Abstract
We examined the extent to which orexin measured during smoking and the early phase of abstinence was related to craving, withdrawal, stress hormones, and risk for smoking relapse in men and women. Considering its role in modulating nicotine-related reward, we predicted that a reduction in circulating orexin during withdrawal would be associated with increased craving and risk for smoking relapse. Two hundred and eighty five participants provided biological samples and self-report information to identify predictors of smoking relapse. All participants attended two laboratory sessions, which were before and after a period of required abstinence from smoking. After quitting, participants also attended four weekly sessions to track smoking relapse. Only smokers who relapsed within the follow-up period exhibited reduced orexin levels during the initial withdrawal period; ACTH, but not craving nor cortisol, increased across the abstinence period for successful abstainers but not for relapsers. Sex differences in orexin and craving or withdrawal associations also emerged. Adding sex, HPA hormones, and self-reported measures of craving and withdrawal as potential mediators had minimal effects on the above abstinence and orexin effects. These results provide the first evidence that circulating orexin may be a useful marker of risk for relapse; and sex, adrenal hormones, and self-reported craving and withdrawal were not mediators of this effect. The results point to a promising pathway to investigate objective biological markers for craving and smoking relapse and highlight the complexity of the neurobiology of relapse.
Keywords: craving, gender, mood, orexin, relapse, smoking
INTRODUCTION
Craving is a strong motivational factor in tobacco dependence (Ferguson & Shiffman, 2009; Tiffany, Warthen, & Goedeker, 2009; West, Baker, Cappelleri, & Bushmakin, 2008), and, as such, it is a risk factor for smoking relapse (al’Absi, Hatsukami, Davis, & Wittmers, 2004; Killen & Fortmann, 1997; Roche et al., 2014; Van Zundert, Ferguson, Shiffman, & Engels, 2012). Research has demonstrated that the intensity of craving during the initial few days of abstinence predicts increased risk for relapse (al’Absi, Carr, & Bongard, 2007; Killen & Fortmann, 1997; Nakajima & al’Absi, 2012). Further research to identify biobehavioral markers and predictors of craving and relapse could enhance our abilities to identify those at high risk for early relapse and to develop appropriate therapeutic contingencies to improve treatment outcomes.
Identifying specific biological markers of cigarette craving and risk for relapse is in its infancy but encouraging. For example, smokers at risk for early relapse exhibit an exaggerated drop in morning cortisol during abstinence relative to levels measured during ad libitum smoking (al’Absi et al., 2004). Risk for smoking relapse has also been associated with attenuated adrenocorticotropin (ACTH) levels and cardiovascular responses, as well as with heightened craving during acute stress (al’Absi, Hatsukami, & Davis, 2005; Frederick et al., 1998; Ussher et al., 2006). Additionally, appetite and energy regulating hormones have recently been recognized as potential biomarkers of craving and relapse (al’Absi et al., 2011; Kiefer et al., 2005; Koopmann et al., 2012; von der Goltz et al., 2010). For example, we recently showed that ghrelin, but not peptide YY, assessed during ad libitum smoking is directly linked to relapse (al’Absi, Lemieux, & Nakajima, 2014). Further, we have shown that both heightened leptin and ghrelin leave a smoker at increased risk of relapse (Harris, Wimmer, & Aston-Jones, 2005; Lemieux, Nakajima, Hatsukami, Allen, & al’Absi, 2015). Orexin is an energy-regulating hormone released by neurons within the lateral hypothalamus, which stimulates dopamine transmission in the ventral tegmental area (VTA) (Hollander, Lu, Cameron, Kamenecka, & Kenny, 2008; Narita et al., 2006). This dopamine stimulation suggests a role for orexin in reinforcement, potentially including the effects of drug use. For example, blocking orexin transmission decreased intravenous nicotine self-administration and diminished nicotine seeking in rats (Hollander et al., 2008), supporting orexin as a mediator of nicotine-related rewards. The present paper extends this research by examining orexin, for the first time, as a potential marker of relapse in a large sample of smokers. We measured orexin during ad libitum smoking and during verified abstinence in a smoking cessation attempt.
To date, no research has investigated the extent to which orexin is associated with craving during smoking cessation and with risk for smoking relapse. Furthermore, the role of adrenal hormones in the association between craving and orexin has not been examined during smoking cessation. The present study addressed these questions and examined whether the orexin peptide predicts relapse over a 4 week follow-up period. We used a cross-sectional prospective, repeated measures design to track changes in mood, smoking withdrawal, and hormones. We obtained measures of craving and withdrawal symptoms as well as circulating levels of orexin, cortisol, and ACTH in smokers and nonsmokers. The nonsmokers provided a reference point for “normal” (i.e. nonsmoking) measures of orexin, HPA, and mood changes over time, all of which can be rather variable. We hypothesized that smokers would show a change in orexin from regular (ad libitum) smoking to 24 hours of monitored abstinence, and that nonsmokers would show no change over a similar period. Further, we hypothesized that this change would differ based on relapse status over the next 28 days such that enhanced levels of orexin would be evident in those who relapse to regular smoking and that orexin would be linked to elevated craving. Based on previous research (al’Absi et al., 2004, 2005), we expected a significant decline in ACTH and cortisol from ad libitum to abstinence, and we expected that these declines would be steeper in smokers who relapsed.
METHODS AND MATERIALS
Participants
A total of 285 participants (200 smokers, 85 nonsmokers) are included in this paper. All participants were recruited through flyers, postings in the community, and social media advertisements (Craigslist). The inclusion criteria required that each participant be free from major physical illness and active psychiatric disorders (new or changed treatment within the past year). Smokers had to report an average > 10 cigarettes per day for at least 2 years and high motivation to quit (score of ≥6 on a 7 point scale, where 1 = no desire to quit; 7 = very strong desire to quit). Nonsmokers had fewer than 100 cigarettes in their lifetime and a normal exhaled carbon monoxide (CO) test (< 8 ppm). Self-reported consumption of 2 or more alcoholic beverages per day and use of other drugs excluded participants. Research assistants were trained to carefully assess alcohol use at the upper limit and daily alcohol consumption was also grounds for exclusion. At each session, participants were asked if they had consumed alcohol in the past 24 hours. Any participant acknowledging use was re-interviewed regarding use patterns and rescheduled with strict instructions to limit alcohol intake prior to appointments. No biological confirmation of alcohol nor other drug consumption was conducted prior to each lab session. All participants received monetary compensation for their time ($15/hour) and signed a written consent form approved by the Institutional Review Board of the University of Minnesota.
