Abstract
A strategy is introduced for enhancing the cellular selectivity of Amphotericin B (AmB) and other classes of membrane-disrupting agents. This strategy involves attaching the agent to a molecular umbrella to minimize the disruptive power of aggregated forms. Based on this approach, AmB has been coupled to a molecular umbrella derived from one spermidine and two cholic acid molecules and found to have antifungal activities approaching that of the native drug. However, in sharp contrast to AmB, the hemolytic activity and the cytotoxcity of this conjugate toward HEK293 T cells have been dramatically reduced.
Graphical Abstract

Amphotericin B (AmB) has been widely used to treat systemic fungal infections and fungal infections in the central nervous system for more than 50 years (Chart 1).1–11 Despite its clinical importance and its stature as the “gold standard” for antifungal chemotherapy, this natural heptaene macrolide antibiotic is generally regarded as one of the most toxic drugs that is used in modern medicine. Very recently, it has been reported that the insertion of an NH unit between the C16 and the C41 (carboxyl) carbons leads to a dramatic reduction in toxicity.12 The basis for this remarkable behavior, however, remains to be established.
Chart 1.

The mechanism by which AmB kills fungal cells continues to be debated.2 In the classic barrel stave model, several AmB molecules combine with ergosterol to form pores. Subsequent alignment of two such pores across the plasma membrane, or a thinning of the membrane around individual pores, is thought to produce lethal water-filled channels through which ions are free to pass (Figure 1A and B). Recently, however, evidence has begun to emerge in support of an entirely different mechanism—one in which AmB extracts ergosterol from the hydrocarbon interior of fungal membrane and deposits it on the cell’s surface as a single complex or a “pile” of complexes; i.e., the “sterol sponge” model (Figure 1C and 1D, respectively).13,14
Figure 1.
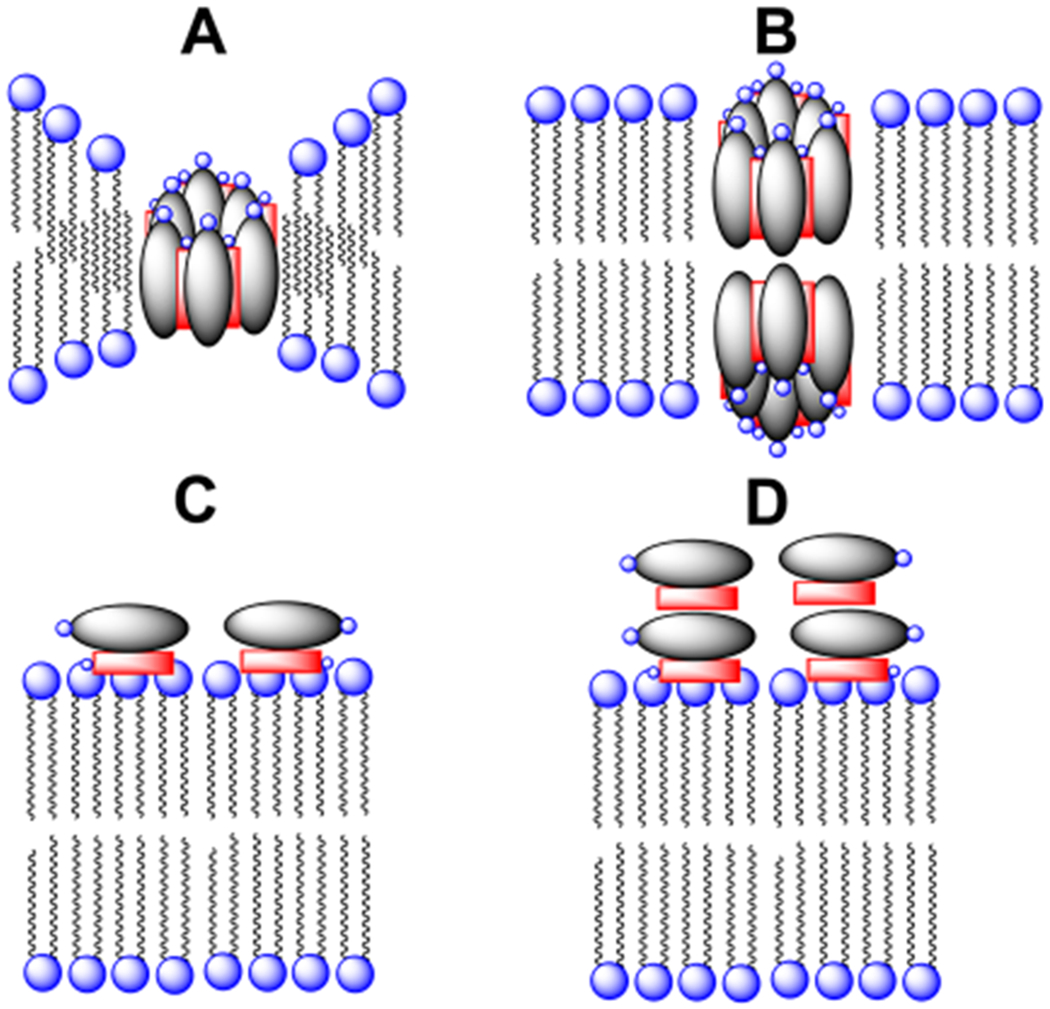
Barrel stave model in which AmB (gray oval) combines with ergosterol (red rectangle) to form (A) a single pore, (B) two aligned water-filled pores, (C) a single adsorbed complex, and (D) a “pile” of complexes.
What has complicated virtually all mechanistic investigations of AmB is the fact that this antibiotic exists in two discrete forms, having different biological properties. Specifically, Bolard and co-workers have shown that, whereas water-soluble aggregates of AmB are toxic to erythrocytes and fungal cells, the monomers are toxic only to fungal cells.15 Similar complexity is likely to exist with all derivatives of AmB that have been reported to date.
