Abstract
The response of the adult mammalian heart to injury such as myocardial infarction has long been described as primarily fibrotic scarring and adverse remodeling with little to no regeneration of cardiomyocytes. Emerging studies have challenged this paradigm by demonstrating that, indeed, adult mammalian cardiomyocytes are capable of completing cytokinesis albeit at levels vastly insufficient to compensate for the loss of functional cardiomyocytes following ischemic injury. Thus, there is great interest in identifying mechanisms to guide adult cardiomyocyte cell cycle re-entry and facilitate endogenous heart regeneration. The Hippo signaling pathway is a core kinase cascade that functions to suppress the transcriptional co-activators Yap and Taz by phosphorylation and therefore cytoplasmic retention or phospho-degradation. This pathway has recently sparked interest in the field of cardiac regeneration as inhibition of Hippo kinase signaling or overdriving the transcriptional co-activator, Yap, significantly promotes proliferation of terminally differentiated adult mammalian cardiomyocytes and can restore function in failing mouse hearts. Thus, the Hippo pathway is an attractive therapeutic target for promoting cardiomyocyte renewal and cardiac regeneration. Although the core kinases and transcriptional activators of the Hippo pathway have been studied extensively over the last twenty years, the regulatory inputs of this pathway, particularly in vertebrates, are poorly understood. Recent studies have elucidated several upstream regulatory inputs to the Hippo pathway in adult mammalian cardiomyocytes that influence cell proliferation and heart regeneration. Considering upstream inputs to the Hippo pathway are thought to be context and cell type specific, targeting these various components could serve as a therapeutic approach for refining Hippo-Yap signaling in the heart. Here, we provide an overview of the emerging regulatory inputs to the Hippo pathway as they relate to mammalian cardiomyocytes and heart regeneration.
Keywords: Cardiomyocyte, Regeneration, Hippo signaling
1. Introduction
The majority of patients who endure a myocardial infarction (MI) survive the initial event, however, the resulting loss of functional cardiomyocytes (CMs) coupled with the poor regenerative ability of the adult mammalian heart can lead to the development of systolic heart failure [1,2]. Heart failure is the number one cause of death in the United States with a staggering 50% of patients dying within 3 years of diagnosis [3]. Once systolic heart failure occurs, current therapies delay but do not prevent progression to end-stage heart failure. Thus, there is a compelling need to explore new therapeutic approaches to treat heart failure due to the loss of functional CMs. Contrary to the long-held dogma that adult mammalian CMs are unable to proliferate, recent studies have demonstrated that adult human and mouse CMs retain limited proliferative capacity, turning over at a rate of approximately 1% per year [4,5]. Additionally, the rate of cardiomyocyte (CM) proliferation marginally increases following experimental MI in the mouse [5]. However, the relatively low frequency of CM turnover in the adult myocardium is clearly insufficient to compensate for loss of billions of CMs following insult such as MI. Due to such findings, there is currently great interest in uncovering genetic and molecular mechanisms that can drive proliferation of pre-existing adult mammalian CMs as a means to promote endogenous heart regeneration. Recent advances in the cardiac regeneration field suggest that this goal might be attainable.
Lower vertebrates such as zebrafish and newts have historically been leveraged as models for studying tissue regeneration as these species retain an extraordinary capacity to regenerate terminally differentiated tissues and organs throughout adulthood including limbs [6], retinas [7], and hearts [8,9]. Although mammals have diminished regenerative capacity of these terminally differentiated tissues, recent work has demonstrated that neonatal mice and rats retain the capacity to regenerate their hearts shortly after birth [10,11]. Within the first week of life in mice, the decline of heart regenerative capacity coincides with CM cell cycle exit [10], suggesting a permissive CM cell cycle state is a prerequisite for cardiac regenerative potential. Neonatal mammalian model systems present an invaluable opportunity to identify genetic mechanisms that facilitate CM proliferation and heart regeneration with the goal of modifying these candidate pathways in the adult to promote endogenous heart regeneration following injury such as MI. Over the past decade or so, several candidate pathways have been identified as important regulators of CM hyperplasia during the developmental or neonatal regenerative period and by extension show promise for stimulating pro-proliferative and pro-regenerative pathways in the adult heart. Arguably, the most striking example of a developmental pathway that has proven to regulate both neonatal and adult CM proliferation is the Hippo-Yap signaling pathway (herein referred to as “Hippo-Yap”). Hippo-Yap is a highly conserved signaling pathway that was originally described to suppress tissue growth in Drosophila. Activation of Hippo pathway kinases function to inhibit the transcriptional co-activators Yap and Taz via phosphorylation and therefore exclusion from the nucleus, or via phosphorylation mediated degradation. Yap transcriptional activity is required for CM proliferation during fetal and neonatal stages of mouse heart development, and interestingly, suppressing the Hippo kinase cascade in adult mammalian CMs or overdriving the transcriptional regulator, Yap, has a prominent effect of inducing post-mitotic CM proliferation in mice. Collectively, the Hippo-Yap pathway has sparked great interest in the field of cardiac biology as emerging evidence suggests this pathway is central to the function of not only CMs but also fibroblasts, epicardial cells, and vascular cells during cardiac development and regeneration. Ultimately, by understanding how Hippo-Yap signaling is mediated in CMs and non-CMs in the heart, we can potentially tailor therapeutic strategies for heart repair in humans. The core kinases of the Hippo-Yap pathway are fairly well understood, yet the upstream regulatory components that feed into the Hippo-Yap pathway remain relatively underexplored, particularly in vertebrates. Identification and characterization of upstream regulatory inputs into the Hippo-Yap pathway will provide insight into how this pathway is regulated in various cell types during development and following injury, shedding light on mechanisms by which this pathway can be fine-tuned in a cell specific manner. Here, we summarize the role of Hippo-Yap core components in heart development and regeneration and describe upstream regulators of Hippo-Yap in mammalian CMs that are emerging with ongoing research.
2. The core components of the Hippo/Yap signaling pathway
The Hippo-Yap signaling pathway is a highly conserved ancient pathway that controls organ size development across species [12]. This pathway was first discovered in Drosophila using genetic screening to characterize its various components and their influence on cell proliferation and apoptosis [13–15]. Hippo kinase is homologous to mammalian members of the Ste20 family, Stk3 and Stk4 (also known as Mst2 and Mst1 respectively; herein referred to as “Stk3/4”). Stk3/4 kinase activity is enabled through phosphorylation of its activation loop by homodimerization and subsequent autophosphorylation or phosphorylation by TAO kinase 1 or 3 (TOAK1/3) [16–18]. Stk3/4 heterodimerizes with the scaffolding protein Salvador (Salv, also known as WW45) by the c-terminal coiled coil domains of each protein. Phosphorylation of Stk3/4 at its activation loop allows it to phosphorylate and in turn activate kinase activity of large tumor suppressor kinase 1 and 2 (Lats1/2). Along with the scaffolding proteins MOB kinase activator 1A and B (Mob1a/b) which coactivates Lats1/2, these proteins form the Hippo pathway core kinase cascade. Phosphorylation of the transcriptional co-activators Yes Associated Protein 1 (Yap) and WW Domain Containing Transcription Regulator 1 (Wwtr1 or Taz, herein referred to as “Wwrt1”) at HXRXXS motifs by Lats1/2 suppresses nuclear localization through either phosphodegradation or 14-3-3 binding which results in in cytoplasmic retention [19–21] (core kinase cascade illustrated in Fig. 1). Hindering Yap/Wwtr1 localization from the nucleus prevents the activity of their binding partners, while non-phosphorylated Yap/Wwtr1 translocate to the nucleus and activate transcription factors such as TEA Domain Transcription Factor 1, 2, 3, and 4 (TEAD1–4), Runt Related Transcription Factors (Runx), and Paired Like Homeodomain 2 (Pitx2), among others, which drive the expression genes related to, but not limited to; proliferation, pro-survival, angiogenesis, and anti-fibrogenesis [22–24]. Subsequent work in higher organisms has led to a growing field of study dedicated to assessing the pathway’s involvement in various forms of cancer, development, and tissue regeneration [25,26].
Fig. 1.