Measures
Biological markers of stress included salivary cortisol, plasma cortisol, and plasma ACTH. Plasma cortisol and ACTH were assayed with chemiluminescent immunoassays using the ADVIA Centaur® cortisol kit and the Immulite® 2000 ACTH kit (Siemens Medical Solutions USA, Malvern, PA). Salivary cortisol was assayed using ELISA and luminescence immunoassay (DSL, Sinsheim, Germany or IBL-International, Hamburg, Germany). The lower limits of detection (lowest sensitivity) were 0.1 ug/dl for plasma cortisol and 0.46 pg/ml for ACTH, both with intra- and inter-assay variability less than 10%. Orexin was measured using commercial RIA assays. The sensitivity range for these RIAs was 0.01 to 1.28 ng/ml (orexin-A, catalog no. S-2030; Bachem Peninsula Laboratories, LLC; San Carlos, CA). Salivary cotinine concentrations were assayed using enzyme immunoassay (EIA; DRG Diagnostics, Marburg, Germany). The inter- and intra-assay coefficients of variation were between 4 and 10%. Changes to kits between studies were verified to produce equivalent results using in-house standardized samples. Expired CO was measured using MicroCO™ (Micro Direct Inc., Auburn, Maine) or Bedfont Micro+™ (Kent, UK) monitors. These monitors were also calibrated and adjusted every six months to show equivalent results.
A questionnaire was used to collect demographic information and smoking history; and nicotine dependence was quantified using the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991). The Subjective State Scale (SSS) is a 24-item adjective checklist created to quantify withdrawal symptoms, including the Minnesota Nicotine Withdrawal Scale (MNWS) (J. Hughes & Hatsukami, 1998; J. R. Hughes & Hatsukami, 1986), positive affect, and distress subscales. These withdrawal symptoms were assessed in this study as well as craving for nicotine, which was assessed using a single item from the SSS. All items on the SSS are rated on a scale from 0 (not at all) to 7 (very strong). We also administered the abbreviated Questionnaire of Smoking Urges (QSU-brief; Cox, Tiffany, & Christen, 2001; Tiffany & Drobes, 1991) to assess craving in the form of appetitive desire to smoke (F1) and the desire to avoid the aversive experience of withdrawal (F2). The QSU-brief is a 10-item questionnaire rated on a scale from 1 (strongly disagree) to 7 (strongly agree). Baseline mood, such as depressive affect and trait anxiety, were also measured using the Patient Health Questionnaire 9-item version (Kroenke, Spitzer, & Williams, 2001) and the State Trait Anxiety Inventory trait scale (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983).
Procedures
In this prospective study, all participants attended two laboratory sessions scheduled within a 2 hour window (early afternoon) to control for diurnal variations in hormones. The first session occurred prior to quitting when the smokers were smoking at their normal, ad libitum rate (see Figure 1). The second session was scheduled immediately at, or after, their initial 24-hour abstinence period. The protocol required that all sessions be scheduled within the same 2-hour window, thus the elapsed time between the quit and 24-hour abstinence sessions could vary at most by 2 hours. During the first 24 hours of abstinence, smokers experience their most intense cravings; and the majority of smokers who relapse will do so within the first 24–48 hours. In this study, however, before they were allowed to complete the second session, all smokers were required to be abstinent, which was confirmed with CO monitoring. Similar elapsed time between the two sessions was maintained for nonsmoking controls. All participants were provided a standard lunch before each session, and food consumption before the lab session was discussed to confirm compliance with dietary instructions. The saliva for cortisol and the blood for cortisol (plasma), orexin, and ACTH were collected after a 30-minute resting baseline period. Blood was sampled into 10 ml serum separation and 10 ml EDTA pretreated vacutainer tubes (Becton Dickinson, New Jersey) and saliva was collected using Salivettes® (Sarstedt, Nümbrecht, Germany). Each blood sample was centrifuged and stored at −80°C until analysis. Self-report baseline mood, craving, and withdrawal measures were also collected at both lab sessions (pre-quit and post-quit). Following the 24-hour abstinence lab, all participants attended four weekly follow-up sessions for social support and assessment of abstinence. CO and saliva samples (one per visit for 4 weeks, ~28 days) were collected weekly to track short-term abstinence. Pharmacological aids were not provided to participants. Verification of self-reported smoking abstinence at the 28 day follow-up session was done using expired CO and cotinine assayed from saliva samples to track longer term abstinence.
Figure 1.
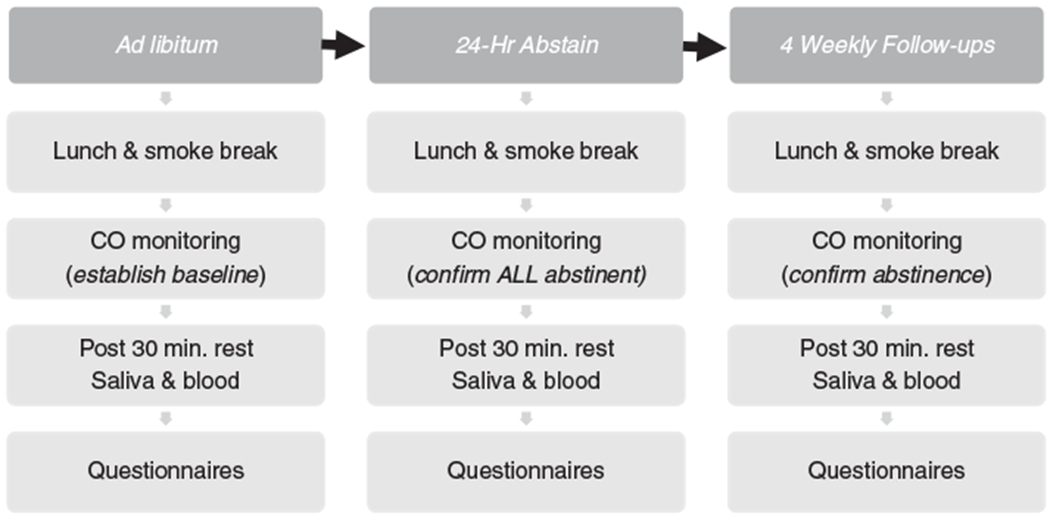
The study time line is depicted
Data analysis
Relapse was defined as smoking at least one cigarette for 7 consecutive days (J. R. Hughes et al., 2003). This is a well-established definition of relapse (Harris & Aston-Jones, 2006) that reflects a return to daily smoking. A smoker who smoked occasionally, but not continuously, for 7 days would have been categorized as abstinent given this definition. Smoking participants were categorized as relapsed or abstinent at 28 days post-quit based on cotinine verified self-report of abstinence. Orexin was normally distributed and did not require transformation before analysis. The background demographics (age, body mass index (BMI), education, sleep during withdrawal) were compared using two-way analysis of covariance (ANCOVA) with sex and smoking group (nonsmoker, relapsers, and successful abstainers) as the between-subjects factors and age as a covariate. Similar analyses were done for smokers only, comparing relapse status groups (successful abstainers, relapsers) on smoking specific measures (cigarettes per day, FTND, and years smoking at the current rate). Pearson’s chi-squared test was used to compare smoking groups and sex on self-reported alcohol use per day (never vs yes). Pearson’s correlation (r) was used to assess potential variables for inclusion in prediction models. Orexin concentrations were examined using general linear models with design 2 (sex) X 3 (smoking group: nonsmokers, relapsers, successful abstainers) X 2 (session: ad libitum, abstinence). Craving and withdrawal variables were also assessed using general linear models with design 2 (sex) X 2 (smoking group: successful abstainers, relapsers) X 2 (session: ad libitum, abstinence). Age was assessed as a potentially important covariate as well. Significant group and group-by-time interactions were followed up using univariate models and Bonferonni post hoc analysis as appropriate.
