To The Editor:
Polyethylene glycols (PEG), or macrogols, are employed in high molecular weight (HMW) (> 1000 g/mol) forms as active ingredients or drug excipients, such as bowel preparations and parenteral steroids.1 We and others have previously reported immediate and life-threatening hypersensitivity reactions to PEG of increasing importance.1 Here, we report on a case of anaphylaxis to PEG 5000-conjugated liposomal perflutren, a perfluorocarbon gas used as echocardiography contrast.
The two existing formulations of perflutren, human albumin conjugated (Optison™) and PEGylated liposomal (Definity®), were approved by the Food and Drug Administration (FDA) in 1997 and 2001, respectively.2, 3 In 2007, the FDA issued a black box warning for perflutren due to 4 deaths within 30 minutes of administration during post-marketing surveillance, without reference to a mechanism; therefore, contraindicating their use in patients with serious cardiopulmonary conditions.2
Based on subsequent safety data, the FDA warning has been revised twice, first in 2008 when the cardiopulmonary contraindication was changed to a warning.4 This revision was based in part upon a multicenter, retrospective analysis of 78,383 doses of perflutren (66,164 PEGylated liposomal and 12,219 human albumin conjugated), that reported only 8 probably-related serious adverse events within 30 minutes of administration, of which all were associated with PEGylated liposomal perflutren.5 Anaphylaxis accounted for 4 of these reactions.5 In 2011, the warning was changed to a statement that cardiopulmonary events were uncommon.6 A prior proposed mechanism for the observed hypersensitivity reactions has been complement activation-related pseudoallergy.7 However, we previously outlined 2 cases of PEG 3350 anaphylaxis that were confirmed by skin prick testing (SPT) and the detection of specific IgE to PEG, suggesting the importance of IgE-mediated as opposed to non-IgE mediated mechanisms for some HMW PEG reactions1 (Figure E1, available in this article’s Online Repository at www.jaci-inpractice.org).
Our patient was a 58-year-old white male with an occupational history as a car audio mechanic and paint department manager, who presented to our drug allergy clinic at Vanderbilt University Medical Center for evaluation of suspected prior immediate reactions associated with PEG-containing medications. One month prior to his clinic visit, he was given PEGylated liposomal perflutren as part of a stress myocardial perfusion scan. Within minutes, he developed shortness of breath, urticaria and hypotension. He was given intramuscular epinephrine, steroids, and antihistamines, but ultimately required intravenous epinephrine drip and intensive care unit admission for continued hypotension. Stabilization over the 4 hours after admission led to wean of his vasopressors, and he was discharged the following day.
Eight months prior, he had anaphylaxis to oral PEG 3350 while undergoing colonoscopy preparation. Within 15 minutes of drinking the first cup of PEG 3350, he developed urticaria, shortness of breath, angioedema, and hypotensive syncope. He was treated with intramuscular epinephrine, steroids and antihistamines, observed in the emergency department, and discharged several hours later. Days before this colonoscopy preparation, while using paints containing HMW PEG, he developed pruritic, swollen hands. Three years prior to consultation, he reported an immediate reaction to a steroid knee injection, characterized by lightheadedness and throat tightness that abated with antihistamine administration.
