summary
Objective:
The aim of this study was to assess cross-sectional and longitudinal effects of meniscal lesions on adjacent cartilage T1ρ and T2 relaxation times, patient-reported outcomes and gait biomechanics.
Design:
Thirty patients with no cartilage morphological defects reported by Whole Organ MRI Score (WORMS) magnetic resonance imaging (MRI) grading and no radiographic osteoarthritis (OA) (Kellgren–Lawrence (KL) ≤ 1) were selected, 15 with posterior meniscus horn lesions and 15 matched controls without meniscal lesions. All were imaged on a 3T MR scanner for three consecutive years, except those who dropped from the study. Sagittal and frontal plane kinematic gait data were acquired at baseline. The Knee Injury and Osteoarthritis Outcome Score (KOOS) survey was taken each time. All images were automatically segmented and registered to an atlas for voxel-by-voxel cross-sectional and longitudinal analyses.
Results:
Relaxation time comparisons between groups showed elevated T1ρ of the lateral tibia (LP) and elevated T2 of the medial tibia (MT) and LT at 1 and 2 years in the lesion group. Longitudinal comparisons within each group revealed greater relaxation time elevations over one and 2 years in the group with lesions. KOOS Quality of Life (QOL) was significantly different between the groups at all time points (P < 0.05), as were other KOOS subcategories. No significant differences in the frontal or sagittal biomechanics were observed between the groups at baseline.
Conclusions:
Individuals with healthy cartilage and posterior meniscal horn lesions have increased relaxation times when compared to matched controls, increased relaxation time changes over 2 years, and consistently report a lower KOOS QOL, yet show no difference in gait biomechanics.
Keywords: T1ρ, T2, KOOS, Gait analysis, Voxel-based relaxometry, Meniscus lesion
Introduction
Damage to the meniscus has been proposed as an initiatory event for osteoarthritis (OA), as meniscus pathology has been shown to precede osteoarthritic change in imaging studies1–3. Meniscus degeneration, tears, and extrusion are associated with joint space narrowing, a radiographic sign of OA just 2 years after injury4,5. Damaged menisci assessed by magnetic resonance imaging (MRI)-based grading have been associated with greater odds of longitudinal cartilage loss than intact menisci6. The posterior meniscus horns are the most common sites for tears; quantitative MRI studies have shown increased cartilage relaxation times and altered trabecular bone in subjects with damage to the posterior meniscus horns, particularly the medial posterior horn (MPH)7–9. In a recent study by Roemer et al., several MRI features of the knee were suggested as precursors to OA onset, particularly highlighting meniscal pathology3.
It is also well known that biochemical compositional changes could precede morphological degeneration. Compositional MRI has become widely used in OA research to detect and quantify early signs of cartilage biochemical alterations. T1ρ and T2 relaxation are two complementary techniques widely used. T2 relaxation times are primarily affected by collagen structure and hydration due to dipolar interactions34. By using spin-lock techniques, cartilage T1ρ relaxation reduces dipolar interactions, and the chemical exchange between OH and NH protons of proteoglycan (PG) and water is suggested to contribute to T1ρ in cartilage10,11. While T1ρ changes in cartilage may also be influenced by hydration and collagen structure, T1ρ has been reported to be more sensitive to PG content than T2 35. Elevated T1ρ and T2 relaxation time has been associated with matrix degradation36.
Analysis of T1ρ and T2 in cartilage divisions based on contact area, meniscus position, and proximity to the subchondral bone or the articular surface have revealed subregions of interest to the degenerative process12,13. Prior studies have investigated joint changes at different stages of OA or in a mixed cohort of OA and healthy individuals. However, a detailed study on the effect of posterior meniscus lesions on adjacent cartilage that did not show any morphological defect detectable using radiological MRI grading, could provide perspective on early changes in OA and inform post-injury recovery to slow or reduce disease progression. As OA onset occurs slowly and cartilage composition alterations occur prior to radiographic changes, a local and unbiased technique must be adopted to identify early, differences in cartilage relaxation times. Voxel-based relaxometry (VBR) is a fully automatic technique used to study local changes of relaxation times14. VBR has been previously employed to assess cartilage relaxation times following anterior cruciate ligament (ACL) injury as well as in the femoral head and acetabulum of subjects with hip OA15,16.
Considering that the meniscus is critical for load transmission and shock absorption, biomechanical variables are crucial factors to consider when assessing the effects of meniscal lesions. Sagittal plane biomechanics have been implicated in patellofemoral joint OA (PFJOA), while frontal plane biomechanical changes, such as the misalignment (varus/valgus angles) and knee adduction moment (KAM) have been connected to tibiofemoral OA17–19. Thus, analyzing the gait patterns and biomechanics of subjects with and without meniscal lesions is necessary to fully understand the effects of these lesions on subjects and their potential to progress to OA. Lastly, patient-reported outcome measures (PROMs) are tangible means to quantify the day-to-day burden of living with knee pain, and are frequently used in Quality of Life (QOL) studies. For example, the Knee Injury and Osteoarthritis Outcome Score (KOOS) survey is a commonly used PROM in OA studies that has demonstrated internal consistency, validity, and test-retest reliability20.
The goal of this study was to assess the relationship between meniscal lesions and adjacent cartilage status in the early onset of cartilage degeneration at three levels: cartilage quality, patient-reported outcomes, and biomechanical changes. We analyzed cartilage T1ρ and T2 relaxation times in two groups, with and without adjacent posterior meniscus tears, as well as compared KOOS subcategory scores and several biomechanical factors between the two groups. Both groups were followed for 2 years, and cross-sectional differences were analyzed at each year following the baseline scan. Within each group, longitudinal differences were also calculated. It was hypothesized that intact cartilage adjacent to posterior meniscus horn lesions would show increased T1ρ and T2 relaxation times, overall worse PROMs and altered biomechanical functions when compared to matched controls. Furthermore, it was hypothesized that relaxation times would longitudinally increase within the lesion group.
Methods
Subjects
A total of 181 subjects were recruited for a larger study on early knee OA. The inclusion criteria for this study were: age >35 years, no use of medications for knee pain in the last year, and no radiographic evidence of OA [Kellgren–Lawrence (KL) grade 0–1] on either knee. The exclusion criteria for all subjects were: concurrent use of an investigational drug, history of fracture or surgical intervention in the study knee, or contraindications to MRI. All subjects signed a written informed consent form approved by the Committee on Human Research of the home institution. To determine KL score, all subjects underwent bilateral weight-bearing, fixed-flexion postero-anterior knee X-ray with the aid of a Synaflexer device (Synarc, Newark, CA, USA). A radiologist with 20 years of experience in musculoskeletal imaging performed the KL scoring from these radiographs21.
