Abstract
Degenerative lumbar spine disease (DLSD) is a heterogenous group of conditions that can significantly affect patients’ quality of life. Lateral lumbar interbody fusion (LLIF) is one of the treatment modalities for DLSD that has been increasing in popularity over the past decade. The treatment of DLSD should be individualized based on patients’ symptoms and characteristics to maximize outcomes.
Methods:
Literature review, invited review.
Results:
In this article, we will (1) review the use of the LLIF technique in the treatment of degenerative lumbar spine disease, (2) review the current concepts of LLIF, and (3) explore the evidence to date that will allow the reader to maximize the benefits of this technique.
Conclusions:
LLIF is an alternative for the treatment of degenerative pathologies of the lumbar spine via indirect decompression.
Keywords: lateral Surgery, XLIF, OLIF, ALIF, lumbar spine
Background
After almost 20 years since it was first described, the lateral lumbar interbody fusion (LLIF) technique is still gaining popularity.1 Initially, LLIF was described as alternative for indirect decompression of the neural elements as well as a treatment for associated low back pain and degenerative disc disease via disc height restoration and ligamentotaxis.2 Ever since its inception, the LLIF technique has faced opposition from the spine surgery community mainly due to initial reports of postoperative thigh pain ranging from 14.3% to 20%.3,4 Additionally, several other patient and surgical factors were identified as potential limitations for LLIF success.5 Over the past decade, some of these factors have been studied by different international spine groups and were shown to be nondetrimental to both clinical and radiological outcomes of LLIF, and did not outweigh the benefits of LLIF. These factors include cage dimensions (16-22 mm), cage positioning (anterior-mid-posterior), approach (concave, convex), location of stenosis (central stenosis, foraminal stenosis, lateral recess stenosis), and presence of facet degeneration (locked facets).6-13
Today, some of the initially described “relative contraindications” such as preoperative thigh pain and facet degeneration have been overcome and satisfactory results have been replicated worldwide.12-14
Despite these successes, a predictive model for patient and surgical technique selection to obtain successful indirect decompression outcomes via LLIF has yet to be described, especially for patients with lateral recess stenosis, most probably because the lateral recess stenosis is not well addressed by ligamentotaxis.5,13,15 The purpose of this review is to summarize the advantages, limitations, and peculiarities of the LLIF technique while giving the reader an update on the most current patient and surgical factors which are known to be relevant for maximizing outcomes after indirect decompression via LLIF. Finally, this review will briefly summarize the role of LLIF in comparison with other similar indirect decompression techniques for the lumbar spine.
Indirect Decompression After LLIF
LLIF has been shown to be effective in achieving indirect decompression of the lumbar spine and relieving symptoms of lumbar spinal stenosis. Furthermore, it has been shown to reduce length of stay, estimated blood loss, and the risk of developing, cerebrospinal leaks, vascular or nerve injury compared with conventional posterior techniques.2,6,16-20 Several studies have looked at the impact of LLIF on restoration of disc height as well as increasing central canal and foraminal surface area and volume using radiographic parameters on plain x-rays and cross-sectional imaging.6,16,21 It has also been shown that technical factors such as approach (concavity-convexity), cage dimensions (height and shape) and cage positioning (anterior-posterior) did not affect the clinical and radiological outcomes after LLIF.6,16
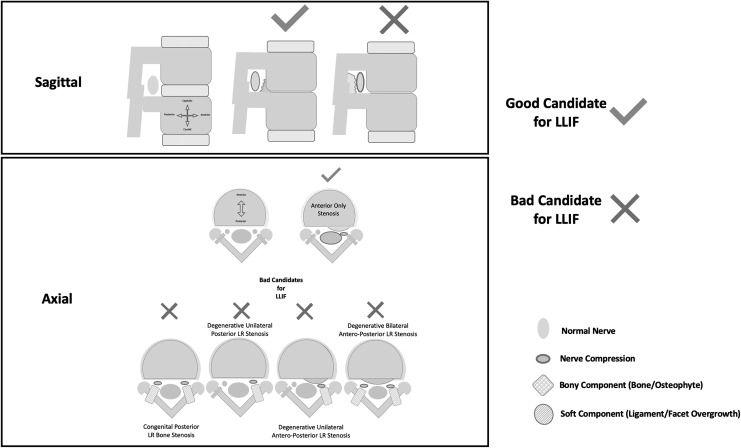
However, there is still a subgroup of patients whose neurological symptoms fail to improve following indirect decompression via LLIF. Wang et al13 and Nakashima et al22 recently raised concerns about using LLIF for lateral recess stenosis due to its particular bony anatomy (not well address by ligamentotaxis), as well as in severe spinal stenosis.13,22 Wang et al13 conducted a multivariate analysis, which showed that “bony lateral recess” stenosis was the only significant predictor of failed indirect decompression after LLIF. Based on the previously mentioned factors we created a cartoon to illustrate the ideal candidates for LLIF based on the anatomical location of “compression” (Figure 1).
