Abstract
Study Design:
Multicenter, prospective, randomized, and double-blinded study.
Objectives:
To compare tubular and endoscopic interlaminar approach.
Methods:
Patients with lumbar spinal stenosis and neurogenic claudication of were randomized to tubular or endoscopic technique. Enrollment period was 12 months. Clinical follow up at 1, 3, 6 months after surgery with visual analogue scale (VAS), Oswestry Disability Index (ODI), and Japanese Orthopedic Association (JOA) score. Radiologic evaluation with magnetic resonance pre- and postsurgery.
Results:
Twenty patients were enrolled: 10 in tubular approach (12 levels) and 10 in endoscopic approach (11 levels). The percentage of enlargement of the spinal canal was higher in endoscopic approach (202%) compared with tubular approach (189%) but was not statistically significant (P = .777). The enlargement of the dural sac was higher in endoscopic group (209%) compared with tubular group (203%) but no difference was found between the 2 groups (P = .628). A modest significant correlation was found between the percentage of spinal canal decompression and enlargement of the dural sac (r = 0.5, P = .023). Both groups reported a significant clinical improvement postsurgery. However, no significant association was found between the percentage of enlargement of the spinal canal or the dural sac and clinical improvement as determined by scales scores. Endoscopic group had lower intrasurgical bleeding (P < .001) and lower disability at 6 months of follow-up than tubular group (p=0.037).
Conclusions:
In the treatment of lumbar spinal stenosis, endoscopic technique allows similar decompression of the spinal canal and the dural sac, lower intrasurgical bleeding, similar symptoms improvement, and lower disability at 6 months of follow-up, as compared with the tubular technique.
Keywords: spinal canal decompression, endoscopic technique, spinal canal, prospective, magnetic resonance, follow-up
Introduction
Lumbar spinal stenosis (LSS) was descriped by Verbiest1 in 1954 as a clinical condition occasioned by compression of nerve roots on walking and standing. Actually, it is a frequent cause of lumbar and leg pain in elderly adults and is responsible of 1.2 million consultations every year in the United States. It is for this reason, the surgery of LSS probably is the most frequent in people older than 65 years.2 The surgery in this condition is performed in patients with moderate or severe symptoms in whom medical treatment fails. Many studies have shown that surgery is better than conservative treatment.3-7 The gold standard in the spinal lumbar stenosis has been traditionally the open laminectomy, with good outcome in 56% to 85% of patients. In this approach, bilateral dissection of paraspinal muscle and resection of spinous process, ligaments supra, and interspinous is done to perform laminectomy.
In the past years, the minimally invasive spine surgery (MISS) has gained in popularity among spine surgeons because several studies have shown similar results as open laminectomy. With MISS, the bilateral decompression is made through an unilateral approach with less muscle damage allowing patients to a fast recovery without risk of iatrogenic instability because spinous process and ligaments are preserved. In addition, MISS is superior to traditional surgery in terms of less tissue trauma, less rate of complications, less blood loss, short hospital stay, and early patient recovery.8-12
It has been previously in some studies that compared radiologically the grade of decompression between MISS and traditional surgery. Fessler et al13 in 2002 compared traditional versus endoscopic surgery in a cadaveric study and concluded, after postoperative evaluation with tomography, that these measurements are similar. Heo et al14 in 2018 compared the pre and postoperative dural sac cross-section area (DSCSA) and concluded that there is no significant difference in grade of decompression between traditional and biportal endoscopic laminectomy.
We propose a comparative study of MISS between tubular and endoscopic techniques with a unilateral and interlaminar approach for LSS.
Methods
A multicenter, prospective, randomized, double-blind study was performed from April 2017 to April 2018. The patients were treated in 2 hospitals of Madrid, Spain (Hospital Clínico San Carlos and Hospital Puerta de Hierro) with approval of ethics commit of both institutions. Randomization was made by 1:1 method.
Enrollment period was 12 months, and patients were clinically followed at least 6 months after surgery. The inclusion criteria were neurogenic claudication, failure of conservative treatment in past 3 months, evidence of central lumbar stenosis in MRI (magnetic resonance imaging), and no previous spinal surgery. Exclusion criteria were degenerative spondylolisthesis grade II or higher, degenerative scoliosis greater than 30 grades, significant axial back pain (visual analogue scale [VAS] >3/10), neurological or osteoarticular disease, symptomatic disc herniation on the same or other level, and segmental instability on dynamic radiographs.
We performed interlaminar approach with bilateral decompression through unilateral approach with tubular retractors and under microscope view or through a uniportal endoscope. We describe the surgical techniques step by step.
Tubular Decompression
Equipment
We used an 18-mm diameter tubular approach, serial dilators, bipolar, dissectors, hooks, spherical bur, Kerrison punches (90° and 45°), and disc forceps.
Operative Procedure
The procedures were performed under general anesthesia, with the patient in prone position with rolls in thoracic region and hips for avoid the pressure of the abdominal organs and venous drainage defect that would favor lumbar epidural bleeding. The distribution of the C-arm and the monitors is important to allow a comfortable view of the surgeon during the procedure. The surgical field was prepared with chlorhexidine solution and a waterproof surgical drape was applied after induction of anesthesia. Anteroposterior and lateral fluoroscopy were used to localize the correct position. An 18-mm paramedian incision is made in the skin making sure that the muscular fascia is opened to introduce the dilator tubes. The muscle dilated sequentially, after which we placed a working channel of 18 mm in diameter and with a length of depth that varied depending on the subcutaneous adipose tissue. The surgical microscope was moved to the field and the lower edge of the lamina was identified. A laminotomy was performed, cranially, until the insertion point of the flavum ligament is found in the upper lamina and caudally to include a smaller portion of the upper aspect of the inferior lamina. A careful dissection of the yellow ligament to the dural surface was performed and with the help of Kerrison punches, ipsilateral flavectomy was performed. The working channel tilts medially and the surgical table is angled about 20° in the opposite direction for the surgeon to allow access to the contralateral side. This maneuver exposes the spinous process (which is removed using a drill) and the contralateral side where the residual lamina and the flavum ligament could be resected using the drill, Kerrison punches, and curettes. Successful decompression is achieved under direct visualization. The incision was closed in layers with Vicryl and intradermal suture.
