Abstract
Stachybotrys chartarum is a fungal contaminant within the built environment and a respiratory health concern in the United States. The objective of this study was to characterize the mechanisms influencing pulmonary immune responses to repeatedly inhaled S. chartarum. Groups of B6C3F1/N mice repeatedly inhaled viable trichothecene-producing S. chartarum conidia (strain A or strain B), heat-inactivated conidia, or high-efficiency particulate absolute–filtered air twice per week for 4 and 13 weeks. Strain A was found to produce higher amounts of respirable fragments than strain B. Lung tissue, serum, and BAL fluid were collected at 24 and 48 hours after final exposure and processed for histology, flow cytometry, and RNA and proteomic analyses. At 4 weeks after exposure, a T-helper cell type 2–mediated response was observed. After 13 weeks, a mixed T-cell response was observed after exposure to strain A compared with a T-helper cell type 2–mediated response after strain B exposure. After exposure, both strains induced pulmonary arterial remodeling at 13 weeks; however, strain A–exposed mice progressed more quickly than strain B–exposed mice. BAL fluid was composed primarily of eosinophils, neutrophils, and macrophages. Both the immune response and the observed pulmonary arterial remodeling were supported by specific cellular, molecular, and proteomic profiles. The immunopathological responses occurred earlier in mice exposed to high fragment-producing strain A. The rather striking induction of pulmonary remodeling by S. chartarum appears to be related to the presence of fungal fragments during exposure.
Keywords: Stachybotrys chartarum fungal fragmentation, pulmonary arterial remodeling, fungal exposure
Clinical Relevance
Stachybotrys chartarum, or black mold, is found in damp indoor environments and is a respiratory health concern in the United States. Two strains of S. chartarum were used in the present study, with one strain fragmenting to a greater extent. Repeated exposure resulted in inflammation and pulmonary arterial remodeling and suggested that the presence of fungal fragments induced an earlier response.
Fungal contamination in indoor environments is a public health concern in the United States. Indoor fungal bioaerosols are generated by fungal disturbances, such as vibrations or airflow through contaminated areas within buildings, and exposure to fungal bioaerosols has been associated with human disease (1, 2). Among species that are frequently observed, Stachybotrys chartarum exposure has become an area of heightened interest. Since a case study in 1994 identified S. chartarum as a potential cause of acute idiopathic pulmonary hemosiderosis among infants (3, 4), evidence has accumulated for the epidemiologic association of S. chartarum with health effects; however, this association with acute idiopathic pulmonary hemosiderosis is less convincing (5). Although recent consensus documents have confirmed associations between damp indoor environments and adverse respiratory symptoms (1, 2), the contribution of S. chartarum to adverse pulmonary immune responses requires further delineation.
S. chartarum is a black, macroscopic, saprophytic, filamentous fungus that requires high amounts of moisture and cellulose for optimal growth (6–8). S. chartarum spores are ∼3–6 μm in diameter and produce mycotoxins (9, 10) and allergens (11, 12). There are several chemotypes of S. chartarum, including macrocyclic trichothecene-producing and atranone-producing strains. Although this fungus has toxigenic properties, the fungal components common to other fungal species are believed to be more critical to its pathophysiology (13–16).
Fungal bioaerosols are composed of aerosolized fungal spores and fragments. These fragments are derived from fragmented spores or hyphae and have been shown to aerosolize in higher concentrations than spores (17). S. chartarum has previously been shown to fragment (18, 19); however, the influence of S. chartarum fungal fragment exposure has not been fully elucidated. The present study used two different S. chartarum strains that included a high-fragmenting isolate and a low-fragmenting isolate. Aerosolized S. chartarum was repeatedly delivered to mice housed in a nose-only chamber to more closely mimic natural human exposure, a documented methodological advancement over studies using intranasal instillations, intratracheal instillations, or liquid aerosol inhalations (20–26). This model has been validated by characterizing the immune response and genetic profile after Aspergillus fumigatus exposure (27–29).
Previous data from our laboratory and others demonstrate pulmonary pathology after S. chartarum exposure (24, 25, 30, 31); however, the underlying mechanisms need further clarification. The aim of this study was to characterize the mechanisms contributing to the pulmonary immune responses after repeated exposure to S. chartarum. Two different strains of S. chartarum exhibiting varying degrees of fragmentation were individually employed. After exposure, lung tissue, serum, and BAL fluid (BALF) were collected at 24 and 48 hours after final exposure and processed for histology, flow cytometry, and RNA and proteomic analyses to characterize immune mechanisms. The use of the two strains that fragmented to a varying extent helped to differentiate the role of fungal fragmentation in the development of immune response and disease pathology.
Methods
Fungal Cultures
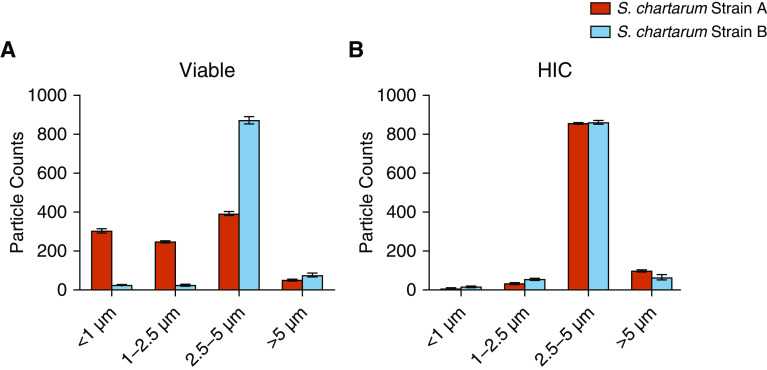
The two macrocyclic trichothecene-producing strains of S. chartarum used in this study were IBT 9460 (strain A) and IBT 7711 (strain B). Strain A produced sixfold higher concentrations of verrucarol and fragmented to a greater extent (<0.5–2 μm in aerodynamic diameter) compared with strain B (Figure 1). Fungal test articles were aerosolized using a computer-controlled acoustical generator system as previously documented (28). See Data Supplement E1 for further details.
Figure 1.
Fragmentation of Stachybotrys chartarum. Aerodynamic particle size distributions of (A) viable and (B) heat-inactivated S. chartarum conidia (HIC) collected during exposures. Data are a representation of the particle size distribution observed over multiple exposures and have been normalized to 1,000 particle counts for comparison. S. chartarum = Stachybotrys chartarum.
Animals
Female B6C3F1/N mice, 5–6 weeks old, were acquired from the National Toxicology Program mouse colony housed at Taconic Biosciences, Inc. Mice were housed in the Assessment and Accreditation of Laboratory Animal Care International–accredited animal facility at the National Institute for Occupational Safety and Health (NIOSH) and were provided an NTP-2000 diet (Harlan Laboratories) and water ad libitum. The mice appeared healthy throughout the study (Figure E2 in the data supplement). All animal procedures were performed under a NIOSH Animal Care and Use Committee–approved protocol.
Fungal Exposures
Mice were randomly separated into six exposure groups with 15 mice per group: Groups 1 and 2 were S. chartarum strain A, viable or heat-inactivated conidia (HIC); groups 3 and 4 were S. chartarum strain B, viable or HIC; and groups 5 and 6 were control groups of high-efficiency particulate absolute–filtered air only. The S. chartarum–exposed mice inhaled 1 × 104 particles (estimated pulmonary deposition/exposure), which was the highest aerosolized dose that reproducibly induced a lymphoproliferative response. Mice were exposed twice per week for 4 or 13 weeks as previously documented (28). Samples were collected 24 hours and 48 hours after final exposure to assess temporal changes in immune responses. Mice were killed via intraperitoneal injection of 200 mg/kg sodium pentobarbital solution (Fatal-Plus Solution; Vortech Pharmaceuticals, Ltd.) and exsanguinated via cardiac puncture. The exposure scheme and harvesting time points were chosen on the basis of preliminary immune response data (27, 29).
Histopathology
Left lung lobes (n = 3/group) were inflated with fixative and collected. The tissue was then embedded, sectioned, and stained with hematoxylin and eosin for routine histopathological evaluation as previously described (29). For additional details, see Data Supplement E2.
Flow Cytometric and Cell Differential Analyses
Cells collected from BAL (n = 7/group) were enumerated using a Cellometer Vision (Nexcelom Bioscience) and prepared for flow cytometric analysis as previously described (29). Cells were blocked and stained using fluorochrome-conjugated antibodies (Table E1). See Data Supplement E3 for additional details.
