Abstract
Study Design:
Technical note, retrospective case series.
Objective:
Lumbar stenosis can be effectively treated using tubular unilateral laminotomy for bilateral decompression (ULBD). For multilevel stenosis, a multilevel ULBD through separate, alternating crossover approaches has been described as the “slalom technique.” To increase efficacy, we introduced this approach with 2 microscopes simultaneously.
Methods:
We collected data on 13 patients, with multilevel lumbar stenosis, operated at our institution between 2015 and 2016 by the aforementioned technique. We assessed surgical time (ST), estimated blood loss (EBL), complications, and revision surgeries. Furthermore, we provide a stepwise instruction for performing the tandem microscopic slalom technique in a safe and efficient manner.
Results:
The mean age of the patients was 68 ± 8 years. The ST per level was 68 ± 19 minutes with an EBL per level of 39 ± 30 mL. We had no intraoperative complications and none of our patients required a revision surgery during a mean follow-up of 12 months.
Conclusions:
We have shown that this technique is feasible and can be performed safely for multisegmental lumbar spinal stenosis with minimal tissue trauma and low EBL. Furthermore, randomized controlled studies with a larger sample size may be necessary to drive any final conclusions.
Keywords: minimally invasive decompression, unilateral laminotomy for bilateral decompression (ULBD), laminectomy, laminotomy, tubular decompression, slalom technique, lumbar stenosis, spinal stenosis
Introduction
Lumbar spinal stenosis (LSS) is a large public health concern due to its high prevalence and economic burden.1 It is the most common indication for spine surgery in patients older than 65 years.2,3 Traditionally, total laminectomy has been considered the gold standard treatment for symptomatic LSS refractory to conservative management, allowing for surgical decompression of the spinal canal.4-6 However, the conventional laminectomy technique has several drawbacks. Open total laminectomy is associated with complications such as extensive tissue trauma, infection, increased intraoperative blood loss and postoperative instability7 associated with high reoperation and secondary fusion rates.8 Less invasive alternatives to laminectomy have been developed over the past few decades.9 In particular, unilateral and bilateral laminotomy have gained popularity as microsurgical decompression techniques for LSS.6 The unilateral laminotomy for bilateral decompression (ULBD) has been reported as safe and effective while minimizing tissue damage and iatrogenic injuries.4,10-15
Mayer et al16 introduced the bilateral multisegmental microsurgical decompression through separate, alternating unilateral crossover approaches. This so-called “slalom” technique reduced the high tissue trauma that occurs with multisegmental open decompression, thereby preserving the microsurgical advantages of unilateral over-the-top decompression over multiple levels.16
More recently, we modified the slalom technique by having 2 surgeons operate with 2 microscopes or 1 microscope and contralateral loupe magnification simultaneously.
The aim of the present article was to provide a technical description simultaneous multilevel decompression with two microscopes. Furthermore, we are reporting about our first clinical experiences in using the 2-microscope slalom technique.
Materials and Methods
Retrospective Case Series
We performed a retrospective analysis of patients who underwent microsurgical multilevel decompression of symptomatic LSS at our institution from January 2016 to December 2016. Data was collected using the patients’ digital health records, including demographics, treatment details, and outcomes of the patients. Specifically, we assessed surgical time (ST) per operated level, estimated blood loss (EBL) per level, intra- and postoperative complications as well as clinical outcomes over a follow-up period of 12 months. Clinical outcomes were determined by pre- and postoperative visual analogue scale (VAS) and Oswestry Disability Index (ODI).
All included patients (n = 13) suffered from radiographic and clinically symptomatic multilevel LSS without segmental instability as determined by preoperative flexion and extension X-rays. Surgery was indicated after the symptoms did not respond to conservative treatment, including pain medication and physical therapy for more than 3 months. All procedures were performed by the same attending neurosurgeon who is the senior author of this article, along with 2 spine fellows. Statistical significance between the pre- and postoperative clinical parameters was evaluated using the Student’s t test or Mann-Whitney U test, respectively.
