Abstract
In addition to the roles of endothelial cells (ECs) in physiological processes, ECs actively participate in both innate and adaptive immune responses. We previously reported that, in comparison to macrophages, a prototypic innate immune cell type, ECs have many innate immune functions that macrophages carry out, including cytokine secretion, phagocytic function, antigen presentation, pathogen associated molecular patterns (PAMPs)-, and danger associated molecular patterns (DAMPs)-sensing, pro-inflammatory, immune-enhancing, anti-inflammatory, immunosuppression, migration, heterogeneity, and plasticity. In this highlight, we introduce recent advances published in both ATVB and many other journals: 1) Several significant characters classify ECs as novel immune cells not only in infections and allograft transplantation but also in metabolic diseases; 2) Several new receptor systems including conditional DAMP receptors, non-pattern receptors, and homeostasis associated molecular patterns (HAMPs) receptors contribute to innate immune functions of ECs; 3) Immunometabolism and innate immune memory determine the innate immune functions of ECs; 4) a great induction of the immune checkpoint receptors (ICRs) in ECs during inflammations suggests the immune tolerogenic functions of ECs; and 5) association of immune checkpoint inhibitors with cardiovascular adverse events (CVAEs) and cardio-oncology indicates the potential contributions of ECs as innate immune cells.
Keywords: innate immunity, endothelial cells, innate immune cells, vascular inflammation, cytokines
1. Introduction
Under physiological conditions1, 2, ECs are involved in the modulations of metabolic homeostasis (trophic functions), vascular hemodynamics (tonic functions)3, vascular permeability, coagulation, and cell extravasation (trafficking)2. In a quiescent state, ECs balance the release of various vasodilating or vasoconstricting factors such as nitric oxide, prostacyclins, and endothelin to maintain vascular tone, blood pressure, and blood flow4. In addition, ECs secrete numerous cytokines and growth factors including interleukin-6 (IL-6)5–7, thrombospondin, frizzled-related protein 3, insulin-like growth factor-1 (IGF-1), connective tissue growth factor (CTGF)8, bone morphogenetic protein (BMP)-99, interleukin (IL)-1α10, 11, IL-1β7, 12, placental growth factor, leukemia inhibitory factor (LIF), Wnt family member 1 (WNT1)-inducible signaling pathway protein 1 (WISP-1), midkine, and adrenomedullin to facilitate cardiac performance and remodeling13. Furthermore, the endothelium is crucial in regulating coagulation, utilizing both anti-coagulation and pro-coagulation mechanisms14–16. ECs have an essential role in modulating vascular permeability17. During states of acute and chronic inflammation18, hyperglycemia9, ECs display an excessive or prolonged increase in permeability, allowing for additional trafficking of immune cells and consequently deleterious effects resulting in tissue edema19. Of note, low dose mitochondrial reactive oxygen species (mtROS) generation, uncoupled from ATP production and promoted by proton leak20, 21, drove upregulation of endothelial cell (EC) adhesion molecule, intercellular adhesion molecule-1 (ICAM-1)20–23. This physiological ECs activation status may facilitate non-classical patrolling monocyte migration for immune-surveillance function in tissues24. The inability of ECs to adequately carry out these functions, which is termed as endothelial dysfunction, causes an elevating risk of cardiovascular events11, 25–27.
Under hypoxic conditions, thrombus-derived monocytes collected from patients with acute coronary artery disease could be transdifferentiated into ECs28. ECs can also be transdifferentiated from fibroblasts via innate immune signaling of a glycolytic switch29. In atherogenic processes, the endothelium is a source for plaque-associated mesenchymal cells through endothelial-to-mesenchymal transition (EndoMT)30. A recent study also demonstrated the presence of EndoMT in human adipose tissue in obesity; and EndoMT reduced mitochondrial oxidative phosphorylation and glycolytic capacity of EC31. In addition, cardiovascular disorders, including atherosclerosis, are considered as premature aging32. The underlying mechanisms of a concept termed inflammaging33 include genetic susceptibility, central obesity, increased gut permeability, changes to microbiota composition, cellular senescence, nucleotide-binding oligomerization domain-like (NOD)-, leucine-rich repeat (LRR)- and pyrin domain-containing protein 3 (NLRP3) inflammasome activation, and oxidative stress. Chronic senescent cells lead to their deleterious effects through a secretory phenotype34 known as the senescence-associated secretory phenotype (SASP)35, 36. Proteomic analysis of endothelial particulate secretome represented by extracellular vesicles (EV) in the proinflammatory conditions exhibite the presence of pro-inflammatory and immune proteins involved in signal transduction, immune and inflammatory responses, and angiogenesis31.
ECs also have important immunological functions. The innate immune system37 including ECs mediates non-specific immunity, which is immediate and antigen-independent. Innate immune interactions between the cardiovascular system and the immune system are a well-accepted mechanism underlying metabolic cardiovascular diseases, which has been emphasized by the success of CANTOS trial (Canakinumab Anti-Inflammatory Thrombosis Outcome Study), a therapeutic monoclonal antibody targeting IL-1β38. Therefore, vascular ECs are innate immune cells1 in many physiological and pathophysiological conditions, including infection, transplantation conditions39–41 metabolic disorders such as hyperlipidemia42, 43, hyperglycemia44, 45, hyperhomocysteinemia46–48, metabolic syndrome, obesity49, 50, or hypertension, and cigarette smoke51, 52. This review will highlight the recent publications to support that endothelial cells are multifunctional innate immune cells.
2. ECs are novel immune cells.
Historically, cardiovascular immunology has focused on the interactions between the cardiovascular and immune systems, which determine how immune cells promote53, 54 and suppress55–58 cardiovascular diseases by modulating pathophysiological responses of cardiovascular cells. Additionally, immunological features of cardiovascular cells have been gradually recognized. Cell interactions have a few formats: i) resident cell-resident cell, ii) migrated cell-migrated cell, iii) migrated cell-resident cell, and iv) migrated cell-structural cell via direct and indirect (secretions and extracellular vesicles) manners59. When investigators had to use microscopy to examine the morphology of migrated cell types in the inflammation sites and immunohistology, classical innate immune cells were identified including neutrophils, monocytes/macrophages, T cells, and mast cells60, which emphasized the roles of cell migration in the cellular interactions during inflammation and immune processes. However, immune regulatory functions are not unique to migrated cells. The roles of structural cells and cardiovascular resident cells such as ECs in cellular interaction and immune regulation, when trans-endothelial migration of immune and inflammatory cells, have been under-appreciated for a long time. Besides the historical reasons, potential assumption that endothelial cells have no immune regulatory effects on migrated immune cells, inflammatory cells, vascular smooth muscle cells and other vascular cells may not be correct3. Now there are strong evidences61 that ECs and other structural cells such as lymphatic ECs62, 63, epithelial cells64–67, stromal cells66, 68–70, Sca1+ progenitor cells71, vascular smooth muscle cells (VSMC)72–78, Kupffer cells in the liver, adipocytes, and others79 play significant roles in regulating innate and adaptive immune functions1, 40, 65, 80–82. Of note, even adaptive immune cells such as CD8+ T cells83, γδT cells84, innate lymphoid cells85, innate B cells86, tissue-resident memory T cells87, type 1 T helper cell (Th1)-like CD4+Foxp3+ regulatory T cells (Treg), Th2-like Treg, Th17-like Treg, and Tfh-like Treg88, antigen-presenting cell (APC)-like Treg, have innate immune functions89.
As we reviewed in 2013, eleven innate immune functions that macrophages carry out can also be performed by ECs, including cytokine secretion, phagocytic function, antigen presentation, PAMPs and DAMPs sensing, proinflammatory, immune-enhancing, anti-inflammatory, immunosuppression, migration, heterogeneity, and plasticity1. A few principles in determining innate immune cell identity are summarized in Figure 1: First, the cells are capable of sensing the stimulations and danger signals by various PAMPs, DAMPs, proinflammatory and anti-inflammatory cytokines, growth factors, exosomes and extracellular vesicles90; Second, in responding to stimuli, the cells are capable of secreting cytokines, chemokines, growth factors, other secretory proteins, microparticles91, exosomes90, circular RNAs92, microRNAs49, 93–95, and other noncoding RNAs49, 92, 96–98, upregulating co-signaling receptors (co-stimulation and immune checkpoint receptors) and major histocompatibility complex II (MHC II) to directly or indirectly interact with adaptive immune cells81, 99; Third, the cells are capable of presenting antigens via MHC II to CD4+ T helper cells80, 100, 101; Fourth, the cells are capable of memorizing the challenges (trained immunity)102, 103 they encountered and enhancing response when encounter challenges again104; and Fifth, the cells are capable of maintaining cellular homeostasis (trained immune tolerance)105 from PAMPs, and DAMPs stimulations106, which is similar to the CRISPR/Cas (clustered regularly interspaced short palindromic repeats/CRISPR-associated protein) immune function in bacteria and archaea from foreign DNA attacks107. Of note, the class defining criteria of being able to present antigens via MHC II is not very restricted. It was recently reported using new single-cell RNA sequencing technique to separate antigenpresenting MHC II-high dendritic cell (DC) population from inflammatory function-high DC population108, suggesting that not all the DC have high antigen presenting capacity. Other recent reports demonstrated that some professional innate immune cells including B cells, macrophages, natural killer cells (NK), monocytes, plasmacytoid dendritic cells (pDC), DC1 and DC2 have 1,300 innate immune gene expression differences, suggesting huge heterogeneities109, 110. Therefore, it may not be optimal to use antigen-presenting capacity111, 112 as the essential criterion for judging the innate immune function of ECs.
Figure.1.