Proposed mediators (hormones and self-report) were examined following significant predictor (group, visit) and outcome (orexin) effects. First, mediators were evaluated for the possibility of combining them in an index rather than using multiple individual predictors. Adrenal hormones (log transformed) were submitted to a principal components analysis using standardized values (i.e., subtracting the average and dividing by the standard deviation, to give a unit-less quantity with average 0 and standard deviation 1). This resulted in two principal components. The first was effectively an average of the two standardized cortisol factors while the second consisted effectively of ACTH alone. The two log cortisol measures had a correlation of 0.77 with each other and the first principal component accounted for 88% of the variance. The second principal component, ACTH, had low correlation with either salivary or plasma cortisol and thus was appropriate as its own component. The same approach was used to combine 4 craving and withdrawal indices (craving item, QSU-B F1, QSU-B F2, and MNWS). The first craving principal component consisted of the average of the four standardized measures. The second craving component consisted of the standardized MNWS minus the weighted average of the other three factors (craving item, QSU-B F1, QSU-B F2). After examining the effects of abstinence on orexin, the two sets of principal components (2 for hormones, 2 for self-reported craving and withdrawal), sex and age were entered into the above general linear models as potential covariates. Consistent with established mediation modeling procedures, a significant change in the predictor (group, visit) to outcome (orexin) relationship would signal significant mediation. In all cases, a significance threshold of p = 0.05 was used.
RESULTS
Group descriptive and smoking history variables
Among the 200 smokers, 115 relapsed during the 28 day follow-up assessment period (relapser group) while 85 remained abstinent through 28 days after the quit (successful abstainers). On average the participants were 36 years old (SD = 13.0) and were normal weight (mean BMI 26.5, SD = 5.6; see Table 1). Males were younger than females in the nonsmoking group (F(1, 83) = 6.63, p < 0.05), but males and females were similarly aged in the two smoking groups (p > 0.1). BMI did not differ by smoking group or sex. The nonsmokers had more education than both relapsers and successful abstainers (p < 0.01 for both main and post-hoc comparisons). Caffeine consumption measured as cups of coffee per day also differed across the three groups (F(2, 272) = 11.46, p < 0.001): the nonsmokers drank less coffee than either the relapsers or successful abstainers (p < 0.001). The smoking groups (relapsers vs. successful abstainers) did not differ in coffee consumption.
Table 1.
Smoking and relapse group differences in basic demographics and smoking variables
| Nonsmokers | Successful Abstainers | Relapsers | ||||
|---|---|---|---|---|---|---|
| Female (n = 48) | Male (n = 37) | Female (n = 41) | Male (n = 44) | Female (n = 50) | Male (n = 65) | |
| Agea,b | 36.48 (14.91)* | 28.81 (11.72) | 35.93 (12.29) | 40.64 (12.50) | 36.96 (11.92) | 37.06 (12.53) |
| Body Mass Index | 25.13 (5.22) | 25.44 (4.37) | 26.36 (7.00) | 27.82 (5.63) | 26.82 (5.86) | 27.19 (5.13) |
| Educationc,a | 15.76 (2.55)** | 15.24 (4.39)** | 13.85 (2.67) | 14.09 (2.92) | 14.35 (2.28) | 13.79 (2.75) |
| Caffeinea | 0.90 (1.10)*** | 0.90 (1.40)*** | 2.10 (2.30) | 2.50 (3.40) | 2.40 (2.30) | 2.70 (3.00) |
| Carbon Monoxidea | 1.74 (1.28) | 2.85 (1.57) | 13.85 (7.61) | 14.27 (7.88) | 15.26 (7.79) | 15.61 (7.90) |
| Years smoked at this rate | 10.07 (10.08) | 13.16 (11.40) | 11.32 (9.93) | 13.56 (11.97) | ||
| Average cigarettes/day | 14.08 (6.13) | 16.88 (7.13) | 16.05 (7.57) | 16.58 (5.47) | ||
| Dependency (FTND) | 4.96 (1.85) | 5.33 (2.04) | 5.27 (2.13) | 5.32 (1.96) | ||
The data are presented as average (SD)
Significant group effect,
Significant sex by group interaction, no main effects of sex were seen
Education represents total years of schooling completed from grade 1 to final year of education completed.
p < 0.05,
p < 0.01,
p < 0.001
Relapsers and successful abstainers did not differ significantly in nicotine dependency, years smoking at the current rate, or average number of cigarettes per day at study entry. The dependency scores (FTND) ranged from 4.96 to 5.33 and did not differ by sex (see Table 1 for details). As expected, smokers had higher CO at study entry than nonsmokers (F(1, 226) = 41.66, p < 0.000), but sex did not differ (p > 0.10).
Manipulation check across sessions
In this study, the critical assumption was that the transition from ad libitum (first session) to 24-hour abstinence (second session) was stressful and biologically relevant. We demonstrate this by examining the change in three outcomes from ad libitum to abstinence: HPA hormones, abstinence-related craving and withdrawal, and orexin.
Confirmation of change in HPA hormones
Analysis of the two adrenal cortisol principal components (combined plasma and saliva cortisol component and ACTH component) found a main effect of session for the cortisol component (F(1, 266) = 18.97, p < 0.0001; see Figure 2) but not for ACTH (p > 0.10). Cortisol declined from an average ad libitum level of 0.50 (SE = 0.05) to an average 24-hour abstinence level of 0.26 (SE = 0.05). The session-by-smoking group interaction was significant for ACTH (F(2, 266) = 3.19, p < 0.05), but not for the cortisol component (p > 0.10). As shown in Figure 3 (a), ACTH increased for the successful abstainers, but it did not change significantly across sessions for either the nonsmokers or relapsers.
