Abstract
Study Design:
Literature review.
Objective:
To provide an overview of the recent advances in minimal access surgery (MAS) for spinal metastases.
Methods:
Literature review.
Results:
Experience gained from MAS in the trauma, degenerative and deformity settings has paved the road for MAS techniques for spinal cancer. Current MAS techniques for the treatment of spinal metastases include percutaneous instrumentation, mini-open approaches for decompression and tumor resection with or without tubular/expandable retractors and thoracoscopy/endoscopy. Cancer care requires a multidisciplinary effort and adherence to treatment algorithms facilitates decision making, ultimately improving patient outcomes. Specific algorithms exist to help guide decisions for MAS for extradural spinal metastases. One major paradigm shift has been the implementation of percutaneous stabilization for treatment of neoplastic spinal instability. Percutaneous stabilization can be enhanced with cement augmentation for increased durability and pain palliation. Unlike osteoporotic fractures, kyphoplasty and vertebroplasty are known to be effective therapies for symptomatic pathologic compression fractures as supported by high level evidence. The integration of systemic body radiation therapy for spinal metastases has eliminated the need for aggressive tumor resection allowing implementation of MAS epidural tumor decompression via tubular or expandable retractors and preliminary data exist regarding laser interstitial thermal therapy and radiofrequency ablation for tumor control. Neuronavigation and robotic systems offer increased precision, facilitating the role of MAS for spinal metastases.
Conclusions:
MAS has a significant role in the treatment of spinal metastases. This review highlights the current utilization of minimally invasive surgical strategies for treatment of spinal metastases.
Keywords: spine, tumor, minimally invasive surgery, minimal access surgery, surgery
Introduction
Over the past 2 decades, we have witnessed an increase in utilization of minimal access surgery (MAS) for the treatment of spinal pathologies. The expertise gained from surgery for spinal trauma, deformity, and degenerative disease has stemmed the adoption of minimally invasive surgeries for spinal cancer. Patients with spinal tumors generally require a combination of surgical, radiation, and systemic therapies making rapid postoperative healing and return to treatment of paramount importance. Comparative data evaluating the benefit of MIS approaches versus open surgeries in spinal metastatic disease are still limited and a systematic review of surgical approaches for spinal metastases concluded that although some studies showed superiority of MAS approaches, data is low quality and strong recommendations cannot be made.1 Nevertheless, multiple studies have demonstrated decreased blood loss, transfusion rates, and hospitalization length with MAS stabilization techniques for spinal tumors.2-5 As an example, a retrospective study comparing outcomes of 25 MAS operations versus 25 open decompressions for spinal metastases, showed a mean of 340 versus 714 mL of blood loss, 3 versus 10 cases requiring transfusions and 2 versus 3.6 days of hospital stay, respectively.3 Moreover, postoperative radiation can occasionally be started within a minimally invasive surgery (MIS) compared with open surgeries where the risk of wound complications frequently delay radiation therapy.6,7 These benefits of MAS surgeries along with other potential benefits for patients with metastatic spinal disease result in expeditious recovery and return to multimodality cancer therapy and are thus becoming a more widely used. Current MAS techniques for the treatment of spinal metastases include percutaneous instrumentation, mini-open approaches for decompression, and tumor removal with or without tubular/expandable retractors and thoracoscopy/endoscopy. This review highlights the current available data on minimally invasive surgical strategies for treatment of metastatic extradural spinal metastases.
Treatment Algorithms
For treatment of spinal tumors, patients benefit from an evaluation by a multidisciplinary team, including neurosurgeons, radiation and medical oncologists, interventional radiologists, and pain specialists.8 The NOMS framework assesses 4 key factors to facilitate the decision making for patients with metastatic tumors: Neurologic, Oncologic, Mechanical, and Systemic.9 Neurologic consideration includes the presence of myelopathy or radiculopathy as well the degree of epidural spinal cord compression.10,11 The epidural spinal compression score facilitates this evaluation as scores of 2 (spinal cord displacement) and 3 (absence of cerebrospinal fluid around the spinal cord due to tumor extension) denote patients with high-grade epidural disease. Oncologic consideration evaluates the predicted tumor response to current available treatments, currently primarily reflecting tumor radiosensitivity.12 Mechanical assessment evaluates the stability of the spine, as facilitated by the Spinal Instability Neoplastic Score (SINS).13 Systemic refers to a comprehensive risk assessment of the patient’s ability to withstand the proposed treatment and the extent of the systemic tumor burden. This paradigm was the basis for development of a treatment algorithm for thoracolumbar MIS spine stabilization and decompression for spinal metastases.14 This algorithm facilitates the selection of optimal MAS strategy for patients requiring surgical treatment of thoracolumbar fractures (Figure 1). The 2 main surgical indications for patients with extradural spinal metastases include symptomatic spinal cord compression by radioresistant tumors and mechanical instability, both of which can be treated using MAS techniques.
Figure 1.
