Abstract
Glucose, a key nutrient utilized by human cells to provide cellular energy and a carbon source for biomass synthesis, is internalized in cells via glucose transporters that regulate glucose homeostasis throughout the human body. Glucose transporters have been used as important targets for the discovery of new drugs to treat cancer, diabetes, and heart disease, owing to their abnormal expression during these disease conditions. Thus far, several glucose transport inhibitors have been used in clinical trials, and increasing numbers of natural products have been characterized as potential anticancer agents targeting glucose transport. The present review focuses on natural product glucose transport inhibitors of plant origin, including alkaloids, flavonoids and other phenolic compounds, and isoprenoids, with their potential antitumor properties also discussed.
Keywords: alkaloids, antitumor agents, glucose transport inhibitors, natural products, flavonoids, isoprenoids
1. INTRODUCTION AND SIGNIFICANCE
Glucose is consumed in our daily diet and involved in several biological processes as a precursor for the biosynthesis of the carbon skeleton (Warburg, 1956). It is metabolized initially to pyruvate that can either be converted to lactate or enter the tricarboxylic acid (TCA) cycle [TCA cycle, also named as citric acid cycle (CAC) or Krebs cycle] in mitochondria to produce adenosine triphosphate (ATP) (Fothergill-Gilmore & Michels, 1993; Saraste, 1999). Glucose is the primary source for producing chemical energy in the form of ATP in the majority of human cells, and glucose uptake plays a critical role in cellular survival and growth (Zierler, 1999). However, glucose cannot diffuse passively via the plasma membrane, and it has to be internalized via plasma membrane spanning proteins, namely, transporters of glucose. These include glucose transporters (GLUTs), sodium glucose cotransporters (SGLTs), and the recently discovered sugar will eventually be exported transporters (SWEETs) (Deng & Yan, 2016).
GLUTs, the uniporters encoded by solute carrier 2 (SLC2) to facilitate sugar transport along a concentration gradient, belong to a family of 14 transmembrane proteins. Of these, 11 are glucose transporters, with GLUTs1–4 having been investigated the most comprehensively (Mueckler & Thorens, 2013). GLUT1 is a basal glucose transporter expressed throughout the human body and the primary transporter in human erythrocytes. It is expressed in the majority of human cells to intake glucose, regardless of the intracellular glucose concentration (Burant & Bell, 1992). GLUT2 is expressed mainly in the liver, pancreas, small intestine, and kidney. It has a higher capacity and lower affinity than GLUT1 to transport glucose. The liver takes glucose up rapidly only when the blood glucose level is high, while the pancreas secretes insulin with a lower affinity (Uldry, Ibberson, Hosokawa, & Thorens, 2002). GLUT3 is expressed mainly in the neurons to ensure a constant rate of glucose uptake irrespective of blood glucose levels (Burant & Bell, 1992; Simpson et al., 2008). As the sole member sensitive to insulin in the GLUT family, GLUT4 is expressed primarily in fat tissue and skeletal muscles and stored in intracellular GLUT4 storage vesicles (Huang & Czech, 2007). When insulin binds to and activates insulin receptors in the target cells, GLUT4 is translocated to the plasma membrane to increase the glucose uptake, and then glucose is stored as glycogen in the skeletal muscle (Govers, 2014).
SGLTs are another group of glucose transporters that contain 12 proteins, of which SGLTs1, 2, 4, and 5 are sugar transporters, but SGLT3 is a glucose sensor (Wright, Loo, & Hirayama, 2011). SGLTs are expressed by intestinal brush border cells (enterocytes) and by the cells of proximal tubules in the kidney, and the ratio of sodium coupling to glucose for SGLT1 was found to be different from that for SGLT2 (Hummel et al., 2010). It was evidenced that glucose binds to a glucose sensor that resides on the external face of the enterocyte luminal membrane and generates an intracellular signal to enhance the expression of SGLT1 (Dyer, Vayro, & Shirazi-Beechev, 2003). Inhibition of SGLT2 provides potentially a new option for the treatment of diabetes (Jabbour & Goldstein, 2008).
The ubiquitous SWEETs are new members in the family of glucose transporters. Compared to humans who have one SWEET gene, plants have around 20 different SWEET genes. SWEETs in plants are sugar translocators and play a critical role in suspecting pathogens (Chen et al., 2010; Feng & Frommer, 2015). However, based on our best knowledge, the function of SWEETs in humans is not yet reported.
It is well known that glucose transport plays a critical role in maintaining glucose homeostasis in the heart (Sohn et al., 2013; Ware et al., 2011), and several natural SGLT2 inhibitors have been characterized to benefit to the treatment of cardiovascular and diabetes (Andrianesis, Glykofridi, & Doupis, 2016; Hsia, Grove, & Cefalu, 2017). Moreover, glucose is used to produce energy and to synthesize biomass (Warburg, Wind, & Negelein, 1927), and glucose-uptake is found to be increased during malignancies. Also, glucose-deprivation is toxic to tumors and sensitizes cancer cells to chemotherapy (Schroll, LaBonia, Ludwig, & Hummon, 2017), and cancer cells take up a majority of their glucose in via GLUTs. Thus, these proteins have become attractive targets for the potential treatment or diagnosis of cancer (Granchi, Fancelli, & Minutolo, 2014). For example, a GLUT-specific radioactive analog of glucose, 2-[18F] fluoro-2-deoxy-D-glucose, namely, F-18 FDG, has been used in PET scans, for the diagnosis of cancers (Kim et al., 1992; Ben-Haim & Ell, 2009). GLUT1 has been found to be upregulated in the majority of cancer types, and GLUT2 expression is increased drastically in liver, pancreatic, gastric and colon cancers. In addition, GLUT3 is upregulated in lung, neck, head, ovarian, breast, and bladder cancers, while GLUT4 is overexpressed in colon, lymphoid, breast, and pancreatic tumors (Macheda, Rogers, & Best, 2005; Qian, Wang, & Chen, 2014).
Other GLUTs have been recently found to be upregulated in several cancer types, and many GLUT-inhibitors have shown cancer-related potency, even though no GLUT-inhibitor has been approved as an anticancer agent by the U.S. FDA thus far (Barron, Bilan, Tsakiridis, & Tsiani, 2016; Granchi, Fortunato, & Minutolo, 2014; Granchi, Fortunato, & Minutolo, 2016). In our search for glucose transport inhibitors, the synthetic compound WZB115 was found to exhibit glucose transport inhibitory activity and selective cytotoxicity against MCF7 human breast cancer cells (Liu, Zhang, Cao, Liu, Bergmeier, & Chen, 2010; Zhang, Liu, Chen, & Bergmeier, 2010), and WZB117 has been characterized (Liu et al., 2012). Interestingly, WZB117 reduced the self-renewing capability of pancreatic Panc1, ovarian A2780, and GS-Y03 cancer stem cells (CSC) (Shibuya et al., 2015). It targets GLUT1, GLUT3, and GLUT4 and binds to GLUT4 and the endofacial site of GLUT1 to compete with cytochalasin B (Ojelabi, Lloyd, Simon, De Zutter, & Carruthers, 2016). Recently, SGLT1 and SGLT2 have been found to be overexpressed in mouse models of pancreatic and prostate adenocarcinomas, and canaglifozin, a FDA-approved synthetic SGLT2 inhibitor, was found to potentiate the antitumor efficacy of gemcitabine and to increase tumor necrosis (Scafoglio et al., 2015).
In previous review articles, the overall information included on glucose transport and metabolism in cancer cells and the progress of new therapeutic developments for glucose transport inhibitors focused on small molecules, with only a limit number of natural products, were summarized (Qian, Wang, & Chen, 2014). Natural product sodium glucose cotransporter (SGLT) inhibitors and their potential antidiabetic activity have been reviewed (Blaschek, 2017; Choi, 2016). In addition, synthetic and naturally derived glucose transport inhibitors and their potential anticancer activities have been discussed (Granchi, Fortunato, & Minutolo, 2014; Granchi, Fortunato, & Minutolo, 2016). As an extension of these previous reviews, in the present contribution, plant-derived constituents showing antitumor potential mediated in part through glucose transport inhibition are included, with their plant origin, structures, activities, and mechanisms of action discussed.
2. GLUCOSE TRANSPORT INHIBITORS FROM EDIBLE PLANTS
Increasing numbers of plant-derived natural products showing clinical implications in cancer, cardiovascular diseases, and diabetes have been characterized as GLUT and SGLT inhibitors. Of these, phlorizin, the first naturally occurring sodium glucose co-transport (SGLT) inhibitor, has been used to identify the SGLT mechanism (Vick, Diedrich, & Bauman, 1973). Following this, many natural product glucose transport inhibitors have been characterized, of which several compounds have been tested in vitro and in vivo, and in some cases have reached clinical trials as potential cancer chemotherapeutic agents (Blaschek, 2017; Choi, 2016; Granchi et al., 2016, Qian, Wang, & Chen, 2014).
Edible plants are well known to contribute to the improvement of overall human health, and many edible plant extracts have been investigated recently for their glucose transport inhibitory activity. After an in vitro intestinal glucose transport system was established, in which glucose uptake was measured between apical and basolateral sides of Caco-2 human colon cancer cells, guava [Psidium guajava L. (Myrtaceae)] fruit and leaf extracts were identified as sodium-dependent and -independent glucose transport inhibitors (Müller et al., 2018). An apple [Malus domestica Borkh. (Rosaceae)] extract was found to inhibit methyl-α-D-glucopyranoside (αMDG) transport via hSGLT1 in a dose-dependent manner. Glucose transport was found to be inhibited when everted sacs, segments of the small intestine of male C57BL/6N mice, were treated with radioactive αMDG followed by an apple (M. domestica) extract (Schulze et al., 2014). Similarly, glucose uptake was reduced when the everted gut sacs obtained from male albino rats were treated with an aqueous extract (3.6 mg/ml) of bitter melon [Momordica charantia L. (Cucurbitaceae) (Mahomoodally, Fakim, & Subratty, 2004). Plant phenol-containing seed extracts from the legumes, Vicia faba L. var. equina and Vicia faba L. var. minor (Fabaceae), were found to reduce intestinal glucose transport in male white Wistar rats (Sobrini, Martinez, Ilundain, & Larralde, 1983), and a similar seed extract from the common bean [Phaseolus vulgaris L. (Fabaceae)] reduced glucose transport in the rat ileum (Motilva, Martinez, Ilundain, & Larralde, 1983). Delphinol®, a standardized extract of maqui berries [Aristotelia chilensis (Molina) Stuntz (Elaeocarpaceae)], was found to decrease glucose uptake in sections of the mouse jejunum by inhibition of a sodium glucose transporter, which suppressed glucose increase in the post-prandial blood of individuals who suffered from impaired glucose regulation (Hidalgo et al., 2014). Also, extracts of both Matricaria recutita L. (Asteraceae) (chamomile) and Camellia sinensis (L.) Kuntze (Theaceae) (green tea) reduced glucose uptake in Caco-2-TC7 differentiated cells, with the M. recutita extract found to target GLUT2 in Na+-free conditions and GLUT5-mediated fructose transport (Villa-Rodriguez et al., 2017). The aqueous extract of black tea (C. sinensis) was found to decrease mucosal glucose uptake in male Sprague-Dawley rats by an average of 44%, over a 45-minute period (Kreydiyyeh, Baydoun, & Churukian, 1994).