Proteomic and RNA Analyses
Right lung lobes (n = 3/group) were sent to MS Bioworks for quantitative proteomic analysis as previously described (29). Total RNA was extracted for microRNA (miRNA) (Exiqon Services) and mRNA analyses as previously described (28). Additional analyzed genes are reported in Table E2. T-helper cell type 1 (Th1), Th2, and Th17 gene expression data were normalized to gusb (β-glucuronidase) and additional genes to gapdh (glyceraldehyde 3-phosphate dehydrogenase).
Proteomic and RNA data were analyzed by using Ingenuity Pathway Analysis (IPA) software (Qiagen) for core, comparative, and miRNA target filter analyses (28). For additional details, see Data Supplement E4.
Statistics
Statistical power tests were conducted to determine the appropriate number of samples needed for each endpoint measurement. Fold changes greater than or equal to 2 or less than or equal to −2 were considered altered. P ≤ 0.05 was considered statistically significant (28, 29). See Data Supplement E5 for additional details.
Results
Histopathological Assessment
Inflammation and arterial remodeling were observed in murine lungs after repeated exposure to viable S. chartarum (Figure 2). Differences in lesion development between the two strains were most evident microscopically after 4 weeks (Table 1). No microscopic changes were observed in lungs exposed to HIC or high-efficiency particulate absolute–filtered air controls (Figure E2).
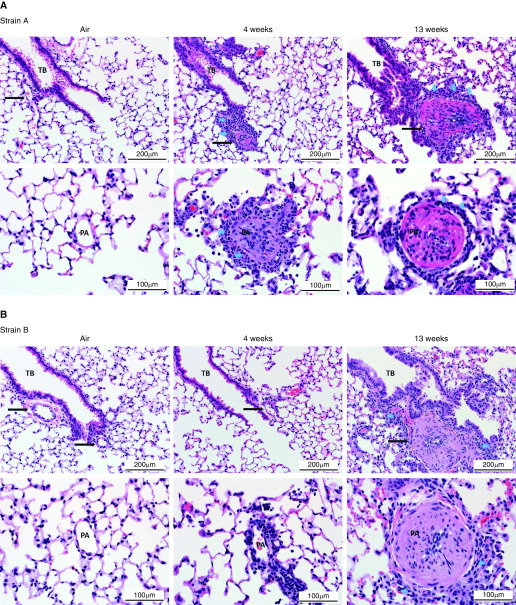
Figure 2.
Pulmonary arterial remodeling. Representative photomicrographs of hematoxylin and eosin–stained sections of lung from mice exposed to viable (A) strain A and (B) strain B. The mice were killed at 24 hours after 4 or 13 weeks of exposure. Lung from an air-exposed mouse is shown for reference. (C) Representative photomicrographs showing the range of changes seen in pulmonary arteries in lungs of mice exposed to viable fungal conidia. The blue arrows indicate different inflammatory cells (i.e., eosinophils and macrophages). Scale bars: 200 μm and 100 μm. PA = pulmonary arteries (indicated by black arrows); TB = terminal bronchiole.
Table 1.
Summary of Observations from Mice Exposed to Viable Stachybotrys chartarum
| Arterial Remodeling |
Airway Changes |
Alveolar Changes |
||||||
|---|---|---|---|---|---|---|---|---|
| Early | Continuum | Advanced | Thrombi | Inflammation | Mucous Metaplasia | Histiocytosis | M2 Histiocytosis | |
| 4 wk | ||||||||
| Strain A | 0/6 | 3/6 | 2/6 | 4/6 | 5/6 | 5/6 | 5/6 | 0/6 |
| Strain B | 3/6 | 2/6 | 0/6 | 0/6 | 4/6 | 0/6 | 0/6 | 0/6 |
| 13 wk | ||||||||
| Strain A | 0/6 | 0/6 | 6/6 | 2/6 | 6/6 | 6/6 | 1/6 | 5/6 |
| Strain B | 0/6 | 1/6 | 5/6 | 2/6 | 5/6 | 5/6 | 2/6 | 4/6 |
Lungs (n = 3/group) from 24- and 48-hour time points were pooled together for n = 6.
At 4 weeks, strain A exposure produced a robust peribronchiolar and perivascular inflammatory response characterized by eosinophilic and neutrophilic infiltrates centered at terminal bronchioles with extension into adjacent alveoli (Figure 2A). Similar inflammatory cells were also observed surrounding smaller pulmonary arteries and within the vessel wall. Remodeling of pulmonary arteries near the terminal bronchiole was evident after 4 weeks of exposure to strain A (Figure 2A). Additional histological changes observed after 4-week strain A exposure included alveolar histiocytosis, bronchiolar epithelial cell hyperplasia with mucous metaplasia, and vascular thrombi (Table 1), whereas strain B exposure resulted in minimal airway inflammation (Figure 2B).
After 13 weeks of exposure, histopathology was nearly indistinguishable between the two strains. Bronchiolitis and pulmonary arterial remodeling were observed in the lungs; however, there was a shift in inflammatory cell composition from eosinophils and neutrophils at 4 weeks to lymphocytes and macrophages at 13 weeks (Figures 2A and 2B), indicating the mice were further along in the pulmonary arterial remodeling process.
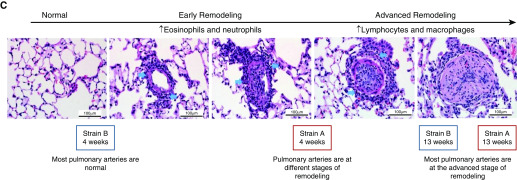
A summary of the histopathology showing a continuum of microscopic changes ranging from arteritis to pulmonary arterial remodeling is presented in Figure 2C. Early changes in pulmonary arteries were characterized by inflammation, whereas later changes in vessels were more severe and characterized by arterial remodeling. Remodeling in affected vessels was characterized by symmetrical thickening of the artery wall and narrowing of the lumen, affecting the small to medium-sized vessels at or below the level of the terminal bronchiole. With increasing exposure duration, there was a shift in inflammatory cell types from polymorphonuclear cells to mononuclear cells.
Flow Cytometry
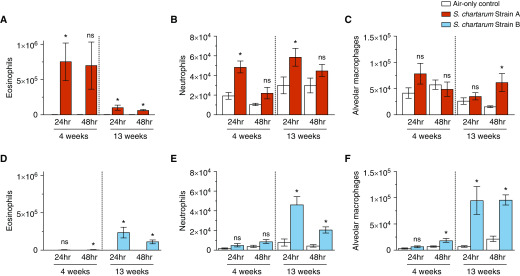
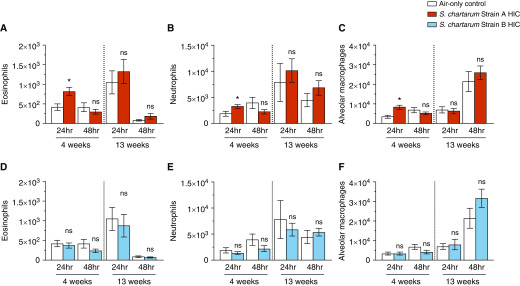
The BALF was composed primarily of eosinophils, neutrophils, and macrophages (Figure 3). Compared with air-only controls, strain A exposure showed increased eosinophils and neutrophils 24 hours after 4 and 13 weeks (Figures 3A and 3B). Alveolar macrophages were significantly increased 48 hours after 13-week strain A exposure (Figure 3C). Only after 4-week strain A HIC exposure were eosinophils, neutrophils, and alveolar macrophages initially increased (Figures 4A–4C).
Figure 3.
Cell populations of BAL fluid (BALF) after exposure to strain A and strain B. (A–F) BALF collected at 24 and 48 hours after 4- and 13-week exposures to strain A (A–C) or strain B (D–F) consisted of eosinophils (A and D), neutrophils (B and E), and macrophages (C and F). Values are expressed as mean ± SEM; n = 7 per group. *P ≤ 0.05 for exposed group versus air-only control group. ns = not significant.
Figure 4.
Cell populations of BAL fluid after exposure to strain A HIC and strain B HIC. (A–F) BALF collected at 24 and 48 hours after a 4- and 13-week exposure to strain A HIC (A–C) or strain B HIC (D–F) consisted of eosinophils (A and D), neutrophils (B and E), and macrophages (C and F). Values are expressed as mean ± SEM; n = 7 per group. *P ≤ 0.05 for exposed group versus air-only control group.
Strain B–exposed mice showed significantly increased eosinophils and alveolar macrophages 48 hours after 4-week exposure (Figures 3D and 3F). After 13-week exposure, eosinophils, neutrophils, and alveolar macrophages were significantly increased (Figures 3D–3F). Strain B HIC exposure did not result in significant cell population changes (Figures 4D–4F).