Figure 1.
Cartoon representing in 2 sections; sagittal (top) and axial (bottom) marked with and “X” those not-ideal candidates for lateral lumbar interbody fusion (LLIF). Emphasizing that all those patients with “bony” posterior compression elements are poor candidates according to different authors around the world and only those with soft tissue bulking/folding should be considered optimal candidates for ligamentotaxis.
LLIF Potential
LLIF is a minimally invasive procedure that can achieve a powerful correction of spinal radiographic parameters. A systematic review of 1080 patients by Lang et al5 showed that LLIF can increase disc height by almost 75%. They also showed that a LLIF procedure can increase the surface area of the foramen and central canal by 36.4 and 25.4% respectively (Table 1). Furthermore, they found that LLIF can increase foraminal height by an average of 29.5% (Table 1), more than the correction achievable using the minimally invasive surgical TILF, which is reported to range from 10% to 19%.6-13
Table 1.
Mean Radiological Outcomes After Lateral Lumbar Interbody Fusion.
| Parameter | Number of Studies | Change Mean | Change % |
|---|---|---|---|
| Disc height (mm) | 13 | 4.1 | 74.8 |
| Foraminal area (mm2) | 8 | 31.6 | 36.4 |
| Foraminal height (mm) | 12 | 4.3 | 29.5 |
| Central canal area (mm2) | 6 | 28.5 | 25.4 |
Although several authors have addressed the effect of LLIF on disc height restoration and subsequent central canal and foraminal decompression,6-13 little has been described regarding the effect of LLIF on back pain. LLIF is a prevalent and effective treatment to improve back pain and disability.1,2 Indirect decompression through LLIF has been shown to achieve similar or better outcomes in terms of pain relief compared with direct approaches.13-17 In addition, many surgeons believe that LLIF is an excellent minimally invasive alternative to direct posterior decompression correlating with less blood loss, shorter lengths of hospital stay,17-19 low incidence of vascular or plexus injury,20-22 and decreased costs23 compared with conventional posterior interbody fusion surgery.
Although LLIF has significant advantages over open procedures, identifying patients at risk for poor clinical outcomes could facilitate patient selection and surgical planning, ultimately improving outcomes and reducing hospital costs associated with continued disability. Wang et al13 described that for patients undergoing indirect decompression, preoperative and postoperative films showed significant improvement in subarticular diameter (at L3-L5), central canal diameter (at L2-L5), mean foraminal area, mean foraminal height, and disk height (at all levels except L2-L3). However, in the same group certain factors were associated with a higher likelihood of indirect decompression failure including smaller central canal diameter and small foraminal height. However, after performing a multivariate analysis, “bony” lateral recess stenosis was the only significant independent predictor of indirect decompression failure.
Impact of Bony Lateral Recess Stenosis on LLIF Success
Lumbar spinal stenosis can be caused by solid/hard elements (calcified ligamentum flavum, calcified facet cysts, facet overgrowth, posterior disc degeneration with calcification) or soft tissue elements (soft disc herniations, ligamentum flavum folding/hypertrophy, facet cysts) (Figure 1).