3. ALKALOID GLUCOSE TRANSPORT INHIBITORS
Alkaloids are found widely in bacteria, fungi, plants, and animals, and exhibit many different types of biological activities (de Sousa Falcão, 2008). Several alkaloids have been found to exhibit glucose transport inhibitory activity. For example, vinblastine (1) (FIGURE 1), a bisindole alkaloid obtained from the Madagascar periwinkle plant, Catharanthus roseus (L.) G.Don (Apocynaceae), was approved by the U.S. FDA in the 1960s as an anticancer drug for the treatment of breast cancer and Hodgkin’s and non-Hodgkin’s lymphomas. It targets β-tubulin to prevent tubulin congregation and suppresses microtubule dynamics at the mitotic spindle leading to M-phase arrest during cell cycle progression (Moudi, Go, Yien, & Nazre, 2013). After a phase I study conducted with 38 patients (17–68 years old) who suffered from an early stage of Hodgkin’s lymphoma (HL) and were treated with injection of ABVD [adriamycin (doxorubicin, 25 mg/m2), bleomycin (10 mg/m2), vinblastine (6 mg/m2), and dacarbazine (375 mg/m2)] on days 1 and 15 (of a 28-day cycle) for 173 cycles, 35 of the patients were in a state of complete remission from HL (Boleti and Maed, 2007). Also, a combination of mitomycin C, vinblastine, and cisplatin (MVP) has been used effectively to treat stage III NSCLC (Ellis et al., 1995). Interestingly, vinblastine was found to inhibit glucose transport through reducing 2-DG uptake in glioma C6 cells (Singh, Gao, Singh, Kunapuli, & Ravindra, 1998).
FIGURE 1.
Structures of alkaloids showing glucose transport inhibitory and potential antitumor activities
Several other indole alkaloids derived from the leaves of Alstonia macrophylla Wall. (Apocynaceae) were found to inhibit SGLT1 and SGLT2 in COS-1 African green monkey kidney fibroblast-like cells, of which 10-methoxy-N(1)-methylburnamine-17-O-veratrate (2) and alstiphyllanine D (3) showed the most potent activity, with IC50 values of 4.0 μM (2) and 5.0 μM (3) against SGLT1 and 0.5 μM (2) and 2.0 μM (3) against SGLT2 (Arai et al., 2010). For these indole alkaloids, substitutions at N1, N4, and C-17 proved to be important for the mediation of SGLT inhibitory activity. Introducing a methyl group at N1 and an aromatic ester unit at the C-17 position enhanced the inhibitory potency, but changing 2 or 3 to a N(4)-oxide resulted in the activity being abolished.
4. FLAVONOID GLUCOSE TRANSPORT INHIBITORS
Flavonoids are a very large group of secondary metabolites found in fruits, vegetables and flowers (Yao et al., 2004). Substitutions in the flavan structure by hydroxy and methyl groups and sugar units create a wide range of flavonoid derivatives (Nijveldt et al., 2001), which may be classified into several major structural subtypes. The flavonoids are well- known for their anti-inflammation and effects on the reduction of nitric oxide synthase (thus reducing ischemia-reperfusion injury) (Nijveldt et al., 2001), and several of these compounds have been identified as GLUT and/or SGLT inhibitors.
4.1. Anthocyanins
Anthocyanins are phenolic compounds found in various berry fruits, of which pelargonidin-3-O-glucoside (4) (FIGURE 2), characterized from strawberries [Fragaria virginiana Duchesne (Rosaceae)], was found to exhibit glucose-uptake inhibitory activity in Caco-2 human colon cancer cells, with an IC50 value of 705 μM (Manzano & Williamson, 2010).
FIGURE 2.
Structures of an anthocyanin, chalcones, and a modified chalcone lactone showing glucose transport inhibitory and potential antitumor activities
4.2. Chalcones
Many chalcones (1,3-diaryl-2-propen-1-ones) isolated from edible plants exhibit potential antimalarial, antiviral, and antiinflammatory activities (Nowakowska, 2007). Of these, phloretin (5) (Figure 2), a dihydrochalcone derived from the apple tree [Malus domestica Borkh. (Rosaceae)] (Gosch, Halbwirth, & Stich, 2010), was found to inhibit competitively 3-O-methyl-D-glucose uptake via GLUT1 in human erythrocytes (Martin, Kornmann, & Fuhrmann, 2003). Phlorizin (6), a glucoside of phloretin (5), was obtained as the first SGLT2 inhibitor from the apple tree (Malus domestica) (Andrianesis, Glykofridi, & Doupis, 2016). Both phloretin (5) and phlorizin (6) inhibited GLUT4-mediated glucose transport, with IC50 values of 9.4 μM (5) and 140 μM (6), indicating that introducing a glucose unit results in the GLUT4 inhibitory activity being decreased (Kasahara & Kasahara, 1997). The potential antitumor activity of phloretin (5) has been reviewed recently (Choi, 2019). Also, it potentiated the antiproliferative effect of paclitaxel in HepG2 human liver cancer cells. Tumor growth was inhibited when six–seven-week-old NOD.CB17-PRKDC(SCID)/J (NOD-SCID) mice were inoculated with HepG2 cells and treated (i.p.) with 5 (10 mg/kg) plus paclitaxel (1 mg/kg) thrice a week for six weeks (Yang et al., 2009).
Xanthohumol (7), a prenylated chalcone isolated from hops [Humulus lupulus L. (Cannabaceae)], reduced [3H-2-DG] uptake in HTR-8/SVneo human first-trimester extravillous trophoblast cells, with an IC50 value of 3.6 μM. This activity was proposed to be mediated through three major intracellular signaling pathways, namely, the mTOR, tyrosine kinases (TKs), and c-Jun N-terminal kinases (JNK) pathways (Correia-Branco et al., 2015). The potential antitumor activity of xanthohumol (7) has been reviewed recently (Jiang, Sun, Xiang, Wei, & Li, 2018). For example, pancreatic tumor growth was inhibited when nude mice were inoculated with Panc1 human pancreatic cancer cells and treated (i.p.) daily with 7 (25 mg/kg) for 27 days (Jiang et al., 2015).
(+)-Cryptocaryone (8), a modified chalcone lactone isolated from Cryptocarya rubra C.R. Skeels. (Lauraceae), was found to exhibit potent cytotoxicity against HT-29 human colon cancer cells, with an IC50 value of 0.32 μM. At a concentration of 30 μM, this compound inhibited significantly glucose transport in H1299 human lung cancer cells, indicating that it may mediate its cytotoxicity at least in part through interaction with glucose transporters (Ren et al., 2014).
4.3. Flavan Derivatives
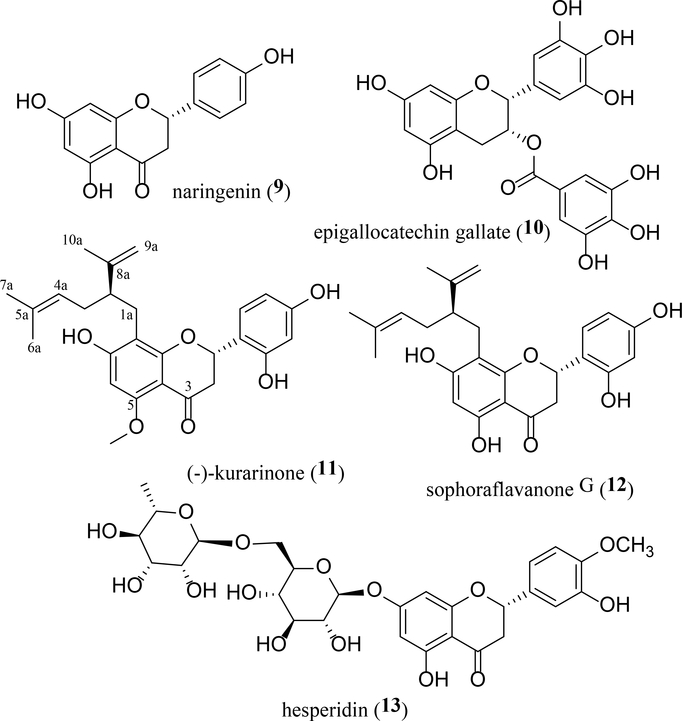
Flavanones are distributed widely in citrus fruits and exhibit inhibitory activity towards chemically induced colon cancer progression (Nijveldt et al., 2001; Yao et al., 2004). Naringenin (9) (FIGURE 3), a flavanone isolated from grapefruit [Citrus paradisa Macfad. (Rutaceae)], was found to decrease the 2-DG uptake in differentiated 3T3-L1 cells, with IC50 values of 61 μM and 71 μM under basal and insulin stimulated conditions, respectively (Claussnitzer, Skurk, Hauner, Daniel, & Rist, 2011). The potential antitumor activity of naringenin (9) has been discussed in a recent review article (Salehi et al., 2019). In an in vivo investigation, tumor metastasis was inhibited when four-week-old female BALB/c mice were inoculated with 4T1 mouse breast cancer cells that were transduced with a TGF-β1 generating 4T1/TGF-β1 cells and treated (orally) daily with 9 (200 mg/kg suspended in 1% carboxyl methyl cellulose) for 30 days (Zhang et al., 2016).
FIGURE 3.
Structures of flavan derivatives showing glucose transport inhibitory and potential antitumor activities
Epigallocatechin gallate (EGCG, 10), a flavan ester found in green tea [Camellia sinensis (L.) Kuntze (Theaceae)], inhibited hGLUTs 1, 3, and 4 by binding to their exofacial site (Ojelabi, Lloyd, De Zutter, & Carruthers, 2018) and decreased glucose and fructose transport via GLUT2. It also inhibited fructose uptake via GLUT5 in Xenopus laevis oocytes that were overexpressed with these GLUTs (Gauer, Tumova, Lippiat, Kerimi, & Williamson, 2018). As reviewed previously, EGCG (10) shows potential antitumor activity (Gan, Li, Sui, & Corke, 2018). In an in vivo study, tumor growth was inhibited when six–eight-week-old female BALB/c nude mice were inoculated with SGC-7901 human gastric cancer cells and treated (i.p.) daily with 10 (1.5 mg/per mice) for four weeks (Zhu et al., 2007).