Endoscopic Decompression
Equipment
We used a 10-mm diameter endoscope with 6-mm worker channel, a work tube, a bipolar flexible radiofrequency probe, serial dilators, and specially designed dissectors, hooks, spherical bur, bullet bur, olive bur with unilateral protection, Kerrison punches (90° and 40°), and disc forceps (iLESSYS interLaminar Endoscopic Surgical System).
Operative Procedure
The procedures were performed under general anesthesia, with the patient in prone position with rolls in thoracic region and hips for avoid the pressure of the abdominal organs and venous drainage defect that would favor lumbar epidural bleeding. The distribution of the C-arm and the monitors is important to allow a comfortable view of the surgeon during the procedure. The surgical field was prepared with chlorhexidine solution and a waterproof surgical drape was applied after induction of anesthesia. Anteroposterior and lateral fluoroscopy are used to localize the correct position. Stab incision of 10 mm is made, at this point we check the fascia opening to allow the entrance of dilators tubes. It is very important that the incision be of the size of the working tube so that the muscles hold it and keep it stable, since unlike the tubular approach, the endoscopic does not have a holding handle. The guide cannula was introduced under radioscopic guidance and sequential dilators were then used to aim at the interlaminar space. The working tube is screwed and confirmed with radioscope the correct position and the rigid laminoscope was inserted through the working tube.
Under endoscopic view, a high-speed endoscopic drill with 4.5-mm outer diameter head was used for laminotomy and endoscopic Kerrison punches with 1.5- and 3.0-mm footprints were used to remove additional bone of lamina, medial facet, and ligament flavum (Video 1, available in the online version of the article). With aim of avoid a dural tear, meticulous dissection of the interface between the ligament flavum and dura was performed with blunt dissector and hook. Also, the pressure of irrigation with saline solution could be turned up for aid in the retraction of dura away from ligament flavum in the decompression. Hemostasis was achieved with bipolar flexible radiofrequency probe. Once the ipsilateral ligament and medial aspect of facet was removed the endoscope is turned to the opposite side for central and contralateral decompression. If the maneuver was interrupted by the spinous process, the basal aspect could be drilled.
Adequate decompression is determined by observing the dural sac pulsation and nerve root mobility (Video 1, available in the online version of the article), as well as the final fluoroscopic revision to confirm the magnitude of decompression. At this point, the working channel and scope were removed, and fascia and skin were closed with a single suture.
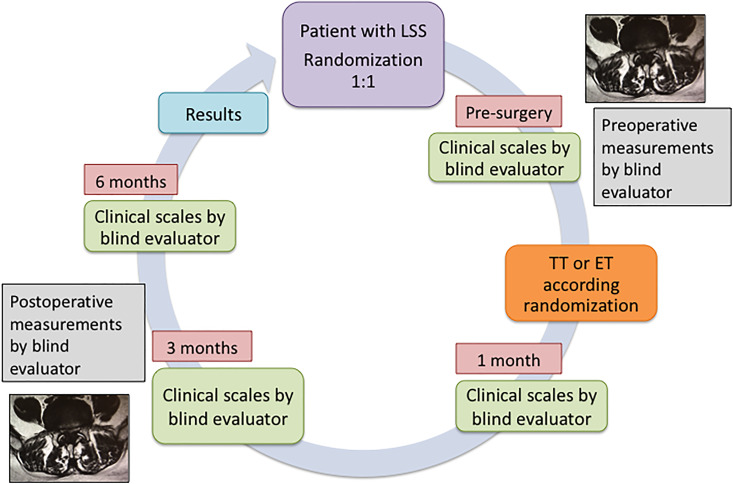
Before surgery, patient symptoms and functional limitation were evaluated with scales for leg pain (VAS), disability (Oswestry Disability Index [ODI]), and the Japanese Orthopedic Association (JOA) score. All scales were repeated at 30 days, 3 months, and 6 months after surgery in a blinded manner regarding type of minimally invasive surgical technique (MIST; Figure 1). Other clinical aspect evaluated was the Walking Claudication Distance (WCD), which was measured in the preoperative stage and at 6 months of treatment.
Figure 1.
Schedule.
All patients were evaluated with MRI before and after surgical treatment. The spinal canal cross-sectional area (SCCSA) and dural sac cross-sectional area (DSCSA) were measured using the area function of the image file communication system IMPAX (Madrid, Spain). The area of dural sac and spinal canal could be automatically calculated by drawing a line along the outer wall of the dura and inner wall of spinal canal respectively (Figure 2a and b). In this sense, an evaluator blinded to the type of MIST performed the measurements. It is important to clarify that the surgical access approach is observed in postsurgical MRI; however, the evaluator cannot differentiate whether the approach was performed through an 18-mm dilator tube or with a 10-mm endoscope, so the measurement of the areas of the dural sac and the spinal canal were performed without knowledge of the technique used.
Figure 2.
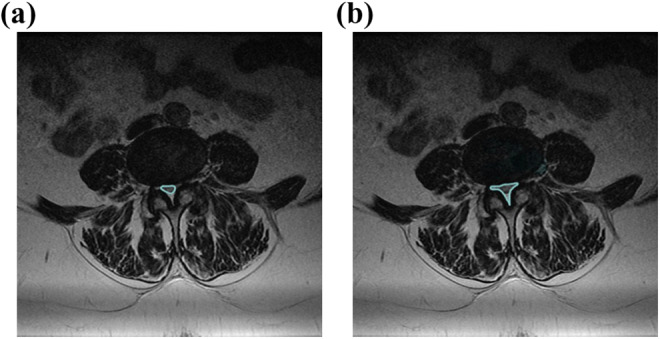
(a) Dural sac cross-sectional area. (b) Spinal canal cross-sectional area.
