Abstract
Study Design:
Surgical technical note.
Objectives:
Describe the preoperative evaluation, approach, and technical considerations for an oblique lumbar interbody fusion using neuronavigation.
Methods:
A thorough review of previous technical and anatomic descriptions for pre- and transpsoas interbody techniques was performed and incorporated into the technical considerations warranting discussion for a navigated oblique lateral interbody fusion.
Results:
The prepsoas technique, also known as an oblique lumbar interbody fusion (OLIF), is an alternative approach for lumbar interbody fusion that utilizes a retroperitoneal corridor between the aorta/inferior vena cava. This corridor is devoid of neurovascular structures and obviates the need for real time electromyography monitoring. This approach spares the psoas and provides direct visualization of key structures and minimizes risk of injury to the great vessels, ureter, and lumbar plexus.
Conclusions:
A navigated prepsoas retroperitoneal approach is an effective minimally invasive technique for lumbar interbody fusion that may help mitigate some of the vascular and neurologic complications present with anterior lumbar interbody fusion or lateral lumbar interbody fusion and minimize radiation exposure to the surgeon.
Keywords: OLIF, oblique interbody fusion, surgical technique, navigation
Introduction
Lumbar interbody fusion is a commonly used technique for a wide variety of pathologies including degenerative disc disease, spondylolisthesis, and spinal deformity. Two commonly used approaches include anterior lumbar interbody fusion (ALIF) and lateral lumbar interbody fusion (LLIF). Compared with posterior approaches, these approaches circumvent the risk of injury to nerve roots and the dura, and they improve the ability to restore disc height necessary to achieve indirect nerve root decompression. While ALIF provides direct access to the disc space, it is associated with an approach-specific risk profile that includes injury to the iliac vessels, peritoneum, ureter, and abdominal wall integrity resulting in abdominal hernias.1,2 In contrast, a direct lateral approach via an LLIF requires dissection through the psoas, which can result in hip flexor weakness postoperatively. In addition, because of proximity to the lumbar plexus, this approach requires real-time electromyography (EMG) monitoring to minimize risk of neural injury.3,4
The prepsoas technique, also known as an oblique lumbar interbody fusion (OLIF), is an alternative approach for lumbar interbody fusion that utilizes a retroperitoneal corridor between the aorta and inferior vena cava.5-8 This corridor is devoid of neurovascular structures and obviates the need for real-time EMG monitoring.9 This approach spares the psoas and provides direct visualization of key structures while minimizing risk of injury to the great vessels, ureter, and lumbar plexus. This review highlights the technical nuances of a minimally invasive OLIF technique utilizing intraoperative neuronavigation and neuromonitoring. Additionally, the authors review the operative outcomes and complications of OLIF as reported in the literature.
Preoperative Evaluation
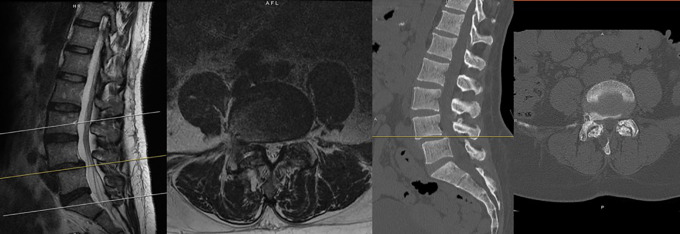
Prior to surgery, magnetic resonance imaging (MRI) and 36-inch standing AP and lateral scoliosis radiographs should be obtained (Figure 1). Extent of canal and foraminal stenosis, as well as disc degeneration, can be additionally be evaluated on MRI. MRI allows for evaluation of the retroperitoneal oblique corridor between the great vessels and the psoas muscle to the spine. While a computed tomography (CT) angiogram can be obtained to evaluate the great vessels, MRI alone is sufficient to assess the corridor the intervertebral disc spaces from L2 to S1 for the majority of patients.6 Thirty-six-inch radiographs allow for the evaluation of spinal alignment to evaluate the extent of sagittal and coronal correction desired (Figure 2). Additionally, these radiographs reveal the position of the iliac crest relative to the intervertebral disc spaces. Specifically, a high iliac crest may limit the surgeon’s ability to access L4-5. Flexion and extension X-rays can additionally be obtained to evaluate mobile spondylolisthesis. If careful evaluation of specific bony anatomy is needed, a preoperative CT of the lumbar spine can also be obtained. Institutional review board approval was utilized for all patients included in this study.