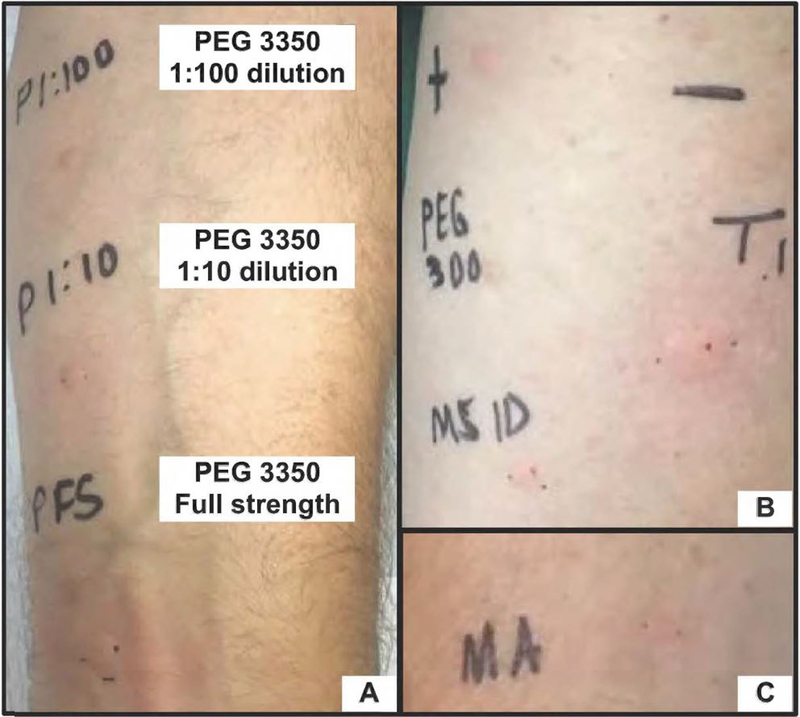
In our clinic, he underwent a previously described skin test protocol and was positive to PEG 3350 at all concentrations, as well as methylprednisolone acetate and triamcinolone acetonide that contain PEG 3350 and polysorbate 80, respectively1 (Figure 1, Table E1, available in this article’s Online Repository at www.jaci-inpractice.org). He was SPT and oral challenge negative to PEG 300, a lower MW macrogol and methylprednisolone sodium succinate which does not contain PEG 3350. Based on his clinical history and SPT results, we concluded his prior immediate reactions to PEG were likely IgE-mediated.
Figure 1.
Selected skin testing images for the patient. In panel A, skin prick testing (SPT) read at 15 minutes had positive responses to polyethylene glycol (PEG) 3350 at all 3 concentrations tested. In panel B, intradermal testing (IDT) is positive for triamcinolone acetonide (contains polysorbate 80), SPT is negative for PEG 300, and IDT is negative for methylprednisolone sodium succinate, all read at 15 minutes. In panel C, SPT is positive for methylprednisolone acetate (contains PEG 3350). (Measurements are recorded in Table E1). Key: + = histamine control (0.1 mg/mL), − = saline, PEG 300 = PEG 300 full strength, MS ID = methylprednisolone sodium succinate intradermal test, TI = triamcinolone acetonide intradermal test, MA = methylprednisolone acetate
Electrochemiluminescent immunoassay was used to detect anti-PEG sIgE.1 The samples were processed with Protein G Plus Agarose then incubated at 1:10 dilution in PEGylated bovine catalase coated Meso-Scale Multi Array plate. A biotin-conjugated goat anti-human IgE (BioRad) antibody was added at 1:10,000 dilution. SULFO-TAG labeled Streptavidin was used as the detection reagent. Plates were read with a Sector Imager 6000 Analyzer (Meso Scale Discovery, Rockville, MD). Anti-PEG sIgE from the patient’s serum capable of binding to PEGylated catalase was detected with a measured luminescence intensity of 347.5. This was comparable in magnitude to previous PEG allergic patients, and significantly higher than PEG exposed controls assayed previously (99% confidence interval [CI] = 54.3 ± 9.3).1
To evaluate the scope at which the differing formulations of perflutren might be associated with anaphylaxis in the United States, we reviewed the FDA Adverse Event Reporting System (FAERS) database from 1989 through 2019. Using the search terms “perflutren” and “anaphylactic shock” or “anaphylactic reaction,” we encountered 90 unique cases of anaphylactic shock or reaction in which perflutren was reported as the primary agent suspected as causal (Table E2, available in this article’s Online Repository at www.jaci-inpractice.org). The formulations of perflutren reported were PEGylated liposomal 84/90 (94%), human albumin 4/90 (4%) and unspecified 2/90 (2%).