Quantitative Proteomic Analysis
Proteomic analysis of whole-lung homogenate detected 3,465 proteins in total (Table E3). Table 2 shows differentially expressed proteins involved in tissue remodeling and inflammation. Chil3 and chil4 (chitinase-like proteins 3 and 4, respectively) were upregulated after 4-week and 13-week strain A exposures, but only after 13-week strain B exposure. Only chil3 was decreased after 4-week strain A HIC exposure. Lgals3 (galectin-3) was upregulated after 4-week and 13-week strain A exposures. After 4-week strain B exposure, lgals3 was decreased, but it was increased after 13 weeks. In contrast, lgals3 was downregulated after 4-week strains A and B HIC exposure. Von Willebrand factor was upregulated after 13-week strain A exposure but downregulated after strain B exposure. Strains A and B HIC exposure resulted in a downregulation of von Willebrand factor after 4-week exposure. Strain A–exposed mice showed increased sftpa (pulmonary surfactant protein A) after 4-week and 13-week exposures. In contrast, both sftpa and sftpd were decreased after 4-week strain B exposure and after 4-week strains A and B HIC exposure.
Table 2.
Alterations in Proteomic Profiles
| Proteins | Symbol | Strain A |
Strain B |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 4 wk |
13 wk |
4 wk |
13 wk |
||||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | ||
| Viable | |||||||||
| Chitinase-like protein 3 | chil3 | 14.8* | — | — | 157.7* | — | — | 149.0* | 77.6* |
| Chitinase-like protein 4 | chil4 | 129.5* | — | 171.8* | 860.1* | — | — | 98.1* | — |
| Galectin-3 | lgals3 | 2.1* | — | 8.0* | 15.1* | — | −2.0* | — | 2.7* |
| Von Willebrand factor | vWF | — | — | 2.3* | — | — | −2.3 | — | — |
| Resistin-like α | retnla | NC* | — | — | NC* | — | — | 87.4* | NC* |
| Macrophage mannose receptor 1 | mrc1 | 3.5* | — | 3.7* | 7.5* | — | −2.1* | 2.1* | — |
| Eosinophil cationic protein 1 | ear1 | 30.5* | — | — | 162.9* | — | — | 9.3* | — |
| Eosinophil cationic protein 2 | ear2 | 25.6* | — | 29.4* | 111.1* | — | — | 8.5* | — |
| C-type lectins domain family | clec1a | — | — | — | — | — | — | −5.4* | — |
| Tetranectin | clec3b | — | — | 2.6* | — | — | −3.0* | — | — |
| Dectin-1 | clec7a | NC* | — | — | — | 14.6* | — | — | — |
| C-type lectin domain family | clec10a | NC* | — | 44.7* | 38.6* | — | — | 17.5* | — |
| C-type lectin domain family | clec14a | — | — | — | — | — | −6.7* | −3.3* | — |
| Surfactant protein A | sftpa | 2.1* | — | 3.1* | 6.0* | — | −2.2* | — | — |
| Surfactant protein D | sftpd | — | — | — | — | — | −3.0* | — | — |
| HIC | |||||||||
| Chitinase-like protein | chil3 | −2.2* | — | — | — | — | — | — | — |
| Chitinase-like protein | chil4 | — | — | — | — | — | — | — | — |
| Galectin-3 | lgals3 | −4.7* | −2.6* | — | — | — | −2.2* | — | — |
| Von Willebrand factor | vWF | −2.3* | −2.3* | — | — | — | −2.2* | — | — |
| Resistin-like α | retnla | — | — | — | — | — | — | — | — |
| Macrophage mannose receptor 1 | mrc1 | — | −2.8* | — | — | — | −3.5* | — | — |
| Eosinophil cationic protein 1 | ear1 | −7.3* | — | — | — | — | −15.8* | — | — |
| Eosinophil cationic protein 2 | ear2 | −8.0* | — | — | — | — | — | — | — |
| C-type lectins domain family | clec1a | −2.3* | — | — | — | — | — | −2.2* | — |
| Tetranectin | clec3b | — | −2.3* | — | — | — | −3.2* | — | — |
| Dectin-1 | clec7a | — | — | — | — | — | −4.8* | — | — |
| C-type lectin domain family | clec10a | — | — | — | — | — | — | — | — |
| C-type lectin domain family | clec14a | −2.7* | −3.7* | — | 2.2* | — | — | — | — |
| Surfactant protein A | sftpa | −6.4* | −2.2* | — | — | — | −2.4* | — | — |
| Surfactant protein D | sftpd | −14.0* | −5.6* | — | — | — | −5.0* | — | — |
Definition of abbreviations: HIC = heat-inactivated Stachybotrys chartarum conidia; NC = protein was detected in exposure group but not in air-only control group.
Values are reported as fold change between the exposed group and the air-only control group. Dashes indicate unchanged protein expression.
P ≤ 0.05; n = 3.
Inflammation-associated proteins retnla (resistin-like α) and mrc1 (macrophage mannose receptor 1) were significantly increased after 4-week and 13-week strain A exposures. After 13-week strain B exposure, retnla was upregulated. Although mrc1 was downregulated at 4 weeks, expression was slightly increased after 13-week strain B exposure. Retnla was unaltered in mice exposed to either HIC strain; however, mrc1 was decreased after 4-week strains A and B HIC exposure. Located in the eosinophil matrix, ear1 and ear2 (eosinophil cationic proteins 1 and 2 receptors, respectively) were significantly increased after 4-week strain A exposure. After 13 weeks, both ear1 and ear2 were increased over 100-fold at 48 hours. With strain B, ear1 and ear2 were only upregulated after 13-week exposure, but not to the same degree as with strain A. For HIC exposures, ear1 and ear2 were decreased after 4-week strain A exposure, and ear1 was decreased after 13-week strain B exposure.
Carbohydrate-binding clec proteins (C-type lectins) are nonspecific pathogen recognition receptors. After strain A exposure, clec7a and clec10a were increased after 4 weeks, and after 13 weeks, clec10a and clec3b were increased. Strain B–exposed mice showed increased clec7a after 4 weeks and increased clec10a after 13 weeks. Unaltered after strain A HIC exposure, clec7a was decreased after 4-week strain B HIC exposure. Clec3b was also decreased after 4-week strains A and B HIC exposure.
Core proteomic analysis conducted using IPA identified associations between altered proteins and canonical pathways, diseases and disorders, and physiological system development and functions, as well as the top cardiotoxicity pathways. These top relationships, mechanisms, functions, and pathways relevant to a dataset are determined by having the highest number of proteins that overlap those included in the specific pathway, disease, or function. As reported in Tables 3 and 4, one of the top canonical pathways identified after exposure to strain A is eIF2 (eukaryotic initiation factor 2) signaling, a component of protein synthesis. After strain A exposure, organismal injury and abnormalities were identified as the top diseases and disorders at all time points, followed by the inflammatory response and neurological disease. The top physiological system developments and functions involving the proteins altered after strain A exposure were the hematological system development and function, as well as immune cell trafficking and tissue morphology. The top cardiotoxicity pathways included cardiac enlargement, dysfunction, and fibrosis.
Table 3.