Figure 1.
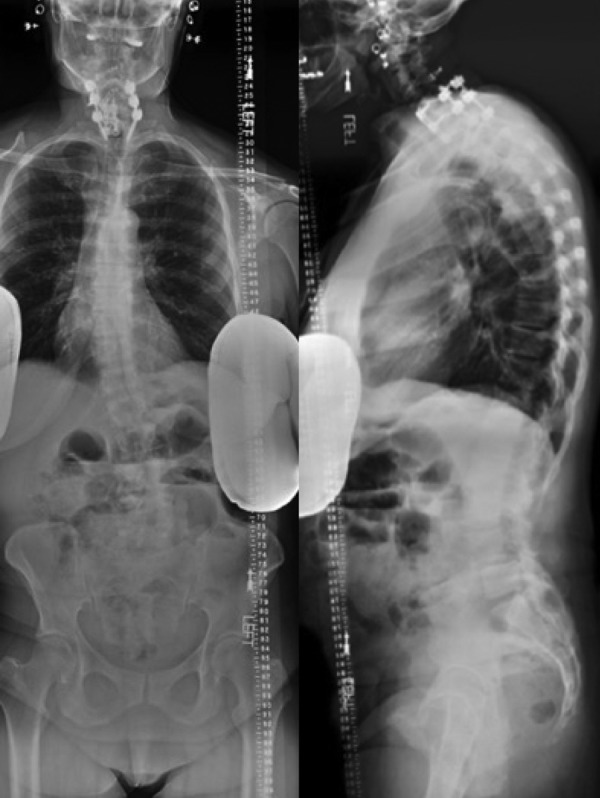
Preoperative standing 36-inch scoliosis X-rays in a female with history of cervical fusion and scoliosis with spondylolisthesis.
Figure 2.
Preoperative magnetic resonance imaging and computed tomography demonstrating degenerative disease and spondylolisthesis at L4-5 associated with canal and foraminal stenosis.
Patient Positioning and Neuromonitoring
Following induction with general anesthesia, the patient is placed in the right lateral decubitus position, with the patient’s left side up (Figure 3). The left side only is used because the vena cava on the right side either blocks or is close to the OLIF corridor. The right side has a significantly higher risk of devastating vascular injury, and because of this risk, only the left side is used for the OLIF. The left side has a natural corridor between the aorta and the psoas muscle, making the left-sided approach much safer.
Figure 3.
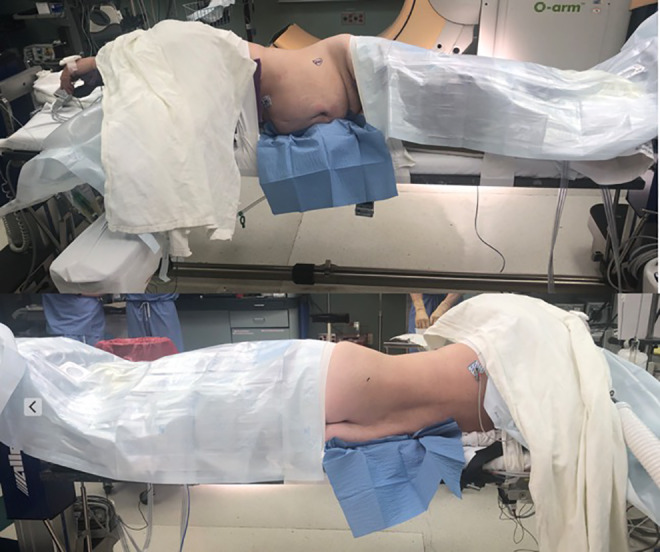
For positioning, the patient is placed in the right lateral decubitus position, with the patient’s left side up.