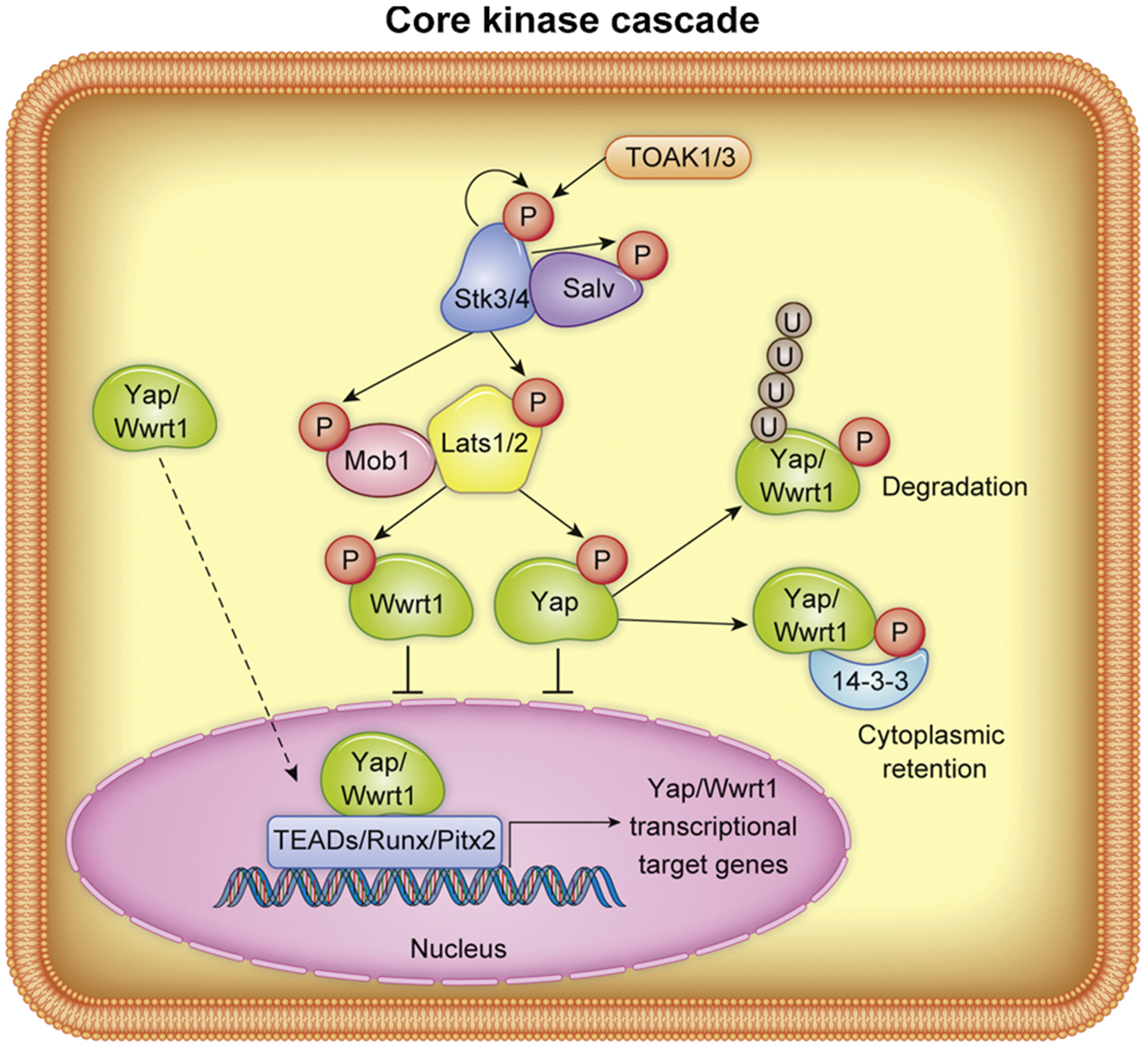
Representation of Hippo-Yap core kinase signaling cascade. “P” indicates protein phosphorylation, “U” indicates ubiquitination. Non-phosphorylated Yap/Wwrt1 translocates to the nucleus, as indicated by dashed arrow.
Although the core kinases and transcriptional outputs of the Hippo-Yap pathway are highly conserved across tissues and cell types, the regulatory inputs for Hippo-Yap signaling are thought to be context or cell type specific [27]. Thus, identifying cell specific inputs to the Hippo-Yap pathways is an attractive approach for therapeutically targeting this pathway and remains an intense area of research. Several cell surface transmembrane proteins have been identified as influencing Hippo-Yap signaling or kinase cascade independent of Yap activity, but the cell type specificity and physiological consequence of modulating regulatory inputs remains underexplored. These regulatory inputs to the Hippo-Yap pathway could serve as therapeutic targets to modulate Hippo-Yap signaling, particularly in the myocardium where Hippo-Yap is an enticing candidate for therapeutic intervention.
3. Hippo-Yap signaling in CMs during embryonic development
Development of the vertebrate heart is an intricate process involving specific spatiotemporal coordination of multiple cell types to form a dynamically functioning organ. By understanding the basic cues governing how the heart forms, we may appreciate how “restarting” this developmental state and inducing cell proliferation and patterning of heart cells in normally quiescent tissue could facilitate cardiac regeneration. Detailed reviews defining cardiac development can be found in Melihac and Buckingham [28], van Eif et al. [29], and Wang et al. [24]. The Hippo-Yap pathway, which to date has been shown to regulate development of the myocardium, epicardium, and endocardium [30–32], is crucial for proper cardiogenesis. Depletion of Salv, a scaffolding protein required for Stk3/4 kinase activity, results in increased Yap translocation to the nucleus and by extension augments Yap-TEAD transcriptional activity [33]. Conditional depletion of Salv and consequently enhanced Yap/Wwrt1 transcriptional activity in Nkx2.5 positive cells, which is expressed in cardiac progenitors starting at E7.5, results in increased CM proliferation, thickened ventricular walls, ventricular septal defects, and severe cardiomegaly at birth. Corroborating these findings in Salv conditional knockout mice, direct overexpression of the transcriptional regulator Yap in fetal CMs either constitutively driven by the myosin heavy chain 7 (Myh7) promoter [34] or inducibly driven by the cardiac troponin (Tnnt2) promoter beginning at E8.5 [35] significantly increases CM proliferation and results in thickened myocardium. Thus, either indirect activation of Yap/ Wwrt1 achieved by deletion of endogenous Salv or directly overexpressing Yap during embryonic development results in gross CM proliferation and cardiomegaly. On the other hand, genetic deletion of Yap in fetal mouse CMs causes embryonic lethality by E16.5 accompanied by pericardial effusion and left, or both left and right, ventricular hypoplasia [35], demonstrating that endogenous Yap activity is indeed required for CM proliferation during embryonic heart development.
Interest in the Hippo-Yap pathway has primarily focused on the role of CM proliferation during developmental stages, however, Hippo-Yap is also a critical regulator of developmental processes in non-CM cell types. For example, depletion of Yap and Wwtr1 in the proepicardial organ during embryonic development diminishes epicardial cell proliferation and impairs epicardial epithelial-to-mesenchymal transition (EMT), ultimately inhibiting coronary vasculature development and resulting in embryonic lethality [31]. Furthermore, endocardial Yap/ Wwtr1 regulates myocardial growth by secretion of Neuregulin, which signals in a paracrine fashion to promote CM hyperplasia during embryonic development [30]. Thus, Hippo-Yap is a central mediator of cardiac development across species and is leveraged by various cardiac cell types including CMs.
4. Hippo signaling in the adult heart
Shortly after birth, mammalian CMs exit the cell cycle and subsequent heart growth is achieved primarily by hypertrophy of existing CMs [36]. The lack of robust CM cell cycle activity significantly contributes to the inability of the adult heart to regenerate; however, substantial evidence suggests that even terminally differentiated adult CMs retain some limited ability for cell division [5,37]. The biochemical, genetic, and mechanical signals that facilitate transition to CM terminal differentiation and cell cycle exit during maturation are currently under intense investigation. Studies from embryonic and neonatal mice uncovered a critical role for the Hippo-Yap pathway in modulating CM proliferation and heart growth begging the question as to whether endogenous Stk3/4 activity normally suppresses CM proliferation in adult mammals, and if so, could modulation of Hippo-Yap components drive adult CM regeneration? Indeed, transgenic or viral overexpression of wildtype Yap stimulated post-natal and adult CM proliferation and improved heart function following experimental MI in mice [35,38]. Furthermore, depletion of Salv, and therefore enhanced Yap/Wwrt1 transcriptional activity, in adult CMs resulted in improved functional outcome following experimental MI which was attributed to CM cell cycle re-entry as assessed by (EdU) incorporation, cell cycle markers, and lineage tracing [39]. Thus, although under normal conditions the adult mammalian heart has minimal regenerative capacity, the hyperplastic window can be extended into adulthood by inhibiting Hippo kinase activity or direct overexpression of Yap. Overexpression of phosphostable Yap (Yap5SA), a variant containing serine to alanine mutations at 5 highly conserved residues that are phosphorylated by Lats1/2 to prime degradation and promote cytoplasmic retention, induced multiple rounds of CM proliferation in unoperated adult mice. Yap5SA overexpression substantially surpassed the pro-proliferative effect of either Salv conditional deletion or overexpression of wildtype Yap in adult CMs. Although robust CM proliferation was achieved following Yap5SA overexpression, all mice died within 1 week of induction, likely due to diminished ventricular chamber size resulting from profound wall thickness [40]. This observation illustrates that precise regulation of the Hippo-Yap pathway will be required to tailor therapeutic approaches to heart regeneration.
The comprehensive cell types that leverage the Hippo-Yap pathway and the specific role Hippo-Yap plays in respective cell types during development and disease are still under investigation. Well established roles for Hippo-Yap signaling are primarily related to cell proliferation and protection form apoptosis and in the heart much focus has been placed on CM centric role of Hippo-Yap signaling. Interestingly, Hippo-Yap appears to be involved in immune cell orchestration in the heart as well. Hippo signaling in the adult epicardium regulates the inflammatory response, and when Yap and Wwtr1 are genetically depleted from the epicardium excessive pericardial inflammation occurs accompanied by adverse ventricular remodeling and increased mortality [41]. Analysis of global Yap deletion in zebrafish elucidated a critical role for Yap in matrix protein secretion and immune cell modulation during scar formation in the regenerating zebrafish heart following cryoinjury [42], which is thought to be in part attributed to Yap activity in myofibroblasts. Although multiple studies confirm a critical role for the Hippo-Yap core kinases and transcriptional regulators in driving proliferation and differentiation in various cell types in the heart, primarily CMs, the physiological consequence of driving or suppressing Hippo signaling in different cell types has not been comprehensively explored. The non-CM roles of the Hippo-Yap pathway is an important area of research for the heart regeneration community, as aberrant Yap/Wwrt1 activation across cell types by global pharmacological activation or inhibition of core pathway components might produce overall negative consequences. Targeting upstream regulatory inputs to this pathway, which perhaps are cell type and context specific, could improve precision and long-term efficacy in targeting the Hippo-Yap pathway for therapeutic purposes.