MRI procedures
Knee images were acquired on a 3.0 T GE MR 750 Scanner (General Electric, Milwaukee, WI, USA) using an eight-channel knee coil (Invivo, Orlando, FL, USA). All subjects returned for scanning 1 year and 2 years after the baseline scan. Subjects were positioned supine with the knee in neutral rotation and fully extended. Images were acquired from one random knee after a 30-min period of sitting. The following sequences were acquired: (1) a high-resolution 3D fast spin-echo (FSE) CUBE sequence for clinical grading and cartilage segmentation (Repetition Time (TR)/Echo Time (TE) = 1500/26.69 ms, Field of View (FOV) = 14 cm, matrix = 384 × 384, slice thickness = 0.5 mm, echo train length = 32, bandwidth = 50.0 kHz, Number of Excitations (NEX) = 0.5, acquisition time = 10.5 min); (2) T1ρ sequence (TR/TE = 9/2.6 ms, time of recovery = 1500 ms, FOV = 14 cm, matrix = 256 × 128, slice thickness = 4 mm, bandwidth = 62.5 kHz, Time of Spin-Lock (TSL) = 0/2/4/8/12/20/40/80 ms, Frequency of Spin-Lock (FSL) = 500 Hz, acquisition time = 11 min); and (3) T2 sequence (same as the T1ρ quantification except magnetization preparation TE = 0/1.8/3.6/7.3/14.5/29.¼3.6/58.2, acquisition time = 11 min)22,38.
PROMs
At each visit, subjects completed the KOOS survey to record their perceived levels of pain, activity, etc. The KOOS subcategories are divided into: Pain, Symptoms, Activity of Daily Life, Sport, and Quality of Daily Life20.
Cartilage grading and group determination
A board-certified radiologist with 5 years of experience graded the tibiofemoral cartilage compartments [medial femoral condyle (MFC), medial tibial plateau (MTP), lateral femoral condyle (LFC), and lateral tibial plateau (LTP)] and the posterior horns of the meniscus [lateral posterior horn (LPH) and MPH] on the sagittal 3D FSE CUBE images using a modified Whole Organ MRI Score (mWORMS) grading system23,24. Meniscus WORMS grade 0 indicates no lesion, grade 1 indicates intrasubstance abnormalities, grade 2 is assigned to non-displaced tears, grade 3 to displaced or complex tears without deformity and grade 4 in cases of maceration of the meniscus.
Of the 181 subjects from initial enrollment, 115 had KL score 1. From these 115 subjects, 98 had no focal morphological defects in any of the tibiofemoral compartments (mWORMS ≤1), establishing macroscopically healthy cartilage. Out of the 98 with healthy cartilage, 15 (six male, Age = 56.1 ± 8.48 years, body mass index (BMI) = 23.7 ± 2.3 kg/m2) possessed a lateral and/or medial posterior meniscal horn lesion (meniscus mWORMS 2); five with a lateral lesion, eight with medial, and two with both. Out of the seven subjects with lateral posterior meniscus tears, two subjects had meniscal cysts and one had a meniscocapsular separation. Out of the 10 subjects with medial posterior meniscus tear one had a root tear and one had a meniscal flap tear.
These 15 subjects were individually matched for age, gender, knee side, and BMI with 15 control subjects (six male, Age = 56.9 ± 8.88 years, BMI = 24.1 ± 2.5 kg/m2) with no meniscal tears or lesions (meniscus mWORMS <2) from the previously determined 98 subjects with macroscopically healthy cartilage (Table I). The study design and subject determination can be seen in Fig. 1. Details on lesion mWORMS classifications can be found in Table II. From initial enrollment, a few subjects in both groups dropped out of the study; however, this did not significantly change the age or BMI demographical information, as seen in Table I.
Table I.
Subject demographic characteristics and KOOS
| Characteristic | Lesion | No lesion | P-value | |
|---|---|---|---|---|
| Baseline | Total a | n = 15 | n = 15 | |
| Male | 6 (40%) | 6 (40%) | ||
| Femalea | 9 (60%) | 9(60%) | ||
| Lefta | 7 (47%) | 7 (47%) | ||
| Righta | 8 (53% | 8 (53%) | ||
| Age (years)b | 56.1 ± 8.5 | 56.9 ± 8.9 | 0.79 | |
| BMI (kg/m2)b | 23.7 ± 2.3 | 24.1± 2.5 | 0.61 | |
| KOOS Painb | 81.5 ± 14.9 | 90.7 ± 11.2 | 0.07* | |
| KOOS Symptomb | 81.4 ± 11.9 | 88.8 ± 10.2 | 0.08* | |
| KOOS Activity Of Daily Lifeb | 89.1 ± 14.8 | 94.0 ± 8.7 | 0.28 | |
| KOOS Sportb | 75.3 ± 19.5 | 86.3 ± 14.2 | 0.09* | |
| KOOS QOLb | 69.2 ± 22.6 | 83.8 ± 16.3 | 0.05** | |
| 1 Year | Total a | n = 12 | n = 13 | |
| Male | 5 (42%) | 6 (46%) | ||
| Femalea | 7 (58%) | 7 (54%) | ||
| Lefta | 5 (42%) | 7 (53%) | ||
| Righta | 7 (58%) | 6 (47%) | ||
| Age (years)b | 57.4 ± 7.9 | 56.5 ± 9.3 | 0.79 | |
| BMI (kg/m2)b | 23.4 ± 2.4 | 23.9 ± 2.5 | 0.63 | |
| KOOS Painb | 78.5 ± 16.1 | 91.0 ± 10.2 | 0.03** | |
| KOOS Symptomb | 82.7 ± 17.7 | 90.4 ± 7.1 | 0.16 | |
| KOOS Activity of Daily Lifeb | 88.0 ± 12.4 | 95.9 ± 5.9 | 0.05** | |
| KOOS Sportb | 72.9 ± 22.9 | 86.5 ± 16.9 | 0.10 | |
| KOOS QOLb | 66.7 ± 19.3 | 84.1 ± 15.9 | 0.02** | |
| 2 Year | Total a | n = 9 | n = 9 | |
| Male | 4 (44%) | 5 (56%) | ||
| Femalea | 5 (56%) | 4 (44%) | ||
| Lefta | 5 (56%) | 5 (56%) | ||
| Righta | 4 (44%) | 4 (44%) | ||
| Age (years)b | 59.9 ± 6.3 | 56.3 ± 11.0 | 0.41 | |
| BMI (kg/m2)b | 22.6 ± 1.9 | 23.9 ± 1.7 | 0.15 | |
| KOOS Painb | 79.5 ± 17.2 | 92.0 ± 11.5 | 0.11 | |
| KOOS Symptomb | 86.9 ± 13.8 | 89.7 ± 9.5 | 0.63 | |
| KOOS Activity of Daily Lifeb | 87.9 ± 18.5 | 95.4 ± 8.6 | 0.28 | |
| KOOS Sportb | 78.3 ± 24.4 | 92.2 ± 15.0 | 0.16 | |
| KOOS QOLb | 68.1 ± 23.7 | 89.6 ± 16.2 | 0.04** | |
Data expressed as Count (Percentage %).