(−)–Kurarinone (11) and sophoraflavanone G (12) (FIGURE 3) are prenylated flavanones characterized as potent Na+-glucose cotransporter (SGLT) inhibitors from the roots of Sophora flavescens Aiton (Fabaceae). In COS-1 cells, both 11 and 12 inhibited SGLT1, with IC50 values of 10.4 and 18.7 μM, respectively. They also showed SGLT2 inhibitory activity, with IC50 values of 1.7 μM (11) and 4.1 μM (12) (Sato, Takeo, Aoyama, & Kawahara, 2007). Investigation of the activity of these flavans and their analogues (Sato, Takeo, Aoyama, & Kawahara, 2007) indicates that the SGLT inhibitory effect decreases slightly when the C-5 methoxy group is replaced by a hydroxy group or when the dihydropyrone C ring opens to convert the molecule to an analogous chalcone. In addition, the activity is weakened when a hydroxy group is introduced at the C-3 position, and the activity declines greatly when the C-4a/5a double bond is saturated followed by introduction of a hydroxy group at the C-5a position.
Interestingly, both (−)–kurarinone (11) and sophoraflavanone G (12) were found to exhibit cytotoxicity toward a panel of human cancer cell lines, with IC50 values being in the range 2–27 μg/mL, of which the potency was weakened slightly by methylation of the hydoxy group at the C-5 or C-2’ position (Sun et al., 2007). In an in vivo study, lung tumor growth was inhibited when four–six-week-old athymic nu/nu (BALB/c) mice were inoculated with A549 human lung cancer cells and treated (i.p.) daily with 11 (20 or 40 mg/kg) for 27 days (Yang et al., 2018). Mechanistically, (−)–kurarinone (11) was found to mediate its cytotoxicity toward H1688 human SCLC cells through the mitochondrial- and receptor-mediated apoptotic pathways (Chung, Lin, Lin, Chan, & Yang, 2019).
Hesperidin (13), a flavanone glycoside identified from the orange [Citrus sinensis (L.) Osbeck (Rutaceae)], was found to reduce the uptake of 14C-glucose in Caco-2-TC7 cells at concentrations 80 and 800 μM, and transport of both 14C-glucose (via GLUT2) and 14C-fructose (via GLUT5) was inhibited by 13 in Xenopus laevis oocytes that were microinjected with GLUT2 and GLUT5 mRNAs (Kerimi et al., 2019). Also, hesperidin (13) induced significantly a reduced mouse hepatic GLUT2 expression and an elevated the mouse adipocyte GLUT4 level when male five-week-old C57BL/KsJ-db/db mice were fed with food supplemented with 13 (0.2 g/kg) for five weeks (Jung, Lee, Park, Kang, & Choi, 2006).
As reviewed previously, hesperidin (13) showed potential antitumor efficacy (Devi et at., 2015). In a phase clinical study conducted on healthy human subjects (18–75 years old) in three independent investigations using different amounts of orange juice supplemented with hesperidin, hesperidin-containing orange juice was found to modulate postprandial blood glucose levels by partially inhibiting intestinal GLUTs (Kerimi et al., 2019). Also, this compound was shown cytotoxicity toward various types of human cancer cells and other evidences of potential antitumor activity, as summarized in a previous review (Devi et al., 2015).
4.4. Flavones
Apigenin (14) (FIGURE 4), one of the flavone components of apple skin [Malus domestica Borkh. (Rosaceae)] (Nijveldt et al., 2001), inhibited 2-DG uptake in CD18 and S2–013 human pancreatic cells at a concentration of 25 μM, with both GLUT1 gene and protein expression reduced in these cells (Melstrom et al., 2008). The cytotoxicity against Hep-2 human laryngeal carcinoma cells of cisplatin was enhanced by 14, owing to the reduced expression of GLUT1 and p-Aκt [p-protein kinase B (PKB)] proteins in Hep-2 cells (Xu et al., 2014). Also, [14C]-glucose uptake via GLUT2 and GLUT7 was inhibited when Xenopus laevis oocytes were transfected with mRNAs of these GLUTs and treated with 14 (Gauer, Tumova, Lippiat, Kerimi, & Williamson, 2018).
FIGURE 4.
Structures of flavones showing glucose transport inhibitory and potential antitumor activities
Luteolin (15), a flavone component of celery [Apium graveolens L. (Apiaceae)] (Nijveldt et al., 2001), reduced 2-DG uptake at a concentration of 10 μM in differentiated mouse MC3T3-G2/PA6 adipose cells via GLUT4 and decreased the insulin stimulated phosphorylation of insulin receptor B and Aκt activation, to lower the levels of plasma membrane-translocated GLUT4. Structure-activity relationship studies indicated that the C-2 and C-3 double bond and 4’- or 3’,4’-hydroxy groups are critical for 15 to mediate its GLUT4 inhibitory activity (Nomura et al., 2008).
As concluded in a recent review article, luteolin (15) is a promising antitumor agent (Imran et al., 2019). It inhibited MDA-MB-231 human breast cancer cell growth and reduced cell migratory and invasive capabilities at a concentration of 100 μM. Both mRNA and protein expression of Notch-1, MMP2, MMP9, VEGF in MDA-MB-231 cells were reduced after 48 hour-treatment with 15 (Sun et al., 2015). Tumor growth was inhibited when six–eight-week-old BALB/c mice were inoculated with MDA-MB-231 cells and treated daily with 15 (20 mg/kg) via tail vein injection for 14 consecutive days (Sun et al., 2015), while tumor growth was inhibited when six–eight-week-old BALB/c mice were inoculated with LoVo human colon cancer cells and treated (i.p.) daily with 15 (20 mg/kg, alternate days) for one month (Chen, Zhang, Gao, & Shi, 2018).
Quercetin (16) is a flavone found in lettuce [Lactuca sativa L. (Asteraceae)], olives [Olea europaea L. (Oleaceae)], onions [Allium cepa L. (Amaryllidaceae)], and parsley [Petroselinum crispum (Mill.) Fuss (Apiaceae)] (Nijveldt et al., 2001). It inhibited GLUT1-, 3-, and 4-mediated 2-DG uptake in HEK 293 human embryonic kidney cells, with respective IC50 values of 2.0 μM (GLUT1), 17.7 μM (GLUT3), and 1.7 μM (GLUT4) (Ojelabi et al., 2018). It also inhibited 2-DG transport in L929 mouse fibroblasts by binding to an exofacial site on GLUT1, with an IC50 value of 8.5 μM (Hamilton et al., 2018).
The potential antitumor activity of quercetin (16) has been reviewed recently (Rauf et al., 2018). Earlier, it has been tested as a potential anticancer drug in a phase I clinical trial. Fifty-one patients (18–75 years old) who were suffering from different cancers were selected for the study. Quercetin dehydrate powder was dissolved in DMSO at 50 mg/ml for doses up to 945 mg/m2 or 100 mg/ml for higher doses (up to 1700 mg/m2). The rate of intravenous (i.v.) injection was rapid (in 30 seconds) for the initial 60 mg/m2 dose, but the doses above 945 mg/m2 were given in five minutes. Dose levels from the level 1 (60 mg/m2) to the level 10 (1700 mg/m2) were decided based on the amount of quercetin (16) used. A bolus dose of 1400 mg/m2 was proposed either weekly or at a three-week interval for a possible phase II clinical trial (Ferry et al., 1996).
Fisetin (17), a flavone derived from citrus fruits (Nijveldt et al., 2001), inhibited 2-DG uptake in myelocytic U937 and lymphocytic Jurkat cells in a dose-dependent manner when tested at 1–100 μM concentrations, which is more potent than either apigenin (14) or quercetin (16) (Park, 1999). As reviewed previously, fisetin (17) showed antitumor efficacy against several different types of animal models (Lall, Adhami, & Mukhtar, 2016). Fisetin (17) suppressed 451Lu human melanoma cell growth, with the IC50 values being estimated as 80 μM, 37.2 μM, and 17.5 μM for the 24-, 48-, and 72-h treatments, respectively. Tumor growth was inhibited when athymic (nu/nu) female nude mice were inoculated with 451Lu cells and treated (i.p.) with 17 (45 mg/kg, twice a week) for 45 days (Syed et al., 2011). Mechanistically, fisetin (17) mediates its cytotoxicity toward 451Lu cells through reducing the key G1 phase cell cycle regulatory proteins cyclin-dependent-kinases (cdk-2, −4, and −6) and down-regulating proteins in the Wnt pathway (Syed et al., 2011). In addition, fisetin (17) showed a synergistic effect with sorafenib when five-week-old BALB/c female nude mice were inoculated with HeLa human cervical cancer cells and treated orally with sorafenib (10 mg/kg) or sorafenib (10 mg/kg) plus 17 (4 mg/kg) twice a week for five weeks (Lin et al., 2016).
The blood brain barrier (BBB) is a key regulator of glucose availability to glial and neuronal cells in the brain, and hCMEC/D3, an endothelial BBB cell line, is a good model to investigate the BBB mediated glucose transport in vitro. Myricetin (18), a flavone derived from fruit peels (Nijveldt et al., 2001), decreased 2-DG uptake in hCMEC/D3 cells as an inhibitor of glucose transport in a concentration-dependent manner when tested at concentration of 30, 100, and 300 μM (Meireles et al., 2013).
As summarized previously, myricetin (18) is a potential antitumor lead compound (Devi, Rajavel, Habtemariam, Nabavi, & Nabavi, 2015). It exhibited cytotoxicity toward T24 human bladder cancer cells (IC50 85 μM with 24 h treatment) by increasing the number of cells in the G2/M phase of the cell cycle and decreased the migratory capacity of these cells. Tumor growth was suppressed when four-week-old female BALB/c nude mice were inoculated with T24 cells and treated (i.p.) daily with 18 (5 mg/kg) for five days (Sun et al., 2012).
4.5. Isoflavones
Isoflavones are found especially in soy [Glycine max (L.) Merr. (Fabaceae)] and in other leguminous seeds. They function as a suppressive agent for the treatment of chemically induced mammary cancer without apparent reproductive or endocrinological toxicities (Zaheer & Akhtar, 2017). Two isoflavonoids, genistein (19) and daidzein (20) (FIGURE 5) derived from soy (Glycine max) (Taylor, Levy, Elliot, & Burnett, 2009), were found to inhibit glucose uptake competitively via GLUT1 in human erythrocytes (Martin, Kornmann, & Fuhrmann, 2003).
FIGURE 5.
Structures of isoflavones, a stilbenoid, an arylheptanoid, a flavonolignan, and an isoprenoid showing glucose transport inhibitory and potential antitumor activities
The potential antitumor efficacy of genistein (19) has been reviewed previously (Taylor, Levy, Elliot, & Burnett, 2009). It has also been tested in clinical trials either alone or with certain FDA-approved anticancer drugs. For example, in a block-randomized double blind phase II clinical trial to determine the efficacy and safety of short-term genistein intervention in patients with localized prostate cancer, 23 patients were selected and treated with genistein (19), and possible therapeutic effects in the early stage localized prostate cancer were predicted for this isoflavone (Lazarevic et al., 2011).