The measurement of intraoperative bleeding was performed in each surgical technique. In the endoscopic approach that is performed under continuous irrigation we have used nonabsorbable surgical fields with drainage bags directly connected to the aspiration system to perform the calculation of intraoperative bleeding. In addition to the irrigation that was not possible to collect through this route, we have used the floor suction device (Puddle Vac). The Puddle Vac is easily moved wherever it is needed (Figure 3).
Figure 3.

Floor suction device.
Statistical Analysis
All data was analyzed using IBM SPSS Statistics Software Application 23.0 version for Mac. The continuous variables are presented as the mean and standard deviation (SD), with discontinuous variables as percentages. Clinical differences evaluated by preoperative and postoperative VAS, ODI, and JOA scales were evaluated by the Wilcoxon rank-sum test, and its correlation with percentages of decompression with Spearman’s rank correlation coefficient. The difference of enlargement between one technique and other were compared with Mann-Whitney U test. P < .05 was considered significant.
Results
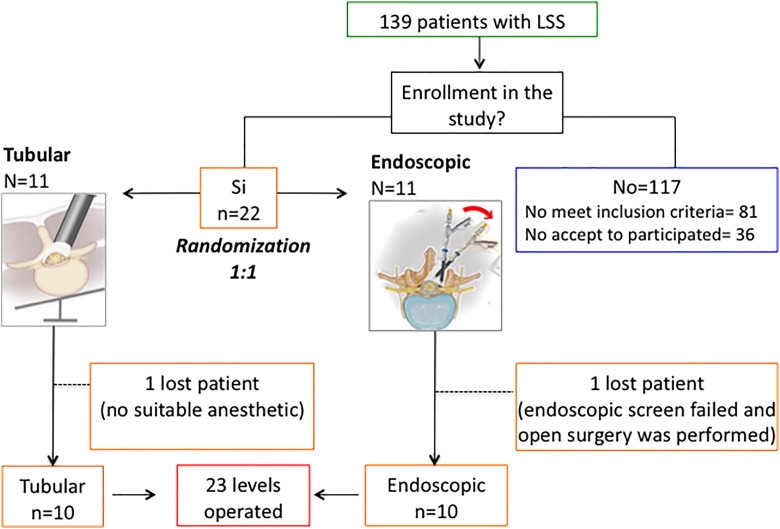
A total 139 patients were enrolled in this study but just 22 met the inclusion criteria and 2 of them were excluded; one for anesthetic problems and other for instrumental failure (the image on the endoscope screen was not clear). The distribution of the patients was as follows: of the 139 patients, 81 did not meet the inclusion criteria (40 required laminectomy and fusion, 20 required laminectomy and discectomy in the same level, 6 had scoliosis, 4 patients were operated from a hernia on another level, 2 had neurological disease, and 7 had osteoarticular disease in the lower limbs (which affected the assessment of clinical scales), 1 patient operated upon at another center and 1 patient presented with congenital canal stenosis. On the other hand, 36 did not participate in the study either because the surgeon only performed traditional open surgery or because the patient preferred traditional surgery; in 28 an open laminectomy was performed, in 7 interspinous device was implanted, and 1 refused to undergo a control magnetic resonance imaging.
Then 10 patients who underwent tubular decompression and 10 patients who underwent endoscopic decompression were included in this study. Twenty-three spinal levels were treated because 2 patients treated with tubular technique (T) and one treated with endoscopic technique (E) had 2 levels affected (Figure 4).
Figure 4.
Flowchart.
All patients were followed up at least 6 months after surgery. There were no significant differences in age, sex, body mass index (BMI), levels treated, and complications using the Fisher exact test (Table 1).
Table 1.
Demographic Characteristics and Clinical Outcome.
| Characteristics | Tubular Technique | Endoscopic Technique | P |
|---|---|---|---|
| Gender, male/female (total) | 4/6 (10) | 4/6 (10) | NS |
| Age, years | 69.7 (SD = 8.62) | 73.5 (SD = 10.51) | NS |
| BMI, kg/m2 | 29.3 (SD = 2.45) | 30.1 (SD = 3.41) | NS |
| Total levels treated, n (%) | 12 | 11 | |
| L2-L3 | 2 (17) | 0 (0) | |
| L3-L4 | 4 (33) | 2 (18) | |
| L4-L5 | 5 (42) | 9 (82) | |
| L5-S1 | 1 (8) | 0 (0) | |
| Surgery time, minutes | 117.5 (IQR 90-132.5) | 125 (IQR 85-145) | NS |
| Surgical blood loss, mL | 106 (IQR 90-126.2) | 18 (IQR 14-21) | <.001 |
| Preoperative SCCSA, mm2 | 87.4 (IQR 64.2-104.2) | 65.3 (IQR 48.3-97.4) | |
| Postoperative SCCSA (mm2) | 161.6 (IQR 123-210.2) | 157.3 (IQR 101.4-191.1) | |
| Preoperative DSCSA, mm2 | 48.5 (IQR 38.9-64.3) | 48 (IQR 32-60) | |
| Postoperative DSCSA (mm2) | 113.8 (IQR 77.1-139.5) | 95 (IQR 53-123) | |
| Lumbar canal decompression, % | 189 (IQR 163-275) | 202 (IQR 144-282) | NS |
| Dural sac enlargement, % | 203 (IQR 143-259) | 209 (IQR 114-251) | NS |
| Preoperative VAS | 9 (IQR 7-10) | 8 (IQR 7.5-10) | |
| Postoperative VAS at 6 months | 0.5 (IQR 0.0-3.25) | 0 (IQR 0-1) | NS |
| Preoperative ODI | 49.5 (IQR 40.2-65) | 72 (IQR 50-77) | |
| Postoperative ODI at 6 months | 3 (IQR 0.0-19.5) | 6 (IQR 0.0-10.5) | .037 |
| Preoperative JOA score | 11.5 (IQR 3-14.25) | 8 (IQR 1-13.5) | |
| Postoperative JOA score at 6 months | 26.5 (IQR 21-29) | 28 (IQR 26.5-29) | NS |
| Complications | |||
| Durotomy | 0 | 2 | NS |
| Wound dehiscence | 1 | 0 | NS |
| Postoperative hematoma | 1 | 0 | NS |
| Urinary tract infection | 1 | 1 | NS |
| Pulmonary embolism | 1 | 0 | NS |
Abbreviations: SCCSA, spinal canal cross-sectional area; DSCSA, dural sac cross-sectional area; NS, not significant; VAS, visual analogue scale; ODI, Oswestry Disability Index; JOA, Japanese Orthopedic Association; IQR, interquartile range.
