Abstract
Background & Purpose:
We aim to determine the potential impact on stroke thrombolysis of “drip and ship” helicopter flights, and specifically of their low frequency vibrations (LFV).
Methods:
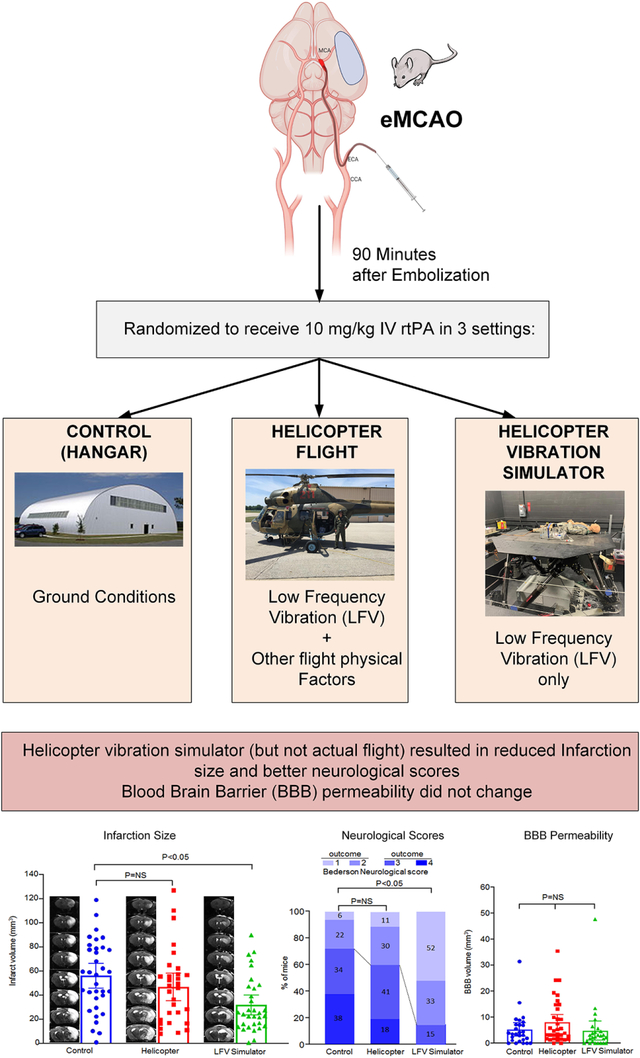
Mice with a middle cerebral artery autologous thromboembolic occlusion (eMCAO) were randomized to receive rtPA (or saline) 90 minutes later in three different settings: 1) A motion platform simulator that reproduced the LFV signature of the helicopter, 2) a standardized actual helicopter flight, and 3) a ground control.
Results:
Mice assigned to the LFV simulation while receiving tPA had smaller infarctions (31.6 vs. 54.9 mmᶟ; p=0.007) and increased favorable neurological outcomes (86 vs. 28%; p=0.0001) when compared to ground controls. Surprisingly, mice receiving tPA in the helicopter did not exhibit smaller infarction (47.8 vs. 54.9 mmᶟ; p=0.58) nor improved neurological outcomes (37 vs. 28%; p=0.71). This could be due to a causative effect of the 20–30 Hz band, which was inadvertently attenuated during actual flights. Mice using saline showed no differences between the LFV simulator and controls with respect to infarct size (80.9 vs. 95.3; p=0.81) or neurological outcomes (25 vs. 11%; p=0.24), ruling out an effect of LFV alone. There were no differences in blood barrier permeability (BBB) between LFV simulator or helicopter, compared to controls (2.45, 3.02 vs. 4.82 mmᶟ; p=0.14).
Conclusion:
Vibration in the low (0.5–120 Hz) frequency range is synergistic with rtPA, significantly improving the effectiveness of thrombolysis without impairing BBB permeability. Our findings reveal LFV as a novel, safe, and simple to deliver intervention that could improve the outcomes of patients.