In another phase II clinical trial, 60 patients (median age 71 years) with bladder cancer were selected and treated (orally) daily with genistein (19) in capsules (G-2535) for 14 or 21 days or up to 30 days if surgery was delayed. G-2535 was found to be well-tolerated, and no significant differences were found in the treatment group (G-2535) and the placebo in terms of adverse effects. Also, the phosphorylated epidermal growth factor receptor was significantly reduced in 19-treated group compared to placebo (Messing et al., 2012).
4.6. Stilbenoid
Stilbenes are 1,2-diarylethene derivatives found exclusively in liverworts and higher plants, with ring A substituted with two hydroxy groups in the meta-position and with ring B substituted with hydroxy and methoxy groups in the ortho-, meta- and/or para-positions (Cassidy, Hanley, & Lamuela-Raventos, 2000). trans-Resveratrol (21), a stilbene occurring in peanuts [Arachis hypogaea L. (Fabaceae)] and grapes [Vitis vinifera L. (Vitaceae)], exhibits antibacterial, antifungal, and antitumor activities (Aluyen et al., 2012). As a GLUT1 and GLUT3 inhibitor, trans-resveratrol (21) inhibited competitively 2-DG uptake in human U937 histiocytic lymphoma and HL-60 leukemia cells, both in a dose-dependent manner (20–120 μM) (Park, 2001). It binds to the endofacial site of GLUT1 to reduce the amount of this glucose transporter bound to cytochalasin B (Salas, et al., 2013). It reduced [3H-2-DG] uptake in A2780 human ovarian cancer cells and further reduced ATP and lactate production, owing to its induction of autophagy and decrease of cellular metabolic activities (Kueck et al., 2007). Importantly, this stilbene was found to inhibit insulin-stimulated glucose uptake in human fat cell suspensions (Gomez-Zorita, Tréguer, Mercader, & Carpéné, 2013).
trans-Resveratrol (21) also showed potential antitumor efficacy and has been tested in several cancer clinical trial studies (Berman, Motechin, Wiesenfeld, & Hold, 2017). In a phase I randomized double-blind clinical trial, nine subjects who had confirmed stage IV colorectal cancer and hepatic metastases were selected and administered with trans-resveratrol (21) at 5.0 g as SRT501, a mixture of 21 in an aliquot of 4 ml sodium docusate solution, for six weeks. The dose was deemed to be safe and tolerated, and the further immunohistochemistry analysis of tumor tissue revealed that caspase-3 was increased in the SRT501 treated group compared to placebo (Howells et al., 2011).
5. OTHER OXYGEN HETEROCYCLIC AND PHENOLIC COMPOUDS
Several plant-derived oxygen heterocyclic and phenolic compounds exhibit both glucose transport inhibitory and potential antitumor activities, including curcumin (22) and silibinin (23). These two compounds showed promising in vivo antitumor efficacy, and silibinin (23) has also been evaluated in clinical trials as a potential anticancer drug for the treatment of prostate and hepatocellular cancers.
5.1. Diarylheptanoid
Turmeric [Curcuma longa L. (Zingiberaceae)] is a major source of various curcuminoids, of which curcumin (22) shows antitumor, radioprotective, and cardioprotective effects (Amalraj, Pius, Gopi, & Gopi, 2017). It was found to reduce GLUT1 gene and protein expression in NSCLC A549 cells transfected with pcDNA3.1-GLUT1 vector, and it also decreased the invasive capability of A549 cells by reducing expression of matrix metalloproteinases (MMPs) 1 and 2. This ultimately resulted in reduction of proliferation rates of untransfected and GLUT1 transfected A549 cells (Liao, Wang, Deng, Ren, & Li, 2015). Curcumin (22) has been subjected to many in vivo studies to date relative to its potential antitumor efficacy (Amalraj, Pius, Gopi, & Gopi, 2017). For example, it reduced metastasis when five–six-week-old BALB/c nude mice were inoculated with untransfected A549 cells and treated (i.p.) daily with 22 (200 μg/kg) for four weeks (Liao, Wang, Deng, Ren, & Li, 2015).
5.2. Flavonolignan
As a competitive inhibitor of GLUT4, silibinin (23, also known as silibin or silybin) (FIGURE 5), a flavonolignan obtained from the extract of the milk thistle Silybum marianum (L.) Gaertn. (Asteraceae), reduced basal and insulin-dependent 2-DG uptake in differentiated 3T3-L1 adipocytes at a concentration of 40 μM (Zhan, Digel, Küch, Stremmel, & Füllekrug, 2011). In addition, silymarin, an extract of S. marianum with 23 as a key active component, inhibited cell growth through increasing cell cycle regulation proteins in DU145 human prostate cancer cells (Zi, Grasso, Kung, & Agarwal, 1998). Tumor growth was inhibited when athymic nude (nu/nu) male mice were inoculated with DU145 cells and fed with 0.05% or 0.1% of 23 (w/w) containing diet for 60 days, or when athymic nude mice were fed with 0.05% or 0.1% of 23 (w/w) for three weeks, inoculated by DU145-cells, and then fed with 0.05% or 0.1% of 23 (w/w) for additional six weeks. No apparent sign of toxicity was observed in mice, and insulin-like growth factor-binding protein-3 (IGFBP-3) levels were found increased in mouse plasma (Singh et al., 2002). These in vivo studies indicate that silibinin (23) exhibits both antitumor and tumor preventive properties, and these activities might be mediated mechanistically through an IGFBP-related pathway, such as cell survival (antiapoptotic) signaling via IGFBP-IGF-1/IGF-1R pathway (Singh et al., 2002).
In a phase I clinical trial for toxicity of high-dose silibinin in a phytosome [silybin-phytosome (Siliphos®)] and a dose recommended for a phase II study, 13 prostate cancer patients with the median age of 70 years were selected and administered orally with 2.5, 5, 10, 13, 15, and 20 g of Siliphos® for a total of 91 courses (four weeks for each course). Dose limiting toxicity was defined as grade 3 or 4 non-hematologic toxicity, or grade 4 hematologic toxicity, and a daily oral dosage of 13 g in three divided doses of Siliphos® was recommended for a phase II clinical trial for the treatment of prostate cancer (Flaig et al., 2007). However, a phase II clinical trial that was conducted on 12 prostate cancer patients with the median age of 57 years who were given orally with 13 g of Siliphos® for 14–31 days was not continued further, owing to the low tissue penetration of 23 (Flaig et al., 2010).
Silibinin (23) was also tested in a phase I clinical trial for advanced hepatocellular carcinoma (AHC). Three male patients (mean age 53 years) suffering from AHC were enrolled for the treatment during a span of 12 weeks with Siliphos®. However, the trial ended without conclusions, owing to deaths of all patients (Siegel et al., 2014).
6. Isoprenoids
Isoprenoids, including terpenoids as a major group, are a large and diverse class of natural products composed of two or more isoprenyl groups that connect each other in different modes. Among these compounds, gossypol is a dimeric sesquiterpene derived from species of the Malvaceae family, including Thespesia populnea (L.) Sol. ex Corrêa (Boonsri, Karalai, Ponglimanont, Chantrapromma, & Kanjana-opas, 2008) and Gossypium barbadense L. (extra-long staple cotton) (Malvaceae) (Dowd, & Pelitire, 2006). Gossypol is an axial chiral compound and exists naturally as an enantiomeric mixture, owing to the restricted rotation of its internaphthyl 2,2’-bond, which results in helical M [(–)-gossypol] and P [(+)-gossypol (24)] isomers (Freedman, Cao, Oliveira, Cass, & Nafie, 2003). Gossypol showed GLUT1 inhibitory potency to reduce 2-DG in HL-60 human leukemia cells and human erythrocytes (Pérez et al., 2009), and it also exhibits a broad spectrum of cytotoxicity toward various human cancer cells. For example, all of (±)-gossypol, (+)-gossypol, and (–)-gossypol were found to show non-selective antiproliferative activity against a panel of human cancer cell lines, with IC50 values being in the range 0.3–6.1 μg/mL, which was attributable primarily to the content of (–)-gossypol (Band et al., 1989). Apoptosis was induced in HL60 human promyelocytic leukemia cells when cells were treated with gossypol acetic acid at concentrations of 50 μM and 100 μM (Balci, Sahin, & Ekmekci, 1999). Interestingly, (+)-gossypol (24), isolated from the wood of Thespesia populnea, showed potent cytotoxicity toward human HeLa cervical and KB oral epidermoid cancer cells, with IC50 values of 80 and 40 ng/mL, respectively. However, an analogue of 24, (+)-6,6′-methoxygossypol, did not show such activity, indicating that methylation of hydroxy group at the C-6 and C-6’ position of 24 results in its cytotoxic potency against HeLa and KB cells being greatly decreased (Boonsri, Karalai, Ponglimanont, Chantrapromma, & Kanjana-Opas, 2008).
In a phase I/II clinical trial study, 20 women patients who suffered with metastatic breast cancer refractory to doxorubicin and paclitaxel received oral gossypol daily for four weeks, using doses between 30 and 50 mg. The maximal tolerated dose (MTD) of 40 mg/day with a median serum gossypol concentration of 271 ng/ml was found to be tolerated, but no therapeutic responses were observed. However, alterations in cyclin D1 and Rb expression and a decrease in serial serum tumor marker values (CEA, BR2729 or CA15–3) observed in these patients suggest a potential role for gossypol to be used in conjunction with other cell cycle-specific compounds (Van Poznak et al., 2001).
Oridonin (25), a diterpene derived from the whole tea plant of Rabdosia rubescens (Hemsl.) H.Hara (Lamiaceae) (Yang, Lin, & Wei, 2017), was found to decrease the glucose uptake and subsequently reduced the expression of GLUT1 mRNA and protein in SW480 human colorectal cancer cells (Yao et al., 2017). It inhibited the growth of multiple myeloma, acute lymphoblastic T-cell leukemia (Jurkat), and adult T-cell leukemia (MT-1) cells, with an ED50 value being in the range 0.75–2.7 μg/mL (Ikezoe et al., 2005). In addition, oridonin (25) reduced expression of p-AMPK (an ATP-sensor), induced autophagy, and inhibited the proliferation of SW480 cells. Tumor growth was inhibited when six-week-old BALB/c nude mice were inoculated with SW480 cells and treated (i.p.) with 25 (15 mg/kg, every alternate day) for two weeks (Yao et al., 2017). Mechanistically, oridonin (25) caused apoptosis of MT-1 cells, down-regulated levels of Mcl-1 and BCL-xL, but not Bcl-2 protein, in both MT-1 and RPMI8226 cells, and inhibited NF-κB activity in MT-1 cells, indicating that this diterpenoid might be useful as adjunct therapy for individuals with lymphoid malignancies, including the lethal disease, adult T-cell leukemia (Ikezoe et al., 2005).
