Abstract
Innovative technology and techniques have revolutionized minimally invasive spine surgery (MIS) within the past decade. The introduction of navigation and image-guided surgery has greatly affected spinal surgery and will continue to make surgery safer and more efficient. Eventually, it is conceivable that fluoroscopy will be completely replaced with image guidance. These advancements, among others such as robotics and virtual and augmented reality technology, will continue to drive the value of 3-dimensional navigation in MIS. In this review, we cover pertinent features of navigation in MIS and explore their evolution over time. Moreover, we aim to discuss the key features germane to surgical advancement, including technique and technology development, accuracy, overall health care costs, operating room time efficiency, and radiation exposure.
Keywords: navigation, 3-dimensional navigation, minimally invasive surgery (MIS), robotics, intraoperative CT, augmented reality
Introduction
Minimally invasive spine surgery (MIS) has witnessed rapid development over the past 2 decades concomitant with advances in navigation techniques and technology. These advances have enabled surgeons to achieve the same operative goal of instrumented stabilization and arthrodesis in patients that would otherwise require traditional open approaches with freehand screw placement. While traditional approaches are still indicated in a number of different scenarios, MIS alternatives have resulted in decreased intraoperative blood loss, inpatient hospital stays, and postoperative narcotic use while resulting in comparable outcomes.1,2
In traditional open surgery, a midline incision is utilized with subperiosteal dissection to expose the relevant landmarks for pedicle screw entry based on the segment in the spine being instrumented. For thoracic and lumbar pedicle screws, the transverse processes and superior articular facets must be exposed or palpable. Screw trajectory can be assessed based on known anatomic variation or the angular relation to the spinous processes. However, with MIS approaches utilizing percutaneous pedicle screw placement, accuracy relies on imaging-based landmarks, which are not easily visualized or palpable. Furthermore, given the lack of these landmarks, screw orientation can be drastically different than what would be expected, even by experienced surgeons, by minor alterations in patient positioning or from friction along the screw shaft and insertion device from skin, fascia, or muscle. While open approaches may provide an advantage in this respect, these advantages can be negated in patients with pathologic anatomy due to severe degenerative disease, congenital abnormalities, trauma, neoplastic involvement, and extensive rotary deformities.
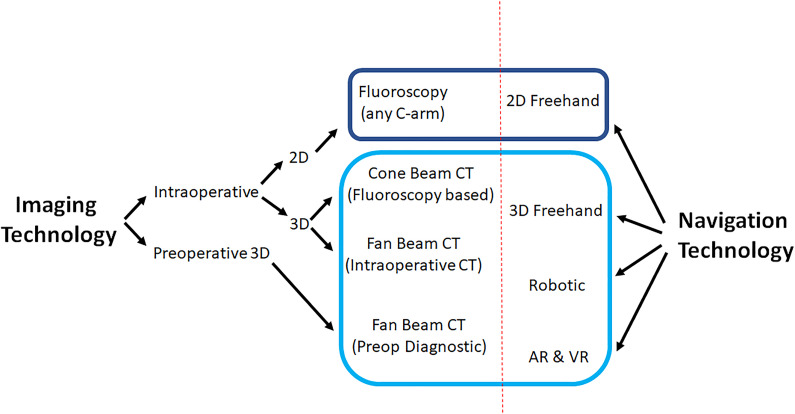
Initial techniques relied on anteroposterior (AP)/lateral fluoroscopy with the use of K-wires for MIS. Percutaneous placement of lumbar pedicle screws were the first studies described utilizing this approach. This relied on new generation instrumentation, including extension sleeves for remote manipulation of polyaxial screw head and rod insertion devices.3-5 This technique was eventually adopted to thoracic spine stabilization, which carried higher risk given the presence of spinal cord and smaller pedicles. Improvements in technology advanced to 2-dimensional (2D)-based systems that had AP and lateral X-rays loaded into a computer navigation system (with or without K-wires), and finally to 3D-based navigation with intraoperative or preoperative imaging (Figure 1). Navigation software programming and applications, developed by various companies, have been key to developing virtual reconstructions with these intraoperative scans that can then be used for real-time navigation guidance
Figure 1.
Navigation techniques and technologies.
In this review, we cover pertinent features of navigation in MIS and explore their evolution over time. Newer technologies such as robotics and augmented reality are also increasingly being utilized and developed with MIS techniques. Moreover, we aim to discuss the key features germane to surgical advancement, including technique and technology development, accuracy, overall health care costs, operating room time efficiency, and radiation exposure (Table 1).
Table 1.
Relative Comparison of Operative Factors in 2-Dimensional Versus 3-Dimensional Minimally Invasive Spine Surgery.