Keywords: Air ambulances, thrombolytic therapy, acute stroke, vibration, models, animal
Graphical Abstract
Introduction
Every year 17 million people in the world suffer a stroke, 800,000 of those in the United States where is an important contributor to death and the main cause of disability. Intravenous recombinant tissue plasminogen activator (rtPA) remains the only approved medical treatment for acute ischemic stroke, and it has limited success. There is a critical need for additional interventions to further enhance the efficacy of rtPA1. Patients with stroke in remote2 or congested areas typically receive the rtPA infusion while being evacuated by helicopter emergency medical services to a tertiary stroke centers (HEMS), known colloquially as “drip & ship”3. Those helicopter flights generate complex physical factors that have plausible effects on stroke and reperfusion that heretofore have been ignored. These factors include predominately low frequency vibrations (LFV)4, but also extreme noise, accelerations in three-axes, and rapid barometric changes5. Importantly, any of these factors can be plausibly harmful or beneficial5. It is therefore critical to understand the overall effects of HEMS-related physical factors, and LFV in particular. Mitigating any harmful effect could lead to optimization of those drip & ship HEMS transfers. But even more importantly, the identification of beneficial HEMS physical factors could lead to the development of interventions to improve patient outcomes in all care settings1. Because, randomized studies of the effect of HEMS flights in stroke patients would be problematic logistically and ethically, we previously reported the feasibility of conducting translational animal research during actual helicopter flights6. Here, we report the actual effect of helicopter flights, and the isolated effect of helicopter-like LFV, in a murine model of acute ischemic thromboembolic stroke.
Methods:
The data that support the findings of this study are available from the corresponding author upon reasonable request. This was a prospective randomized controlled study conducted between February 2018 and February 2019. Mice with a middle cerebral artery autologous thromboembolic occlusion (eMCAO) were randomized to receive rtPA 90 minutes later in three different settings: 1) a standardized actual helicopter flight, 2) A motion platform simulator that reproduced the LFV signature of the helicopter without the other flight physical factors, and 3) a ground control (Fig 1). Main outcome measures were residual infarct size on MRI, and % of good neurological outcomes. Hyperacute blood brain barrier (BBB) permeability was a secondary outcome. Pre-scheduled missions proceeded to surgery only if weather conditions permitted, and the animal laboratory was simultaneously available along with the helicopter, vibration simulator and neuroimaging teams and equipment. A subsequent study using saline was planned in case of positive results to explore possible interactions with rtPA.
Fig. 1.

Consort-style diagram of the study
eMCAO procedures
We previously optimized the murine model in regards to maximal number of simultaneous surgeries and clot size, and determined that two simultaneous surgeries and a 30 mm clot achieved eMCAO less than 15 minutes apart with adequate infarction size. Pairs of 10–14 week old C57BL/6J male mice underwent the method described by Overgaard et al with slight modification7. Animals were anesthetized with 1–1.5% isoflurane during the surgery. The catheter containing a single 30 mm fibrin-rich clot was then introduced into the external carotid artery and advanced to the internal carotid artery. After embolization, the catheter was removed and the external carotid artery was blocked by cauterization. Laser Doppler flow monitoring (Perimed Instruments, Stockholm, Sweden) was used to confirm the induction of brain hypoperfusion. The body temperature of the mouse was maintained at 37 ± 1C° during the entire procedure. Buprenorphine (0.1 mg/kg, SC) was administered as an analgesic agent every 6–12 hours for 48 hours post-surgery. The right jugular vein was cannulated and connected to the pump for the administration of rtPA (Cathflo, Genentech), 10 mg/kg, 10% volume by bolus and remaining slow infusion for 45 minutes, or same volume of saline, 90 minutes post-embolization.