Data expressed as mean ± standard deviation.
Approaching significance (P < 0.1).
Significant (P < 0.05).
Fig. 1.
Diagramed are the overall study design and subject selection criteria for the 15 subjects with meniscus lesions and their matched controls.
Table II.
Baseline clinical characteristics of the knee as Assessed by mWORMS* Meniscus WORMS grade 0 indicates no lesion, grade 1 indicates intrasubstance abnormalities, grade 2 is assigned to non-displaced tears, grade 3 to displaced or complex tears without deformity and grade 4 in cases of maceration of the meniscus (Lesion n = 15; No lesion n = 15)
| Lesion | No Lesion | Lesion | No lesion | ||
|---|---|---|---|---|---|
| LPH meniscus lesion |
MPH meniscus lesion |
||||
| mWORMS = 0 | 6 (40%) | 11 (73%) | mWORMS = 0 | 2 (13%) | 7 (47%) |
| mWORMS = 1 | 2 (13%) | 4 (27%) | mWORMS = 1 | 3 (20%) | 8 (53%) |
| mWORMS = 2 | 6 (40%) | 0 (0%) | mWORMS = 2 | 7 (47%) | 0 (0%) |
| mWORMS = 3 | 1 (7%) | 0 (0%) | mWORMS = 3 | 3 (20%) | 0 (0%) |
| mWORMS ≥ 4 | 0 (0%) | 0 (0%) | mWORMS ≥ 4 | 0 (0%) | 0 (0%) |
| LF cartilage lesion |
LT cartilage lesion |
||||
| mWORMS = 0 | 14 (93%) | 15 (100%) | mWORMS = 0 | 11 (73%) | 12 (80%) |
| mWORMS = 1 | 1 (7%) | 0 (0%) | mWORMS = 1 | 4 (27%) | 3 (20%) |
| mWORMS = 2 | 0 (0%) | 0 (0%) | mWORMS = 2 | 0 (0%) | 0 (0%) |
| mWORMS = 3 | 0 (0%) | 0 (0%) | mWORMS = 3 | 0 (0%) | 0 (0%) |
| mWORMS ≥ 4 | 0 (0%) | 0 (0%) | mWORMS ≥ 4 | 0 (0%) | 0 (0%) |
| MF cartilage lesion |
MT cartilage lesion |
||||
| mWORMS = 0 | 14 (93%) | 14 (93%) | mWORMS = 0 | 14 (93%) | 15 (100%) |
| mWORMS = 1 | 1 (7%) | 1 (7%) | mWORMS = 1 | 1 (7%) | 0 (0%) |
| mWORMS = 2 | 0 (0%) | 0 (0%) | mWORMS = 2 | 0 (0%) | 0 (0%) |
| mWORMS = 3 | 0 (0%) | 0 (0%) | mWORMS = 3 | 0 (0%) | 0 (0%) |
| mWORMS ≥ 4 | 0 (0%) | 0 (0%) | mWORMS ≥ 4 | 0 (0%) | 0 (0%) |
| Trochlea cartilage lesion |
Patella cartilage lesion |
||||
| mWORMS = 0 | 11 (73%) | 9 (60%) | mWORMS = 0 | 5 (33%) | 6 (40%) |
| mWORMS = 1 | 1 (7%) | 3 (20%) | mWORMS = 1 | 3 (20%) | 1 (7%) |
| mWORMS = 2 | 1 (7%) | 0 (0%) | mWORMS = 2 | 2 (13%) | 3 (20%) |
| mWORMS = 3 | 2 (13%) | 2 (13%) | mWORMS = 3 | 4 (27%) | 3 (20%) |
| mWORMS ≥ 4 | 0 (0%) | 0 (0%) | mWORMS ≥ 4 | 1 (7%) | 2 (13%) |
Data expressed as Count (Percentage %).
Biomechanical gait analysis
Three-dimensional kinematic data were collected with a passive 10-camera system (VICON, Oxford Metrics, UK) at 250 Hz, while kinetic data were collected from two embedded force plat-forms at a sampling rate of 1000 Hz (AMTI, Watertown, MA, USA). Retro-reflective markers were placed on body landmarks of the lower extremities and rigid clusters on lateral surfaces of the thighs, legs and heel shoe counters to track motions during the walking test25. Kinematic and kinetics were calculated using Visual3D (C-motion, Germantown, MD, USA). Net joint moments were determined as external moments, normalized to the body mass (kg) and height (m) of each subject17. Frontal and sagittal plane kinematics at baseline were both assessed, including: Peak Knee Flexion and Adduction Angles, Peak Knee Flexion and Adduction Moments, and Peak Knee Flexion and Adduction Moment Impulses during the stance phase of walking. All biomechanical variables can be seen in Table III.
Table III.
Biomechanical testing resultsa
| Lesion | No lesion | P-value | |||
|---|---|---|---|---|---|
| Baseline | Frontal plane mechanics | KAM 1 (Nm/kg*m) | 0.33 ± 0.12 | 0.35 ± 0.10 | 0.71 |
| KAM 2 (Nm/kg*m) | 0.21 ± 0.10 | 0.17 ± 0.04 | 0.30 | ||
| KAM impulse (Nm*ms/kg*m) | 81.6 ± 20.3 | 82.7 ± 12.4 | 0.90 | ||
| Varus (Deg) | 0.08 ± 2.1 | 1.3 ± 3.7 | 0.42 | ||
| Valgus (Deg) | 5.4 ± 2.3 | 5.3 ± 3.0 | 0.99 | ||
| Sagittal plane mechanics | Peak Knee Flexion 1 (Deg) | 21.9 ± 4.9 | 19.4 ± 3.1 | 0.23 | |
| Peak Knee Flexion 2 (Deg) | 44.1 ± 2.5 | 41.8 ± 5.3 | 0.30 | ||
| Peak Knee Flexion Moment (Nm/kg*m) | 0.39 ± 0.19 | 0.32 ± 0.12 | 0.45 | ||
| Peak Knee Flexion Impulse (Nm*ms/kg*m) | 55.7 ± 25.4 | 50.9 ± 25.2 | 0.71 | ||
| Peak Knee Flexion Impulse (Nm*ms/kg*m) | 55.7 ± 25.4 | 50.9 ± 25.2 | 0.71 | ||
Data expressed as mean ± standard deviation.