| Factor | 2D Navigation | 3D Navigation |
|---|---|---|
| Upfront economic burden | + | +++ |
| Surgical revision rates | +++ | + |
| Pedicle screw accuracy | + | +++ |
| Operative time | ++ | + |
| Flexibility of utilization | + | +++ |
| Radiation exposure to patient | ++ | +++ |
| Radiation exposure to staff | ++ | 0 |
| Overall economic health care burden | +++ | ++ |
Fluoroscopic Guidance Without Navigation
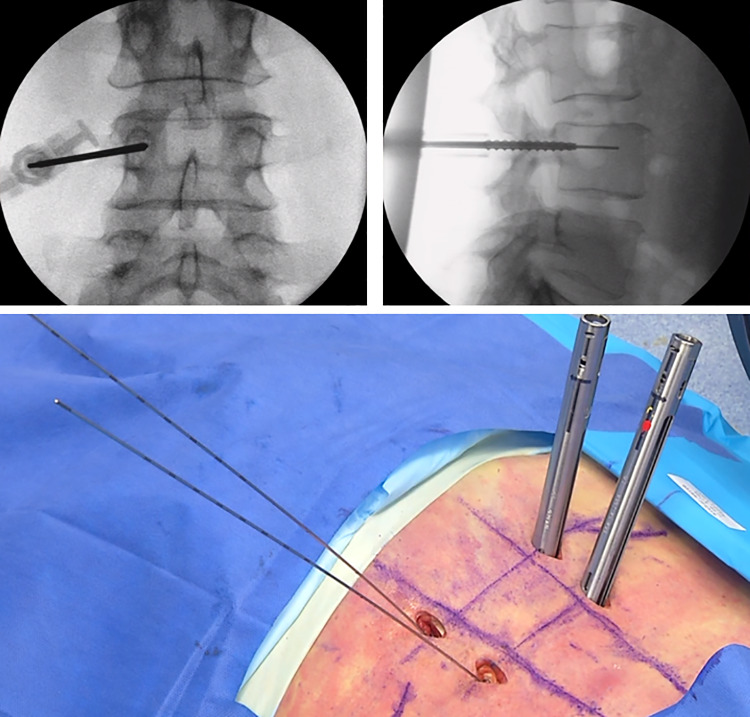
C-arm fluoroscopy is the most commonly used modality for image-guided percutaneous pedicle screw placement. Initial AP images should achieve the following prior to instrumentation: even endplates of the vertebral body of interest, midline spinous process, and well-defined circular or ovoid pedicles that are relatively symmetric in the context of the vertebral body. Once these anatomic constraints are acceptable, sequential use of Jamshidi needles docked on the lateral margin of the pedicle on AP view are used, and directly in line with the sagittal midline of the pedicle on lateral shots. A Jamshidi needle is then used and carefully advanced until at or just passed the midpoint of the pedicle on AP views and into the vertebral body on lateral X-rays (Figure 2). At this point, cannulated instruments, including pedicle probe, tap, and finally screw are placed along the K-wire. Pedicle breaches using this technique can be readily apparent on close examination of serial X-rays.
Figure 2.
Anteroposterior/lateral fluoroscopic-guidance with K-wires for pedicle screw placement.
While an accurate method, there are a number of limitations with AP and lateral fluoroscopic-guided placement of thoracolumbar pedicle screw instrumentation using traditional K-wire guidance techniques. First, multiple X-rays in both the lateral and AP positions are required to ensure that the alignment of the spine is correct for accurate instrument insertion. This involves adjusting the C-rotation, tilt, and wag of the C-arm machine to achieve the proper view. Subsequent X-rays are usually taken in both planes with passage of the different cannulated instruments, to ensure there is no unintentional K-wire migration and to confirm the depth of instrumentation into the vertebral body prior to and after final screw placement. Second, the increased frequency of X-rays taken results in increased radiation exposure. Tian et al6 performed a meta-analysis of MIS to open freehand techniques and found that standard AP and lateral fluoroscopic guidance resulted in twice as long X-ray exposure time. In some situations, the borders of the pedicle are not readily apparent or can overlap with the superior articulating facet, making the initial docking with the Jamshidi needle inaccurate. Subsequent advancement of the K-wire in these situations can result in inadvertent lateral or worse, medial breaches, which can injure the thecal sac and neural structures. Furthermore, unintentional advancement of K-wires while passing cannulated instruments back and forth or in situations of osteoporotic bone can have catastrophic results. Dural lacerations, bladder and abdominal viscera injury, retroperitoneal hematoma, and cardiac tamponade have been reported.7,8 Last, K-wire fractures may result in retained hardware, which then presents a contraindication for placing a pedicle screw at that level/side due to the risk of advancing the retained fragment anteriorly out of the vertebral body.9
Two-Dimensional Navigation
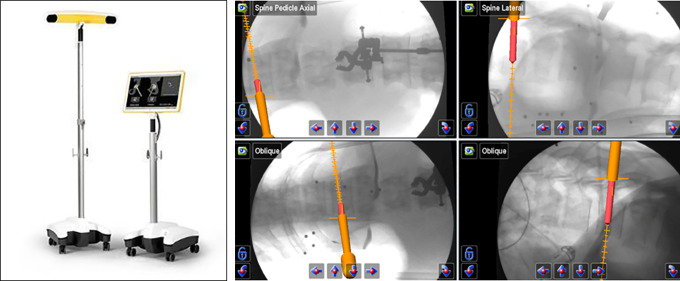
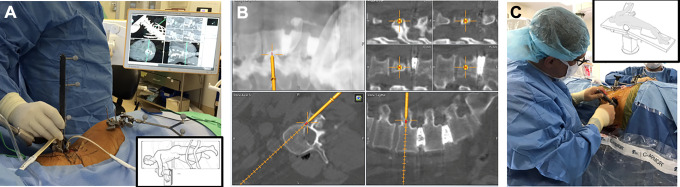
Given the limitations presented by radiation exposure with conventional AP and lateral fluoroscopic guidance, 2D computer guidance was the next step in navigation development. These techniques relied on the placement of a fixed array to the patient (iliac crest or spinous process) and devices that were then calibrated to a tracking array including awl, tap, and screws as necessary, with on-screen image guidance (Figure 3). Kim et al10 initially studied various factors using 2D computer navigation to fluoroscopy for MIS–transforaminal interbody fusion (TLIF) procedures in cadavers and real patients. Despite an increase in setup time attributed to the novelty of devices used, there was near complete elimination of detectable radiation exposure to the patient and staff.10 Njoku et al11 utilized the 2D Kick system (BrainLab AG, Munich, Germany) for percutaneous stabilization of a traumatic burst fracture (Figure 3), and furthermore explored its use in developing countries that are technologically limited in the present day, such as Tanzania.11
Figure 3.
Two-dimensional fluoroscopic-based K-wireless navigation for pedicle screw placement with the Brainlab Kick system.
