Abstract
Background:
In patients with symptomatic lumbar stenosis undergoing lateral transpsoas approach for lumbar interbody fusion (LLIF) surgery, it is not always clear when indirect decompression is sufficient in order to achieve symptom resolution. Indirect decompression failure (IDF), defined as “postoperative persistent symptoms of nerve compression with or without a second direct decompression surgery to reach adequate symptom resolution,” is not widely reported. This information, however, is critical to better understand the indications, the potential, and the limitations of indirect decompression.
Objective:
The purpose of this study was to systematically review the current literature on IDF after LLIF.
Methods:
A literature search was performed on PubMed. We included randomized controlled trials and prospective, retrospective, case-control studies, and case reports. Information on sample size, demographics, procedure, number and location of involved levels, follow-up time, and complications were extracted.
Results:
After applying the exclusion criteria, we included 9 of the 268 screened articles that reported failure. A total of 632 patients were screened in these articles and detailed information was provided. Average follow-up time was 21 months. Overall reported incidence of IDF was 9%.
Conclusion:
Failures of decompression via LLIF are inconsistently reported and the incidence is approximately 9%. IDF failure in LLIF may be underreported or misinterpreted as a complication. We propose to include the term “IDF” as described in this article to differentiate them from complications for future studies. A better understanding of why IDF occurs will allow surgeons to better plan surgical intervention and will avoid revision surgery.
Keywords: transpsoas surgery, XLIF, ELIF, DLIF, MIS-LIF, complication
Introduction
Symptomatic lumbar spinal stenosis can be treated surgically with direct or indirect decompression.1-5 Patients who present with lumbar spinal stenosis and mechanical instability or deformity will sometimes require a stabilization of the segment in addition to the decompression.
The lateral transpsoas approach for lumbar interbody fusion (LLIF) also known as extreme lateral interbody fusion (XLIF; NuVasive, San Diego, CA, or ELIF) was developed in the early 2000s as an alternative to direct anterior or posterior approaches to indirectly decompress and fuse the spine through a muscle-sparing and minimally disruptive procedure.6 LLIF has advantages over traditional open procedures such as decreased blood loss, sparing of the posterior musculature, reduced postoperative pain, faster return to productive activities, and shorter hospital stay.7,8 LLIF achieves indirect decompression of the neural elements of the spine by restoring disc height and foraminal height.4,5 LLIF has become increasingly popular over the last decade,9 and a recent systematic review demonstrated that LLIF is an effective and safe technique for decompression and stabilization of the lumbar spine.10
Unfortunately, it is difficult to predict how much indirect decompression will be achieved and which patients may still require direct decompression. We and others5,6,11-14 have published data on radiographic changes after indirect decompression such as disc height changes, foraminal and central canal volume, and so on, but clinical data are not available. Therefore, some surgeons anecdotally and in publications propose indirect decompression followed by a postoperative clinical evaluation of the patient to see if direct decompression is still required in order to achieve symptom resolution.15,16 For example, Anand and Baron described performing LLIF first followed by a clinical reevaluation to determine the need for direct decompression during planned second-stage surgery.15 In our opinion, this approach is not satisfying because it may put patients through a second operation that could or should have been avoided with better planning. Clinical data on indirect decompression failure (IDF) are necessary to fill the knowledge gap.
IDF after LLIF can be defined as the inability to resolve symptoms from either central canal, or foraminal compression. This may result in the need for an additional surgery to address the initial symptomatology. Failure to address the initial symptomatology is clinically and economically unfavorable; therefore, there should be emphasis on optimizing patient selection or planning for combined surgeries involving direct decompression.15,16
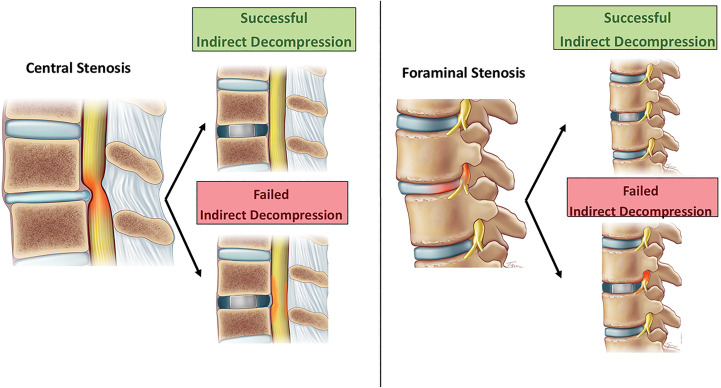
The purpose of this study was to systematically review the current literature on the IDF after LLIF per the specific definition described, detected during the postoperative period (Figure 1).
