Abstract
OBJECTIVE.
The purpose of this study is to describe postoperative MRI findings after femoroacetabular impingement surgery in correlation with pain changes and surgical findings.
SUBJECTS AND METHODS.
We prospectively enrolled 42 patients (43 hips) who were scheduled for FAI surgery. Pre- and postoperative MR images were obtained using a 3-T MRI system. Changes in pain scores were assessed using the hip dysfunction and osteoarthritis outcome score. MR images were evaluated for the presence of acetabuloplasty or femoroplasty, presence of chondral and labral repair surgery, bone marrow edema, subchondral cysts, chondral defects, labral tears, capsular defects, and effusion. The optimal orientation to detect these changes was noted. Imaging findings were compared with pain score changes using linear regression analysis. Sensitivity and specificity were assessed using surgical correlation as the reference standard.
RESULTS.
Increased acetabular bony débridement length was associated with decreased improvement in pain scores (coefficient, −2.07; 95% CI, −3.53 to −0.62; p = 0.008), whereas other imaging findings were not significantly different. Femoroplasty and capsular alterations were best detected on oblique axial sequences; acetabuloplasty and cartilage and labral repair were best seen on sagittal sequences. MRI showed excellent sensitivity (100%) and specificity (100%) for detecting labral repair and excellent sensitivity for detecting femoroplasty (98%). Sensitivity and specificity were lower for detecting acetabuloplasty (83% and 80%, respectively) and chondral repair (75% and 54%, respectively).
CONCLUSION.
Arthroscopic acetabuloplasty showed a greater association with postoperative pain than did other aspects of surgical correction for femoroacetabular impingement. Femoroplasty and labral repair were reliably diagnosed on 3-T MRI; however, limitations were found in the evaluation of acetabular chondral repair.
Keywords: arthroscopy, femoroacetabular impingement, MRI
Femoroacetabular impingement (FAI) is a common source of hip pain and is frequently associated with impaired hip joint biomechanics, acetabular chondral delamination, cartilage defects, and labral tearing [1–10]. Arthroscopy of the hip is increasingly performed for FAI treatment, with the rate of surgery increasing from 3.6 procedures per 100,000 population in 2005 to 16.7 procedures per 100,000 population in 2013 [11]. The aim of arthroscopic surgery for FAI is to reduce the abnormal contact between the acetabulum and the femur to prevent further damage to the hip joint, alleviate pain, and potentially delay or obviate prosthetic hip replacement in later disease stages [12–15]. Moreover, it has previously been shown that hip arthroscopy is associated with a shorter hospital stay and improved short-term outcome in comparison with surgical hip dislocation [16]. During this surgery, the osseous prominence along the femoral head-neck junction is débrided (in femoroplasty) or the overcoverage of the acetabulum is debrided (acetabuloplasty), and labral tears are repaired, sometimes debriding the unstable components of a torn labrum [17]. Chondral lesions can be treated by adhesive reattachment of the cartilaginous surface, microfracture (in cases of exposed subchondral bone), or débridement of chondral flaps [10, 18].
For nonsurgical patients, the use of nonarthrographic hip MRI has been shown to reliably identify abnormalities with respect to cartilage and labral abnormalities [19]; however, postoperative hip MRI findings in patients who have undergone FAI surgery have not been well studied. To date, the available literature on this topic consists mainly of reviews and case reports. To our knowledge, only two original studies have been conducted for postperative MR arthrographic findings after FAI surgery: one focused primarily on the labrum and recurrent tears [20], and the other focused on imaging abnormalities seen on 1.5-T MR arthrography after arthroscopic hip surgery but without surgical correlation [21].
Given the limitations of previous research, the present study attempts to provide a global assessment of postoperative findings using nonarthrographic 3-T MRI in correlation with changes in pain and surgical findings. The aim of our study was to assess the postoperative MRI appearance after FAI surgery of the hip, including a detailed assessment of the acetabular and femoral bone, the cartilage, the labrum, and additional findings such as joint effusion and capsular alterations. Moreover, we wanted to evaluate the association of morphologic findings and pre- and postoperative changes in the pain score. Because accurately detecting and documenting postoperative changes after FAI surgery is challenging, our goal was to assess the sensitivity and specificity of detecting postoperative changes on MR images, additionally noting the optimal sequence orientation for detecting these changes.
Subjects and Methods
Our study was approved by the committee on human research at the University of California, San Francisco. All patients were given written information about the study and provided their written consent.
Study Subjects
We prospectively enrolled patients scheduled for FAI surgery from the hip preservation clinic at our institution. All patients were 18–50 years old and had a body mass index (BMI; weight in kilograms dividing by the square of height in meters) of less than 35. All patients had hip pain that was functionally limiting on a daily basis and was refractory to nonoperative measures such as physical therapy, corticosteroid injections, or both treatments. Pre- and postoperative MR images of the surgical hip were obtained for all patients. We excluded patients with previous hip surgery, radiographic hip osteoarthritis (Kellgren-Lawrence radiographic score, ≥ 2), dysplastic hip joints, or contraindications for MRI. Patients with metal implants close to the hip joint that could potentially compromise MR image quality were also excluded.
Pain assessment was done at the time of the preoperative MRI examination and again at the time of the postoperative MRI examination, with use of the hip dysfunction and osteoarthritis outcome pain score (HOOS) [22], with a score of 100 indicating no symptoms and 0 indicating extreme symptoms. The change in pain scores was calculated by subtracting the preoperative HOOS pain score from the postoperative HOOS pain score.