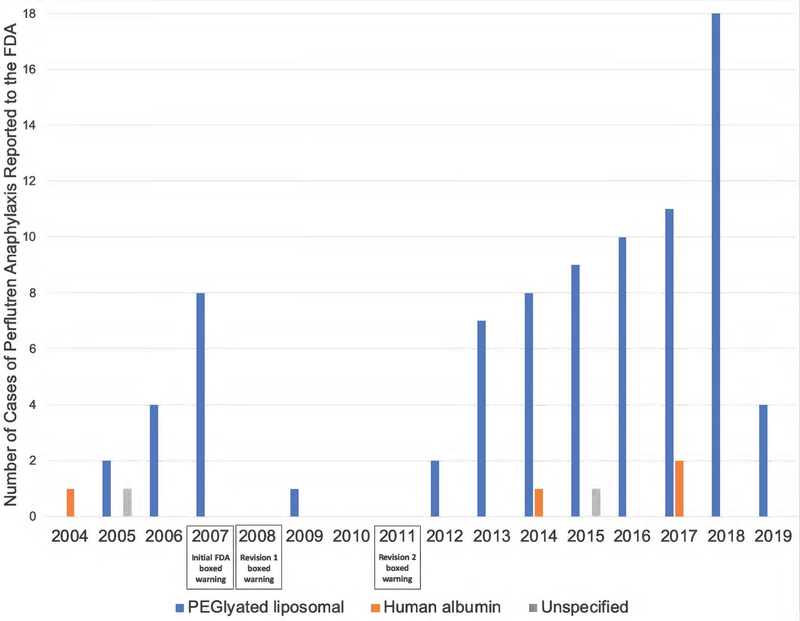
For PEGylated liposomal perflutren, the average age at reaction was 61.2 years (11% missing data), and 64% of those who reacted were male (8% missing data). At the time of reaction, 35% reported that PEGlyated liposomal perflutren was the sole agent administered before anaphylaxis. The other 65% were taking concomitant medications at the time of their reaction. Reactions for PEGylated liposomal perflutren were distributed from 2005 to 2019, with an average of 6 cases per year (Figure 2). There were no reports of PEGylated liposomal perflutren anaphylactic reactions before 2005.
Figure 2.
Cases of anaphylaxis reported to the FDA (FAERS) implicating PEGylated liposomal, human albumin, and unspecified formulations of perflutren echocardiography contrast, by year, along with annotations of key FDA boxed warning revisions. FAERS, FDA Adverse Event Reporting System; FDA, US Food and Drug Administration; PEG, polyethylene glycol.
In this report we demonstrate the presence of anti-PEG specific IgE and positive SPT to HMW PEG in a patient with anaphylaxis to PEGylated liposomal perflutren and two prior immediate hypersensitivity reactions to HMW PEG-containing medications. This patient’s occupational history, of repeated exposures to mechanical fluids and paints, is similar to other patients with repeated cutaneous exposure to PEG-containing products who subsequently develop anaphylaxis to PEG, adding support for a possible cutaneous mode of sensitization.1
In our literature review, only 2 cases focusing on immediate hypersensitivity reactions to perflutren have been reported, and no mechanism has been elucidated thus far.8, 9 Our findings are therefore novel, as they suggest that an IgE-mediated type I hypersensitivity to PEG underlies at least some cases of immediate hypersensitivity to PEGylated liposomal perflutren. We were limited by being unable to devise an assay to prove that our patient’s PEG specific antibodies bind directly to PEGylated liposomal perflutren, due to its gaseous formulation. Further, while we did not use PEG 5000 for SPT because we could not find it in a medical grade source, we believe based upon previous studies that PEG 3350 is representative of PEG 5000 and that IgE-mediated reaction potential to HMW PEG may increase with increasing side chain polyether repeats. This is supported by our previous observation that increasing molecular weight correlates with increasing PEG-specific antibody avidity, since the primary determinant of molecular weight with PEGs is the number of side chain polyether repeats.1 In the future, studies are needed to demonstrate whether preexisting PEG-specific antibodies are behind the disproportionate number of cases of anaphylaxis reported to PEGylated liposomal perflutren compared to human albumin conjugated perflutren.
Anaphylactic reactions to PEGylated liposomal perflutren appear to be rare overall, reported by Wei et al. as occurring in 0.006% of patients.5 We encountered only 84 cases of anaphylaxis to PEGylated liposomal perflutren reported in FAERS. However, self-reported databases are potentially limited due to under-recognition and reporting.
In conclusion, given the severity of these reactions, allergists, cardiologists, and radiologists should become aware of the possibility of hypersensitivity reactions to macrogols to facilitate prompt diagnosis, treatment, and future avoidance. We suggest that use of PEGylated liposomal perflutren and other drugs and devices containing HMW PEG should be avoided in favor of alternatives when patients report prior immediate hypersensitivity reactions consistent with severe macrogol allergy. Further studies are needed to assess how often this emerging drug allergy mechanism is responsible for reactions when they do occur.