Three-Dimensional Navigation
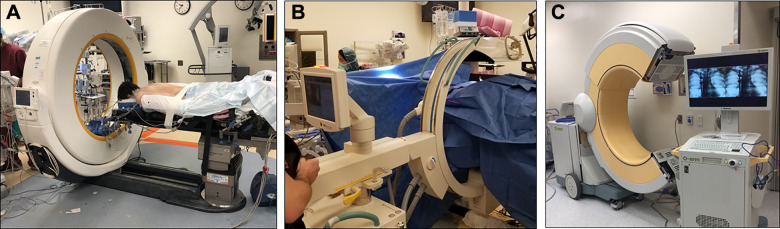
A number of 3D navigation platforms have been developed simultaneously with software that has drastically altered the workflow and benefits of MIS techniques, particularly for placement of thoracolumbar instrumentation. These studies have shown increased accuracy and lower likelihood of unsafe breaches of the pedicle cortical wall compared with fluoroscopically guided placement of pedicle screws.12,13 Among the most commonly used of these systems include the intraoperative computed tomography (CT)–based Airo (Mobius Imaging, Shirley, MA, USA) with navigation software (Brainlab, Munich, Germany), which is a fan beam-based CT system. There are also cone beam–based CT platforms such as the O-arm (open C-arm based system that goes around the patient then closes to a CT-based closed configuration) with associated Stealth Station (Medtronic, Minneapolis, MN, USA), and the C-arm based Ziehm Vision FD Vario 3-D with open navigation software integration capabilities (Ziehm Imaging, Orlando, FL, USA)14 (Figure 4). These machines rely on a stationary array that is attached to the patients to serve as a reference. Typically for lumbosacral fixation, Steinmann pins placed into the iliac crest are used, or conversely for thoracic or thoracolumbar junction fixation, spinous process clamps can be used. For cervical pathologies, there are also arrays available that can be attached directly to pinned skull clamps.
Figure 4.
Intraoperative computed tomography (CT)–based 3-dimensional navigation technology systems: (A) fan beam CT, (B, C) cone beam CT.
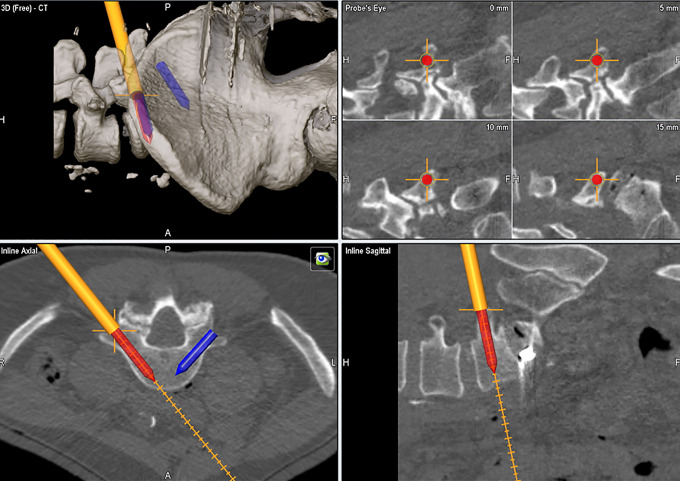
These imaging platforms acquire a 3D fluoroscopic or CT-based reconstruction of the patient’s spine over the region of interest (Figure 5). Once the image has been acquired, it is imperative that the references array is not manipulated or moved as this can alter accuracy of subsequently used navigated instruments. Star arrays can be attached to a number of modified instruments, including a drill guide, pedicle probe, tap, and finally screw shaft. Calibration of all instruments is required prior to use to ensure accuracy. While 2D navigation techniques have been described without the use of K-wires, the definitive elimination of K-wires and associated complications is achieved by 3D navigation techniques. To this end, Shin et al15 described the use of a navigated guide through which drilling, tapping, and screw insertion could be performed without the complications related to conventional 2D navigated screw insertion with K-wires. Furthermore, Nottmeier et al16 used 3D guidance without K-wires using tubular retractors to isolate the initial pilot hole and docking site for the pedicle probe and had no pedicle breaches in 15 patients while exposing the surgeon and operating room staff to minimal raditation.16
Figure 5.
Intraoperative computed tomography (iCT)–based 3-dimensional reconstruction and freehand navigation for lumbar pedicle screw trajectory/size planning and placement.
Accuracy
Reports vary in the accuracy of free hand pedicle screws placed in the thoracolumbar spine based on the indication for surgery (trauma, degenerative disease, scoliosis) and also by the particular definition of “accuracy” used by the study. Various breach classifications systems and definitions have been described, which makes direct comparison between techniques difficult to perform. Studies from the 1990s demonstrated overall accuracy rates ranging from 72% to 75% in large series.17,18 More recent studies report accuracy ranging from 88% to 94% using the freehand technique.19-22 Mason et al23 performed a study comparing the accuracy of pedicle screws placed using conventional fluoroscopy, 2D navigation, and 3D navigation systems, and found accuracy rates of 68%, 84%, and 95.5%, respectively. Fan et al24 compared conventional fluoroscopy to O-arm guided pedicle screw placement and found an improvement in accuracy rates from 78% to 90%. Accuracy with 3D navigation is 91% using first generation fluoroscopy-based cone beam CT navigation technologies in the setting of MIS TLIFs.25 The same group found an increase in pedicle screw placement accuracy to 98.6% with the use of new generation intraoperative CT (iCT)–based (fan beam CT) guidance given the improvement in image resolution and surgical team workflow.26 Lasron et al27 used CT navigation guidance for congenital spine deformities, which is a notoriously high-risk patient population due to abnormal pathology and dysmorphic pedicles, and were able to achieve 99.3% accuracy with pedicle screw placement. Collectively, these studies suggest that the evolution to 3D navigation has been vital for improving screw placement accuracy. Even in studies which there are a small percentage of screws with pedicle breaches, the rates of clinically relevant misplaced screws or those that require revision surgery is exceedingly low. Indeed, Bydon et al28 performed one of the largest series of iCT-based navigation guidance for the placement of screws at any level of the spine and found that only 0.99% required reoperations due to undetected misplaced screws.