5. Classical regulatory elements of the Hippo-Yap pathway
Initial genetic screens conducted in Drosophila identified a multitude of upstream regulatory elements responsible for modulating activity of the Hippo core kinase cascade. Subsequent work in vertebrates characterizing orthologs of these classical regulatory elements revealed similar effects governing the activity of the Hippo pathway. The exact mechanism by which these elements regulate the core kinase cascade is not fully understood. Nonetheless, some of these regulatory elements have been shown to modulate cardiac development and repair in a Hippo pathway-dependent manner.
In Drosophila, the tumor suppressor protein Merlin promotes activity of the Hippo kinase cascade thereby suppressing activity of Yorkie, the Drosophila ortholog of Yap [43]. Merlin is a FERM (4.1 protein/Ezrin/Radixin/Moesin) domain adaptor protein which functions in conjunction with other classical regulatory elements Kibra, Expanded, and Crumbs at sub-apical cell-cell junctions [44–46]. The co-localization of these classical elements facilitates localization and phosphorylation of the core kinase cascade. Recent findings demonstrate mechanical flattening of epithelial cells during follicular development drives Yorkie activity [44]. Furthermore, Merlin’s association with Kibra, Expanded, and Crumbs at the sub-apical region is mediated by cellular stretching, likely in conjunction with the spectrin cytoskeleton [44,47]. Dilution of these classical regulatory elements due to stretching of the sub-apical domain results in increased nuclear localization of Yorkie which can be reversed with overexpression of these regulatory elements. The vertebrate ortholog of Merlin, neurofibromin 2 (NF2 in humans or Nf2 in mouse and rat), has a similar function in negatively regulating the activity of Yap in various tissues [48,49]. In both human and mouse CMs, which lack a traditional apical domain, NF2 localizes to the intercalated discs, suggesting an analogous regulatory role for its subcellular distribution in CMs [50]. While it has yet to be shown whether vertebrate homologs of the Drosophila classical regulatory elements colocalize and facilitate activation of the core kinase cascade in CMs, overexpression of Nf2 in cultured neonatal rat ventricular CMs promotes apoptosis in CMs by activating Stk4 and inhibiting Yap [48]. in vivo, Nf2 is dephosphorylated by Myosin Light Chain Phosphatase (Mypt-1) in mouse hearts following ischemia reperfusion (I/R), likely as a result of increased oxidative stress [48]. Dephosphorylation at residue Ser518 promotes a closed conformation of NF2 [51] and results in an increased affinity of NF2 with Stk4 and Lats2 [48]. Additionally, CM specific depletion of Nf2 improves heart function after I/R injury in a Yap-dependent manner [48]. Thus, Nf2 mediates Yap activity in response to oxidative stress and could therefore serve as therapeutic target to promote Yap/Wwtr1 mediated cardioprotection following ischemic events.
While the PPxY motif of Expanded functions to sequester Yki to the apical cortex via its WW domain in Drosophila, the vertebrate ortholog Frmd6, which lacks a PPxY domain, does not sequester Yap [52–54]. It is suspected that the role of Expanded in the Hippo-Yap pathway is unique to arthopods due to evolutionary divergence. In vertebrates, Angiomotin (Amot) family proteins facilitate the function of sequestering Yap/Wwtr1 to the cell cortex, suppressing Yap/Wwtr1 activity independent of direct phosphorylation by Lats [55,56]. Amot was first identified as an Angiostatin mediated cell mobility and angiogenic factor through yeast two-hybrid screening [57]. Angiomotin family members Amot, Amotl1, and Amotl2 are adaptor proteins known to localize with Yap and Wwtr1 at tight junctions through interactions of their N-terminal PPxY and LPxY motifs with the WW domains of Yap and Wwtr1. Similar to Expanded in Drosophila, Angiomotin proteins form a complex with Crb [55]. Angiomotin family members also function as a scaffold between Stk3/4-Sav1 and Lats1/2, promoting the activation of Lats1/2 [58] while Amot itself is the target of Lats1/2 kinase activity at residue Ser175 in humans [59] and Ser176 in mice [60], which mediates localization of Amot to the cell cortex. However, work in HEK293 cells demonstrated non-phosphorylated Amot is able to stabilize nuclear localization Yap, forming a complex between the Yap and Amot [61](Amot regulation of Yap illustrated in Fig. 2). In mouse CMs, nuclear localization of the Amotl1-Yap complex promotes proliferation [62]. Upstream of Amotl1 in mouse CMs, Fat4, an atypical cadherin, inhibits nuclear localization of Amotl1-Yap through Amotl1 binding independent of Lats1/2 phosphorylation of Yap. Consequentially, knockout of Fat4 promotes Amotl1-Yap nuclear localization, Yap transcriptional activity, and CM proliferation [62]. Collectively, data in both flies and mammalian models suggest Angiomotin family members act to modulate core kinase function as well as chaperone Yap and Wwtr1. Angiomotin family member not only offer an intriguing mechanism to promote Yap/ Wwtr1 nuclear localization but also illustrate an underappreciated means of regulating Yap/ Wwtr1 activity through plasma membrane sequestration.
Fig. 2.
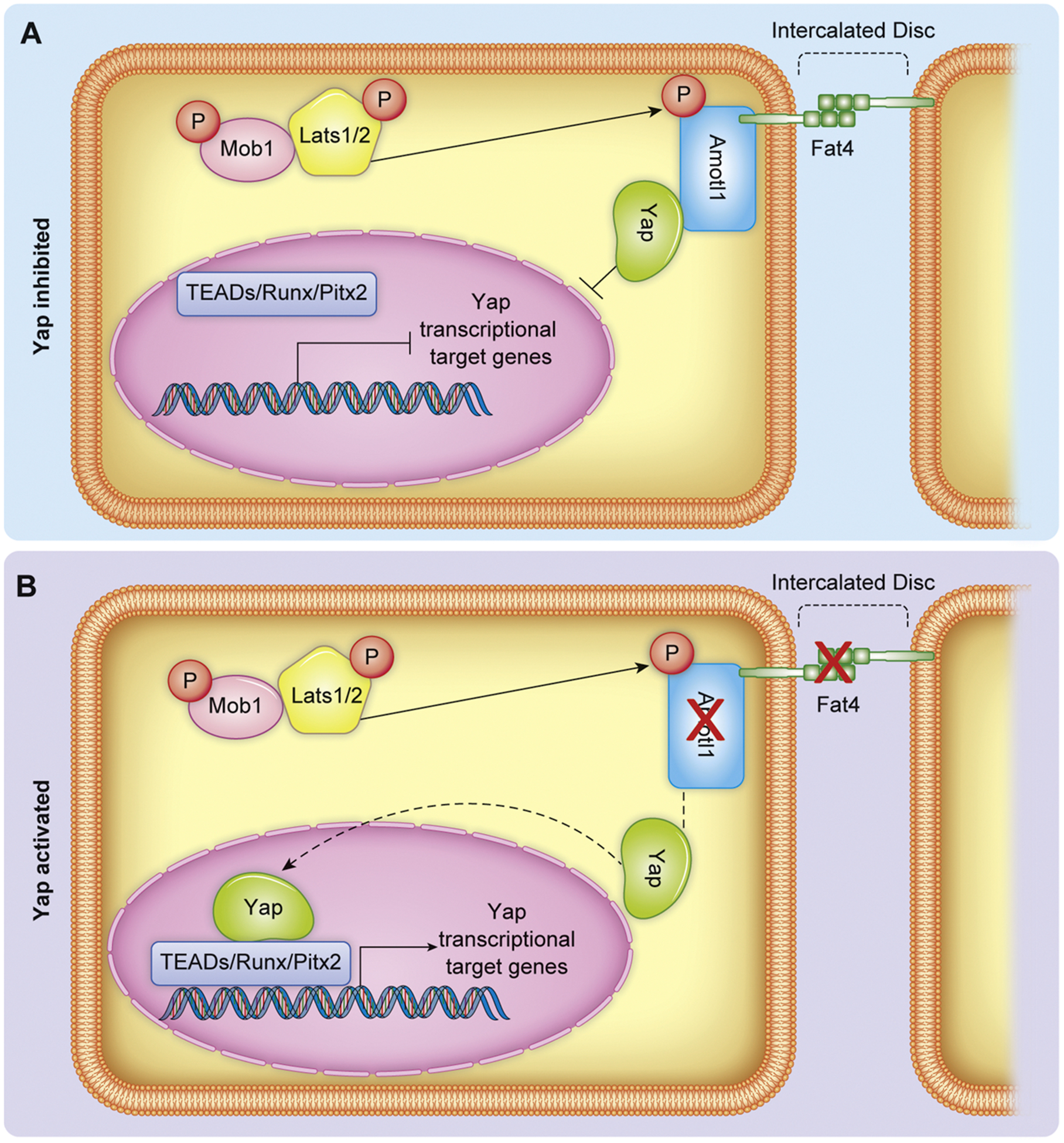
Representation of Yap regulation at the intercalated disc by Amot/Fat4. A) Amot/Fat4 binds and sequesters Yap, preventing nuclear localization and transcriptional activity. B) Genetic depletion of Amotl1 or Fat4 (indicated by red X) releases Yap membrane sequestration and therefore increases Yap nuclear translocation and transcriptional activity. Dashed lines indicate change in Yap localization following Amotl1 or Fat4 genetic depletion.