Figure 2.
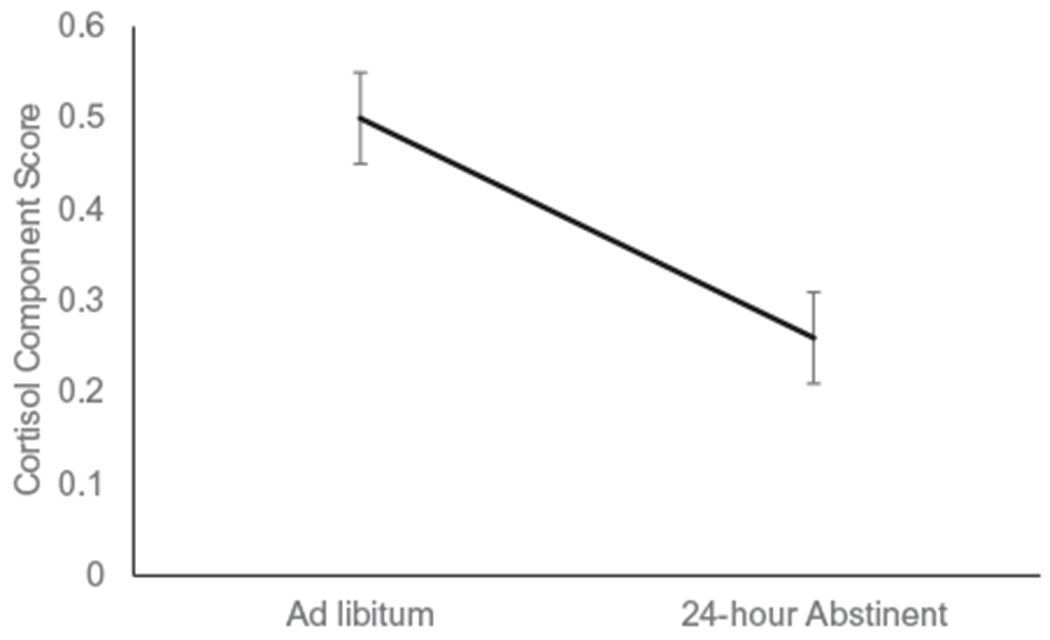
The decline in the cortisol component score from ad libitum to 24-hour abstinent is depicted (estimated marginal mean & SE)
Figure 3.
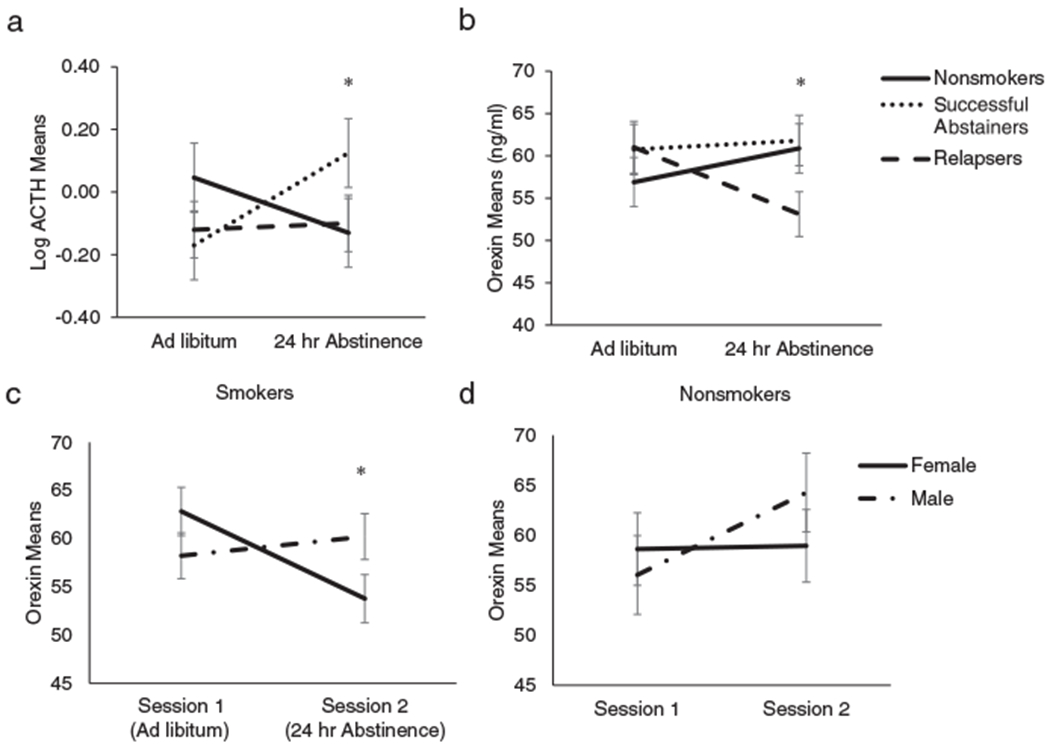
Orexin is depicted across the ad libitum and 24-hour abstinence sessions (3b). Unlike either of the nonsmokers or the successful abstainers, the relapsers (determined at 28 days post-cessation via weekly follow-up self-report and expired CO) show a significant decrease in orexin across time (p < 0.05). ACTH is depicted across the ad libitum and 24-hour abstinence sessions (3a). Unlike either of the nonsmokers orthe relapsers, the successful abstainers show a significant increase in ACTH across time (p < 0.05). Analysis shows that the session-by-smoking group persists even after including both adrenal hormones and the symptoms of craving and withdrawal (p < 0.05). The relapsers show a significant decrease in orexin across time (p < 0.01) but the successful abstainers do not (3b). Orexin means are depicted across the two sessions for male and female smokers (3c) and nonsmokers (3d) for reference. Analysis shows that that males were lower than females at the ad libitum session (p < 0.05), but at the abstinence session the males were higher than the females (3c, p < 0.05). There was no significant change in the nonsmokers across the two sessions (3d, p < 0.05)
Confirmation of changes in craving and withdrawal
This analysis assessed only the smokers (relapsers and successful abstainers) to characterize changes in craving and withdrawal symptoms in the two smoking groups. When the first principal component, which largely captured craving (QSU + MNWS), was assessed for change over time, there were no significant main effects for smoking group or time, nor was the session-by-smoking group interaction significant. The second principal component, which was specific to withdrawal symptoms, increased between the two sessions (F(1, 193) = 22.90, p < 0.0001) but smoking groups did not differ either in overall level (main effect) or in change between visit (interaction).