Figure 1.
Successful versus failed indirect decompression.
Materials and Methods
Definition of IDF
Our definition of failure was based solely on clinical assessment and not on radiographic findings. Thus, we defined IDF as follows:
Lack of resolution of the initial compressive symptoms during the postoperative period due to persistent central, lateral recess, and/or foraminal stenosis after LLIF.
This may or may not be associated with the need for a second surgical procedure with direct decompression after LLIF.
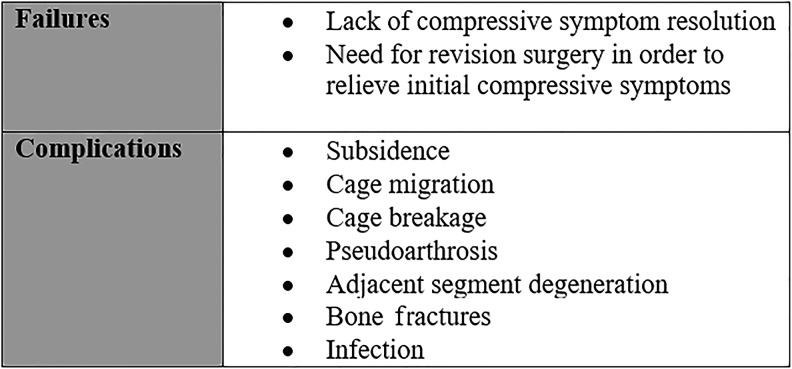
Complications After LLIF
Complications can arise due to patient- or procedure-related factors, such as poor bone quality leading to vertebral endplate fractures, cage subsidence and migration, pseudoarthrosis, and adjacent segment degeneration. According to our aforementioned definition, unsuccessful surgery due to these factors were not included as failure in this study (Figure 2).
Figure 2.
Definition of failures and complications.
Literature Search Strategy and Inclusion Criteria
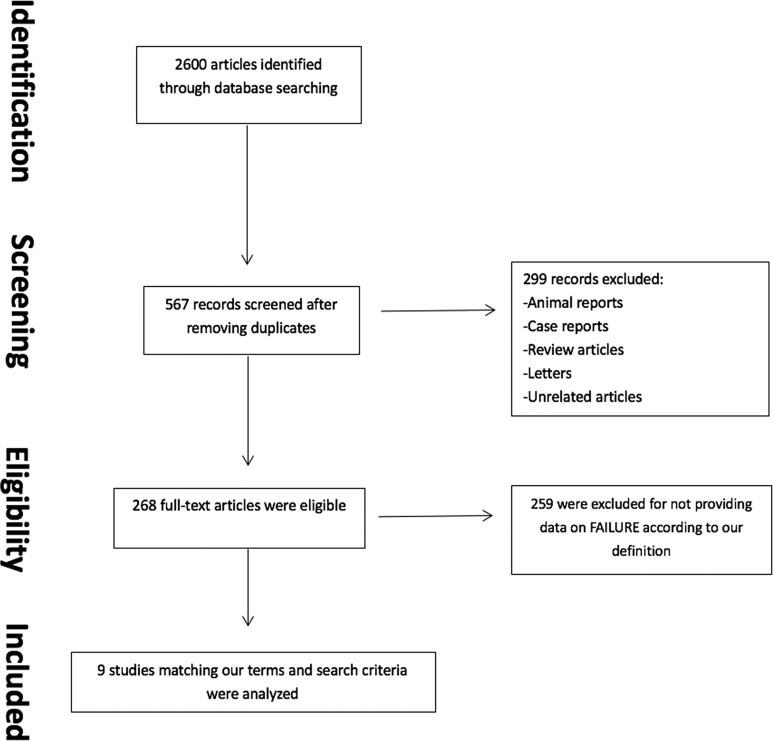
A comprehensive literature search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria using the electronic databases of PubMed, Cochrane Library, and Science Direct for articles published up to July 2017. Key search words included “XLIF,” “ELIF,” “LLIF,” “DLIF,” “extreme lateral interbody fusion,” “minimally invasive surgery lateral interbody fusion,” “MIS-LIF,” “transpsoas surgery,” “lateral interbody fusion,” “indirect decompression,” “foraminal stenosis,” “central canal stenosis,” and “lateral recess stenosis.” Only articles written in English were included (Figure 3).