Approaching significance (P < 0.1).
Significant (P < 0.05).
Image post-processing
Image processing was done with in-house programs written in MatLab (MathWorks, Natick, MA), integrated with the elastix toolbox for non-rigid image registration14,26,27. All images were registered and aligned to an atlas knee, allowing for voxel-by-voxel statistical comparisons in a technique previously described by Pedoia et al.14. Relaxation maps were acquired by fitting the morphed images from different TSLs, employing a Levenberg–Marquardt mono-exponential (S(TSL) ∝ exp(−TSL/T1ρ) and S(TE) ∝ exp(−TE/T2)) applied to each voxel28.
Statistical analysis
Statistical Parametric Mapping (SPM) was conducted to assess the local cross-sectional differences between the two groups and longitudinal differences within groups. Voxel-based summary statistics, such as mean and standard deviation, were calculated. Group comparisons, including all KOOS and biomechanics results, were performed with paired student t-tests, with significance determined as P < 0.05. Percentages of voxels showing significance (PSV), average P-values in the overall compartment (P-value) and average percentage differences (APD) for each compartment were summarized by SPMs for the T1ρ and T2 cross-sectional and longitudinal changes. Age, gender and BMI were considered as adjusting factors in statistical analyses. Random Field Theory correction was used to take in to account possible false positives due to multiple comparisons37. For visualization, an in-house program was used to construct a 3D bone mesh segmented from the first echo (TSL = 0). The six knee compartments were stitched together and interpolated from the 2D sagittal images, creating a color map of the desired statistical parameter or relaxation time, and then overlaid on the 3D bone mesh.
Results
Cross-sectional analyses
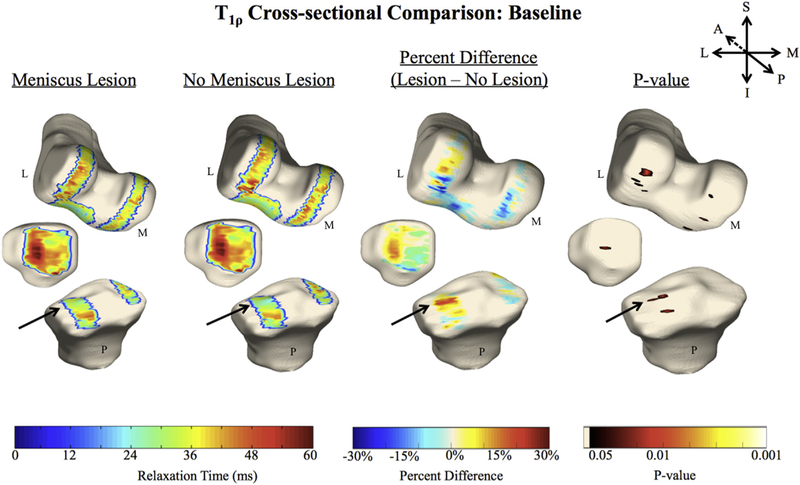
A summary of all the numerical statistical comparisons can be found in Table IVAA. At baseline, subjects with a posterior meniscal horn lesion (LPH and/or MPH) displayed elevated T1ρ in the LT cartilage adjacent to the meniscus (Fig. 2) compared to the matched controls without meniscal lesions (PSV = 9.2%, APD = 23.6%, P-value = 0.03). No significant cross-sectional differences were observed at the 1 year or 2 year follow-ups (Table IVA). No significant cross-sectional differences in T2 times between groups were observed at baseline (Table IVA). However at later time points, significant differences in the MT cartilage adjacent to the meniscus lesion were observed at 1 year (PSV = 17.6%, APD = 30.5%, P-value = 0.02) as well as at 2 years in the MT (PSV = 11.5%, APD = 29.1%, P-value = 0.03) and LT (PSV = 8.6%, APD = 27.7%, P-value = 0.02), with subjects possessing meniscal lesions having elevated relaxation times compared to the matched controls (Table IVA).
Table IVA.
Cross-sectional results*
| Baseline |
1 Year |
2 Years |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PSV | APD | P | PSV | APD | P | PSV | APD | P | |||
| T1ρ cross-sectional | No lesion > Lesion | MF | 1.2% | 15.0% | 0.03 | 3.8% | 18.9% | 0.02 | <1% | – | – |
| MT | 3.8% | 19.8% | 0.03 | 4.6% | 27.9% | 0.03 | 4.3% | 24.8% | 0.02 | ||
| LF | 4.2% | 29.1% | 0.02 | 1.5% | 32.6% | 0.02 | 3.0% | 40.0% | 0.03 | ||
| LT | 1.0% | 25.5% | 0.02 | <1% | – | – | <1% | – | – | ||
| TrF | 6.9% | 16.4% | 0.03 | 1.3% | 25.2% | 0.04 | <1% | – | – | ||
| P | 0.2% | 16.3% | 0.03 | 0.0% | – | – | 0.0% | – | – | ||
| No lesion < Lesion | MF | <1% | – | – | <1% | – | – | 1.2% | 28.9% | 0.03 | |
| MT | 3.1% | 17.6% | 0.03 | <1% | – | – | 1.5% | 22.6% | 0.03 | ||
| LF | 3.7% | 17.9% | 0.03 | <1% | – | – | 1.2% | 20.8% | 0.04 | ||
| LT | 9.2% | 23.6% | 0.03 | 4.1% | 31.4% | 0.03 | 1.7% | 30.8% | 0.03 | ||
| TrF | <1% | – | – | <1% | – | – | <1% | – | – | ||
| P | 1.1% | 25.9% | 0.04 | <1% | – | – | 1.6% | 26.9% | 0.04 | ||
| T2 cross-sectional | No lesion > Lesion | MF | 6.9% | 17.5% | 0.03 | <1% | – | – | 1.4% | 32.1% | 0.03 |
| MT | 3.8% | 15.2% | 0.03 | <1% | – | – | 5.3% | 28.4% | 0.02 | ||
| LF | <1% | – | – | <1% | – | – | 6.1% | 40.8% | 0.02 | ||
| LT | 1.0% | 22.1% | 0.03 | <1% | – | – | 0% | – | – | ||
| TrF | <1% | – | – | <1% | – | – | 1.1% | 34.2% | 0.04 | ||
| P | <1% | – | – | <1% | – | – | <1% | – | – | ||
| No lesion < Lesion | MF | 0% | – | – | 1.0% | 21.9% | 0.03 | <1% | – | – | |
| MT | 0% | – | – | 17.6% | 30.5% | 0.02 | 11.5% | 29.1% | 0.03 | ||
| LF | 2.2% | 18.0% | 0.03 | 1.5% | 20.7% | 0.03 | <1% | – | – | ||
| LT | 2.8% | 25.8% | 0.02 | 8.6% | 27.7% | 0.02 | 4.3% | 33.0% | 0.03 | ||
| TrF | 1.9% | 15.8% | 0.03 | 1.6% | 17.6% | 0.03 | <1% | – | – | ||
| P | <1% | – | – | <1% | – | – | <1% | – | – | ||
P, patella.