Arthroscopic Techniques
For FAI surgery, patients were positioned, and a two-portal technique was used for dynamic arthroscopic visualization of the hip using fluoroscopic guidance. After careful evaluation, acetabular rim trimming and osteochondroplasty of the cam lesion were performed if judged to be appropriate, with the hip flexed and rotated to confirm adequate resection using fluoroscopy to ensure no residual impingement. If judged to be appropriate, repair of labral tears was performed with suture anchors (Nanotack, Stryker) used to tie down the labrum to the acetabulum and with unstable components of the labrum débrided, if necessary. In case of treatment requiring chondral lesions, loose flaps were removed, and in some cases microfracture of the exposed subchondral bone was performed. All loose bone debris ultimately was removed via suction, and the wounds were copiously irrigated and closed.
MRI Protocol
All patients underwent a nonarthrographic MRI examination performed using a 3-T system (Discovery MR750, GE Healthcare) with a 16-channel large flex array coil placed over the hip region. Patients were scanned within 3 months before FAI surgery and within 6 months to 1 year after FAI surgery. The imaging protocol included triplanar 2D fat-suppressed, intermediate-weighted fast spin-echo sequences (slice thickness, 4.0 mm) in the coronal, sagittal, and oblique axial planes (FOV, 18.0 × 18.0 cm; TR/TE, 2400–3700/60) and a 3D FOV-optimized and constrained undistorted single-shot (FOCUS) fat-suppressed fast spin-echo sequence (TR/TE, 1200/20; FOV, 15.3 × 15.3 cm; slice thickness, 0.8 mm) reformatted in coronal, sagittal, and oblique axial planes with a slice thickness of 4.0 mm.
Image Analysis
Under the supervision of a musculoskeletal radiologist with 25 years of experience, a radiologist with 4 years of experience in musculoskeletal imaging evaluated all MR images by comparing preoperative and postoperative MR images in side-by-side readings. Radiologists were unblinded to the timely sequence of the images, as is standard in clinical image evaluations, but were blinded to operative reports and clinical information.
The following preoperative FAI types were documented for all hips: primarily cam (α angle > 55°, measured as previously described by Nötzli et al. [23]), primarily pincer (lateral center edge angle ≥ 40°, measured as previously described by Wiberg [24]), or mixed FAI type.
Moreover, the following categories were evaluated: the femur and acetabulum were assessed for the presence or absence of imaging signs of femoroplasty or acetabuloplasty, respectively, and the maximum length and depth of the resection were measured to compare pre- and postoperative MR images. All measurements were obtained from the maximum diameter. The pre- and postoperative alpha angles were measured on oblique axial slices, and the difference in the angle measurements was calculated. Moreover, we assessed the presence or absence of the bone marrow edema pattern and subchondral cysts of the femur and the acetabulum, respectively.
Cartilage was analyzed for signs of chondral treatment, and abnormalities were documented, including cartilage débridement, change in morphologic features and signal, irregularity of the cartilage surface, thinning of the cartilage (which can be seen in the setting of microfracture), and susceptibility artifacts related to surgery. In addition, we graded cartilage lesions according to the scoring hip osteoarthritis with MRI (SHOMRI) score [25], using a 3 -point scale to grade six regions of the femur and four regions of the acetabulum, respectively, with a score of 0 denoting that no lesion was present; 1, that partial thickness defects were present; and 2, that full-thickness defects were present. A cartilage sum score of all regions was calculated for the femur and the acetabulum.
Imaging signs for labral surgery, which were defined as imaging signs for labral débridement, change in morphologic findings, labral repair, or a combination of these findings, were graded as present or absent. The presence of imaging signs for labral débridement and the presence of imaging signs for suture anchors were assessed by comparing pre- and postoperative MR images. We also described the presence of signal abnormalities suggestive of new tears, although differentiation between repaired tears, residual tears, and retears is challenging. According to a previous study, however, recurrent labral tears are frequently found in patients with symptoms (95% of such patients) [20].
Presence or absence of joint effusion, defined as fluid signal at the femoral neck region greater 0.7 cm in thickness, was assessed [25]. Presence or absence of capsular defects was noted, including partial- or full-thickness tears along the anterior aspect of the joint capsule. Moreover, presence of capsular adhesion at the anterior femoral neck and obliteration of the paralabral sulcus were assessed as previously described by Kim et al. [21].
In addition, the optimal orientation to detect each of the following features was noted: femoroplasty, acetabuloplasty, labral surgery, acetabular chondral treatment, and capsular alterations.
Statistical Analysis
Statistical analysis was performed with the SPSS statistical package (version 23, IBM), using a two-sided level of significance of p < 0.05. Descriptive statistics were used to assess the prevalence of imaging findings. Linear regression analysis was used to evaluate the following associations: the association of preoperative FAI type (primarily cam, primarily pincer, or mixed) and pre- and postoperative HOOS pain score changes; the association of MRI findings and changes in HOOS pain scores; and the association of type of chondral repair surgery performed and changes in HOOS pain scores. True-positive, false-positive, true-negative, and false-negative test results were calculated using two-by-two tables. The sensitivity, specificity, positive predictive value, and negative predictive value for presence or absence of femoroplasty, presence or absence of acetabuloplasty, presence or absence of labral surgery (including either signs for labral débridement, labral repair, or both), and presence or absence of chondral treatment were calculated. The intraarthroscopic evaluation as stated in the operative report and as discussed with our hip surgeon served as our reference standard.