Radiation Exposure
Radiation exposure with CT-based 3D navigation systems were initially controversial, given the exponential increase in radiation exposure compared with standard fluoroscopy. For example, Kingler et al29 performed a study specifically looking at eye lens radiation exposure comparing the Iso-C machine and the Ziehm Vision FD Vario 3D and found nearly a 2-fold increase with the Ziehm machine during lumbar 3D scans (11.2 and 22.5 mSv, respectively). However, a number of important developments over time have quelled these fears. First, CT-based 3D navigation systems require a single upfront registration scan in order for a case to be performed. During this initial scan, all operating room personnel and surgeons exit the room and can remain behind lead shields in lead-lined substerile rooms, essentially eliminating radiation exposure to all individuals except the patient.
For the Airo machine, the effective dose of radiation given per scan for patients ranges from 5.5 to 7.4 mSv per scan based on patient weight.30 Given that a postinstrumentation scan is also usually performed to confirm accurate screw placement, the effective average radiation dose to the patient is 13.4 mSv per case and nearly 0 mSv to surgeon and operating room staff. Furthermore, the Airo machine allows graded radiation exposure settings, including 25%, 50%, 75% of typical radiation exposure from a CT scan. These reduced radiation settings must be balanced with poorer resolution images, however, which is particularly limiting in obese patients or those with hardware given increased streak artifact. The O-arm similarly has radiation exposure equivalent of 9 mSv of radiation per scan, which is half of the radiation dose as a traditional 64 multisclice CT scanner.31-33 Other studies have reported doses up to 31 mSv when 3 scans or less are performed during a procedure.34
Economics
Two-dimensional navigation requires basic C-arm fluoroscopy and additional instruments, including K-wires, Jamshidi needles, and computer navigation software station when used. However, 3D navigation systems require far greater costs for acquisition and implementation. First- and second-generation 3D fluoroscopy-based cone beam CT machines (including the Orbic 3D, O-arm, and Ziehm RFD 3D) range in costs from $300 000 to $600 000.35 Intraoperative CT scanners (eg, Airo) can cost up to $1.2 million.35 These costs must be coupled with navigation systems software and in-hospital personnel, which can add an additional $500 000 to $700 000 to initial costs.35 Varying from geographic region and institution to institution, there may be additional costs associated with maintenance and upgrades of these products. However, the cost benefits are seen over a long-term basis in the sense of lower likelihood of misplaced screws, revision surgery, readmissions, operative time, and hospital length of stay.36 Early studies by Zausinger et al37 found a decrease in rates of revision surgeries using navigation-guidance compared with fluoroscopy from 4.4% to 0%, with an average cost saving per revision surgery of just over $27 000. Another study performed by Watkins et al38 utilized the Arcadis Orbic 3D (Siemens Medical, Munich, Germany) and NaviVision (BrainLAB AG, Munich, Germany) image-guided system for the placement of thoracolumbar screws in 100 patients compared to historical control group of 100 patients who had screws placed using fluoroscopy and found a reduction in revision surgeries from 3% to 0%, with the estimated cost savings of revision surgery to the institution of almost $24 000.
Another important factor in determining the overall benefits of health care economics leveraging 3D navigation lies in the operating room utilization time. Specifically, this refers to the time it takes to complete the actual operation excluding anesthetic induction and emergence. Sasso et al39 used computer-navigation guidance for placement of lumbosacral pedicle screws and found this group to be 22 minutes faster per case over conventional fluoroscopic-guidance, even when factoring in the learning curve of the surgeon and staff. Houten et al40 determined that using O-arm navigation guidance resulted in operating room utilization time savings of 21 minutes for single-level surgeries. Surveys of hospitals have demonstrated ranges in operating room cost short of surgeon fee, anesthesia, and equipment to range from $62 to $80 per minute based on the level of complexity.41 With a potential savings of $1760 per case, the annual savings from shorter operating times at high-volume centers due to the use of 3D navigation technology in MIS can easily become profitable, essentially “buying-back” or exceeding the upfront purchasing costs within 1 year of use.
Concept of 3D “Total Navigation”
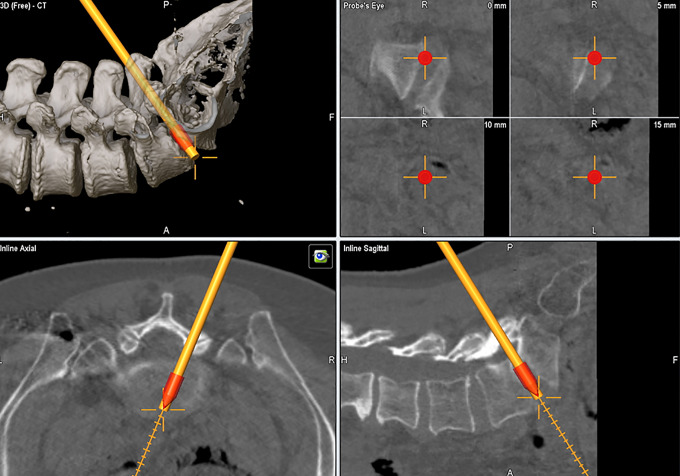
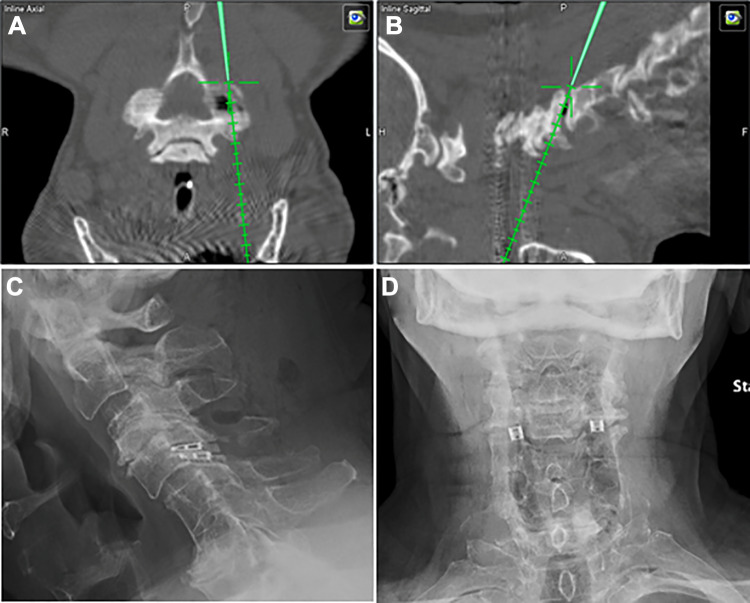
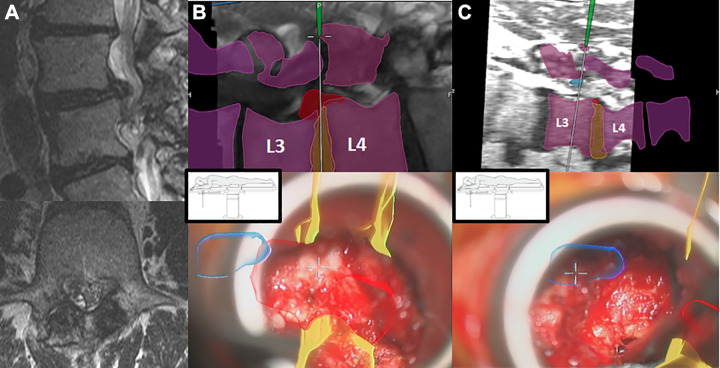