Compartments with <1% of PSV do not have APD and P values displayed. Compartments with the largest PSV are bolded.
Fig. 2.
In these 3D renderings, the average cartilage T1ρ for each group (n = 15, 15) is overlaid onto a bone mesh constructed from the first echo in the two figures on the left. Voxel-based statistics, such as the average percent difference (second from the right) and average P-value (right) are also imaged. The arrows point to the region of significant difference between the groups in the LT.
Regarding PROMs, overall the group with lesions tended to have lower KOOS scores, and thus worse reported outcomes than the group without lesions; see Table I for a summary of all KOOS sub-categories with indicated significance between groups. KOOS QOL was significantly different between the two groups at all three time points. At 1 year after baseline, KOOS Pain and Activity were also significantly different between the groups. There were no significant differences in any kinematic or kinetic variables when assessing frontal and sagittal plane biomechanics collected at baseline between the two groups (Table III).
Longitudinal analyses
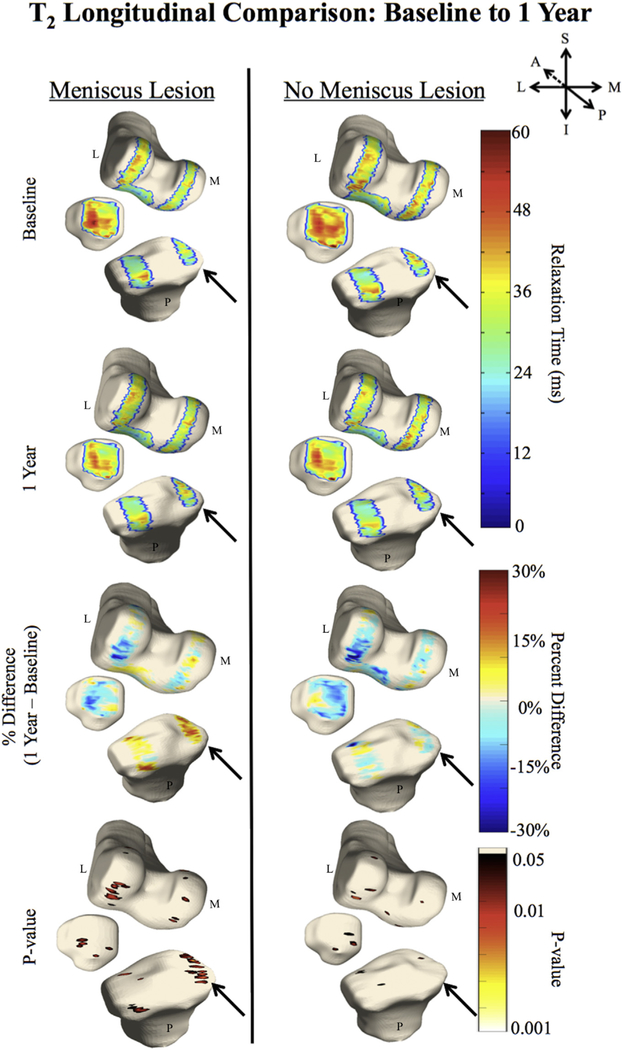
Longitudinally, significant relaxation time elevations were observed in the group with lesions between baseline and 1 year follow-up (Fig. 3), particularly in the MF (PSV = 25.5%, APD = 13.8%, P-value = 0.02) and trochlea (TrF; PSV = 10.6%, APD = 13.4%, P-value = 0.02). Elevations at 1 year were also observed in the group without lesions in the LF (PSV = 10.3%, APD = 14.6%, P-value = 0.02). At the 2-year mark, longitudinal elevations compared to baseline were primarily observed in the group with lesions, particularly in the MF (PSV = 12.7%, APD = 14.3%, P-value = 0.02), MT (PSV = 12.5%, APD = 18.3%, P-value = 0.03) and LF (PSV = 14.9%, APD = 15.3%, P-value = 0.02). See Table IVBB for a summary of other numerical statistical comparisons. Similar to the T1ρ analysis, significant T2 elevations at 1 year compared to baseline were observed in the group with posterior meniscal horn lesions (Fig. 4), namely in the MT (PSV = 38.4%, APD = 21.4%, P-value = 0.02), MF (PSV = 10.6%, APD = 13.0%, P-value = 0.02) and LT (PSV = 10.0%, APD = 24.2%, P-value = 0.02). At 2 years, T2 elevations were observed in the MT of those with lesions (PSV = 17.3%, APD = 27.6%, P-value = 0.03), while a decrease in T2 was observed in the LF (PSV = 13.7%, APD = 18.4%, P-value = 0.02). See Table IVB for a summary of other numerical statistical comparisons. There were no significant longitudinal differences in any KOOS subcategories within each of the two groups.
Fig. 3.
In these 3D renderings, the average cartilage T1ρ for each group is overlaid onto a bone mesh constructed from the first echo in the top four images. The arrow points to the longitudinal difference in the MFC, which is present in the group with meniscal lesions, but not in the group without.
Table IVB.