Surgical Technique
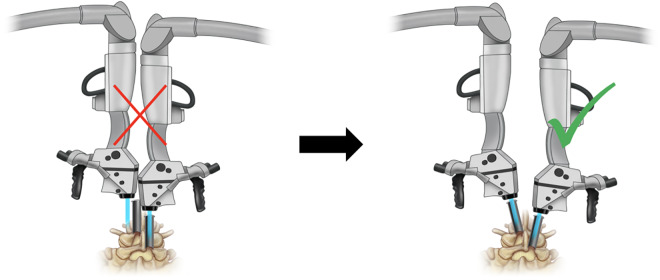
The patient is placed in prone position under general anesthesia. Lateral fluoroscopy is used to mark the localization for the skin incision. Both surgeons set the incisions consistent with the pattern described as the “slalom technique” by Mayer et al (Figure 1).16 It is critical to perform the dilatation for the tube placement simultaneously to avoid losing accuracy due to excessive retraction of the skin marks (Figure 2).
Figure 1.

Postoperative image showing healed scars from alternating incisions after lumbar decompression using the “slalom” technique.
Figure 2.

Intraoperative lateral X-ray showing simultaneous serial dilatation for both sides to avoid losing accuracy of the skin marks due to excessive retraction
The detailed 10-step minimally invasive surgery (MIS) technique for ULBD was previously described by our group.17
The bilateral decompression through a unilateral tubular approach is begun with placing a tube over the inferior edge of the medial ipsilateral lamina using sequential dilation. At this point, it is critical to perform incisions and dilation on both sides at the same time in order to avoid excessive skin retraction leading to inaccuracy of the skin marks.
Afterward, soft tissue is removed and the inferior edge of the lamina as well as the base of the spinous process is identified. To perform the ipsilateral laminotomy, the medial portion of the lamina is drilled to access the medial yellow ligament and bone removal is accomplished. During this step, there may not be enough space for a second microscope in some cases. To overcome this limitation, one surgeon starts the ipsilateral decompression with loops until the second surgeon angles the tube to decompress the contralateral side. After completion of this step, the tube is angled medially toward the midline (Figure 3). As the contralateral decompression requires a more lateral positioning of the microscope, it allows the surgeon to introduce the second contralateral microscope (Figure 3). After introduction of both microscopes, 2 surgeons simultaneously carry out the subsequent steps (Figure 4). Contralateral and undercut drilling of the bone “behind” the contralateral yellow ligament is performed as well as the following removal of the contralateral yellow ligament.17 The contralateral traversing nerve is identified, followed inferiorly, and can be decompressed if necessary. The goal is the performance of a complete flavectomy all the way from the caudal pedicle to the cranial insertion of the yellow ligament. After completion, the tube and table are tilted back, the ipsilateral drilling and removal of the yellow ligament is completed. The laminotomy procedure, including over-the-top decompression of the contralateral side, is performed through 18-mm tubes.
Figure 3.
Schematic drawing of tubular decompression using the tandem microscopic slalom technique. (Left) Tubular trajectory for ipsilateral decompression offering suboptimal conditions for the use of 2 microscopes simultaneously. (Middle) For contralateral decompression, tubulars retractor are angled approximately by 3 cm from position A to B. (Right): After angling the tubes toward the contralateral side, both microscopes can be used, allowing 2 surgeons to work simultaneously.
Figure 4.

Intraoperative setup with 2 surgeons working simultaneously with 2 microscopes, performing the tandem microscopic slalom technique for multilevel lumbar stenosis.