Experimental Group Assignment
In each day of the experiment, three pairs of mice were sequentially assigned to receive the three exposures (helicopter, LFV simulator or ground control) in a random order 2h apart (Fig 1). Following surgery, each pair of mice was protected by conical restraints and placed on the floor of a customized 0.6 mm thick acrylic Plexiglas box (48.5×20.5×34.6 cm) equipped with accelerometers, and pre-programmed syringe pumps attached to IV lines (Fig 1). The surgeon, blinded to randomization status, handed the animal box to an unblinded investigator (ECL) who transported it to the assigned exposure in an Institutional Animal Care and Use Committee (IACUC)-approved ground vehicle (Fig 1). The driving was kept constant for the three groups by using a triangular route of 5 miles total (animal lab-LFV simulator-airport-animal lab). In all three exposures, the IV rtPA pumps were manually started exactly 90 minutes from the half-way point between the two mice eMCAO times. The box was equipped with fan, air cooling unit, heating pads, which were manually operated as needed aiming for a target of 25 C°. All operational times and atmospheric variables were recorded.
The flight exposure consisted of a soviet-era Mi2 HEMS/military dual turbine (298 kW, 400 sHP) helicopter certified and instrumented as an airborne laboratory (Fig 1). The animal box was secured to the floor of the helicopter in a consistent way for reproducibility of the LFV exposure. Engine start time always coordinated with IV pump initiation 90 minutes after embolization. A 1-hour local standardized flight was conducted in visual meteorological conditions at an altitude of 1000 feet (309 meters) above ground in and out of Iowa City Municipal Airport, Iowa (KIOW), following a triangular path with Muscatine, Iowa (KMUT) and Washington, Iowa (KAWG) as waypoints (total distance: 71.3 nm, 132 Km).
The LFV simulation exposure (also 1h) consisted of a modified six-degree-of-freedom man-rated Moog-FCS motion platform simulator capable of consistent reproduction motions up to 30 Hz. A tactile transducer was added to the animal box to extend the vibration to the 60 Hz range (Fig 1). This simulator reproduced with fidelity the LFV signature of a Mi2 flight that was previously recorded with accelerometers (X16, Gulf Coast Data Concepts) in three directional axes, and which involved multiple waveforms in the 1–120 Hz range (Fig 2). Simulated engine start was adjusted to always coincide with IV pump initiation 90 minutes after embolization.
Fig 2.

A: Time based LFV profile of a typical experiment with the three sequential missions (Simulator-Control-Flight). Colors indicate X Y and Z axes (fore-aft, lateral, vertical). Handling and driving seen at around 10 minutes (brief spikes), LFV simulator motion 45–105 minutes, then handling and driving (brief spikes), control setting from 155–215, handling and driving (brief spikes), then Mi2 flight 280–350, followed by handling and driving (brief spikes). B: Average mean LFV vibration (95% CI) by frequency bands between the LFV simulator and Helicopter flights.
The animal box assigned to ground control received the IV infusion in an office inside the hangar of the research facility at KIOW.
Blinded MRI and Clinical Outcome Analyses
After each exposure, animals were returned to the laboratory where MRIs were immediately performed, per established protocol8, to analyze acute changes in BBB permeability expressed as percentage of Gd-DTPA enhancement within the ischemic lesion9. Hyperacute infarction size was also analyzed to minimize loss of data from animal death. The MRI was repeated at 24h and used for a more precise infarction determination. Animals were anesthetized with isoflurane (2.5% induction, 1.2% maintenance) and placed in the bore of the 7.0 Tesla MRI (Agilent Technologies Inc., Santa Clara, CA, USA) with a two-channel receive-only surface coil.
Following scout scans, high-resolution images were acquired with a 9-minute T2-weighted 2D fast spin-echo sequence oriented coronally. Imaging parameters included TR/TE = 6380 ms/83 ms, echo train length of 12, and 7 signal averages to achieve voxel resolution 0.10 mm × 0.10 mm × 0.50 mm with no gaps. This was followed by intraperitoneal injection of gadobuterol (Gadavist, Bayer HealthCare) at a dose of 0.3 mmol/kg and subsequent multiple 3D gradient echo acquisitions in the same coronal plane (TR/TE = 25/3 ms, flip angle 30°, resolution 0.1mm × 0.1mm × 0.25mm, 3 minutes per scan) acquired over 25 minutes. The area of infarction was quantified by a blinded operator using NIH Image J software by outlining the zone with abnormally T2 hyperintense regions in each brain slice, and total infarct volume was obtained by summation of the infarcted areas multiplied by the slice thickness. To correct for brain swelling due to edema after ischemia, the corrected total infarct volume (%) was calculated. The BBB permeability was quantified by a blinded operator using NIH Image J software by outlining the zone with abnormally hyperintense regions in each brain slice, and total BBB leakage volume was obtained by summation of the hyperintense areas multiplied by the slice thickness. The Bederson scale, which is a global neurological assessment in experimental models10, was used to by a blinded observer to measure neurological impairment. A score of 0–2 was categorized as “good”.