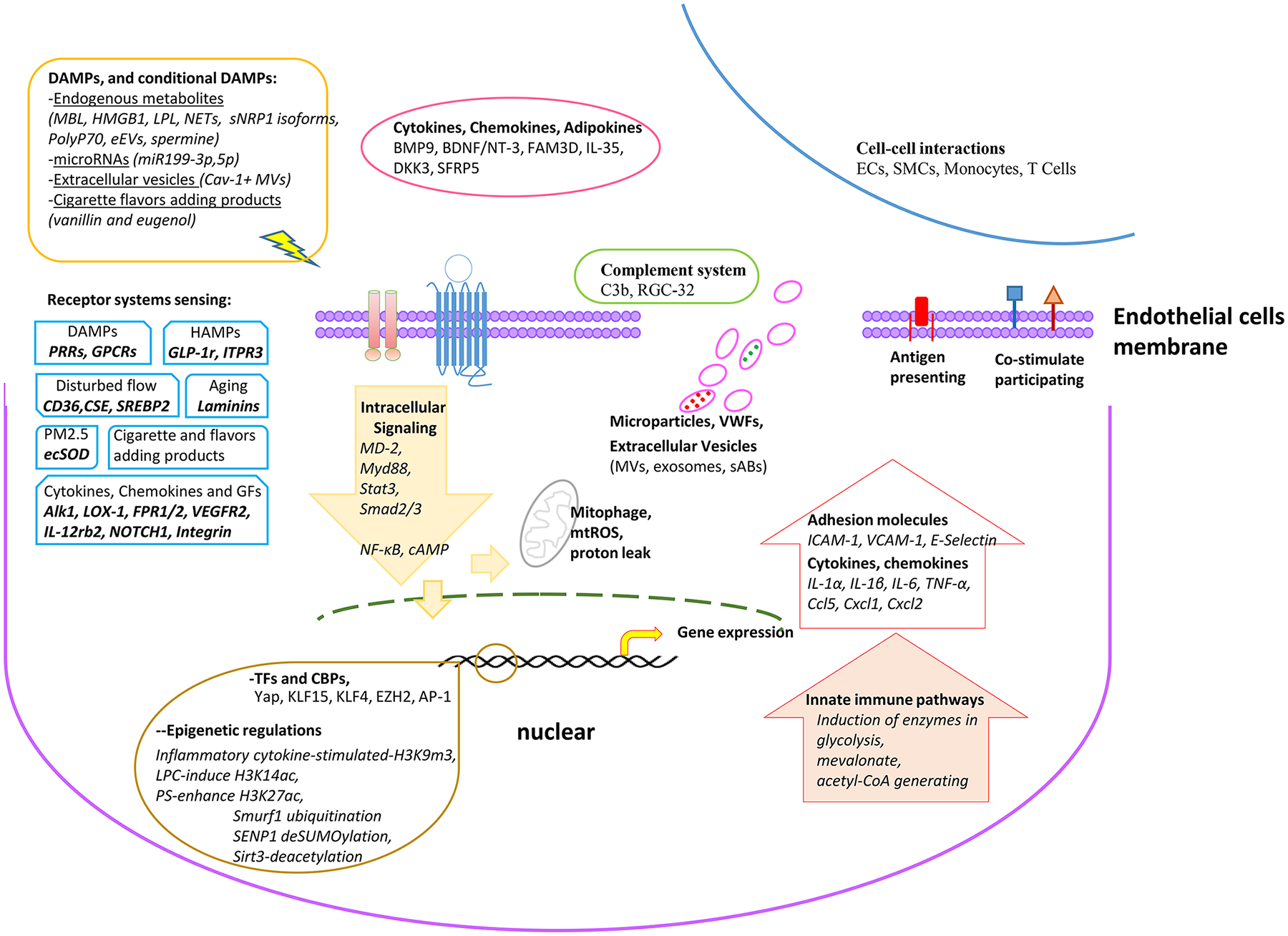
Schematic Model of Endothelial Cells Are Innate Immune Cells
ECs have classical DAMPs sensing systems. Traditional innate immune cells that patrol the blood, such as DCs, and Ly6Clow monocytes24, 113, is equipped with a series of PAMPs receptors including Tolllike receptors (TLRs)114 and NOD-like receptors (NLRs)52, 115. TLRs, NLRs, retinoic acid-inducible gene 1 (RIG-I)-like receptors (RLRs), absent in melanoma 2 (AIM2)-like receptors (ALRs) and C-type lectin receptors (CLRs) are pattern recognition receptors (PRRs). These receptors are part of the innate immune system and are known to be expressed on immune cells as well as non-immune cells116 including a few vascular cell types such as aortic ECs43, angiogenic ECs42, Sca-1+ vascular progenitor cells71, and VSMCs72, 73. PRRs can sense components of exogenous microbes as well as harmful endogenous components. These findings suggest a novel concept of conditional danger receptors that endogenous metabolites, when elevated to pathological concentrations, can trigger inflammation by binding to their intrinsic receptors rather than DAMPs/PAMPs such as TLRs or NLRs. This type of intrinsic receptors for elevated endogenous metabolites are conditional danger receptors since they carry out physiological signaling function when metabolites are in physiological concentrations117, 118.
The cellular “receptors, trouble-detectors and metabolic sensors”79, which can recognize the risk factors for atherogenesis119 such as hyperlipidemia120 and hyperhomocysteinemia121, contribute significantly to the innate immune functions of ECs. The roles of PRRs have been characterized recently as bridging innate immune sensory systems for exogenous infectious agents and endogenous metabolic DAMPs to initiation of inflammation51, 122, 123. In addition to TLRs and NLRs, four additional DAMP receptor categories have been characterized124, 125: first, transmembrane C-type lectin receptors (dendritic cell natural killer lectin group receptor 1 (DNGR1, receptor for F-actin), macrophage-inducible C-type lectin (MINCLE, receptor for spliceosome-associated protein 130 (SAP130), β-glycosylceramide), Dectin-1 (receptor for N-glycans) and Dectin-2116; second, retinoid acid inducible gene I (RIG-I, melanoma differentiation-associated protein 5 (MDA5) and RIG-like receptor dsRNA helicase (LGP2)116), third, cytosolic DNA sensors such as AIM2 (absent in melanoma 2), cyclic GMP–AMP synthase (cGAS) and stimulator of interferon genes (STING)76, and fourth, receptor for advanced glycation end products (RAGE, a receptor for AGEs, high mobility group box 1 (HMGB1)126, S100s, β-amyloid (Aβ) and DNA)127. Herein, we refer to the six categories above-mentioned as classical DAMP receptors. Early hyperlipidemia activates NLR/inflammasome-caspase-1 pathway in endothelial cells, which is responsible for increased atherogenesis43, decreased angiogenesis42, and weakened progenitor cell vessel repair128, 129. Oxidized LDL (oxLDL)-activated human aortic endothelial cells –(HAECs)- undergo EC inflammation and EC inflammatory cell death stage (pyroptosis) as judged by caspase-1 activation43. In addition, ECs secrete the pro-inflammatory cytokine interleukin-8 (IL-8) in a NOD1-dependent response to microbial stimulation130, 131, and NOD2-dependent response to bacterial peptidoglycan muramyl dipeptide132,89, 133. IL-17 activates HAECs by inducing IL-6, granulocyte-macrophage colony stimulating factor (GM-CSF), chemokine (C-X-C) ligand 1 (CXCL1) and CXCL2134, which may suggest an interaction mode between T cells and ECs. Besides, a very recent study revealed that in pulmonary vascular cells, EC-derived HMGB1 activate RAGE in ECs and SMCs via HIMF (Hypoxia-Induced Mitogenic Factor) to form a positive feedback loop, accelerating the secretion and release of more HMGB1 and fueling the vascular-immune milieu of PH development126.
Inflamed endothelium and endothelium of atherosclerotic lesions have significant upregulation of TLR2 and TLR4 expression135, and induce ECs responses such as the production of IL-1, IL-8, and monocyte chemotactic protein-1 (MCP-1) via TLR4136–139. Similarly, LPS, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) can induce TLR2 expression140. ECs also express CD14 which is also a known receptor for LPS141. TLR3, TLR7, and TLR8 are important in detecting viral RNA and activating innate immune responses against viruses. Human umbilical vein endothelial cells (HUVECs) do express TLR3. In fact, ECs also express IFN-α, which is an important cytokine in regulating innate immune responses against viruses and is shown to strongly induce TLR3 expression142. Moreover, ECs also express TLR9 which recognizes viral and bacterial DNA135, 143. Aside from the expression of PRRs, ECs also express important downstream adaptor molecules for PRR signaling including myeloid differentiation-2 (MD2) and myeloid differentiation primary response protein 88 (MyD88)144, 145. ECs express the lectin-like oxLDL receptor (LOX-1) at low levels. Strong evidence has suggested a pathological role of LOX-1 in atherosclerosis, a chronic autoimmune inflammatory disease in response to stimulation by oxLDL170, 171,146, proinflammatory cytokines, and proatherogenic factors such as angiotensin II147. LOX-1 is also important in endothelial-mediated vascular homeostasis and coagulation prevention under physiological conditions148.
3. Receptors or signaling pathways contribute to innate immune functions of endothelial cells.
Following principles can be “extracted” underlying the current classification of DAMPs149 and their receptors as: (1) endogenous metabolites released under the stresses could serve as the DAMPs; (2) DAMPs bind to specific receptors; (3) DAMPs receptor initiates proinflammatory transcription factor-mediated signaling150; and (4) DAMPs receptor genes are evolutionally conserved from mice to humans151. However, the current classification and paradigm of DAMPs receptors have several problems. First, whether endogenous lysophospholipids (LPL) receptors that fit all the above-discussed principles can be classified as novel DAMPs receptors in initiating inflammation-modulating signaling; and second, the current DAMP receptor model emphasizes only the danger signals generated from endogenous metabolic processes but fails in recognizing the roles of potential endogenous metabolites in anti-inflammatory responses, inflammation resolution and maintenance of homeostasis. Therefore, LPLs can use their intrinsic receptors but not classical DAMP receptors to initiate innate immune signaling, which we termed conditional DAMP receptors118, 151. Similarly, various types of non-PRR (non-pattern recognition) transmembrane proteins including TREMs (triggering receptors expressed on myeloid cells 1 (TREM1) and TREM2), G-proteincoupled receptors (N-formyl peptide receptor (FPR)1, FPR2, P2Y2 purinoceptor receptor (P2Y2R)52, P2Y6R, P2Y12R, calcium-sensing receptor (CaSR), G-protein-coupled receptor family C group 6 member A (GPRC6A)) and ion channels (transient receptor potential cation channel subfamily member 2 (TRPM2), other transient receptor potentials (TRPs), P2X7R) have been reported to sense DAMPs125. Recently, significant progress has been reported in identifying additional membrane receptor systems in regulating EC activation in innate and adaptive immune responses as summarized in Table 1.
Table1.
Recent reports identified new evidence to support the model that endothelial cells have innate immune functions in metabolic diseases. (Part I)
| Innate immune relevance | Associated metabolic situlations | Target and experimental model | Responses on ECs | Interaction between ECs and other cells | Reference |
|---|---|---|---|---|---|
| Complement system | Early ischemia Late ischemia |
MBL (mannose-binding lectin)-deposition (MBL−/− mice) | Induces the binding of IL-1α with IL-1R1 Increases C3b and induces ICAM-1 |
10 | |
| Atherogenesis | RGC-32 (response gene to complement 32) in resident in vascular cells, but not macrophage (RGC-32−/− mice) | induces ICAM-1, VCAM-1 through directly interacting with NF-KB | RGC-32 deficiency decreases TNF-α-induced monocyte-endothelial cell interaction | 196 | |
| Reguatlion Of cytokines, chemokines and adipokines | Diabetic macular edema | BMP9/Alk1 (activin-like kinase receptor type I) (HUVECs and streptozotocin-induced mice) | Prevents VEGF-induced phosphorylation of VE-cadherin, induces occludin and strengthens vascular barrier functions | 9 | |
| Angiogenesis | BDNF (brain-derived neurotrophic factor) /NT-3 (neurotrophin-3) (ESCs, embryonic stem cells) | Promotes ESCs differentiation to ECs in a BDNF/NT3 receptors dependent way | 197 | ||
| Abdominal aortic aneurysm (AAA) | FAM3D (Mouse Models of AAA, and FAM3D−/− mice) | FAM3D is upregulated in ECs by vascular pathogenic stimuli | Contributes to neutrophil recruitment via FPR-Gi Protein/β-Arrestin-Mac-1 Signaling | 198 | |
| Atherogenesis | IL-35 (HAECs and APOE−/− mice) | Inhibits mtROS-H3K14 acetylation-activator protein 1-mediated EC activation | 199 | ||
| Vascualr inflammation | NOTCH1 signaling (HUVECs and RbpjiΔEC, NICD (Notch intracellular domain)iEC-OE mice) | Modulates the transcriptional response to inflammatory cytokines; Supports the expression of a subset of inflammatory genes at the enhancer level | Increases leukocyte recruitment to the inflamed lesion | 200 | |
| Endothelial regeneration | Cytokine-like protein dikkopf-3 (DKK3) (Human embryonic lung fibroblasts) | Drives human fibroblasts to differentiate to functional ECs via mesenchymal-to-epithelial transition and VEGF-microRNA-Stat3 pathways | 201 | ||
| Atherosclerosis and arterial stiffness | SFRP5 (secreted frizzled-related protein 5) (HUVECs, HAECs and patients with type 2 diabetes) | Restors Wnt5 (wingless-type family member 5a)-reduced NO production via eNOS | 202 | ||
| Part II | |||||
| Innate immune relevance | Associated metabolic situlations | Target and experimental model | Responses on ECs | Interaction between ECs and other cells | Reference |
| Receptor systems sensing (DMAPs) | Erosion-associated thrombosis | Neutrophil extracellular traps (HSVECs (human saphenous vein ECs) or HUVECs) | Augments ICAM-1, VCAM-1 and transcription factors through concerted action of IL-1α and cathepsin G, but not IL-1β | 11 | |
| Angiogenesis | Posttranslational proteolytic cleavage of VEGF receptors (HUVECs) | Upregulates neuropilin-1 (NRP1) species in an ADAM(a disintegrin and metalloproteinase)9/10-dependent manner resulting in inhibition of VEGF-induced EC motility and angiogenesis | 203 | ||
| Angiogenesis | MicroRNA-199a-3p/5p (bovine aortic endothelial cells) | Redundantly decreases eNOS activity and induces its degradation, thereby supporting VEGF-induced endothelial tubulogenesis | 97 | ||
| Atherogenesis | Dislipidemia and disturbed flow (HAECs and CD36−/− mice) | Increases oxLDL uptake and enhences endothelial stiffening via CD36 | 204 | ||
| Thrombophilia | Hypoxic trophoblasts derived HMGB1 (HUVECs and Pregnant mice) | Stimulates the generation and release of EC-oringin microparticles and enhances blood coagulation | Triggers neutrophil activation | 205 | |