The media of preoperative SCCSA were 87.4 mm2 (interquartile range [IQR] = 64.2-104.2 mm2) in T and 65.3 mm2 (IQR = 48.3-97.4 mm2) in E. In postoperative stage, the values were 161.6 mm2 (IQR = 123-210.2 mm2) and 157.3 mm2 (IQR = 101.4-191.1 mm2), respectively. The improvement in SCCSA was 189% (IQR 163-275, P = .001) in T and 202% (IQR 144-282, P < .001) in E. Given that the endoscopy group apparently started from a smaller area of the canal than the tubular group, a comparison of statistics was made by comparing with the Mann-Whitney U test where no significant differences were found between the presurgical areas of both groups (P = .777).
The media of preoperative DSCSA were 48.5 mm2 (IQR = 38.9-64.3 mm2) in T and 48 mm2 (IQR = 32-60 mm2) in ET. In postoperative stage, the values were 113.8 mm2 (IQR = 77.1-139.5 mm2) and 95 mm2 (IQR = 53-123 mm2), respectively. The improvement in DSCSA was 203% (IQR 143-259, P < .001) in T and 209% (IQR 114-251, P = .015) in E. With Mann-Whitney U test we did not find significant differences between the techniques (P = .628).
The preoperative stenotic SCCSA and DSCSA were significantly increased in both groups after surgery (P < .05).
There was no difference at 6 months in postoperative scales VAS (P = .558) and JOA (P = .119) in both techniques, the only significant difference found was a better improvement in ODI at 6 months in endoscopic group (P = .037; Table 1).
There is no correlation between the value of SCCSA and DSCSA and improvement of clinical scales with Spearman’s rank correlation coefficient (P > .05). A modest significant correlation was found between the percentage of spinal canal decompression and enlargement of the dural sac (r = 0.5, P = .023).
The mean preoperative WCD was 121.9 m (SD = 143.9) in tubular group and 263.3 m (SD = 314) in endoscopic group whereas the mean of postoperative WCD was 2450 m (SD = 2543.5) and 3444.4 m (SD = 2242.27), respectively. We found good outcome in both groups but without significant statistical difference between them (P = .141).
The mean operation times were 125 minutes (IQR 85-145 minutes) in the E group and 117.5 minutes (IQR 90-132.5 minutes) in the T group. There was no significant difference in the mean operation time between the 2 techniques (P > .05).
The blood loss values were 106 mL (IQR = 90-126.25 m L) in the T group and 18 mL (IQR = 14-21 mL) in the E group. We found a significant difference with less blood loss in the E group (P < .001, confidence interval [CI] = 99%).
There was no significant difference in incidence of complications between the 2 groups, in E there were 2 dural tears and in T there was 1 wound dehiscence and 1 postoperative epidural hematoma evacuated by the same tubular approach. No infections or cerebrospinal fistulas was found (Table 1).
Discussion
LSS is a frequent pathology in patients over the sixth decade. Given that life expectancy has increased in past years, it is expected that the incidence of this pathology will increase too and therefore the number of procedures performed as well.15 It is of vital importance to remember that with more age, the patients have more possibility of multipathology; for that reason, it is essential to handle less invasive surgical techniques that offer good clinical outcomes with a faster recovery in these patients.
In the elderly and the obese, there are no contraindications for surgical treatment. Patients older than 80 years show a postoperative improvement similar to youngers and commoncomplications in them were urinary infections like in our case.16-20
The mean goal of surgery is to decrease pain and disability of patients to decompress canal spinal stenosis and thus improve their quality of life. Several studies have tested safety and effectiveness of minimally invasive surgery for LSS.21-25
The endoscopic11,22,23,26-35 and tubular10,36-42 approach have yielded good results in comparatives studies with open traditional approach.24
However, to our knowledge, there are no prospective studies that compare these 2 techniques both clinically and radiologically. Recently, McGrath et al25 performed a retrospective study in 95 patients comparing both techniques and finding superiority in the endoscopic technique in terms of shorter hospital stay, lower complication rate, lower blood loss, and lower ODI and VAS at 1-year follow-up. Our findings are similar, finding less intraoperative bleeding and less ODI at the end of follow-up. In our opinion, the statistically significant result of the disability scale at 6 months is the most important finding of this study. The pain scale only provides us with a numerical value; on the contrary, the JOA scale and the ODI give us much more information about the clinical improvement of the patients. ODI also gives us information that the JOA scale does not give us, such as the patient’s ability to travel, his social and sexual activity, personal care, and nighttime rest. Although the JOA scale gives us much more information compared with the numerical VAS, it does not include the parameters mentioned above.