Extended Data
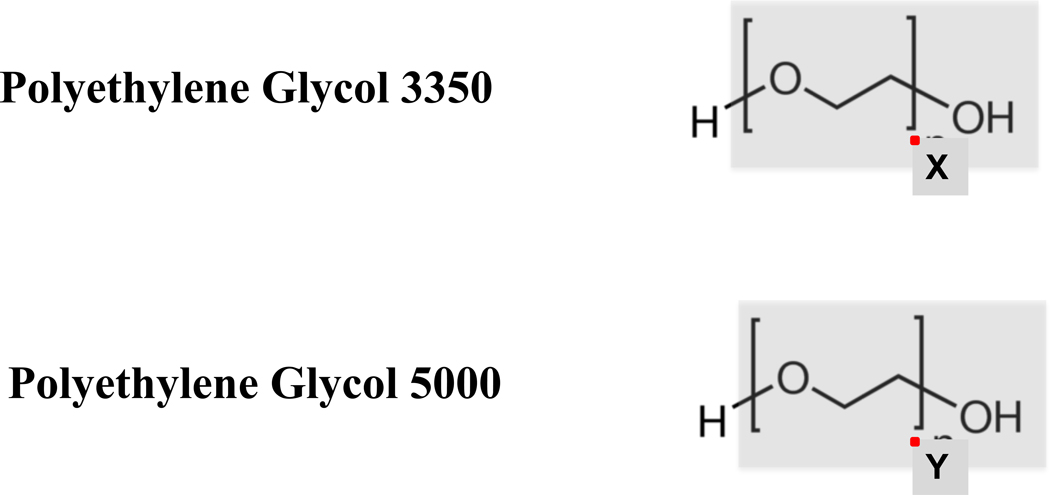
Figure E1.
X and Y indicate the repeating oxyethylene group. The number of repeats determines the length of the molecule’s chain, and thereby determines the molecular weight. A polyethylene glycol of molecular weight 3350 would be a mixture of molecules with enough oxyethylene repeats to have an average molecular weight of 3350 g/mol.
Table E1.
Skin prick and intradermal testing with corticosteroids and polyethylene glycols
| Agent (concentration) | Wheal (mm) | Flare (mm) | Interpretation |
|---|---|---|---|
| Skin Prick Test Results | |||
| Histamine control (0.1 mg/mL) | 6 | 30 | Positive |
| Saline | 3 | 3 | Negative |
| PEG 3350 | 11 | 45 | Positive |
| PEG 3350 (1:10 dilution) | 7 | 40 | Positive |
| PEG 3350 (1:100 dilution) | 7 | 40 | Positive |
| PEG 300 | 0 | 0 | Negative |
| Betamethasone (0.6 mg/mL) | 4 | 5 | Negative |
| Dexamethasone (0.4 mg/mL) | 3 | 3 | Negative |
| Hydrocortisone (5 mg/mL) | 4 | 4 | Negative |
| Methylprednisolone acetate (4 mg/mL) | 9 | 15 | Positive |
| Methylprednisolone sodium succinate (5 mg/mL) | 4 | 4 | Negative |
| Triamcinolone acteonide (1 mg/mL) | 4 | 4 | Negative |
| Intradermal Skin Test Results | |||
| Triamcinolone acetonide (0.1 mg/mL) | 17 | 36 | Positive |
Table E2.