Minimal access treatment algorithm for metastatic thoracolumbar compression fractures. Adapted from Barzilai et al.14
MIS Decompression
Patients with high-grade spinal cord compression (ie, epidural spinal cord compression [ESCC] scores of 2 and 3) secondary to radioresistant tumors generally require surgical decompression and stabilization followed by radiation treatment (Figure 2).
Below the level of the conus, mechanical radiculopathy (ie, radicular pain secondary to axial loading) represents the primary indication for decompression, and patients are known to benefit from stabilization and decompression15 (Figure 3).
Figure 2.
A 43-year-old woman who presented with newly diagnosed squamous cell carcinoma of thymic origin. Evaluated for severe biologic pain, no evidence of mechanical instability and with high-grade spinal cord compression at T2. She underwent a minimally invasive tubular decompression with percutaneous stabilization followed by stereotactic body radiation therapy (SBRT). At 3 months postoperatively, the patient was neurologically intact, pain free, no evidence of viable tumor on magnetic resonance imaging. (Left) Preoperative axial T2 demonstrating high-grade epidural spinal cord compression. (Right) Three-month follow-up, postoperative changes demonstrated with no residual cord compression.
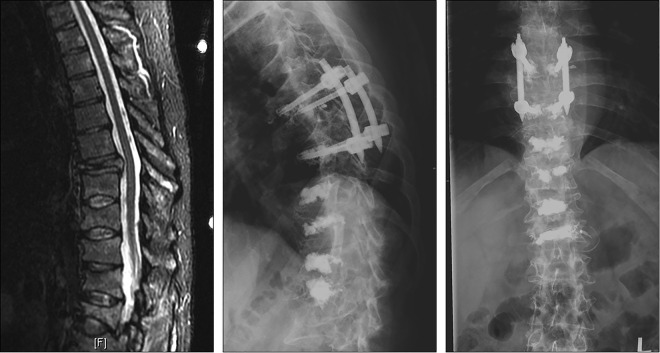
Figure 3.
A 58-year-old woman with widely metastatic breast cancer to the lymph nodes, bone, liver, and pleura presented with progressive lower back pain secondary to a previously irradiated L5 metastatic lesion. She developed significant mechanical radiculopathy in the left L5 distribution and magnetic resonance imaging (MRI) demonstrated progression of a compression fracture with severe foraminal stenosis. She underwent a minimally invasive left L5-S1 left hemifacetectomy along with L4-S1 instrumented stabilization with cement augmentation. At 3-month follow-up, her preoperative pain has significantly decreased and patient regained full ambulation. (Left) Preoperative MRI; (top) axial T1 with contrast demonstrating the foraminal disease and (bottom) T1 without contrast demonstrating the fracture compressing the exiting nerve root. (Right) (top) postoperative computed tomography demonstrating the left sided hemifacetectomy and (bottom) postoperative x-ray showing the stabilizing construct with cement augmentation.
The integration of stereotactic body radiation therapy has revolutionized surgery for spine cancer since it eliminates the purpose of cytoreductive or gross total resections. Since the introduction of spinal stereotactic body radiation therapy (SBRT), an abundance of data has established the safety and efficacy of SBRT demonstrating high rates of tumor control with low complication profiles.16-18 Postoperative SBRT provides durable and consistent local control irrespective of tumor volume or tumor histology.19 Therefore, spinal SBRT diminishes the need for extensive tumor excision, with patients undergoing decompressive separation surgery to provide circumferential spinal cord or cauda equina decompression in order to optimize SBRT dosimetry.19 Among patients with lumbar radiculopathy manifested by severe radicular pain exacerbated by axial loads in the setting of lumbar burst fracture with tumor extension into the pedicle, facetectomy with fractured pedicle excision and instrumented stabilization provide reliable symptom relief.
As SBRT eliminated the need for extensive excisional operations, this strategy allowed exploration of even less invasive surgeries with goals of rapid continuation of concomitant cancer therapies. Chou et al,20 described the “mini-open” approach, using minimally invasive laminectomy and transpedicular ventral epidural decompression with trans-fascial instrumented stabilization. Others have since described case series demonstrating the safety and efficacy with minimal access approaches using tubular and expandable retractors for either circumferential spinal cord decompression or facetectomy and nerve root decompression in the setting of mechanical radiculopathy.21,22 Our current MAS decompression strategy involves the use of a tubular or expandable retractor placed though one of the pedicle screw incisions, allowing us to perform a hemilaminotomy and/or facetectomy. For patients requiring a midline or bilateral decompression, an expandable midline retractor provides excellent exposure while minimizing the approach-related soft tissue injury. Spinal endoscopy is an emerging field for degenerative spine disease and considering the minimal access entailed, coupled with direct target visualization, it will likely play a role in surgery for spinal metastases in the future.