Our results of percentage of area enlargement were significant for spinal canal and dural sac and we do not found difference statistical between both techniques as was also found in the study carried out by Lee et al.24
There are some studies that compared measurement of pre and postoperative spinal canal and dural sac area. A study made by Mariconda et al43 in 2002 found a preoperative DSCSA of 70.76 ± 28.2 mm2 and postoperative DSCSA of 108.12 ± 31.5 mm2 in tubular approach of minimally invasive technique. Our results are little better than those maybe because our preoperative DSCSA was smaller.
A recent retrospective study published by Akbary et al,31 in September 2018, assessed the endoscopic biportal approach in 30 patients finding an enlargement from SCCSA of 99 mm2 to 186 mm2 with a conclusion that the technique offers a good decompression. These results are similar to those in our patients who underwent a decompression with a uniportal endoscopic technique. Both techniques show good clinical outcomes in correlation with literature.21-25,28-31,37-39,44-48
We do not find correlation between the grade of decompression of spinal canal and dural sac with clinical improvement in patients, a finding that correlates with most published studies. Many authors conclude that there is no relation between radiologic stenosis grade and symptoms of patients.43,49-56 That is maybe because the spinal canal stenosis is not just an anatomical disease and have a physiopathology background. It is believed that the intermittent hypoxia of cauda equine roots that results from venous congestion and the lack of arterial vasodilatation of the congested roots offers a physiopathological mechanism to neurogenic claudication.57,58 In addition, the canal diameter has a dynamic variation with flexion and extension movements and the routine MRI is a static study. Also, the difference in sensibility at pain between patients could be a conditioning factor for these discrepancy like Kim et al59 found in their study.
Our mean surgical time in the E group was 125 minutes, which is similar to other studies like Wada et al21 with 144 minutes and Lee et al35 with 105 minutes. On the other hand, the mean of surgical time in the T group was 117 minutes that is more than what is published, perhaps because we are in our learning curve and because the 2 patients operated with a 2-level tubular approach, are the patients who recorded the longest surgical time of all the series. An explanation could be the advanced age they were (79 and 77 years old) and a severe stenosis of the canal with areas of the presurgical dural sac of 36.8 and 45.4 mm2 in the first level, and 71.6 and 25.6 mm2 in the second level, which made the surgery long and cumbersome. Unfortunately, we have not collected the surgical time by level intervened.
The blood loss was significantly less in the E group (18 mL, P < .001). This result is similar to that found by Nomura et al.60 The blood loss in the T group was 106 mL, which shows superiority of the endoscopic approach against blood loss.
Our main complication in the T group were 1 epidural hematoma. Our search for an explanation found that Fujita et al61 in 2018 identified that hypertension, multilevel surgery, and lordosis less than 25° were considered risk factors. On the other hand, Fujiwara et al62 found in 2017 that hypertension and lack of debit in surgical drainage were also risk factors. Our patient had hypertension, a lordosis of 23°, and was without drainage, maybe for that reason the patient was at more risk to develop this complication.
In the E group, the main complication was dural tear observed in 2 patients. Durotomy is the most frequent complication in LSS. One study published by Strömqvist et al63 in August 2018 with 64,431 patients showed an incidence of 5%, and that is more frequent in older patients and in surgery of LSS than discectomy. In addition, during the learning curve of endoscopic surgery, it is frequent to get durotomy as complication.21 We resolved this with autologous muscle and biological sealant through working channel of endoscope. The durotomies had an approximate diameter between 2 and 3 mm. However, since they are such small defects and make the closure with materials of the very small size, large areas of hard adhesions to these materials are not expected. This management was described by Oertel et al64 in 2017 proving that endoscopic surgery is a faster, effective, and safe way to resolve a dural tear. If a lumbar reintervention is required, a transforaminal approach could be considered to avoid fibrosis caused by previous durotomy and repair.
Recently, the use of a double layer of TachoSil65 and suturing of the dura mater through the endoscope for incidental durotomy was described.66
We did not have cerebrospinal fistulas or pseudomeningocele (all patients had MRI postoperative), maybe because the small incision in MISS decreases the death space in tissues with the consequent pseudomeningocele formation.64 Moreover, a good closure of the wound makes for a very low probability of fistula.
Conclusion
There is no statistically significant difference in percentage of decompression between tubular and endoscopic technique in our study. Both procedures yielded good clinical outcomes at follow-up with a slightly better grade of disability in endoscopic technique at 6 months. The MISS techniques were shown to be safe and effective in the treatment of LSS. We did not find correlation between the grade of decompression of spinal canal and dural sac with clinical improvement in patients. The blood loss is statistically significantly low in endoscopic approach but could be clinically irrelevant. However, because this is a pilot study, we think that full trial would provide greater confidence in these results.
Supplementary Material
Footnotes
Author Contributions: Conception and design: Angela Carrascosa-Granada, Willian Velazquez. Acquisition of data: Angela Carrascosa-Granada, Andrés Vargas-Jimenez. Analysis and interpretation of data: Angela Carrascosa-Granada, Willian Velazquez. Drafting the article: Angela Carrascosa-Granada, Willian Velazquez. Critically revising the article: Ralf Wagner. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors. Statistical analysis: Angela Carrascosa-Granada. Administrative/technical/material support: Ralf Wagner. Study supervision: Ralf Wagner
Authors’ Note: This article is part of PhD thesis of Dra. Angela Carrascosa-Granada, Universidad Complutense de Madrid, Spain.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Ralf Wagner, consulting and teaching for Joimax.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This supplement was supported by funding from the Carl Zeiss Meditec Group.
ORCID iD: Angela Carrascosa-Granada, MD, PhD  https://orcid.org/0000-0003-2231-8346
https://orcid.org/0000-0003-2231-8346
Supplemental Material: The supplemental material is available in the online version of the article.