Confirmation of orexin changes
The analyses predicting orexin based on session (ad libitum, 24-hour abstain), smoking group (nonsmoker, relapser, abstainer), and an interaction term (session-by-smoking group), found a significant session-by-smoking group interaction (F(2, 268) = 4.29, p < 0.05) but non-significant main effects of session and smoking group (ps > 0.10). In follow-up analysis of the interaction (see Figure 3(b)), relapsers showed a decline in orexin from the ad libitum session to the 24-hour abstinence session (p < 0.05), while neither the nonsmokers nor abstainers showed a significant change in orexin between these time points (ps > 0.10).
HPA hormones, craving, and withdrawal as mediators of orexin change
Primary analysis
The previous results supported that the transition from ad libitum smoking to abstinence was stressful and neurobiologically activating, particularly for those who relapsed. To determine whether these changes in HPA activation, craving and withdrawal mediated the change in orexin, the two HPA hormone composite scores (representing cortisol and ACTH) and the composite scores for craving and withdrawal were entered as potential mediators in the full model with the two smoking groups (successful abstainers, relapsers), session (ad libitum, 24 hr abstain), and the interaction of smoking group and session. In this mediation model, the cortisol component was positively associated with orexin (F(1, 472.5) = 4.79, p < 0.05) but cortisol was not associated with relapse. As in the model with only adrenal hormones as potential mediators, the interaction between smoking group and session remained significant (F(1,257.2) = 4.48, p < 0.05) but no other main effect or interaction was significant. As shown in Figure 3(b), the relapsers showed a decline in orexin from the ad libitum to 24-hour abstinence session while the abstainers did not change. This suggests that neither stress hormones nor craving and withdrawal mediate the smoking group-by-session interaction.
Sex and age as additional adjusters
To determine the role of sex in the above findings, analyses were run as before predicting orexin with the three groups (nonsmokers, relapsers, and successful abstainers), session (ad libitum, 24 hr abstinence), with the addition of sex and age as adjusters, and with all interactions. The smoking group-by-session interaction maintained its significance (F(2, 265.8) = 3.95, p < 0.05), and the session-by-sex interaction was also significant (F(1, 261.8) = 4.09, p < 0.05). As shown in Figure 3(c), males were lower in orexin than females during the ad libitum session (p < 0.05), but during abstinence males were higher in orexin than females (p < 0.05). The main effect of sex was not significant, nor was the interaction between smoking group and sex. While the main effect of age was significant (F(1, 276.5) = 8.28, p < 0.01; slope = 0.27), age did not interact significantly with smoking group, session, or sex (ps > 0.10).
Orexin as a predictor of relapse
Having examined potential mediators of change in orexin, we then completed an analysis to determine the relevance of the shift in orexin from ad libitum to abstinence in predicting relapse. A Cox regression with days until relapse (up to 28 days) as the dependent variable, with the primary predictor being the continuous orexin change score (abstinence session minus ad libitum; higher scores represent an increase between the two sessions), and with adjustment for age and sex found that orexin change predicts days until relapse (hazard ratio (HR) = 0.71, 95% confidence interval. 51–.99, p < 0.05). For ease of interpretation, Kaplan–Meier curves were created using the median split of the orexin change score. As shown in Figure 4, the risk of relapse is 29% lower in participants with orexin change above the median compared to below the median. This is consistent with the analysis of group differences in relapse described earlier. Additional correlation analyses are included in the supplementary materials.
Figure 4.
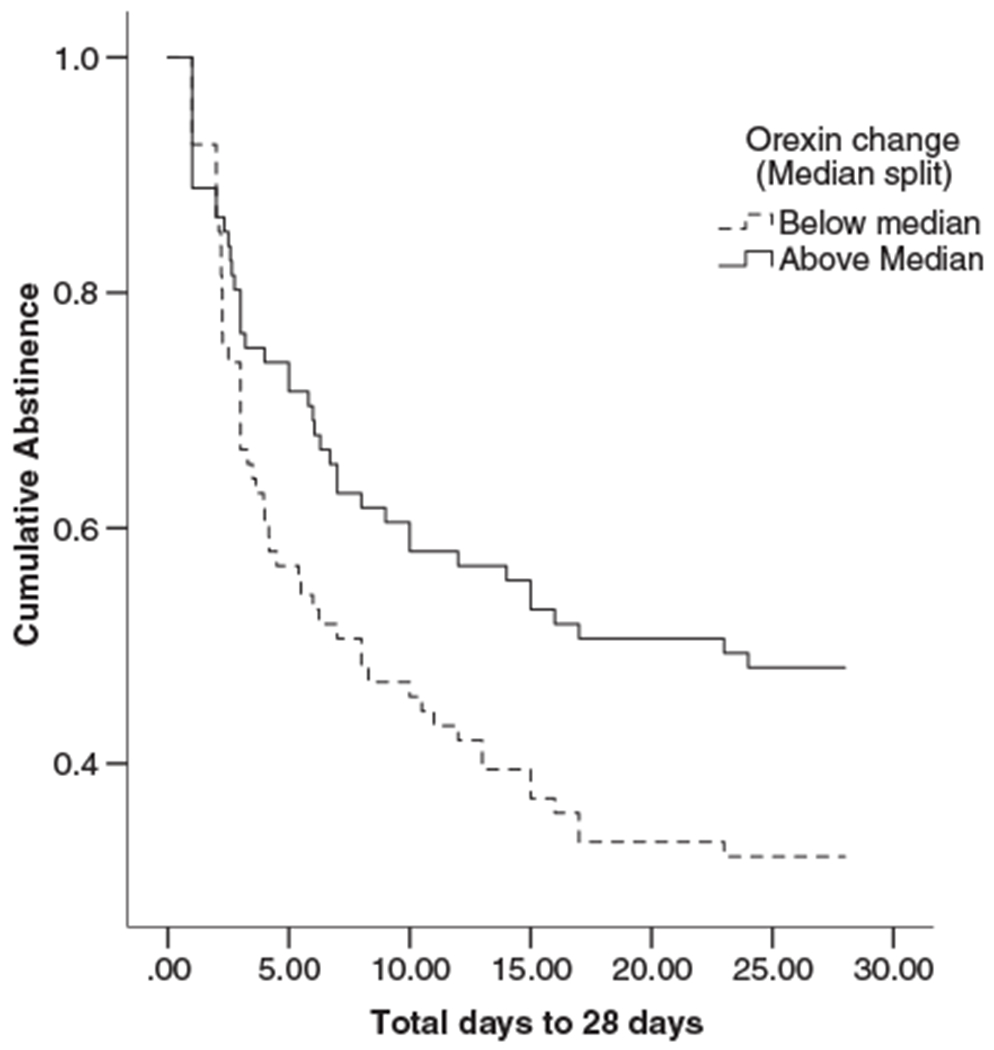
Survival curves for smokers’ orexin change score below the median (dashed lines) and above the median (solid line). Those with low orexin change scores relapsed faster than those with high orexin (HR = .71, 95% CI = .51–.99, p < 0.05)
DISCUSSION
This study’s primary findings are that relative to ad libitum smoking: 1) smokers who relapsed in the first 4 weeks exhibited a decline in orexin during the initial withdrawal period (24 hours of abstinence); 2) cortisol declined during the withdrawal period in all smokers, but ACTH was lower only in those who relapsed; 3) orexin was positively associated with cortisol, and this association was independent of relapse and withdrawal effect; and 4) lower orexin levels during withdrawal predicted shorter time to relapse even after accounting for multiple potential confounders and mediators.