Results
The mean age of patients at the time of surgery was 68 ± 8 years. Thirteen patients, including 10 males (77%) and 3 females (23%) with a mean body mass index of 30 ±7 kg/m2, underwent a lumbar decompression using the slalom technique. Demographic details are shown in Table 1. Tubular ULBD was performed on 35 spinal levels in 13 patients between L1 and L5. On average, 3 ± 1 levels were treated per patient. The ST per level was 68 ± 9 minutes, and EBL per level averaged 39 ± 30 mL. We experienced 1 cerebrospinal fluid leak (8%). The hospitalization time averaged 2 ± 1 days. From preoperatively to the 12 months follow-up patients showed significant improvement in VAS and ODI (P < .05) (Table 2). On flexion-extension radiography, none of the patients showed signs of segmental instability in the index levels. None of the patients required a revision or secondary fusion surgery during the 12-month follow-up period.
Table 1.
Demographic Patient Data (Mean and Standard Deviation)
| Demographic Data | |
|---|---|
| No. of patients | 13 |
| Age, years, mean ± SD | 68 ± 8 |
| Gender, n | |
| Female | 3 |
| Male | 10 |
| Body mass index, kg/m2, mean ± SD | 30 ± 7 |
| Operated levels, n | 3 ± 1 |
Table 2.
Outcome Parameters (Mean and Standard Deviation).
| Results | |
|---|---|
| Operated levels, n | 35 |
| Operated levels per patient, mean ± SD | 3 ± 1 |
| Surgical time per level, minutes, mean ± SD | 68 ± 19 |
| Estimated blood loss, mL, mean ± SD | 39 ± 30 |
| Length of hospitalization, days, mean ± SD | 2 ± 1 |
| VAS score, mean ± SD | |
| Preoperative | 6 ± 2 |
| Follow-up | 2 ± 3 |
| Improvement | 4* |
| ODI, mean ± SD | |
| Preoperative | 47 ± 16 |
| Postoperative | 23 ± 16 |
| Improvement | 24* |
Abbreviations: VAS, visual analogue scale; ODI, Oswestry Disability Index.
*P < .05.
Discussion
Our initial experience with the 2-microscope slalom technique indicates that this technique is a feasible alternative for multilevel LSS. Performing the slalom technique provides all the benefits of an MIS approach in terms of reduced tissue damage and less risk of postoperative instability.8,18-20 Apart from the general benefits of MIS surgery in terms of minimized tissue trauma, the slalom technique balances tissue trauma by alternating the approach side.16
In the conventional unilateral MIS treatment of multisegmental stenosis, the aggregate of multiple same-sided interlaminar exposures may lead to an extensive unilateral muscle trauma and possible iatrogenic destabilization. With the described slalom technique tissue trauma and bone/ligamentous removal is spread over a wider bilateral area and may therefore result in less overall damage.
As described by Shamji et al,21 minimally invasive spinal procedures are known to be a safe and effective treatment especially in the elderly population. Given the mean age of our patients, this information matches with the findings of our case series. Not only is collateral trauma reduced, but also surgery times, which may lead to faster recovery. Obese and overweight patients may benefit from the above-described technique as the average body mass index of our patients is considered as obese. Overall complication rates among spinal surgery are typically significantly higher in obese patients, mostly due to wound-related issues (ie, infection or persistent wound drainage) and minimally invasive noninstrumented surgery is known to minimize postoperative infection rates.22 This is particularly applicable when tissue damage and blood loss are kept low and surgery time is reduced to a minimum.23,24
In our study, we attempted to enhance the efficiency of the slalom technique by introducing the simultaneous use of 2 microscopes, or 1 microscope and a second surgeon performing the initial approach with loupe magnification. As shown in Table 3, the outcomes of our first 2-microscope slalom surgeries reveal results comparable to the current literature.
Table 3.