Results
Study Subjects
A total of 42 patients and 43 hips that underwent FAI surgery were included. One patient first had FAI surgery performed on the left hip and then had FAI surgery performed on the right hip half a year later. The mean (± SD) patient age was 35 ± 8.9 years (range, 19–56 years), and the mean BMI was 24 ± 2.9. More men (n = 27) than women (n = 15) were included. With regard to FAI type, 15 of 43 hips (35%) were primarily cam, four (9%) were primarily pincer, and 24 (56%) were mixed. The mean change in the HOOS pain score was 25.9 ± 17.2 (range, −12.5 to 60). Thirty-eight patients had improved postoperative HOOS pain scores, compared with preoperative HOOS pain scores, with the scores of 27 patients improving by more than 20 points. Only four patients had worse postoperative HOOS pain scores (negative change in pain scores). One patient did not complete the HOOS questionnaire. In terms of the arthroscopic techniques used, all hips included in the study underwent femoroplasty, and 42% (18/43) underwent acetabuloplasty. Nineteen percent of hips (8/43) received chondral treatment, with loose cartilage flaps removed in seven of eight hips and microfracture of the exposed subchondral bone performed for two of eight hips. Labral repair surgery was performed for 98% of hips (42/43). The characteristics of the subjects are summarized in Table 1.
TABLE 1:
Demographic and Clinical Characteristics of 42 Subjects With 43 Hips That Underwent Femoroacetabular Impingement (FAI) Surgery
| Characteristic | Value |
|---|---|
| Age (y), mean ± SD | 35 ± 8.9 |
| Sex, no. (%) of patients | |
| Female | 15 (36) |
| Male | 27 (64) |
| Body mass indexa | 24 ± 2.9 |
| Race, no. (%) of patients | |
| White | 38 (90) |
| African American | 0 (0) |
| Asian | 4 (10) |
| Other | 0 (0) |
| FAI type, no. (%) of hips | |
| Primarily cam | 15 (35) |
| Primarily pincer | 4 (9) |
| Mixed | 24 (56) |
| Arthroscopic technique, no. (%) of hips | |
| Femoroplasty | 43 (100) |
| Acetabuloplasty | 18 (42) |
| Chondral treatment | 8 (19) |
| Débridement of loose flaps | 7 (16) |
| Microfracture | 2 (5) |
| Labral repair surgery | 42 (98) |
Weight in kilograms divided by the square of height in meters.
Postoperative MRI Findings
Patients were scanned within 3 months before undergoing FAI surgery and between 6 months to 1 year after undergoing FAI surgery. On postoperative MR images, 98% of hips (42/43) were evaluated and found to have a morphologic imaging appearance consistent with femoroplasty, compared with preoperative images, as shown in Figure 1. The mean measured resection length and depth were 16.0 ± 3.3 mm and 3.2 ± 1.3 mm, respectively. The mean change in pre- and postoperative alpha angles was 5.6° ± 6.4°. The change in alpha angle measurements was less than 5° in 20 hips, 10° or less in 13 hips, and greater than 10° in 10 hips. In 19% of hips (8/43), there was a mild bone marrow edema pattern associated with the area of bony débridement, and associated subchondral cysts (< 0.5 cm) were seen in 5% (2/43).
Fig. 1—
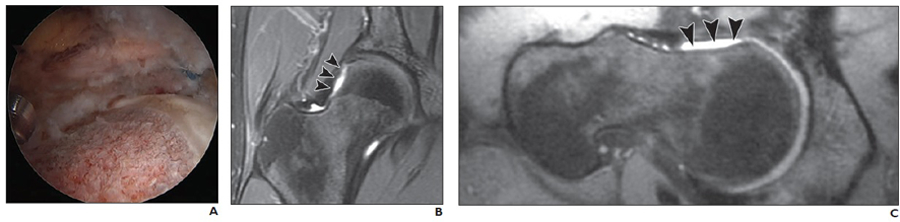
31-year-old man who underwent femoral osteochondroplasty of right hip.
A, Intraarthroscopic image shows femoral osteochondroplasty in process.
B and C, Postoperative coronal fast spin-echo (TR/TE, 2400–3700/60) (B) and oblique axial fast spin-echo (TR/TE, 2400–3700/60) (C) MR images show postoperative MR appearance of femur after bony débridement of cam-type lesion. Slightly concave-shaped area of anterolateral femur head–neck junction (arrowheads) is seen on coronal (B) and oblique axial (C) MR images.
Forty-seven percent of hips (20/43) were evaluated as having a morphologic imaging appearance consistent with acetabuloplasty, as shown in Figure 2. The mean measured resection length and depth were 9.7 ± 5.2 mm and 5.9 ± 2.6 mm, respectively. In 40% of hips (17/43), there was a mild bone marrow edema pattern associated with the area of bony débridement, and associated subchondral cysts were observed in 9% (4/43).
Fig. 2—
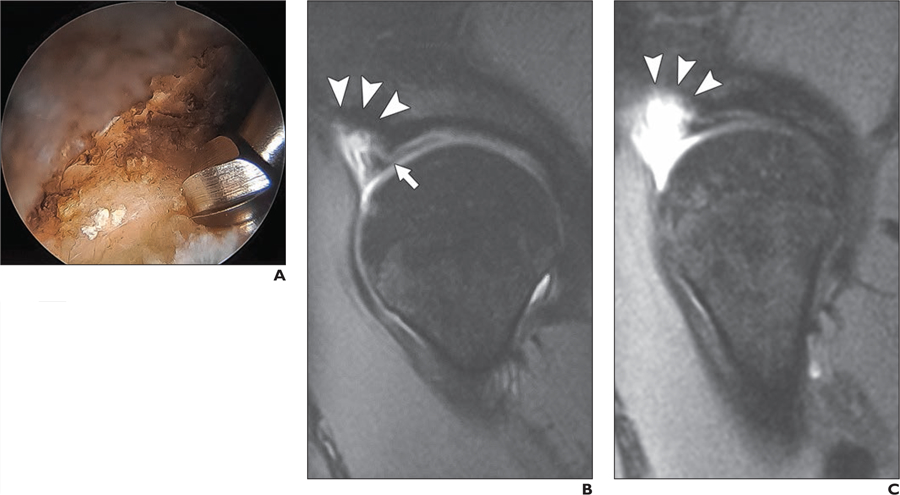
34-year-old woman who underwent acetabular rim resection.