LLIF relies on indirect decompression by restoring disc height, reducing bulky soft tissue, increasing the canal and foramina volumes thus releasing pressure from the dura and nerves, which is usually achieved via ligamentotaxis. When bony stenosis is present, ligamentotaxis might not be as effective (Figure 1). Wang et al,13 in 2017, showed that bony lateral recess stenosis was the only significant independent predictor associated to failed indirect decompression after LLIF. Malham et al10 also showed similar results in a prospective study of 122 consecutive patients undergoing indirect decompression through LLIF. In the study by Malham et al,10 11 patients failed indirect decompression (presented same symptoms after surgery) and of the 11 failed cases, 3 required subsequent direct decompression due to residual bony lateral recess stenosis. Based on these findings, the authors concluded that patients with bony lateral recess stenosis should be considered for direct posterior decompression. More recently, Nakashima et al,22 after analyzing 158 cases of LLIF, suggested that in patients with severe preoperative canal stenosis, motor deficits and/or ligament ossification, indirect decompression should be “contraindicated.” However, none of these studies explicitly define the radiographic parameters/findings that they used to categorize the patient as having “bony” lateral recess stenosis. Further work is therefore needed to create specific cutoffs for these parameters in order to improve consensus amongst spine surgeons. In figure 1, we aim to offer a theoretical approach to which candidates would be ideal for an indirect decompression via an LLIF procedure.
Indirect Decompression Using LLIF, OLIF, and ALIF
Although not the main goal of this article, these days we cannot exclude mentioning where LLIF fits in relationship with other indirect decompression techniques to treat degenerative lumbar spine disease such as oblique lumbar interbody fusion (OLIF) and anterior lumbar interbody fusion (ALIF). Here, we will only present a summary of what was already published by Xu et al.23 There remains a considerable debate about which technique to use when treating lumbar spine conditions. Our group, much like other experts, believe that these procedures are not directly comparable as they are best suited to treat different pathologies and different parts of the lumbar spine. LLIF is ideally suited for L1-L4 pathology, whereas OLIF is more suitable for L5-S1 pathology. OLIF was initially described as an alternative for LLIF for L5-S1 disease when ALIF was not a good option. ALIF remains the gold standard for treatment of L5-S1 pathology in terms of disc height restoration and power on deformity correction however, OLIF is a valid option when ALIF is not possible due to previous anterior surgery and/or complex vascular anatomy.
Based on what is presented in Table 2, we are sure that the 3 techniques described above can and should coexist when indirect decompression is considered rather than being seen as substitutes for one another.
Table 2.
Comparison of Indirect Decompression Techniques for the Lumbar Spine.
| Treatment Modality | Contraindications | Ideal Segments | Disc Access Point | Percentage Change in Neuroforaminal Area | Risk of Subsidence | Complications |
|---|---|---|---|---|---|---|
| LLIF | Prior retroperitoneal surgery, transitional anatomy, osteoporosis | T12-L4 | Lateral | 24.7 | 11%-30% | 0.7%-30% transient motor weakness 0.56% risk of vascular injury |
| OLIF | Severe spondylolisthesis, high grade central canal stenosis | L1-S1 | Anterolateral | 30.0 | No studies have reported on the risk of subsidence, but this technique is typically supplemented with pedicle screw fixation | 6.1%-21.4% transient motor weakness 1.6% risk of vascular injury |
| ALIF | Prior abdominal or retroperitoneal surgery, peripheral vascular disease, transitional anatomy at L5-S1 | L4-L5, L5-S1 (ideally below the bifurcation of the great vessels) | Anterior | 67 | Lowest (10.2%) | 1.9%-4.6% vascular injury 1.7%-2% retrograde ejaculation (open ALIF approach) |
Abbreviations: LLIF, lateral lumbar interbody fusion; OLIF, oblique lumbar interbody fusion; ALIF, anterior lumbar interbody fusion.