References
- 1. Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. 1954. Clin Orthop Relat Res. 2001;(384):3–9. [DOI] [PubMed] [Google Scholar]
- 2. Joaquim AF, Sansur CA, Hamilton DK, Shaffrey CI. Degenerative lumbar stenosis: update. Arq Neuropsiquiatr. 2009;67(2B):553–558. [DOI] [PubMed] [Google Scholar]
- 3. Amundsen T, Weber H, Nordal HJ, Magnaes B, Abdelnoor M, Lilleâs F. Lumbar spinal stenosis: conservative or surgical management? A prospective 10-year study. Spine (Phila Pa 1976). 2000;25:1424–1436. [DOI] [PubMed] [Google Scholar]
- 4. Atlas SJ, Deyo RA, Keller RB, et al. The Maine Lumbar Spine Study, Part III. 1-year outcomes of surgical and nonsurgical management of lumbar spinal stenosis. Spine (Phila Pa 1976). 1996;21:1787–1795. [DOI] [PubMed] [Google Scholar]
- 5. Weinstein JN, Tosteson TD, Lurie JD, et al. ; SPORT Investigators. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358:794–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Atlas SJ, Keller RB, Robson D, Deyo RA, Singer DE. Surgical and nonsurgical management of lumbar spinal stenosis: four-year outcomes from the Maine lumbar spine study. Spine (Phila Pa 1976). 2000;25:556–562. [DOI] [PubMed] [Google Scholar]
- 7. Malmivaara A, Slätis P, Heliövaara M, et al. ; Finnish Lumbar Spinal Research Group. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine (Phila Pa 1976). 2007;32:1–8. [DOI] [PubMed] [Google Scholar]
- 8. Kovacs FM, Urrútia G, Alarcón JD. Surgery versus conservative treatment for symptomatic lumbar spinal stenosis: a systematic review of randomized controlled trials. Spine (Phila Pa 1976). 2011;36:E1335–E1351. [DOI] [PubMed] [Google Scholar]
- 9. Turner JA, Ersek M, Herron L, Deyo R. Surgery for lumbar spinal stenosis. Attempted meta-analysis of the literature. Spine (Phila Pa 1976). 1992;17:1–8. [DOI] [PubMed] [Google Scholar]
- 10. Thomé C, Zevgaridis D, Leheta O, et al. Outcome after less-invasive decompression of lumbar spinal stenosis: a randomized comparison of unilateral laminotomy, bilateral laminotomy, and laminectomy. J Neurosurg Spine. 2005;3:129–141. [DOI] [PubMed] [Google Scholar]
- 11. Khoo LT, Fessler RG. Microendoscopic decompressive laminotomy for the treatment of lumbar stenosis. Neurosurgery. 2002;51(5 suppl): S146–S154. [PubMed] [Google Scholar]
- 12. Ng KKM, Cheung JPY. Is minimally invasive surgery superior to open surgery for treatment of lumbar spinal stenosis? A systematic review. J Orthop Surg (Hong Kong). 2017;25:2309499017716254. [DOI] [PubMed] [Google Scholar]
- 13. Polikandriotis JA, Hudak EM, Perry MW. Minimally invasive surgery through endoscopic laminotomy and foraminotomy for the treatment of lumbar spinal stenosis. J Orthop. 2013;10:13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yadav YR, Parihar V, Kher Y, Bhatele PR. Endoscopic inter laminar management of lumbar disease. Asian J Neurosurg. 2016;11:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosen DS, O’Toole JE, Eichholz KM, et al. Minimally invasive lumbar spinal decompression in the elderly: outcomes of 50 patients aged 75 years and older. Neurosurgery. 2007;60:503–510. [DOI] [PubMed] [Google Scholar]
- 16. Phan K, Mobbs RJ. Minimally invasive versus open laminectomy for lumbar stenosis: a systematic review and meta-analysis. Spine (Phila Pa 1976). 2016;41:E91–E100. [DOI] [PubMed] [Google Scholar]
- 17. Hopp E, Tsou PM. Postdecompression lumbar instability. Clin Orthop Relat Res. 1988;227:143–151. [PubMed] [Google Scholar]
- 18. Jansson KA, Németh G, Granath F, Blomqvist P. Spinal stenosis re-operation rate in Sweden is 11% at 10 years—a national analysis of 9,664 operations. Eur Spine J. 2005;14:659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jansson KA, Blomqvist P, Granath F, Németh G. Spinal stenosis surgery in Sweden 1987-1999. Eur Spine J. 2003;12:535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fredman B, Arinzon Z, Zohar E, et al. Observations on the safety and efficacy of surgical decompression for lumbar spinal stenosis in geriatric patients. Eur Spine J. 2002;11:571–574. [DOI] [PubMed] [Google Scholar]
- 21. Wada K, Sairyo K, Sakai T, Yasui N. Minimally invasive endoscopic bilateral decompression with a unilateral approach (endo-BiDUA) for elderly patients with lumbar spinal canal stenosis. Minim Invasive Neurosurg. 2010;53:65–68. [DOI] [PubMed] [Google Scholar]
- 22. Komp M, Hahn P, Oezdemir S, et al. Bilateral spinal decompression of lumbar central stenosis with the full endoscopic interlaminar versus microsurgical laminotomy technique: a prospective, randomized, controlled study. Pain Physician. 2015;18:61–70. [PubMed] [Google Scholar]
- 23. Pao JL, Chen WC, Chen PQ. Clinical outcomes of microendoscopic decompressive laminotomy for degenerative lumbar spinal stenosis. Eur Spine J. 2009;18:672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee CW, Yoo KJ, Ha SS. Comparative analysis between three different lumbar decompression techniques (microscopic, tubular, and endoscopic) in lumbar canal and lateral recess stenosis: preliminary report. Biomed Res Int. 2019;2019:6078469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McGrath LB, White-Dzuro GA, Hofstetter CP. Comparison of clinical outcomes following minimally invasive or lumbar endoscopic unilateral laminotomy for bilateral decompression [published online January 11, 2019]. J Neurosurg Spine. doi:10.3171/2018.9.SPINE18689 [DOI] [PubMed] [Google Scholar]