7. Conclusions
Glucose is a key nutrient utilized for the production of cellular energy in the form of ATP and for the synthesis of biomass, and glucose transporter proteins, including GLUTs, SGLTs, and SWEETs, are responsible for the uptake of glucose in human cells and have become attractive targets for the discovery of potential anticancer agents. Thus far, many plant-derived inhibitors of glucose transport have been discovered, which target mainly GLUTs 1–4, 5, and 7 and SGLTs 1 and 2 (TABLE 1). Interestingly, most of these glucose cotransport inhibitors are either major components of edible plants or analogues of these plant-derived compounds, indicating that these natural inhibitors may be used to address the management of the toxicities observed from the currently-used anticancer drugs. Thus, characterization of novel small-molecule naturally occurring glucose transport inhibitors, especially SWEET inhibitors, should be a promising strategy for the development of new anticancer drugs.
TABLE 1.
Plant-derived glucose transport inhibitors with potential antitumor activity
| No | Compound | Plant Origin | Biological Potency | Reference |
|---|---|---|---|---|
| 1 | Vinblastine | Catharanthus roseus | Reduced 2-DG uptake in glioma C6 cells and has been used effectively to treat stage III NSCLC | Boleti et al. (2007), Moudi et al. (2013), Ellis et al. (1995), Singh et al. (1998) |
| 2 | 10-methoxy-N(1)-methylburnamine-17-O-veratrate | Alstonia macrophylla | Inhibited SGLT1 (IC50 4.0 μM) and SGLT2 (IC50 0.5 μM) in COS-1 cells | Arai et al., 2010 |
| 3 | Alstiphyllanine D | Alstonia macrophylla | Inhibited SGLT1 (IC50 5.0 μM) and SGLT2 (IC50 2.0 μM) in COS-1 cells | Arai et al. (2010) |
| 4 | Pelargonidin-3-O-glucoside | Fragaria virginiana | Inhibited glucose uptake in Caco-2 cells (IC50 705 μM) | Manzano et al. (2010) |
| 5 | Phloretin | Malus domestica | Inhibited GLUT4-mediated glucose transport (IC50 9.4 μM) in GLUT4 cells and suppressed HepG2 xenograft tumor growth (10 mg/kg) | Andrianesis et al. (2016), Gosch et al. (2010), Kasahara et al. (1997), Yang et al. (2009) |
| 6 | Phlorizin | Malus domestica | Inhibited GLUT4-mediated glucose transport (IC50 140 μM) in GLUT4 cells | Andrianesis et al. (2016), Kasahara et al. (1997) |
| 7 | Xanthohumol | Humulus lupulus | Reduced [3H-2-DG] uptake in HTR-8/SVneo cells (IC50 3.6 μM) and suppressed pancreatic tumor growth (25 mg/kg) | Correia-Branco et al. (2015), Jiang et al. (2015) |
| 8 | (+)-Cryptocaryone | Cryptocarya rubra | Reduced glucose uptake in H1299 cells and showed cytotoxicity against HT-29 cells (IC50 0.32 μM) | Ren et al. (2014) |
| 9 | Naringenin | Citrus paradisa | Decreased the 2-DG uptake in differentiated 3T3-L1 cells and inhibited 4T1 breast mouse tumor metastasis (200 mg/kg) | Claussnitzer et al. (2011), Salehi et al. (2019), Zhang et al. (2016) |
| 10 | Epigallocatechin gallate | Camellia sinensis | Inhibited GLUT2 and GLUT5 in Xenopus ooctyes and suppressed SGC-7901 gastric xenograft tumor growth (1.5 mg/kg) | Ojelabi et al. (2018), Gauer et al. (2018), Zhu et al. (2007) |
| 11 | (−)-Kurarinone | Sophora flavescens | Inhibited SGLT1 (IC50 10.4 μM) and SGLT2 (IC50 1.7 μM) in COS-1 cells and showed cytotoxicity against a panel of human cancer cell lines (IC50 2–27 μg/mL) | Sato et al. (2007), Sun et al. (2007) |
| 12 | Sophoraflavanone G | Sophora flavescens | Inhibited SGLT1 (IC50 18.7 μM) and SGLT2 (IC50 4.1 μM) in COS-1 cells and showed cytotoxicity against a panel of human cancer cell lines (IC50 2–27 μg/mL) | Sato et al. (2007), Sun et al. (2007) |
| 13 | Hesperidin | Citrus sinensis | Inhibited GLUT2 and GLUT5 in Xenopus ooctyes and showed potential antitumor activity | Kerimi et al. (2019), Jung et al. (2006) |
| 14 | Apigenin | Malus domestica | Inhibited GLUT2 and GLUT7 in Xenopus ooctyes and enhanced cytotoxicity against Hep-2 cells of cisplatin | Nijveldt et al. (2001), Melstrom et al. (2008) |
| 15 | Luteolin | Apium graveolens | Inhibited GLUT4 in MC3T3-G2/PA6 adipose cells and invasive capability of MDA-MB-231 cells | Imran et al. (2019), Nijveldt et al. (2001), Nomura et al. (2008), Sun et al. (2015) |
| 16 | Quercetin | Allium cepa Lactuca sativa Petroselinum crispum Olea europaea | Inhibited GLUT1 (IC50 2.0 μM), GLUT3 (IC50 17.7 μM), and GLUT4 (IC50 1.7 μM) in HEK293 cells and showed anticancer potentials in a phase I clinical trial (1400 mg/m2) | Nijveldt et al. (2001), Ojelabi et al. (2018), Rauf et al. (2018), Ferry et al. (1996) |
| 17 | Fisetin | Citrus fruits | Reduced 2-DG uptake in U937 and Jurkat cells and showed cytotoxicity against 451Lu cells | Nijveldt et al. (2001), Park (1999), Lall et al. (2016), Syed et al. (2011) |
| 18 | Myricetin | Fruit peels | Decreased 2-DG uptake in hCMEC/D3 cells, showed cytotoxicity against T24 cells (IC50 85 μM), and suppressed T24 bladder tumor growth (5 mg/kg) | Nijveldt et al. (2001), Meireles et al. (2013), Devi et al. (2015), Sun et al. (2012) |
| 19 | Genistein | Glycine max | Inhibited GLUT1 in human erythrocytes and showed anticancer potential in a phase I clinical trial for the treatment of prostate cancer and in a phase II clinical trial for the treatment of bladder cancer | Lazarevic et al. (2011), Martin et al. (2003), Messing et al. (2012), Taylor et al. (2009) |
| 20 | Daidzein | Glycine max | Inhibited GLUT1 in human erythrocytes | Taylor et al. (2009), Martin et al. (2003) |
| 21 | trans-Resveratrol | Arachis hypogaea Vitis vinifera | Inhibited 2-DG uptake in U937 and HL-60 cells and showed anticancer potential in a phase I clinical trial for the treatment of colon cancer | Aluyen et al. (2012), Salas, et al. (2013), Howells et al. (2011) |
| 22 | Curcumin | Curcuma longa | Inhibited GLUT1 in A549 cells and suppressed A549 xenograft lung tumor growth (200 μg/kg) | Amalraj et al. (2017), Liao et al. (2015) |
| 23 | Silibinin | Silybum marianum | Reduce basal and insulin-dependent 2-DG uptake in differentiated 3T3-L1 adipocytes at 40 μM and suppressed DU145 xenograft tumor growth | Singh et al. (2002), Zhan et al. (2011), Zi et al. (1998) |
| 24 | (+)-Gossypol | Thespesia populnea Gossypium barbadense | Inhibited GLUT1 in HL-60 cells and in human erythrocytes and showed cytotoxicity against a panel of human cancer cell lines (IC50 0.3–6.1 μg/mL) | Band et al. (1989), Balci et al. (1999), Boonsri et al. (2008), Freedman et al. (2003), Pérez et al. (2009) |
| 25 | Oridonin | Rabdosia rubescens | Inhibited GLUT1 in human SW480 cells and showed cytotoxicity against Jurkat/MT-1 cells (ED50 0.75–2.7 μg/mL) | Ikezoe et al. (2005), Yang et al. (2017), Yao et al. (2017) |
ACKNOWLEDGMENTS
The experimental studies by our group mentioned in this article were supported by an Administrative Supplement from NCCIH, NIH, to grant P01 CA125066, awarded to Professor A. Douglas Kinghorn, by the National Cancer Institute, NIH, Bethesda, MD, and also supported partially by John J. Kopchick Internship grant awarded to Pratik Shriwas, the Edison Biotechnology Institute of Ohio University and Ohio University Heritage College of Osteopathic Medicine. We are very grateful to many faculty colleagues, research staff, postdoctoral fellows, and graduate students who have contributed to this work.
Abbreviations
- αMDG
Methyl-α-D-glucopyranoside
- Aκt
Protein kinase B (PKB)
- ATP
Adenosine triphosphate
- BBB
Blood brain barrier
- Caco-2 cells
human colon cancer cells
- CHO cells
Chinese hamster ovary cells
- COS-1 cells
African green monkey kidney fibroblast-like cells
- CSC
Cancer stem cells
- 2-DG
2-Deoxy-glucose
- GLUT
Glucose transporter
- HL
Hodgkin’s lymphoma
- IC50
The concentration of a compound required for 50% inhibition of cell viability
- NSCLC
Non-small cell lung cancer
- SGLT1
Sodium glucose cotransporter-1
- SLC2
Solute carrier 2
- SWEET
Sugar will eventually be exported transporter
- T2DM
Type 2 diabetes mellitus
- TCA
Tricarboxylic acid
Footnotes
The authors declare no competing financial interest.