Longitudinal results*
| Lesion |
No lesion |
|||||||
|---|---|---|---|---|---|---|---|---|
| PSV | APD | P | PSV | APD | P | |||
| T1ρ longitudinal: baseline and 2 year | Baseline >2 year | MF | 0% | – | – | <1% | – | – |
| MT | 0% | – | – | 0% | – | – | ||
| LF | <1% | – | – | <1% | – | – | ||
| LT | 1.9% | 18.5% | 0.03 | 6.5% | 29.5% | 0.02 | ||
| TrF | 1.0% | 11.5% | 0.03 | 1.9% | 22.3% | 0.03 | ||
| P | <1% | – | – | <1% | – | – | ||
| Baseline <2 year | MF | 12.7% | 14.3% | 0.02 | <1% | – | – | |
| MT | 12.5% | 18.3% | 0.03 | 4.1% | 31.3% | 0.03 | ||
| LF | 14.9% | 15.3% | 0.02 | 9.3% | 24.6% | 0.03 | ||
| LT | 1.5% | 23.5% | 0.03 | <1% | – | – | ||
| TrF | 6.9% | 12.3% | 0.02 | <1% | – | – | ||
| P | 2.4% | 17.8% | 0.02 | <1% | – | – | ||
| T2 longitudinal: baseline and 2 year | Baseline >2 year | MF | <1% | 11.1% | 0.04 | 4.1% | 22.1% | 0.03 |
| MT | 0% | – | – | 0% | – | – | ||
| LF | 13.7% | 18.4% | 0.02 | <1% | – | – | ||
| LT | <1% | – | – | 6.0% | 34.5% | 0.3 | ||
| TrF | 2.3% | 11.1% | 0.02 | <1% | – | – | ||
| P | 6.5% | 25.7% | 0.03 | 5.3% | 26.0% | 0.03 | ||
| Baseline <2 year | MF | 2.1% | 21.5% | 0.02 | 0% | – | – | |
| MT | 17.3% | 27.6% | 0.03 | 2.3% | 28.0% | 0.03 | ||
| LF | 1.6% | 21.8% | 0.02 | <1% | – | – | ||
| LT | 6.2% | 24.8% | 0.03 | 0% | – | – | ||
| TrF | <1% | – | – | <1% | – | – | ||
| P | <1% | – | – | 0% | – | – | ||
P, patella.
Compartments with <1% of PSV do not have APD and P values displayed. Compartments with the largest PSV are bolded.
Fig. 4.
In these 3D renderings, the average cartilage T2 for each group is overlaid onto a bone mesh constructed from the first echo in the top four images. The arrow points to the longitudinal difference in the MT, which is present in the group with meniscal lesions, but not in the group without.
Considering the association between the T1ρ and T2 change between baseline and 2-year and KOOS subcategories, negative weak to moderate associations were observed, confirming simultaneous accelerated cartilage degeneration and worse PROMs in the lesion group [Supplemental Fig. 5(a)]. Specifically, significant negative correlation was observed between longitudinal changes in the LF and KOOS QOL (PSV = 7.92%, average R-value = 0.67, P-value = 0.03).
An individualized analysis of the cartilage compositional progression in comparison with KOOS QOL demonstrated that two subjects, belonging to the lesion group, drove the observed correlations. These subjects showed significantly lower QOL scores (both 37.5) compared with the overall group (78.81 ± 23.28) and a marked longitudinal change in T1ρ. Baseline and 2-year follow up T1ρ maps of one of these subjects are shown in Supplemental Fig. 5(b) (first row). It also worth noting that, although higher values of KOOS QOL scores (better QOL) correspond to smaller longitudinal changes in cartilage relaxation times, as shown in group correlation analysis, single patient qualitative observations showed high variability. For example, cartilage T1ρ progression in two subjects that reported high QOL scores [Supplemental Fig. 5(b) second and third row], show very different patterns of increase over time. These results highlight how, even in the absence of reported degradation of QOL scores, notable cartilage compositional changes could still occur in subject with posterior meniscus lesions.
Discussion
In this quantitative MRI study, we investigated T1ρ and T2 relaxation times in non-osteoarthritic cartilage with and without adjacent posterior meniscal lesions using VBR, and further assessed PROMs and biomechanical data. The cohort of 30 subjects was carefully selected so that the only apparent difference between the subjects and controls was the presence of posterior meniscal horn lesions. From the relaxation time analysis, it appeared that macroscopically healthy cartilage adjacent to posterior horn lesions revealed elevated T1ρ and T2 relaxation times compared to cartilage next to intact menisci. As seen in Fig. 2, the LT shows the largest difference (9.2%) in T1ρ times between cohorts. This region of elevation in the LT is localized to the superficial chondral layer adjacent to the meniscus. Even more prevalent are the T2 cross-sectional differences observed at one and 2 years after baseline, where the MT and LT continue to highlight elevated relaxation times of regions adjacent to meniscal lesions. One possible explanation for the early T1ρ changes and the later T2 changes is that the PG may be altered before the collagen in the early onset of cartilage degeneration29.
In a previous study, post-menopausal women asymptomatic of OA were analyzed for meniscal tears, and it was found that tears were relatively common; furthermore, a greater tibial plateau bone area was observed to correlate with meniscal tears30. From the study, it was undetermined whether the bone shape or biomechanical gait alterations led to the meniscal tears. Souza et al. also previously noted elevated relaxation times in the cartilage adjacent to the posterior meniscal horn in a study that assessed T1ρ and T2, though in subjects with posterior horn tears following a partial meniscectomy31. In our study, we have observed these elevations without the invasive surgery, thus pointing to the meniscal lesions as a potential source of cartilage change.
Longitudinally, the majority of elevated relaxation times are within the tibiofemoral cartilage compartments of the group with posterior horn lesions. This is particularly clear in the MT of Fig. 4, the longitudinal progression of T2 from baseline to 1 year after baseline. Here it is apparent that the T2 relaxation times are increasing more rapidly in the group with lesions than the controls. A longitudinal increase was also observed in the LF of those without lesions after 1 year. However, this increase was only demonstrated in roughly 10% of the voxels, whereas the MF of the group with lesions at the same time point had an elevation in about 25% of the voxels. The T1ρ and T2 elevations observed at both time points in the medial femur and tibia of the subjects with lesions were further not seen in the controls, indicating the importance these posterior meniscal horn lesions have on the quality of adjacent cartilage.