Figure 3.
Comprehensive literature search.
We included every article that had LLIF as the primary surgical procedure in their study. Abstracts were first evaluated for duplicates. Thus, publications from the same institution or corresponding author were reviewed in their entirety for duplication potential. References from each reviewed article in our search were examined to avoid the inclusion of unidentified resources. Letters to the editor, editorials, reviews without case-series, meta-analyses, guidelines, meeting presentations, and expert opinions were excluded. The remaining articles were reviewed in their entirety.
The number of randomized controlled trials for LLIF was limited; all nonrandomized studies with a concurrent control group, randomized controlled trials with unclear randomization, prospective, retrospective, and case-control studies were included. If an abstract did not provide sufficient information, the entire article was reviewed. The final decision on article eligibility was made after reviewing the entire article.
The primary inclusion criteria was information on IDF after LLIF. This information must have included the following: (1) the number of cases with IDF in the whole sample and/or (2) the treatment strategy for patients with failed LLIF decompression (second surgery). Only articles in which LLIF was used to achieve neural decompression were included in this study (Table 1). Studies involving patients suffering from trauma or tumors were excluded. We also excluded the articles that did not report the IDF based on our definition.
Table 1.
Details From The Manuscripts Used in This Review.
| Author | Year | Country | Research Support From Companies | No. of Patients | No. of Levels | Mean Age (Years) | Mean BMI (kg/m2) | Gender | Average Operation Time (Minutes) | Length of Stay | Blood Loss | Follow-up (Months) | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alimi et al7 | 2014 | USA | No | 90 | 145 | 64.4 | 27.6 | 55 F/35 M | NR | 4.5 days | NR | 3-50.4m | DDD (32); spondylolisthesis (24); degenerative scoliosis (22); adjacent segment disease (7); post-laminectomy syndrome (3); instrumentation failure/non-fusion (2) |
| Malham et al5 | 2014 | Australia | No | 52 | 79 | 66.4 | 26.1 | 34 F/18 M | NR | NR | NR | NR | Spondylolisthesis(14); DDD (20); scoliosis (12); stenosis (2); herniated nucleus pulposus (3); adjacent segment disease (1) |
| Dominguez et al17 | 2017 | Spain | No | 97 | 138 | 68 | NR | NR | 35 mL per level (+57 if additional fixation) | 3.2 days | 40 mL per level | 12-44 | Adjacent segment disease (30%); deformity (11%); lumbar disc disease (21%) |
| Marchi et al11 | 2011 | Brazil | No | 52 | 52 | 67.6 | 27.4 | 38 F/14 M | 73.2 mL per level | NR | <50 mL per level | 24 | Low-grade spondylolisthesis |
| Grimm et al18 | 2017 | USA | No | 108 | 193 | 59 | NR | 71 F/37 M | 151 mL (1 level); 174 mL (2 levels); 253 mL (>2 levels) | 3 days | 268 ± 88 | 12 | Degenerative scoliosis (33); DDD (27); degenerative spondylolisthesis (24); stenosis(12); recurrent disc herniation (6); post-laminectomy syndrome (6) |
| Phillips et al19 | 2013 | USA | NuVasive | 107 | 344 | 68 | NR | 78 F/29 M | 57.9 mL per level | 2.9 days | <100 mL | 24 | Degenerative scoliosis |
| Wang et al12 | 2017 | USA | No | 45 | 101 | 65 | NR | 31 F/14 M | NR | NR | NR | 6 | Revision (4); spondylosis (29); DDD(4); adjacent segment disease (1); instability (3); trauma (1); scoliosis (24); central canal stenosis (37) |
| Khajavi et al20 | 2015 | USA | NuVasive | 60 | 71 | 64.5 | 29.1 | 45 F/15 M | 206 mL (per case) | 1.3 days | 83 mL | 20.3 | Degenerative spondylolisthesis: Grade I (47); Grade II (13) |
| Oliveira et al21 | 2010 | USA | NuVasive | 21 | 43 | 67.6 | NR | 14 F/7 M | 47 mL per level | 1.2 days | 23 mL per level | NR | Degenerative spondylolisthesis (8); degenerative scoliosis (19) |
Abbreviations: F, female; M, male; BMI, body mass index; DDD, degenerative disc disease.