Strain A and Strain B 4-Week Exposure: Proteomic Core Analysis Summary Report Generated Using Ingenuity Pathway Analysis
| 4 wk | Strain A |
Strain B |
||
|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | |
| Canonical pathways | Oxidative phosphorylation (2.8 × 10−22) | eIF2 signaling (4.4 × 10−04) | Mitochondrial dysfunction (1.1 × 10−02) | eIF2 signaling (1.3 × 10−25) |
| Mitochondrial damage (9.1 × 10−22) | Antigen presentation pathway (1.6 × 10−03) | CDP-diacylglycerol biosynthesis I (7.6 × 10−03) | Integrin signaling (1.6 × 10−26) | |
| Diseases and disorders | Organismal injury and abnormalities (1.3 × 10−02 to 1.2 × 10−15) | Organismal injury and abnormalities (3.6 × 10−02 to 5.1 × 10−06) | Inflammatory response (4.8 × 10−02 to 5.0 × 10−03) | Organismal injury and abnormalities (1.2 × 10−04 to 6.9 × 10−35) |
| Inflammatory response (1.3 × 10−02 to 1.4 × 10−07) | Neurological disease (3.6 × 10−02 to 2.2 × 10−04) | Immunological disease (1.6 × 10−02 to 1.2 × 10−03) | Inflammatory response (8.9 × 10−05 to 2.1 × 10−11) | |
| Physiological system development and function | Tissue morphology (1.3 × 10−02 to 5.3 × 10−07) | Hematological system development and function (3.6 × 10−02 to 3.2 × 10−04) | Organismal development (4.8 × 10−02 to 2.9 × 10−04) | Organismal survival (9.5 × 10−05 to 1.5 × 10−29) |
| Hematological system development and function (1.3 × 10−02 to 1.4 × 10−07) | Lymphoid tissue structure and development (3.6 × 10−02 to 3.2 × 10−04) | Tissue development (3.3 × 10−03 to 2.9 × 10−04) | Cardiovascular system development and function (8.9 × 10−05 to 1.6 × 10−17) | |
| Immune cell trafficking (1.1 × 10−02 to 1.4 × 10−07) | Cell-mediated immune response (1.8 × 10−02 to 3.2 × 10−04) | Organ development (4.3 × 10−02 to 2.9 × 10−04) | Tissue development (1.1 × 10−04 to 1.7 × 10−18) | |
| Cardiotoxicity | Cardiac enlargement (5.2 × 10−01 to 2.2 × 10−02) | Cardiac fibrosis (4.4 × 10−01 to 2.9 × 10−01) | Cardiac enlargement (2.8 × 10−01 to 5.5 × 10−03) | Cardiac enlargement (5.1 × 10−01 to 5.1 × 10−05) |
| Cardiac dysfunction (1.0 × 1000 to 8.0 × 10−03) | Cardiac enlargement (1.1 × 1000 to 8.8 × 10−02) | Cardiac dysfunction (2.0 × 10−01 to 4.8 × 10−02) | Cardiac fibrosis (5.8 × 10−01 to 1.4 × 10−05) | |
| Cardiac proliferation (1.7 × 10−01 to 2.2 × 10−02) | Cardiac damage (5.8 × 10−02 to 5.4 × 10−02) | Cardiac inflammation (1.8 × 10−01 to 1.8 × 10−01) | Cardiac damage (9.1 × 10−02 to 7.6 × 10−05) | |
Definition of abbreviations: CDP = cytidine diphosphate; eIF2 = eukaryotic initiation factor 2.
P values associated with each pathway/disease are shown in parentheses.
Table 4.
Strain A and Strain B 13-Week Exposure: Proteomic Core Analysis Summary Report Generated Using Ingenuity Pathway Analysis
| 13 wk | Strain A |
Strain B |
||
|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | |
| Canonical pathways | eIF2 signaling (1.4 × 10−30) | eIF2 signaling (2.2 × 10−42) | Gβγ signaling (9.3 × 10−08) | eIF2 signaling (4.5 × 10−25) |
| Protein ubiquitination (1.0 × 10−19) | Mitochondrial dysfunction (1.1 × 10−29) | Integrin signaling (2.5 × 10−07) | Mitochondrial dysfunction (1.6 × 10−18) | |
| Diseases and disorders | Organismal injury and abnormalities (7.3 × 10−03 to 4.0 × 10−31) | Organismal injury and abnormalities (1.2 × 10−03 to 3.6 × 10−39) | Organismal injury and abnormalities (7.6 × 10−03 to 1.6 × 10−06) | Organismal injury and abnormalities (5.4 × 10−03 to 5.4 × 10−28) |
| Inflammatory response (7.3 × 10−03 to 1.3 × 10−09) | Inflammatory response (1.1 × 10−03 to 3.5 × 10−11) | Cardiovascular disease (7.5 × 10−03 to 1.6 × 10−06) | Inflammatory response (4.6 × 10−03 to 2.3 × 10−08) | |
| Physiological system development and function | Hematological system development and function (7.3 × 10−03 to 5.5 × 10−06) | Hematological system development and function (1.3 × 10−03 to 2.1 × 10−09) | Organismal development (6.9 × 10−03 to 1.8 × 10−09) | Hematological system development and function (4.7 × 10−03 to 3.2 × 10−09) |
| Tissue morphology (6.6 × 10−03 to 8.9 × 10−06) | Immune cell trafficking (1.3 × 10−03 to 2.2 × 10−09) | Cardiovascular system development and function (7.6 × 10−03 to 9.6 × 10−08) | Tissue morphology (5.3 × 10−03 to 9.3 × 10−09) | |
| Immune cell trafficking (7.3 × 10−03 to 5.6 × 10−06) | Tissue development (1.3 × 10−03 to 5.6 × 10−07) | Tissue morphology (7.6 × 10−03 to 1.3 × 10−06) | Lymphoid tissue structure and development (4.6 × 10−03 to 9.3 × 10−09) | |
| Cardiotoxicity | Cardiac infarction (5.1 × 10−05 to 5.1 × 10−05) | Cardiac enlargement (4.6 × 10−01 to 7.9 × 10−04) | Cardiac fibrosis (4.5 × 10−01 to 1.1 × 10−03) | Cardiac enlargement (5.3 × 10−01 to 1.3 × 10−03) |
| Cardiac dysfunction (4.7 × 10−01 to 9.5 × 10−03) | Cardiac fibrosis (5.4 × 10−01 to 2.8 × 10−03) | Cardiac inflammation (1.8 × 10−01 to 1.0 × 10−02) | Cardiac dysfunction (2.9 × 10−01 to 2.5 × 10−02) | |
| Cardiac inflammation (3.1 × 10−01 to 9.1 × 10−03) | Cardiac dysfunction (3.6 × 10−01 to 3.5 × 10−04) | Cardiac damage (1.4 × 10−01 to 1.8 × 10−03) | Heart failure (4.9 × 10−01 to 2.8 × 10−02) | |
P values associated with each pathway/disease are shown in parentheses.
The core proteomic analysis identified eIF2 signaling as well as mitochondrial dysfunction and cytidine diphosphate (CDP)-diacylglycerol biosynthesis I as the top canonical pathways associated with the proteins altered after exposure to strain B (Tables 3 and 4). CDP-diacylglycerol biosynthesis results in the synthesis of CDP-diacylglycerol, which is an intermediate product in the synthesis of cardiolipin, a phospholipid primarily located in the inner mitochondrial membrane. After 13 weeks of exposure to strain B, Gβγ signaling, a contributor to heart failure and inflammation, was one of the top canonical pathways identified. In addition, organismal injury and abnormalities and inflammatory response were among the top diseases and disorders associated with the proteins altered after exposure to strain B.
Gene Expression Profiles
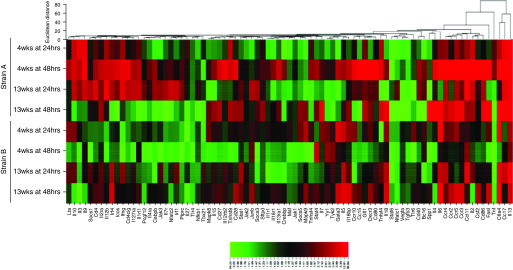
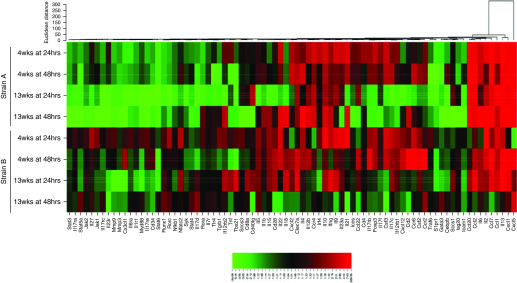
The RT2 Profiler plates (Qiagen) measured genes involved in the Th1, Th2, or Th17 response pathways. Heat maps illustrate fold changes between viable exposed groups versus the air-only control group for both time points after 4-week and 13-week exposures (Figures 5 and 6). The majority of differentially expressed genes associated with Th1/Th2 immune responses were upregulated after strains A and B exposure (Figure 5), although not to the same extent. Many Th17-associated genes were downregulated after strain A versus strain B exposure (Figure 6). Overall, the Th1/Th2 and Th17 gene expression profiles were altered to a greater extent in lungs from strain A–exposed mice than in those from strain B–exposed mice.
Figure 5.
Heat map of genes involved in the T-helper cell type 1 (Th1) and Th2 immune response pathways. Altered genes involved in the Th1 and Th2 response pathways after 4 weeks and 13 weeks of exposure to strain A and to strain B. Genes are color coded (red and green for up- and downregulation, respectively) and are arranged by Euclidean distance; n = 3–5 per group.
Figure 6.
Heat map of genes involved in the Th17 immune response pathway. Altered genes involved in the Th17 response pathways after 4-week and 13-week exposures to strain A and to strain B. Genes are color coded (red and green for up- and downregulation, respectively) and are arranged by Euclidean distance; n = 3–5 per group.