6. Membrane sequestration as a mechanism of Yap regulation by extracellular matrix and dystrophin-glycoprotein complex factors
Physical sequestration of effector proteins, Yap and Wwtr1, at the cell cortex has been demonstrated as a means to negatively regulate their transcriptional abilities, and in some cases protect from phosphodegradation. Yap localizes to tight junctions in epithelia cells as a result of interaction with a variety of effectors such as Amot family members (described in Section 5), Claudin-18 (Cldn18), and Zonula Occludens-1 (Zo-1) [61,63,64]. In mouse CMs, Yap appears to be localized to either the adherens junction-like intercalated discs or the lateral surface by distinct mechanisms. Localization of Yap to the lateral surface is achieved at least in party by the dystrophin-glycoprotein complex (DGC), which serves to transmit mechanical and biochemical signals between intracellular cytoskeleton and the extracellular matrix. The DGC is a multicomponent complex that functions to bridge ECM components such as Agrin and laminins to the cytoskeleton [65] and is exclusively present in cardiac and skeletal muscle [66,67]. Mutations in DGC components cause several kinds of muscular dystrophy [68]. Among them, Duchenne muscular dystrophy (DMD), a progressive myopathy affecting both skeletal and cardiac muscle. DMD is most commonly associated with mutations to the gene encoding the DGC component Dystrophin [68]. However, the mechanisms leading to progressive disease remain elusive [69].
Components of the DGC, sarcoglycan delta (Sgcd) and syntrophin B1 (Sntb1), are differentially expressed in hearts following CM specific depletion of Salv [70] suggesting a link between the structural DGC components and the Hippo-Yap pathway. The interaction between Yap and the DGC was directly demonstrated using the mdx mouse, an animal model DMD. The mdx mouse arose from spontaneous mutation in exon 23 of the large sarcolemma-associated Dystrophin [67]. While Salv conditional depletion in CMs promoted CM cell cycle activity and cardiac regeneration past the neonatal regenerative window, Salv CKO on the mdx genetic background (Salv;Mdx CKO) significantly potentiated the CM regenerative effect which correlated with increased Yap nuclear translocation [67]. In adult mdx CMs Yap localization to the lateral surface, but not the intercalated discs, was diminished suggesting the DGC physically interacts with Yap to sequester it to the lateral surface [67]. Immunoprecipitation assays confirmed protein-protein interaction between the transmembrane component of the DGC, Dag1, via its PPxY domain and phosphorylated Yap, whereas DGC components did not interact with Lats1/2 [67]. Interestingly, Lats phosphorylation of Yap is required to promote the interaction between DGC components and Yap suggesting an interaction between the core Hippo kinase elements and physical membrane sequestration to regulate Yap activity.
Regulation of Yap can also be attributed to extracellular components that interact with the DGC. Dag1 is a known receptor for the extracellular heparan sulfate proteoglycan, Agrin, which is expressed at high levels in the neonatal heart extracellular matrix [65]. Agrin binding to Dag1 causes a conformational change in and partial disassembly of the DGC. The compromised DGC releases Yap which can be translocated to the nucleus to promote CM cell cycle activity (DGC and Agrin regulation of Yap illustrated in Fig. 3). As Agrin levels decline with age in the mouse heart, the DGC binds other stabilizing matrix proteins likely contributing to CM cell cycle arrest in the adult heart [65]. In cancer cells, Agrin similarly functions to enhance Yap activity. Agrin stabilizes focal adhesion integrity via binding to lipoprotein-related receptor-4 (LRP4) and Muscle-specific kinase (MuSK) which inhibits Yap interaction with Amot via 14-3-3 binding site thereby promoting Yap nuclear localization and transcriptional activity. Agrin depletion leads to increased cytoplasmic retention of Yap which is attributed to its binding to Amot [71]. Thus, in both CMs and cancer cells Yap activity is enhanced by the presence of the extracellular matrix protein Agrin.
Fig. 3.
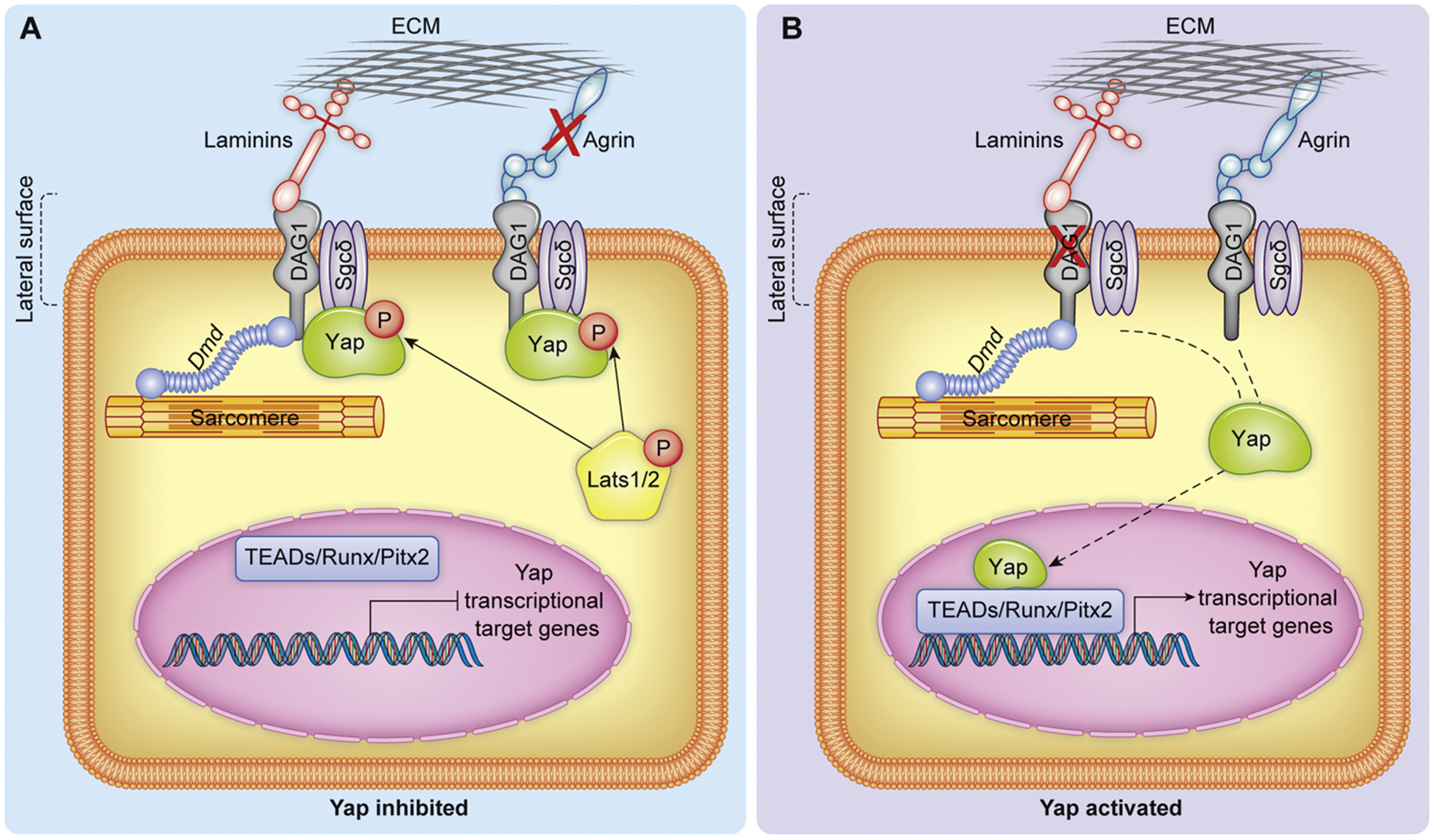
Representation of Yap regulation by the matrix interacting DGC and Agrin. A) phosphorylated Yap physically interacts with the DGC at the lateral surface of CMs thereby preventing Yap nuclear activity. The DGC is stabilized by lack of Agrin (indicated by red X). B) genetic depletion of DAG1 or Agrin overexpression both result in decreased sequestration of Yap to the lateral surface by the DGC, resulting in increased Yap nuclear activity. Dashed lines indicate change in Yap localization following destabilization of the DGC.
7. Interaction between the extracellular matrix and Hippo-Yap signaling via catenin/cadherin complexes
Cells respond to their extracellular environment in part through mechanotransduction, a process by which cells convert mechanical stimulus to biochemical signals. Mechanotransduction can influence cell shape, proliferation, and differentiation as well as communication between neighboring cells [72]. The modulation of the ECM and how its composition affects Hippo-Yap signaling in CMs or other cardiac cell types to date has not been well defined, but a considerable body of literature demonstrates Yap/Wwtr1 activity in various cell types dynamically responds to matrix stiffness. Yap/Wwtr1 translocation to the nucleus and transcriptional activity is enhances upon increased ECM stiffness, whereas decreased ECM stiffness or increased cell density results in Yap/ Wwtr1 cytoplasmic retention [73]. This response to ECM composition is facilitated in part by cell junctions, cadherin-catenin complexes, mechanotransduction regulated by cell shape [74], and nuclear flattening [75].