Data Collection and Extraction
Two independent reviewers (RNR and JG) assessed titles and abstracts of all articles in accordance to our search algorithm to determine if they met the inclusion criteria. Any discrepancies were resolved after group discussion with the 2 aforementioned reviewers (Figure 3). Full text review was performed on all included articles. Data was extracted in a standardized fashion. The collected information included author information (institution affiliation, sponsoring bodies), methods, patient demographics (eg, age, gender, ethnicity, body mass index [BMI]), comorbidities, indication for surgery, diagnoses, and surgical details (levels involved, operating time, blood loss, length of hospital stay, additional instrumentation, and follow-up period). Clinical and radiographic parameters included Visual Analog Scale (VAS), Oswestry Disability Index (ODI), disc height, and foraminal and central canal area. As we included all eligible studies in the analysis, it was not necessary to assess the risk of bias of the selected searches.
Results
Initially, 567 articles were screened after removing duplicates. Following exclusion of case reports, reviews, and other unrelated articles, 268 publications were eligible. Of these, 9 articles matched our inclusion criteria and were reviewed in full length providing information about LLIF failure based on our specific criteria (Figure 3).5,7,11,12,17-21 Most articles were published in the United States (6 of 9).7,12,18-21 Literature in English was investigated, and most articles were published after 2014 (6/9).5,7,12,17,18,20 The follow-up period reported in the included studies were between 3 and 50.4 months (Table 1). Average follow-up time was 21 months.
Study Population
A total of 632 patients were included in this analysis. Mean age was 65.2 years, and the majority of patients was female (68%). The most common indications for initial LLIF surgery were degenerative disc disease (DDD), spinal stenosis (central or foraminal), degenerative spondylolisthesis with focal neural compression (low grade), and degenerative scoliosis with focal neural compression. The total number of operative levels was 1166 (ranging from 1 to 6 levels per patient). Most operations addressed a single level (46.8%). The most common levels treated were L4-5 and L3-4 (46.7% and 31.4%, respectively). Only 57 patients out of 632 were reported as failure that matched our criteria. More surgical details are provided in Table 2.
Table 2.
Reported Surgical Detailsa.
| Parameter | Value |
|---|---|
| Total patients | 632 (100%)5,7,11,12,17-21 |
| Failed reported patients | 57 (9%) |
| Gender (%) | 5,7,11,12,17-21 |
| Male | 169 (32%) |
| Female | 366 (68%) |
| Mean age | 65.2 years5,7,11,12,17-21 |
| Mean BMI | 27.6 kg/m2 5,7,11,20 |
| Mean operative time | 169.4 minutes11,17-21 |
| Mean follow-up | 21 months7,11,17-21 |
| Instrument | 7,17-20 |
| No | 77 (17%) |
| Yes | 378 (83%) |
| Instrument details | 7,17,18 |
| Unilateral screw | 70 (30%) |
| Bilateral screw | 104 (45%) |
| Lateral plate | 59 (25%) |
| Total number of levels | 11665,7,11,12,17-21 |
| Levels/patient, range | 1-6 |
| Number of levels | 10215,7,11,12,20,21 |
| 1 | 251 (46.8%) |
| 2 | 141 (26.3%) |
| 3 | 97 (18.1%) |
| 4 | 40 (7.5%) |
| 5 | 5 (1%) |
| 6 | 2 (0.4%) |
| Level treated | 4915,7,11,12,18-21 |
| Above T11 | 1 (0.2%) |
| T12-L1 | 2 (0.4%) |
| L1-2 | 26 (5.3%) |
| L2-3 | 81 (16.5%) |
| L3-4 | 154 (31.4%) |
| L4-5 | 227 (46.7%) |
| Total number of levels | 11665,7,11,12,17-21 |
| Levels/patient, range | 1-6 |
Abbreviation: BMI, body mass index.
aThe numbers presented in this table are only a transcript of a pool of data from each article. The variation in the total number of patients and their single procedure characteristics is due to lack of information provided by the authors in their original manuscripts. Superscript numbers (references) represent the articles that included this type of information.
Implant Characteristics
Out of the 9 articles included in this study, only 5 authors provided information about the cage used. Two authors stated that they used only 18-mm anteroposterior width cages.12,21 The other 3 authors either used 18 mm or 22 mm anteroposterior width cages.7,11,17 Laterolateral diameters of cages were mentioned in 2 articles, and its range was from 45 to 60 mm.11,17 Additionally, 2 authors reported cage heights from 8 to 14 mm in 2-mm increments.7,17
Radiological and Clinical Outcomes
Although the comparison of radiological and clinical outcome parameters was not the purpose of this study, we included them if provided (Tables 3 and 4). The average reported increase in disc height, foraminal height, axial central canal area, and sagittal central canal diameter was 68%, 19%, 15%, and 32%, respectively. VAS Back, VAS Leg, and ODI improvement after surgery were 54.3% 58.4%, and 45.5%, respectively.