Genes involved in tissue remodeling and the inflammatory response were increased after 4-week and 13-week strain A exposures, such as retnla, chil3, arg1 (arginase 1), and mrc1 (Table 5). These genes were upregulated after 13-week strain B exposure, but not to the extent of strain A. HIC exposure did not alter retnla and chil3 expression by either strain; however, arg1 and mrc1 were slightly upregulated after 4-week strain A HIC exposure. Monocyte chemoattractant protein 1, or ccl2, was also significantly increased after 4-week and 13-week strain A exposures (Figure 6), but it was only significantly increased after 13-week strain B exposure.
Table 5.
Differentially Expressed Genes after Exposure
| Genes | Symbol | Strain A |
Strain B |
Strain A |
Strain B |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 wk |
13 wk |
4 wk |
13 wk |
4 wk |
13 wk |
4 wk |
13 wk |
|||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |||
| Viable |
|
|||||||||
| Arginase-1 | arg1 | 13.4* | 12.5* | 3.8 | 9.8* | — |
— | 6.0* | 2.1 | |
| Cluster of differentiation 163 | cd163 | — | — | — | 8.9* | −2.6 |
— | — | — | |
| Cluster of differentiation 163 | cd163 | — | — | — | 67.3* | — |
— | — | — | |
| Chitinase-like 3 | chil3 | 20.9* | 15.0* | 81.8* | — | −2.3 |
2.8* | 32.1* | — | |
| C-type lectin domain family 4(n) | clec4n | 2.2* | — | 5.1* | — | −3.8* |
— | — | — | |
| Interleukin-33 | il33 | 3.9* | 2.8* | 5.2* | — | — |
— | 4.8* | — | |
| Mannose receptor | mrc1 | 5.8* | — | 4.2* | — | — |
— | 3.8* | −6.0 | |
| Resistin-like α | retnla | 247.6* | 60.6* | 145.1* | −3.9 | 9.0* |
39.0* | 108.2* | −3.5 | |
| Thymic stromal lymphopoietin | tslp | 3.3* | −2.6 | — | — | −3.4* |
−2.2* | 7.1* | — | |
| HIC |
|
|||||||||
| Arginase-1 | arg1 | 4.8* | — | — | 3.0 | — |
−4.3* | 2.6 | — | |
| C-C chemokine receptor 7 | ccr7 | — | — | — | 2.2 | — |
— | — | — | |
| Cluster of differentiation 163 | cd163 | 2.5* | — | 4.0* | 3.0 | — |
— | — | 2.3 | |
| Chitinase-like 3 | chil3 | — | — | — | −4.0* | — |
−2.1 | — | — | |
| C-type lectin domain family 4(n) | clec4n | — | — | — | — | — |
— | — | — | |
| Interleukin-33 | il33 | — | — | −2.8* | −3.8 | — |
— | — | — | |
| Mannose receptor | mrc1 | 2.1* | — | — | — | — |
— | — | −4.1 | |
| Resistin-like α | retnla | — | — | — | — | 2.3 |
— | — | −3.8* | |
| Thymic stromal lymphopoietin | tslp | 3.1* | — | — | — | 2.0 | — | — | — | |
Values are reported as fold change between the exposed group compared with air-only control group. Dashes indicate unchanged gene expression.
P ≤ 0.05; n = 3–5.
Strain A increased il13 at both time points after 4 weeks, together with il4 and il6 at 48 hours. After 13-week strain A exposure, ifng (IFN-γ), il2, and il13 expression was increased at both time points, il4 and il6 expression was increased at 48 hours (Figures 5 and 6). In contrast, strain B–exposed mice showed upregulated il6 expression, together with significantly increased il13 expression, after 4 weeks. After 13 weeks, ifng expression was slightly increased, together with significantly upregulated il13 and il5 at 24 hours and increased il6 at 48 hours (Figures 5 and 6).
Involved with eosinophil recruitment, il33 expression was initially upregulated after 4-week and 13-week strain A exposures compared with only after 13-week strain B exposure (Table 5). Ccl7 (monocyte chemotactic protein 3) and ccr3 were upregulated after 4-week and 13-week strain A exposures (Figure 5). Strain B exposure resulted in increased ccl7 expression 48 hours after 4-week exposure and 24 hours after 13-week exposure (Figure 5). Inflammatory mediator cxcl5 (Chemokine [C-X-C motif] ligand 5) and cxcl1 expression was significantly increased after 4-week and 13-week strain A exposures (Figure 6). In strain B–exposed mice, cxcl1 and cxcl5 were upregulated 48 hours after 4 weeks, but not significantly. After 13 weeks, cxcl1 was significantly upregulated (Figure 6).
miRNA Profiles
miRNA profiling analysis identified 531 (strain A) and 512 (strain B) miRNAs in the lung samples (Table E4). After 4-week strain A exposure, 116 (24 h) and 138 (48 h) miRNAs were significantly altered compared with air-only controls. After 13-week strain A exposure, 15 (24 h) and 49 (48 h) miRNAs were significantly differentially expressed, the majority of which were downregulated (Table E4). In contrast, no miRNAs were altered after 4-week strain B exposure, and only 14 (24 h) and 35 (48 h) miRNAs were differentially expressed after 13 weeks.
Using IPA, the miRNA target filter predicts miRNAs and target genes included in this study. A regulator of il13 and il33, miR-339-5p was decreased after strain A and strain A HIC exposures (Table 6). Downregulation of miR-365 after 4-week strain A viable and HIC exposure was predicted to regulate il6. Expression of il10 was predicted to increase due to decreased miR-144-5p or miR-374b-5p expression after strain A viable and HIC exposure. After strain B viable and HIC exposure, the expression of these miRNAs was unchanged (Table 6). Expression of Th1 cytokines, such as il2 and tnf, may be regulated by miR-181b-5p (il2), miR-21a-5p (tnf), and miR-669d-5p (tnf). miR-181b-5b was decreased after 4-week strain A exposure and at 4-week and 13-week strain A HIC exposure. miR-21a-5p was upregulated only after 13-week strain A exposure, but it was downregulated 24 hours after 13-week strain A HIC exposure. After strain A viable and HIC exposure, miR-669d-5p expression was increased. After strain B viable and HIC exposure, the expression of these miRNAs was unchanged. Critical to eosinophilic recruitment, miR-133a-3p and miR-335-5p were downregulated after strain A viable and HIC exposure, but unchanged after strain B exposure. miR-23b-3p, a regulator of arg1 and mrc1, was downregulated after strain A exposure, but not after HIC exposure. In contrast to downregulation after 13-week strain B exposure, miR-23b-3p was unchanged after HIC exposure.
Table 6.
Altered MicroRNAs after Exposure
| miRNAs | Strain A |
Strain B |
Strain A |
Strain B |
||||
|---|---|---|---|---|---|---|---|---|
| 4 wk |
13 wk |
4 wk |
13 wk |
4 wk |
13 wk |
4 wk |
13 wk |
|
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| Viable | ||||||||
| mmu-miR-133a-3p | −3.2* | −3.0* | −3.3* | −2.4* | — | — | — | −2.3 |
| mmu-miR-144-5p | −2.2* | −2.1* | −2.2* | −3.0* | — | — | — | — |
| mmu-miR-181b-5p | −2.2* | −2.1* | — | — | — | — | — | — |
| mmu-miR-21a-5p | — | — | 2.9* | 1.9* | — | — | — | — |
| mmu-miR-23b-3p | −3.4* | −2.3* | — | −2.1* | — | — | −2.0* | — |
| mmu-miR-335-5p | −2.2* | −2.7* | — | −2.3 | — | — | — | — |
| mmu-miR-339-5p | −2.0* | −2.9* | −2.0* | −2.4* | — | — | — | — |
| mmu-miR-365-3p | −2.2* | −2.4* | — | −1.7* | — | — | — | — |
| mmu-miR-374b-5p | −2.2* | −2.3* | — | −2.0* | — | — | — | −1.8* |
| mmu-miR-491-3p | — | 3.0* | — | 2.2* | — | — | −1.7* | — |
| mmu-miR-669d-5p | 2.7* | 2.8* | — | 2.0* | — | — | — | — |
| mmu-miR-706 | 4.7* | 7.4* | 2.2* | 3.2* | — | — | — | 2.1* |
| HIC | ||||||||
| mmu-miR-133a-3p | −4.2* | −3.4* | −5.9* | −3.2* | — | 2.1 | — | — |
| mmu-miR-144-5p | — | — | −4.4* | −2.3* | — | — | — | — |
| mmu-miR-181b-5p | −2.7* | −2.4* | −3.1* | −2.2* | — | — | — | — |
| mmu-miR-21a-5p | — | — | −3.1* | — | — | — | — | — |
| mmu-miR-23b-3p | −1.9* | — | — | — | — | — | — | — |
| mmu-miR-335-5p | −2.8* | −2.3* | −3.0* | −2.3* | — | — | — | — |
| mmu-miR-339-5p | −2.2* | −2.4* | −3.3* | −2.5* | — | — | — | — |
| mmu-miR-365-3p | −4.3* | −3.6* | −4.6* | −3.2* | — | — | — | — |
| mmu-miR-374b-5p | −2.3* | −2.0* | −3.3* | −2.1* | — | — | — | — |
| mmu-miR-491-3p | 2.7* | 5.9* | 3.3* | 3.9* | — | — | — | — |
| mmu-miR-669d-5p | 3.6* | 3.1* | 3.9* | 2.4* | — | — | — | — |
| mmu-miR-706 | 7.4* | 7.5* | 6.3* | 3.7* | — | — | — | — |
Definition of abbreviations: miR and miRNA = microRNA.