A, Intraarthroscopic image shows acetabular rim resection in process.
B and C, Postoperative sagittal reconstructed 3D FOV-optimized and constrained undistorted single-shot (FOCUS) MR images (TR/TE, 1200/20) of two consecutive slices depict area of acetabular rim resection (arrowheads). Labrum was surgically repaired; however, labrum has morphologic appearance consistent with simple tear (white arrow, B).
Cartilage was evaluated and was found to have changes consistent with chondral treatment, including débridement or microfracture, in 51% of hips (22/43) on postoperative MR images. The postoperative SHOMRI cartilage sum score of the acetabulum was 1.9 ± 1.4, and that of the femur was 2.5 ± 2.1.
In 98% of hips (42/43), the labrum was graded as having changes consistent with surgery compared with preoperative MR images. In 77% of hips (33/43), the labrum was evaluated to be débrided, and in 77% of hips (33/43), imaging signs of suture anchors were detected, as shown in Figures 3 and 4. Eighty-four percent of all postoperative hips (36/43) were graded as having a morphologic appearance consistent with a labral tear.
Fig. 3—
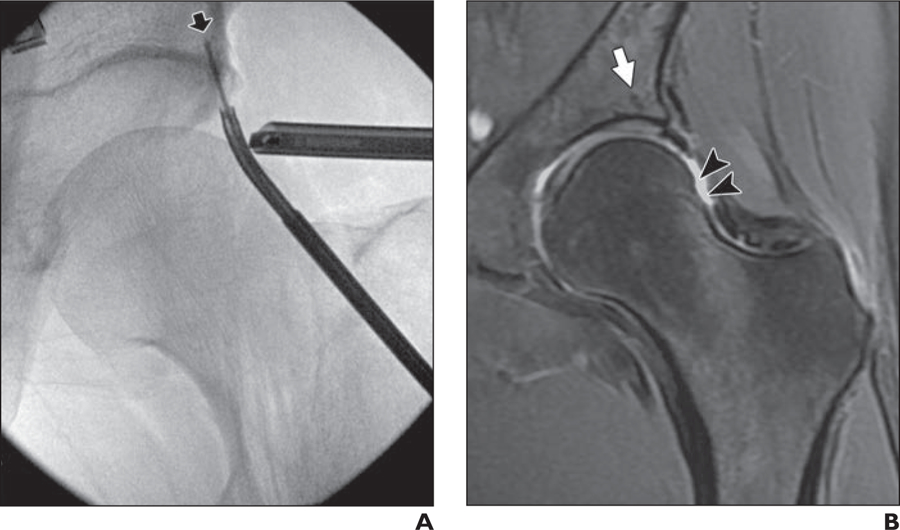
34-year-old woman who underwent femoral osteochondroplasty of left hip.
A, Intraarthroscopic radiograph of left hip obtained using fluoroscopic guidance shows placement of suture anchor in subchondral acetabular bone (arrow).
B, Postoperative coronal fast spin-echo MR image (TR/TE, 2400–3700/60) shows hypointense thin line in same area, which is typical finding after labral repair with suture anchors (arrow). Slightly concave shape of femoral head-neck junction is seen on postoperative MR images (arrowheads), which is typical of femoral osteochondroplasty.
Fig. 4—
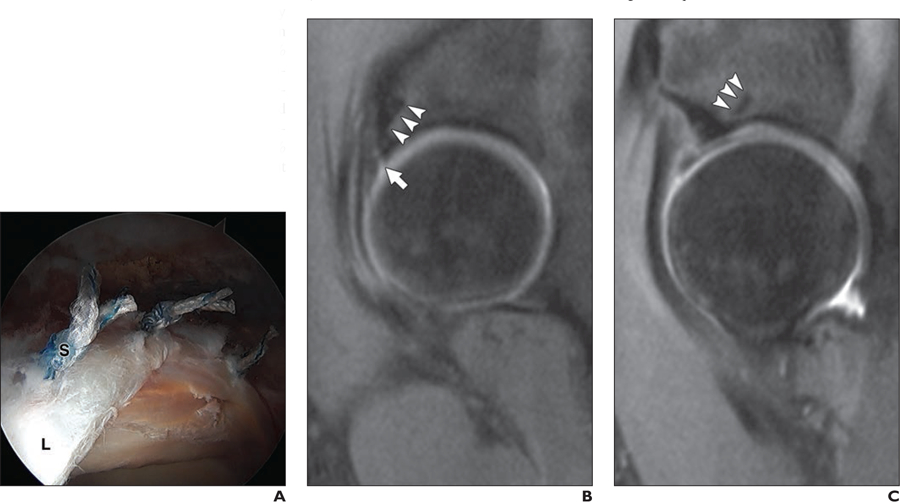
35-year-old woman who underwent surgery for femoroacetabular impingement of right hip.
A, Intraarthroscoic image of hip shows appearance of three suture anchors (S) used to tie down labrum (L) to acetabulum.
B and C, Postoperative sagittal reconstructed 3D FOV-optimized and constrained undistorted single-shot (FOCUS) MR images (TR/TE, 1200/20) of two subsequently obtained slices show postoperative appearance of suture anchors, which appear as thin hypointense lines in subchondral acetabular bone (arrowheads). Note presence of hyperintense signal abnormalities in labrum, suggestive of repaired tear, residual tear, or retear (arrow, B).
Joint effusion was present in 47% of hips (20/43). Capsular defects were present in 61% (26/43). All patients with capsular defects had postoperative T2-weighted hyperintense signal alterations along the anterior aspect of the joint capsule; however, none of the patients included in the present study had larger capsular defects. No capsular adhesions at the anterior femoral neck or obliterations of the paralabral sulcus were detected.