In a past study, we provided additional evidence that AmB monomers exhibit much greater cellular selectivity than aggregated forms by showing that the attachment of poly-(ethylene glycol) chains allows one to separate antifungal from hemolytic activity. Specfically, we showed that the attachment of poly(ethylene glycol) chains to AmB increases its critical aggregation concentration (cac), and that hemolytic activity is observed only at concentrations that are in excess of these concentrations—where aggregates and monomers coexist.16 Below their cac concentration, where monomers are dominant, only antifungal activity was significant. As previously discussed, this behavior closely resembles the action of a common detergent (Triton X-100) on cholesterol-rich liposomes where attack by aggregates results in a catastrophic rupture of the membrane, while attack by monomers leads to mild leakage.17 In fact, this dichotomy in membrane-disrupting behavior (catastrophic rupture by aggregates versus mild perturbation by monomers) can account for the reduced toxicity of AmB that has been found with liposomal formulations; i.e., the liposomes merely serve as a reservoir that release highly cell-selective monomers.17,18
Based on these earlier studies, our working hypothesis has been that (i) the “sponge” mechanism of action is the dominant pathway by which AmB monomers destroy fungal cells, and (ii) nonselective membrane disruption by aggregates is responsible for the high toxicity of this antibiotic. This hypothesis has also led us to speculate that if one could reduce the ability of AmB to insert into cell membranes, the rupturing power of its aggregates would be greatly reduced, while the monomers would retain significant antifungal activity. In other words, AmB would be “tamed”.
To explore this line of reasoning, we chose molecular umbrella-AmB conjugates 1a and 2a as synthetic targets (Chart 2). Our rationale was based on previous fluorescence quenching measurements that were made with certain diwalled and tetrawalled “molecular umbrellas” bearing Cascade blue; i.e., 1b and 2b, respectively.19 Specifically, these measurements provided support for a model in which such molecules favor binding at the surface as opposed to the interior of lipid membranes (Chart 2 and Figure 2). Thus, we posited that analogous molecules would localize a pendant AmB moiety close to the cell surface, thereby favoring the killing of fungal cells via a “sponge” mechanism.
Chart 2.

Figure 2.

