findings. Finally, the sample size limited our ability to compare QOL scores across individual neck disability levels. Future studies could provide a more detailed picture of QOL scores stratified by more discriminate levels of disability.
Conclusion
This study demonstrates a high prevalence of neck disability and pain after nonsurgical treatment with RT or CRT only. We found that neck disability significantly affects QOL beyond physical impairment alone. Neck disability represents a substantial treatment-related burden, even in the absence of surgery. In the longitudinal care of HNC survivors, more comprehensive screening is warranted, particularly among those treated with cytotoxic and radiation modalities. Finally, these results merit further study of mechanisms of toxicities and development of targeted medical and biological interventions to mitigate these toxicities.
Acknowledgments
Funding source: Beckwith Institute (Nilsen, Johnson), 5T32DC000066 NIH/NIDCD Research Training in Otolaryngology (Johnson, Mady).
Footnotes
This article was presented at the meeting of the American Head and Neck Society; April 18-19, 2018; National Harbor, Maryland, USA.
Competing interests: None.
References
- 1.Bozec A, Poissonnet G, Chamorey E, et al. Quality of life after oral and oropharyngeal reconstruction with a radial forearm free flap: prospective study. J Otolaryngol Head Neck Surg. 2009;38:401–408. [PubMed] [Google Scholar]
- 2.Mittal BB, Kepka A, Mahadevan A, et al. Tissue/dose compensation to reduce toxicity from combined radiation and chemotherapy for advanced head and neck cancers. Int J Cancer. 2001;96:61–70. [DOI] [PubMed] [Google Scholar]
- 3.Karatzanis AD, Psychogios G, Zenk J, et al. Evaluation of available surgical management options for early supraglottic cancer. Head Neck. 2010;32:1048–1055. [DOI] [PubMed] [Google Scholar]
- 4.Byers RM. Neck dissection: concepts, controversies, and technique. Semin Surg Oncol. 1991;7:9–13. [DOI] [PubMed] [Google Scholar]
- 5.Eisbruch A, Dawson L, Kim H, et al. Conformal and intensity modulated irradiation of head and neck cancer: the potential for improved target irradiation, salivary gland function, and quality of life. Acta Otorhinolaryngol Belg. 1999;53:271–275. [PubMed] [Google Scholar]
- 6.Tschiesner U Changing the perspective: current trends in the assessment of functional outcome in patients with head and neck cancer. Curr Oncol Rep. 2011;13:126–131. [DOI] [PubMed] [Google Scholar]
- 7.Ghiam MK, Mannion K, Dietrich MS, Stevens KL, Gilbert J, Murphy BA. Assessment of musculoskeletal impairment in head and neck cancer patients. Support Care Cancer. 2017;25: 2085–2092. [DOI] [PubMed] [Google Scholar]
- 8.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J lin Oncol. 2008;26:3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martino R, Ringash J. Evaluation of quality of life and organ function in head and neck squamous cell carcinoma. Hematol Oncol Clin North Am. 2008;22:1239–1256. [DOI] [PubMed] [Google Scholar]
- 11.Sehlen S, Lenk M, Herschbach P, et al. Depressive symptoms during and after radiotherapy for head and neck cancer. Head Neck. 2003;25:1004–1018. [DOI] [PubMed] [Google Scholar]
- 12.Koch R, Wittekindt C, Altendorf–Hofmann A, Singer S, Guntinas-Lichius O. Employment pathways and work-related issues in head and neck cancer survivors. Head Neck. 2015;37: 585–593. [DOI] [PubMed] [Google Scholar]
- 13.Pulte D, Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. Oncologist. 2010;15:994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Argiris A, Harrington KJ, Tahara M, et al. Evidence-based treatment options in recurrent and/or metastatic squamous cell carcinoma of the head and neck. Front Oncol. 2017;7:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fakhry C, Zhang Q, Nguyen-Tan PF, et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol. 2014;32:3365–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trotti A, Pajak TF, Gwede CK, et al. TAME: development of a new method for summarising adverse events of cancer treatment by the Radiation Therapy Oncology Group. Lancet Oncol. 2007;8:613–624. [DOI] [PubMed] [Google Scholar]