Laser Interstitial Thermotherapy
The search for less invasive methods for epidural tumor decompression has brought forth the adoption of image-guided laser interstitial thermal therapy (LITT) as an alternative to open surgery. LITT delivers thermal energy, under real-time magnetic resonance imaging (MRI) monitoring. The technical safety and feasibility of LITT, initially developed for ablation of intracranial pathologies, has been described by Tatsui et al.23-26 In combination with SBRT, LITT reduced the epidural tumor volume while improving pain control and health-related quality of life (HRQoL).23,27 In brief, the laser probe is inserted under navigation to the affected epidural space, typically via a transpedicular, vertical, or translaminar approach.26 Thermal energy is then delivered under real-time MRI monitoring. A dedicated MRI sequence shows both intensity and spread of heat within the involved tissue providing real-time monitoring of the thermal damage.23 Despite this method’s promising potential, it has not been widely adopted likely due to significant technological and time requirements.
MAS Stabilization
Systemic and radiation therapy are the primary treatment modalities for spinal metastases, yet they do not treat neoplastic spinal instability. Hence, spinal stabilization serves as a separate surgical indication, regardless of oncologic or local control goals. To simplify the assessment of mechanical stability and to unify decision making and reporting across institutions, the spine oncology study group developed a scoring system—the SINS.28 SINS has become widely accepted and is used to determine stable, unstable, and intermediate scores ultimately expediting referrals for evaluation and treatment of spinal instability.
Patients with mechanical instability but without high-grade epidural extension of radioresistant tumors and without mechanical radiculopathy do not require surgical decompression and can be treated with stabilization alone. Cement and instrumented stabilization serve as the dominant modalities for spinal stabilization. Fracture morphology determines whether vertebral cement augmentation alone would suffice or whether additional instrumented stabilization is indicated.
Patients with mechanically unstable compression fractures without significant epidural extension, extensive posterior cortical destruction, or posterior element involvement can be treated with balloon kyphoplasty or vertebroplasty. The tumor requires postprocedure treatment with SBRT or cEBRT, with the tumor histology determining the choice of radiotherapy modality.
Patients with mechanically unstable fractures with extensive posterior cortical destruction and/or fracture extension into the posterior elements benefit from percutaneous stabilization combined with balloon kyphoplasty. The addition of percutaneous instrumentation provides stabilization of the posterior elements in addition to more robust stabilization of the vertebral body fracture. The tumor requires post-procedure treatment with SBRT or conventional external beam radiation therapy (cEBRT), with the tumor histology determining the choice of radiotherapy modality (Figure 4).
Patients with mechanically unstable fractures with significant epidural retropulsion caused by radiosensitive tumors (ie, lymphoma, multiple myeloma, or breast and prostate adenocarcinoma) benefit from percutaneous stabilization without kyphoplasty followed by cEBRT. Kyphoplasty should be avoided at the level of the fracture to avoid the risk of exacerbation of the epidural disease and spinal cord compression. The tumor requires postprocedure cEBRT with the expectation of resolution of the epidural tumor component.
Figure 4.
A 43-year-old man with newly diagnosed IgG-lambda multiple myeloma with anemia, hypercalcemia, acute renal failure (ARF), and bone lesions at initial presentation. He presented with severe, progressive, and debilitating movement-related back pain localized in his mid to lower thoracic region. His magnetic resonance imaging (MRI) demonstrated multilevel compression fractures most notably a T8 planum burst fracture with a mild kyphotic deformity but without significant spinal cord compression. He was not able to tolerate transport into the hospital for oncologic therapy and hence pain palliation was necessary. He underwent T7-T9 percutaneous instrumentation with cement-augmented screws, and kyphoplasty at T10, T11, T12, and L1. He went on to chemotherapy and bone marrow transplantation and at 6-month follow up reported minimal (1/10) back discomfort. (Left) Preoperative sagittal MRI STIR (short tau inversion recovery) demonstrating the multilevel compression fractures. (Center) Sagittal standing postoperative x-ray. (Right) Anterior-posterior standing postoperative x-ray.
Traditionally, stabilization was achieved via open surgery with low risk of hardware failure requiring surgical revision.29 Over time, with improvement in cancer care and prolonged survivals, long-term analyses show that these rates increase, yet remain acceptable.30 Implementation of MIS percutaneous stabilization in the cancer population, has allowed minimization of approach-related soft tissue injury and systemic stress, leading to preservation of muscle attachments, improved wound healing and shorter recovery times.31 Percutaneous stabilization can be performed using intraoperative fluoroscopic guidance or navigation systems.
Cement Augmentation
High-quality evidence strongly supports the use of balloon-assisted kyphoplasty and/or vertebroplasty in order to treat symptomatic tumor-related compression fractures.32-35 Berenson et al34 conducted a prospective randomized trial and found that patients who underwent balloon kyphoplasty experienced significantly better pain reduction and improvement in disability indexes that persist for up to 6 months compared with patients treated in the noninterventional control arm. Other, lower level evidence also support kyphoplasty for symptomatic osteolytic tumors with goal of pain palliation.32,33 Similarly, for vertebroplasty, pain reduction has been shown in patients treated for spinal metastases.36
However, since cement injection only provide stability in the vertebral body, fractures that extend into the pedicles and joints require instrumented stabilization in order to provide support to the posterior elements in the spine. Therefore, such fractures require a combination of percutaneously placed pedicle instrumentation anchored above and below the fracture, and kyphoplasty at the level of the fracture. Of note, since kyphoplasty may result in retropulsion of fracture fragments and tumor into the spinal canal, thereby exacerbating the epidural tumor extension, kyphoplasty should be avoided in patients with high-grade epidural tumors.