The “Total Navigation” concept refers to the newest iteration of 3D navigation-guided spinal instrumentation in which there is complete elimination of fluoroscopy for device implants that were typically guided by X-rays even when computer navigation guidance was utilized for screw placement. More specifically, this refers to interbody cage implants placed through lateral or transforaminal routes. In these scenarios, cage measurements can be made before insertion and followed through navigation to the appropriate depth and location relative to the vertebral bodies. For transpsoas interbody fusion with the patients positioned in the lateral position, this is particularly advantageous. The navigation array can be firmly implanted along the lateral margin of the iliac crest (Figure 6A). Once dilators and retractors have been localized over the interbody space of interest, 3D navigation guidance can be used for implanting the cage to the appropriate depth (Figure 6B). Moreover, when posterior pedicle screw stabilization is also indicated, the guidance system can also be used for pedicle screw placement while still in the lateral position (Figure 6B and C). This saves the staff the second stage of repositioning the patient, which adds to the operating room utilization time and furthermore presents an opportunity for unintended anesthetic complications related to turning prone from the lateral position frequently onto a different operating table type (eg, open Jackson). During TLIF, the cage size and position can be performed seamlessly after placing pedicle screws and performing the decompression, using a single registration scan (Figure 7). Recently, our group has also adopted 3D navigation techniques for the implantation of percutaneous cervical interfacet joint cages (DTRAX Cervical Cage, Providence Medical Technology; Lafayette, CA, USA). This severs an alternative treatment options for patients requiring posterior arthrodesis who otherwise have medical comorbidities that make them suboptimal candidates for traditional open lateral mass screw fixation (Figure 8).
Figure 6.
Intraoperative computed tomography (iCT) 3-dimensional “Total Navigation” for single lateral position transpsoas surgery. (A) Lateral cage placement with 3D navigation. (B) Pedicle screw placement in lateral position. (C) Tubular direct decompression in lateral position.
Figure 7.
Intraoperative computed tomography (iCT) 3-dimensional “Total Navigation” for minimally invasive surgery (MIS) transforaminal lumbar interbody fusion (TLIF) with interbody cage size and trajectory planning and freehand tracking.
Figure 8.
Example of intraoperative computed tomography (iCT) 3-dimensional navigation-based placement of percutaneous interfacet joint cages for posterior cervical spine arthrodesis.
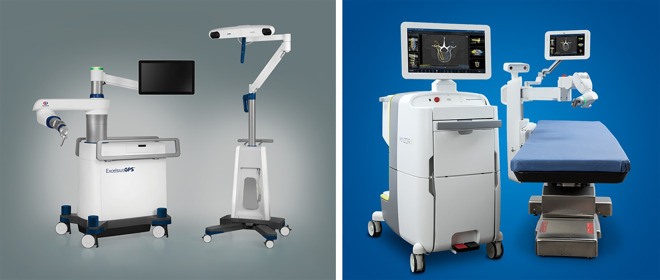
Robotics
The arrival of navigation for pedicle screw placement and other instrumentation implantation has revolutionized the safety and efficiency of MIS surgery. Future technological development will continue to build upon these advances. Robotic surgery is considered a special application of 3D navigation and is the newest iteration of navigation-based systems, which are coupled with existing software systems and array-based instruments. Floor-based (eg, MazorX, Medtronic, Minneapolis, MN, USA) and table-based systems (eg, Globus Excelsius GPS, Globus Medical, Audubon, PA, USA) (Figure 9) have already faced similar hurdles as iCT 3D navigation systems faced in their infancy, in the sense that they require increase set up time, personnel training, and upfront costs (up to $1.5 million). However, very accurate instrumentation placement can be achieved, with recent reports demonstrating >95% accuracy and negligible increase in operating room times as learning curves are overcome.42-44 Menger et al45 reported a 1-year experience with robotic spine surgery at a high volume academic center and found time savings of 3.4 minutes per level instrumented translating to $5700 in annual savings, and improved screw accuracy that wound have avoided 9.5 revisions with resultant savings of $314 661. When also accounting for patients that would have required open surgery, a reduction in infection, and reduction in hospital admissions days, conservative estimates of $608 546/year dollars were demonstrated with the use of robotics.45 The main disadvantage of robotic navigation however is that it is a passive-imaging based system that does not allow for active navigation. It requires preoperative scans and screw trajectories, in contrast to intraoperatively acquired imaging used by the systems mentioned previously. For example, it does not allow for intraoperative identification of anatomy and pathology and cage placement, which is typically required for 3D total navigation in MIS TLIF procedures. Therefore, next generations of robotic systems will aim to combine passive robotic navigation with active 3D image guidance.
Figure 9.
Examples of spinal surgical robots: (left) Globus Excelsius and (right) Mazor X.