REFERENCES
- Aluyen JK, Ton QN, Tran T, Yang AE, Gottlieb HB, & Bellanger RA (2012). Resveratrol: potential as anticancer agent. Journal of dietary supplements, 9(1), 45–56 [DOI] [PubMed] [Google Scholar]
- Amalraj A, Pius A, Gopi S, & Gopi S (2017). Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives–A review. Journal of Traditional and Complementary Medicine, 7(2), 205–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrianesis V, Glykofridi S, & Doupis J (2016). The renal effects of SGLT2 inhibitors and a mini-review of the literature. Therapeutic Advances in Endocrinology and Metabolism, 7(5–6), 212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai H, Hirasawa Y, Rahman A, Kusumawati I, Zaini NC, Sato S, … & Morita H (2010). Alstiphyllanines E–H, picraline and ajmaline-type alkaloids from Alstonia macrophylla inhibiting sodium glucose cotransporter. Bioorganic & Medicinal Chemistry, 18(6), 2152–2158. [DOI] [PubMed] [Google Scholar]
- Balci A, Sahin FI, & Ekmekci A (1999). Gossypol induced apoptosis in the human promyelocytic leukemia cell line HL 60. Tohoku Journal of Experimental Medicine, 189(1), 51–57. [DOI] [PubMed] [Google Scholar]
- Band V, Hoffer AP, Band H, Rhinehardt AE, Knapp RC, Matlin SA, & Anderson DJ (1989). Antiproliferative effect of gossypol and its optical isomers on human reproductive cancer cell lines. Gynecologic Oncology, 32(3), 273–277. [DOI] [PubMed] [Google Scholar]
- Barron CC, Bilan PJ, Tsakiridis T, & Tsiani E (2016). Facilitative glucose transporters: Implications for cancer detection, prognosis and treatment. Metabolism: Clinical and Experimental, 65(2), 124–139. [DOI] [PubMed] [Google Scholar]
- Ben-Haim S, & Ell P (2009). 18F-FDG PET and PET/CT in the evaluation of cancer treatment response. The Journal of Nuclear Medicine, 50(1), 88–99. [DOI] [PubMed] [Google Scholar]
- Berman AY, Motechin RA, Wiesenfeld MY, & Holz MK (2017). The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precision Oncology, 1, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschek W (2017). Natural products as lead compounds for sodium glucose cotransporter (SGLT) inhibitors. Planta Medica, 83(12/13), 985–993. [DOI] [PubMed] [Google Scholar]
- Boletí E, & Mead GM (2007). ABVD for Hodgkin’s lymphoma: Full-dose chemotherapy without dose reductions or growth factors. Annals of Oncology, 18(2), 376–380. [DOI] [PubMed] [Google Scholar]
- Boonsri S, Karalai C, Ponglimanont C, Chantrapromma S, Kanjana-Opas A (2008). Cytotoxic and antibacterial sesquiterpenes from Thespesia populnea. Journal of Natural Products, 71(7), 1173–1177. [DOI] [PubMed] [Google Scholar]
- Burant CF, & Bell GI (1992). Mammalian facilitative glucose transporters: Evidence for similar substrate recognition sites in functionally monomeric proteins. Biochemistry, 31(42), 10414–10420. [DOI] [PubMed] [Google Scholar]
- Cassidy A, Hanley B, & Lamuela-Raventos RM (2000). Isoflavones, lignans and stilbenes–origins, metabolism and potential importance to human health. Journal of the Science of Food and Agriculture, 80(7), 1044–1062. [Google Scholar]
- Chen L-Q, Hou B-H, Lalonde S, Takanaga H, Hartung ML, Qu X-Q, … & Frommer WB (2010). Sugar transporters for intercellular exchange and nutrition of pathogens. Nature, 468(7323), 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zhang B, Gao F, & Shi R (2018). Modulation of G2/M cell cycle arrest and apoptosis by luteolin in human colon cancer cells and xenografts. Oncology Letters, 15(2), 1559–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C-I (2016). Sodium-glucose cotransporter 2 (SGLT2) inhibitors from natural products: Discovery of next-generation antihyperglycemic agents. Molecules, 21(9), 1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BY (2019). Biochemical basis of anti-cancer-effects of phloretin—a natural dihydrochalcone. Molecules, 24(2), 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T-W, Lin C-C, Lin S-C, Chan H-L, Yang C-C (2019). Antitumor effect of kurarinone and underlying mechanism in small cell lung carcinoma cells. OncoTargets and Therapy, 12, 6119–6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claussnitzer M, Skurk T, Hauner H, Daniel H, & Rist MJ (2011). Effect of flavonoids on basal and insulin-stimulated 2-deoxyglucose uptake in adipocytes. Molecular Nutrition & Food Research, 55(S1), S26–S34. [DOI] [PubMed] [Google Scholar]
- Correia-Branco A, Azevedo CF, Araújo JR, Guimarães JT, Faria A, Keating E, & Martel F (2015). Xanthohumol impairs glucose uptake by a human first-trimester extravillous trophoblast cell line (HTR-8/SVneo cells) and impacts the process of placentation. Molecular Human Reproduction, 21(10), 803–815. [DOI] [PubMed] [Google Scholar]
- de Sousa Falcão H, Leite JA, Barbosa-Filho JM, de Athayde-Filho PF, de Oliveira Chaves MC, Moura MD, … & Batista LM (2008). Gastric and duodenal antiulcer activity of alkaloids: A review. Molecules, 13(12), 3198–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng D, & Yan N (2016). GLUT, SGLT, and SWEET: Structural and mechanistic investigations of the glucose transporters. Protein Science, 25(3), 546–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi KP, Rajavel T, Habtemariam S, Nabavi SF, & Nabavi SM (2015). Molecular mechanisms underlying anticancer effects of myricetin. Life Sciences, 142, 19–25. [DOI] [PubMed] [Google Scholar]
- Devi KP, Rajavel T, Nabavi SF, Setzer WN, Ahmadi A, Mansouri K, & Nabavi SM (2015). Hesperidin: A promising anticancer agent from nature. Industrial Crops and Products, 76, 582–589. [Google Scholar]
- Dowd MK, Pelitire SM (2006). Isolation of 6-methoxy gossypol and 6,6’-dimethoxy gossypol from Gossypium barbadense Sea Island cotton. Journal of Agricultural and Food Chemistry, 54(9), 3265–3270. [DOI] [PubMed] [Google Scholar]
- Dyer J, Vayro S, & Shirazi-Beechey SP (2003). Mechanism of glucose sensing in the small intestine. Biochemical Society Transactions, 31(part 6), 1140–1142. [DOI] [PubMed] [Google Scholar]
- Ellis PA, Smith IE, Hardy JR, Nicolson MC, Talbot DC, Ashley SE, & Priest K (1995). Symptom relief with MVP (mitomycin C, vinblastine and cisplatin) chemotherapy in advanced non-small-cell lung cancer. British Journal of Cancer, 71(2), 366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, & Frommer WB (2015). Structure and function of SemiSWEET and SWEET sugar transporters. Trends in Biochemical Sciences, 40(8), 480–486. [DOI] [PubMed] [Google Scholar]
- Ferry DR, Smith A, Malkhandi J, Fyfe DW, deTakats PG, Anderson D, … & Kerr DJ (1996). Phase I clinical trial of the flavonoid quercetin: Pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clinical Cancer Research, 2(4), 659–668. [PubMed] [Google Scholar]
- Flaig TW, Glodé M, Gustafson D, van Bokhoven A, Tao Y, Wilson S, … & Pollak M (2010). A study of high-dose oral silybin-phytosome followed by prostatectomy in patients with localized prostate cancer. The Prostate, 70(8), 848–855. [DOI] [PubMed] [Google Scholar]
- Flaig TW, Gustafson DL, Su L-J, Zirrolli JA, Crighton F, Harrison GS, … & Glodé LM (2007). A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Investigational New Drugs, 25(2), 139–146. [DOI] [PubMed] [Google Scholar]
- Fothergill-Gilmore LA, & Michels PA (1993). Evolution of glycolysis. Progress in Biophysics and Molecular Biology, 59(2), 105–235. [DOI] [PubMed] [Google Scholar]
- Freedman TB, Cao X, Oliveira RV, Cass QB, Nafie LA (2003). Determination of the absolute configuration and solution conformation of gossypol by vibrational circular dichroism. Chirality, 15(2), 196–200. [DOI] [PubMed] [Google Scholar]
- Gan R-Y, Li H-B, Sui Z-Q, & Corke H (2018). Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Critical Reviews in Food Science and Nutrition, 58(6), 924–941. [DOI] [PubMed] [Google Scholar]
- Gauer JS, Tumova S, Lippiat JD, Kerimi A, & Williamson G (2018). Differential patterns of inhibition of the sugar transporters GLUT2, GLUT5 and GLUT7 by flavonoids. Biochemical Pharmacology, 152, 11–20. [DOI] [PubMed] [Google Scholar]
- Gomez-Zorita S, Tréguer K, Mercader J, & Carpéné C (2013). Resveratrol directly affects in vitro lipolysis and glucose transport in human fat cells. Journal of Physiology and Biochemistry, 69(3), 585–593. [DOI] [PubMed] [Google Scholar]
- Gosch C, Halbwirth H, & Stich K (2010). Phloridzin: Biosynthesis, distribution and physiological relevance in plants. Phytochemistry, 71(8–9), 838–843. [DOI] [PubMed] [Google Scholar]
- Govers R (2014). Cellular regulation of glucose uptake by glucose transporter GLUT4 In Advances in Clinical Chemistry, Makowski G (ed). Elsevier: Amsterdam, Netherlands; 173–240. [DOI] [PubMed] [Google Scholar]
- Granchi C, Fancelli D, & Minutolo F (2014). An update on therapeutic opportunities offered by cancer glycolytic metabolism. Bioorganic & Medicinal Chemistry Letters, 24(21), 4915–4925. [DOI] [PubMed] [Google Scholar]
- Granchi C, Fortunato S, & Minutolo F (2016). Anticancer agents interacting with membrane glucose transporters. Medchemcomm, 7(9), 1716–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KE, Rekman JF, Gunnink LK, Busscher BM, Scott JL, Tidball AM, … & Louters LL (2018). Quercetin inhibits glucose transport by binding to an exofacial site on GLUT1. Biochimie, 151, 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo J, Flores C, Hidalgo MA, Perez M, Yañez A, Quiñones L, … & Burgos RA (2014). Delphinol® standardized maqui berry extract reduces postprandial blood glucose increase in individuals with impaired glucose regulation by novel mechanism of sodium glucose cotransporter inhibition. Panminerva Medicine, 56(S3), 2. [PubMed] [Google Scholar]
- Howells LM, Berry DP, Elliott PJ, Jacobson EW, Hoffmann E, Hegarty B, … & Gescher AJ (2011). Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases—safety, pharmacokinetics, and pharmacodynamics. Cancer Prevention Research, 4(9), 1419–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia DS, Grove O, & Cefalu WT (2017). An update on SGLT2 inhibitors for the treatment of diabetes mellitus. Current Opinion in Endocrinology, Diabetes, and Obesity, 24(1), 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, & Czech MP (2007). The GLUT4 glucose transporter. Cell Metabolism, 5(4), 237–252. [DOI] [PubMed] [Google Scholar]