Because of the osteolytic tumors, chemotherapy, radiation therapy, nutritional status, and other comorbidities, the expectation of achieving bony fusion is low. Hence, stabilizing constructs for both open and minimally invasive surgeries rely on heavily on the instrumentation. Bone cement improves the osseous purchase of the pedicle screws and the advent of fenestrated screws has greatly facilitated screw cement augmentation. Fenestrated screws allow injection of polymethylmethacrylate (PMMA) bone cement through fenestrations in the screw shaft, simplifying screw cement augmentation and may be utilized in order to improve osseous purchase in osteoporotic patients with cancer. It is important to inject the cement under fluoroscopic, real-time, guidance to avoid cement leak into the spinal canal, foramen or distal embolus.
Radiofrequency Ablation
Radiofrequency ablation (RFA) can provide rapid relief (including for painful but benign lesions) and can be used synergistically with both cement augmentation and concurrent radiation therapy.37-40 RFA is a percutaneous procedure in which an electrode is percutaneously inserted into the involved vertebral body to deliver high-frequency alternating current into the lesion, resulting in heating, protein denaturation, and subsequent coagulative necrosis.41,42 Technically similar to pedicle cannulation for kyphoplasty or vertebroplasty, insertion of RFA catheters is achieved under fluoroscopic and computed tomography image guidance.38,42 Several studies demonstrated the palliative benefit for local pain control of bone metastases using RFA.43,44 The utility of RFA for purpose of local tumor control requires further study though it has been shown to provide some short-term benefit.39 RFA has traditionally been limited in posterior vertebral body lesions due to the close anatomical proximity to spinal cord and nerve roots45,46 with new bipolar devices attempting to overcome this shortcoming. Long-term outcomes are unclear, but this technology may be a useful addition to the minimally invasive methods available for palliative treatment.47 While combined RFA and vertebral augmentation have theoretical benefits, comparative trials have not been performed to establish superiority of combined therapy.48
Navigation and Robotics
Spinal navigation is gaining popularity in the degenerative, deformity, and trauma populations and has been shown to reduce screw placement time, improve hardware placement accuracy and decrease risk of reoperation.49-51 For minimally invasive surgeries, intraoperative navigation has been particularly impactful for hardware placement.51 Given the increased risk for surgical morbidity and complications, modern surgical treatment of spinal metastases aims to minimize surgical exposure, operative time, and complications. Thus, the role for neuronavigation in spine cancer is currently under exploration. A variety of 2- or 3-dimensional intraoperative navigation systems are currently used is spine tumor surgery. These systems register to an intraoperative scan from either fluoroscopic based systems or, more recently, intraoperative computed tomography devices. To date, the key role of navigation in surgery for spinal metastases is to aid in instrumented stabilization while minimalizing staff and patient exposure to radiation.52 Unlike for primary bone tumors where navigation can be used for planning osteotomies, the role for navigated decompression in metastatic disease is limited since there is a very limited role for cytoreduction or gross total resection, except for institutions in which SBRT is not readily available. Still, navigated drills, probes and curettes in conjunction with intraoperative ultrasound may be useful in facilitating ventral decompression.53,54
Another promising area of innovation is the integration of robotic technologies in spine surgery.55 Though limited data and experience exist with these devices in general, as they become more available in spine surgery, it is likely that they will be used in minimally invasive surgery for spinal metastases as well. Robotic systems such as SpineAssist (MAZOR Robotics Ltd, Cesarea, Israel) and ROSA (Zimmer Biomet, Warsaw, IN) have been used for accurate placement of pedicle screws.56,57 In essence, these robots assist the surgeon in finding and maintaining an accurate screw placement trajectory. In the future, with technological advances such as force and torque sensors, pedicle screw placement may one day be automated.58
Patient-Reported Outcomes (and Other Outcomes Using MAS)
Patient-reported outcomes (PROs) and other HRQoL measures are increasingly recognized as an important method to evaluate treatment outcomes As mentioned, minimally invasive spine surgery has demonstrated benefit in reduction of blood loss, operating times and length of stay, even when dealing with spinal metastases.2-5 While PRO improvement after MAS has been illustrated, comparative data of PRO after open and MAS techniques are lacking.59-61 Prospective62,63 as well as large-scale multicenter retrospective64 data show that surgery, along with radiation and systemic therapy, provides improvement in PRO and other HRQoL measures with acceptable risks and complications. Similarly, prospective data demonstrate MAS for the treatment of spinal metastases results in significant decrease in pain severity and symptom interference with daily activities.14 There are currently no comparative PRO data evaluating open versus MAS for extradural metastases and future studies will determine whether there is a clinically meaningful difference between the approaches.