- 26. Heo DH, Quillo-Olvera J, Park CK. Can percutaneous biportal endoscopic surgery achieve enough canal decompression for degenerative lumbar stenosis? Prospective case-control study. World Neurosurg. 2018;120:e684–e689. [DOI] [PubMed] [Google Scholar]
- 27. Kim JE, Choi DJ. Clinical and radiological outcomes of unilateral biportal endoscopic decompression by 30° arthroscopy in lumbar spinal stenosis: minimum 2-year follow-up. Clin Orthop Surg. 2018;10:328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang B, Chen R, Xie P, Liu B, Dong J, Rong L. Microendoscopic decompression via unilateral approach for lumbar spinal stenosis [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011;25:1158–1163. [PubMed] [Google Scholar]
- 29. Ruetten S, Komp M, Merk H, Godolias G. Surgical treatment for lumbar lateral recess stenosis with the full-endoscopic interlaminar approach versus conventional microsurgical technique: a prospective, randomized, controlled study. J Neurosurg Spine. 2009;10:476–485. [DOI] [PubMed] [Google Scholar]
- 30. Wong AP, Smith ZA, Lall RR, Bresnahan LE, Fessler RG. The microendoscopic decompression of lumbar stenosis: a review of the current literature and clinical results. Minim Invasive Surg. 2012;2012:325095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Akbary K, Kim JS, Park CW, Jun SG, Hwang JH. Biportal endoscopic decompression of exiting and traversing nerve roots through a single interlaminar window using a contralateral approach: technical feasibilities and morphometric changes of the lumbar canal and foramen. World Neurosurg. 2018;117:153–161. [DOI] [PubMed] [Google Scholar]
- 32. Minamide A, Yoshida M, Yamada H, et al. Endoscope-assisted spinal decompression surgery for lumbar spinal stenosis. J Neurosurg Spine. 2013;19:664–671. [DOI] [PubMed] [Google Scholar]
- 33. Eum JH, Heo DH, Son SK, Park CK. Percutaneous biportal endoscopic decompression for lumbar spinal stenosis: a technical note and preliminary clinical results. J Neurosurg Spine. 2016;24:602–607. [DOI] [PubMed] [Google Scholar]
- 34. Kim HS, Paudel B, Jang JS, et al. Percutaneous full endoscopic bilateral lumbar decompression of spinal stenosis through uniportal-contralateral approach: techniques and preliminary results. World Neurosurg. 2017;103:201–209. [DOI] [PubMed] [Google Scholar]
- 35. Lee CW, Yoon KJ, Jun JH. Percutaneous endoscopic laminotomy with flavectomy by uniportal, unilateral approach for the lumbar canal or lateral recess stenosis. World Neurosurg. 2018;113:e129–e137. [DOI] [PubMed] [Google Scholar]
- 36. Hamanishi C, Matukura N, Fujita M, Tomihara M, Tanaka S. Cross-sectional area of the stenotic lumbar dural tube measured from the transverse views of magnetic resonance imaging. J Spinal Disord. 1994;7:388–393. [PubMed] [Google Scholar]
- 37. Alimi M, Hofstetter CP, Torres-Campa JM, et al. Unilateral tubular approach for bilateral laminotomy: effect on ipsilateral and contralateral buttock and leg pain. Eur Spine J. 2017;26:389–396. [DOI] [PubMed] [Google Scholar]
- 38. Alimi M, Njoku I, Jr, Cong GT, et al. Minimally invasive foraminotomy through tubular retractors via a contralateral approach in patients with unilateral radiculopathy. Neurosurgery. 2014;10(suppl 3):436–447. [DOI] [PubMed] [Google Scholar]
- 39. Alimi M, Hofstetter CP, Pyo SY, Paulo D, Härtl R. Minimally invasive laminectomy for lumbar spinal stenosis in patients with and without preoperative spondylolisthesis: clinical outcome and reoperation rates. J Neurosurg Spine. 2015;22:339–352. [DOI] [PubMed] [Google Scholar]
- 40. Ha S, Hong Y, Lee S. Minimally invasive lumbar spinal decompression in elderly patients with magnetic resonance imaging morphological analysis. Asian Spine J. 2018;12:285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mobbs RJ, Li J, Sivabalan P, Raley D, Rao PJ. Outcomes after decompressive laminectomy for lumbar spinal stenosis: comparison between minimally invasive unilateral laminectomy for bilateral decompression and open laminectomy: clinical article. J Neurosurg Spine. 2014;21:179–186. [DOI] [PubMed] [Google Scholar]
- 42. Yagi M, Okada E, Ninomiya K, Kihara M. Postoperative outcome after modified unilateral-approach microendoscopic midline decompression for degenerative spinal stenosis. J Neurosurg Spine. 2009;10:293–299. [DOI] [PubMed] [Google Scholar]
- 43. Mariconda M, Fava R, Gatto A, Longo C, Milano C. Unilateral laminectomy for bilateral decompression of lumbar spinal stenosis: a prospective comparative study with conservatively treated patients. J Spinal Disord Tech. 2002;15:39–46. [DOI] [PubMed] [Google Scholar]
- 44. Celik SE, Celik S, Göksu K, Kara A, Ince I. Microdecompressive laminatomy with a 5-year follow-up period for severe lumbar spinal stenosis. J Spinal Disord Tech. 2010;23:229–235. [DOI] [PubMed] [Google Scholar]