Stylized illustration of a diwalled molecular umbrella bearing Cascade Blue (X) adsorbed on the surface of a lipid bilayer. The open and filled rectangles represent the hydrophilic and hydrophobic face of facially amphiphilic moieties, e.g., a choloyl group.
In synthesizing derivatives of AmB, we have found an Fmoc-carbamate of AmB that has been activated by N,N,N′,N′-tetramethyl-O-(3,4-dihydro-4-oxo-1,2,3-benzotriazin-3-yl)-uronium tetrafluoroborate (TDBTU) (i.e., 3) to be a convenient and stable precursor (Chart 2).12 Thus, direct coupling of 3 with a diwalled molecular umbrella bearing a pendant amine group (4), followed by removal of Fmoc with piperidine afforded 1a (Scheme 1). A similar sequence of reactions that was carried out using lysine dicholamide in place of cholic acid afforded 2a (not shown).
Scheme 1.

To assess the antifungal properties of 1a and 2a, we examined their in vitro activity toward four clinically relevant microbes, i.e., C. albicans, C. glabrata, C. neoformans, and C. gatti (Table 1). On a molar basis, 1a exhibits a potency that approaches that of the native antibiotic. However, 2a showed negligible antifungal activity at concentrations as high as 11 μM. We suspect that strong intramolecular association of the pendant AmB moeity with the molecule’s four choloyl groups limits its ability to bind ergosterol, thereby reducing its activity. Our attention then focused on 1a for more detailed investigation.
Table 1.
Antifungal Activities
| MIC/MFCa (μM) | |||
|---|---|---|---|
| Microbe | AmB | 1b | 2b |
| C. albicans | 0.5/1 | 1/2 | >11/-- |
| C. glabrata | 0.5/1 | 2/4 | >11/-- |
| C. neoformans | 0.3/0.5 | 1/2 | >11/-- |
| C. gatti | 0.3/0.5 | 1/2 | >11/-- |
MIC and MFC values are the lowest concentrations required for completely inhibiting growth, and killing at least 99% of the fungi, respectively.
Similar to AmB, 1a exhibits a characteristic absorption at 409 nm with an apparent molar absorptivity that decreases upon aggregation.16 If one lets (i) T, m, and P represent the total, the monomeric, and the aggregate concentrations of this macrolide, respectively, (ii) ε represent the apparent molar asorptivity and (iii) εm and εp represent the molar absorptivity for the monomeric and aggregate components, respectively, then it can be shown that ε = εp + (εm − εp)m/T.16 At concentrations that are in excess of its cac, m is constant, and ε is expected to be inversely proportional to T. Thus, by measuring the apparent molar absorptivity as a function of the reciprocal of the concentration of 1a, its cac value is estimated to be 0.9 μM (from the intercept of two straight lines). This is essentially the same cac as that of AmB (Figure 3).16
Figure 3.

Plot of molar absorptivity (λmax 409 nm) as a function of the reciprocal concentration of 1a [C ]in PBS at 37 °C.
Significantly, aggregates of 1a showed dramatically reduced hemolytic activity relative to aggregates of AmB (Figure 4). Thus, whereas the concentration of AmB that was required to induce 50% release of hemoglobin from erythrocytes was 4 μM, the concentration of 1a that was required for such release was ca. 2 orders of magnitude higher, i.e., 375 μM.
Figure 4.

Plot of percent release of hemoglobin from sheep red blood cells as a function of concentration of AmB (○) and 1a (●) at 37 °C in saline, pH 7.4.
As further evidence that AmB has been tamed in the form of 1a, we have compared its toxicity toward HEK293 T cells with that of the native AmB molecule. As shown in Figure 5, its toxicity toward these cells has been dramatically reduced.
Figure 5.

Bar graph showing the viability HEK293 T cells in the presence of 1, 10, 50, and 100 μM concentrations of AmB and 1a.