Conclusions
MAS techniques gained prominence in the treatment of extradural spinal metastases and have a clear role in the treatment of neoplastic spinal instability and metastatic ESCC. The purported advantages include reduced blood loss, shorter length of stay, decreased systemic stress of surgery, lower risk of complications, and most important, rapid return to systemic and radiation therapy. Percutaneous instrumented and cement stabilization has been widely used for the treatment of neoplastic instability. Furthermore, MAS muscle-sparing approaches may be used for decompression of the spinal cord and nerve roots. While PRO data illustrate the benefit of MAS in the treatment of metastatic spine disease, high-quality data comparing MAS and open surgical techniques in the treatment of spinal metastases are lacking. Initial efforts focused on identifying appropriate candidates and current efforts aim to improve surgical techniques and continue to minimize surgical extent with greater precision. Neuronavigation and robotics improve accuracy and are likely to continue to improve MIS surgical outcomes.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Barzilai has nothing to disclose. Dr Bilsky reports speaker’s bureau from Globus, Varian, and BrainLab, outside the submitted work. In addition, Dr Bilsky has a patent Globus CREO with royalties paid, and a patent Depuy PEEK/carbon fiber cage with royalties paid. Dr Laufer reports consulting fees from Globus, DePuy/Synthes, BrainLab, Medtronic, Inc, and SpineWave, outside the submitted work.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This supplement was supported by funding from the Carl Zeiss Meditec Group.
ORCID iD: Ori Barzilai, MD  https://orcid.org/0000-0002-4037-8716
https://orcid.org/0000-0002-4037-8716
References
- 1. Zuckerman SL, Laufer I, Sahgal A, et al. When less is more: the indications for MIS techniques and separation surgery in metastatic spine disease. Spine (Phila Pa 1976). 2016;41(suppl 20):S246–S253. doi:10.1097/BRS.0000000000001824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hansen-Algenstaedt N, Kwan MK, Algenstaedt P, et al. Comparison between minimally invasive surgery and conventional open surgery for patients with spinal metastasis: a prospective propensity score-matched study. Spine (Phila Pa 1976). 2017;42:789–797. doi:10.1097/BRS.0000000000001893 [DOI] [PubMed] [Google Scholar]
- 3. Hikata T, Isogai N, Shiono Y, et al. A retrospective cohort study comparing the safety and efficacy of minimally invasive versus open surgical techniques in the treatment of spinal metastases. Clin Spine Surg. 2017;30:e1082–e1087. doi:10.1097/BSD.0000000000000460 [DOI] [PubMed] [Google Scholar]
- 4. Rao PJ, Thayaparan GK, Fairhall JM, Mobs RJ. Minimally invasive percutaneous fixation techniques for metastatic spinal disease. Orthop Surg. 2014;6:187–195. doi:10.1111/os.12114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kumar N, Malhotra R, Maharajan K, et al. Metastatic spine tumor surgery: a comparative study of minimally invasive approach using percutaneous pedicle screws fixation versus open approach. Clin Spine Surg. 2017;30:E1015–E1021. doi:10.1097/BSD.0000000000000400 [DOI] [PubMed] [Google Scholar]
- 6. Disa JJ, Smith AW, Bilsky MH. Management of radiated reoperative wounds of the cervicothoracic spine: the role of the trapezius turnover flap. Ann Plast Surg. 2001;47:394–397. [DOI] [PubMed] [Google Scholar]
- 7. Yang Z, Yang Y, Zhang Y, et al. Minimal access versus open spinal surgery in treating painful spine metastasis: a systematic review. World J Surg Oncol. 2015;13:68 doi:10.1186/s12957-015-0468-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barzilai O, Fisher CG, Bilsky MH. State of the art treatment of spinal metastatic disease. Neurosurgery. 2018;82:757–769. doi:10.1093/neuros/nyx567 [DOI] [PubMed] [Google Scholar]
- 9. Laufer I, Rubin DG, Lis E, et al. The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist. 2013;18:744–751. doi:10.1634/theoncologist.2012-0293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bilsky MH, Laufer I, Fourney DR, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine. 2010;13:324–328. doi:10.3171/2010.3.SPINE09459 [DOI] [PubMed] [Google Scholar]
- 11. Fourney DR, Frangou EM, Ryken TC, et al. Spinal instability neoplastic score: an analysis of reliability and validity from the spine oncology study group. J Clin Oncol. 2011;29:3072–3077. doi:10.1200/JCO.2010.34.3897 [DOI] [PubMed] [Google Scholar]