The results of this study provide initial evidence of a possible role for orexin in craving, withdrawal, and risk for smoking relapse. Contrary to our hypothesized positive relationship between orexin and craving, we found a steeper decline in orexin from the ad libitum to the withdrawal session in relapsers. This was further supported by the observation that this group was the only one that exhibited orexin declines from ad libitum smoking to the withdrawal session (24 hours of abstinence) relative to smokers who were successful abstainers, a finding that persisted after accounting for differences in the stress hormones and other covariates. It is important to note that the change in orexin from ad libitum smoking to the first 24 hours of abstinence predates the relapse event. All participants were verified to be abstinent at this second session, thus the drop was specific to smokers who would subsequently relapse. This methodological detail may also explain why, at 24 hours abstinent, there was no difference between the nonsmokers and those who abstained. The persistence of the finding after accounting for stress hormones or other covariates suggests a direct and independent link between orexin and risk for smoking relapse. While no previous human research has addressed this association, these results are consistent with basic research implicating this neuropeptide in the reward and motivational properties of nicotine (Harris et al., 2005; Hollander et al., 2008). Preclinical research shows that nicotine activates orexin neurons in the lateral hypothalamus (Pasumarthi & Fadel, 2008), implicating orexin as a potential mediator of nicotine reinforcement. Consistent findings have also been obtained in other drug administration studies (Lawrence, Cowen, Yang, Chen, & Oldfield, 2006).
Findings from the correlation analyses (see supplementary materials) support the association between orexin, craving, and withdrawal during both ad libitum and abstinence period, (24–48 hrs). These associations were not evident when considering general negative trait measures, such as anxiety or depressive trait. Our inclusion of nonsmokers demonstrates that both of these dynamic features (mood and orexin levels) show varying patterns across time, but it is the consistency of findings within the smokers during the 24-hour abstinence period that lends strength to the conclusion of sex differences. For example, the consistent opposing associations between orexin and withdrawal in abstaining men and women (negative for women and positive for men) as well as between orexin and craving in smoking men and women (positive for women and negative for men) suggest that sex is important to consider as a moderator of any association between relapse and orexin change. It is known that the dopaminergic reward system is tightly linked to craving-associated positive reinforcement, while craving driven by the desire to relieve withdrawal symptoms may involve stress-related pathways including the corticotrophin-releasing factor (CRF) system (Harris & Aston-Jones, 2006; Koob & Le Moal, 2008). The extent to which these patterns of association vary between the sexes has not been clearly defined. The meaning of the opposing relationships in the two sexes (positive orexin-aversive urges correlation in abstaining women, negative orexin-craving correlation in abstaining men) is also unclear, but may be related to sex differences in CRF sensitization of nicotine (O’Dell & Torres, 2014). Additionally, the influence of orexin may represent a dispositional trait that contributes to reduced mesolimbic dopaminergic transmission and may, therefore, contribute to maintenance of or increased risk for relapse to tobacco use, independent of acute craving measures. These results are also partially consistent with a recent characterization of orexin as a stress promoting peptide (Bali, Singh, & Jaggi, 2014), though our results indicate that the presence of other cofactors such as sex hormones, nicotine, and glucocorticoid exposure may further define this relationship.
This study confirmed the decline in cortisol concentration in the withdrawal phase (24-hour abstinent period) relative to the ad libitum phase, and extended previous research showing that steeper decline in cortisol levels during this initial withdrawal period is associated with increased risk for early relapse (al’Absi et al., 2004). The study also confirmed earlier findings showing a similar pattern with ACTH, which was lower during withdrawal in those who relapsed relative to smokers who successfully abstained (al’Absi et al., 2005). These findings may reflect long-term alteration of the HPA axis and central dopaminergic systems as a function of chronic exposure to tobacco or they may be related to preexisting vulnerability in the relapsing group (Koob & Le Moal, 1997; Lemieux & al’Absi, 2016). It is worth noting that these differences in HPA hormones did not influence the association between orexin and relapse; analyses that included all hormones confirmed that relapsers exhibited a steeper decline in orexin during the withdrawal phase (24-hour abstinent period) relative to the ad libitum measures.
Our results add to the growing evidence that appetite-regulating hormones relate to drug craving and reward through multiple pathways including the dopaminergic pathway (Arditti, Grzywacz, & Gallimore, 2013; Kalra & Kalra, 2004; Palmiter, 2007; Sustkova-Fiserova, Jerabek, Havlickova, Kacer, & Krsiak, 2014; Tan, Bishop, Lauzon, Sun, & Laviolette, 2009; Volkow et al., 2008). Changes due to long-term nicotine exposure may reduce dopaminergic receptor density (Adermark et al., 2016; Dagher et al., 2001; Ehlinger et al., 2016; Exley et al., 2013; Wittekind & Kluge, 2015), leading, in turn, to rebound changes during cessation, possibly contributing to increased craving, mood changes, and risk for relapse. These changes may also alter the effectiveness of nicotine’s rewarding properties. Beyond effects on receptor density, other mechanisms mediating the link between relapse and reduced orexin during withdrawal are yet to be established, but may include GABA (Fehr et al., 2008) or glutamate (Davoudi, Azizi, Mirnajafi-Zadeh, & Semnanian, 2016).