Statistical Consideration and Sample Size
The differences in the effect of the three exposures of interest (ground control, helicopter, LFV simulator) on the outcome measures was assessed using linear mixed model analysis for infarction, and BBB permeability, and by logistic regression fitted by generalized estimating equations (GEE) method for favorable neurological outcome (0–2 of 4 point scale). The p-values for pairwise comparison between exposures were adjusted using Tukey-Kramer method. The models were expanded to include covariates to account for potential effect of confounders, which included time to rtPA infusion start, outside temperature, barometric pressure, dew point, total vibration during exposure, and vibration during ground transportation. Based on infarction size data from our pilot study, assuming an infarct difference of 33 and standard deviation of 95, we determined that a sample size of at least 25 per group would detect a 25% reduction between groups with 0.80 power at a significance level of 0.05. All experimental procedures were approved by the University of IACUC.
Results
Figure 1 shows a CONSORT-style11 study diagram. One hundred and one mice survived the MCAO surgical procedure and were included. The 24h mortality of 19–22% was the same in all three groups (Fig 1), and consistent with the expected perioperative mortality of the thromboembolic model12. Fig 2B shows the box accelerometer amplitude measurements between the helicopter flight and the LFV simulator by frequency band. The two groups delivered comparable LFV energy, with the exception of the 20–30 Hz band which was overrepresented in the simulator group. The raw data results of the study are shown in Fig 3. From the linear mixed model and GEE analysis, mice in the helicopter group did not show a reduction in infarct volume (47.8 vs. 54.9 mmᶟ, difference of −7.1; 95% CI: −24.8, 10.5; p=0.58) or better neurological outcomes when compared to the control group (37 vs. 28%, odds ratio of 1.52; 95% CI: 0.43, 5.30; p=0.71). On the other hand, mice assigned to the LFV simulator had significantly smaller infarction volumes than ground controls (31.6 vs. 54.9 mmᶟ, difference of −23.4; 95% CI: −40.8, −6.0; p=0.007), and also a significantly higher percentage of good neurological outcomes (86 vs. 28%, odds ratio of 15.08; 95% CI: 3.22, 70.60; p=0.0001). The adjusted linear mixed model to account for confounding effects of the covariates with respect to infarct volume, continued to show a significant effect of group exposure assignment (p=0.027). There was no significant difference in blood-brain barrier (BBB) permeability between the helicopter or ground LFV simulator, compared to controls (2.45, 3.02 vs. 4.82 mmᶟ; difference of −2.37; 95% CI: −4.03, 0.84; p=0.14 for helicopter, and difference of −1.80; 95% CI: −3.74, 1.96; p=0.36 for simulator).
Fig 3.

Raw data results of the main rtPA experiment in regard to (A) MRI infarct volume, (B) % “good” (0–2) outcomes in the Bederson Neurological scores, and (C) hyperacute BBB permeability on MRI.
A subsequent similar study using saline was conducted after these positive results to determine whether LFV alone had an effect on stroke outcomes independently of rtPA. Here, we conducted 4 missions comparing LFV simulator and control using saline (total n=18) (Raw data shown in Fig 4). The linear mixed model and GEE analysis showed no differences between the LFV simulator group and control group with respect to infarct size (80.9 vs. 95.3, difference of −14.4; 95% CI: −58.3, 49.6; p=0.81) or good neurological outcomes (25 vs. 11%, odds ratio of 2.65; 95% CI: 0.53, 13.29; p=0.24).