Conclusion
LLIF remains an alternative for the treatment of degenerative pathologies of the lumbar spine via indirectly decompression together with OLIF and ALIF. It has not been found to be limited by approach, cage positioning or facet degeneration. Current research efforts are focused on the role of “bony” lateral recess stenosis on surgical outcomes. Maximizing indirect decompression after LLIF will depend on meticulous patient and surgical technique selection.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This supplement was supported by funding from the Carl Zeiss Meditec Group.
ORCID iD: Rodrigo Navarro-Ramirez, MD, MSc  https://orcid.org/0000-0003-2543-9063
https://orcid.org/0000-0003-2543-9063
References
- 1. Pimenta L. Lateral endoscopic transpsoas retroperitoneal approach for lumbar spine surgery Paper presented at: VIII Brazilian Spine Society Meeting.; May 4, 2001; Belo Horizonte, Brazil. [Google Scholar]
- 2. Ozgur BM, Aryan HE, Pimenta L, Taylor ER. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6:435–443. doi:10.1016/j.spinee.2005.08.012 [DOI] [PubMed] [Google Scholar]
- 3. Elowitz EH, Yanni DS, Chwajol M, Starke RM, Perinet NI. Evaluation of indirect decompression of the lumbar spinal canal following minimally invasive lateral transpsoas interbody fusion: radiographic and outcome analysis. Minim Invasive Neurosurg. 2011;54:201–206. doi:10.1055/s-0031-1286334 [DOI] [PubMed] [Google Scholar]
- 4. Oliveira L, Marchi L, Coutinho E, Pimenta L. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine (Phila Pa 1976). 2010;35(26 suppl):S331–S337. doi:10.1097/BRS.0b013e3182022db0 [DOI] [PubMed] [Google Scholar]
- 5. Lang G, Perrech M, Navarro-Ramirez R, et al. Potential and limitations of neural decompression in extreme lateral interbody fusion—a systematic review. World Neurosurg. 2017;101:99–113. doi:10.1016/j.wneu.2017.01.080 [DOI] [PubMed] [Google Scholar]
- 6. Alimi M, Hofstetter CP, Tsiouris AJ, Elowitz E, Härtl R. Extreme lateral interbody fusion for unilateral symptomatic vertical foraminal stenosis. Eur Spine J. 2015;24(suppl 3):346–352. doi:10.1007/s00586-015-3940 [DOI] [PubMed] [Google Scholar]
- 7. Isaacs RE, Sembrano JN, Tohmeh AG; SOLAS Degenerative Study Group. Two-year comparative outcomes of MIS lateral and MIS transforaminal interbody fusion in the treatment of degenerative spondylolisthesis: part II: radiographic findings. Spine (Phila Pa 1976). 2016;41(suppl 8):S133–S144. doi:10.1097/BRS.0000000000001472 [DOI] [PubMed] [Google Scholar]
- 8. Sembrano JN, Horazdovsky RD, Sharma AK, Yson SC, Santos ERG, Polly DW., Jr Do lordotic cages provide better segmental lordosis versus nonlordotic cages in lateral lumbar interbody fusion (LLIF)? Clin Spine Surg. 2017;30:E338–E343. doi:10.1097/BSD.0000000000000114 [DOI] [PubMed] [Google Scholar]
- 9. Berjano P, Langella F, Damilano M, et al. Fusion rate following extreme lateral lumbar interbody fusion. Eur Spine J. 2015;24(suppl 3):369–371. doi:10.1007/s00586-015-3929-7 [DOI] [PubMed] [Google Scholar]
- 10. Malham GM, Parker RM, Goss B, Blecher CM, Ballok ZE. Indirect foraminal decompression is independent of metabolically active facet arthropathy in extreme lateral interbody fusion. Spine (Phila Pa 1976). 2014;39:E1303–E1310. doi:10.1097/BRS.0000000000000551 [DOI] [PubMed] [Google Scholar]