Values are reported as fold change between the exposed group compared with air-only control group. Dashes indicate miRNA was unaltered.
P ≤ 0.05; n = 5.
Discussion
The results of this study showed that repeated exposure to S. chartarum results in inflammation and pulmonary arterial remodeling. The molecular and immunological markers underlying the inflammatory response, as well as the rate at which the remodeling occurred, varied between the two strains. Strain A, the higher-fragmenting strain, elicited an earlier Th2-mediated immune response after 4 weeks that shifted to a mixed T-cell response after 13 weeks. Arterial remodeling after strain A exposure was observed at an earlier time point than in mice exposed to the lower-fragmenting strain B; however, arterial remodeling was evident after 13 weeks of exposure to both strains. After 4 weeks, strain B exposure also elicited a pulmonary Th2 immune response, which remained after 13 weeks. Although HIC exposure did not result in significant lung pathology, alterations in protein, gene, and miRNA profiles were evident, suggesting a unique fungus-induced profile, regardless of viability. Neither viable strain B nor either HIC strain generated as many fragments as viable strain A; therefore, mice exposed to viable strain A fragments inhaled a greater number of smaller particles, resulting in less mass deposition within the lungs of these mice than in mice exposed to strain B or to either strain of HIC. This difference in exposure suggests that exposure to fungal fragments, despite decreased pulmonary mass deposition, elicits an earlier pulmonary immune response and pathology after repeated S. chartarum exposure.
Using the same concentration of S. chartarum as in the present study, Ochiai and colleagues also reported that repeated intratracheal instillations of S. chartarum resulted in pulmonary arterial hypertension (PAH) as early as 4 weeks (24). In the present study, histopathology showed pulmonary arterial remodeling after strains A and B exposure; however, arterial remodeling developed earlier with strain A than with strain B. In another study of repeated intratracheal instillations of S. chartarum, goblet cell metaplasia was observed after seven exposures, but not after a single exposure, indicative of airway remodeling after S. chartarum exposure (25). In agreement with Fan and colleagues, retnla was increased with arterial remodeling, together with asthma pathogenesis and tissue remodeling–associated proteins (32). These proteins and respective genes were significantly increased after both viable S. chartarum exposures compared with air-only and HIC controls, as well as at an earlier time point in strain A–exposed versus strain B–exposed mice. A potential protein biomarker for PAH, lgals3 (33), and ccl2, involved in pulmonary inflammation and vascular tissue remodeling, were upregulated after 4-week and 13-week strain A exposures, but only after 13-week strain B exposure. Proteomic core analysis indicated associations with inflammatory responses, tissue remodeling, and cardiac abnormalities after exposure to both strains (Tables 3 and 4). Highly affected canonical pathways, such as mitochondrial dysfunction (34) and pathways contributing to heart failure (35, 36) and inflammation (37), suggested that the pulmonary vasculature may be affected after fungal exposures. Pulmonary arterial remodeling is a Th2-mediated response (13, 30, 38) and is in agreement with the Th2-mediated immune response observed in this study, as well as in other studies of repeated exposure to S. chartarum (25, 30). The resultant pulmonary arterial remodeling progressed at strain-specific rates, which is further supported by gene and proteomic alterations.
Increased il13, il4, and il5 gene expression, together with no significant ifng or tnf increase, after 4-week strains A and B exposures indicates a Th2-mediated immune response. Interestingly, 13-week strain B exposure maintained the Th2-dominant response, whereas strain A exposure shifted to a mixed T-cell response. A limitation of our study was that, for unknown reasons, the cycle threshold values for genes from HIC-exposed lungs were reported as undetermined after RT-PCR. Differential staining of BALF cells collected from strain A– and strain B–exposed mice supported il4 gene expression data (not shown); however, BALF volume limited the measurement of IL-13. In another study, increased eosinophils, IL-4, and IL-5, but not IFN-γ, were observed in BALF after intratracheal instillation for 12 weeks (30), which agreed with strain B but differed from strain A. Taken together, the results suggest that different strains of S. chartarum elicit a continuum of immune response, dependent on the duration and composition of exposure.
Histology and BALF collected from S. chartarum–exposed mice showed the presence of eosinophils, neutrophils, and macrophages. In agreement with the present study, a study comparing a single intratracheal instillation of S. chartarum spores with multiple instillations (once per week for 7 wk) showed increased lymphocytes and eosinophils after multiple exposures (25). Released during degranulation of eosinophils, ear1 and ear2 were significantly increased after 4-week and 13-week strain A exposures, compared with only 13-week strain B exposure. Critical to eosinophil commitment, IL-5 is preceded by IL-33 (39), both of which were upregulated after both 4-week and 13-week strain A exposures compared with only after 13-week strain B exposure. Having eosinophilic chemoattractant properties, ccl7 and ccr3 were upregulated after strain A exposure. Eosinophils produce cxcl5, which, together with cxcl1, attracts neutrophils (40). Exposure to both strains resulted in a temporal increase in cxcl5 and cxcl1 expression, indicative of the progressive influx of pulmonary neutrophils. Associated with alternatively activated macrophages, arg1 and mrc1 were upregulated after exposure to both strains. Together with other increased M2 markers such as retnla, chil3, and il33 (41–43), these data correlate with increased macrophages observed in exposed lungs. Overall, the results support the increased presence of inflammatory cell infiltrates and the earlier induction of an immune response after strain A versus strain B exposure.
Clec proteins are critical in the recognition and neutralization of pathogens (44). Exposure to both strains increased clec7a protein expression after 4-week exposure. Gene expression was increased after 4-week strain A and 13-week strain B exposures. Although S. chartarum spores are too large to deposit deep within the lung and subsequently germinate, previous studies highlighted the association between increased clec7a expression with germination after A. fumigatus exposure (28, 29). In addition, gene expression analyses showed increased clec4n after 4-week and 13-week strain A exposures, with decreased expression after 4-week strain B exposure. Mrc1 also contributes to pathogen recognition by recognizing complex carbohydrates (45). Together, increased expression of proteins involved in pathogen recognition supports the hypothesis that fungal fragments enter the lung and elicit an immune response.
Increased gene expression associated with decreased expression of regulating miRNAs suggests a possible mechanism underlying the immune response after S. chartarum exposure. Using the same delivery system, altered miRNAs after A. fumigatus exposure (28) were also found to be altered in the present study (Table 6). miR-23b-3p, one of the highest-altered miRNAs after A. fumigatus exposure, was also altered after S. chartarum exposure. In A. fumigatus–exposed mice, mir-706 expression was increased (28); however, after strain A exposure, miR-706 consistently had the highest fold change for all time points, except 24 hours after 13 weeks, when miR-21a-5p expression was highest (Table 6). miR-706 is involved in liver fibrogenesis (46); however, the present results also suggest involvement in pulmonary tissue remodeling. In agreement with the present study, miR-21a is involved in pulmonary fibrogenesis (47) and was surprisingly decreased after A. fumigatus exposure (28). The difference in miR-21a expression is most likely due to the difference in fungal species; however, research suggests that A. fumigatus elicits an immune response due to fungal germination (28, 29), whereas this study suggests the earlier induction of an immune response is driven by the fungal fragments present in greater numbers in the composition of strain A exposure.