Augmented and Virtual Reality
Augmented and virtual reality systems are also gaining traction with recent technology development ventures, similar to as they have already done in other sectors such as the gaming, aeronautics, and military industries. Virtual reality (VR) refers to the development of a lifelike surface and volume 3D reconstruction that can be oriented or manipulated in any direction. Understanding the relationship of spinal pathology to neurovascular structures, as well as for preoperative planning of optimal approach has proven to be invaluable from an educational and preparatory standpoint. Archavlis et al46 utilized preoperative VR technology for patients requiring thoracic transpedicular corpectomies. They were able to determine the degree of bone removal required, the distance between the pedicle and the spinal cord, and estimated cage implant diameter and positioning, which resulted in no postoperative complciations.46 Gasco et al47 found a 53% reduction in lumbar pedicle screw placement errors in a group of trainees that had previously undergone VR simulation compared with a group that had just visual and verbal instruction. Likewise, Gottschalk et al48 found progressive improvement and accuracy in cervical lateral mass screw placement in groups of residents that had training with VR simulators over a control group with traditional visual/verbal instruction.
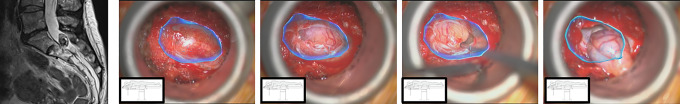
Augmented reality (AR) is similar to VR in the sense that it aims to overlay virtual bony structures and preplanned screw trajectories on patients in the setting of hybrid operating rooms that enable real-time feedback of all instruments in space and also in relation to anatomical structures.49 For example, herniated discs and synovial cysts can be clearly differentiated from surround neural tissue and bony landmarks by color, and allow for the optimal trajectory and laminotomy site to reach the pathology (Figure 10A and B). Intraoperatively through tubular retractors, AR allows for the identification of the pars, pedicle, and disc even when they are not in the exact field of view, which allows the surgeon to perform maneuvers for resection of tissue or bone safer and more confidently. We have also recently used AR for resection of intradural tumors (Figure 11), and further applications may be extended to intramedullary spinal cord tumors. These technologies may also leverage lens or goggle-based visualization,50 allowing the surgeon to focus on the task at hand without having to refer back and forth between navigation screens and the patient. Studies are ongoing leveraging AR technology in live patients, assessing for operating room design, workflow, image acquisition, software planning, radiation exposure, and navigated instrumentation.51-54
Figure 10.
Example of augmented reality–based 3-dimensional reconstruction of the lumbar spine. (A) Preoperative magnetic resonance imaging. (B) Example of approach planning for paracentral disc herniation (red), with relative location to intervertebral disc (yellow) and the synovial cyst (blue). (C) Approach planning for synovial cyst resection.
Figure 11.
Example of an S1-2 intradural schwannoma resected through a minimally invasive approach using a tubular retractor with augmented reality (AR). Even prior to durotomy, the outline of the tumor is denoted by the blue line. Once the durotomy is made, tumor resection is guided by internal debulking working toward the borders visualized through AR. After excision, the remaining space can be explored using the blue AR outline as a perimeter to inspect for confirmation of gross total resection.
Conclusion
The introduction of navigation and image-guided surgery has greatly affected spinal surgery and will continue to make surgery safer and more efficient. Eventually, it is conceivable that fluoroscopy will be completely replaced with image guidance. These advancements, among others such as robotics and virtual and augmented reality technology, will continue to drive the value of 3D navigation in MIS.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RH is a consultant for Ulrich, Brainlab, DePuy-Synthes, and has royalties from Zimmer. The other authors have no disclosures.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This supplement was supported by funding from the Carl Zeiss Meditec Group.
ORCID iD: Murat Cosar, MD  https://orcid.org/0000-0002-8537-0224
https://orcid.org/0000-0002-8537-0224
Sertac Kirnaz, MD  https://orcid.org/0000-0002-4104-3985
https://orcid.org/0000-0002-4104-3985
Christoph Wipplinger, MD  https://orcid.org/0000-0002-9001-6932
https://orcid.org/0000-0002-9001-6932
References
- 1. Nerland US, Jakola AS, Solheim O, et al. Minimally invasive decompression versus open laminectomy for central stenosis of the lumbar spine: pragmatic comparative effectiveness study. BMJ. 2015;350:h1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Imada AO, Huynh TR, Drazin D. Minimally invasive versus open laminectomy/discectomy, transforaminal lumbar, and posterior lumbar interbody fusions: a systematic review. Cureus. 2017;9:e1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foley KT, Gupta SK. Percutaneous pedicle screw fixation of the lumbar spine: preliminary clinical results. J Neurosurg. 2002;97(1 suppl):7–12. [DOI] [PubMed] [Google Scholar]
- 4. Foley KT, Gupta SK, Justis JR, Sherman MC. Percutaneous pedicle screw fixation of the lumbar spine. Neurosurg Focus. 2001;10:E10. [DOI] [PubMed] [Google Scholar]
- 5. Wong AP, Smith ZA, Stadler JA, 3rd, et al. Minimally invasive transforaminal lumbar interbody fusion (MI-TLIF): surgical technique, long-term 4-year prospective outcomes, and complications compared with an open TLIF cohort. Neurosurg Clin N Am. 2014;25:279–304. [DOI] [PubMed] [Google Scholar]