Association of Morphologic Findings and Pain Score Changes
We evaluated the association of preoperative FAI type and pre- and postoperative HOOS pain score changes with a negative value for the change in HOOS pain scores indicating pain worsening, low values for the change in HOOS pain scores indicating minimal improvement, and high values for the change in HOOS pain scores indicating significant improvement in pain. Hips with a preoperative cam or mixed FAI had improved postoperative pain scores; however, results were nonsignificant (for cam type: coefficient, 0.17; 95% CI, −11.19 to 11.53 [p = 0.976]; (for mixed type: coefficient: 4.72; 95% CI, −6.17 to 15.62 [ p = 0.386]), whereas those with preoperative pincer FAI had decreased improvement in pain scores (coefficient, −9.53; 95% CI, −26.06 to 7.00; p = 0.251); however, associations were also nonsignificant.
Table 2 shows the associations of MRI findings and HOOS pain score changes. Although most imaging findings were relatively evenly distributed across hips with different values for the change in pain scores, increased acetabular bony débridement was associated with decreased improvement in pain scores compared with less acetabular bony débridement. Increased length of acetabular bony débridement was significantly associated with decreased improvement in HOOS pain scores (coefficient, −2.07; 95% CI, −3.53 to −0.62; p = 0.008). Moreover, increased depth of acetabular bony débridement was also associated with decreased improvement in HOOS pain scores, albeit not significantly (coefficient, −2.53; 95% CI, −6.1 to 1.05; p = 0.154). A nonsignificant trend was also noted for lower values representing the change in HOOS pain scores in patients with postoperative capsular defects (coefficient, −7.14; 95% CI, −18.1 to 3.83; p = 0.196). No significant associations were found for different degrees of preoperative cartilage damage of the acetabulum or the femur and changes in HOOS pain scores.
TABLE 2:
Association of MRI Findings and Changes in Hip Dysfunction and Osteoarthritis Outcome (HOOS) Pain Scores
| MRI Findings | Coefficient | 95% CI | pa |
|---|---|---|---|
| For acetabulum | |||
| Bony resection length (mm) | −2.07 | −3.53 to −0.62 | 0.008b |
| Bony resection depth (mm) | −2.53 | −6.11 to 1.05 | 0.154 |
| Presence of new BMEP | −3.98 | −15.2 to 7.31 | 0.480 |
| Presence of new subchondral cyst | 22.13 | −12.9 to 57.1 | 0.290 |
| Preoperative cartilage sum score | −1.06 | −5.19 to 3.07 | 0.607 |
| Change in sum scores for cartilage lesions | 1.44 | −6.89 to 9.76 | 0.729 |
| For femur | |||
| Bony resection length (mm) | −0.41 | −2.12 to 1.31 | 0.635 |
| Bony resection depth (mm) | −0.88 | −5.02 to 3.26 | 0.668 |
| Presence of new BMEP | −4.44 | −19.9 to 11.0 | 0.565 |
| Presence of new subchondral cyst | −11.4 | −36.7 to 13.9 | 0.366 |
| Preoperative cartilage sum score | 0.32 | −2.56 to 3.21 | 0.822 |
| Change in sum scores for cartilage lesions | 1.76 | −3.09 to 6.60 | 0.468 |
| Presence of new labral tear morphology | 5.63 | −19.9 to 31.1 | 0.658 |
| Presence of effusion | 3.80 | −7.50 to 15.1 | 0.501 |
| Presence of capsular defects | −7.14 | −18.1 to 3.83 | 0.196 |
Note—Negative values for the change in HOOS pain scores indicate pain worsening, low values for the change in HOOS pain scores indicate minimal improvement, and high values for the change in HOOS pain scores indicate significant improvement. BMEP = bone marrow edema pattern.
By linear regression analysis.
Statistically significant.
Association of Chondral Repair Surgery and Pain Score Changes
No significant associations were found for changes in HOOS pain scores and type of chondral treatment performed: hips with débridement of unstable cartilage flaps had decreased improvement in HOOS pain scores compared with hips without cartilage débridement (coefficient, −5.79; 95% CI, −20.27 to 8.70; p = 0.424), whereas hips with cartilage microfracture of the exposed subchondral bone had improved HOOS pain scores (coefficient, 16.13; 95% CI, −8.91 to 41.16; p = 0.200).
Optimal Sequence Orientation to Detect Postoperative Changes
The orientation evaluated as optimal for detecting specific postoperative changes is shown in Table 3. Femoroplasty and capsular alterations were best detected on oblique axial sequences, whereas acetabuloplasty and cartilage and labral repair were best seen on sagittal sequences.
TABLE 3:
Optimal MRI Sequence Orientation to Detect Postoperative Changes in Patients
| Postoperative Change | Coronal Sequence | Sagittal Sequence | Oblique Axial Sequence |
|---|---|---|---|
| Femur débridement (n = 42) | 3 (7) | 15 (36) | 24 (57) |
| Acetabular débridement (n = 20) | 4 (20) | 16 (80) | 0 (0) |
| Acetabular chondral repair (n = 22) | 3 (14) | 19 (86) | 0 (0) |
| Labral repair surgery (n = 42) | 11 (26) | 30 (71) | 1 (2) |
| Capsular alterations (n = 26) | 1 (4) | 0 (0) | 25 (96) |
Note—Data are number (%) of patients for whom postoperative change were noted.
Detection of Postoperative MRI Findings
Table 4 summarizes the sensitivity, specificity, positive predictive value, and negative predictive value of MRI detection of postoperative abnormalities in correlation with surgical findings. According to the surgical standard of reference, all hips underwent femoroplasty, and 42% (18/43) underwent acetabuloplasty. Chondral treatment was performed for 19% (8/43) of hips, and labral repair surgery was performed for 98% (42/43).