Table 3.
Radiological Outcomes.
Table 4.
Clinical Outcomes.
| Preoperative | Latest Follow-up | Improvement | |
|---|---|---|---|
| VAS Back5,7,11,17,19,20 | 7.7 | 3.2 | 54.3% |
| VAS Leg5,7,11,17,19,20 | 6.8 | 3.1 | 58.4% |
| ODI5,7,11,19,20 | 50.6% | 27.6% | 45.5% |
Abbreviations: VAS, Visual Analog Scale; ODI, Oswestry Disability Index.
Included Studies and Definition of Failure
Fifty-seven out of the 632 patients had IDF following LLIF (9%). Only 5 out of 9 articles that had met our criteria included a specific reason for failure.12,17-19,21 The incidence of IDF was 6.4% for those 5 articles. The reasons behind these failures included severe foraminal stenosis, inadequately restored disc and/or foraminal height, and/or bony lateral recess stenosis (Table 5).
Table 5.
Reported Failures.
| Author | Reported # of Failures | Reported Radiological Reason for Failure | Reported Clinical Reason for Failure | Does It Meet Our Criteria? |
|---|---|---|---|---|
| Alimi et al7 | 1 | NR | NR | Yes |
| Malham et al5 | 4 | NR | NR | Yes |
| Dominguez et al17 | 2 | NR | Persistent symptoms | Yes |
| Marchi et al11 | 2 | NR | NR | Yes |
| Grimm et al18 | 1 | NR | Persistent symptoms | Yes |
| Phillips et al19 | 7 | NR | Persistent symptoms | Yes |
| Wang et al12 | 13 | Bony lateral recess stenosis(9), associated to reduced central canal/foraminal area (4) | Persistent symptoms | Yes |
| Khajavi et al20 | 26 | NR | NR | Yesa |
| Oliveira et al21 | 1 | Disc and foraminal height were not adequately restored | NR | Yes |
| Reported failures (cases) 57 |
Abbreviation: NR, not reported.
aMet our criteria in terms of that 26 patients required second posterior decompression surgery. However, no information was provided about whether these procedures were done either because of failure (unsuccessful indirect decompression) or a complication (subsidence, etc).
Discussion
Advantages of Indirect Decompression
It has been shown that LLIF has several advantages over open and direct decompressive approaches.22-26 The outcomes of indirect decompression via LLIF have shown noninferiority to direct approaches in terms of improvement in pain scales and radiological parameters. Moreover, shorter hospital stays and recovery times, reduced blood loss, decreased postoperative pain, and less soft tissue trauma due to sparing of posterior musculature was observed when compared with other traditional approaches.6,24
The Definition of Failure
The aim of this study was to systematically review the current literature on reasons for “IDF” after LLIF which we defined as the failure to alleviate the compressive symptoms and/or the need for a second surgery to address the unresolved issues. According to our knowledge, this is the first systematic review specifically analyzing current literature on failure of the LLIF procedure based on this specific, clinical definition.
Based on our definition and search terms, “IDF” are only reported by 3.4% of the articles. Phillips et al19 addresses failed cases that match our criteria as “complications.” Thirteen patients required a second surgical procedure. However, only 7 of these 13 patients had second surgeries due to persisting symptoms and not pseudoarthrosis nor subsidence. On the other hand, Khajavi et al20 reported several patients that had second surgeries, but never specified if they were due to unsuccessful indirect decompression or due to radiographic complications such as subsidence, vertebral endplate fractures, cage migration, instability, pseudoarthrosis, or adjacent segment disease. These findings indicate that failures and complications are not appropriately distinguished in the literature.