Emerging literature supports a role for ECM regulation of Yap transcriptional activity in CMs as well. CM specific depletion of Salv in developing mouse hearts revealed that Yap directly regulates expression not only of cell cycle genes in proliferating CMs, but also expression of genes related to the DGC (described in Section 6) and Factin polymerization and factors linking the cytoskeleton components to the ECM [70]. Specifically, cadherin-associate proteins such as αT-catenin (Ctnna3) were transcriptional targets of Yap. Cadherins are transmembrane proteins that are important for the formation of adherens junctions, the protein complexes that facilitate cell-cell connections. The extracellular cadherin domain binds with cadherins of neighboring cells while the intracellular domains complex with proteins such as α- and β- catenins, which function to link cadherins to the actin cytoskeleton [76]. The cadherin-catenin complexes are tension sensing and when strain is applied to the adherens junction, α-catenin undergoes a conformational change promoting interaction with the actin binding protein Vinculin, collectively promoting adherens junction formation and wound closure [77]. During mammalian CM maturation N-cadherin/catenin complexes accumulate at the ends of CM termini contributing to specialized cell-cell contacts called intercalated discs [78]. The intercalated discs are regions that electromechanically couple adjoining CMs through gap junctions, adherens junctions, and desmosomes [79]. Sarcomere organization and establishment of intercalated discs correlates with CM exit from the cell cycle, suggesting regulation of CM terminal differentiation and cell cycle activity are coordinated events [80,81].
Deletion of either αE-catenin (Ctnna1) or αT-catenin (Ctnna3) in the murine heart results in disruption of adherens junction and desmosome proteins, leading to progressive cardiomyopathy [82,83]. Conditional deletion of both Ctnna1/3 causes disruption of the intercalated discs, as assessed by aberrant localization of N-cadherin and increased nuclear localization of Yap and CM proliferation [84]. This increase in proliferation indeed depends on Yap activity as siRNA mediated Yap knockdown in primary rat neonatal CMs suppressed the proliferative ability of Ctnna1/3 depleted cells. Ctnna1/3 are necessary for biomechanical dependent nuclear localization of Yap [78] and mechanistically, Yap has been shown to physically interact with the cadherin-catenin complex. The ubiquitously expressed Ctnna1 has been demonstrated to directly bind Yap and suppress transcriptional activity in other cell types, such as mouse epidermal cells [85]. Resembling the interaction between Dag1 and Yap, this Yap-Ctnna1 interaction is dependent on Lats phosphorylation (Cadherin-Catenin regulation of Yap illustrated in Fig. 4). Phosphorylation of murine Yap at Serine127 and subsequent 14-3-3 binding is critical for Yap-Ctnna1 binding, as determined by immunoprecipitation and mass spectrometry experiments [85]. Thus, similar to but independent of the DGC, Ctnna1/3 sequesters Yap to a plasmalemmal complex, suppressing nuclear translocation.
Fig. 4.
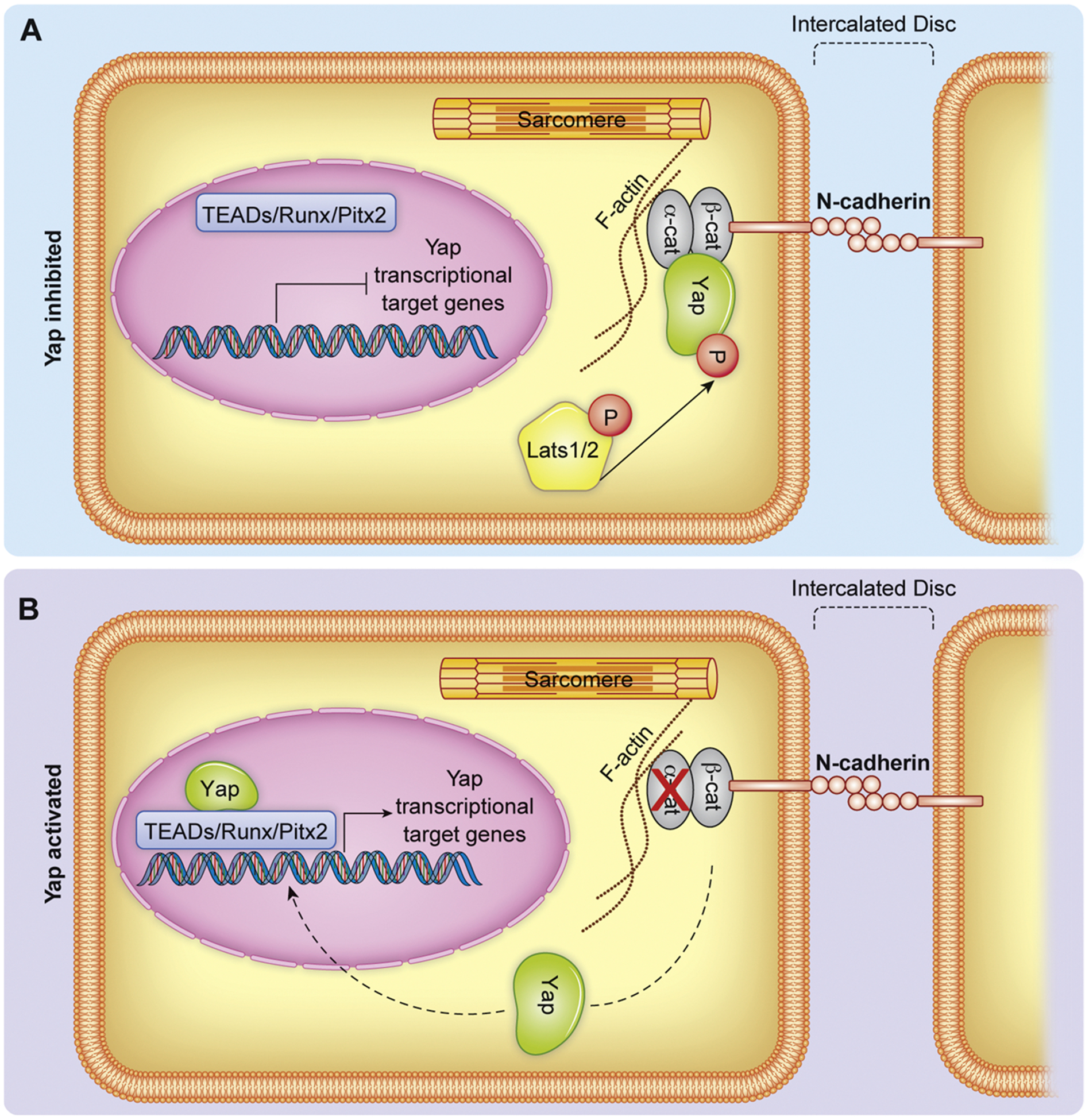
Representation of Yap regulation by Cadherin/Catenin complexes at the intercalated disc. A) Yap is sequestered at the intercalated disc by physical interaction with the catenin/cadherin complex. B) Genetic depletion of α-catenins releases Yap from the cadherin/catenin complex thereby enhancing nuclear translocation of Yap. Dashed lines indicate change in Yap localization following depletion of α-catenins.
8. Signaling pathway crosstalk with Hippo-Yap
Signaling pathways that govern CM development, function, and repair have been shown to crosstalk with or modulate the Hippo-Yap pathway. The Notch pathway is a juxtacrine signaling pathway active in myocardium, endocardium, and epicardium during cardiac development and is necessary for many aspects of cardiogenesis including atrioventricular valve formation, ventricular trabeculation, and coronary vessel formation [86–88]. The Notch pathway promotes gene expression through the binding of a transmembrane Notch with an extracellular ligand, prompting cleavage and nuclear transcriptional activity by the Notch intracellular domain. Crosstalk between the Notch and Hippo-Yap pathways have been demonstrated as Yap directly interacts with the Notch intracellular domain to promote transcription [89]. Yap and Wwtr1 additionally promote transcription of both Notch signaling ligands and inhibitors [90,91]. In the context of CM proliferation, Notch and Hippo-Yap signaling in the endocardium converge to independently drive expression and secretion of Neuregulin (Nrg), which in turn mediates expansion of the myocardium and promotes ventricular trabeculation [30,92]. Furthermore, Wwtr1 drives Notch expression in the compact layer of CMs in a cell-autonomous manner, promoting ventricular trabeculation [87]. Thus, Hippo-Notch pathway interaction controls CM proliferation in both cell-autonomous and nonautonomous manner.
The Wnt/β-catenin pathway is another example of a signaling pathway intertwined with the Hippo-Yap signaling during myocardium development. During cardiogenesis, the Wnt/β-catenin pathway promotes proliferation of cardiomyocytes derived from the second heart field through stabilization of β-catenin, facilitating the function of transcription factors such as T cell factor/ Lymphoid enhancer factor (TCF/LEF) family members [93]. Increased Yap activity in Salv condition knockout utilizing Nkx2.5 driven Cre recombination promotes nuclear β-catenin localization and expression of Wnt/β-catenin target genes in E12.5 hearts [33] demonstrating feedback interaction between Hippo-Yap and Wnt/β-catenin. Furthermore, haploinsufficiency of β- catenin blunts the proliferative effects associated with Salv depletion. Thus, Yap interacts with Wnt/β-catenin to promote CM proliferation at fetal stages. However, the exact mechanism by which Yap drives β-catenin is not clearly understood. In the kidney, Lats phosphorylation of Wwtr1 inhibits phosphorylation of Dishevelled segment polarity protein 2 (DVL2), inhibiting its negative regulation of β-catenin [94]. Additionally, unphosphorylated Yap forms a complex with β-catenin and TCF4 in cultured mouse colonic epithelial cells, promoting nuclear localization and expression of Wnt/β-catenin target genes [95]. Whether the Wnt/β-catenin and Hippo-Yap pathways function by similar mechanisms in cardiomyocytes has yet to be shown.