TABLE 4:
MRI Detection of Postoperative Abnormalities by Surgical Reference Standard
| Type of Surgery (n = 43) | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value |
|---|---|---|---|---|
| Femur débridement | 98 (42/43) | NA | 100 (42/42) | NA |
| Acetabular débridement | 83 (15/18) | 75 (15/20) | 75 (15/20) | 87 (20/23) |
| Chondral treatment | 75 (6/8) | 54 (19/35) | 27 (6/22) | 90 (19/21) |
| Labral surgery | 100 (42/42) | 100 (1/1) | 100 (42/42) | 100 (1/1) |
Note—Data are percentage of abnormalities detected (number of abnormalities detected/total number of hips evaluated). NA = not applicable.
We had excellent sensitivity (100%) and specificity (100%) for detecting labral repair surgery and excellent sensitivity for detecting femoroplasty (98%). Because all hips underwent femoroplasty, specificity could not be assessed for this feature. The sensitivity and specificity were lower for detecting acetabuloplasty (sensitivity, 83%; specificity, 80%) and lower for detecting chondral treatment (sensitivity, 75%; specificity, 54%).
Discussion
In the present study, we assessed the postoperative nonarthrographic 3-T MRI appearance of the hip in patients who underwent FAI surgery. Frequent postoperative MRI findings after FAI surgery included joint effusion, capsular alterations, and apparent labral tear. Patients who required greater bony débridement of the acetabulum had significantly lower improvement in HOOS pain scores, and patients with preoperative pincer FAI had decreased improvement in pain scores, however nonsignificantly. Moreover, a trend was noted for lower values for the change in HOOS pain scores in patients with postoperative capsular defects. Using surgical correlation with postoperative MRI findings, we had excellent sensitivity and specificity for detecting labral surgery and femoroplasty, whereas detecting acetabuloplasty was slightly more challenging but still yielded good sensitivity and specificity. Although the sensitivity for detecting chondral treatment was good, the specificity was comparatively low.
We frequently observed joint effusion and capsular alterations after FAI surgery. Both features were present in approximately every second individual, and patients with capsular alterations had a trend toward decreased improvement in pain scores. With increasing numbers of arthroscopic surgeries being performed, microinstability and postoperative pain caused by capsulotomy and capsulectomy are reported more frequently [26–28]. Symptomatic hip instability is assumed to be caused by underlying bony or soft-tissue abnormalities, trauma or repetitive microtrauma to the capsuloligamentous structures, and iatrogenic capsule injuries [29–31]. Although iatrogenic hip instability is not very common, poor capsular management may predispose patients to pain and joint unsteadiness [31].
To date, only limited original studies are available on postoperative MRI findings after FAI surgery. Kim et al. [21] evaluated the postoperative appearance of the hip after FAI surgery on MR arthrograms of patients with and without symptoms without surgical correlation. They found that the rates of capsular alterations were similar to those in our study cohort but did not evaluate joint effusion [21]. Of interest, the imaging findings most frequently seen in their study were capsular adhesions at the anterior femoral neck and obliteration of the paralabral sulcus [21]. Using nonarthrographic MRI, we did not detect either of these features. Although nonarthrographic 3-T MRI of the hip is highly accurate for detecting bone marrow edema, subchondral cysts, chondrolabral abnormalities, and bone shape [7, 19], capsular adhesions or obliterations of the paralabral sulcus may be visible only if the thick capsular structure of the hip is expanded with intraarticular contrast. Contrasting findings have been published regarding intraarticular adhesions and postoperative pain. Although Kim et al. [21] found no significant differences between patients with and without symptoms and presence of intraarticular adhesions, other studies identified intraarticular adhesions to be a frequent cause of persistent postoperative groin pain [32–34].
Blankenbaker et al. [20] studied MR arthrograms of 20 subjects with suspected recurrent acetabular labral tears after previous arthroscopic labral repair surgery who were scheduled for arthroscopic reevaluation. In the subsequent arthroscopic reevaluation, 95% of these subjects (19/20) had a recurrent labral tear diagnosed, 74% (14/19) of which had been correctly diagnosed on the MR arthrography [20]. Because all included subjects presented with new hip pain after the first labral repair surgery and were highly suspicious for recurrent labral tears, the rate of recurrent labral tears was likely higher than the average postoperative rate of recurrent labral tears [20]. Because none of our patients were scheduled for arthroscopic reevaluation, it remains unclear whether 84% of all hips (36/43) graded as having the morphologic appearance of a labral tear had a real recurrent labral tear or whether this could be the typical postoperative appearance of a repaired labrum, therefore presenting a pitfall about which all radiologists should be aware.
Adequate morphologic bony correction of pincer and cam deformities are technically demanding [16], and patients who required increased bony débridement of the acetabulum had significantly lower values for the change in HOOS pain scores in our study. Moreover, patients with preoperative pincer FAI had decreased improvement in pain scores; however, results were nonsignificant. The amount of femoral bony débridement was not significantly associated with pain score changes. Therefore the outcome for patients requiring arthroscopic acetabuloplasty is likely worse than that for patients requiring other aspects of surgical correction for FAI.
The alpha angle is commonly used to quantify the degree of femoral (cam-type) deformity, and the hips of subjects with impingement typically have significantly less concavity at the femoral head-neck junction than do hips without impingement [23]. However, previous studies have described a substantial overlap in alpha angle measurements between volunteers without symptoms and patients with cam-type deformities [35]. In our study, all subjects underwent femoroplasty, but in 47% the change in the pre- and postoperative alpha angle measurements was less than 5°, a change likely coinciding within the range of measurement error. Because all cases had femoral osteochondroplasty in the anterolateral area of the femoral head-neck junction but the alpha angle is measured in the center of the femoral head on oblique axial sequences [23], the alpha angle may likely not capture the full extent of bony débridement. We therefore conclude that measuring the length and depth of femoroplasty on oblique axial images showing the maximum extent of bony débridement may be a more accurate method to determine the amount of bony resection.