- Hummel CS, Lu C, Loo DDF, Hirayama BA, Voss AA, & Wright EM (2011). Glucose transport by human renal Na+/D-glucose cotransporters SGLT1 and SGLT2. American Journal of Physiology-Cell Physiology, 300(1), C14–C21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezoe T, Yang Y, Bandobashi K, Saito T, Takemoto S, Machida H, … & Taguchi H (2005). Oridonin, a diterpenoid purified from Rabdosia rubescens, inhibits the proliferation of cells from lymphoid malignancies in association with blockade of the NF-κB signal pathways. Molecular Cancer Therapeutics, 4(4), 578–586. [DOI] [PubMed] [Google Scholar]
- Imran M, Rauf A, Abu-Izneid T, Nadeem M, Shariati MA, Khan IA, … & Mubarak MS (2019). Luteolin, a flavonoid, as an anticancer agent: A review. Biomedicine & Pharmacotherapy, 112, 108612. [DOI] [PubMed] [Google Scholar]
- Jabbour SA, & Goldstein BJ (2008). Sodium glucose co-transporter 2 inhibitors: Blocking renal tubular reabsorption of glucose to improve glycaemic control in patients with diabetes. International Journal of Clinical Practice, 62(8), 1279–1284. [DOI] [PubMed] [Google Scholar]
- Jiang C-H, Sun T-L, Xiang D-X, Wei S-S, & Li WQ (2018). Anticancer activity and mechanism of xanthohumol: A prenylated flavonoid from hops (Humulus lupulus L.). Frontiers in Pharmacology, 9, 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Zhao S, Xu L, Lu Y, Lu Z, Chen C, … & Yang L (2015). The inhibitory effects of xanthohumol, a prenylated chalcone derived from hops, on cell growth and tumorigenesis in human pancreatic cancer. Biomedicine & Pharmacotherapy, 73, 40–47. [DOI] [PubMed] [Google Scholar]
- Jung UJ, Lee M-K, Park YB, Kang MA, & Choi M-S (2006). Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. The International Journal of Biochemistry & Cell Biology, 38(7), 1134–1145. [DOI] [PubMed] [Google Scholar]
- Kasahara T, & Kasahara M (1997). Characterization of rat Glut4 glucose transporter expressed in the yeast Saccharomyces cerevisiae: Comparison with Glut1 glucose transporter. Biochimica et Biophysica Acta, 1324(1), 111–119. [DOI] [PubMed] [Google Scholar]
- Kerimi A, Gauer JS, Crabbe S, Cheah JW, Lau J, Walsh R, … & Williamson G (2019). Effect of the flavonoid hesperidin on glucose and fructose transport, sucrase activity and glycaemic response to orange juice in a crossover trial on healthy volunteers. British Journal of Nutrition, 121(7), 782–792. [DOI] [PubMed] [Google Scholar]
- Kim EE, Chung S-K, Haynie TP, Kim C-G, Cho B-J, Podoloff DA, … & Ajani JA (1992). Differentiation of residual or recurrent tumors from post-treatment changes with F-18 FDG PET. RadioGraphics, 12(2), 269–279. [DOI] [PubMed] [Google Scholar]
- Kreydiyyeh SI, Baydoun EA-H, & Churukian ZM (1994). Tea extract inhibits intestinal absorption of glucose and sodium in rats. Comparative Biochemistry and Physiology Part C: Pharmacology, 108(3), 359–365. [PubMed] [Google Scholar]
- Kueck A, Opipari AW, Griffith KA, Tan L, Choi M, Huang J, … & Liu JR (2007). Resveratrol inhibits glucose metabolism in human ovarian cancer cells. Gynecologic Oncology, 107(3), 450–457. [DOI] [PubMed] [Google Scholar]
- Lall RK, Adhami VM, & Mukhtar H (2016). Dietary flavonoid fisetin for cancer prevention and treatment. Molecular Nutrition & Food Research, 60(6), 1396–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarevic B, Boezelijn G, Diep LM, Kvernrod K, Ogren O, Ramberg H, … & Karlsen SJ (2011). Efficacy and safety of short-term genistein intervention in patients with localized prostate cancer prior to radical prostatectomy: A randomized, placebo-controlled, double-blind Phase 2 clinical trial. Nutrition and Cancer, 63(6), 889–898. [DOI] [PubMed] [Google Scholar]
- Liao H, Wang Z, Deng Z, Ren H, & Li X (2015). Curcumin inhibits lung cancer invasion and metastasis by attenuating GLUT1/MT1-MMP/MMP2 pathway. International Journal of Clinical and Experimental Medicine, 8(6), 8948–8957. [PMC free article] [PubMed] [Google Scholar]
- Lin M-T, Lin C-L, Lin T-Y, Cheng C-W, Yang S-F, Lin CL, … & Tsai J-P (2016). Synergistic effect of fisetin combined with sorafenib in human cervical cancer HeLa cells through activation of death receptor-5 mediated caspase-8/caspase-3 and the mitochondria-dependent apoptotic pathway. Tumor Biology, 37(5), 6987–6996. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cao Y, Zhang W, Bergmeier S, Qian Y, Akbar H, … & Chen X (2012). A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Molecular Cancer Therapeutics, 11(8), 1672–1682. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang W, Cao Y, Liu Y, Bergmeier S, & Chen X (2010). Small compound inhibitors of basal glucose transport inhibit cell proliferation and induce apoptosis in cancer cells via glucose-deprivation-like mechanisms. Cancer Letters, 298(2), 176–185. [DOI] [PubMed] [Google Scholar]
- Macheda ML, Rogers S, & Best JD (2005). Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. Journal of Cellular Physiology, 202(3), 654–662. [DOI] [PubMed] [Google Scholar]
- Mahomoodally MF, Fakim AG, & Subratty AH (2004). Momordica charantia extracts inhibit uptake of monosaccharide and amino acid across rat everted gut sacs in vitro. Biological and Pharmaceutical Bulletin, 27(2), 216–218. [DOI] [PubMed] [Google Scholar]
- Manzano S, & Williamson G (2010). Polyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal Caco-2 cells. Molecular Nutrition & Food Research, 54(12), 1773–1780. [DOI] [PubMed] [Google Scholar]
- Martin H-J, Kornmann F, & Fuhrmann GF (2003). The inhibitory effects of flavonoids and antiestrogens on the Glut1 glucose transporter in human erythrocytes. Chemico-Biological Interactions, 146(3), 225–235. [DOI] [PubMed] [Google Scholar]
- Meireles M, Martel F, Araújo J, Santos-Buelga C, Gonzalez-Manzano S, Dueñas M, … & Faria A (2013). Characterization and modulation of glucose uptake in a human blood–brain barrier model. The Journal of Membrane Biology, 246(9), 669–677. [DOI] [PubMed] [Google Scholar]
- Melstrom LG, Salabat MR, Ding X-Z, Milam BM, Strouch M, Pelling JC, & Bentrem DJ (2008). Apigenin inhibits the GLUT-1 glucose transporter and the phosphoinositide 3-kinase/Aκt pathway in human pancreatic cancer cells. Pancreas, 37(4), 426–431. [DOI] [PubMed] [Google Scholar]
- Messing E, Gee JR, Saltzstein DR, Kim K, diSant’Agnese A, Kolesar J, … & Bailey H (2012). A phase 2 cancer chemoprevention biomarker trial of isoflavone G-2535 (genistein) in presurgical bladder cancer patients. Cancer Prevention Research, 5(4), 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motilva MJ, Martínez JA, Ilundain A, & Larralde J (1983). Effect of extracts from bean (Phaseolus vulgaris) and field bean (Vicia faba) varieties on intestinal D-glucose transport in rat in vivo. Journal of the Science of Food and Agriculture, 34(3), 239–246. [DOI] [PubMed] [Google Scholar]
- Moudi M, Go R, Yien CYS, & Nazre M (2013). Vinca alkaloids. International Journal of Preventive Medicine, 4(11), 1231–1235. [PMC free article] [PubMed] [Google Scholar]
- Mueckler M, & Thorens B (2013). The SLC2 (GLUT) family of membrane transporters. Molecular Aspects of Medicine, 34(2–3), 121–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U, Stübl F, Schwarzinger B, Sandner G, Iken M, Himmelsbach M, … & Weghuber J (2018). In vitro and in vivo inhibition of intestinal glucose transport by guava (Psidium guajava) extracts. Molecular Nutrition & Food Research, 62(11), 1701012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, & van Leeuwen PA (2001). Flavonoids: A review of probable mechanisms of action and potential applications. The American Journal of Clinical Nutrition, 74(4), 418–425. [DOI] [PubMed] [Google Scholar]
- Nomura M, Takahashi T, Nagata N, Tsutsumi K, Kobayashi S, Akiba T, … & Miyamoto K-I (2008). Inhibitory mechanisms of flavonoids on insulin-stimulated glucose uptake in MC3T3-G2/PA6 adipose cells. Biological and Pharmaceutical Bulletin, 31(7), 1403–1409. [DOI] [PubMed] [Google Scholar]
- Nowakowska Z (2007). A review of anti-infective and anti-inflammatory chalcones. European Journal of Medicinal Chemistry, 42(2), 125–137. [DOI] [PubMed] [Google Scholar]
- Ojelabi OA, Lloyd KP, De Zutter JK, & Carruthers A (2018). Red wine and green tea flavonoids are cis-allosteric activators and competitive inhibitors of glucose transporter 1 (GLUT1)-mediated sugar uptake. The Journal of Biological Chemistry, 293(51), 19823–19834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojelabi OA, Lloyd KP, Simon AH, De Zutter JK, & Carruthers A (2016). WZB117 (2-fluoro-6-(m-hydroxybenzoyloxy) phenyl m-hydroxybenzoate) inhibits GLUT1-mediated sugar transport by binding reversibly at the exofacial sugar binding site. The Journal of Biological Chemistry, 291(52), 26762–26772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JB (1999). Flavonoids are potential inhibitors of glucose uptake in U937 cells. Biochemical and Biophysical Research Communications, 260(2), 568–574. [DOI] [PubMed] [Google Scholar]
- Park JB (2001). Inhibition of glucose and dehydroascorbic acid uptakes by resveratrol in human transformed myelocytic cells. Journal of Natural Products, 64(3), 381–384. [DOI] [PubMed] [Google Scholar]
- Pérez A, Ojeda P, Valenzuela X, Ortega M, Sánchez C, Ojeda L, … & Reyes AM (2009). Endofacial competitive inhibition of the glucose transporter 1 activity by gossypol. American Journal of Physiology-Cell Physiology, 297(1), C86–C93. [DOI] [PubMed] [Google Scholar]
- Qian Y, Wang X, & Chen X (2014). Inhibitors of glucose transport and glycolysis as novel anticancer therapeutics. World Journal of Translational Medicine, 3(2), 37–57. [Google Scholar]
- Rauf A, Imran M, Khan IA, Mujeeb-ur-Rehman, Gilani SA, Mehmood Z, & Mubarak MS (2018). Anticancer potential of quercetin: A comprehensive review. Phytotherapy Research, 32(11), 2109–2130. [DOI] [PubMed] [Google Scholar]