Comparative Summary of Outcomes in Similar Studies.
| Authors | Year | Technique | ST/Level (min) | EBL/Level (mL) | VAS Improvement |
|---|---|---|---|---|---|
| Arai et al25 | 2014 | MIS ULBD | 108 | 67.9 | 4 |
| MIS MILD | 118 | 73.7 | 2 | ||
| Liu et al26 | 2013 | Open | 43.5 | 59.5 | 4 |
| MIS | 48.9 | 40.9 | 5 | ||
| Celik et al7 | 2010 | Open | 53.5 | 113.5 | 4 |
| MIS | 41.5 | 89 | 5 | ||
| Rajasekaran et al12 | 2013 | Open | 37.6 | 40 | 2 |
| Split laminectomy | 35.6 | 49 | 3 | ||
| Palmer et al27 | 2012 | MIS | 55 | 37 | 3 |
| Musluman et al28 | 2012 | MIS | N/A | 118.7 | 2 |
| Watanabe et al29 | 2011 | Open | 59 | 39 | N/A |
| Split laminectomy | 49 | 31 | N/A | ||
| Current study | MIS ULBD | 68 | 39 | 4 |
Abbreviations: ST, surgical time; EBL, estimated blood loss; VAS, visual analogue scale; MIS, minimally invasive surgery; ULBD, unilateral laminotomy for bilateral decompression; MILD, muscle-preserving interlaminar decompression; N/A, not applicable.
Limitations and Advantages
Since these results represent the first experience with a new technique, the operating room times are longer than one may expect if simultaneous procedures are performed. The surgical time is likely to decrease with an increasing learning curve.30 The goal of the present article was therefore to provide a technical note with the nuances necessary to perform spinal decompression with 2 microscopes rather than to proof that this technique is superior to single-microscope, single surgeon procedures.
The variability in the length of stay can be explained by the fact that some of our elderly patients with significant comorbidities were observed for several days following the operation. Our technique seems to lead to favorable outcomes especially in terms of blood loss.
An advantage of this approach is that it facilitates the teaching and training of surgeons in MIS. The resident/fellow can start with the exposure while the attending surgeon is completing the adjacent level. The attending surgeon can observe the procedure on the screen of the trainee’s screen and take over at critical portions of the surgery. This can reduce the surgical time required for a procedure performed by a surgeon-in-training.
However, we only recommend this technique for teaching senior residents or postgraduate fellows who already possess a certain amount of surgical experience. An obvious limitation and challenge of this technique is the increased need for resources. The tandem microscope slalom technique requires an increased amount of resources such as a second microscope, a second set of surgical equipment and a second surgeon. Therefore, this technique is only justifiable if a second microscope and additional equipment are readily available and a surgical trainee at a senior level is participating in the procedure. Additional operating room staff, other than a second surgeon was not required in the above-described case series. The acquisition of an additional microscope solely for this technique would likely not be cost-efficient. However, if a second microscope is readily available, the additional costs only involve a second set of surgical instruments and an additional sterile drape for the second microscope.
Further studies with a larger sample size will be necessary to demonstrate whether this technique is superior to conventional single-microscope ULBD.
Conclusion
The presented data summarizes our first experience with the simultaneous use of 2 microscopes for MIS slalom decompression for lumbar spinal stenosis. We have shown that it is feasible and can be performed safely for multisegmental lumbar spinal stenosis with minimal tissue trauma and low EBL. To derive final conclusions, a longer follow-up and a comparative randomized study will be necessary to demonstrate if this technique is superior to conventional ULBD.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Roger Härtl receives consultant fees from DePuy Synthes, Brainlab, and Ulrich and royalties from Zimmer Biomet.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This supplement was supported by funding from the Carl Zeiss Meditec Group.