The common peroneal nerve and bony prominences are carefully padded, and an axillary roll is placed underneath the lateral chest wall. Of note, sufficient room for an intraoperative CT scanner passing beneath the table must be made when positioning the patient’s arm. Motor evoked potentials and free-running EMG is used for neuromonitoring. Paralytic anesthetic agents should be avoided as it can lead to false negative readings in the setting of triggered EMG monitoring.
Procedure
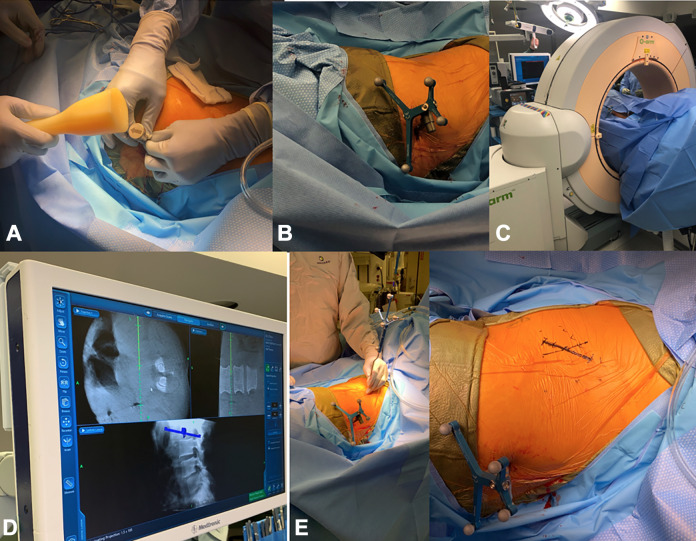
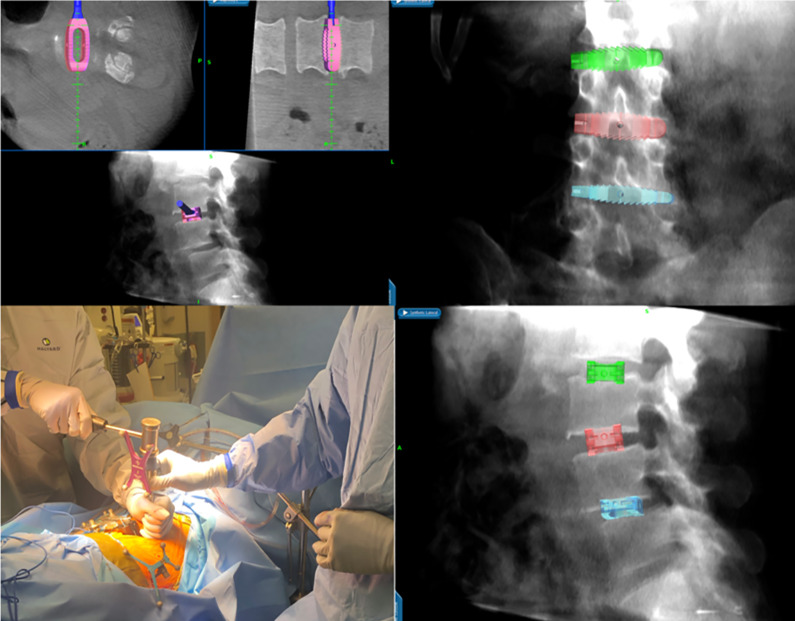
After the patient is prepped and draped in sterile fashion, a 1-cm incision is made over the iliac crest for the placement of a reference arc for intraoperative navigation, which is malleted into the iliac crest. The incision is made 2 inches superolateral to the posterior superior iliac spine (Figure 4).
Figure 4.
A reference arc for intraoperative navigation is placed into the iliac crest, 2 inches superolateral to the posterior superior iliac spine (A and B). Using navigation (C), the incision is marked out in a true lateral fashion over the pathological disc space (D and E).