- 45. Costa F, Sassi M, Cardia A, et al. Degenerative lumbar spinal stenosis: analysis of results in a series of 374 patients treated with unilateral laminotomy for bilateral microdecompression. J Neurosurg Spine. 2007;7:579–586. [DOI] [PubMed] [Google Scholar]
- 46. Cavusoglu H, Kaya RA, Türkmenoglu ON, Tuncer C, Colak I, Aydin Y. Midterm outcome after unilateral approach for bilateral decompression of lumbar spinal stenosis: 5-year prospective study. Eur Spine J. 2007;16:2133–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Birjandian Z, Emerson S, Telfeian AE, Hofstetter CP. Interlaminar endoscopic lateral recess decompression—surgical technique and early clinical results. J Spine Surg. 2017;3:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Staats PS, Chafin TB, Golovac S, et al. Long term-safety and efficacy of minimally invasive lumbar decompression procedure for the treatment of lumbar spinal stenosis with neurogenic claudication: 2-year results of MiDAS ENCORE. Reg Anesth Pain Med. 2018;43:789–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403–408. [PubMed] [Google Scholar]
- 50. Schönström N, Lindahl S, Willén J, Hansson T. Dynamic changes in the dimensions of the lumbar spinal canal: an experimental study in vitro. J Orthop Res. 1989;7:115–121. [DOI] [PubMed] [Google Scholar]
- 51. Matsumoto M, Watanabe K, Tsuji T, et al. Nocturnal leg cramps: a common complaint in patients with lumbar spinal canal stenosis. Spine (Phila Pa 1976). 2009;34:E189–E194. [DOI] [PubMed] [Google Scholar]
- 52. Inui Y, Doita M, Ouchi K, Tsukuda M, Fujita N, Kurosaka M. Clinical and radiologic features of lumbar spinal stenosis and disc herniation with neuropathic bladder. Spine (Phila Pa 1976). 2004;29:869–873. [DOI] [PubMed] [Google Scholar]
- 53. Herno A, Airaksinen O, Saari T. Computed tomography after laminectomy for lumbar spinal stenosis. Patients’ pain patterns, walking capacity, and subjective disability had no correlation with computed tomography findings. Spine (Phila Pa 1976). 1994;19:1975–1978. [PubMed] [Google Scholar]
- 54. Herno A, Saari T, Suomalainen O, Airaksinen O. The degree of decompressive relief and its relation to clinical outcome in patients undergoing surgery for lumbar spinal stenosis. Spine (Phila Pa 1976). 1999;24:1010–1014. [DOI] [PubMed] [Google Scholar]
- 55. Weber C, Giannadakis C, Rao V, et al. Is there an association between radiological severity of lumbar spinal stenosis and disability, pain, or surgical outcome? A multicenter observational study. Spine (Phila Pa 1976). 2016;41:E78–E83. [DOI] [PubMed] [Google Scholar]
- 56. Zeifang F, Schiltenwolf M, Abel R, Moradi B. Gait analysis does not correlate with clinical and MR imaging parameters in patients with symptomatic lumbar spinal stenosis. BMC Musculoskelet Disord. 2008;9:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Porter RW. Spinal stenosis and neurogenic claudication. Spine (Phila Pa 1976). 1996;21:2046–2052. [DOI] [PubMed] [Google Scholar]
- 58. Andrasinova T, Adamova B, Buskova J, Kerkovsky M, Jarkovsky J, Bednarik J. Is there a correlation between degree of radiologic lumbar spinal stenosis and its clinical manifestation? Clin Spine Surg. 2018;31:E403–E408. [DOI] [PubMed] [Google Scholar]
- 59. Kim HJ, Suh BG, Lee DB, et al. The influence of pain sensitivity on the symptom severity in patients with lumbar spinal stenosis. Pain Physician. 2013;16:135–144. [PubMed] [Google Scholar]
- 60. Nomura K, Yoshida M. Assessment of the learning curve for microendoscopic decompression surgery for lumbar spinal canal stenosis through an analysis of 480 cases involving a single surgeon. Global Spine J. 2017;7:54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fujita N, Michikawa T, Yagi M, et al. Impact of lumbar hypolordosis on the incidence of symptomatic postoperative spinal epidural hematoma after decompression surgery for lumbar spinal canal stenosis. Eur Spine J. 2019;28:87–93. [DOI] [PubMed] [Google Scholar]
- 62. Fujiwara Y, Manabe H, Izumi B, et al. The impact of hypertension on the occurrence of postoperative spinal epidural hematoma following single level microscopic posterior lumbar decompression surgery in a single institute. Eur Spine J. 2017;26:2606–2615. [DOI] [PubMed] [Google Scholar]
- 63. Strömqvist F, Sigmundsson FG, Strömqvist B, Jönsson B, Karlsson MK. Incidental durotomy in degenerative lumbar spine surgery—a register study of 64 431 operations. Spine J. 2019;19:624–630. [DOI] [PubMed] [Google Scholar]
- 64. Oertel JM, Burkhardt BW. Full endoscopic treatment of dural tears in lumbar spine surgery. Eur Spine J. 2017;26:2496–2503. [DOI] [PubMed] [Google Scholar]
- 65. Nam HGW, Kim HS, Park JS, Lee DK, Park CK, Lim KT. Double-layer TachoSil packing for management of incidental durotomy during percutaneous stenoscopic lumbar decompression. World Neurosurg. 2018;120:448–456. [DOI] [PubMed] [Google Scholar]
- 66. Shin JK, Youn MS, Seong YJ, Goh TS, Lee JS. Iatrogenic dural tear in endoscopic lumbar spinal surgery: full endoscopic dural suture repair (Youn’s technique). Eur Spine J. 2018;27(Suppl 3):544–548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.