| Thrombotic and inflammatory disorders | PolyP70 (Inorganic polyphosphate 70) (HUVECs) | Amplified HMGB1-mediated VWF release via binding to RAGE and P2Y1 receptors | Promotes VWF-platelet string formation on ECs | 15 | |
| Flow-dependent vascular remodeling | Lack of cystathionine γ-lyase (CSE−/− mice) | limits disturbed flow-induced ICAM-1, VCAM-1 through altering NO availability | Decreases monocyte infiltration | 206 | |
| Pulmonary Hypertension (PH) | HIMF Signaling (human pulmonary microvascular ECs and pulmonary arteries of patients with idiopathic PH and PH Models in mice and rats) | Triggers the HMGB1 pathway and RAGE | EC-derived HMGB1 induces an autophagic response, BMPR2 defects, and subsequent apoptosis-resistant proliferation in smooth muscle cells | 126 | |
| Pulmonary Hypertension | EC-specific caveolin-1 (Cav-1) depletion (EC-Cav1−/− mice) | exhibits a non-EC phenotype and contributes to vascular remodeling via TGF-β/pSmad2/3 signaling | With Increasing Cav-1+ extracellular vesicle shedding into the circulation and decreasing circulating monocytes | 207 | |
| Pulmonary Hypertension | Cigarette Smoke Exposure (PAECs and rats model) | Increases endothelial extracellular vesicles (eEV) generation and the spermine content in eEV | Triggers eEV migration into SMCs, and contributes to pulmonary artery smooth muscle constriction and proliferation via CaSR | 208 | |
| Vascular aging and acute myocardial infarction (AMI) | Switch in Lamb2 to Lamb1 (HUVECs and Mouse models of AMI) | Impaires the functional properties and phenotype of endothelial cell via integrin receptors | 209 | ||
| ECs Dysfunction | Nine flavors added to tobacco products (HEACs) | Iimpairs eNOS agonist-stimulated NO production and triggers inflammation, even cell death, such as vanillin and eugenol. | 5 | ||
| ECs Dysfunction | Exposure to fine particulate matter (PM2.5) (Endothelial progenitor cells, EPCs) | Impairs EPC abundance and function and prevents EPC-mediated vascular recovery after hindlimb ischemia via vascular VEGF resistance and a decrement in NO bioavailability | 210 | ||
| Hypertension | GLP-1 (glucagon-like peptide-1) analogs (Global, EC-and myelomonocytic-specific Glp1r −/− mice) | Reduces blood pressure and protects endothelial function through endothelial but not myeloid cell GLP-1 receptor | Prevents Ly6G−Ly6C+ and Ly6G+ Ly6C+ cell infiltration to the vessel wall | 211 | |
| Part III | |||||
| Innate immune relevance | Associated metabolic situlations | Target and experimental model | Responses on ECs | Interaction between ECs and other cells | Reference |
| Receptor systems sensing (HAMPs) | Organ specificity | Cardiac ECs (transcriptome) | Highly expresses key regulators in fatty acid uptake, such as Meox2/Tcf15, Fabp4, and Cd36 | 212 | |
| Pathological angiogenesis | EC-specific Atg5 deletion (HUVECs, HRMECs (human retinal microvascular ECs), MLECs (murine lung ECs) and Mice with EC-specific inactivation of Atg5) | Impairs mitochondrial function, diminishes production of mtROS, decreased oxidative inactivation of PTPs (phospho-tyrosine phosphatases)and hence, decreasing phosphorylation of the VEGFR2 | 167 | ||
| Atherogenesis | Atheroprotective pulsatile shear stress (HUVECs) | Activates ITPR3 transcription via KLF4-regulated H3K27ac (acetylation of histone 3 lysine 27) enrichment and chromatin accessibility, contributes to the Ca2+ - dependent eNOS activation and EC homeostasis. | 168 | ||
| Atherogenesis and CAD (coronary artery disease) | JCAD, CAD-associated variants at 10p11.23, knockdown (HUVECs) | Decreases ICAM-1, VCAM-1 and Selectin E by negatively regulates YAP activity and Hippo signaling | Reduces monocyte adhesion | 26 | |
| Abdominal aortic aneurysm | Cilostazol, a selective inhibitor of phosphodiesterase III (PDEIII) (MAECs and APOE−/− mice) | Reduces MCP-1 and ICAM-1 via increasing intracellular cAMP | Reduces medial disruption and macrophage infiltration in angiotensin II-Induced AAA, but no effect on atherosclerosis | 213 | |
| Pulmonary hypertension | Hypoxia (HPMEC) | Induces SENP1 (sentrin-specific protease 1)and deprivates KLF15 by SUMOylation, lossing repression on arginase 2 promoter and impairing NO production | 169 | ||
| Inflammation/Stress | A shift from AIP1A to AIP1B isoform (HUVECs) | Localizes to the mitochondria and augments TNFα-induced mtROS generation and EC activation | 170 | ||
| Hypertension | Knockdown of SIRT3 (sirtuin 3) (EPCs) | Contributes to the decline in reendothelialization capacity | Results in mitochondrial oxidative damage, hyperacetylation of SOD2 (superoxide dismutase 2) in EPCs | 171 |
The significance of solving these problems is that a new paradigm will encourage investigators152 to search for anti-inflammatory and homeostatic signals derived from endogenous metabolites. Recent progress in immunology has clearly demonstrated the well-published “two arms model.” This model states that there are several immunotolerance and anti-inflammatory mechanisms, including T cell coinhibition/immune checkpoint pathways153, T cell anergy154, Treg155, and anti-inflammatory/immunosuppressive cytokines. Anti-inflammatory/immunosuppressive cytokines include transforming growth factor-β (TGF-β), IL-10, IL-35, and IL-37 as we and others reported156–159, and specialized pro-resolving mediators (SPMs) such as lipoxins, E-series and D-series resolvins, protectins, and maresins160, etc. Following the same logic of “two arms”, lysophosphatidylserine161, lysophosphatidylenthaolamine162 and IL-35163 were identified as the signals that are generated from endogenous metabolic processes and have anti-inflammatory and homeostatic functions via pattern-dependent manners78, 164. Therefore, HAMPs receptors are the receptors for binding to signals that are generated from endogenous metabolic processes and can initiate anti-inflammatory/homeostatic signaling and promote inflammation resolution151, 157, 165, 166. Taking advantages of cell type-specific gene knockdown techniques and selective inhibitors, recent studies updated several molecules and receptors, as well as their mechanism in maintaining ECs homeostasis, such as Atg5 (autophagy protein 5)167, ITPR3 (inositol 1,4,5-trisphosphate receptor 3)168, GATA (GATA zinc finger transcription factor family)-68, KLF (Kruppel-like factor)15169, AIP1A (ASK1 [apoptosis signal-regulating kinase 1]-interacting protein-1 form A)170 and sirtuin 3 (SIRT3)171. On the contrary, endothelial-specific deletion of the mineralocorticoid receptor protects against vascular inflammation in atherosclerosis in a sex-specific manner172. In addition, a novel model of endothelial dysfunction, that uses isogenic human induced pluripotent stem cell-derived cells harboring different alleles of the APOE gene and identifies ApoE4 expression by endothelial cells, results in cellular dysfunction. This new model exhibits a proinflammatory state and prothrombotic state, evidenced by enhanced secretion of Aβ (amyloid-β) 40 and 42, increased release of cytokines, and overexpression of the platelet-binding protein VWF (von Willebrand factor)173.
4. Innate immune functions of endothelial cells are determined by immunometabolism and innate immune memory.
Highly ordered interactions between immune and metabolic responses are evolutionarily conserved and paramount for tissue and organismal health. Tissue immunometabolism emphasizes that tissue accessory cells such as immune cells, stromal cells and ECs communicate with their clients, tissue parenchymal cells, to optimize the metabolic process for environmental adaptation174. Disruption of these interactions underlies the emergence of many pathologies, particularly chronic non-communicable diseases such as obesity and diabetes175. The proinflammatory and anti-inflammatory functions of these immune cells are determined by the metabolic stage of the immune cells. The metabolic process of immune cells is called immunometabolism and its shift determined by inflammatory stimuli is called immunometabolic reprogramming176. The recent report that 20 novel disease group-specific and 12 new shared macrophage pathways in eight groups of 34 diseases including 24 inflammatory organ diseases and 10 types of tumors177, suggests that disease-related immune microenvironments shape immunometabolism and signaling pathways of immune cells.
In atherogenesis, cholesterol crystals and apolipoprotein B-peptides have been shown to activate macrophages and T helper cells, respectively178. Meanwhile, lipoproteins are also important modulators of regulatory T cells that can hamper vascular inflammation178, 179. As indicated in the comprehensive MetaCye Metabolic Pathway Dataset, which collected 2766 documented metabolic pathways, pro-inflammatory and anti-inflammatory pathways are highly specific and resulted from extensive metabolic remodeling and re-focusing180. Indeed, two recent studies revealed unlike oxLDL/CD36 signaling in macrophages links dysregulated fatty acid metabolism to oxidative stress from the mitochondria, which triggers cell patrolling to drive chronic inflammation181, ECs present a CD36-independent regulation of a non-classical subset of monocytes, which function in an atheroprotective manner during early atherogenesis182. In diabetes, hyperglycemia and hyperlipidemia downregulate of TFEB (transcription factor EB) expression in aortic ECs, attenuating its anti-inflammatory effects via inhibiting IKK (inhibitor of nuclear factor kappa-B kinase) activity and increasing IκBα level to suppress NF-κB activity183. Compared with the report numbers immunometabolism in macrophages (232 publications at PubMed), ECs immunometabolism is at the early stage (13 publications at PubMed). There is novel transcriptomic evidence that HAECs could be transdifferentiated into innate immune cells by exposing them to hyperlipidemia-up-regulated DAMP molecules, i.e. lysophospholipids. In RNA-seq analysis, lysophosphatidylcholine (LPC) up-regulated genes are involved in cholesterol biosynthesis, presumably through sterol regulatory element-binding protein 2 (SREBP2). Of note, SREBP2 activation mediates atheroprone flow-induced NLRP3 inflammasome function in ECs184. By contrast, lysophosphatidylinositol (LPI) up-regulate gene transcripts critical for the metabolism of glucose, lipids, and amino acids. Of note, we reported that, in HAECs, LPC and LPI both induce adhesion molecules, cytokines, and chemokines, which are all classic markers of endothelial activation. Moreover, LPC and LPI share the ability to transdifferentiate HAECs into innate immune cells, including induction of potent DAMP receptors, such as CD36, T-cell costimulation-, coinhibition/immune checkpoint receptors, and MHC-II proteins. The induction of these innate-immunity signatures by lysophospholipids correlates with their ability to induce up-regulation of cytosolic calcium and mitochondrial ROS. Hence, lysophospholipids such as LPC and LPI induce innate immune cell transdifferentiation in HAECs, supporting a new concept that innate immune cells transdifferentiation of ECs confers a status of prolonged endothelial activation99.