- 6. Tian NF, Wu YS, Zhang XL, Xu HZ, Chi YL, Mao FM. Minimally invasive versus open transforaminal lumbar interbody fusion: a meta-analysis based on the current evidence. Eur Spine J. 2013;22:1741–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heini P, Scholl E, Wyler D, Eggli S. Fatal cardiac tamponade associated with posterior spinal instrumentation. A case report. Spine (Phila Pa 1976). 1998;23:2226–2230. [DOI] [PubMed] [Google Scholar]
- 8. Mobbs RJ, Raley DA. Complications with K-wire insertion for percutaneous pedicle screws. J Spinal Disord Tech. 2014;27:390–394. [DOI] [PubMed] [Google Scholar]
- 9. Scheer JK, Harvey MJ, Dahdaleh NS, Smith ZA, Fessler RG. K-Wire fracture during minimally invasive transforaminal lumbar interbody fusion: Report of six cases and recommendations for avoidance and management. Surg Neurol Int. 2014;5(suppl 15):S520–S522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim CW, Lee YP, Taylor W, Oygar A, Kim WK. Use of navigation-assisted fluoroscopy to decrease radiation exposure during minimally invasive spine surgery. Spine J. 2008;8:584–590. [DOI] [PubMed] [Google Scholar]
- 11. Njoku I, Wanin O, Assey A, et al. Minimally invasive 2D navigation-assisted treatment of thoracolumbar spinal fractures in East Africa: a case report. Cureus. 2016;8:e507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shin MH, Ryu KS, Park CK. Accuracy and safety in pedicle screw placement in the thoracic and lumbar spines: comparison study between conventional C-arm fluoroscopy and navigation coupled with O-arm® guided methods. J Korean Neurosurg Soc. 2012;52:204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shin MH, Hur JW, Ryu KS, Park CK. Prospective comparison study between the fluoroscopy-guided and navigation coupled with O-arm-guided pedicle screw placement in the thoracic and lumbosacral spines. J Spinal Disord Tech. 2015;28:E347–E351. [DOI] [PubMed] [Google Scholar]
- 14. Overley SC, Cho SK, Mehta AI, Arnold PM. Navigation and robotics in spinal surgery: where are we now? Neurosurgery. 2017;80(3 suppl):S86–S99. [DOI] [PubMed] [Google Scholar]
- 15. Shin BJ, Njoku IU, Tsiouris AJ, Hartl R. Navigated guide tube for the placement of mini-open pedicle screws using stereotactic 3D navigation without the use of K-wires: technical note. J Neurosurg Spine. 2013;18:178–183. [DOI] [PubMed] [Google Scholar]
- 16. Nottmeier EW, Fenton D. Three-dimensional image-guided placement of percutaneous pedicle screws without the use of biplanar fluoroscopy or Kirschner wires: technical note. Int J Med Robot. 2010;6:483–488. [DOI] [PubMed] [Google Scholar]
- 17. Gertzbein SD, Robbins SE. Accuracy of pedicular screw placement in vivo. Spine (Phila Pa 1976). 1990;15:11–14. [DOI] [PubMed] [Google Scholar]
- 18. Liljenqvist UR, Halm HF, Link TM. Pedicle screw instrumentation of the thoracic spine in idiopathic scoliosis. Spine (Phila Pa 1976). 1997;22:2239–2245. [DOI] [PubMed] [Google Scholar]
- 19. Parker SL, McGirt MJ, Farber SH, et al. Accuracy of free-hand pedicle screws in the thoracic and lumbar spine: analysis of 6816 consecutive screws. Neurosurgery. 2011;68:170–178. [DOI] [PubMed] [Google Scholar]
- 20. Karapinar L, Erel N, Ozturk H, Altay T, Kaya A. Pedicle screw placement with a free hand technique in thoracolumbar spine: is it safe? J Spinal Disord Tech. 2008;21:63–67. [DOI] [PubMed] [Google Scholar]
- 21. Schizas C, Theumann N, Kosmopoulos V. Inserting pedicle screws in the upper thoracic spine without the use of fluoroscopy or image guidance. Is it safe? Eur Spine J. 2007;16:625–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kotil K, Bilge T. Accuracy of pedicle and mass screw placement in the spine without using fluoroscopy: a prospective clinical study. Spine J. 2008;8(4):591–596. [DOI] [PubMed] [Google Scholar]
- 23. Mason A, Paulsen R, Babuska JM, et al. The accuracy of pedicle screw placement using intraoperative image guidance systems. J Neurosurg Spine. 2014;20:196–203. [DOI] [PubMed] [Google Scholar]
- 24. Fan Y, Du J, Zhang J, et al. Comparison of accuracy of pedicle screw insertion among 4 guided technologies in spine surgery. Med Sci Monit. 2017;23:5960–5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Torres J, James AR, Alimi M, Tsiouris AJ, Geannette C, Hartl R. Screw placement accuracy for minimally invasive transforaminal lumbar interbody fusion surgery: a study on 3-D neuronavigation-guided surgery. Global Spine J. 2012;2:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lian X, Navarro-Ramirez R, Berlin C, et al. Total 3D Airo® navigation for minimally invasive transforaminal lumbar interbody fusion. Biomed Res Int. 2016;2016:5027340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Larson AN, Polly DW, Jr, Guidera KJ, et al. The accuracy of navigation and 3D image-guided placement for the placement of pedicle screws in congenital spine deformity. J Pediatr Orthop. 2012;32(6): e23–29. [DOI] [PubMed] [Google Scholar]
- 28. Bydon M, Xu R, Amin AG, et al. Safety and efficacy of pedicle screw placement using intraoperative computed tomography: consecutive series of 1148 pedicle screws. J Neurosurg Spine. 2014;21:320–328. [DOI] [PubMed] [Google Scholar]
- 29. Klingler JH, Sircar R, Scheiwe C, et al. Comparative study of C-Arms for intraoperative 3-dimensional imaging and navigation in minimally invasive spine surgery part II: radiation exposure. Clin Spine Surg. 2017;30:E669–E676. [DOI] [PubMed] [Google Scholar]
- 30. Navarro-Ramirez R, Lang G, Lian X, et al. Total navigation in spine surgery; a concise guide to eliminate fluoroscopy using a portable intraoperative computed tomography 3-dimensional navigation system. World Neurosurg. 2017;100:325–335. [DOI] [PubMed] [Google Scholar]