- 11. Caputo AM, Michael KW, Chapman TM, Jr, et al. Clinical outcomes of extreme lateral interbody fusion in the treatment of adult degenerative scoliosis. ScientificWorldJournal. 2012;2012:680643 doi:10.1100/2012/680643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Navarro-Ramirez R, Lang G, Moriguchi Y, et al. Are locked facets a contraindication for extreme lateral interbody fusion? World Neurosurg. 2017;100:607–618. doi:10.1016/j.wneu.2016.11.059 [DOI] [PubMed] [Google Scholar]
- 13. Wang TY, Nayar G, Brown CR, Pimenta L, Karikari IO, Isaacs RE. Bony lateral recess stenosis and other radiographic predictors of failed indirect decompression via extreme lateral interbody fusion: multi-institutional analysis of 101 consecutive spinal levels. World Neurosurg. 2017;106:819–826. doi:10.1016/j.wneu.2017.07.045 [DOI] [PubMed] [Google Scholar]
- 14. Bendersky M, Sola C, Muntadas J, et al. Monitoring lumbar plexus integrity in extreme lateral transpsoas approaches to the lumbar spine: a new protocol with anatomical bases. Eur Spine J. 2015;24:1051–1057. doi:10.1007/s00586-015-3801-9 [DOI] [PubMed] [Google Scholar]
- 15. Gabel BC, Hoshide R, Taylor W. An algorithm to predict success of indirect decompression using the extreme lateral lumbar interbody fusion procedure. Cureus. 2015;7:e317 doi:10.7759/cureus.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sharma AK, Kepler CK, Girardi FP, Cammisa FP, Huang RC, Sama AA. Lateral lumbar interbody fusion: clinical and radiographic outcomes at 1 year: a preliminary report. J Spinal Disord Tech. 2011;24:242–250. doi:10.1097/BSD.0b013e3181ecf995 [DOI] [PubMed] [Google Scholar]
- 17. Sembrano JN, Tohmeh A, Isaacs R; SOLAS Degenerative Study Group. Two-year comparative outcomes of MIS lateral and MIS transforaminal interbody fusion in the treatment of degenerative spondylolisthesis: part I: clinical findings. Spine (Phila Pa 1976). 2016;41(suppl 8):S123–S132. doi:10.1097/BRS.0000000000001471 [DOI] [PubMed] [Google Scholar]
- 18. Youssef JA, McAfee PC, Patty CA, et al. Minimally invasive surgery: lateral approach interbody fusion: results and review. Spine (Phila Pa 1976). 2010;35(26 suppl):S302–S311. doi:10.1097/BRS.0b013e3182023438 [DOI] [PubMed] [Google Scholar]
- 19. Rodgers WB, Gerber EJ, Patterson J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: an analysis of 600 cases. Spine (Phila Pa 1976). 2011;36:26–32. doi:10.1097/BRS.0b013e3181e1040a [DOI] [PubMed] [Google Scholar]
- 20. Winder MJ, Gambhir S. Comparison of ALIF vs. XLIF for L4/5 interbody fusion: pros, cons, and literature review. J Spine Surg. 2016;2:2–8. doi:10.21037/jss.2015.12.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Navarro-Ramirez R, Berlin C, Lang G, et al. A new volumetric radiologic method to assess indirect decompression after extreme lateral interbody fusion using high-resolution intraoperative computed tomography. World Neurosurg. 2018;109:59–67. doi:10.1016/j.wneu.2017.07.155 [DOI] [PubMed] [Google Scholar]
- 22. Nakashima H, Kanemura T, Satake K, et al. Unplanned second-stage decompression for neurological deterioration caused by central canal stenosis after indirect lumbar decompression surgery. Asian Spine J. 2019;13:584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu D, Walker C, Godzik J, Turner J, Smith W, Uribe JS. Minimally invasive anterior, lateral and oblique lumbar interbody fusion: a literature review. Ann Transl Med. 2018;6:104. [DOI] [PMC free article] [PubMed] [Google Scholar]