Trained immunity (also termed as innate immune memory) is an emerging concept describing a prolonged hyper-activation of the innate immune system after exposure to certain stimuli, leading to an augmented immune response to a secondary stimulus. Innate immune cells such as monocytes, macrophages, dendritic cells, and NK cells and some non-immune cells185 have been shown to develop trained immunity by undergoing functional reprogramming when exposed to inflammatory stimuli, that elicit changed responses to subsequent inflammatory challenges. This long-term reprogramming depends on the rewiring of cell metabolism and epigenetic processes186, and they stay at the basis of induction of both innate immune memory (also termed trained immunity) and innate immune tolerance187. It has been identified that three metabolic pathways (trained immunity pathways, TIP) including glycolysis pathway, mevalonate pathway and acetyl coenzyme A (acetyl-CoA) generation are responsible for initiating innate immune memory formation. Inductions of trained immunity regulators are a new category of qualification markers for chronic disease risk factors and conditional DAMPs and potential mechanisms for acute inflammation transition to chronic ones. Increased acetylation of histone 3 lysine 14 (H3K14) in the genomic regions that encode TIP genes in comparison to that of endothelial activation genes such as ICAM-1102. Of note, among all the 26,625 compounds identified in the foods (http://foodb.ca/compounds) and environmental compounds (for example, more than 7,000 compounds in cigarette smoke (https://www.lung.org/stop-smoking/smoking-facts/whats-in-a-cigarette.html), therefore, only those compounds stimulations that induce trained immunity have potential to become cardiovascular disease (CVD) risk factors. This conceptual advance on identification of the key features of CVD risk factors will facilitate the future characterization of novel risk factors. Moreover, anti-inflammatory cytokines IL-10 and IL-35 inhibit endothelial activation gene expressions but spare the trained immunity enzyme gene expressions188. The five new training programs have been reported including (i) β-glucan-induced, (ii) Bacillus Calmette-Guérin (BCG)-induced, (iii) oxLDL-induced, (iv) LPS-induced, and (v) aldosterone-induced103. The future work will be needed to determine whether and how each of these training programs regulate innate immune functions of vascular cells in CVD104.
5. Immune tolerogenic functions of ECs, immune checkpoint receptors (ICRs), and cardio-oncology.
Antigen-specific immunity requires regulated trafficking of T cells in and out of diverse tissues in order to orchestrate lymphocyte development, immune surveillance, responses, and memory. ECs serve as a unique barrier, as well as a sentinel, between the blood and the tissues, and as such, they play an essential locally tuned role in regulating T cell migration and information exchange. In addition to providing trafficking cues, intimate cell-cell interaction between lymphocytes and ECs provides instruction to T cells, which influences their activation and differentiation states189. Aside from aiding T cells in playing a proinflammatory role in immune responses (also see the above-discussed sections on cytokines, chemokines, and secretory proteins), ECs can also have an immune tolerogenic function and induce suppressive immune function in T cells. Mouse ECs activated by IFN-γ and co-cultured with allogeneic CD4+ T cells are shown to induce the generation of immunosuppressive Treg190. Furthermore, after contact with ECs, Treg upregulate the expression of ICR, programmed death-1 receptor (PD-1), and increase the production of anti-inflammatory cytokines IL-10 and TGF-β191.
Chronic kidney disease induces inflammatory CD40+ monocyte differentiation192, suggesting that reverse signaling via co-stimulation receptor CD40 promotes vascular inflammation. ECs and VSMCs upregulate 28 co-signaling receptors for T cell activation including 14 co-stimulation receptors (CSRs), 4 dual-function receptors and 10 co-inhibition receptors (CIRs) in pathologies81, 153. ECs upregulate four CSRs such as inducible T cell costimulator ligand (B7-H2, CD275), CD40, Semaphorin 4A (SEMA4A) and CD112, and four CIRs including Galectin 9, TNF superfamily member 14 (HVEM, CD258), programmed cell death 1 ligand 2 (B7-DC, CD273), and programmed cell death 1 ligand 1 (B7-H1, PD-L1, CD274) after stimulation with TNF-α and IFN-γ193. Forward and reverse signaling of three out of 18 CSRs, CD275, CD40 and SEMA4A (16.7%), play significant roles in vascular cells (including VSMCs) in response to proinflammatory cytokine TNF-α and IFN-γ stimulations. TNF-α and IFN-γ also upregulate five out of ten CIRs (50%) in ECs, suggesting that ECs play significant roles in immune tolerance, anti-inflammatory responses, and inflammation resolution81.
Recently, immune checkpoint inhibitors (ICIs) have been an important therapeutic advance in the field of cancer medicine, resulting in a significant improvement in survival of patients with advanced malignancies194. Recent reports provided greater insights into the incidence of cardiovascular adverse events (CVAEs) with ICI use, which leads to the new development of cardio-oncology. Myocarditis is the most common CVAE associated with ICI. Pericardial diseases, Takotsubo syndrome, arrhythmias, and vasculitis constitute other significant adverse events (AEs)195. ECs upregulation of multiple ICRs after the stimulation of proinflammatory cytokines TNF-α and IFN-γ make ECs as significant contributors to CVAEs associated with ICI therapies.
6. Summary
The current literature supports a conceptual innovation that ECs are innate immune cells not only in infections and allograft transplantation but also in metabolic cardiovascular diseases, other sterile inflammations and cancers. Continuous improvement of our understanding on innate immune features of vascular endothelial cells will lead to identification of novel targets for the development of the future therapeutics to treat cardiovascular diseases, cerebrovascular diseases, peripheral artery disease, metabolic diseases, inflammations, infections, neurodegenerative diseases and cancers.
Acknowledgements
YS carried out the primary literature search and drafted the manuscript. Others provided material input and helped revise the manuscript. XFY conceived the study and provided field expertise. The authors are very grateful to Dr. Hong Lu in ATVB Editorial Office for generous guidance and advices. All authors read and approved the final manuscript.
Sources of Funding
Our research activities are supported by grants from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (HL131460, HL132399, HL138749, HL147565, HL130233, DK104116, and DK113775). The content in this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- DAMPs
damage-associated molecular patterns
- DC
dendritic cell
- ECs
endothelial cells
- HAECs
human aortic endothelial cells
- HMGB1
high mobility group box 1
- HUVECs
human umbilical vein endothelial cells
- ICAM-1
intercellular adhesion molecule 1
- PAMPs
pathogen associated molecular patterns
- PRRs
pattern recognition receptors
- SMCs
smooth muscle cells
- VCAM-1
vascular cell adhesion molecule 1
- VEGF
vascular endothelial growth factor
Footnotes
Disclosure
None
References
- 1.Mai J, Virtue A, Shen J, Wang H, Yang XF. An evolving new paradigm: Endothelial cells--conditional innate immune cells. J Hematol Oncol. 2013;6:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davidson SM. Endothelial mitochondria and heart disease. Cardiovascular research. 2010;88:58–66 [DOI] [PubMed] [Google Scholar]
- 3.Shao Y, Cheng Z, Li X, Chernaya V, Wang H, Yang X-f. Immunosuppressive/anti-inflammatory cytokines directly and indirectly inhibit endothelial dysfunction-a novel mechanism for maintaining vascular function. Journal of hematology & oncology. 2014;7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moncada S, Higgs EA. Nitric oxide and the vascular endothelium. Springer Berlin Heidelberg; 2006. [DOI] [PubMed] [Google Scholar]
- 5.Fetterman JL, Weisbrod RM, Feng B, Bastin R, Tuttle ST, Holbrook M, Baker G, Robertson RM, Conklin DJ, Bhatnagar A, Hamburg NM. Flavorings in tobacco products induce endothelial cell dysfunction. Arterioscler Thromb Vasc Biol. 2018;38:1607–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Wang DW, Chen Y, et al. Genome-wide association and functional studies identify scml4 and thsd7a as novel susceptibility genes for coronary artery disease. Arterioscler Thromb Vasc Biol. 2018;38:964–975 [DOI] [PubMed] [Google Scholar]
- 7.Garshick MS, Barrett TJ, Wechter T, Azarchi S, Scher JU, Neimann A, Katz S, Fuentes-Duculan J, Cannizzaro MV, Jelic S, Fisher EA, Krueger JG, Berger JS. Inflammasome signaling and impaired vascular health in psoriasis. Arterioscler Thromb Vasc Biol. 2019;39:787–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhuang T, Liu J, Chen X, et al. Cell-specific effects of gata (gata zinc finger transcription factor family)-6 in vascular smooth muscle and endothelial cells on vascular injury neointimal formation. Arterioscler Thromb Vasc Biol. 2019;39:888–901 [DOI] [PubMed] [Google Scholar]
- 9.Akla N, Viallard C, Popovic N, Lora Gil C, Sapieha P, Larrivee B. Bmp9 (bone morphogenetic protein-9)/alk1 (activin-like kinase receptor type i) signaling prevents hyperglycemia-induced vascular permeability. Arterioscler Thromb Vasc Biol. 2018;38:1821–1836 [DOI] [PubMed] [Google Scholar]
- 10.Orsini F, Fumagalli S, Csaszar E, Toth K, De Blasio D, Zangari R, Lenart N, Denes A, De Simoni MG. Mannose-binding lectin drives platelet inflammatory phenotype and vascular damage after cerebral ischemia in mice via il (interleukin)-1alpha. Arterioscler Thromb Vasc Biol. 2018;38:2678–2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folco EJ, Mawson TL, Vromman A, Bernardes-Souza B, Franck G, Persson O, Nakamura M, Newton G, Luscinskas FW, Libby P. Neutrophil extracellular traps induce endothelial cell activation and tissue factor production through interleukin-1alpha and cathepsin g. Arterioscler Thromb Vasc Biol. 2018;38:1901–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sikura KE, Potor L, Szerafin T, et al. Potential role of h-ferritin in mitigating valvular mineralization. Arterioscler Thromb Vasc Biol. 2019;39:413–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segers VFM, Brutsaert DL, De Keulenaer GW. Cardiac remodeling: Endothelial cells have more to say than just no. Front Physiol. 2018;9:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cines DB, Pollak ES, Buck CA, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561 [PubMed] [Google Scholar]
- 15.Biswas I, Panicker SR, Cai X, Mehta-D’souza P, Rezaie AR. Inorganic polyphosphate amplifies high mobility group box 1-mediated von willebrand factor release and platelet string formation on endothelial cells. Arterioscler Thromb Vasc Biol. 2018;38:1868–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rondaij MG, Bierings R, Kragt A, van Mourik JA, Voorberg J. Dynamics and plasticity of weibel-palade bodies in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:1002–1007 [DOI] [PubMed] [Google Scholar]
- 17.Peng Z, Shu B, Zhang Y, Wang M. Endothelial response to pathophysiological stress. Arterioscler Thromb Vasc Biol. 2019;39:e233–e243 [DOI] [PubMed] [Google Scholar]