In addition to fellow developmental pathways such as Notch and Wnt/β-catenin, various cardioprotective pathways have also been demonstrated to interact with the Hippo-Yap pathway. Signaling through the purinergic receptor P2Y2 (P2RY2) has been shown to modulate the Hippo-Yap pathway through suppression of the core kinase cascade, promoting Yap activity. Upon ischemic injury, intracellular pyrimidine nucleotides are released into the intercellular space where they bind to and activate the P2RY2, prompting a cardioprotective response [96]. In cultured human cardiac progenitor cells, P2RY2 over expression enhances Yap transcriptional activity through downregulation of STK4 and LATS1 [97]. Treatment of these culture cells with the P2RY2 agonist uridine triphosphate (UTP) promoted proliferation and migration in a Yap dependent manner as administration of verteporfin, a small molecule inhibitor of Yap-TEAD interaction [98], subdued these responses (P2Y2 regulation of Yap illustrated in Fig. 5).
Fig. 5.
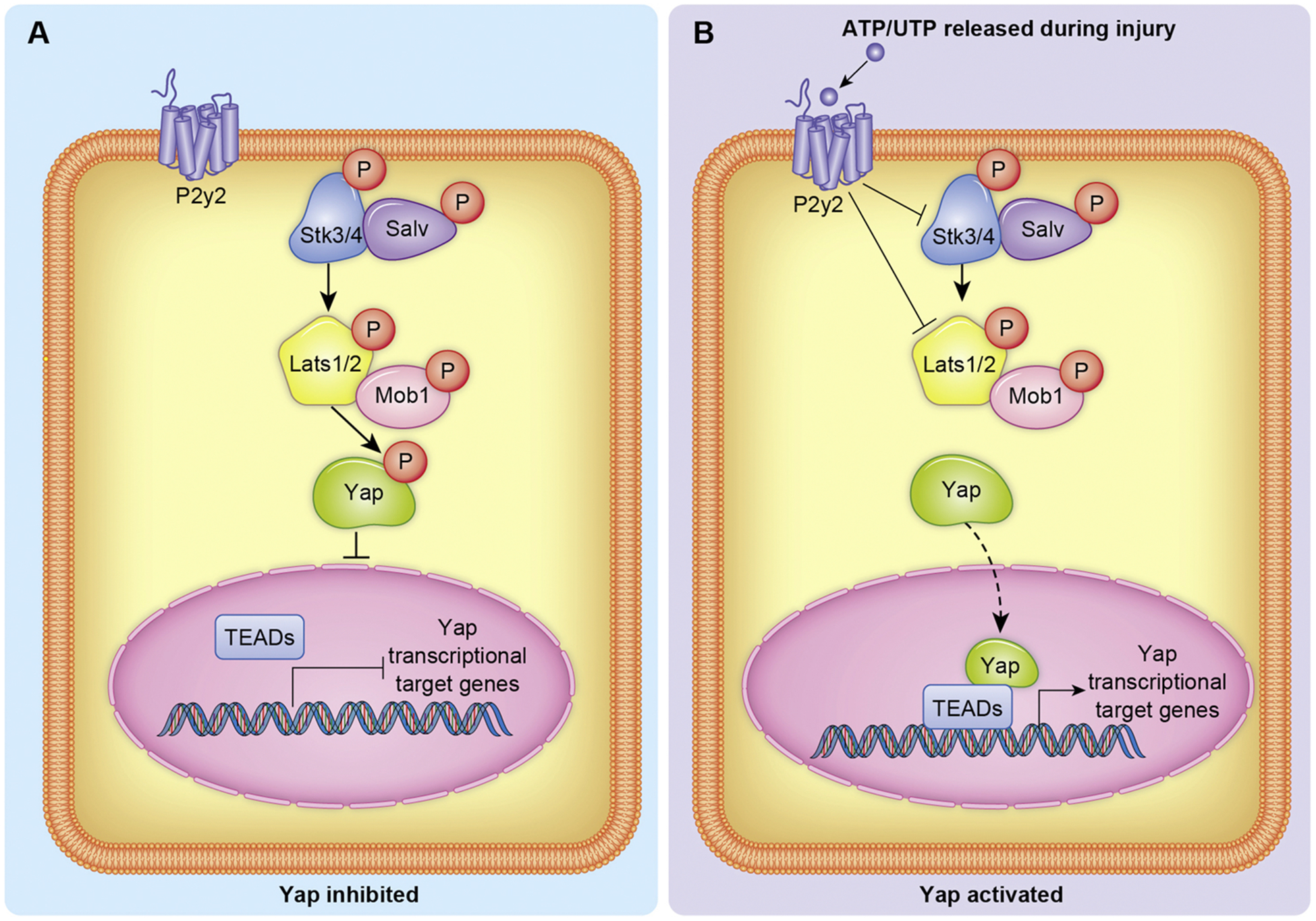
Representation of Yap regulation by P2y2 signaling. A) The absence of P2y2 signaling enables robust phosphorylation of Yap by the core kinase cascade. B) ATP/UTP are ligands for the P2y2 receptor, and when activated inhibits Skt3/4 and Lats1/2 kinase activity. Inhibited core kinase activity results in less Yap phosphorylation and therefore increased Yap translocation to the nucleus.
The homeodomain transcription factor Pitx2 acts in both a developmental and cardiac protective manner. Pitx2 is necessary for proper cardiac morphogenesis, regulating left-right asymmetry, while in the neonatal mouse model it promotes the expression of genes that protect CMs from reactive oxygen species [99,100]. Pitx2 expression diminishes postnatally but substantially increases in CMs upon ischemic injury [100]. In neonatal mice, CM expression of Pitx2 is required for regeneration after apex resection [100]. Immunoprecipitation experiments revealed Yap and Pitx2 form a complex while ChIP-seq of injured hearts show Yap is enriched in roughly half of Pitx2-targeted gene promoters. ChIP-re-ChIP assays further demonstrate Yap and Pitx2 cooperatively promote the expression of redox genes while genetic deletion of Pitx2 in CMs prevents the regenerative response of Salv CKO P8 mice [100]. Thus, Pitx2 and Yap are necessary for cardiac regeneration and as a potential mechanism, cooperatively promote the transcriptional response to oxidative stress. Collectively, these data demonstrate how several pathways affecting CM function do so in conjunction with the Hippo-Yap pathway. While this is not an exhaustive synopsis, the literature describing how various signaling pathways crosstalk with the Hippo-Yap pathway is growing, permitting potential new avenues to control cardiac function and repair post injury.
9. Discussion
The Hippo-Yap pathway has become a central area of focus in the field of cardiac regeneration due to the remarkable impact that modulation of core elements of this pathway has on post-mitotic CM cell cycle re-entry. Emerging evidence has revealed that non-CM cell types depend heavily on Hippo-Yap signaling during development, regeneration, and disease [30,31,41,101]. Thus, while CM promotion of Yap is intriguing from a therapeutic standpoint, global modulation of Hippo-Yap signaling may have unknown consequences in the heart. Investigating how the Hippo-Yap pathway can be temporally and spatial regulated is an area of research that must be addressed prior to implementing therapeutic approaches targeting this pathway. For example, activation of Stk4 has an apoptotic and deleterious effect in CMs resulting in progressive cardiomyopathy [102] but similar activation of Stk4 in fibroblasts inhibits proliferation and protects against adverse remodeling following pressure overload in mice [103]. Furthermore, depletion of Yap/ Wwtr1 transcriptional activity in the epicardium results in a deleterious inflammatory response after ischemic injury [41], whereas the role of Yap/Wwtr1 in the endothelium is necessary for Nrg1 expression and Nrg1-ErbB signaling [30]. Thus, one can appreciate how global activation or inhibition of Hippo-Yap pathway components could have beneficial or detrimental effects on the heart, depending on the cell type that is activated. We propose that targeting various upstream regulatory elements differentially expressed in cardiac cell types could be utilized to modulate the Hippo-Yap pathway in a cell type specific manner to tailor therapeutic strategies for heart repair in humans.
Here, we have discussed several upstream regulators to the Hippo-Yap pathway that have recently been verified in CMs. Additional cell surface receptors and extracellular inputs such as G protein coupled receptors [104], cell polarity [105], and cell tension [106], are known to feed into the Hippo-Yap pathway in various cell types. With the vast majority of in vivo research performed in adult mice, we have likely only scratched the surface of characterizing the comprehensive Hippo-Yap upstream regulatory inputs in mammalian cardiac cells, particularly throughout various developmental timepoints. To fully realize the potential for endogenous heart regeneration there is a need to understand mechanisms by which we can fine tune Hippo-Yap activity in CM populations, and also understand how non-myocyte cell types such as cardiac myofibroblasts, endothelial cells, and immune cells leverage the Hippo-Yap pathway. Additional studies aimed at distinguishing cell type specific role of Hippo-Yap core signaling in the heart and upstream regulatory inputs will shed light on therapeutic strategies that could be implemented to target this pathway to promote cardiac regenerative healing.