ORCID iD: Christoph Wipplinger, MD  https://orcid.org/0000-0002-9001-6932
https://orcid.org/0000-0002-9001-6932
Sara Lener, MD  https://orcid.org/0000-0002-5644-2399
https://orcid.org/0000-0002-5644-2399
References
- 1. Parker SL, Anderson LH, Nelson T, Patel VV. Cost-effectiveness of three treatment strategies for lumbar spinal stenosis: conservative care, laminectomy, and the Superion interspinous spacer. Int J Spine Surg. 2015;9:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schneider MJ, Terhorst L, Murphy D, Stevans JM, Hoffman R, Cambron JA. Exploratory analysis of clinical predictors of outcomes of nonsurgical treatment in patients with lumbar spinal stenosis. J Manipulative Physiol Ther. 2016;39:88–94. [DOI] [PubMed] [Google Scholar]
- 3. Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303:1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oertel MF, Ryang YM, Korinth MC, Gilsbach JM, Rohde V. Long-term results of microsurgical treatment of lumbar spinal stenosis by unilateral laminotomy for bilateral decompression. Neurosurgery. 2006;59:1264–1270. [DOI] [PubMed] [Google Scholar]
- 5. Thome C, Zevgaridis D, Leheta O, et al. Outcome after less-invasive decompression of lumbar spinal stenosis: a randomized comparison of unilateral laminotomy, bilateral laminotomy, and laminectomy. J Neurosurg Spine. 2005;3:129–141. [DOI] [PubMed] [Google Scholar]
- 6. Overdevest GM, Jacobs W, Vleggeert-Lankamp C, Thome C, Gunzburg R, Peul W. Effectiveness of posterior decompression techniques compared with conventional laminectomy for lumbar stenosis. Cochrane Database Syst Rev. 2015;(3):CD010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Celik SE, Celik S, Goksu K, Kara A, Ince I. Microdecompressive laminatomy with a 5-year follow-up period for severe lumbar spinal stenosis. J Spinal Disord Tech. 2010;23:229–235. [DOI] [PubMed] [Google Scholar]
- 8. Schöller K, Alimi M, Cong GT, Christos P, Härtl R. Lumbar spinal stenosis associated with degenerative lumbar spondylolisthesis: a systematic review and meta-analysis of secondary fusion rates following open vs minimally invasive decompression. Neurosurgery. 2017;80:355–367. [DOI] [PubMed] [Google Scholar]
- 9. Machado GC, Ferreira PH, Yoo RI, et al. Surgical options for lumbar spinal stenosis. Cochrane Database Syst Rev. 2016;(11):CD012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Usman M, Ali M, Khanzada K, et al. Unilateral approach for bilateral decompression of lumbar spinal stenosis: a minimal invasive surgery. J Coll Physicians Surg Pak. 2013;23:852–856. [PubMed] [Google Scholar]
- 11. Gurelik M, Bozkina C, Kars Z, Karadag O, Ozum U, Bayrakli F. Unilateral laminotomy for decompression of lumbar stenosis is effective and safe: a prospective randomized comparative study. J Neurol Sci. 2012;29:744–753. [Google Scholar]
- 12. Rajasekaran S, Thomas A, Kanna RM, Prasad Shetty A. Lumbar spinous process splitting decompression provides equivalent outcomes to conventional midline decompression in degenerative lumbar canal stenosis: a prospective, randomized controlled study of 51 patients. Spine (Phila Pa 1976). 2013;38:1737–1743. [DOI] [PubMed] [Google Scholar]
- 13. Scholler K, Steingruber T, Stein M, et al. Microsurgical unilateral laminotomy for decompression of lumbar spinal stenosis: long-term results and predictive factors. Acta Neurochir (Wien). 2016;158:1103–1113. [DOI] [PubMed] [Google Scholar]
- 14. Costa F, Sassi M, Cardia A, et al. Degenerative lumbar spinal stenosis: analysis of results in a series of 374 patients treated with unilateral laminotomy for bilateral microdecompression. J Neurosurg Spine. 2007;7:579–586. [DOI] [PubMed] [Google Scholar]