- 18.Aikawa M, Manabe I, Marx N. Editorial: New trends in vascular inflammation research: From biology to therapy. Front Cardiovasc Med. 2019;6:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang P, Li X, Shan H, Saredy JJ, Cueto R, Xia J, Jiang X, Yang XF, Wang H. Ly6c(+) inflammatory monocyte differentiation partially mediates hyperhomocysteinemia-induced vascular dysfunction in type 2 diabetic db/db mice. Arterioscler Thromb Vasc Biol. 2019;39:2097–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nanayakkara GK, Wang H, Yang X. Proton leak regulates mitochondrial reactive oxygen species generation in endothelial cell activation and inflammation - a novel concept. Arch Biochem Biophys. 2019;662:68–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng J, Nanayakkara G, Shao Y, Cueto R, Wang L, Yang WY, Tian Y, Wang H, Yang X. Mitochondrial proton leak plays a critical role in pathogenesis of cardiovascular diseases. Adv Exp Med Biol. 2017;982:359–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Fang P, Li Y, et al. Mitochondrial reactive oxygen species mediate lysophosphatidylcholine-induced endothelial cell activation. Arterioscler Thromb Vasc Biol. 2016;36:1090–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Fang P, Yang WY, Chan K, Lavallee M, Xu K, Gao T, Wang H, Yang X. Mitochondrial ros, uncoupled from atp synthesis, determine endothelial activation for both physiological recruitment of patrolling cells and pathological recruitment of inflammatory cells. Can J Physiol Pharmacol. 2017;95:247–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas G, Tacke R, Hedrick CC, Hanna RN. Nonclassical patrolling monocyte function in the vasculature. Arterioscler Thromb Vasc Biol. 2015;35:1306–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao Y, Cheng Z, Li X, Chernaya V, Wang H, Yang XF. Immunosuppressive/anti-inflammatory cytokines directly and indirectly inhibit endothelial dysfunction--a novel mechanism for maintaining vascular function. J Hematol Oncol. 2014;7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones PD, Kaiser MA, Ghaderi Najafabadi M, et al. Jcad, a gene at the 10p11 coronary artery disease locus, regulates hippo signaling in endothelial cells. Arterioscler Thromb Vasc Biol. 2018;38:1711–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan L, Lin Z, Tang X, Tian J, Zheng Q, Jing J, Xie L, Chen H, Lu Q, Wang H, Li Q, Han Y, Ji Y. S-nitrosylation of plastin-3 exacerbates thoracic aortic dissection formation via endothelial barrier dysfunction. Arterioscler Thromb Vasc Biol. 2020;40:175–188 [DOI] [PubMed] [Google Scholar]
- 28.Fu H, Vadalia N, Xue ER, Johnson C, Wang L, Yang WY, Sanchez C, Nelson J, Chen Q, Choi ET, Ma JX, Yu J, Wang H, Yang X. Thrombus leukocytes exhibit more endothelial cell-specific angiogenic markers than peripheral blood leukocytes do in acute coronary syndrome patients, suggesting a possibility of trans-differentiation: A comprehensive database mining study. J Hematol Oncol. 2017;10:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai L, Reineke E, Hamilton DJ, Cooke JP. Glycolytic switch is required for transdifferentiation to endothelial lineage. Circulation. 2019;139:119–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Souilhol C, Harmsen MC, Evans PC, Krenning G. Endothelial-mesenchymal transition in atherosclerosis. Cardiovascular research. 2018;114:565–577 [DOI] [PubMed] [Google Scholar]
- 31.Haynes BA, Yang LF, Huyck RW, Lehrer EJ, Turner JM, Barabutis N, Correll VL, Mathiesen A, McPheat W, Semmes OJ, Dobrian AD. Endothelial-to-mesenchymal transition in human adipose tissue vasculature alters the particulate secretome and induces endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2019;39:2168–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alique M, Sanchez-Lopez E, Bodega G, Giannarelli C, Carracedo J, Ramirez R. Hypoxia-inducible factor-1alpha: The master regulator of endothelial cell senescence in vascular aging. Cells. 2020;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrucci L, Fabbri E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sturmlechner I, Durik M, Sieben CJ, Baker DJ, van Deursen JM. Cellular senescence in renal ageing and disease. Nat Rev Nephrol. 2017;13:77–89 [DOI] [PubMed] [Google Scholar]
- 35.Ritschka B, Storer M, Mas A, Heinzmann F, Ortells MC, Morton JP, Sansom OJ, Zender L, Keyes WM. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes & development. 2017;31:172–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang R, Saredy J, Shao Y, et al. End-stage renal disease is different from chronic kidney disease in upregulating ros-modulated proinflammatory secretome in pbmcs - a novel multiple-hit model for disease progression. Redox Biol. 2020:101460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.An D, Hao F, Zhang F, Kong W, Chun J, Xu X, Cui MZ. Cd14 is a key mediator of both lysophosphatidic acid and lipopolysaccharide induction of foam cell formation. J Biol Chem. 2017;292:14391–14400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131 [DOI] [PubMed] [Google Scholar]
- 39.Wood KJ, Zaitsu M, Goto R. Cell mediated rejection. Methods Mol Biol. 2013;1034:71–83 [DOI] [PubMed] [Google Scholar]
- 40.Pober JS, Merola J, Liu R, Manes TD. Antigen presentation by vascular cells. Front Immunol. 2017;8:1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pober JS, Bothwell AL, Lorber MI, McNiff JM, Schechner JS, Tellides G. Immunopathology of human t cell responses to skin, artery and endothelial cell grafts in the human peripheral blood lymphocyte/severe combined immunodeficient mouse. Springer Semin Immunopathol. 2003;25:167–180 [DOI] [PubMed] [Google Scholar]
- 42.Lopez-Pastrana J, Ferrer LM, Li YF, et al. Inhibition of caspase-1 activation in endothelial cells improves angiogenesis: A novel therapeutic potential for ischemia. J Biol Chem. 2015;290:17485–17494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin Y, Li X, Sha X, et al. Early hyperlipidemia promotes endothelial activation via a caspase-1-sirtuin 1 pathway. Arterioscler Thromb Vasc Biol. 2015;35:804–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fang P, Zhang D, Cheng Z, Yan C, Jiang X, Kruger WD, Meng S, Arning E, Bottiglieri T, Choi ET. Hyperhomocysteinemia potentiates hyperglycemia-induced inflammatory monocyte differentiation and atherosclerosis. Diabetes. 2014:DB_140809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang P, Li X, Shan H, Saredy JJ, Cueto R, Xia J, Jiang X, Yang XF, Wang H. Ly6c(+) inflammatory monocyte differentiation partially mediates hyperhomocysteinemia-induced vascular dysfunction in type 2 diabetic db/db mice. Arterioscler Thromb Vasc Biol. 2019:ATVBAHA119313138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang D, Fang P, Jiang X, Nelson J, Moore JK, Kruger WD, Berretta RM, Houser SR, Yang X, Wang H. Severe hyperhomocysteinemia promotes bone marrow-derived and resident inflammatory monocyte differentiation and atherosclerosis in ldlr/cbs-deficient mice. Circulation research. 2012;111:37–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang D, Jiang X, Fang P, Yan Y, Song J, Gupta S, Schafer AI, Durante W, Kruger WD, Yang X, Wang H. Hyperhomocysteinemia promotes inflammatory monocyte generation and accelerates atherosclerosis in transgenic cystathionine beta-synthase-deficient mice. Circulation. 2009;120:1893–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xi H, Zhang Y, Xu Y, Yang WY, Jiang X, Sha X, Cheng X, Wang J, Qin X, Yu J, Ji Y, Yang X, Wang H. Caspase-1 inflammasome activation mediates homocysteine-induced pyrop-apoptosis in endothelial cells. Circulation research. 2016;118:1525–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Virtue A, Johnson C, Lopez-Pastrana J, et al. Microrna-155 deficiency leads to decreased atherosclerosis, increased white adipose tissue obesity, and non-alcoholic fatty liver disease: A novel mouse model of obesity paradox. J Biol Chem. 2017;292:1267–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson C, Drummer Ct, Virtue A, Gao T, Wu S, Hernandez M, Singh L, Wang H, Yang XF. Increased expression of resistin in microrna-155-deficient white adipose tissues may be a possible driver of metabolically healthy obesity transition to classical obesity. Front Physiol. 2018;9:1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang XF, Yin Y, Wang H. Vascular inflammation and atherogenesis are activated via receptors for pamps and suppressed by regulatory t cells. Drug Discov Today Ther Strateg. 2008;5:125–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yin Y, Pastrana JL, Li X, Huang X, Mallilankaraman K, Choi ET, Madesh M, Wang H, Yang XF. Inflammasomes: Sensors of metabolic stresses for vascular inflammation. Front Biosci (Landmark Ed). 2013;18:638–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J, McArdle S, Gholami A, Kimura T, Wolf D, Gerhardt T, Miller J, Weber C, Ley K. Ccr5+t-bet+foxp3+ effector cd4 t cells drive atherosclerosis. Circulation research. 2016;118:1540–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baylis RA, Gomez D, Mallat Z, Pasterkamp G, Owens GK. The cantos trial: One important step for clinical cardiology but a giant leap for vascular biology. Arterioscler Thromb Vasc Biol. 2017;37:e174–e177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kimura T, Kobiyama K, Winkels H, et al. Regulatory cd4(+) t cells recognize major histocompatibility complex class ii molecule-restricted peptide epitopes of apolipoprotein b. Circulation. 2018;138:1130–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiong Z, Song J, Yan Y, Huang Y, Cowan A, Wang H, Yang XF. Higher expression of bax in regulatory t cells increases vascular inflammation. Front Biosci. 2008;13:7143–7155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiong Z, Yan Y, Song J, Fang P, Yin Y, Yang Y, Cowan A, Wang H, Yang XF. Expression of tctp antisense in cd25(high) regulatory t cells aggravates cuff-injured vascular inflammation. Atherosclerosis. 2009;203:401–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abadier M, Pramod AB, McArdle S, Marki A, Fan Z, Gutierrez E, Groisman A, Ley K. Effector and regulatory t cells roll at high shear stress by inducible tether and sling formation. Cell Rep. 2017;21:3885–3899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang R, Jason Saredy, Ying Shao, Tian Yao, Lu Liu, Fatma Saaoud, William Y.Yang, Yu Sun, Candice Johnson, Charles Drummer, Hangfei Fu, Yifan Lu, Keman Xu, Ming Liu, Jirong Wang, Elizabeth Cutler, Daohai Yu, Xiaohua Jiang, Yafeng Li, Rongshan Li, Lihua Wang, Eric T. Choi, Hong Wang, Xiaofeng Yang. End-stage renal disease is different from chronic kidney disease in upregulating ros-modulated proinflammatory secretome in pbmcs - a novel multiple-hit model for disease progression. REDOX Biology. 2020:REDOX 101460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stramer BM, Mori R, Martin P. The inflammation-fibrosis link? A jekyll and hyde role for blood cells during wound repair. J Invest Dermatol. 2007;127:1009–1017 [DOI] [PubMed] [Google Scholar]
- 61.Al-Soudi A, Kaaij MH, Tas SW. Endothelial cells: From innocent bystanders to active participants in immune responses. Autoimmun Rev. 2017;16:951–962 [DOI] [PubMed] [Google Scholar]
- 62.Onder L, Ludewig B. A fresh view on lymph node organogenesis. Trends Immunol. 2018;39:775–787 [DOI] [PubMed] [Google Scholar]
- 63.Humbert M, Hugues S, Dubrot J. Shaping of peripheral t cell responses by lymphatic endothelial cells. Front Immunol. 2016;7:684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peterson LW, Artis D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153 [DOI] [PubMed] [Google Scholar]
- 65.Jain A, Pasare C. Innate control of adaptive immunity: Beyond the three-signal paradigm. J Immunol. 2017;198:3791–3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nowarski R, Jackson R, Flavell RA. The stromal intervention: Regulation of immunity and inflammation at the epithelial-mesenchymal barrier. Cell. 2017;168:362–375 [DOI] [PubMed] [Google Scholar]
- 67.Schleimer RP, Kato A, Kern R, Kuperman D, Avila PC. Epithelium: At the interface of innate and adaptive immune responses. J Allergy Clin Immunol. 2007;120:1279–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Malhotra D, Fletcher AL, Turley SJ. Stromal and hematopoietic cells in secondary lymphoid organs: Partners in immunity. Immunol Rev. 2013;251:160–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Owens BM. Inflammation, innate immunity, and the intestinal stromal cell niche: Opportunities and challenges. Front Immunol. 2015;6:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buckley CD, Barone F, Nayar S, Benezech C, Caamano J. Stromal cells in chronic inflammation and tertiary lymphoid organ formation. Annu Rev Immunol. 2015;33:715–745 [DOI] [PubMed] [Google Scholar]