- 31. Kim TT, Drazin D, Shweikeh F, Pashman R, Johnson JP. Clinical and radiographic outcomes of minimally invasive percutaneous pedicle screw placement with intraoperative CT (O-arm) image guidance navigation. Neurosurg Focus. 2014;36:E1. [DOI] [PubMed] [Google Scholar]
- 32. Oertel MF, Hobart J, Stein M, Schreiber V, Scharbrodt W. Clinical and methodological precision of spinal navigation assisted by 3D intraoperative O-arm radiographic imaging. J Neurosurg Spine. 2011;14:532–536. [DOI] [PubMed] [Google Scholar]
- 33. Van de Kelft E, Costa F, Van der Planken D, Schils F. A prospective multicenter registry on the accuracy of pedicle screw placement in the thoracic, lumbar, and sacral levels with the use of the O-arm imaging system and StealthStation Navigation. Spine (Phila Pa 1976). 2012;37:E1580–1587. [DOI] [PubMed] [Google Scholar]
- 34. Lange J, Karellas A, Street J, et al. Estimating the effective radiation dose imparted to patients by intraoperative cone-beam computed tomography in thoracolumbar spinal surgery. Spine (Phila Pa 1976). 2013;38:E306–E312. [DOI] [PubMed] [Google Scholar]
- 35. Malham GM, Wells-Quinn T. What should my hospital buy next? Guidelines for the acquisition and application of imaging, navigation, and robotics for spine surgery. J Spine Surg. 2019;5:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Al-Khouja LT, Baron EM, Johnson JP, Kim TT, Drazin D. Cost-effectiveness analysis in minimally invasive spine surgery. Neurosurg Focus. 2014;36:E4. [DOI] [PubMed] [Google Scholar]
- 37. Zausinger S, Scheder B, Uhl E, Heigl T, Morhard D, Tonn JC. Intraoperative computed tomography with integrated navigation system in spinal stabilizations. Spine (Phila Pa 1976). 2009;34:2919–2926. [DOI] [PubMed] [Google Scholar]
- 38. Watkins RG, Gupta A, Watkins RG. Cost-effectiveness of image-guided spine surgery. Open Orthop J. 2010;4:228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sasso RC, Garrido BJ. Computer-assisted spinal navigation versus serial radiography and operative time for posterior spinal fusion at L5-S1. J Spinal Disord Tech. 2007;20:118–122. [DOI] [PubMed] [Google Scholar]
- 40. Houten JK, Nasser R, Baxi N. Clinical assessment of percutaneous lumbar pedicle screw placement using the O-arm multidimensional surgical imaging system. Neurosurgery. 2012;70:990–995. [DOI] [PubMed] [Google Scholar]
- 41. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22:233–236. [DOI] [PubMed] [Google Scholar]
- 42. Han X, Tian W, Liu Y, et al. Safety and accuracy of robot-assisted versus fluoroscopy-assisted pedicle screw insertion in thoracolumbar spinal surgery: a prospective randomized controlled trial [published online February 8, 2019]. J Neurosurg Spine. doi:10.3171/2018.10.SPINE18487 [DOI] [PubMed] [Google Scholar]
- 43. Zhang Q, Han XG, Xu YF, et al. Robot-assisted versus fluoroscopy-guided pedicle screw placement in transforaminal lumbar interbody fusion for lumbar degenerative disease. World Neurosurg. 2019;125:e429–e434. [DOI] [PubMed] [Google Scholar]
- 44. Staartjes VE, Klukowska AM, Schroder ML. Pedicle screw revision in robot-guided, navigated, and freehand thoracolumbar instrumentation: a systematic review and meta-analysis. World Neurosurg. 2018;116:433–443.e8. [DOI] [PubMed] [Google Scholar]
- 45. Menger RP, Savardekar AR, Farokhi F, Sin A. A cost-effectiveness analysis of the integration of robotic spine technology in spine surgery. Neurospine. 2018;15:216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Archavlis E, Schwandt E, Kosterhon M, et al. A modified microsurgical endoscopic-assisted transpedicular corpectomy of the thoracic spine based on virtual 3-dimensional planning. World Neurosurg. 2016;91:424–433. [DOI] [PubMed] [Google Scholar]
- 47. Gasco J, Patel A, Ortega-Barnett J, et al. Virtual reality spine surgery simulation: an empirical study of its usefulness. Neurol Res. 2014;36:968–973. [DOI] [PubMed] [Google Scholar]
- 48. Gottschalk MB, Yoon ST, Park DK, Rhee JM, Mitchell PM. Surgical training using three-dimensional simulation in placement of cervical lateral mass screws: a blinded randomized control trial. Spine J. 2015;15:168–175. [DOI] [PubMed] [Google Scholar]
- 49. Burstrom G, Nachabe R, Persson O, Edstrom E, Terander AE. Augmented and virtual reality instrument tracking for minimally invasive spine surgery: a feasibility and accuracy study. Spine (Phila Pa 1976). 2019;44:1097–1104. [DOI] [PubMed] [Google Scholar]
- 50. Urakov TM, Wang MY, Levi AD. Workflow caveats in augmented reality-assisted pedicle instrumentation: cadaver lab. World Neurosurg. 2019;126:e1449–e1455. [DOI] [PubMed] [Google Scholar]
- 51. Edstrom E, Burstrom G, Nachabe R, Gerdhem P, Terander AE. A novel augmented-reality-based surgical navigation system for spine surgery in a hybrid operating room: design, workflow, and clinical applications [published online August 27, 2019]. Oper Neurosurg (Hagerstown). doi:10.1093/ons/opz236 [DOI] [PubMed] [Google Scholar]
- 52. Edstrom E, Burstrom G, Omar A, et al. Augmented reality surgical navigation in spine surgery to minimize staff radiation exposure. Spine (Phila Pa 1976). 2019;45:E45–E53. [DOI] [PubMed] [Google Scholar]
- 53. Elmi-Terander A, Burstrom G, Nachabe R, et al. Pedicle screw placement using augmented reality surgical navigation with intraoperative 3D imaging: a first in-human prospective cohort study. Spine (Phila Pa 1976). 2019;44:517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carl B, Bopp M, Saß B, Nimsky C. Microscope-based augmented reality in degenerative spine surgery: initial experience. World Neurosurg. 2019;128:e541–e551. [DOI] [PubMed] [Google Scholar]