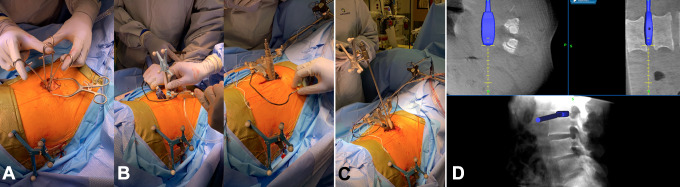
CT imaging is obtained and the reference array is registered for intraoperative navigation. Navigation is subsequently used to mark out a 3-inch incision in a true lateral fashion over the mid-portion of the desired intervertebral disc space. A point 5 cm anterior to this is marked out. A prepsoas incision is then made at this point parallel to the left abdominal wall nerve root trajectory. Care is taken to avoid entering the peritoneal cavity, and the trajectory can be confirmed with navigation. Handheld retractors can be used to assist in the visualization of this corridor. Blunt dissection is used through the external oblique, internal oblique, and transversus abdominus fascia. Retroperitoneal fat is identified and is mobilized ventrally to allow for direct visualization of the psoas muscle. Navigation is then used to identify the correct disc space and the entry point into the disc space (Figure 5). This entry point will be anterior to the psoas. Blunt dissection is then used to clear the remaining soft tissue.
Figure 5.
Blunt dissection is used (A), and the psoas muscle is visualized. The correct disc space is identified with navigation (B) and sequential dilators are placed at the desired disc space. Triggered EMG monitoring is used (C). Navigation is used during localization and sequential dilation over disc space (D).
Using triggered EMG monitoring at each step, sequential dilators are placed at the desired disc space. Navigation should be used again to confirm the correct level. Following placement of the retractor, an annulotomy is performed with a disc knife. The disc is then removed with navigated instruments to the contralateral side. A straight oblique trajectory is critical in minimizing risk of injuring the contralateral nerve roots or the spinal canal. To ensure a true lateral positioning of the instruments, the surgeon should move his hand dorsally. The endplates are prepared and care is taken to preserve the anterior longitudinal ligament.
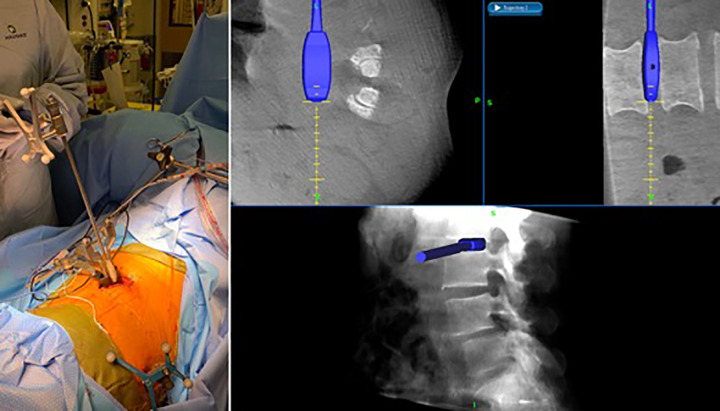
Interbody trial sizers are utilized to select the correct cage size (Figure 6). The cage is loaded with graft material and placed with navigation for guidance. The implant should ideally span the entire apophyseal ring laterally and not be located either too ventral or too dorsal near the spinal canal (Figure 7). To confirm this ideal positioning of the implant, intraoperative AP and lateral X-rays are taken in case intraoperative navigation was not accurate. The wound is then closed by layers.
Figure 6.
Following discectomy, interbody trial sizers are utilized to select the correct cage size under navigation.
Figure 7.
The cage is placed with navigation for guidance. The implant should ideally span the entire apophyseal ring laterally.