While promising, this study has limitations that need to be addressed in future research. These include a short follow-up period and an absence of published clinical research on the acute effects of tobacco use on orexin. We note that alcohol and other drugs were not biologically confirmed prior to each lab session. This will be particularly important to address in future work given the consistent finding of orexin’s role in motivating alcohol and other drug consumption (James, Mahler, Moorman, & Aston-Jones, 2017). It is noteworthy that in this study no causality link can be clearly made as the effects may be due to the stress of withdrawal or other nonspecific factors. Future studies are needed to examine the effect of stress on orexin under both ad libitum and withdrawal phases (24-hour abstinent period). In addition, it would be beneficial for establishing causality if the study used an ABA design whereby smokers return to ad libitum smoking after the 24-hours of verified abstinence. Nevertheless, the current study’s design and methods have several strengths and novel features, including the use of multiple measures of craving, the well-controlled, repeated-measure assessment after smoking abstinence, the relatively large sample size, and the inclusion of a nonsmoking control group. Although other, less conservative, definitions of relapse could have been used (J. R. Hughes et al., 2003), the current study used the stringent definition of relapse to reflect a return to addictive nicotine use. Although measurement of orexin in the periphery may not directly reflect central levels, orexin passively crosses the blood-brain barrier (Plaza-Zabala et al., 2013), which enhances the relevance of peripheral levels to central functions. This work is also clinically relevant. It may inform future research examining hormonal and behavioral changes during smoking abstinence that may contribute to appetite changes and weight gain, a widely cited deterrent for quitting (Emery, Levine, Cheng, & Marcus, 2014; Kastin & Akerstrom, 1999; Levine, Cheng, Kalarchian, Perkins, & Marcus, 2012; O’Hara et al., 1998; Williamson et al., 1991).
In summary, these results provide the first evidence of a potential role for orexin in the risk for smoking relapse. The results affirm the potential usefulness of orexin as a marker of craving and relapse, and they underscore the conclusion that smoking relapse is a complex neurobiological process. Future research should address the extent to which orexin contributes to post-cessation changes in mood, dietary behaviors, and weight gain.
Supplementary Material
Supplementary Table 1. Correlations between orexin and craving and withdrawal symptoms, by gender and group.
Supplement Figure 1. A scatter plot depicts the association between orexin and withdrawal symptoms (MNWS) in smoking females (1a.) and smoking males (1b.).
Acknowledgement and declarations of interest
This research was supported in part by National Institutes of Health grants R01DA016351 and R01DA027232. We would like to thank the following individuals for their help with collecting (Barbara Gay, Elizabeth Ford, Dayna Schleppenbach, Soni Rraklli, Angie Forsberg) and managing (Motohiro Nakajima and Jie Gooder) the data for this study. Nikki Neumann, Christopher Schweiger, and Dan Vuicich helped with the conducting the assays. We would like to thank Dr. Susan Raatz for help in providing guidance on nutrition during the implementation of the protocol. We also would like to thank Briana DeAngelis for her editorial help with this manuscript. The authors have no conflict of interest in this manuscript.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- Adermark L, Morud J, Lotfi A, Danielsson K, Ulenius L, Soderpalm B, Ericson M (2016) Temporal Rewiring of Striatal Circuits Initiated by Nicotine. Neuropsychopharmacology 41:3051–3059. 10.1038/npp.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al’Absi M, Carr SB, Bongard S (2007) Anger and psychobiological changes during smoking abstinence and in response to acute stress: prediction of smoking relapse. Int J Psychophysiol 66:109–115. 10.1016/j.ijpsycho.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al’Absi M, Hatsukami D, Davis GL (2005) Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology (Berl) 181:107–117. 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Hatsukami D, Davis GL, Wittmers LE (2004) Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug Alcohol Depend 73:267–278. 10.1016/j.drugalcdep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Hooker S, Fujiwara K, Kiefer F, von der Goltz C, Cragin T, Wittmers LE (2011) Circulating leptin levels are associated with increased craving to smoke in abstinent smokers. Pharmacol Biochem Behav 97:509–513. 10.1016/j.pbb.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al’Absi M, Lemieux A, Nakajima M (2014) Peptide YY and ghrelin predict craving and risk for relapse in abstinent smokers. Psychoneuroendocrinology 49c:253–259. 10.1016/j.psyneuen.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arditti JA, Grzywacz JG, Gallimore SW (2013) A demedicalized view of maternal distress: conceptualization and instrument development. Psychol Serv 10:386–394. 10.1037/a0029954. [DOI] [PubMed] [Google Scholar]
- Bali A, Singh N, Jaggi AS (2014) Neuropeptides as therapeutic targets to combat stress-associated behavioral and neuroendocrinological effects. CNS Neurol Disord Drug Targets 13:347–368. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG (2001) Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res 3:7–16. 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Dagher A, Bleicher C, Aston JA, Gunn RN, Clarke PB, Cumming P (2001) Reduced dopamine D1 receptor binding in the ventral striatum of cigarette smokers. Synapse 42:48–53. 10.1002/syn.1098. [DOI] [PubMed] [Google Scholar]
- Davoudi M, Azizi H, Mirnajafi-Zadeh J, Semnanian S (2016) The blockade of GABAA receptors attenuates the inhibitory effect of orexin type 1 receptors antagonist on morphine withdrawal syndrome in rats. Neurosci Lett 617:201–206. 10.1016/j.neulet.2016.02.022. [DOI] [PubMed] [Google Scholar]
- Ehlinger DG, Bergstrom HC, Burke JC, Fernandez GM, McDonald CG, Smith RF (2016) Adolescent nicotine-induced dendrite remodeling in the nucleus accumbens is rapid, persistent, and D1-dopamine receptor dependent. Brain Struct Funct 221:133–145. 10.1007/s00429-014-0897-3. [DOI] [PubMed] [Google Scholar]
- Emery RL, Levine MD, Cheng Y, Marcus MD (2014) Change in Body Weight Does not Mediate the Relationship Between Exercise and Smoking Cessation Among Weight-Concerned Women Smokers. Nicotine Tob Res 10.1093/ntr/ntu284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, McIntosh JM, Franklin M, Bermudez I, Cragg SJ (2013) Striatal dopamine transmission is reduced after chronic nicotine with a decrease in alpha6-nicotinic receptor control in nucleus accumbens. Eur J Neurosci 38:3036–3043. 10.1111/ejn.12298. [DOI] [PubMed] [Google Scholar]
- Fehr C, Yakushev I, Hohmann N, Buchholz HG, Landvogt C, Deckers H, Eberhardt A, Klager M, Smolka MN, Scheurich A, Dielentheis T, Schmidt LG, Rosch F, Bartenstein P, Grander G, Schreckenberger M (2008) Association of low striatal dopamine d2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am J Psychiatry 165:507–514. 10.1176/appi.ajp.2007.07020352. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S (2009) The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat 36:235–243. 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Frederick SL, Reus VI, Ginsberg D, Hall SM, Munoz RF, Ellman G (1998) Cortisol and response to dexamethasone as predictors of withdrawal distress and abstinence success in smokers. Biol Psychiatry 43:525–530. 10.1016/s0006-3223(97)00423-x. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G (2006) Arousal and reward: a dichotomy in orexin function. Trends Neurosci 29:571–577. 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G (2005) A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437:556–559. 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991) The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 86:1119–1127. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ (2008) Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci U S A 105:19480–19485. 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J, Hatsukami DK (1998) Errors in using tobacco withdrawal scale. Tob Control 7:92–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D (1986) Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry 43:289–294. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE (2003) Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res 5:13–25. [PubMed] [Google Scholar]