- 71.Li YF, Huang X, Li X, et al. Caspase-1 mediates hyperlipidemia-weakened progenitor cell vessel repair. Front Biosci (Landmark Ed). 2016;21:178–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferrer LM, Monroy AM, Lopez-Pastrana J, Nanayakkara G, Cueto R, Li YF, Li X, Wang H, Yang XF, Choi ET. Caspase-1 plays a critical role in accelerating chronic kidney disease-promoted neointimal hyperplasia in the carotid artery. J Cardiovasc Transl Res. 2016;9:135–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Monroy MA, Fang J, Li S, Ferrer L, Birkenbach MP, Lee IJ, Wang H, Yang XF, Choi ET. Chronic kidney disease alters vascular smooth muscle cell phenotype. Front Biosci (Landmark Ed). 2015;20:784–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circulation research. 2016;118:692–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ren J, Zhou T, Pilli VSS, Phan N, Wang Q, Gupta K, Liu Z, Sheibani N, Liu B. Novel paracrine functions of smooth muscle cells in supporting endothelial regeneration following arterial injury. Circulation research. 2019;124:1253–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luo W, Wang Y, Zhang L, et al. Critical role of cytosolic DNA and its sensing adaptor sting in aortic degeneration, dissection, and rupture. Circulation. 2020;141:42–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herman AB, Silva Afonso M, Kelemen SE, Ray M, Vrakas CN, Burke AC, Scalia RG, Moore K, Autieri MV. Regulation of stress granule formation by inflammation, vascular injury, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2019;39:2014–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Byon CH, Heath JM, Chen Y. Redox signaling in cardiovascular pathophysiology: A focus on hydrogen peroxide and vascular smooth muscle cells. Redox Biol. 2016;9:244–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–823 [DOI] [PubMed] [Google Scholar]
- 80.Danese S, Dejana E, Fiocchi C. Immune regulation by microvascular endothelial cells: Directing innate and adaptive immunity, coagulation, and inflammation. J Immunol. 2007;178:6017–6022 [DOI] [PubMed] [Google Scholar]
- 81.Shen H, Wu N, Nanayakkara G, et al. Co-signaling receptors regulate t-cell plasticity and immune tolerance. Front Biosci (Landmark Ed). 2019;24:96–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815 [DOI] [PubMed] [Google Scholar]
- 83.Berg RE, Forman J. The role of cd8 t cells in innate immunity and in antigen non-specific protection. Curr Opin Immunol. 2006;18:338–343 [DOI] [PubMed] [Google Scholar]
- 84.Beetz S, Wesch D, Marischen L, Welte S, Oberg HH, Kabelitz D. Innate immune functions of human gammadelta t cells. Immunobiology. 2008;213:173–182 [DOI] [PubMed] [Google Scholar]
- 85.Fan H, Wang A, Wang Y, Sun Y, Han J, Chen W, Wang S, Wu Y, Lu Y. Innate lymphoid cells: Regulators of gut barrier function and immune homeostasis. J Immunol Res. 2019;2019:2525984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grasseau A, Boudigou M, Le Pottier L, Chriti N, Cornec D, Pers JO, Renaudineau Y, Hillion S. Innate b cells: The archetype of protective immune cells. Clin Rev Allergy Immunol. 2020;58:92–106 [DOI] [PubMed] [Google Scholar]
- 87.Ardain A, Marakalala MJ, Leslie A. Tissue-resident innate immunity in the lung. Immunology. 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qiu R, Zhou L, Ma Y, Zhou L, Liang T, Shi L, Long J, Yuan D. Regulatory t cell plasticity and stability and autoimmune diseases. Clin Rev Allergy Immunol. 2020;58:52–70 [DOI] [PubMed] [Google Scholar]
- 89.Xu K, Yang WY, Nanayakkara GK, Shao Y, Yang F, Hu W, Choi ET, Wang H, Yang X. Gata3, hdac6, and bcl6 regulate foxp3+ treg plasticity and determine treg conversion into either novel antigen-presenting cell-like treg or th1-treg. Frontiers in immunology. 2018;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang Q, Nanayakkara GK, Drummer C, et al. Low-intensity ultrasound-induced anti-inflammatory effects are mediated by several new mechanisms including gene induction, immunosuppressor cell promotion, and enhancement of exosome biogenesis and docking. Front Physiol. 2017;8:818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tripathi D, Biswas B, Manhas A, Singh A, Goyal D, Gaestel M, Jagavelu K. Proinflammatory effect of endothelial microparticles is mitochondria mediated and modulated through mapkapk2 (mapk-activated protein kinase 2) leading to attenuation of cardiac hypertrophy. Arterioscler Thromb Vasc Biol. 2019;39:1100–1112 [DOI] [PubMed] [Google Scholar]
- 92.Li A, Sun Y, Drummer Ct, et al. Increasing upstream chromatin long-range interactions may favor induction of circular rnas in lysopc-activated human aortic endothelial cells. Front Physiol. 2019;10:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang YJ, Li YS, Wu CC, Wang KC, Huang TC, Chen Z, Chien S. Extracellular microrna-92a mediates endothelial cell-macrophage communication. Arterioscler Thromb Vasc Biol. 2019;39:2492–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Icli B, Wu W, Ozdemir D, et al. Microrna-615–5p regulates angiogenesis and tissue repair by targeting akt/enos (protein kinase b/endothelial nitric oxide synthase) signaling in endothelial cells. Arterioscler Thromb Vasc Biol. 2019;39:1458–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fernandez Esmerats J, Villa-Roel N, Kumar S, Gu L, Salim MT, Ohh M, Taylor WR, Nerem RM, Yoganathan AP, Jo H. Disturbed flow increases ube2c (ubiquitin e2 ligase c) via loss of mir-483–3p, inducing aortic valve calcification by the pvhl (von hippel-lindau protein) and hif-1alpha (hypoxia-inducible factor-1alpha) pathway in endothelial cells. Arterioscler Thromb Vasc Biol. 2019;39:467–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, Ofrecio JM, Wollam J, Hernandez-Carretero A, Fu W, Li P, Olefsky JM. Adipose tissue macrophage-derived exosomal mirnas can modulate in vivo and in vitro insulin sensitivity. Cell. 2017;171:372–384 e312 [DOI] [PubMed] [Google Scholar]
- 97.Joris V, Gomez EL, Menchi L, Lobysheva I, Di Mauro V, Esfahani H, Condorelli G, Balligand JL, Catalucci D, Dessy C. Microrna-199a-3p and microrna-199a-5p take part to a redundant network of regulation of the nos (no synthase)/no pathway in the endothelium. Arterioscler Thromb Vasc Biol. 2018;38:2345–2357 [DOI] [PubMed] [Google Scholar]
- 98.Simion V, Haemmig S, Feinberg MW. Lncrnas in vascular biology and disease. Vascul Pharmacol. 2019;114:145–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li X, Wang L, Fang P, Sun Y, Jiang X, Wang H, Yang XF. Lysophospholipids induce innate immune transdifferentiation of endothelial cells, resulting in prolonged endothelial activation. J Biol Chem. 2018;293:11033–11045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marelli-Berg FM, Jarmin SJ. Antigen presentation by the endothelium: A green light for antigen-specific t cell trafficking? Immunol Lett. 2004;93:109–113 [DOI] [PubMed] [Google Scholar]
- 101.Rothermel AL, Wang Y, Schechner J, Mook-Kanamori B, Aird WC, Pober JS, Tellides G, Johnson DR. Endothelial cells present antigens in vivo. BMC Immunol. 2004;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu Y, Sun Y, Drummer Ct, et al. Increased acetylation of h3k14 in the genomic regions that encode trained immunity enzymes in lysophosphatidylcholine-activated human aortic endothelial cells - novel qualification markers for chronic disease risk factors and conditional damps. Redox Biol. 2019;24:101221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhong C, Xiao-feng Yang, Yulin Feng and Jun Yu. Trained immunity: An underlying driver of inflammatory atherosclerosis Frontiers in Immunology 2020;| 10.3389/fimmu.2020.00284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cf Zhong. Trained immunity: An underlying driver of inflammatory atherosclerosis. Frontiers in Immunology. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mulder WJM, Ochando J, Joosten LAB, Fayad ZA, Netea MG. Therapeutic targeting of trained immunity. Nat Rev Drug Discov. 2019;18:553–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marques RE, Marques PE, Guabiraba R, Teixeira MM. Exploring the homeostatic and sensory roles of the immune system. Front Immunol. 2016;7:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Horvath P, Barrangou R. Crispr/cas, the immune system of bacteria and archaea. Science. 2010;327:167–170 [DOI] [PubMed] [Google Scholar]
- 108.Villani AC, Satija R, Reynolds G, et al. Single-cell rna-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. 2017;356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jaitin DA, Kenigsberg E, Keren-Shaul H, Elefant N, Paul F, Zaretsky I, Mildner A, Cohen N, Jung S, Tanay A, Amit I. Massively parallel single-cell rna-seq for marker-free decomposition of tissues into cell types. Science. 2014;343:776–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Papalexi E, Satija R. Single-cell rna sequencing to explore immune cell heterogeneity. Nat Rev Immunol. 2018;18:35–45 [DOI] [PubMed] [Google Scholar]
- 111.Yang XF, Mirkovic D, Zhang S, Zhang QE, Yan Y, Xiong Z, Yang F, Chen IH, Li L, Wang H. Processing sites are different in the generation of hla-a2.1-restricted, t cell reactive tumor antigen epitopes and viral epitopes. Int J Immunopathol Pharmacol. 2006;19:853–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Carrasco YR, Batista FD. B cell recognition of membrane-bound antigen: An exquisite way of sensing ligands. Curr Opin Immunol. 2006;18:286–291 [DOI] [PubMed] [Google Scholar]
- 113.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, Hedrick CC, Cook HT, Diebold S, Geissmann F. Nr4a1-dependent ly6c(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153:362–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hamakawa Y, Omori N, Ouchida M, Nagase M, Sato K, Nagano I, Shoji M, Fujita T, Abe K. Severity dependent up-regulations of lox-1 and mcp-1 in early sclerotic changes of common carotid arteries in spontaneously hypertensive rats. Neurological research. 2004;26:767–773 [DOI] [PubMed] [Google Scholar]
- 115.Xu S, Liu Z, Huang Y, Le K, Tang F, Huang H, Ogura S, Little PJ, Shen X, Liu P. Tanshinone ii-a inhibits oxidized ldl-induced lox-1 expression in macrophages by reducing intracellular superoxide radical generation and nf-kappab activation. Translational research : the journal of laboratory and clinical medicine. 2012;160:114–124 [DOI] [PubMed] [Google Scholar]
- 116.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820 [DOI] [PubMed] [Google Scholar]
- 117.Wang X, Li YF, Nanayakkara G, Shao Y, Liang B, Cole L, Yang WY, Li X, Cueto R, Yu J, Wang H, Yang XF. Lysophospholipid receptors, as novel conditional danger receptors and homeostatic receptors modulate inflammation-novel paradigm and therapeutic potential. J Cardiovasc Transl Res. 2016;9:343–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shao Y, Nanayakkara G, Cheng J, Cueto R, Yang WY, Park JY, Wang H, Yang X. Lysophospholipids and their receptors serve as conditional damps and damp receptors in tissue oxidative and inflammatory injury. Antioxid Redox Signal. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu C, Daugherty A, Lu HS. Updates on approaches for studying atherosclerosis. Arterioscler Thromb Vasc Biol. 2019;39:e108–e117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Preston KJ, Rom I, Vrakas C, Landesberg G, Etwebi Z, Muraoka S, Autieri M, Eguchi S, Scalia R. Postprandial activation of leukocyte-endothelium interaction by fatty acids in the visceral adipose tissue microcirculation. FASEB J. 2019;33:11993–12007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shen W, Gao C, Cueto R, Liu L, Fu H, Shao Y, Yang WY, Fang P, Choi ET, Wu Q, Yang X, Wang H. Homocysteine-methionine cycle is a metabolic sensor system controlling methylation-regulated pathological signaling. Redox Biol. 2020;28:101322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang L, Fu H, Nanayakkara G, et al. Novel extracellular and nuclear caspase-1 and inflammasomes propagate inflammation and regulate gene expression: A comprehensive database mining study. J Hematol Oncol. 2016;9:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li YF, Nanayakkara G, Sun Y, et al. Analyses of caspase-1-regulated transcriptomes in various tissues lead to identification of novel il-1beta-, il-18- and sirtuin-1-independent pathways. J Hematol Oncol. 2017;10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schaefer L Complexity of danger: The diverse nature of damage-associated molecular patterns. J Biol Chem. 2014;289:35237–35245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gong T, Liu L, Jiang W, Zhou R. Damp-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20:95–112 [DOI] [PubMed] [Google Scholar]