The Limitations of LLIF
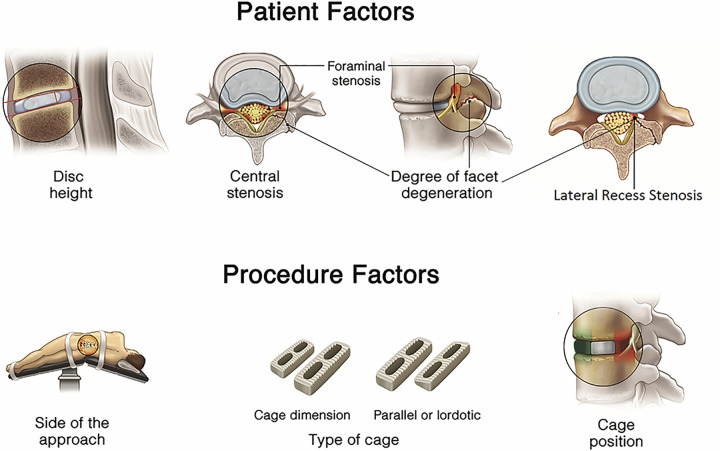
Indirect decompression via LLIF has shown to achieve clinical outcomes similar to direct approaches, especially in terms relieving foraminal stenosis.10 However, the underreporting of “IDF” complicates the identification of factors that make patients prone to insufficient indirect decompression. More important, it makes it difficult for surgeons to understand the limitations of LLIF. This cycle perpetuates inappropriate patient selection, potentially increasing LLIF procedure failures. Studies published by our group and others have shown that there is no obvious anatomical, radiologic, or clinical correlation that can accurately predict LLIF failure or success.5,6,11-14 Furthermore, other potential factors related to LLIF outcomes have been investigated over the past decade, including the presence of “locked facets” due to facet degeneration. This radiographic feature was originally believed to limit restoration of disc height during LLIF,11,12 but was later shown not to be a limitation.12,13 In a previous study by our group,10 we investigated potential patient-related and procedure-related factors that could affect the outcomes of indirect decompression (Figure 4). It was reported that cage size, particularly cage width, is the most crucial procedure-related factor to restore and maintain disc and foraminal height, and to avoid cage subsidence.7,27,28 Other investigated patient-related and procedure-related factors such as side of the approach, level of spinal segments, number of spinal levels, presence of facet arthropathy, cage type, cage height, and cage position were less likely to influence success of indirect decompression.5,7,13,28-31
Figure 4.
Potential patient-related and procedure-related factors that determine the success of indirect decompression.
Reasons for Failure
Recently, a new patient-related factor has been identified by Wang et al.12 According to this study, bony lateral recess stenosis was the only independent factor associated with insufficient indirect decompression after LLIF. Figure 4 represents a hypothetical case with either central and/or lateral recess compression due to a posterolateral component (eg, facet arthropathy, synovial cyst, or ligamentum flavum infolding) which would demonstrate a case not suitable for LLIF.
Based on both published findings and our own surgical experience, we recommend indirect decompression for patients who have symptomatic foraminal stenosis as long as we can confirm the source of the pain by eliciting radicular symptoms with a Kemp’s test. Indirect decompression can also be safely used for the patients who have central canal stenosis with neurologic claudication. On the other hand, we recommend direct decompression as an alternative or in addition to indirect decompression for the patients with severe lateral recess stenosis.
In order to gather more accurate information on failure incidence and risks, we propose to include the term “IDF” as described in this article as a category separate from complications for future studies on LLIF. As more authors become aware of this term and report their failures, new patient-related and procedure-related predictive factors of LLIF failure could be identified through the literature. Furthermore, this may help identify an algorithm to avoid failure and maximize successful indirect decompression via LLIF.
Limitations
Most studies included in this review were of retrospective nature. Only one study aimed to investigate and report the cause of failure.12 The rest considered failure only as part of their surgical complications. Information on causes of failure is limited in the studies meeting our inclusion criteria (Table 5).
Our definition of IDF does not include patient-reported outcome measures such as ODI or VAS scores. However, we are limited by the details that authors have reported on patients’ symptoms in their articles. Therefore, we propose to study this phenomenon prospectively with a more objective definition. Moreover, there were many deformity patients who underwent multi-segment surgery in some of the included articles and this situation makes it difficult to identify the IDF among the other symptoms in this patient population.
Conclusion
In this article, we introduce the concept of IDF, which is defined as “postoperative persistent symptoms of nerve compression with or without a second direct decompression surgery to reach adequate symptom resolution.” Current information is limited, but based on the best currently available evidence the incidence of IDF is approximately 9%. Future studies should introduce and monitor “clinical IDF” including the reasons for failure, in order to develop an algorithm that allows determining a reliable preoperative surgical plan.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Roger Härtl is a consultant for Ulrich, Brainlab, DePuy-Synthes, and he has royalties from Zimmer. The other authors have no conflicts of interest to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This supplement was supported by funding from the Carl Zeiss Meditec Group.