In a broader context, this taming strategy that we have described offers a unique opportunity for enhancing the cellular selectivity and therapeutic potential of a variety of membrane-disrupting agents. As we noted previously, those agents that operate at the membrane level are particularly attractive as drugs because they circumvent two of the more common mechanisms of drug resistance, i.e., export mechanisms and enzymatic degradation within the cell.20
The ability of molecular umbrellas to cross lipid membranes raises the possibility that 1a may allow for more efficient transport of this heptaene macrolide antibiotic across the blood-brain-barrier via passive diffusion.21–24 Studies currently in progress are aimed at assessing the in vivo toxicity and efficacy of 1a and related analogs in treating systemic fungal infections and fungal infections in the central nervous system. Efforts are also underway to explore the scope of this taming strategy by examining its applicability to other classes of membrane-disrupting agents (e.g., the polymyxins, the magainins, and quaternary ammonium compounds) using molecular umbrellas and a variety of other facially amphiphilic molecules.25,26 In this regard, it should be noted that there are recent indications that monomers of quaternary ammonium compounds are much more selective in killing bacterial cells than aggregated forms.27 Thus, this taming strategy could lead the way to important new classes of therapeutic agents in the fight against drug-resistant bacteria.28
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the National Institutes of Health (PHS GM100962).
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.bioconjchem.5b00463.
Experimental procedures used for chemical synthesis and physical measurements (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Falci DR, da Rosa FB, and Pasqualotto AC (2015) Comparison of nephrotoxicity associated to different lipid formulations of amphotericin b: a real-life study. Mycoses 58, 104–112. [DOI] [PubMed] [Google Scholar]
- (2).Kaminski DM (2014) Recent progress in the study of the interactions of amphotericin B with cholesterol and ergosterol in lipid environments. Eur. Biophys. J. 43, 453–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Tevyashova AS, Olsufyeva EN, Solovieva SE, Printsevskaya SS, Reznikova MI, Trenin AS, Galatenko OA, Treshalin ID, Pereverzeva ER, Mirchink EP, et al. (2013) Structure-antifungal activity relationships of polyene antibiotics of the amphotericin b group. Antimicrob. Agents Chemother 57, 3815–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Wilcox BC, Endo MM, Uno BE, and Burke MD (2013) C2′-OH of amphotericin b plays an important role in binding the primary sterol of human cells but not yeast cells. J. Am. Chem. Soc 135, 8488–8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, and White TC (2012) Hidden killers: human fungal infections. Sci. Transl. Med 4, 1–9. [DOI] [PubMed] [Google Scholar]
- (6).Palacios DS, Dailey I, Siebert DM, Wilcock BC, and Burke MD (2011) Synthesis-enabled functional group deletions reveal key underpinnings of amphotericin B ion channel and antifungal activities. Proc. Natl. Acad. Sci. U. S. A 108, 6733–6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Volmer AA, Szpilman AM, and Carreira EM (2010) Synthesis and biological evaluation of amphotericin B derivatives. Nat. Prod. Rep 27, 1329–1349. [DOI] [PubMed] [Google Scholar]
- (8).Neumann A, Baginski M, and Czub J (2010) How do sterols determine the antifungal activity of amphotericin B? Free energy of binding between the drug and its membrane targets. J. Am. Chem. Soc 132, 18266–18272. [DOI] [PubMed] [Google Scholar]
- (9).Sedlak M (2009) Amphotericin B: from derivatives to covalent targeted conjugates. Mini-Rev. Med. Chem 9, 1306–1316. [DOI] [PubMed] [Google Scholar]
- (10).Monk BC, and Goffeau A (2008) Outwitting multidrug resistance to antifungals. Science 321, 367–369. [DOI] [PubMed] [Google Scholar]