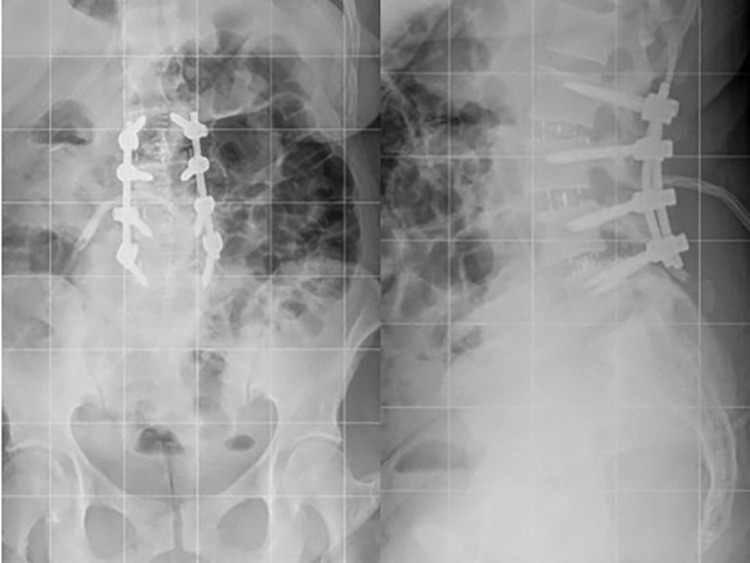
Posterior instrumentation is often placed. This can be completed in a staged fashion following the OLIF (Figure 8). Staging the posterior portion of the case following the anterior approach provides time for a clinical assessment of indirect foraminal decompression. Evaluation of radicular symptoms can subsequently guide the posterior approach. If radicular symptoms resolve, percutaneous fixation can be adequate. However, if there is continued radiculopathy, decompression in addition for posterior fixation is indicated.
Figure 8.
Postoperative scoliosis following lumbar 2-5 OLIF and a second stage lumbar 2-5 posterior spinal fusion with posterior column osteotomies.
With regards to different-sized patients and their body mass indices (BMI), when operating on underweight patients, it can be very easy to inadvertently enter the peritoneum because of the lack of fat. Thus, extreme care and slow, meticulous dissection should be performed in underweight patients. With regards to very large patients, deeper retractors and a larger skin incision are often necessary. The main issue with larger patients is that despite retraction, there is so much retroperitoneal fat, that the fat will migrate around the retractor, obscuring visualization. Thus, wider bladed retractors to prevent fat migration or a larger skin incision allowing for wider retraction may be useful in larger patients.
Postoperative Management
The patient is mobilized on postoperative day 1. The diet is advanced from a clear liquid diet once flatus has returned. While uncommon, postoperative ileus can occur. If a second staged procedure is planned for posterior fixation, the patient is encouraged to mobilize to evaluate for persistent radicular symptoms that may need to be addressed with additional decompression. Patients are ready for discharge once pain is controlled, the patient is ambulating well, and bowel function has returned.
Discussion
The prepsoas approach has a number of advantages compared with a transpsoas approach. The prepsoas approach allows for access to the L5-S1 disc space. This can be achieved with careful assessment of an adequate surgical corridor in relation to the vascular anatomy, the sacral slope, and position of the iliac crest on preoperative imaging.6,10 The approach eliminates the need for dissection through the psoas, minimizing injury to the psoas, which can occasionally result in permanent weakened hip flexion. Additionally, there is a reduced risk for injury to the lumbar plexus.8 Although the lumbar plexus is avoided and the nerves themselves are not encountered, the local practice environment may dictate whether or not neuromonitoring is used. However, there are surgeons who do not use monitoring during the OLIF, but this decision should be based on one’s local practice environment.
In a review of 49 patients undergoing a prepsoas approach for lumbar interbody fusion, DiGiorgio et al noted one case of morbidity related to the surgical approach.10 This was a postoperative psoas hematoma in a patient undergoing 3-level fusion who subsequently developed transient hip flexor weakness. Six patients experienced expected sensory deficits to the left thigh from this approach, which resolved in 3 patients. Transient postoperative ileus was noted in 3 patients. In a meta-analysis, incidence of intraoperative complications was 1.5%.5 Of these, the most common was major vessel injury in 0.9%. The rate of postoperative complications was 9.9%, comprising transient sensory deficits to the thigh in 3% and transient hip flexion weakness in 1.2% of patients. In contrast, a meta-analysis by Joseph et al reported a 9.4% and 2.5% rate of temporary and permanent neurological deficits with a transpsoas approach and a 27.1% rate of sensory deficits.11
There is also an increased theoretical risk for injury to the vessels and the bowel given a more anterior corridor with the prepsoas approach.12,13 Silvestre et al reported incisional pain, lower extremity symptoms secondary to sympathetic chain injury, iliac vein injury, and iliolumbar vein injury as the most common complications.2 Other complications reported include male sexual dysfunction, cerebrovascular accidents, peritoneal laceration, ileus, psoas paresis, and groin numbness.