ORCID iD: Sertac Kirnaz, MD  https://orcid.org/0000-0002-4104-3985
https://orcid.org/0000-0002-4104-3985
Christoph Wipplinger, MD  https://orcid.org/0000-0002-9001-6932
https://orcid.org/0000-0002-9001-6932
References
- 1. Khechen B, Haws BE, Patel DV, et al. Comparison of postoperative outcomes between primary MIS TLIF and MIS TLIF as a revision procedure to primary decompression [published online June 22, 2018]. Spine (Phila Pa 1976). doi:10.1097/BRS.0000000000002759 [DOI] [PubMed] [Google Scholar]
- 2. Lee SH, Choi WG, Lim SR, Kang HY, Shin SW. Minimally invasive anterior lumbar interbody fusion followed by percutaneous pedicle screw fixation for isthmic spondylolisthesis. Spine J. 2004;4:644–649. [DOI] [PubMed] [Google Scholar]
- 3. Phan K, Maharaj M, Assem Y, Mobbs RJ. Review of early clinical results and complications associated with oblique lumbar interbody fusion (OLIF). J Clin Neurosci. 2016;31:23–29. [DOI] [PubMed] [Google Scholar]
- 4. Elowitz EH, Yanni DS, Chwajol M, Starke RM, Perin NI. Evaluation of indirect decompression of the lumbar spinal canal following minimally invasive lateral transpsoas interbody fusion: radiographic and outcome analysis. Minim Invasive Neurosurg. 2011;54:201–206. [DOI] [PubMed] [Google Scholar]
- 5. Malham GM, Parker RM, Goss B, Blecher CM, Ballok ZE. Indirect foraminal decompression is independent of metabolically active facet arthropathy in extreme lateral interbody fusion. Spine (Phila Pa 1976). 2014;39:E1303–E1310. [DOI] [PubMed] [Google Scholar]
- 6. Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6:435–443. [DOI] [PubMed] [Google Scholar]
- 7. Alimi M, Hofstetter CP, Cong GT, et al. Radiological and clinical outcomes following extreme lateral interbody fusion. J Neurosurg Spine. 2014;20:623–635. [DOI] [PubMed] [Google Scholar]
- 8. Joseph JR, Smith BW, La Marca F, Park P. Comparison of complication rates of minimally invasive transforaminal lumbar interbody fusion and lateral lumbar interbody fusion: a systematic review of the literature. Neurosurg Focus. 2015;39:E4. [DOI] [PubMed] [Google Scholar]
- 9. Arnold PM, Anderson KK, McGuire RA., Jr The lateral transpsoas approach to the lumbar and thoracic spine: a review. Surg Neurol Int. 2012;3(suppl 3):S198–S215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lang G, Perrech M, Navarro-Ramirez R, et al. Potential and limitations of neural decompression in extreme lateral interbody fusion—a systematic review. World Neurosurg. 2017;101:99–113. [DOI] [PubMed] [Google Scholar]
- 11. Marchi L, Abdala N, Oliveira L, Amaral R, Coutinho E, Pimenta L. Stand-alone lateral interbody fusion for the treatment of low-grade degenerative spondylolisthesis. ScientificWorldJournal. 2012;2012:456346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang TY, Nayar G, Brown CR, Pimenta L, Karikari IO, Isaacs RE. Bony lateral recess stenosis and other radiographic predictors of failed indirect decompression via extreme lateral interbody fusion: multi-institutional analysis of 101 consecutive spinal levels. World Neurosurg. 2017;106:819–826. [DOI] [PubMed] [Google Scholar]
- 13. Navarro-Ramirez R, Lang G, Moriguchi Y, et al. Are locked facets a contraindication for extreme lateral interbody fusion? World Neurosurg. 2017;100:607–618. [DOI] [PubMed] [Google Scholar]
- 14. Gabel BC, Hoshide R, Taylor W. An algorithm to predict success of indirect decompression using the extreme lateral lumbar interbody fusion procedure. Cureus. 2015;7:e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anand N, Baron EM. Minimally invasive approaches for the correction of adult spinal deformity. Eur Spine J. 2013;22(suppl 2):S232–S241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dangelmajer S, Zadnik PL, Rodriguez ST, Gokaslan ZL, Sciubba DM. Minimally invasive spine surgery for adult degenerative lumbar scoliosis. Neurosurgl Focus. 2014;36:E7. [DOI] [PubMed] [Google Scholar]