The repeated fungal exposure model presented in this study reproducibly elicited pulmonary arterial remodeling in both S. chartarum strains. Other studies that have induced PAH through well-known mechanisms, such as hypoxic conditions or monocrotaline injury, have several limitations, as discussed by Provencher and colleagues (48), including less stringent study designs. The model in the present study appears to overcome several of the described methodological limitations (48, 49); however, future studies focusing on critical parameters required to identify PAH, such as cardiac measurements and pulmonary artery smooth muscle thickness, will need to be conducted to better understand the initiation and recovery of PAH using fungal test articles such as S. chartarum.
Respiratory morbidities in patients exposed to S. chartarum have been reported (50, 51), highlighting the importance of characterizing mechanisms influencing the pulmonary immune response and disease pathology. Published studies have identified fungal particles aerosolized from moldy building materials (52, 53), but those studies were unable to isolate and characterize the effect of individual fungal species. Having different mycotoxin profiles, both A. fumigatus (29) and S. chartarum induce pulmonary arterial remodeling; therefore, it appears more likely that common fungal antigens present on the respirable S. chartarum fragments are inducing the early pathophysiological response. Collectively, the results of this study demonstrate that exposure to S. chartarum elicits pulmonary immune responses and pulmonary arterial remodeling; however, it is the presence of fungal fragments that induces these responses.
Supplementary Material
Acknowledgments
Acknowledgment
The authors acknowledge Benjamin P. Jackson, M.D., NIOSH, for his contribution in fungal cultivation.
Footnotes
Supported in part by an interagency agreement between the National Institute for Occupational Safety and Health (NIOSH) and the National Institute of Environmental Health Sciences (AES12007001-1-0-6) as a collaborative National Toxicology Program research activity. This study was also funded in part by Centers for Disease Control and Prevention/NIOSH intramural funds (927ZLCT). The findings and conclusions in this study are those of the authors and do not necessarily represent the official position of NIOSH, Centers for Disease Control and Prevention.
Author Contributions: T.L.C., A.R.L., A.P.N., B.J.G., and D.H.B. conceived of and designed the research. T.L.C., A.R.L., and A.P.N. cultured the test articles. W.T.G. conducted the exposures. T.L.C., A.R.L., M.A.B., and A.P.N. performed the experiments. T.L.C. and M.A.B. analyzed the data. T.L.C., M.S.O., B.J.G., and D.H.B. interpreted results of the experiments. T.L.C., A.R.L., M.A.B., and M.S.O. prepared the figures. T.L.C. drafted the manuscript. T.L.C., A.R.L., M.A.B., M.S.O., A.P.N., D.R.G., B.J.G., and D.H.B. edited and revised the manuscript. T.L.C., A.R.L., M.A.B., W.T.G., M.S.O., A.P.N., D.R.G., B.J.G., and D.H.B. approved the final version of the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0221OC on October 31, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.World Health Organization (WHO) WHO guidelines for indoor air quality: dampness and mould. Geneva, Switzerland: WHO Regional Office for Europe; 2009. [PubMed] [Google Scholar]
- 2.Institute of Medicine. Damp indoor spaces and health. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- 3.Montaña E, Etzel RA, Allan T, Horgan TE, Dearborn DG. Environmental risk factors associated with pediatric idiopathic pulmonary hemorrhage and hemosiderosis in a Cleveland community. Pediatrics. 1997;99:e5. doi: 10.1542/peds.99.1.e5. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Acute pulmonary hemorrhage/hemosiderosis among infants: Cleveland, January 1993–November 1994. MMWR Morb Mortal Wkly Rep. 1994;43:881–883. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Update: pulmonary hemorrhage/hemosiderosis among infants—Cleveland, Ohio, 1993–1996. MMWR Morb Mortal Wkly Rep. 2000;49:180–184. [PubMed] [Google Scholar]
- 6.Pestka JJ, Yike I, Dearborn DG, Ward MD, Harkema JR. Stachybotrys chartarum, trichothecene mycotoxins, and damp building-related illness: new insights into a public health enigma. Toxicol Sci. 2008;104:4–26. doi: 10.1093/toxsci/kfm284. [DOI] [PubMed] [Google Scholar]
- 7.Andersson MA, Nikulin M, Köljalg U, Andersson MC, Rainey F, Reijula K, et al. Bacteria, molds, and toxins in water-damaged building materials. Appl Environ Microbiol. 1997;63:387–393. doi: 10.1128/aem.63.2.387-393.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boutin-Forzano S, Charpin-Kadouch C, Chabbi S, Bennedjai N, Dumon H, Charpin D. Wall relative humidity: a simple and reliable index for predicting Stachybotrys chartarum infestation in dwellings. Indoor Air. 2004;14:196–199. doi: 10.1111/j.1600-0668.2004.00233.x. [DOI] [PubMed] [Google Scholar]
- 9.Brasel TL, Martin JM, Carriker CG, Wilson SC, Straus DC. Detection of airborne Stachybotrys chartarum macrocyclic trichothecene mycotoxins in the indoor environment. Appl Environ Microbiol. 2005;71:7376–7388. doi: 10.1128/AEM.71.11.7376-7388.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarvis BB, Sorenson WG, Hintikka EL, Nikulin M, Zhou Y, Jiang J, et al. Study of toxin production by isolates of Stachybotrys chartarum and Memnoniella echinata isolated during a study of pulmonary hemosiderosis in infants. Appl Environ Microbiol. 1998;64:3620–3625. doi: 10.1128/aem.64.10.3620-3625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes C, Buckley S, Pacheco F, Portnoy J. IgE-reactive proteins from Stachybotrys chartarum. Ann Allergy Asthma Immunol. 2002;89:29–33. doi: 10.1016/S1081-1206(10)61907-2. [DOI] [PubMed] [Google Scholar]
- 12.Chung YJ, Copeland LB, Doerfler DL, Ward MD. The relative allergenicity of Stachybotrys chartarum compared to house dust mite extracts in a mouse model. Inhal Toxicol. 2010;22:460–468. doi: 10.3109/08958370903380712. [DOI] [PubMed] [Google Scholar]
- 13.Yike I, Dearborn D. Guest editorial: novel insights into the pathology of Stachybotrys chartarum. Mycopathologia. 2011;172:1–3. doi: 10.1007/s11046-011-9426-6. [DOI] [PubMed] [Google Scholar]
- 14.Rand TG, Sun M, Gilyan A, Downey J, Miller JD. Dectin-1 and inflammation-associated gene transcription and expression in mouse lungs by a toxic (1,3)-β-d glucan. Arch Toxicol. 2010;84:205–220. doi: 10.1007/s00204-009-0481-4. [DOI] [PubMed] [Google Scholar]
- 15.Vesper SJ, Magnuson ML, Dearborn DG, Yike I, Haugland RA. Initial characterization of the hemolysin stachylysin from Stachybotrys chartarum. Infect Immun. 2001;69:912–916. doi: 10.1128/IAI.69.2.912-916.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller JD, Sun M, Gilyan A, Roy J, Rand TG. Inflammation-associated gene transcription and expression in mouse lungs induced by low molecular weight compounds from fungi from the built environment. Chem Biol Interact. 2010;183:113–124. doi: 10.1016/j.cbi.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Górny RL, Reponen T, Willeke K, Schmechel D, Robine E, Boissier M, et al. Fungal fragments as indoor air biocontaminants. Appl Environ Microbiol. 2002;68:3522–3531. doi: 10.1128/AEM.68.7.3522-3531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho SH, Seo SC, Schmechel D, Grinshpun SA, Reponen T. Aerodynamic characteristics and respiratory deposition of fungal fragments. Atmos Environ. 2005;39:5454–5465. [Google Scholar]
- 19.Green BJ, Tovey ER, Sercombe JK, Blachere FM, Beezhold DH, Schmechel D. Airborne fungal fragments and allergenicity. Med Mycol. 2006;44(Suppl 1):S245–S255. doi: 10.1080/13693780600776308. [DOI] [PubMed] [Google Scholar]
- 20.Yike I, Rand TG, Dearborn DG. Acute inflammatory responses to Stachybotrys chartarum in the lungs of infant rats: time course and possible mechanisms. Toxicol Sci. 2005;84:408–417. doi: 10.1093/toxsci/kfi080. [DOI] [PubMed] [Google Scholar]