Conclusion
A prepsoas retroperitoneal approach is an effective minimally invasive technique for lumbar interbody fusion. By offering the surgeon real-time anatomical guidance, intraoperative navigation can improve the ease and accuracy of an OLIF, and minimize exposure to radiation.14 This technique represents a unique approach in the surgeon’s arsenal of interbody fusion techniques that can mitigate the risk of vascular or neurological complications compared with an ALIF or LLIF.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This supplement was supported by funding from the Carl Zeiss Meditec Group.
ORCID iD: Rory Richard Mayer, MD  https://orcid.org/0000-0002-9813-0027
https://orcid.org/0000-0002-9813-0027
References
- 1. Mummaneni PV, Haid RW, Rodts GE. Lumbar interbody fusion: state-of-the-art technical advances. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1:24–30. [DOI] [PubMed] [Google Scholar]
- 2. Silvestre C, Mac-Thiong JM, Hilmi R, Roussouly P. Complications and morbidities of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lumbar interbody fusion in 179 patients. Asian Spine J. 2012;6:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang YH, White I, Potts E, Mobasser JP, Chou D. Comparison perioperative factors during minimally invasive pre-psoas lateral interbody fusion of the lumbar spine using either navigation or conventional fluoroscopy. Global Spine J. 2017;7:657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lykissas MG, Aichmair A, Hughes AP, et al. Nerve injury after lateral lumbar interbody fusion: a review of 919 treated levels with identification of risk factors. Spine J. 2014;14:749–758. [DOI] [PubMed] [Google Scholar]
- 5. Li JX, Phan K, Mobbs R. Oblique Lumbar interbody fusion: technical aspects, operative outcomes, and complications. World Neurosurg. 2017;98:113–123. [DOI] [PubMed] [Google Scholar]
- 6. Molinares DM, Davis TT, Fung DA. Retroperitoneal oblique corridor to the L2-S1 intervertebral discs: an MRI study. J Neurosurg Spine. 2016;24:248–255. [DOI] [PubMed] [Google Scholar]
- 7. Hartl R, Joeris A, McGuire RA. Comparison of the safety outcomes between two surgical approaches for anterior lumbar fusion surgery: anterior lumbar interbody fusion (ALIF) and extreme lateral interbody fusion (ELIF). Eur Spine J. 2016;25:1484–1521. [DOI] [PubMed] [Google Scholar]
- 8. Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg. 2015;1:2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ohtori S, Orita S, Yamauchi K, et al. Mini-open anterior retroperitoneal lumbar interbody fusion: oblique lateral interbody fusion for lumbar spinal degeneration disease. Yonsei Med J. 2015;56:1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DiGiorgio AM, Edwards CS, Virk MS, Mummaneni PV, Chou D. Stereotactic navigation for the prepsoas oblique lateral lumbar interbody fusion: technical note and case series. Neurosurg Focus. 2017;43:E14. [DOI] [PubMed] [Google Scholar]
- 11. Joseph JR, Smith BW, La Marca F, Park P. Comparison of complication rates of minimally invasive transforaminal lumbar interbody fusion and lateral lumbar interbody fusion: a systematic review of the literature. Neurosurg Focus. 2015;39:E4. [DOI] [PubMed] [Google Scholar]
- 12. Choy W, Miller CA, Chan AK, Fu KM, Park P, Mummaneni PV. Evolution of the minimally invasive spinal deformity surgery algorithm: an evidence-based approach to surgical strategies for deformity correction. Neurosurg Clin N Am. 2018;29:399–406. [DOI] [PubMed] [Google Scholar]
- 13. Phan K, Maharaj M, Assem Y, Mobbs RJ. Review of early clinical results and complications associated with oblique lumbar interbody fusion (OLIF). J Clin Neurosci. 2016;31:23–29. [DOI] [PubMed] [Google Scholar]
- 14. Villard J, Ryang YM, Demetriades AK, et al. Radiation exposure to the surgeon and the patient during posterior lumbar spinal instrumentation: a prospective randomized comparison of navigated versus non-navigated freehand techniques. Spine (Phila Pa 1976). 2014;39:1004–1009. [DOI] [PubMed] [Google Scholar]