- Ren Y, Yuan C, Qian Y, Chai H-B, Chen X, Goetz M, & Kinghorn AD (2014). Constituents of an extract of Cryptocarya rubra housed in a repository with cytotoxic and glucose transport inhibitory effects. Journal of Natural Products, 77(3), 550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas M, Obando P, Ojeda L, Ojeda P, Pérez A, Vargas-Uribe M, … & Reyes AM (2013). Resolution of the direct interaction with and inhibition of the human GLUT1 hexose transporter by resveratrol from its effect on glucose accumulation. American Journal of Physiology-Cell Physiology, 305(1), C90–C99. [DOI] [PubMed] [Google Scholar]
- Salehi B, Fokou PVT, Sharifi-Rad M, Zucca P, Pezzani R, Martins N, & Sharifi-Rad J (2019). The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals, 12(1), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M (1999). Oxidative phosphorylation at the fin de siècle. Science, 283(5407), 1488–1493. [DOI] [PubMed] [Google Scholar]
- Sato S, Takeo J, Aoyama C, & Kawahara H (2007). Na+-glucose cotransporter (SGLT) inhibitory flavonoids from the roots of Sophora flavescens. Bioorganic & Medicinal Chemistry, 15(10), 3445–3449. [DOI] [PubMed] [Google Scholar]
- Scafoglio C, Hirayama BA, Kepe V, Liu J, Ghezzi C, Satyamurthy N, … & Wright EM (2015). Functional expression of sodium-glucose transporters in cancer. Proceedings of the National Academy of Sciences USA, 112(30), E4111–E4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroll MM, LaBonia GJ, Ludwig KR, & Hummon AB (2017). Glucose restriction combined with autophagy inhibition and chemotherapy in HCT 116 spheroids decreases cell clonogenicity and viability regulated by tumor suppressor genes. Journal of Proteome Research, 16(8), 3009–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze C, Bangert A, Kottra G, Geillinger KE, Schwanck B, Vollert H, … & Daniel H (2014). Inhibition of the intestinal sodium-coupled glucose transporter 1 (SGLT1) by extracts and polyphenols from apple reduces postprandial blood glucose levels in mice and humans. Molecular Nutrition & Food Research, 58(9), 1795–1808. [DOI] [PubMed] [Google Scholar]
- Shibuya K, Okada M, Suzuki S, Seino M, Seino S, Takeda H, & Kitanaka C (2015). Targeting the facilitative glucose transporter GLUT1 inhibits the self-renewal and tumor-initiating capacity of cancer stem cells. Oncotarget, 6(2), 651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel AB, Narayan R, Rodriguez R, Goyal A, Jacobson JS, Kelly K, … & Greenlee H (2014). A phase I dose-finding study of silybin phosphatidylcholine (milk thistle) in patients with advanced hepatocellular carcinoma. Integrative Cancer Therapies, 13(1), 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson IA, Dwyer D, Malide D, Moley KH, Travis A, & Vannucci SJ (2008). The facilitative glucose transporter GLUT3: 20 Years of distinction. American Journal of Physiology-Endocrinology and Metabolism, 295(2), E242–E253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RP, Dhanalakshmi S, Tyagi AK, Chan DCF, Agarwal C, & Agarwal R (2002). Dietary feeding of silibinin inhibits advance human prostate carcinoma growth in athymic nude mice and increases plasma insulin-like growth factor-binding protein-3 levels. Cancer Research, 62(11), 3063–3069. [PubMed] [Google Scholar]
- Singh SP, Gao Y, Singh LD, Kunapuli SP, & Ravindra R (1998). Role of microtubules in glucose uptake by C6 glioma cells. Pharmacology & Toxicology, 83(2), 83–89. [DOI] [PubMed] [Google Scholar]
- Sobrini FJ, Martinez JA, Ilundain A, & Larralde J (1983). The effects of ‘Vicia faba L.’ polyphenols on absorption, growth and metabolism in the rat. Qualitas Plantarum Plant Foods for Human Nutrition, 33(2–3), 231–235. [Google Scholar]
- Sohn K, Wende AR, Abel ED, Moreno AP, Sachse FB, & Punske BB (2013). Absence of glucose transporter 4 diminishes electrical activity of mouse hearts during hypoxia. Experimental Physiology, 98(3), 746–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D-W, Zhang H-D, Mao L, Mao C-F, Chen W, Cui M, … & Tang J,-H (2015). Luteolin inhibits breast cancer development and progression in vitro and in vivo by suppressing notch signaling and regulating MiRNAs. Cellular Physiology and Biochemistry, 37(5), 1693–1711. [DOI] [PubMed] [Google Scholar]
- Sun F, Zheng XY, Ye J, Wu TT, Wang JL, & Chen W (2012). Potential anticancer activity of myricetin in human T24 bladder cancer cells both in vitro and in vivo. Nutrition and Cancer, 64(4), 599–606. [DOI] [PubMed] [Google Scholar]
- Sun M, Han J, Duan J, Cui Y, Wang T, Zhang W, … & Yan X (2007). Novel antitumor activities of Kushen flavonoids in vitro and in vivo. Phytotherapy Research, 21(3), 269–277. [DOI] [PubMed] [Google Scholar]
- Syed DN, Afaq F, Maddodi N, Johnson JJ, Sarfaraz S, Ahmad A, … & Mukhtar H (2011). Inhibition of human melanoma cell growth by dietary flavonoid fisetin is associated with disruption of Wnt/β-catenin signaling and decreased Mitf levels. Journal of Investigative Dermatology, 131(6), 1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CK, Levy RM, Elliott JC, & Burnett BP (2009). The effect of genistein aglycone on cancer and cancer risk: A review of in vitro, preclinical, and clinical studies. Nutrition Reviews, 67(7), 398–415. [DOI] [PubMed] [Google Scholar]
- Uldry M, Ibberson M, Hosokawa M, & Thorens B (2002). GLUT2 is a high affinity glucosamine transporter. FEBS Letters, 524(1–3), 199–203. [DOI] [PubMed] [Google Scholar]
- Van Poznak C, Seidman AD, Reidenberg MM, Moasser MM, Sklarin N, Van Zee K, … & Hudis C (2001). Oral gossypol in the treatment of patients with refractory metastatic breast cancer: A phase I/II clinical trial. Breast Cancer Research and Treatment, 66(3), 239–248. [DOI] [PubMed] [Google Scholar]
- Vick H, Diedrich DF, & Baumann K (1973). Reevaluation of renal tubular glucose transport inhibition by phlorizin analogs. American Journal of Physiology, 224(3), 552–557. [DOI] [PubMed] [Google Scholar]
- Villa-Rodriguez JA, Aydin E, Gauer JS, Pyner A, Williamson G, & Kerimi A (2017). Green and chamomile teas, but not acarbose, attenuate glucose and fructose transport via inhibition of GLUT2 and GLUT5. Molecular Nutrition & Food Research, 61, 1700566. [DOI] [PubMed] [Google Scholar]
- Warburg O (1956). On the origin of cancer cells. Science, 123(3191), 309–314. [DOI] [PubMed] [Google Scholar]
- Warburg O, Wind F, & Negelein E (1927). The metabolism of tumors in the body. The Journal of General Physiology, 8(6), 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware B, Bevier M, Nishijima Y, Rogers S, Carnes CA, & Lacombe VA (2011). Chronic heart failure selectively induces regional heterogeneity of insulin-responsive glucose transporters. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 301(5), R1300–R1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright EM, Loo DDF, & Hirayama BA (2011). Biology of human sodium glucose transporters. Physiological Reviews, 91(2), 733–794. [DOI] [PubMed] [Google Scholar]
- Xu Y-Y, Wu T-T, Zhou S-H, Bao Y-Y, Wang Q-Y, Fan J, & Huang Y-P (2014). Apigenin suppresses GLUT-1 and p-Aκt expression to enhance the chemosensitivity to cisplatin of laryngeal carcinoma Hep-2 cells: An in vitro study. International Journal of Clinical and Experimental Pathology, 7(7), 3938–3947. [PMC free article] [PubMed] [Google Scholar]
- Yang J, Chen H, Wang Q, Deng S, Huang M, Ma X, … & Yang X (2018). Inhibitory effect of kurarinone on growth of human non-small cell lung cancer: An experimental study both in vitro and in vivo studies. Frontiers in Pharmacology, 9, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K-C, Tsai C-Y, Wang Y-J, Wei P-L, Lee C-H, Chen J-H, … & Ho Y-S (2009). Apple polyphenol phloretin potentiates the anticancer actions of paclitaxel through induction of apoptosis in human hep G2 cells. Molecular Carcinogenesis, 48(5), 420–431. [DOI] [PubMed] [Google Scholar]
- Yang Y-C, Lin P-H, & Wei M-C (2017). Production of oridonin-rich extracts from Rabdosia rubescens using hyphenated ultrasound-assisted supercritical carbon dioxide extraction. Journal of the Science of Food and Agriculture, 97(10), 3323–3332. [DOI] [PubMed] [Google Scholar]
- Yao LH, Jiang YM, Shi J, Tomás-Barberán FA, Datta N, Singanusong R, & Chen SS (2004). Flavonoids in food and their health benefits. Plant Foods for Human Nutrition, 59(3), 113–122. [DOI] [PubMed] [Google Scholar]
- Yao Z, Xie F, Li M, Liang Z, Xu W, Yang J, … & Qu L-H (2017). Oridonin induces autophagy via inhibition of glucose metabolism in p53-mutated colorectal cancer cells. Cell Death & Disease, 8(2), e2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaheer K, & Akhtar MH (2017). An updated review of dietary isoflavones: Nutrition, processing, bioavailability and impacts on human health. Critical Reviews in Food Science and Nutrition, 57(6), 1280–1293. [DOI] [PubMed] [Google Scholar]
- Zhan T, Digel M, Küch E-M, Stremmel W, & Füllekrug J (2011). Silybin and dehydrosilybin decrease glucose uptake by inhibiting GLUT proteins. Journal of Cellular Biochemistry, 112(3), 849–859. [DOI] [PubMed] [Google Scholar]
- Zhang F, Dong W, Zeng W, Zhang L, Zhang C, Qiu Y, … & Liang W (2016). Naringenin prevents TGF-β1 secretion from breast cancer and suppresses pulmonary metastasis by inhibiting PKC activation. Breast Cancer Research, 18(1), 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Liu Y, Chen X, & Bergmeier SC (2010). Novel inhibitors of basal glucose transport as potential anticancer agents. Bioorganic & Medicinal Chemistry Letters, 20(7), 2191–2194. [DOI] [PubMed] [Google Scholar]
- Zhu B-H, Zhan W-H, Li Z-R, Wang Z, He Y-L, Peng J-S, … & Zhang C-H (2007). (–)-Epigallocatechin-3-gallate inhibits growth of gastric cancer by reducing VEGF production and angiogenesis. World Journal of Gastroenterology, 13(8), 1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zi X, Grasso AW, Kung H-J, & Agarwal R (1998). A flavonoid antioxidant, silymarin, inhibits activation of erbB1 signaling and induces cyclin-dependent kinase inhibitors, G1 arrest, and anticarcinogenic effects in human prostate carcinoma DU145 cells. Cancer Research, 58(9), 1920–1929. [PubMed] [Google Scholar]
- Zierler K (1999). Whole body glucose metabolism. American Journal of Physiology-Endocrinology and Metabolism, 276(3), E409–E426. [DOI] [PubMed] [Google Scholar]