- (11).Bolard J (1986) How do polyene macrolide antibiotics affect the cellular membrane properties? Biochim. Biophys. Acta, Rev. Biomembr 864, 257–304. [DOI] [PubMed] [Google Scholar]
- (12).Davis SA, Vincent BM, Endo MM, Whitesell L, Marchillo K, Andes DR, Lindquist S, and Burke MD (2015) Nontoxic antimicrobials that evade drug resistance. Nat. Chem. Biol 11, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Gray KC, Palacios DS, Dailey I, Endo MM, Uno BE, Wilcock BC, and Burke MD (2012) Amphotericin primarily kills yeast by simply binding ergosterol. Proc. Natl. Acad. Sci. U. S. A 109, 2234–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Anderson TM, Clay MC, Cioffi AG, Diaz KA, Hisao GS, Tuttle MD, Nieuwkoop AJ, Comellas G, Maryum N, Wang S., et al. (2014) Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol 10, 400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Legrand P, Romero EA, Cohen BE, and Bolard J (1992) Effects of aggregation and solvent on the toxicity of amphotericin B to human erythrocytes. Antimicrob. Agents Chemother 36, 2518–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Yamashita K, Janout V, Bernard EM, Armstrong D, and Regen SL (1995) Micelle/monomer control over the membrane-disrupting properties of an amphiphilic antibiotic. J. Am. Chem. Soc 117, 6249–6253. [Google Scholar]
- (17).Liu Y, and Regen SL (1993) Control over vesicle rupture and leakage by membrane packing and by the aggregation state of an attacking surfactant. J. Am. Chem. Soc 115, 708–713. [Google Scholar]
- (18).Juliano RL, Grant CW, Barber KR, and Kalp MA (1987) Mechanism of the selective toxicity of amphotericin B incorporated into liposomes. Mol. Pharmacol 31, 1–11. [PubMed] [Google Scholar]
- (19).Kondo M, Mehiri M, and Regen SL (2008) Viewing membrane-bound molecular umbrellas by parallax analyses. J. Am. Chem. Soc 130, 13771–13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Stadler E, Dedek P, Yamashita K, and Regen SL (1994) Amphotericin B mimics: a sterol-based ionophore. J. Am. Chem. Soc 116, 6677–6682. [Google Scholar]
- (21).Janout V, and Regen SL (2009) Bioconjugate-based molecular umbrellas. Bioconjugate Chem. 20, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Arranz-Gibert P, Guixer B, Malakoutikhah M, Muttenthaler M, Guzman F, Teixido M, and Giralt E (2015) Lipid bilayer crossing—the gate symmetry. Water-soluble phenylproline-based blood-brain barrier shuttles. J. Am. Chem. Soc. 137, 7357–7364. [DOI] [PubMed] [Google Scholar]
- (23).Shao K, Huang R, Li J, Han L, Ye L, Lou J, and Jiang C (2010) Angiopep-2 modified PE-PEG based polymeric micelles for amphotericin B deliery targeted to the brain. J. Controlled Release 147, 118–126. [DOI] [PubMed] [Google Scholar]
- (24).Pyrgos V, Mickiene D, Sein T, Cotton M, Fransesconi A, Mizrahi I, Donoghue M, Bundrant N, Kim SY, Hardwick M, et al. (2010) Effects of immunomodulatory and organism-associated molecules on the permeability of an in vitro blood-brain barrier model to amphotericin B and fluconazole. Antimicrob. Agents Chemother 54, 1305–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Gaspar D, Velga AS, and Castanho MH (2013) From antimicrobial to anticancer peptides. A review. Front. Microbiol. 4, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Jennings MC, Minbiole KPC, and Wuest WM (2015) Quaternary ammonium compounds: an antimicrobial mainstay and platform for innovation to address bacterial resistance. ACS Infect. Dis 1, 288–303. [DOI] [PubMed] [Google Scholar]
- (27).Joondan N, Caumul P, Akerman M, and Jhaumeer-Laullo S (2015) Synthesis, micellization and interaction of novel quaternary ammonium compounds derived from L-phenylalanine with 1,2-dipalmitoyl-sn-glycero-3-phosphocholine as model membrane in relation to their antibacterial activity, and their selectivity over human red blood cells. Bioorg. Chem 58, 117–129. [DOI] [PubMed] [Google Scholar]
- (28).Blaskovich MAT, Zuegg J, Elliott AG, and Cooper MA (2015) Helping chemists discover new antibiotics. ACS Infect. Dis 1, 285–287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