- 18.Van der Molen L, van Rossum MA, Burkhead LM, Smeele LE, Hilgers FJ. Functional outcomes and rehabilitation strategies in patients treated with chemoradiotherapy for advanced head and neck cancer: a systematic review. Eur Arch Otorhinolaryngol. 2009;266:889–900. [DOI] [PubMed] [Google Scholar]
- 19.Forastiere AA, Zhang Q, Weber RS, et al. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013;31:845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moukarbel RV, Fung K, Franklin JH, et al. Neck and shoulder disability following reconstruction with the pectoralis major pedicled flap. Laryngoscope. 2010;120:1129–1134. [DOI] [PubMed] [Google Scholar]
- 21.Gane E, Michaleff Z, Cottrell M, et al. Prevalence, incidence, and risk factors for shoulder and neck dysfunction after neck dissection: a systematic review. Eur J Surg Oncol. 2017;43: 1199–1218. [DOI] [PubMed] [Google Scholar]
- 22.Gane EM, O’Leary SP, Hatton AL, Panizza BJ, McPhail SM. Neck and upper limb dysfunction in patients following neck dissection: looking beyond the shoulder. Otolaryngol Head Neck Surg. 2017;157:631–640. [DOI] [PubMed] [Google Scholar]
- 23.van Wouwe M, de Bree R, Kuik DJ, et al. Shoulder morbidity after non-surgical treatment of the neck. Radiother Oncol. 2009;90:196–201. [DOI] [PubMed] [Google Scholar]
- 24.Kaur P, Pannu A, Singh S. Assessment of swallowing and neck dysfunction post radiotherapy/chemo-radiotherapy in head and neck cancer patients. Biom Biostat Int J. 2016;3:00066. [Google Scholar]
- 25.Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther. 1991;14:409–415. [PubMed] [Google Scholar]
- 26.Hains F, Waalen J, Mior S. Psychometric properties of the neck disability index. J Manipulative Physiol Ther. 1998;21: 75–80. [PubMed] [Google Scholar]
- 27.Saltychev M, Mattie R, McCormick Z, Laimi K. Psychometric properties of the neck disability index amongst patients with chronic neck pain using item response theory. Disabil Rehabil. 2018;40:2116–2121. [DOI] [PubMed] [Google Scholar]
- 28.Rogers SN, Gwanne S, Lowe D, Humphris G, Yueh B, Weymuller EA Jr. The addition of mood and anxiety domains to the University of Washington quality of life scale. Head Neck. 2002;24:521–529. [DOI] [PubMed] [Google Scholar]
- 29.Rogers SN, Lowe D, Yueh B, Weymuller EA Jr. The physical function and social-emotional function subscales of the University of Washington Quality of Life Questionnaire. Arch Otolaryngol Head Neck Surg. 2010;136:352–357. [DOI] [PubMed] [Google Scholar]
- 30.Peddada SD, Prescott KE, Conaway M. Tests for order restrictions in binary data. Biometrics. 2001;57:1219–1227. [DOI] [PubMed] [Google Scholar]
- 31.Jelsema CM, Peddada SD. CLME: an R package for linear mixed effects models under inequality constraints. J Stat Softw. 2016;75:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilgen CPv Dijkstra PU, Laan BFAMvd Plukker JT, Roodenburg JLN. Morbidity of the neck after head and neck cancer therapy. Head Neck. 2004;26:785–791. [DOI] [PubMed] [Google Scholar]
- 33.Cardoso LR, Rizzo CC, de Oliveira CZ, dos Santos CR, Carvalho AL. Myofascial pain syndrome after head and neck cancer treatment: prevalence, risk factors, and influence on quality of life. Head Neck. 2015;37:1733–1737. [DOI] [PubMed] [Google Scholar]
- 34.Inverso G, Mahal BA, Aizer AA, Donoff RB, Chau NG, Haddad RI. Marital status and head and neck cancer outcomes. Cancer. 2015;121:1273–1278. [DOI] [PubMed] [Google Scholar]
- 35.Simpson MC, Challapalli SD, Cass LM, et al. Impact of gender on the association between marital status and head and neck cancer outcomes. Oral Oncol. 2019;89:48–55. [DOI] [PubMed] [Google Scholar]
- 36.Terrell JE, Ronis DL, Fowler KE, et al. Clinical predictors of quality of life in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004;130:401–408. [DOI] [PubMed] [Google Scholar]
- 37.Kuntz AL, Weymuller EA Jr. Impact of neck dissection on quality of life. Laryngoscope. 1999;109:1334–1338. [DOI] [PubMed] [Google Scholar]
- 38.Carvalho AP, Vital FM, Soares BG. Exercise interventions for shoulder dysfunction in patients treated for head and neck cancer. Cochrane Database Syst Rev. 2012;18:CD008693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNeely ML, Parliament MB, Seikaly H, et al. Effect of exercise on upper extremity pain and dysfunction in head and neck cancer survivors. Cancer. 2008;113:214–222. [DOI] [PubMed] [Google Scholar]