Although good communication between orthopedic surgeons and radiologists is essential in clinical practice, it is also highly useful for the radiologist to be able to accurately detect and document postoperative changes. Therefore, we assessed the sensitivity and specificity of detecting operative changes on MR images after FAI surgery, and we also noted the optimal sequence orientation to detect these changes. Our results show that we were able to reliably detect femoroplasty and labral repair surgery on postoperative hip MR images. Our sensitivity and specificity for detecting postoperative changes of the acetabulum were marginally lower, possibly because of partial volume effects related to the marginal location of acetabular débridement and cystic changes of the acetabulum creating the impression of bony débridement. Although our sensitivity for detecting acetabular chondral treatment was good (75%), our specificity was only 54%. Because of the small intraarticular volume, changes in the acetabular cartilage are relatively challenging to assess [19]. Therefore, we conclude that we are not able to detect chondral treatment on postoperative MR images with sufficient diagnostic confidence. However, it should be noted that the sensitivity for detecting femoroplasty may have been higher because of a certain extent of reader bias: because we did not include in our study patients who had not undergone arthroscopic surgery, readers may have assumed that all or almost all patients had undergone femoroplasty at a minimum.
Some limitations are pertinent to this study. First, because we evaluated nonarthrographic hip MR images in this study, the lack of intraarticular contrast is likely the reason why we were not able to detect postoperative features such as obliteration of the paralabral sulcus. However, postoperative follow-up hip MRI examinations performed without intraarticular contrast are frequently conducted as part of standard clinical care because of lower procedural risks, including infection or allergic reaction to contrast medium. Second, because pain assessment was determined on the basis of responses to a questionnaire, we cannot exclude a certain extent of response bias. Third, to determine whether abnormal postoperative MRI findings, such as labral tears, are real recurrent tears or whether they could reflect the typical postoperative appearance after labral repair surgery, a second arthroscopic evaluation would have been necessary. However, because arthroscopic reevaluation after previous FAI surgery is not normally conducted in patients without symptoms, these issues are challenging to investigate. Moreover, because patients underwent scanning within 6 months to 1 year after FAI surgery, this time frame could also be long enough for a recurrent tear to occur after labral repair surgery. Fourth, we included only 43 hips in the present study, and larger studies would be of interest to confirm these findings.
Conclusion
In conclusion, our study shows postoperative MRI findings and associated pain changes in patients undergoing FAI surgery and the diagnostic performance in visualizing these findings. Common MRI findings after FAI surgery included joint effusion and capsular alterations. Arthroscopic acetabuloplasty showed a greater association with postoperative pain than other aspects of surgical correction for femoroacetabular impingement. Postoperative labral abnormalities morphologically consistent with a labral tear may be the typical postoperative appearance of a repaired labrum and therefore could present a pitfall about which radiologists should be aware. The alpha angle may likely not capture the full extent of femoral osteochondroplasty. Femoroplasty, acetabuloplasty, and labral repair surgery were reliably diagnosed, but nonarthrographic 3-T MRI had limitations in evaluating chondral treatment.
Acknowledgments
We thank the study participants, and we also thank the staff involved in this study for their invaluable assistance with patient selection, statistical analysis, and technical support.
Supported by grants R01 AR069006, P0512535, P0058313, and P0505900 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and by Young Investigator Grant YIG-2016–1 from the American Orthopedic Society for Sports Medicine.
References
- 1.Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res 2003; (417):112–120 [DOI] [PubMed] [Google Scholar]
- 2.Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The etiology of osteoarthritis of the hip: an integrated mechanical concept. Clin Orthop Relat Res 2008; 466:264–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casartelli NC, Maffiuletti NA, Item-Glatthorn JF, et al. Hip muscle weakness in patients with symptomatic femoroacetabular impingement. Osteoarthritis Cartilage 2011; 19:816–821 [DOI] [PubMed] [Google Scholar]
- 4.Kennedy MJ, Lamontagne M, Beaulé PE. Femoroacetabular impingement alters hip and pelvic biomechanics during gait walking biomechanics of FAI. Gait Posture 2009; 30:41–44 [DOI] [PubMed] [Google Scholar]
- 5.Lamontagne M, Kennedy MJ, Beaulé PE. The effect of cam FAI on hip and pelvic motion during maximum squat. Clin Orthop Relat Res 2009; 467:645–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jannelli E, Parafioriti A, Acerbi A, Ivone A, Fioruzzi A, Fontana A. Acetabular delamination: epidemiology, histological features, and treatment. Cartilage 2019; 10:314–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linda DD, Naraghi A, Murnaghan L, Whelan D, White LM. Accuracy of non-arthrographic 3T MR imaging in evaluation of intra-articular pathology of the hip in femoroacetabular impingement. Skeletal Radiol 2017; 46:299–308 [DOI] [PubMed] [Google Scholar]