Perhaps the most clinically applicable findings were the significant differences in the PROMs between the two groups. Considering that all 30 subjects were predetermined to show no radiographic signs of OA and possess healthy tibiofemoral cartilage, the baseline time point is a relatively arbitrary point in these sub-jects’ lives. However, at baseline, the KOOS QOL subcategory is significantly lower in the group with lesions, with the Pain, Symptoms and Sport subcategories approaching significant differences (P < 0.10). Thus, despite no radiographic OA and no reported injuries to the knee, the subjects with meniscal lesions were reporting more pain and lower functionality than those without lesions. KOOS QOL continued to be an indicator for distinguishing between the lesion and no lesion groups at the other two time points, and thus was determined to be the best KOOS subcategory to differentiate between the groups. Previously, it was shown that there was no significant difference in pain or WOMAC in a group of 154 subjects with clinically determined OA compared to 49 age-matched controls32. However, our subjects displayed no signs of OA, and the presence of these meniscal lesions in asymptomatic subjects seems to be more correlated with lower KOOS sub-categories than clinically determined OA.
Despite the clear differences in T1ρ and T2 relaxation times between subjects and matched controls, as well as the significantly different patient-reported outcomes, it appears that the presence of posterior meniscal horn lesions does not have any effect on the biomechanics and gait patterns. Considering that all the subjects in this study did not show signs of radiographic OA, yet we have observed elevated cartilage relaxation times in the group with meniscal lesions, this finding supports the argument that meniscal lesions may be one of the first signs of degenerating cartilage2. Thus, nuanced cartilage changes, namely elevated relaxation times associated with degeneration, occur prior to subjects altering their gait. In fact, assessing kinematic gait before and after a partial meniscectomy in subjects with meniscal lesions and no radiographic OA similarly demonstrated no significant differences31.
To the best of our knowledge, this is the first comprehensive study assessing the effects of posterior meniscal horn lesions on cartilage relaxation times, PROMs, and biomechanical variables in a matched-control design. In a compelling double-blind study by Sihvonen et al., it was determined that an arthroscopic partial meniscectomy, the most common orthopedic procedure for treating meniscal lesions, yielded no better results than a sham surgical procedure, where the arthroscopic surgery was simulated, but not in fact conducted, unbeknownst to the subject33. Thus, not only are the effects of meniscal lesions on healthy cartilage not fully understood, but our current treatments must also be evaluated31.
In terms of study limitations, although this study looked at a range of effects from the presence of posterior horn lesions, the small sample size may not be large enough to make conclusive statements; a larger cohort and a longer analysis would bolster these findings. A larger cohort would allow for the division between lateral and medial horn lesions, providing a richer understanding of the nuanced outcomes of meniscal lesions on cartilage and QOL. A better understanding of the presence of meniscus lesions, as well as the comparison of our observations done in this cohort without radiological OA signs, would also be interesting for future studies in subjects with clearly defined OA. A longer study could follow these degenerative lesions into the full development of OA, allowing for a better understanding about the longitudinal path of the observed compositional changes, which would help determine the mechanism for which these lesions are influencing the neighboring cartilage, guiding future preventative treatments.
Supplementary Material
Acknowledgements
This project was supported by Grant Numbers R01AR046905, R01AR062370, and P50AR060752 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, United States of America (NIH-NIAMS). This content is solely the responsibility of the authors and does not necessarily reflect the views of the NIH-NIAMS.
We would like to thank Melissa Lu for help with study coordination, Lorenzo Nardo and Thomas M. Link for assistance in grading, as well as Michael Samaan, Hsiang-Ling Teng and Nathan Calixto for assisting with this study.
Role of the funding source
The funding source had no involvement in the study design, collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
Conflict of interest
The authors received grant funding, which is listed in the Acknowledgements. Otherwise, none of the authors have any relationships that could potentially influence/bias their work and conclusions.
Footnotes
Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.joca.2016.10.025.
References
- 1.Fox AJ, Bedi A, Rodeo SA. The basic science of human knee menisci: structure, composition, and function. Sports Health 2012;4(4):340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Englund M Meniscal tear–a feature of osteoarthritis. Acta Orthop Scand Suppl 2004;75(312):1–45. [PubMed] [Google Scholar]
- 3.Roemer FW, Kwoh CK, Hannon MJ, Hunter DJ, Eckstein F, Fukii T, et al. What comes first? Multitissue involvement leading to radiographic osteoarthritis. Arthritis Rheumatol 2015;67(8):2085–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madan-Sharma R, Kloppenburg M, Kornaat PR, Botha-Scheepers SA, Le Graverand MP, Bloem JL, et al. Do MRI features at baseline predict radiographic joint space narrowing in the medial compartment of the osteoarthritic knee 2 years later? Skelet Radiol 2008;37(9):805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badlani JT, Borrero C, Golla S, Harner CD, Irrgang JJ. The effects of meniscus injury on the development of knee osteoarthritis: data from the osteoarthritis initiative. Am J Sports Med 2013;41(6):1238–44 [DOI] [PubMed] [Google Scholar]
- 6.Felson DT, Lynch J, Guermazi A, Roemer FW, Niu J, McAlindon T, et al. Comparison of BLOKS and WORMS scoring systems part II. Longitudinal assessment of knee MRIs for osteoarthritis and suggested approach based on their performance: data from the osteoarthritis initiative. Osteoarthritis Cartilage 2010;18(11):1402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Englund M, Guermazi A, Gale D, Hunter DJ, Aliabadi P, Clancy M, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med 2008;359(11): 1108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zarins ZA, Bolbos RI, Pialat JB, Link TM, Li X, Souza RB, et al. Cartilage and meniscus assessment using T1rho and T2 measurements in healthy subjects and patients with osteoarthritis. Osteoarthritis Cartilage 2010;18(11):1408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar D, Schooler J, Zuo J, McCulloch CE, Nardo L, Link TM, et al. Trabecular bone structure and spatial differences in articular cartilage MR relaxation times in individuals with posterior horn medial meniscal tears. Osteoarthritis Cartilage 2013;21(1):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duvvuri U, Reddy R, Patel SD, Kaufman JH, Kneeland JB, Leigh JS. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med 1997;38(6):863–7. [DOI] [PubMed] [Google Scholar]