- 21.Rao CY, Burge HA, Brain JD. The time course of responses to intratracheally instilled toxic Stachybotrys chartarum spores in rats. Mycopathologia. 2000;149:27–34. doi: 10.1023/a:1007239017018. [DOI] [PubMed] [Google Scholar]
- 22.Rand TG, Mahoney M, White K, Oulton M. Microanatomical changes in alveolar type II cells in juvenile mice intratracheally exposed to Stachybotrys chartarum spores and toxin. Toxicol Sci. 2002;65:239–245. doi: 10.1093/toxsci/65.2.239. [DOI] [PubMed] [Google Scholar]
- 23.Korpi A, Kasanen JP, Raunio P, Kosma VM, Virtanen T, Pasanen AL. Effects of aerosols from nontoxic Stachybotrys chartarum on murine airways. Inhal Toxicol. 2002;14:521–540. doi: 10.1080/089583701753678607. [DOI] [PubMed] [Google Scholar]
- 24.Ochiai E, Kamei K, Watanabe A, Nagayoshi M, Tada Y, Nagaoka T, et al. Inhalation of Stachybotrys chartarum causes pulmonary arterial hypertension in mice. Int J Exp Pathol. 2008;89:201–208. doi: 10.1111/j.1365-2613.2008.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenblum Lichtenstein JH, Molina RM, Donaghey TC, Hsu YH, Mathews JA, Kasahara DI, et al. Repeated mouse lung exposures to Stachybotrys chartarum shift immune response from type 1 to type 2. Am J Respir Cell Mol Biol. 2016;55:521–531. doi: 10.1165/rcmb.2015-0291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leino MS, Alenius HT, Fyhrquist-Vanni N, Wolff HJ, Reijula KE, Hintikka EL, et al. Intranasal exposure to Stachybotrys chartarum enhances airway inflammation in allergic mice. Am J Respir Crit Care Med. 2006;173:512–518. doi: 10.1164/rccm.200503-466OC. [DOI] [PubMed] [Google Scholar]
- 27.Buskirk AD, Green BJ, Lemons AR, Nayak AP, Goldsmith WT, Kashon ML, et al. A murine inhalation model to characterize pulmonary exposure to dry Aspergillus fumigatus conidia. PLoS One. 2014;9:e109855. doi: 10.1371/journal.pone.0109855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Croston TL, Nayak AP, Lemons AR, Goldsmith WT, Gu JK, Germolec DR, et al. Influence of Aspergillus fumigatus conidia viability on murine pulmonary microRNA and mRNA expression following subchronic inhalation exposure. Clin Exp Allergy. 2016;46:1315–1327. doi: 10.1111/cea.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nayak AP, Croston TL, Lemons AR, Goldsmith WT, Marshall NB, Kashon ML, et al. Aspergillus fumigatus viability drives allergic responses to inhaled conidia. Ann Allergy Asthma Immunol. 2018;121:200–210, e2. doi: 10.1016/j.anai.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagayoshi M, Tada Y, West J, Ochiai E, Watanabe A, Toyotome T, et al. Inhalation of Stachybotrys chartarum evokes pulmonary arterial remodeling in mice, attenuated by Rho-kinase inhibitor. Mycopathologia. 2011;172:5–15. doi: 10.1007/s11046-011-9400-3. [DOI] [PubMed] [Google Scholar]
- 31.Croston TL, Nayak AP, Lemons AR, Goldsmith WT, Germolec DM, Beezhold DH, et al. Pulmonary immune response following subchronic Stachybotrys chartarum exposure [abstract] J Allergy Clin Immunol. 2017;139(2 Suppl):AB75. [Google Scholar]
- 32.Fan C, Meuchel LW, Su Q, Angelini DJ, Zhang A, Cheadle C, et al. Resistin-like molecule α in allergen-induced pulmonary vascular remodeling. Am J Respir Cell Mol Biol. 2015;53:303–313. doi: 10.1165/rcmb.2014-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calvier L, Legchenko E, Grimm L, Sallmon H, Hatch A, Plouffe BD, et al. Galectin-3 and aldosterone as potential tandem biomarkers in pulmonary arterial hypertension. Heart. 2016;102:390–396. doi: 10.1136/heartjnl-2015-308365. [DOI] [PubMed] [Google Scholar]
- 34.Croston TL, Shepherd DL, Thapa D, Nichols CE, Lewis SE, Dabkowski ER, et al. Evaluation of the cardiolipin biosynthetic pathway and its interactions in the diabetic heart. Life Sci. 2013;93:313–322. doi: 10.1016/j.lfs.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams ML, Hata JA, Schroder J, Rampersaud E, Petrofski J, Jakoi A, et al. Targeted β-adrenergic receptor kinase (βARK1) inhibition by gene transfer in failing human hearts. Circulation. 2004;109:1590–1593. doi: 10.1161/01.CIR.0000125521.40985.28. [DOI] [PubMed] [Google Scholar]
- 36.Rockman HA, Chien KR, Choi DJ, Iaccarino G, Hunter JJ, Ross J, Jr, et al. Expression of a β-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc Natl Acad Sci USA. 1998;95:7000–7005. doi: 10.1073/pnas.95.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, et al. Central role for G protein-coupled phosphoinositide 3-kinase γ in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 38.Daley E, Emson C, Guignabert C, de Waal Malefyt R, Louten J, Kurup VP, et al. Pulmonary arterial remodeling induced by a Th2 immune response. J Exp Med. 2008;205:361–372. doi: 10.1084/jem.20071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston LK, Hsu CL, Krier-Burris RA, Chhiba KD, Chien KB, McKenzie A, et al. IL-33 precedes IL-5 in regulating eosinophil commitment and is required for eosinophil homeostasis. J Immunol. 2016;197:3445–3453. doi: 10.4049/jimmunol.1600611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiu Y, Zhu J, Bandi V, Atmar RL, Hattotuwa K, Guntupalli KK, et al. Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168:968–975. doi: 10.1164/rccm.200208-794OC. [DOI] [PubMed] [Google Scholar]
- 41.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bosurgi L, Cao YG, Cabeza-Cabrerizo M, Tucci A, Hughes LD, Kong Y, et al. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science. 2017;356:1072–1076. doi: 10.1126/science.aai8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 44.Weis WI, Taylor ME, Drickamer K. The C-type lectin superfamily in the immune system. Immunol Rev. 1998;163:19–34. doi: 10.1111/j.1600-065x.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]
- 45.Stahl PD, Ezekowitz RAB. The mannose receptor is a pattern recognition receptor involved in host defense. Curr Opin Immunol. 1998;10:50–55. doi: 10.1016/s0952-7915(98)80031-9. [DOI] [PubMed] [Google Scholar]
- 46.Yin R, Guo D, Zhang S, Zhang X. miR-706 inhibits the oxidative stress-induced activation of PKCα/TAOK1 in liver fibrogenesis. Sci Rep. 2016;6:37509. doi: 10.1038/srep37509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rajasekaran S, Rajaguru P, Sudhakar Gandhi PS. MicroRNAs as potential targets for progressive pulmonary fibrosis. Front Pharmacol. 2015;6:254. doi: 10.3389/fphar.2015.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Provencher S, Archer SL, Ramirez FD, Hibbert B, Paulin R, Boucherat O, et al. Standards and methodological rigor in pulmonary arterial hypertension preclinical and translational research. Circ Res. 2018;122:1021–1032. doi: 10.1161/CIRCRESAHA.117.312579. [DOI] [PubMed] [Google Scholar]
- 49.Bonnet S, Provencher S, Guignabert C, Perros F, Boucherat O, Schermuly RT, et al. Translating research into improved patient care in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2017;195:583–595. doi: 10.1164/rccm.201607-1515PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johanning E, Biagini R, Hull D, Morey P, Jarvis B, Landsbergis P. Health and immunology study following exposure to toxigenic fungi (Stachybotrys chartarum) in a water-damaged office environment. Int Arch Occup Environ Health. 1996;68:207–218. doi: 10.1007/BF00381430. [DOI] [PubMed] [Google Scholar]
- 51.Hodgson MJ, Morey P, Leung WY, Morrow L, Miller D, Jarvis BB, et al. Building-associated pulmonary disease from exposure to Stachybotrys chartarum and Aspergillus versicolor. J Occup Environ Med. 1998;40:241–249. doi: 10.1097/00043764-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Madsen AM, Larsen ST, Koponen IK, Kling KI, Barooni A, Karottki DG, et al. Generation and characterization of indoor fungal aerosols for inhalation studies. Appl Environ Microbiol. 2016;82:2479–2493. doi: 10.1128/AEM.04063-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mensah-Attipoe J, Saari S, Veijalainen AM, Pasanen P, Keskinen J, Leskinen JTT, et al. Release and characteristics of fungal fragments in various conditions. Sci Total Environ. 2016;547:234–243. doi: 10.1016/j.scitotenv.2015.12.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.