- 12. Gerszten PC, Mendel E, Yamada Y. Radiotherapy and radiosurgery for metastatic spine disease: what are the options, indications, and outcomes? Spine (Phila Pa 1976). 2009;34(22 suppl):S78–S92. doi:10.1097/BRS.0b013e3181b8b6f5 [DOI] [PubMed] [Google Scholar]
- 13. Fisher CG, Schouten R, Versteeg AL, et al. Reliability of the Spinal Instability Neoplastic Score (SINS) among radiation oncologists: an assessment of instability secondary to spinal metastases. Radiat Oncol. 2014;9:69 doi:10.1186/1748-717X-9-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barzilai O, McLaughlin L, Amato MK, et al. Minimal access surgery for spinal metastases: prospective evaluation of a treatment algorithm using patient-reported outcomes. World Neurosurg. 2018;120:e889–e901. doi:10.1016/j.wneu.2018.08.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moliterno J, Veselis CA, Hershey MA, Lis E, Laufer I, Bilsky MH. Improvement in pain after lumbar surgery in cancer patients with mechanical radiculopathy. Spine J. 2014;14:2434–2439. doi:10.1016/j.spinee.2014.03.006 [DOI] [PubMed] [Google Scholar]
- 16. Yamada Y, Katsoulakis E, Laufer I, et al. The impact of histology and delivered dose on local control of spinal metastases treated with stereotactic radiosurgery. Neurosurg Focus. 2017;42:E6 doi:10.3171/2016.9.FOCUS16369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976). 2007;32:193–199. doi:10.1097/01.brs.0000251863.76595.a2 [DOI] [PubMed] [Google Scholar]
- 18. Guckenberger M, Mantel F, Gerszten PC, et al. Safety and efficacy of stereotactic body radiotherapy as primary treatment for vertebral metastases: a multi-institutional analysis. Radiat Oncol. 2014;9:226 doi:10.1186/s13014-014-0226-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laufer I, Iorgulescu JB, Chapman T, et al. Local disease control for spinal metastases following “separation surgery” and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: outcome analysis in 186 patients. J Neurosurg Spine. 2013;18:207–214. doi:10.3171/2012.11.SPINE12111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chou D, Lu DC. Mini-open transpedicular corpectomies with expandable cage reconstruction. Technical note. J Neurosurg Spine. 2011;14:71–77. doi:10.3171/2010.10.SPINE091009 [DOI] [PubMed] [Google Scholar]
- 21. Zairi F, Arikat A, Allaoui M, Marinho P, Assaker R. Minimally invasive decompression and stabilization for the management of thoracolumbar spine metastasis. J Neurosurg Spine. 2012;17:19–23. doi:10.3171/2012.4.SPINE111108 [DOI] [PubMed] [Google Scholar]
- 22. Donnelly DJ, Abd-El-Barr MM, Lu Y. Minimally invasive muscle sparing posterior-only approach for lumbar circumferential decompression and stabilization to treat spine metastasis—technical report. World Neurosurg. 2015;84:1484–1490. doi:10.1016/j.wneu.2015.06.018 [DOI] [PubMed] [Google Scholar]
- 23. Tatsui CE, Stafford RJ, Li J, et al. Utilization of laser interstitial thermotherapy guided by real-time thermal MRI as an alternative to separation surgery in the management of spinal metastasis. J Neurosurg Spine. 2015;23:400–411. doi:10.3171/2015.2.SPINE141185 [DOI] [PubMed] [Google Scholar]
- 24. Ghia AJ, Rebueno NC, Li J, Brown PD, Rhines LD, Tatsui CE. The use of image guided laser interstitial thermotherapy to supplement spine stereotactic radiosurgery to manage metastatic epidural spinal cord compression: proof of concept and dosimetric analysis. Pract Radiat Oncol. 2016;6:e35–e38. doi:10.1016/j.prro.2015.09.014 [DOI] [PubMed] [Google Scholar]
- 25. Tatsui CE, Belsuzarri TA, Oro M, et al. Percutaneous surgery for treatment of epidural spinal cord compression and spinal instability: technical note. Neurosurg Focus. 2016;41:E2 doi:10.3171/2016.8.FOCUS16175 [DOI] [PubMed] [Google Scholar]
- 26. Tatsui CE, Nascimento CN, Suki D, et al. Image guidance based on MRI for spinal interstitial laser thermotherapy: technical aspects and accuracy. J Neurosurg Spine. 2017;26:605–612. doi:10.3171/2016.9.SPINE16475 28186470 [Google Scholar]
- 27. Tatsui CE, Lee SH, Amini B, et al. Spinal laser interstitial thermal therapy: a novel alternative to surgery for metastatic epidural spinal cord compression. Neurosurgery. 2016;79(suppl 1):S73–S82. doi:10.1227/NEU.0000000000001444 [DOI] [PubMed] [Google Scholar]
- 28. Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976). 2010;35:E1221–E1229. doi:10.1097/BRS.0b013e3181e16ae2 [DOI] [PubMed] [Google Scholar]