- 126.Lin Q, Fan C, Gomez-Arroyo J, Van Raemdonck K, Meuchel LW, Skinner JT, Everett AD, Fang X, Macdonald AA, Yamaji-Kegan K, Johns RA. Himf (hypoxia-induced mitogenic factor) signaling mediates the hmgb1 (high mobility group box 1)-dependent endothelial and smooth muscle cell crosstalk in pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2019;39:2505–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Venereau E, Ceriotti C, Bianchi ME. Damps from cell death to new life. Front Immunol. 2015;6:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li Y-F, Xiao Huang, Xinyuan Li, Ren Gong, Ying Yin, Jun Nelson, Erhe Gao, Hongyu Zhang, Nicholas E. Hoffman, Steven R. Houser, Muniswamy Madesh, Douglas G. Tilley, Eric T. Choi, Xiaohua Jiang, Cong-Xin Huang, Hong Wang, Xiao-Feng Yang. Caspase-1 mediates hyperlipidemia-weakened progenitor cell vessel repair. Frontiers in Bioscience (Landmark Edition). 2016;21:178–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yin Y, Pastrana JL, Li X, Huang X, Mallilankaraman K, Choi ET, Madesh M, Wang H, Yang XF. Inflammasomes: Sensors of metabolic stresses for vascular inflammation. Front Biosci. 2013;18:638–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Opitz B, Forster S, Hocke AC, Maass M, Schmeck B, Hippenstiel S, Suttorp N, Krull M. Nod1-mediated endothelial cell activation by chlamydophila pneumoniae. Circulation research. 2005;96:319–326 [DOI] [PubMed] [Google Scholar]
- 131.Opitz B, Puschel A, Beermann W, Hocke AC, Forster S, Schmeck B, van Laak V, Chakraborty T, Suttorp N, Hippenstiel S. Listeria monocytogenes activated p38 mapk and induced il-8 secretion in a nucleotide-binding oligomerization domain 1-dependent manner in endothelial cells. J Immunol. 2006;176:484–490 [DOI] [PubMed] [Google Scholar]
- 132.Davey MP, Martin TM, Planck SR, Lee J, Zamora D, Rosenbaum JT. Human endothelial cells express nod2/card15 and increase il-6 secretion in response to muramyl dipeptide. Microvascular research. 2006;71:103–107 [DOI] [PubMed] [Google Scholar]
- 133.Manni M, Ding W, Stohl LL, Granstein RD. Muramyl dipeptide induces th17 polarization through activation of endothelial cells. J Immunol. 2011;186:3356–3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mai J, Nanayakkara G, Lopez-Pastrana J, et al. Interleukin-17a promotes aortic endothelial cell activation via transcriptionally and post-translationally activating p38 mitogen-activated protein kinase (mapk) pathway. J Biol Chem. 2016;291:4939–4954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.El Kebir D, Jozsef L, Pan W, Wang L, Filep JG. Bacterial DNA activates endothelial cells and promotes neutrophil adherence through tlr9 signaling. J Immunol. 2009;182:4386–4394 [DOI] [PubMed] [Google Scholar]
- 136.Faure E, Equils O, Sieling PA, Thomas L, Zhang FX, Kirschning CJ, Polentarutti N, Muzio M, Arditi M. Bacterial lipopolysaccharide activates nf-kappab through toll-like receptor 4 (tlr-4) in cultured human dermal endothelial cells. Differential expression of tlr-4 and tlr-2 in endothelial cells. J Biol Chem. 2000;275:11058–11063 [DOI] [PubMed] [Google Scholar]
- 137.Anand AR, Bradley R, Ganju RK. Lps-induced mcp-1 expression in human microvascular endothelial cells is mediated by the tyrosine kinase, pyk2 via the p38 mapk/nf-kappab-dependent pathway. Molecular immunology. 2009;46:962–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Anand AR, Cucchiarini M, Terwilliger EF, Ganju RK. The tyrosine kinase pyk2 mediates lipopolysaccharide-induced il-8 expression in human endothelial cells. J Immunol. 2008;180:5636–5644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Marceau F, Grassi J, Frobert Y, Bergeron C, Poubelle PE. Effects of experimental conditions on the production of interleukin-1 alpha and −1 beta by human endothelial cells cultured in vitro. International journal of immunopharmacology. 1992;14:525–534 [DOI] [PubMed] [Google Scholar]
- 140.Faure E, Thomas L, Xu H, Medvedev A, Equils O, Arditi M. Bacterial lipopolysaccharide and ifn-gamma induce toll-like receptor 2 and toll-like receptor 4 expression in human endothelial cells: Role of nf-kappa b activation. J Immunol. 2001;166:2018–2024 [DOI] [PubMed] [Google Scholar]
- 141.Jersmann HP, Hii CS, Hodge GL, Ferrante A. Synthesis and surface expression of cd14 by human endothelial cells. Infection and immunity. 2001;69:479–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tissari J, Siren J, Meri S, Julkunen I, Matikainen S. Ifn-alpha enhances tlr3-mediated antiviral cytokine expression in human endothelial and epithelial cells by up-regulating tlr3 expression. J Immunol. 2005;174:4289–4294 [DOI] [PubMed] [Google Scholar]
- 143.Li J, Ma Z, Tang ZL, Stevens T, Pitt B, Li S. Cpg DNA-mediated immune response in pulmonary endothelial cells. American journal of physiology. 2004;287:L552–558 [DOI] [PubMed] [Google Scholar]
- 144.Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Laboratory investigation; a journal of technical methods and pathology. 2006;86:9–22 [DOI] [PubMed] [Google Scholar]
- 145.Singer G, Houghton J, Rivera CA, Anthoni C, Granger DN. Role of lps in the hepatic microvascular dysfunction elicited by cecal ligation and puncture in mice. Journal of hepatology. 2007;47:799–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cominacini L, Rigoni A, Pasini AF, Garbin U, Davoli A, Campagnola M, Pastorino AM, Lo Cascio V, Sawamura T. The binding of oxidized low density lipoprotein (ox-ldl) to ox-ldl receptor-1 reduces the intracellular concentration of nitric oxide in endothelial cells through an increased production of superoxide. J Biol Chem. 2001;276:13750–13755 [DOI] [PubMed] [Google Scholar]
- 147.Morawietz H Lox-1 and atherosclerosis: Proof of concept in lox-1-knockout mice. Circulation research. 2007;100:1534–1536 [DOI] [PubMed] [Google Scholar]
- 148.Oka K, Sawamura T, Kikuta K, Itokawa S, Kume N, Kita T, Masaki T. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9535–9540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Matzinger P The danger model: A renewed sense of self. Science. 2002;296:301–305 [DOI] [PubMed] [Google Scholar]
- 150.Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9:692–703 [DOI] [PubMed] [Google Scholar]
- 151.Wang X, Li Y-F, Nanayakkara G, Shao Y, Liang B, Cole L, Yang WY, Li X, Cueto R, Yu J, Wang H, Yang X-F. Lysophospholipid receptors, as novel conditional danger receptors and homeostatic receptors modulate inflammation—novel paradigm and therapeutic potential. Journal of Cardiovascular Translational Research. 2016;9:343–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kuhn TS. The structure of scientific revolutions. Chicago, IL: University of Chicago Press; 1996. [Google Scholar]
- 153.Chen L, Flies DB. Molecular mechanisms of t cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fathman CG, Lineberry NB. Molecular mechanisms of cd4+ t-cell anergy. Nat Rev Immunol. 2007;7:599–609 [DOI] [PubMed] [Google Scholar]
- 155.Yang WY, Ying Shao, Jahaira Lopez-Pastrana, Jietang Mai, Hong Wang and Xiao-feng Yang. Pathological conditions re-shape physiological tregs into pathological tregs. Burns & Trauma. 2015;3:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vignali DA, Kuchroo VK. Il-12 family cytokines: Immunological playmakers. Nature immunology. 2012;13:722–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li X, Mai J, Virtue A, Yin Y, Gong R, Sha X, Gutchigian S, Frisch A, Hodge I, Jiang X, Wang H, Yang XF. Il-35 is a novel responsive anti-inflammatory cytokine--a new system of categorizing anti-inflammatory cytokines. PLoS One. 2012;7:e33628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sha X, Meng S, Li X, Xi H, Maddaloni M, Pascual DW, Shan H, Jiang X, Wang H, Yang XF. Interleukin-35 inhibits endothelial cell activation by suppressing mapk-ap-1 pathway. J Biol Chem. 2015;290:19307–19318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: Back to the future. Immunity. 2013;39:1003–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Basil MC, Levy BD. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat Rev Immunol. 2016;16:51–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Frasch SC, Bratton DL. Emerging roles for lysophosphatidylserine in resolution of inflammation. Progress in lipid research. 2012;51:199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hung ND, Kim MR, Sok DE. 2-polyunsaturated acyl lysophosphatidylethanolamine attenuates inflammatory response in zymosan a-induced peritonitis in mice. Lipids. 2011;46:893–906 [DOI] [PubMed] [Google Scholar]
- 163.Li X, Fang P, Yang WY, Wang H, Yang X. Il-35, as a newly proposed homeostasis-associated molecular pattern, plays three major functions including anti-inflammatory initiator, effector, and blocker in cardiovascular diseases. Cytokine. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Durante W Protective role of heme oxygenase-1 against inflammation in atherosclerosis. Front Biosci (Landmark Ed). 2011;16:2372–2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ng B, Yang F, Huston DP, Yan Y, Yang Y, Xiong Z, Peterson LE, Wang H, Yang XF. Increased noncanonical splicing of autoantigen transcripts provides the structural basis for expression of untolerized epitopes. J Allergy Clin Immunol. 2004;114:1463–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yin Y, Yan Y, Jiang X, Mai J, Chen NC, Wang H, Yang XF. Inflammasomes are differentially expressed in cardiovascular and other tissues. Int J Immunopathol Pharmacol. 2009;22:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sprott D, Poitz DM, Korovina I, Ziogas A, Phieler J, Chatzigeorgiou A, Mitroulis I, Deussen A, Chavakis T, Klotzsche-von Ameln A. Endothelial-specific deficiency of atg5 (autophagy protein 5) attenuates ischemia-related angiogenesis. Arterioscler Thromb Vasc Biol. 2019;39:1137–1148 [DOI] [PubMed] [Google Scholar]
- 168.He M, Huang TS, Li S, et al. Atheroprotective flow upregulates itpr3 (inositol 1,4,5-trisphosphate receptor 3) in vascular endothelium via klf4 (kruppel-like factor 4)-mediated histone modifications. Arterioscler Thromb Vasc Biol. 2019;39:902–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pandey D, Nomura Y, Rossberg MC, Hori D, Bhatta A, Keceli G, Leucker T, Santhanam L, Shimoda LA, Berkowitz D, Romer L. Hypoxia triggers senp1 (sentrin-specific protease 1) modulation of klf15 (kruppel-like factor 15) and transcriptional regulation of arg2 (arginase 2) in pulmonary endothelium. Arterioscler Thromb Vasc Biol. 2018;38:913–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li Z, Li L, Zhang H, Zhou HJ, Ji W, Min W. Short aip1 (ask1-interacting protein-1) isoform localizes to the mitochondria and promotes vascular dysfunction. Arterioscler Thromb Vasc Biol. 2020;40:112–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.He J, Liu X, Su C, Wu F, Sun J, Zhang J, Yang X, Zhang C, Zhou Z, Zhang X, Lin X, Tao J. Inhibition of mitochondrial oxidative damage improves reendothelialization capacity of endothelial progenitor cells via sirt3 (sirtuin 3)-enhanced sod2 (superoxide dismutase 2) deacetylation in hypertension. Arterioscler Thromb Vasc Biol. 2019;39:1682–1698 [DOI] [PubMed] [Google Scholar]