- 15. Papavero L, Thiel M, Fritzsche E, Kunze C, Westphal M, Kothe R. Lumbar spinal stenosis: prognostic factors for bilateral microsurgical decompression using a unilateral approach. Neurosurgery. 2009;65(6 suppl):182–187. [DOI] [PubMed] [Google Scholar]
- 16. Mayer HM, Heider F. “Slalom”: microsurgical cross-over decompression for multilevel degenerative lumbar stenosis. Biomed Res Int. 2016;2016:9074257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boukebir MA, Berlin CD, Navarro-Ramirez R, et al. Ten-step minimally invasive spine lumbar decompression and dural repair through tubular retractors. Oper Neurosurg (Hagerstown). 2017;13:232–245. [DOI] [PubMed] [Google Scholar]
- 18. Alimi M, Hofstetter CP, Pyo SY, Paulo D, Haertl R. Minimally invasive laminectomy for lumbar spinal stenosis in patients with and without preoperative spondylolisthesis: clinical outcome and reoperation rates. J Neurosurg Spine. 2015;22:339–352. [DOI] [PubMed] [Google Scholar]
- 19. Grunert P, Reyes PM, Newcomb AG, et al. Biomechanical evaluation of lumbar decompression adjacent to instrumented segments. Neurosurgery. 2016;79:895–904. [DOI] [PubMed] [Google Scholar]
- 20. Kim CW. Scientific basis of minimally invasive spine surgery: prevention of multifidus muscle injury during posterior lumbar surgery. Spine (Phila Pa 1976). 2010;35(26 suppl):S281–S286. [DOI] [PubMed] [Google Scholar]
- 21. Shamji MF, Goldstein CL, Wang M, Uribe JS, Fehlings MG. Minimally invasive spinal surgery in the elderly: does it make sense? Neurosurgery. 2015;77(suppl 4):S108–S115. [DOI] [PubMed] [Google Scholar]
- 22. Shousha M, Cirovic D, Boehm H. Infection rate after minimally invasive noninstrumented spinal surgery based on 4350 procedures. Spine (Phila Pa 1976). 2015;40:201–205. [DOI] [PubMed] [Google Scholar]
- 23. Bekelis K, Coy S, Simmons N. Operative duration and risk of surgical site infection in neurosurgery. World Neurosurg. 2016;94:551–555.e6. [DOI] [PubMed] [Google Scholar]
- 24. Dubory A, Giorgi H, Walter A, et al. Surgical-site infection in spinal injury: incidence and risk factors in a prospective cohort of 518 patients. Eur Spine J. 2015;24:543–554. [DOI] [PubMed] [Google Scholar]
- 25. Arai Y, Hirai T, Yoshii T, et al. A prospective comparative study of 2 minimally invasive decompression procedures for lumbar spinal canal stenosis: unilateral laminotomy for bilateral decompression (ULBD) versus muscle-preserving interlaminar decompression (MILD). Spine. 2014;39(4):332–340. [DOI] [PubMed] [Google Scholar]
- 26. Liu X, Yuan S, Tian Y. Modified unilateral laminotomy for bilateral decompression for lumbar spinal stenosis: technical note. Spine. 2013;38(12):E732–E737. [DOI] [PubMed] [Google Scholar]
- 27. Palmer S, Davison L. Minimally invasive surgical treatment of lumbar spinal stenosis: Two-year follow-up in 54 patients. Surg Neurol Int. 2012;3:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Müslüman AM, Cansever T, Yılmaz A, Çavuşoğlu H, Yüce İ, Aydın Y. Midterm outcome after a microsurgical unilateral approach for bilateral decompression of lumbar degenerative spondylolisthesis. J Neurosurg Spine. 2012;16(1):68–76. [DOI] [PubMed] [Google Scholar]
- 29. Watanabe K, Matsumoto M, Ikegami T, et al. Reduced postoperative wound pain after lumbar spinous process-splitting laminectomy for lumbar canal stenosis: a randomized controlled study. J Neurosurg Spine. 2011;14(1):51–58. [DOI] [PubMed] [Google Scholar]
- 30. Parikh K, Tomasino A, Knopman J, Boockvar J, Härtl R. Operative results and learning curve: microscope-assisted tubular microsurgery for 1- and 2-level discectomies and laminectomies. Neurosurg Focus. 2008;25:E14. [DOI] [PubMed] [Google Scholar]