- 29. Amankulor NM, Xu R, Iorgulescu JB, et al. The incidence and patterns of hardware failure after separation surgery in patients with spinal metastatic tumors. Spine J. 2014;14:1850–1859. doi:10.1016/j.spinee.2013.10.028 [DOI] [PubMed] [Google Scholar]
- 30. Barzilai O, McLaughlin L, Lis E, Yamada Y, Bilsky MH, Laufer I. Outcome analysis of surgery for symptomatic spinal metastases in long-term cancer survivors [published online April 26, 2019]. J Neurosurg Spine. doi:10.3171/2019.2.SPINE181306 [DOI] [PubMed] [Google Scholar]
- 31. Kim CH, Chung CK, Sohn S, Lee S, Park SB. Less invasive palliative surgery for spinal metastases. J Surg Oncol. 2013;108:499–503. doi:10.1002/jso.23418 [DOI] [PubMed] [Google Scholar]
- 32. Mendel E, Bourekas E, Gerszten P, Golan JD. Percutaneous techniques in the treatment of spine tumors: what are the diagnostic and therapeutic indications and outcomes? Spine (Phila Pa 1976). 2009;34(22 suppl):S93–S100. doi:10.1097/BRS.0b013e3181b77895 [DOI] [PubMed] [Google Scholar]
- 33. Papanastassiou ID, Filis AK, Gerochristou MA, Vrionis FD. Controversial issues in kyphoplasty and vertebroplasty in malignant vertebral fractures. Cancer Control. 2014;21:151–157. [DOI] [PubMed] [Google Scholar]
- 34. Berenson J, Pflugmacher R, Jarzem P, et al. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol. 2011;12:225–235. doi:10.1016/S1470-2045(11)70008-0 [DOI] [PubMed] [Google Scholar]
- 35. Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98(1 suppl):21–30. [DOI] [PubMed] [Google Scholar]
- 36. Xie P, Zhao Y, Li G. Efficacy of percutaneous vertebroplasty in patients with painful vertebral metastases: a retrospective study in 47 cases. Clin Neurol Neurosurg. 2015;138:157–161. doi:10.1016/j.clineuro.2015.08.026 [DOI] [PubMed] [Google Scholar]
- 37. Greenwood TJ, Wallace A, Friedman MV, Hillen TJ, Robinson CG, Jennings JW. Combined ablation and radiation therapy of spinal metastases: a novel multimodality treatment approach. Pain Physician. 2015;18:573–581. [PubMed] [Google Scholar]
- 38. Yu F, Niu XH, Zhang Q, Zhao HT, Xu LH, Deng ZP. Radiofrequency ablation under 3D intraoperative Iso-C C-arm navigation for the treatment of osteoid osteomas. Br J Radiol. 2015;88:20140535 doi:10.1259/bjr.20140535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wallace AN, Tomasian A, Vaswani D, Vyhmeister R, Chang RO, Jennings JW. Radiographic local control of spinal metastases with percutaneous radiofrequency ablation and vertebral augmentation. AJNR Am J Neuroradiol. 2016;37:759–765. doi:10.3174/ajnr.A4595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morassi LG, Kokkinis K, Evangelopoulos DS, et al. Percutaneous radiofrequency ablation of spinal osteoid osteoma under CT guidance. Br J Radiol. 2014;87:20140003 doi:10.1259/bjr.20140003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kurup AN, Callstrom MR. Image-guided percutaneous ablation of bone and soft tissue tumors. Semin Intervent Radiol. 2010;27:276–284. doi:10.1055/s-0030-1261786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ma Y, Wallace AN, Madaelil TP, Jennings JW. Treatment of osseous metastases using the Spinal Tumor Ablation with Radiofrequency (STAR) system. Expert Rev Med Devices. 2016;13:1137–1145. doi:10.1080/17434440.2016.1256772 [DOI] [PubMed] [Google Scholar]
- 43. Callstrom MR, Charboneau JW, Goetz MP, et al. Painful metastases involving bone: feasibility of percutaneous CT- and US-guided radio-frequency ablation. Radiology. 2002;224:87–97. doi:10.1148/radiol.2241011613 [DOI] [PubMed] [Google Scholar]
- 44. Goetz MP, Callstrom MR, Charboneau JW, et al. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multicenter study. J Clin Oncol. 2004;22:300–306. doi:10.1200/JCO.2004.03.097 [DOI] [PubMed] [Google Scholar]
- 45. Zhang C, Han X, Douglas P, Dai Y, Wang G. Bipolar radiofrequency ablation of spinal tumors: the effect of the posterior vertebral cortex defect on temperature distribution in the spinal canal. AJNR Am J Neuroradiol. 2018;39:E1–E2. doi:10.3174/ajnr.A5393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dupuy DE, Hong R, Oliver B, Goldberg SN. Radiofrequency ablation of spinal tumors: temperature distribution in the spinal canal. AJR Am J Roentgenol. 2000;175:1263–1266. doi:10.2214/ajr.175.5.1751263 [DOI] [PubMed] [Google Scholar]
- 47. Choi D, Bilsky M, Fehlings M, Fisher C, Gokaslan Z. Spine oncology-metastatic spine tumors. Neurosurgery. 2017;80(3 suppl):S131–S137. doi:10.1093/neuros/nyw084 [DOI] [PubMed] [Google Scholar]