- 17. Dominguez I, Luque R, Noriega M, Rey J, Alia J, Marco-Martinez F. Extreme lateral lumbar interbody fusion. Surgical technique, outcomes and complications after a minimum of one year follow-up [in Spanish]. Rev Esp Cir Ortop Traumatol. 2017;61:8–18. [DOI] [PubMed] [Google Scholar]
- 18. Grimm BD, Leas DP, Poletti SC, Johnson DR., 2nd Postoperative complications within the first year after extreme lateral interbody fusion: experience of the first 108 patients. Clin Spine Surg. 2016;29:E151–E156. [DOI] [PubMed] [Google Scholar]
- 19. Phillips FM, Isaacs RE, Rodgers WB, et al. Adult degenerative scoliosis treated with XLIF: clinical and radiographical results of a prospective multicenter study with 24-month follow-up. Spine (Phila Pa 1976). 2013;38:1853–1861. [DOI] [PubMed] [Google Scholar]
- 20. Khajavi K, Shen A, Hutchison A. Substantial clinical benefit of minimally invasive lateral interbody fusion for degenerative spondylolisthesis. Eur Spine J. 2015;24(suppl 3):314–321. [DOI] [PubMed] [Google Scholar]
- 21. Oliveira L, Marchi L, Coutinho E, Pimenta L. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine (Phila Pa 1976). 2010;35(26 suppl):S331–S337. [DOI] [PubMed] [Google Scholar]
- 22. Saraph V, Lerch C, Walochnik N, Bach CM, Krismer M, Wimmer C. Comparison of conventional versus minimally invasive extraperitoneal approach for anterior lumbar interbody fusion. Eur spine J. 2004;13:425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sembrano JN, Tohmeh A, Isaacs R; SOLAS Degenerative Study Group. Two-year comparative outcomes of MIS lateral and MIS transforaminal interbody fusion in the treatment of degenerative spondylolisthesis: part I: clinical findings. Spine (Phila Pa 1976). 2016;41(suppl 8):S123–S132. [DOI] [PubMed] [Google Scholar]
- 24. Pereira EA, Farwana M, Lam KS. Extreme lateral interbody fusion relieves symptoms of spinal stenosis and low-grade spondylolisthesis by indirect decompression in complex patients. J Clin Neurosci. 2017;35:56–61. [DOI] [PubMed] [Google Scholar]
- 25. Isaacs RE, Sembrano JN, Tohmeh AG. Two-year comparative outcomes of MIS lateral and MIS transforaminal interbody fusion in the treatment of degenerative spondylolisthesis: part II: radiographic findings. Spine (Phila Pa 1976). 2016;41(suppl 8):S133–S144. [DOI] [PubMed] [Google Scholar]
- 26. Youssef JA, McAfee PC, Patty CA, et al. Minimally invasive surgery: lateral approach interbody fusion: results and review. Spine (Phila Pa 1976). 2010;35(26 suppl):S302–S311. [DOI] [PubMed] [Google Scholar]
- 27. Kim SJ, Lee YS, Kim YB, Park SW, Hung VT. Clinical and radiological outcomes of a new cage for direct lateral lumbar interbody fusion. Korean J Spine. 2014;11:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tohmeh AG, Khorsand D, Watson B, Zielinski X. Radiographical and clinical evaluation of extreme lateral interbody fusion: effects of cage size and instrumentation type with a minimum of 1-year follow-up. Spine (Phila Pa 1976). 2014;39:E1582–E1591. [DOI] [PubMed] [Google Scholar]
- 29. Malham GM, Ellis NJ, Parker RM, et al. Maintenance of segmental lordosis and disk height in stand-alone and instrumented extreme lateral interbody fusion (XLIF). Clin Spine Surg. 2017;30:E90–E98. [DOI] [PubMed] [Google Scholar]
- 30. Karikari IO, Grossi PM, Nimjee SM, et al. Minimally invasive lumbar interbody fusion in patients older than 70 years of age: analysis of peri- and postoperative complications. Neurosurgery. 2011;68:897–902. [DOI] [PubMed] [Google Scholar]
- 31. Pimenta L, Marchi L, Oliveira L, Coutinho E, Amaral R. A prospective, randomized, controlled trial comparing radiographic and clinical outcomes between stand-alone lateral interbody lumbar fusion with either silicate calcium phosphate or rh-BMP2. J Neurol Surg A Cent Eur Neurosurg. 2013;74:343–350. [DOI] [PubMed] [Google Scholar]