Fig 4.
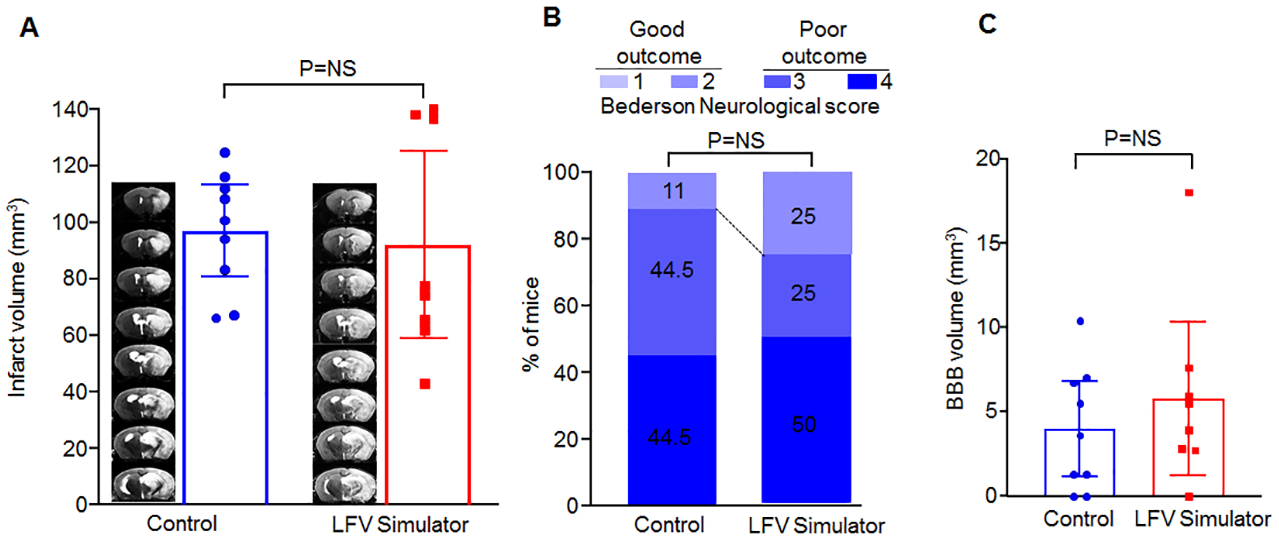
Raw Data results of the subsequent exploratory, saline-only, experiment in regard to (A) MRI infarct volume, (B) % “good” (0–2) outcomes in the Bederson Neurological scores, and (C) hyperacute BBB permeability on MRI.
Discussion
This is the first report of a synergistic effect between a replicated helicopter-like LFV on the ground and rtPA thrombolysis on stroke outcomes. The magnitude of this synergistic effect was substantial, with approximately half the infarct volume and triple the percentage of good neurological outcomes compared to rtPA alone. This study, which involved a large sample with rigorous methodology to eliminate bias, suggests that LFV provides a potential ancillary treatment strategy for patients with stroke undergoing thrombolysis. Although the concept of enhancing rtPA thrombolysis with physical energy has also been proposed with ultrasound13, large studies failed to show an effect14. But unlike transcranial focused ultrasound, LFV could be easily achieved in clinical settings. For example, LFV is omnidirectional and does not require sophisticated equipment or human expertise necessary to deliver focused energy. It could also be easily delivered in hospital and ambulance patient beds. Moreover, LFV is considered safe15 and does not raise concerns of brain cavitation. The lack of effect on BBB permeability is also reassuring.
Our results are also consistent with studies showing LFV-mediated acceleration of reperfusion in in-vitro experiments16–18. LFV has been previously proposed to influence blood flow through convective currents and velocity shifts19, 20,21, which could conceivably facilitate clot erosion and rtPA exposure, nitric oxide release by blood vessels22, or increased rtPA levels23. It is also possible that the effect of LFV is not mediated through increased thrombolysis, but through protecting the ischemic penumbra from an increased sensory stimulation24. We recognize the uncertainty regarding the actual mechanism of action, which should be the subject of future studies along with optimizing the therapeutic effect. Notably, we did not observe an independent effect of LFV in our study, suggesting that the synergism between LFV and rtPA is critical to enhance outcomes.