- 48. Kam NM, Maingard J, Kok HK, et al. Combined vertebral augmentation and radiofrequency ablation in the management of spinal metastases: an update. Curr Treat Options Oncol. 2017;18:74 doi:10.1007/s11864-017-0516-7 [DOI] [PubMed] [Google Scholar]
- 49. Kotani T, Akazawa T, Sakuma T, et al. Accuracy of pedicle screw placement in scoliosis surgery: a comparison between conventional computed tomography-based and O-arm-based navigation techniques. Asian Spine J. 2014;8:331–338. doi:10.4184/asj.2014.8.3.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rajasekaran S, Vidyadhara S, Ramesh P, Shetty AP. Randomized clinical study to compare the accuracy of navigated and non-navigated thoracic pedicle screws in deformity correction surgeries. Spine (Phila Pa 1976). 2007;32:E56–E64. doi:10.1097/01.brs.0000252094.64857.ab [DOI] [PubMed] [Google Scholar]
- 51. Helm PA, Teichman R, Hartmann SL, Simon D. Spinal navigation and imaging: history, trends, and future. IEEE Trans Med Imaging. 2015;34:1738–1746. doi:10.1109/TMI.2015.2391200 [DOI] [PubMed] [Google Scholar]
- 52. Jeys L, Matharu GS, Nandra RS, Grimer RJ. Can computer navigation-assisted surgery reduce the risk of an intralesional margin and reduce the rate of local recurrence in patients with a tumour of the pelvis or sacrum? Bone Joint J. 2013;95-B:1417–1424. doi:10.1302/0301-620X.95B10.31734 [DOI] [PubMed] [Google Scholar]
- 53. Barzilai O, Laufer I, Robin A, Xu R, Yamada Y, Bilsky MH. Hybrid therapy for metastatic epidural spinal cord compression: technique for separation surgery and spine radiosurgery. Oper Neurosurg (Hagerstown). 2019;16:310–318. doi:10.1093/ons/opy137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nasser R, Drazin D, Nakhla J, et al. Resection of spinal column tumors utilizing image-guided navigation: a multicenter analysis. Neurosurg Focus. 2016;41:E15. [DOI] [PubMed] [Google Scholar]
- 55. Overley SC, Cho SK, Mehta AI, Arnold PM. Navigation and robotics in spinal surgery: where are we now? Neurosurgery. 2017;80(3 suppl):S86–S99. [DOI] [PubMed] [Google Scholar]
- 56. Shoham M, Lieberman IH, Benzel EC, et al. Robotic assisted spinal surgery—from concept to clinical practice. Computer Aided Surg. 2007;12:105–115. [DOI] [PubMed] [Google Scholar]
- 57. Lonjon N, Chan-Seng E, Costalat V, Bonnafoux B, Vassal M, Boetto J. Robot-assisted spine surgery: feasibility study through a prospective case-matched analysis. Eur Spine J. 2016;25:947–955. [DOI] [PubMed] [Google Scholar]
- 58. Tian W, Han X, Liu B, et al. A robot-assisted surgical system using a force-image control method for pedicle screw insertion. PLoS One. 2014;9:e86346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Goldstein CL, Macwan K, Sundararajan K, Rampersaud YR. Perioperative outcomes and adverse events of minimally invasive versus open posterior lumbar fusion: meta-analysis and systematic review. J Neurosurg Spine. 2016;24:416–427. doi:10.3171/2015.2.SPINE14973 [DOI] [PubMed] [Google Scholar]
- 60. Mummaneni PV, Bisson EF, Kerezoudis P, et al. Minimally invasive versus open fusion for Grade I degenerative lumbar spondylolisthesis: analysis of the Quality Outcomes Database. Neurosurg Focus. 2017;43:E11 doi:10.3171/2017.5.FOCUS17188 [DOI] [PubMed] [Google Scholar]
- 61. McGirt MJ, Parker SL, Mummaneni P, et al. Is the use of minimally invasive fusion technologies associated with improved outcomes after elective interbody lumbar fusion? Analysis of a nationwide prospective patient-reported outcomes registry. Spine J. 2017;17:922–932. doi:10.1016/j.spinee.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 62. Fehlings MG, Nater A, Tetreault L, et al. Survival and clinical outcomes in surgically treated patients with metastatic epidural spinal cord compression: results of the prospective multicenter AOSpine study. J Clin Oncol. 2016;34:268–276. doi:10.1200/JCO.2015.61.9338 [DOI] [PubMed] [Google Scholar]
- 63. Barzilai O, Amato MK, McLaughlin L, et al. Hybrid surgery-radiosurgery therapy for metastatic epidural spinal cord compression: a prospective evaluation using patient-reported outcomes. Neurooncol Pract. 2018;5:104–113. doi:10.1093/nop/npx017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Choi D, Fox Z, Albert T, et al. Rapid improvements in pain and quality of life are sustained after surgery for spinal metastases in a large prospective cohort. Br J Neurosurg. 2016;30:337–344. doi:10.3109/02688697.2015.1133802 [DOI] [PubMed] [Google Scholar]