- 8.Pfirrmann CW, Duc SR, Zanetti M, Dora C, Hodler J. MR arthrography of acetabular cartilage delamination in femoroacetabular cam impingement. Radiology 2008; 249:236–241 [DOI] [PubMed] [Google Scholar]
- 9.Samaan MA, Pedoia V, Zhang AL, et al. A novel mr-based method for detection of cartilage delamination in femoroacetabular impingement patients. J Orthop Res 2018; 36:971–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gédouin JE. Arthroscopic treatment of femoroacetabular impingement: technical review. Orthop Traumatol Surg Res 2012; 98:583–596 [DOI] [PubMed] [Google Scholar]
- 11.Maradit Kremers H, Schilz SR, Van Houten HK, et al. Trends in utilization and outcomes of hip arthroscopy in the United States between 2005 and 2013. J Arthroplasty 2017; 32:750–755 [DOI] [PubMed] [Google Scholar]
- 12.Bedi A, Kelly BT, Khanduja V. Arthroscopic hip preservation surgery: current concepts and perspective. Bone Joint J 2013; 95-B:10–19 [DOI] [PubMed] [Google Scholar]
- 13.Philippon MJ, Briggs KK, Yen YM, Kuppersmith DA. Outcomes following hip arthroscopy for femoroacetabular impingement with associated chondrolabral dysfunction: minimum two-year follow-up. J Bone Joint Surg Br 2009; 91:16–23 [DOI] [PubMed] [Google Scholar]
- 14.Lung R, O’Brien J, Grebenyuk J, et al. The prevalence of radiographic femoroacetabular impingement in younger individuals undergoing total hip replacement for osteoarthritis. Clin Rheumatol 2012; 31:1239–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas GE, Palmer AJ, Batra RN, et al. Subclinical deformities of the hip are significant predictors of radiographic osteoarthritis and joint replacement in women: a 20 year longitudinal cohort study. Osteoarthritis Cartilage 2014; 22:1504–1510 [DOI] [PubMed] [Google Scholar]
- 16.Zingg PO, Ulbrich EJ, Buehler TC, Kalberer F, Poutawera VR, Dora C. Surgical hip dislocation versus hip arthroscopy for femoroacetabular impingement: clinical and morphological short-term results. Arch Orthop Trauma Surg 2013; 133:69–79 [DOI] [PubMed] [Google Scholar]
- 17.Kelly BT, Weiland DE, Schenker ML, Philippon MJ. Arthroscopic labral repair in the hip: surgical technique and review of the literature. Arthroscopy 2005; 21:1496–1504 [DOI] [PubMed] [Google Scholar]
- 18.Karthikeyan S, Roberts S, Griffin D. Microfracture for acetabular chondral defects in patients with femoroacetabular impingement: results at second-look arthroscopic surgery. Am J Sports Med 2012; 40:2725–2730 [DOI] [PubMed] [Google Scholar]
- 19.Neumann J, Zhang AL, Schwaiger BJ, et al. Validation of scoring hip osteoarthritis with MRI (SHOMRI) scores using hip arthroscopy as a standard of reference. Eur Radiol 2019; 29:578–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blankenbaker DG, De Smet AA, Keene JS. MR arthrographic appearance of the postoperative acetabular labrum in patients with suspected recurrent labral tears. AJR 2011; 197:[web]W1118–W1122 [DOI] [PubMed] [Google Scholar]
- 21.Kim CO, Dietrich TJ, Zingg PO, Dora C, Pfirrmann CWA, Sutter R. Arthroscopic hip surgery: frequency of postoperative MR arthrographic findings in asymptomatic and symptomatic patients. Radiology 2017; 283:779–788 [DOI] [PubMed] [Google Scholar]
- 22.Arbab D, van Ochten JHM, Schnurr C, Bouillon B, König D. Assessment of reliability, validity, responsiveness and minimally important change of the German hip dysfunction and osteoarthritis outcome score (HOOS) in patients with osteoarthritis of the hip. Rheumatol Int 2017; 37:2005–2011 [DOI] [PubMed] [Google Scholar]
- 23.Nötzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br 2002; 84:556–560 [DOI] [PubMed] [Google Scholar]
- 24.Wiberg G Shelf operation in congenital dysplasia of the acetabulum and in subluxation and dislocation of the hip. J Bone Joint Surg Am 1953; 35-A:65–80 [PubMed] [Google Scholar]
- 25.Lee S, Nardo L, Kumar D, et al. Scoring hip osteoarthritis with MRI (SHOMRI): a whole joint osteoarthritis evaluation system. J Magn Reson Imaging 2015; 41:1549–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domb BG, Philippon MJ, Giordano BD. Arthroscopic capsulotomy, capsular repair, and capsular plication of the hip: relation to atraumatic instability. Arthroscopy 2013; 29:162–173 [DOI] [PubMed] [Google Scholar]
- 27.Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy 2009; 25:400–404 [DOI] [PubMed] [Google Scholar]
- 28.Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip: a case report. J Bone Joint Surg Am 2009; 91:192–197 [DOI] [PubMed] [Google Scholar]
- 29.Moorman CT 3rd, Warren RF, Hershman EB, et al. Traumatic posterior hip subluxation in American football. J Bone Joint Surg Am 2003; 85:1190–1196 [DOI] [PubMed] [Google Scholar]
- 30.Guille JT, Pizzutillo PD, MacEwen GD. Development dysplasia of the hip from birth to six months. J Am Acad Orthop Surg 2000; 8:232–242 [DOI] [PubMed] [Google Scholar]
- 31.Kalisvaart MM, Safran MR. Microinstability of the hip-it does exist: etiology, diagnosis and treatment. J Hip Preserv Surg 2015; 2:123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck M Groin pain after open FAI surgery: the role of intraarticular adhesions. Clin Orthop Relat Res 2009; 467:769–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krueger A, Leunig M, Siebenrock KA, Beck M. Hip arthroscopy after previous surgical hip dislocation for femoroacetabular impingement. Arthroscopy 2007; 23:1285–1289 e1281 [DOI] [PubMed] [Google Scholar]
- 34.Philippon MJ, Schenker ML, Briggs KK, Kuppersmith DA, Maxwell RB, Stubbs AJ. Revision hip arthroscopy. Am J Sports Med 2007; 35:1918–1921 [DOI] [PubMed] [Google Scholar]
- 35.Sutter R, Dietrich TJ, Zingg PO, Pfirrmann CW. How useful is the alpha angle for discriminating between symptomatic patients with cam-type femoroacetabular impingement and asymptomatic volunteers? Radiology 2012; 264:514–521 [DOI] [PubMed] [Google Scholar]