- 172.Moss ME, Lu Q, Iyer SL, Engelbertsen D, Marzolla V, Caprio M, Lichtman AH, Jaffe IZ. Endothelial mineralocorticoid receptors contribute to vascular inflammation in atherosclerosis in a sex-specific manner. Arterioscler Thromb Vasc Biol. 2019;39:1588–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rieker C, Migliavacca E, Vaucher A, Baud G, Marquis J, Charpagne A, Hegde N, Guignard L, McLachlan M, Pooler AM. Apolipoprotein e4 expression causes gain of toxic function in isogenic human induced pluripotent stem cell-derived endothelial cells. Arterioscler Thromb Vasc Biol. 2019;39:e195–e207 [DOI] [PubMed] [Google Scholar]
- 174.Man K, Kutyavin VI, Chawla A. Tissue immunometabolism: Development, physiology, and pathobiology. Cell Metab. 2017;25:11–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hotamisligil GS. Foundations of immunometabolism and implications for metabolic health and disease. Immunity. 2017;47:406–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kumar V Inflammation research sails through the sea of immunology to reach immunometabolism. Int Immunopharmacol. 2019;73:128–145 [DOI] [PubMed] [Google Scholar]
- 177.Lai B, Wang J, Fagenson A, et al. Twenty novel disease group-specific and 12 new shared macrophage pathways in eight groups of 34 diseases including 24 inflammatory organ diseases and 10 types of tumors. Front Immunol. 2019;10:2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gistera A, Ketelhuth DFJ. Lipid-driven immunometabolic responses in atherosclerosis. Curr Opin Lipidol. 2018;29:375–380 [DOI] [PubMed] [Google Scholar]
- 179.Sorci-Thomas MG, Thomas MJ. High density lipoprotein biogenesis, cholesterol efflux, and immune cell function. Arterioscler Thromb Vasc Biol. 2012;32:2561–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Caspi R, Billington R, Fulcher CA, Keseler IM, Kothari A, Krummenacker M, Latendresse M, Midford PE, Ong Q, Ong WK, Paley S, Subhraveti P, Karp PD. The metacyc database of metabolic pathways and enzymes. Nucleic Acids Res. 2018;46:D633–D639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chen Y, Yang M, Huang W, et al. Mitochondrial metabolic reprogramming by cd36 signaling drives macrophage inflammatory responses. Circulation research. 2019;125:1087–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Marcovecchio PM, Thomas GD, Mikulski Z, Ehinger E, Mueller KAL, Blatchley A, Wu R, Miller YI, Nguyen AT, Taylor AM, McNamara CA, Ley K, Hedrick CC. Scavenger receptor cd36 directs nonclassical monocyte patrolling along the endothelium during early atherogenesis. Arterioscler Thromb Vasc Biol. 2017;37:2043–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Song W, Zhang CL, Gou L, He L, Gong YY, Qu D, Zhao L, Jin N, Chan TF, Wang L, Tian XY, Luo JY, Huang Y. Endothelial tfeb (transcription factor eb) restrains ikk (ikappab kinase)-p65 pathway to attenuate vascular inflammation in diabetic db/db mice. Arterioscler Thromb Vasc Biol. 2019;39:719–730 [DOI] [PubMed] [Google Scholar]
- 184.Xiao H, Lu M, Lin TY, et al. Sterol regulatory element binding protein 2 activation of nlrp3 inflammasome in endothelium mediates hemodynamic-induced atherosclerosis susceptibility. Circulation. 2013;128:632–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hamada A, Torre C, Drancourt M, Ghigo E. Trained immunity carried by non-immune cells. Front Microbiol. 2018;9:3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shao Y, Chernaya V, Johnson C, Yang WY, Cueto R, Sha X, Zhang Y, Qin X, Sun J, Choi ET, Wang H, Yang XF. Metabolic diseases downregulate the majority of histone modification enzymes, making a few upregulated enzymes novel therapeutic targets--”sand out and gold stays”. J Cardiovasc Transl Res. 2016;9:49–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dominguez-Andres J, Netea MG. Long-term reprogramming of the innate immune system. J Leukoc Biol. 2019;105:329–338 [DOI] [PubMed] [Google Scholar]
- 188.Li X, Fang P, Sun Y, Shao Y, Yang WY, Jiang X, Wang H, Yang X. Anti-inflammatory cytokines il-35 and il-10 block atherogenic lysophosphatidylcholine-induced, mitochondrial ros-mediated innate immune activation, but spare innate immune memory signature in endothelial cells. Redox Biol. 2020;28:101373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Carman CV, Martinelli R. T lymphocyte-endothelial interactions: Emerging understanding of trafficking and antigen-specific immunity. Front Immunol. 2015;6:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Krupnick AS, Gelman AE, Barchet W, Richardson S, Kreisel FH, Turka LA, Colonna M, Patterson GA, Kreisel D. Murine vascular endothelium activates and induces the generation of allogeneic cd4+25+foxp3+ regulatory t cells. J Immunol. 2005;175:6265–6270 [DOI] [PubMed] [Google Scholar]
- 191.Bedke T, Pretsch L, Karakhanova S, Enk AH, Mahnke K. Endothelial cells augment the suppressive function of cd4+ cd25+ foxp3+ regulatory t cells: Involvement of programmed death-1 and il-10. J Immunol. 2010;184:5562–5570 [DOI] [PubMed] [Google Scholar]
- 192.Yang J, Fang P, Yu D, Zhang L, Zhang D, Jiang X, Yang WY, Bottiglieri T, Kunapuli SP, Yu J, Choi ET, Ji Y, Yang X, Wang H. Chronic kidney disease induces inflammatory cd40+ monocyte differentiation via homocysteine elevation and DNA hypomethylation. Circulation research. 2016;119:1226–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Patten DA, Wilson GK, Bailey D, Shaw RK, Jalkanen S, Salmi M, Rot A, Weston CJ, Adams DH, Shetty S. Human liver sinusoidal endothelial cells promote intracellular crawling of lymphocytes during recruitment: A new step in migration. Hepatology. 2017;65:294–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhou YW, Zhu YJ, Wang MN, Xie Y, Chen CY, Zhang T, Xia F, Ding ZY, Liu JY. Immune checkpoint inhibitor-associated cardiotoxicity: Current understanding on its mechanism, diagnosis and management. Front Pharmacol. 2019;10:1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ball S, Ghosh RK, Wongsaengsak S, Bandyopadhyay D, Ghosh GC, Aronow WS, Fonarow GC, Lenihan DJ, Bhatt DL. Cardiovascular toxicities of immune checkpoint inhibitors: Jacc review topic of the week. J Am Coll Cardiol. 2019;74:1714–1727 [DOI] [PubMed] [Google Scholar]
- 196.Cui XB, Luan JN, Dong K, Chen S, Wang Y, Watford WT, Chen SY. Rgc-32 (response gene to complement 32) deficiency protects endothelial cells from inflammation and attenuates atherosclerosis. Arterioscler Thromb Vasc Biol. 2018;38:e36–e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Descamps B, Saif J, Benest AV, Biglino G, Bates DO, Chamorro-Jorganes A, Emanueli C. Bdnf (brain-derived neurotrophic factor) promotes embryonic stem cells differentiation to endothelial cells via a molecular pathway, including microrna-214, ezh2 (enhancer of zeste homolog 2), and enos (endothelial nitric oxide synthase). Arterioscler Thromb Vasc Biol. 2018;38:2117–2125 [DOI] [PubMed] [Google Scholar]
- 198.He L, Fu Y, Deng J, et al. Deficiency of fam3d (family with sequence similarity 3, member d), a novel chemokine, attenuates neutrophil recruitment and ameliorates abdominal aortic aneurysm development. Arterioscler Thromb Vasc Biol. 2018;38:1616–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Li X, Shao Y, Sha X, Fang P, Kuo YM, Andrews AJ, Li Y, Yang WY, Maddaloni M, Pascual DW, Luo JJ, Jiang X, Wang H, Yang X. Il-35 (interleukin-35) suppresses endothelial cell activation by inhibiting mitochondrial reactive oxygen species-mediated site-specific acetylation of h3k14 (histone 3 lysine 14). Arterioscler Thromb Vasc Biol. 2018;38:599–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Poulsen LC, Edelmann RJ, Kruger S, et al. Inhibition of endothelial notch1 signaling attenuates inflammation by reducing cytokine-mediated histone acetylation at inflammatory enhancers. Arterioscler Thromb Vasc Biol. 2018;38:854–869 [DOI] [PubMed] [Google Scholar]
- 201.Chen T, Karamariti E, Hong X, Deng J, Wu Y, Gu W, Simpson R, Wong MM, Yu B, Hu Y, Qu A, Xu Q, Zhang L. Dkk3 (dikkopf-3) transdifferentiates fibroblasts into functional endothelial cells-brief report. Arterioscler Thromb Vasc Biol. 2019;39:765–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Cho YK, Kang YM, Lee SE, Lee Y, Seol SM, Lee WJ, Park JY, Jung CH. Effect of sfrp5 (secreted frizzled-related protein 5) on the wnt5a (wingless-type family member 5a)-induced endothelial dysfunction and its relevance with arterial stiffness in human subjects. Arterioscler Thromb Vasc Biol. 2018;38:1358–1367 [DOI] [PubMed] [Google Scholar]
- 203.Mehta V, Fields L, Evans IM, Yamaji M, Pellet-Many C, Jones T, Mahmoud M, Zachary I. Vegf (vascular endothelial growth factor) induces nrp1 (neuropilin-1) cleavage via adams (a disintegrin and metalloproteinase) 9 and 10 to generate novel carboxy-terminal nrp1 fragments that regulate angiogenic signaling. Arterioscler Thromb Vasc Biol. 2018;38:1845–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Le Master E, Huang RT, Zhang C, et al. Proatherogenic flow increases endothelial stiffness via enhanced cd36-mediated uptake of oxidized low-density lipoproteins. Arterioscler Thromb Vasc Biol. 2018;38:64–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hu Y, Yan R, Zhang C, Zhou Z, Liu M, Wang C, Zhang H, Dong L, Zhou T, Wu Y, Dong N, Wu Q. High-mobility group box 1 from hypoxic trophoblasts promotes endothelial microparticle production and thrombophilia in preeclampsia. Arterioscler Thromb Vasc Biol. 2018;38:1381–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yuan S, Yurdagul A Jr., Peretik JM, Alfaidi M, Al Yafeai Z, Pardue S, Kevil CG, Orr AW. Cystathionine gamma-lyase modulates flow-dependent vascular remodeling. Arterioscler Thromb Vasc Biol. 2018;38:2126–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Oliveira SDS, Chen J, Castellon M, Mao M, Raj JU, Comhair S, Erzurum S, Silva CLM, Machado RF, Bonini MG, Minshall RD. Injury-induced shedding of extracellular vesicles depletes endothelial cells of cav-1 (caveolin-1) and enables tgf-beta (transforming growth factor-beta)-dependent pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2019;39:1191–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhu L, Xiao R, Zhang X, et al. Spermine on endothelial extracellular vesicles mediates smoking-induced pulmonary hypertension partially through calcium-sensing receptor. Arterioscler Thromb Vasc Biol. 2019;39:482–495 [DOI] [PubMed] [Google Scholar]
- 209.Wagner JUG, Chavakis E, Rogg EM, Muhly-Reinholz M, Glaser SF, Gunther S, John D, Bonini F, Zeiher AM, Schaefer L, Hannocks MJ, Boon RA, Dimmeler S. Switch in laminin beta2 to laminin beta1 isoforms during aging controls endothelial cell functions-brief report. Arterioscler Thromb Vasc Biol. 2018;38:1170–1177 [DOI] [PubMed] [Google Scholar]
- 210.Haberzettl P, Conklin DJ, Abplanalp WT, Bhatnagar A, O’Toole TE. Inhalation of fine particulate matter impairs endothelial progenitor cell function via pulmonary oxidative stress. Arterioscler Thromb Vasc Biol. 2018;38:131–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Helmstadter J, Frenis K, Filippou K, et al. Endothelial glp-1 (glucagon-like peptide-1) receptor mediates cardiovascular protection by liraglutide in mice with experimental arterial hypertension. Arterioscler Thromb Vasc Biol. 2020;40:145–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lother A, Bergemann S, Deng L, Moser M, Bode C, Hein L. Cardiac endothelial cell transcriptome. Arterioscler Thromb Vasc Biol. 2018;38:566–574 [DOI] [PubMed] [Google Scholar]
- 213.Umebayashi R, Uchida HA, Kakio Y, Subramanian V, Daugherty A, Wada J. Cilostazol attenuates angiotensin ii-induced abdominal aortic aneurysms but not atherosclerosis in apolipoprotein edeficient mice. Arterioscler Thromb Vasc Biol. 2018;38:903–912 [DOI] [PubMed] [Google Scholar]