- 11.Nieminen MT, Toyras J, Rieppo J, Hakumaki JM, Silvennoinen J, Helminen HJ, et al. Quantitative MR microscopy of enzymatically degraded articular cartilage. Magn Reson Med 2000;43(5):676–81. [DOI] [PubMed] [Google Scholar]
- 12.Liebl H, Joseph G, Nevitt MC, Singh N, Heilmeier U, Subburaj K, et al. Early T2 changes predict onset of radiographic knee osteoarthritis: data from the osteoarthritis initiative. Ann Rheum Dis 2015;74(7):1353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su F, Hilton JF, Nardo L, Wu S, Liang F, Link TM, et al. Cartilage morphology and T1rho and T2 quantification in ACL-reconstructed knees: a 2-year follow-up. Osteoarthritis Carti-lage 2013;21(8):1058–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedoia V, Li X, Su F, Calixto N, Majumdar S. Fully automatic analysis of the knee articular cartilage T1r relaxation time using voxel-based relaxometry. J Magn Reson Imaging 2016;43(4):970–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell C, Pedoia V, Amano K, Potter H, Majumdar S, AF-ACL Consortium. Baseline cartilage quality is associated with voxel-based T1rho and T2 following ACL reconstruction: a multi-center pilot study. J Orthop Res 2016, 10.1002/jor.23277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallo MC, Wyatt C, Pedoia V, Kumar D, Lee S, Nardo L, et al. T1rho and T2 relaxation times are associated with progression of hip osteoarthritis. Osteoarthritis Cartilage 2016, 10.1016/j.joca.2016.03.005. S1063–4584(16)01062–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teng HL, MacLeod TD, Kumar D, Link TM, Majumdar S, Souza RB. Individuals with isolated patellofemoral joint osteoarthritis exhibit higher mechanical loading at the knee during the second half of gait cycle. Clin Biomech (Bristol, Avon) 2015;30(4):383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Debbi EM, Wolf A, Goryachev Y, Rozen N, Haim A. Alterations in sagittal plane knee kinetics in knee osteoarthritis using a biomechanical therapy device. Ann Biomed Eng 2015;43(5): 1089–97. [DOI] [PubMed] [Google Scholar]
- 19.Sharma L, Hurwitz DE, Thonar EJ, Sum JA, Lenz ME, Dunlop DD, et al. Knee addiction moment, serum hyaluronan level, and disease severity in medial tibiofemoral osteoarthritis. Arthritis Rheum 1998;41(7):1233–40. [DOI] [PubMed] [Google Scholar]
- 20.Collins NS, Prinsen CA, Christensen R, Bartels EM, Terwee CB, Roos EM. Knee Injury and Osteoarthritis Outcome Score (KOOS): systematic review and meta-analysis of measurement properties. Osteoarthritis Cartilage 2016, 10.1016/j.joca.2016.03.010. S163–4584(16)01071–2. [DOI] [PubMed] [Google Scholar]
- 21.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis 1957;16(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Han ET, Busse RF, Majumdar S. In vivo T1r mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS). Magn Reson Med 2008;59(2):298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stehling C, Liebl H, Krug R, Lane NE, Nevitt MC, Lynch J, et al. Patellar cartilage: T2 values and morphologic abnormalities at 3.0-T MR imaging in relation to physical activity in asymp-tomatic subjects from the osteoarthritis initiative. Radiology 2010;254(2):509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterfy CG, Guermazi A, Zaim S, Tirman PFJ, Miaux Y, White D, et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 2004;12(3):177–90. [DOI] [PubMed] [Google Scholar]
- 25.Souza RB, Fang C, Luke A, Wu S, Li X, Majumdar S. Relationship between knee kinetics during jumping tasks and knee articular cartilage MRI T1rho and T2 relaxation times. Clin Biomech (Bristol, Avon) 2012;27(4):403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shamonin DP, Bron EE, Lelieveldt BP, Smits M, Klein S, Staring M, et al. Fast parallel image registration on CPU and GPU for diagnostic classification of Alzheimer’s disease. Front Neuroinform 2014;7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein S, Staring M, Murphy K, Biergever MA, Pluim JP. elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging 2010;29(1):196–205. [DOI] [PubMed] [Google Scholar]
- 28.Marquardt D An algorithm for least-squares estimation of nonlinear parameters. J Soc Ind Appl Math 1963;11:431. [Google Scholar]
- 29.Saarakkala S, Julkunen P, Kiviranta P, Makitalo J, Jurvelin JS, Korhonen RK, et al. Depth-wise progression of osteoarthritis in human articular cartilage: investigation of composition, structure and biomechanics. Osteoarthritis Cartilage 2010;18(1):73–81. [DOI] [PubMed] [Google Scholar]
- 30.Daives-Tuck ML, Martel-Pelletier J, Wluka AE, Pelletier JP, Ding C, Jones G, et al. Meniscal tear and increased tibial plateau bone area in healthy post-menopausal women. Osteoarthritis Cartilage 2008;16(2):268–71. [DOI] [PubMed] [Google Scholar]
- 31.Souza RB, Wu SJ, Morse LJ, Subburaj K, Allen CR, Feeley BT. Cartilage MRI relaxation times after arthroscopic partial medial meniscectomy reveal localized degeneration. Knee Surg Sports Traumatol Arthrosc 2015;23(1):188–97. [DOI] [PubMed] [Google Scholar]
- 32.Bhattacharyya T, Gale D, Dewire P, Totterman S, Gale ME, McLaughlin S, et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. J Bone Joint Surg Am 2003;85-A(1):4–9. [DOI] [PubMed] [Google Scholar]
- 33.Sihvonen R, Paavola M, Malmivaara A, Itala A, Joukainen A, Nurmi H, et al. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med 2013;369(26):2515–24. [DOI] [PubMed] [Google Scholar]
- 34.Nieminen MT, Rieppo J, Toyras J, Hakumaki JM, Silvennoinen J, Hyttinen MM, et al. T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med 2001;46(3):487–93. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Cheng J, Lin K, Saadat E, Bolbos RI, Jobke B, et al. Quantitative MRI using T1rho and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging 2011;29(3): 324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X, Pai A, Blumenkrantz G, Carballido-Gamio J, Link TM, Ma CB, et al. Spatial distribution and relationship of T1rho and T2 relaxation times in knee cartilage with osteoarthritis. Magn Reson Med 2009;61(6):1310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marchini J, Presanis A. Comparing methods of analyzing fMRI statistical parametric maps. NeuroImage 2004;22(3):1203–13. [DOI] [PubMed] [Google Scholar]
- 38.Li X, Pedoia V, Kumar D, Rivoire J, Wyatt C, Lansdown D, et al. Cartilage T1ρ and T2 relaxation times: longitudinal reproducibility and variations using different coils, MR systems and sites. Osteoarthritis Cartilage 2015. December;23(12):2214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.