Surprisingly, we did not observe the same benefit in mice exposed to the actual helicopter flights6. While there was a suggested possible benefit in the helicopter group compared with ground, the difference was not significant. The LFV energy that was delivered in the helicopter was comparable to the one generated in the simulator, with the notable exception of the 20–30 Hz band. This band ended up being delivered with larger intensity in the simulator, either due to unforeseen resonance in the platform/speakers or sampling issues with the flight used to elicit the original LFV signature. Therefore, the difference in outcomes between the helicopter and the LFV simulator groups could be related to a possible causative effect of the 20–30 Hz band. This was suggested by exploratory logistic analyses using the different frequency bands where a significant association of 20–30 Hz intensity with infarct volume and neurological outcome was observed (results not shown). Retrospective studies in patients with stroke transported by helicopter have shown mixed clinical outcomes, ranging from no effect25 to a hint of potential benefit26, but there is variability in the LFV signature between different helicopter ambulances used in clinical care. Still, we cannot exclude with this study a negating effect of other as-yet unidentified physical factors present in flight5. Importantly, the potential negative effect of helicopter transportation on the pharmacological integrity of rtPA has been specifically ruled-out27. We recognize that we cannot completely exclude a confounding negative effect of temperature fluctuations during flights despite the efforts to maintain the animal temperature at 25 C°. However, the lack of significance of outside temperature and other atmospheric variables in the multivariate model makes temperature an unlikely confounding factor. However, other factors present in helicopter flight do present plausible negative effects. For example, rapid barometric reductions28 could result in hypoxia in the ischemic penumbra, and accelerations in three axes29, as well as extreme noise (105 db)30, could also negatively influence the outcome. Future work will focus on measuring these factors in helicopter flights and recapitulating them in the ground scenario in order to determine their independent effect.
We also recognize the potential effects of the small vibratory exposure received during ground transportation, which was inevitable given the different locations of the laboratories involved. However, we feel this factor is negligible given that ground transportation was short (Fig 1) and kept constant for the three groups. Furthermore, this ground transportation acceleration was measured (Fig 2), and it did not confound the results as shown in the adjusted multivariate analysis. We are also aware of the human translational concerns regarding stroke models, including scaling issues31, 32. The impact of LFV in a human head may be different, and the composition of clots in patients with stroke is variable. The use of an animal stroke model was needed, however, to rigorously address our question, as it would be unethical to randomize patients with acute stroke to different transport modalities and rtPA/saline at pre-specified points of time5, 6. We also recognize that additional future research is needed to measure the impact of LFV on rtPA reperfusion in other species, sex, and co-morbid conditions. We also recognize the need to assess long-term neurological outcomes and behavioral outcomes as well. Still, the lack of any negative effects of flights on stroke outcomes, including no increase in BBB permeability as previously suggested33, supports the safety of future trials of neuroprotection delivered during helicopter transport.
In summary, we have identified a synergistic effect between recapitulation of the LFV signature of a helicopter flight on the ground with rtPA treatment with respect to reperfusion outcomes in a mouse stroke model. This novel finding appears to form the basis for an ancillary intervention that could potentially improve patient care.
Supplementary Material
Acknowledgments:
We thank Mr. Charles Harris for his assistance with the vibration experiments: Funding: This work was sponsored by the NIH-NINDS grant R21 NS104579-01 to ECL, AKC, AAP, TS and SR. AAP is sponsored by the Brockman Foundation, the Elizabeth Ring Mather & William Gwinn Mather Fund, the S. Livingston Samuel Mather Trust, and project 19PABH134580006 from the American Heart Association/Allen Initiative in Brain Health and Cognitive Impairment.