Funding
This work was supported by American Heart Association [17SDG33370061to C.C.O.], the National Institute of Health [R01HL141159 to C.C.O, R01EY029267 to B.A.L], and the Cardiovascular Center and Research and Education Program Fund at the Medical College of Wisconsin (C.C.O. and B.A.L.).
Abbreviations:
- CM
cardiomyocyte
- CMs
cardiomyocytes
- MI
myocardial infarction
- I/R
ischemia reperfusion
- UTP
uridine triphosphate
Footnotes
Declaration of Competing Interest
None.
References
- [1].Bui AL, Horwich TB, Fonarow GC, Epidemiology and risk profile of heart failure, Nat. Rev. Cardiol 8 (1) (2012) 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].O’Meara CC, et al. , Transcriptional reversion of cardiac myocyte fate during mammalian cardiac regeneration, Circ. Res 116 (5) (2015) 804–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bozkurt B, Mann DL, The treatment of heart failure in the 21st century: is the glass half empty or half full? Methodist Debakey Cardiovasc. J 9 (1) (2013) 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lazar E, Sadek HA, Bergmann O, Cardiomyocyte renewal in the human heart: insights from the fall-out, Eur. Heart J 38 (30) (2017) 2333–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Senyo SE, et al. , Mammalian heart renewal by pre-existing cardiomyocytes, Nature 493 (7432) (2013) p. 433–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stocum DL, Cameron JA, Looking proximally and distally: 100 years of limb regeneration and beyond, Dev. Dyn 240 (5) (2011) 943–968. [DOI] [PubMed] [Google Scholar]
- [7].Wan J, Goldman D, Retina regeneration in zebrafish, Curr. Opin. Genet. Dev 40 (2016) 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Oberpriller JO, Oberpriller JC, Response of the adult newt ventricle to injury, J. Exp. Zool 187 (2) (1974) 249–253. [DOI] [PubMed] [Google Scholar]
- [9].Poss KD, Wilson LG, Keating MT, Heart regeneration in zebrafish, Science 298 (5601) (2002) p. 2188–90. [DOI] [PubMed] [Google Scholar]
- [10].Porrello ER, et al. , Transient regenerative potential of the neonatal mouse heart, Science 331 (6020) (2011) p. 1078–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zogbi C, et al. , Early postnatal rat ventricle resection leads to long-term preserved cardiac function despite tissue hypoperfusion, Physiol. Rep 2 (8) (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hilman D, Gat U, The evolutionary history of YAP and the hippo/YAP pathway, Mol. Biol. Evol 28 (8) (2011) p. 2403–17. [DOI] [PubMed] [Google Scholar]
- [13].Huang J, et al. , The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP, Cell 122 (3) (2005) 421–434. [DOI] [PubMed] [Google Scholar]
- [14].Udan RS, et al. , Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway, Nat. Cell Biol 5 (10) (2003) 914–920. [DOI] [PubMed] [Google Scholar]
- [15].Wu S, et al. , Hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts, Cell 114 (4) (2003) 445–456. [DOI] [PubMed] [Google Scholar]
- [16].Boggiano JC, Vanderzalm PJ, Fehon RG, Tao-1 phosphorylates Hippo/MST kinases to regulate the Hippo-Salvador-Warts tumor suppressor pathway, Dev. Cell 21 (5) (2011) 888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ni L, et al. , Structural basis for autoactivation of human Mst2 kinase and its regulation by RASSF5, Structure 21 (10) (2013) p. 1757–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Poon CL, et al. , The sterile 20-like kinase Tao-1 controls tissue growth by regulating the Salvador-Warts-Hippo pathway, Dev. Cell 21 (5) (2011) 896–906. [DOI] [PubMed] [Google Scholar]
- [19].Liu CY, et al. , The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase, J. Biol. Chem 285 (48) (2010) p. 37159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhao B, et al. , A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP), Genes Dev 24 (1) (2010) 72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhao B, et al. , Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control, Genes Dev 21 (21) (2007) p. 2747–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kim MK, Jang JW, Bae SC, DNA binding partners of YAP/TAZ, BMB Rep 51 (3) (2018) 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu S, Martin JF, The regulation and function of the Hippo pathway in heart regeneration, Wiley Interdiscip. Rev. Dev. Biol 8 (1) (2019) p. e335. [DOI] [PubMed] [Google Scholar]
- [24].Wang J, et al. , The Hippo pathway in the heart: pivotal roles in development, disease, and regeneration, Nat. Rev. Cardiol 15 (11) (2018) 672–684. [DOI] [PubMed] [Google Scholar]
- [25].Camargo FD, et al. , YAP1 increases organ size and expands undifferentiated progenitor cells, Curr. Biol 17 (23) (2007) p. 2054–60. [DOI] [PubMed] [Google Scholar]
- [26].Dong J, et al. , Elucidation of a universal size-control mechanism in Drosophila and mammals, Cell 130 (6) (2007) p. 1120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhao B, Li L, Guan KL, Hippo signaling at a glance, J. Cell. Sci 123 (Pt 23) (2010) p. 4001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Meilhac SM, Buckingham ME, The deployment of cell lineages that form the mammalian heart, Nat. Rev. Cardiol 15 (11) (2018) 705–724. [DOI] [PubMed] [Google Scholar]
- [29].van Eif VWW, et al. , Transcriptional regulation of the cardiac conduction system, Nat. Rev. Cardiol 15 (10) (2018) 617–630. [DOI] [PubMed] [Google Scholar]
- [30].Artap S, et al. , Endocardial Hippo signaling regulates myocardial growth and cardiogenesis, Dev. Biol 440 (1) (2018) 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Singh A, et al. , Hippo signaling mediators yap and taz are required in the epicardium for coronary vasculature development, Cell Rep 15 (7) (2016) 1384–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xin M, et al. , Hippo pathway effector Yap promotes cardiac regeneration, Proc. Natl. Acad. Sci. U. S. A 110 (34) (2013) p. 13839–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Heallen T, et al. , Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size, Science 332 (6028) (2011) 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Xin M, et al. , Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size, Sci. Signal 4 (196) (2011) p. ra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].von Gise A, et al. , YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy, Proc. Natl. Acad. Sci. U. S. A 109 (7) (2012) p. 2394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bicknell KA, Coxon CH, Brooks G, Can the cardiomyocyte cell cycle be reprogrammed? J. Mol. Cell. Cardiol 42 (4) (2007) 706–721. [DOI] [PubMed] [Google Scholar]
- [37].Bergmann O, et al. , Evidence for cardiomyocyte renewal in humans, Science 324 (5923) (2009) 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lin Z, et al. , Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model, Circ. Res 115 (3) (2014) 354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Leach JP, et al. , Hippo pathway deficiency reverses systolic heart failure after infarction, Nature 550 (7675) (2017) 260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Monroe TO, et al. , YAP partially reprograms chromatin accessibility to directly induce adult cardiogenesis in vivo, Dev. Cell 48 (6) (2019) 765–779 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ramjee V, et al. , Epicardial YAP/TAZ orchestrate an immunosuppressive response following myocardial infarction, J. Clin. Invest 127 (3) (2017) 899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Flinn MA, et al. , Yap is required for scar formation but not myocyte proliferation during heart regeneration in zebrafish, Cardiovasc. Res 115 (3) (2019) 570–577. [DOI] [PubMed] [Google Scholar]
- [43].Yin F, et al. , Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2, Cell 154 (6) (2013) p. 1342–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fletcher GC, et al. , Mechanical strain regulates the Hippo pathway in Drosophila, Development 145 (5) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Su T, et al. , Kibra and Merlin activate the hippo pathway spatially distinct from and independent of expanded, Dev. Cell 40 (5) (2017) 478–490 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yu J, et al. , Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded, Dev. Cell 18 (2) (2010) 288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Fletcher GC, et al. , The Spectrin cytoskeleton regulates the Hippo signalling pathway, EMBO J 34 (7) (2015) 940–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Matsuda T, et al. , NF2 activates hippo signaling and promotes Ischemia/Reperfusion injury in the heart, Circ. Res 119 (5) (2016) 596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhang N, et al. , The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals, Dev. Cell 19 (1) (2010) 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chen SN, et al. , The hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy, Circ. Res 114 (3) (2014) 454–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Surace EI, Haipek CA, Gutmann DH, Effect of merlin phosphorylation on neurofibromatosis 2 (NF2) gene function, Oncogene 23 (2) (2004) p. 580–7. [DOI] [PubMed] [Google Scholar]
- [52].Angus L, et al. , Willin/FRMD6 expression activates the Hippo signaling pathway kinases in mammals and antagonizes oncogenic YAP, Oncogene 31 (2) (2012) 238–250. [DOI] [PubMed] [Google Scholar]
- [53].Badouel C, et al. , The FERM-domain protein expanded regulates Hippo pathway activity via direct interactions with the transcriptional activator Yorkie, Dev. Cell 16 (3) (2009) 411–420. [DOI] [PubMed] [Google Scholar]