- James MH, Mahler SV, Moorman DE, Aston-Jones G (2017) A Decade of Orexin/Hypocretin and Addiction: Where Are We Now? Curr Top Behav Neurosci 33:247–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra SP, Kalra PS (2004) Overlapping and interactive pathways regulating appetite and craving. J Addict Dis 23:5–21. 10.1300/J069v23n03_02. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V (1999) Orexin A but not orexin B rapidly enters brain from blood by simple diffusion. J Pharmacol Exp Ther 289:219–223. [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Otte C, Demiralay C, Wolf K, Wiedemann K (2005) Increasing leptin precedes craving and relapse during pharmacological abstinence maintenance treatment of alcoholism. J Psychiatr Res 39:545–551. 10.1016/j.jpsychires.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP (1997) Craving is associated with smoking relapse: findings from three prospective studies. Exp Clin Psychopharmacol 5:137–142. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (1997) Drug abuse: hedonic homeostatic dysregulation. Science 278:52–58. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (2008) Addiction and the brain antireward system. Annu Rev Psychol 59:29–53. 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koopmann A, von der Goltz C, Grosshans M, Dinter C, Vitale M, Wiedemann K, Kiefer F (2012) The association of the appetitive peptide acetylated ghrelin with alcohol craving in early abstinent alcohol dependent individuals. Psychoneuroendocrinology 37:980–986. 10.1016/j.psyneuen.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB (2001) The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B (2006) The orexin system regulates alcohol-seeking in rats. Br J Pharmacol 148:752–759. England. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux A, al’Absi M (2016) Stress psychobiology in the context of addiction medicine: from drugs of abuse to behavioral addictions. Prog Brain Res 223:43–62. 10.1016/bs.pbr.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Lemieux A, Nakajima M, Hatsukami DK, Allen S, al’Absi M (2015) Changes in circulating leptin levels during the initial stage of cessation are associated with smoking relapse. Psychopharmacology (Berl) 232:3355–3361. 10.1007/s00213-015-3989-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MD, Cheng Y, Kalarchian MA, Perkins KA, Marcus MD (2012) Dietary intake after smoking cessation among weight-concerned women smokers. Psychol Addict Behav 26:969–973. 10.1037/a0028948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, al’Absi M (2012) Predictors of risk for smoking relapse in men and women: a prospective examination.Psychol Addict Behav 26:633–637. 10.1037/a0027280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T (2006) Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci 26:398–405. 10.1523/jneurosci.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Torres OV (2014) A mechanistic hypothesis of the factors that enhance vulnerability to nicotine use in females. Neuropharmacology 76 Pt B:566–580. 10.1016/j.neuropharm.2013.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara P, Connett JE, Lee WW, Nides M, Murray R, Wise R (1998) Early and late weight gain following smoking cessation in the Lung Health Study. Am J Epidemiol 148:821–830. [DOI] [PubMed] [Google Scholar]
- Palmiter RD (2007) Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci 30:375–381. 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Pasumarthi RK, Fadel J (2008) Activation of orexin/hypocretin projections to basal forebrain and paraventricular thalamus by acute nicotine. Brain Res Bull 77:367–373. 10.1016/j.brainresbull.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Zabala A, Flores A, Martin-Garcia E, Saravia R, Maldonado R, Berrendero F (2013) A role for hypocretin/orexin receptor-1 in cue-induced reinstatement of nicotine-seeking behavior. Neuropsychopharmacology 38:1724–1736. 10.1038/npp.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche DJ, Bujarski S, Moallem NR, Guzman I, Shapiro JR, Ray LA (2014) Predictors of smoking lapse in a human laboratory paradigm. Psychopharmacology (Berl) 231:2889–2897. 10.1007/s00213-014-3465-x. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA (1983) Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press: Palo Alto, CA. [Google Scholar]
- Sustkova-Fiserova M, Jerabek P, Havlickova T, Kacer P, Krsiak M (2014) Ghrelin receptor antagonism of morphine-induced accumbens dopamine release and behavioral stimulation in rats. Psychopharmacology (Berl) 231:2899–2908. 10.1007/s00213-014-3466-9. [DOI] [PubMed] [Google Scholar]
- Tan H, Bishop SF, Lauzon NM, Sun N, Laviolette SR (2009) Chronic nicotine exposure switches the functional role of mesolimbic dopamine transmission in the processing of nicotine’s rewarding and aversive effects. Neuropharmacology 56:741–751. 10.1016/j.neuropharm.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ (1991) The development and initial validation of a questionnaire on smoking urges. Br J Addict 86:1467–1476. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Warthen MW, Goedeker KC (2009) The functional significance of craving in nicotine dependence. Nebr Symp Motiv 55:171–197. [DOI] [PubMed] [Google Scholar]
- Ussher M, West R, Evans P, Steptoe A, McEwen A, Clow A, Hucklebridge F (2006) Reduction in cortisol after smoking cessation among users of nicotine patches. Psychosom Med 68:299–306. 10.1097/01.psy.0000204926.27215.a1. [DOI] [PubMed] [Google Scholar]
- Van Zundert RM, Ferguson SG, Shiffman S, Engels R (2012) Dynamic effects of craving and negative affect on adolescent smoking relapse. Health Psychol 31:226–234. 10.1037/a0025204. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C (2008) Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage 39:1266–1273. 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Goltz C, Koopmann A, Dinter C, Richter A, Rockenbach C, Grosshans M, et al. (2010) Orexin and leptin are associated with nicotine craving: a link between smoking, appetite and reward. Psychoneuroendocrinology 35:570–577. 10.1016/j.psyneuen.2009.09.005. [DOI] [PubMed] [Google Scholar]
- West R, Baker CL, Cappelleri JC, Bushmakin AG (2008) Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology (Berl) 197:371–377. 10.1007/s00213-007-1041-3. [DOI] [PubMed] [Google Scholar]
- Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T (1991) Smoking cessation and severity of weight gain in a national cohort. N Engl J Med 324:739–745. 10.1056/nejm199103143241106. [DOI] [PubMed] [Google Scholar]
- Wittekind DA, Kluge M (2015) Ghrelin in psychiatric disorders - A review. Psychoneuroendocrinology 52:176–194. 10.1016/j.psyneuen.2014.11.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Correlations between orexin and craving and withdrawal symptoms, by gender and group.
Supplement Figure 1. A scatter plot depicts the association between orexin and withdrawal symptoms (MNWS) in smoking females (1a.) and smoking males (1b.).