Footnotes
Disclosure Statement: Dr. Leira, Schnell, Rahmatalla, Chauhan and Pieper report US patent Application N0 16/783,003 pending.
References:
- 1.Knecht T, Story J, Liu J, Davis W, Borlongan CV and Dela Pena IC. Adjunctive Therapy Approaches for Ischemic Stroke: Innovations to Expand Time Window of Treatment. International journal of molecular sciences. 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carr BG, Branas CC, Metlay JP, Sullivan AF and Camargo CA Jr. Access to emergency care in the United States. AnnEmergMed. 2009;54:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tekle WG, Chaudhry SA, Hassan AE, Rodriguez GJ, Suri MF and Qureshi AI. Drip-and-ship thrombolytic treatment paradigm among acute ischemic stroke patients in the United States. Stroke. 2012;43:1971–4. [DOI] [PubMed] [Google Scholar]
- 4.Andrew Hoffman HG. Externally applied vibration at 50hz facilitates dissolution of blood clots in-vitro. American Journal of Biomedical Sciences. 2012;4:274–284. [Google Scholar]
- 5.Leira EC, Stilley JD, Schnell T, Audebert HJ and Adams HPJ. Helicopter transportation in the era of thrombectomy: The next frontier for acute stroke treatment and research. European Stroke Journal. 2016;1:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leira EC, Zaheer A, Schnell T, Torner JC, Olalde HM, Pieper AA, et al. Effect of helicopter transport on neurological outcomes in a mouse model of embolic stroke with reperfusion: AIR-MICE pilot study. International journal of stroke : official journal of the International Stroke Society. 2015;10 Suppl A100:119–24. [DOI] [PubMed] [Google Scholar]
- 7.Overgaard K, Sereghy T, Pedersen H and Boysen G. Dose-response of rt-PA and its combination with aspirin in a rat embolic stroke model. Neuroreport. 1992;3:925–8. [DOI] [PubMed] [Google Scholar]
- 8.Dhanesha N, Vazquez-Rosa E, Cintron-Perez CJ, Thedens D, Kort AJ, Chuong V, et al. Treatment with Uric Acid Reduces Infarct and Improves Neurologic Function in Female Mice After Transient Cerebral Ischemia. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2018;27:1412–1416. [DOI] [PubMed] [Google Scholar]
- 9.Durukan A, Marinkovic I, Strbian D, Pitkonen M, Pedrono E, Soinne L, et al. Post-ischemic blood-brain barrier leakage in rats: one-week follow-up by MRI. Brain Res. 2009;1280:158–65. [DOI] [PubMed] [Google Scholar]
- 10.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL and Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–6. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. International journal of surgery (London, England). 2012;10:28–55. [DOI] [PubMed] [Google Scholar]
- 12.Hoda MN, Li W, Ahmad A, Ogbi S, Zemskova MA, Johnson MH, et al. Sex-independent neuroprotection with minocycline after experimental thromboembolic stroke. Exp Transl Stroke Med. 2011;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, et al. Ultrasound-Enhanced Systemic Thrombolysis for Acute Ischemic Stroke. N Engl J Med. 2004;351:2170–2178. [DOI] [PubMed] [Google Scholar]
- 14.Alexandrov AV, Kohrmann M, Soinne L, Tsivgoulis G, Barreto AD, Demchuk AM, et al. Safety and efficacy of sonothrombolysis for acute ischaemic stroke: a multicentre, double-blind, phase 3, randomised controlled trial. Lancet neurology. 2019;18:338–347. [DOI] [PubMed] [Google Scholar]
- 15.Brogårdh C, Flansbjer U-B and Lexell J. No Specific Effect of Whole-Body Vibration Training in Chronic Stroke: A Double-Blind Randomized Controlled Study. Arch Phys Med Rehabil. 2012;93:253–258. [DOI] [PubMed] [Google Scholar]
- 16.Yohannes FG and Hoffmann AK. Non-invasive low frequency vibration as a potential emergency adjunctive treatment for heart attack and stroke. An in vitro flow model. J Thromb Thrombolysis. 2008;25:251–8. [DOI] [PubMed] [Google Scholar]