- [54].Oh H, Reddy BV, Irvine KD, Phosphorylation-independent repression of Yorkie in Fat-Hippo signaling, Dev. Biol. (Basel) 335 (1) (2009) 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bossuyt W, et al. , An evolutionary shift in the regulation of the Hippo pathway between mice and flies, Oncogene 33 (10) (2014) p. 1218–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Chan SW, et al. , Hippo pathway-independent restriction of TAZ and YAP by angiomotin, J. Biol. Chem 286 (9) (2011) p. 7018–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Troyanovsky B, et al. , Angiomotin: an angiostatin binding protein that regulates endothelial cell migration and tube formation, J. Cell Biol 152 (6) (2001) p. 1247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mana-Capelli S, McCollum D, Angiomotins stimulate LATS kinase autophosphorylation and act as scaffolds that promote Hippo signaling, J. Biol. Chem 293 (47) (2018) 18230–18241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Chan SW, et al. , Actin-binding and cell proliferation activities of angiomotin family members are regulated by Hippo pathway-mediated phosphorylation, J. Biol. Chem 288 (52) (2013) p. 37296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hirate Y, et al. , Polarity-dependent distribution of angiomotin localizes Hippo signaling in preimplantation embryos, Curr. Biol 23 (13) (2013) p. 1181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Moleirinho S, et al. , Regulation of localization and function of the transcriptional co-activator YAP by angiomotin, Elife (2017) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ragni CV, et al. , Amotl1 mediates sequestration of the Hippo effector Yap1 downstream of Fat4 to restrict heart growth, Nat. Commun 8 (2017) p. 14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Van Itallie CM, et al. , The N and C termini of ZO-1 are surrounded by distinct proteins and functional protein networks, J. Biol. Chem 288 (19) (2013) p. 13775–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhou B, et al. , Claudin-18-mediated YAP activity regulates lung stem and progenitor cell homeostasis and tumorigenesis, J. Clin. Invest 128 (3) (2018) 970–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bassat E, et al. , The extracellular matrix protein agrin promotes heart regeneration in mice, Nature 547 (7662) (2017) 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lapidos KA, Kakkar R, McNally EM, The dystrophin glycoprotein complex: signaling strength and integrity for the sarcolemma, Circ. Res 94 (8) (2004) p. 1023–31. [DOI] [PubMed] [Google Scholar]
- [67].Morikawa Y, et al. , Dystrophin-glycoprotein complex sequesters Yap to inhibit cardiomyocyte proliferation, Nature 547 (7662) (2017) 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Torella A, et al. , One hundred twenty-one dystrophin point mutations detected from stored DNA samples by combinatorial denaturing high-performance liquid chromatography, J. Mol. Diagn 12 (1) (2010) 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Iyer SR, et al. , Differential YAP nuclear signaling in healthy and dystrophic skeletal muscle, Am. J. Physiol., Cell Physiol (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Morikawa Y, et al. , Actin cytoskeletal remodeling with protrusion formation is essential for heart regeneration in Hippo-deficient mice, Sci. Signal 8 (375) (2015) p. ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Chakraborty S, et al. , Agrin as a mechanotransduction signal regulating YAP through the Hippo Pathway, Cell Rep 18 (10) (2017) 2464–2479. [DOI] [PubMed] [Google Scholar]
- [72].Mohri Z, Del Rio Hernandez A, Krams R, The emerging role of YAP/TAZ in mechanotransduction, J. Thorac. Dis 9 (5) (2017) E507–E509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Dupont S, et al. , Role of YAP/TAZ in mechanotransduction, Nature 474 (7350) (2011) 179–183. [DOI] [PubMed] [Google Scholar]
- [74].Gumbiner BM, Kim NG, The Hippo-YAP signaling pathway and contact inhibition of growth, J. Cell. Sci 127 (Pt 4) (2014) 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Elosegui-Artola A, et al. , Force triggers YAP nuclear entry by regulating transport across nuclear pores, Cell 171 (6) (2017) 1397–1410 e14. [DOI] [PubMed] [Google Scholar]
- [76].Conacci-Sorrell M, Zhurinsky J, Ben-Ze’ev A, The cadherin-catenin adhesion system in signaling and cancer, J. Clin. Invest 109 (8) (2002) 987–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Yonemura S, et al. , Alpha-Catenin as a tension transducer that induces adherens junction development, Nat. Cell Biol 12 (6) (2010) 533–542. [DOI] [PubMed] [Google Scholar]
- [78].Vite A, et al. , Alpha-Catenin-dependent cytoskeletal tension controls Yap activity in the heart, Development 145 (5) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Geisler SB, et al. , Ordered assembly of the adhesive and electrochemical connections within newly formed intercalated disks in primary cultures of adult rat cardiomyocytes, J. Biomed. Biotechnol 2010 (2010) p. 624719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kostetskii I, et al. , Induced deletion of the N-cadherin gene in the heart leads to dissolution of the intercalated disc structure, Circ. Res 96 (3) (2005) 346–354. [DOI] [PubMed] [Google Scholar]
- [81].Swope D, et al. , Loss of cadherin-binding proteins beta-catenin and plakoglobin in the heart leads to gap junction remodeling and arrhythmogenesis, Mol. Cell. Biol 32 (6) (2012) p. 1056–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Li J, et al. , Loss of alphaT-catenin alters the hybrid adhering junctions in the heart and leads to dilated cardiomyopathy and ventricular arrhythmia following acute ischemia, J. Cell. Sci 125 (Pt 4) (2012) p. 1058–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Sheikh F, et al. , alpha-E-catenin inactivation disrupts the cardiomyocyte adherens junction, resulting in cardiomyopathy and susceptibility to wall rupture, Circulation 114 (10) (2006) p. 1046–55. [DOI] [PubMed] [Google Scholar]
- [84].Li J, et al. , Alpha-catenins control cardiomyocyte proliferation by regulating Yap activity, Circ. Res 116 (1) (2015) 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Schlegelmilch K, et al. , Yap1 acts downstream of alpha-catenin to control epidermal proliferation, Cell 144 (5) (2011) 782–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].del Monte G, et al. , Differential Notch signaling in the epicardium is required for cardiac inflow development and coronary vessel morphogenesis, Circ. Res 108 (7) (2011) 824–836. [DOI] [PubMed] [Google Scholar]
- [87].Lai JKH, et al. , The Hippo pathway effector Wwtr1 regulates cardiac wall maturation in zebrafish, Development 145 (10) (2018). [DOI] [PubMed] [Google Scholar]
- [88].Wang Y, et al. , Endocardial to myocardial notch-wnt-bmp axis regulates early heart valve development, PLoS One 8 (4) (2013) p. e60244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Manderfield LJ, et al. , Hippo signaling is required for Notch-dependent smooth muscle differentiation of neural crest, Development 142 (17) (2015) p. 2962–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Esteves de Lima J, et al. , Muscle contraction is required to maintain the pool of muscle progenitors via YAP and NOTCH during fetal myogenesis, Elife (2016) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Totaro A, et al. , YAP/TAZ link cell mechanics to Notch signalling to control epidermal stem cell fate, Nat. Commun 8 (2017) p. 15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Grego-Bessa J, et al. , Notch signaling is essential for ventricular chamber development, Dev. Cell 12 (3) (2007) 415–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Klaus A, et al. , Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis, Proc. Natl. Acad. Sci. U. S. A 104 (47) (2007) p. 18531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Varelas X, et al. , The hippo pathway regulates Wnt/beta-catenin signaling, Dev. Cell 18 (4) (2010) 579–591. [DOI] [PubMed] [Google Scholar]
- [95].Deng F, et al. , YAP triggers the Wnt/beta-catenin signalling pathway and promotes enterocyte self-renewal, regeneration and tumorigenesis after DSS-induced injury, Cell Death Dis 9 (2) (2018) 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Hochhauser E, et al. , P2Y2 receptor agonist with enhanced stability protects the heart from ischemic damage in vitro and in vivo, Purinergic Signal 9 (4) (2013) 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Khalafalla FG, et al. , P2Y2 nucleotide receptor prompts human cardiac progenitor cell activation by modulating hippo signaling, Circ. Res 121 (11) (2017) 1224–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Liu-Chittenden Y, et al. , Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP, Genes Dev 26 (12) (2012) p. 1300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Lu MF, et al. , Function of Rieger syndrome gene in left-right asymmetry and craniofacial development, Nature 401 (6750) (1999) p. 276–8. [DOI] [PubMed] [Google Scholar]
- [100].Tao G, et al. , Pitx2 promotes heart repair by activating the antioxidant response after cardiac injury, Nature 534 (7605) (2016) 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Xiao Y, et al. , Hippo signaling plays an essential role in cell state transitions during cardiac fibroblast development, Dev. Cell 45 (2) (2018) 153–169 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Yamamoto S, et al. , Activation of Mst1 causes dilated cardiomyopathy by stimulating apoptosis without compensatory ventricular myocyte hypertrophy, J. Clin. Invest 111 (10) (2003) p. 1463–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Del Re DP, et al. , Proapoptotic Rassf1A/Mst1 signaling in cardiac fibroblasts is protective against pressure overload in mice, J. Clin. Invest 120 (10) (2010) p. 3555–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Yu FX, et al. , Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling, Cell 150 (4) (2012) 780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kono K, Tamashiro DA, Alarcon VB, Inhibition of RHO-ROCK signaling enhances ICM and suppresses TE characteristics through activation of Hippo signaling in the mouse blastocyst, Dev. Biol 394 (1) (2014) 142–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Ibar C, et al. , Tension-dependent regulation of mammalian Hippo signaling through LIMD1, J. Cell. Sci 131 (5) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