- 17.Marzencki M, Kajbafzadeh B, Khosrow-Khavar F, Tavakolian K, Soleimani-Nouri M, Hamburger J, et al. Low frequency mechanical actuation accelerates reperfusion in-vitro. Biomedical engineering online. 2013;12:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang W and Zhou Y. Effect of pulse repetition frequency of high-intensity focused ultrasound on in vitro thrombolysis. Ultrason Sonochem. 2017;35:152–160. [DOI] [PubMed] [Google Scholar]
- 19.Oberti S, Neild A and Wah Ng T. Microfluidic mixing under low frequency vibration. Lab on a chip. 2009;9:1435–8. [DOI] [PubMed] [Google Scholar]
- 20.Francis CW, Blinc A, Lee S and Cox C. Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound Med Biol. 1995;21:419–424. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann A and Gill H. Externally Applied Vibration at 50 Hz Facilitates Dissolution of Blood Clots In-Vitro. American Journal of Biomedical Sciences. 2012:274–284. [Google Scholar]
- 22.Maloney-Hinds C, Petrofsky JS, Zimmerman G and Hessinger DA. The role of nitric oxide in skin blood flow increases due to vibration in healthy adults and adults with type 2 diabetes. Diabetes technology & therapeutics. 2009;11:39–43. [DOI] [PubMed] [Google Scholar]
- 23.Ghazalian F, Hakemi L, Pourkazemi L and Akhoond M. Effects of whole-body vibration training on fibrinolytic and coagulative factors in healthy young men. J Res Med Sci. 2014;19:982–986. [PMC free article] [PubMed] [Google Scholar]
- 24.Murmu RP, Fordsmann JC, Cai C, Brazhe A, Thomsen KJ and Lauritzen M. Sensory Stimulation-Induced Astrocytic Calcium Signaling in Electrically Silent Ischemic Penumbra. Front Aging Neurosci. 2019;11:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olson MD and Rabinstein AA. Does helicopter emergency medical service transfer offer benefit to patients with stroke? Stroke. 2012;43:878–80. [DOI] [PubMed] [Google Scholar]
- 26.Almallouhi E, Al Kasab S, Nahhas M, Harvey JB, Caudill J, Turner N, et al. Outcomes of interfacility helicopter transportation in acute stroke care. Neurology: Clinical Practice. 2019: 10.1212/CPJ.0000000000000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faine BA, Dayal S, Kumar R, Lentz SR and Leira EC. Helicopter “Drip and Ship” Flights Do Not Alter the Pharmacological Integrity of rtPA. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2018;27:2720–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knotts D, Arthur AO, Holder P, Herrington T and Thomas SH. Pneumothorax volume expansion in helicopter emergency medical services transport. Air Med J. 2013;32:138–43. [DOI] [PubMed] [Google Scholar]
- 29.McKinley RA, Tripp LD, Bolia SD and Roark MR. Computer Modeling of Acceleration Effects on Cerebral Oxygen Saturation. Aviation, Space, and Environmental Medicine. 2005;76:733–738. [PubMed] [Google Scholar]
- 30.Munzel T, Schmidt FP, Steven S, Herzog J, Daiber A and Sorensen M. Environmental Noise and the Cardiovascular System. J Am Coll Cardiol. 2018;71:688–697. [DOI] [PubMed] [Google Scholar]
- 31.Lapchak PA. Scientific Rigor Recommendations for Optimizing the Clinical Applicability of Translational Research. Journal of neurology & neurophysiology. 2012;3:e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jean A, Nyein MK, Zheng JQ, Moore DF, Joannopoulos JD and Radovitzky R. An animal-to-human scaling law for blast-induced traumatic brain injury risk assessment. Proceedings of the National Academy of Sciences. 2014;111:15310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iakubovich TG and Getsel’ Kh A. [The effect of vibration on the permeability of the blood-brain barrier]. Fiziol Zh SSSR Im I M Sechenova. 1972;58:845–50. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