Cases of anaphylaxis reported to the FDA from 2004 to 2019 where perflutren contrast was the primary drug suspected, including PEGylated liposomal, human albumin, and unspecified formulations
| FAERS Report ID Number | Age | Sex | Year of report | Formulation of perflutren | Patient taking any other medications concomitantly |
|---|---|---|---|---|---|
| 4305851-X | 57 | Female | 2004 | Human albumin | No |
| 4799863-X | 83 | Female | 2005 | Unspecified | Yes |
| 4854899–5 | 65 | Male | 2005 | PEGylated liposomal | No |
| 4856232–1 | 75 | Male | 2005 | PEGylated liposomal | No |
| 4897423–3 | 64 | Male | 2006 | PEGylated liposomal | No |
| 5098650–5 | 48 | Female | 2006 | PEGylated liposomal | Yes |
| 5143226–4 | 47 | Female | 2006 | PEGylated liposomal | Yes |
| 5169390–9 | N/A | N/A | 2006 | PEGylated liposomal | No |
| 5226997–8 | 35 | Female | 2007 | PEGylated liposomal | Yes |
| 5235427–1 | 51 | Female | 2007 | PEGylated liposomal | Yes |
| 5237726–6 | N/A | N/A | 2007 | PEGylated liposomal | No |
| 5386792–6 | N/A | N/A | 2007 | PEGylated liposomal | No |
| 5444176–6 | 65 | Male | 2007 | PEGylated liposomal | Yes |
| 5456457–0 | N/A | Female | 2007 | PEGylated liposomal | No |
| 5870366–4 | N/A | N/A | 2007 | PEGylated liposomal | No |
| 5870449–9 | N/A | N/A | 2007 | PEGylated liposomal | No |
| 6385659–4 | 47 | Male | 2009 | PEGylated liposomal | Yes |
| 8986382 | 57 | Male | 2012 | PEGylated liposomal | Yes |
| 8991087 | 57 | Male | 2012 | PEGylated liposomal | No |
| 12114192 | 59 | Male | 2013 | PEGylated liposomal | No |
| 9174101 | 87 | Female | 2013 | PEGylated liposomal | Yes |
| 9478127 | 65 | Female | 2013 | PEGylated liposomal | Yes |
| 9496468 | 84 | Female | 2013 | PEGylated liposomal | Yes |
| 9535950 | 31 | Male | 2013 | PEGylated liposomal | Yes |
| 9547479 | 76 | Male | 2013 | PEGylated liposomal | Yes |
| 9758411 | 84 | Male | 2013 | PEGylated liposomal | No |
| 10979100 | 52 | Male | 2014 | PEGylated liposomal | No |
| 10979122 | 57 | Male | 2014 | PEGylated liposomal | Yes |
| 10981361 | 61 | Male | 2014 | PEGylated liposomal | Yes |
| 10981661 | 55 | Male | 2014 | PEGylated liposomal | No |
| 10981674 | 75 | Male | 2014 | PEGylated liposomal | Yes |
| 10983516 | 57 | Male | 2014 | PEGylated liposomal | Yes |
| 12114174 | 60 | Male | 2014 | PEGylated liposomal | No |
| 12173673 | 29 | Female | 2014 | PEGylated liposomal | Yes |
| 9973637 | N/A | N/A | 2014 | Human albumin | No |
| 11115484 | 43 | Female | 2015 | Unspecified | Yes |
| 12111173 | 53 | Male | 2015 | PEGylated liposomal | No |
| 12111176 | 72 | Male | 2015 | PEGylated liposomal | Yes |
| 12111182 | 69 | Male | 2015 | PEGylated liposomal | Yes |
| 12111186 | 48 | Male | 2015 | PEGylated liposomal | No |
| 12111187 | 58 | Female | 2015 | PEGylated liposomal | Yes |
| 12115134 | 40 | Male | 2015 | PEGylated liposomal | Yes |
| 12115143 | 36 | Male | 2015 | PEGylated liposomal | Yes |
| 12115145 | 58 | Male | 2015 | PEGylated liposomal | No |
| 13266056 | 62 | Male | 2015 | PEGylated liposomal | Yes |
| 13265913 | 77 | Male | 2016 | PEGylated liposomal | No |
| 13265929 | 46 | Female | 2016 | PEGylated liposomal | Yes |
| 13265933 | 75 | Female | 2016 | PEGylated liposomal | Yes |
| 13265937 | 68 | Female | 2016 | PEGylated liposomal | Yes |
| 13265956 | 59 | Male | 2016 | PEGylated liposomal | Yes |
| 13266052 | 68 | Male | 2016 | PEGylated liposomal | Yes |
| 13266058 | 67 | Male | 2016 | PEGylated liposomal | Yes |
| 13266071 | 82 | Male | 2016 | PEGylated liposomal | No |
| 13266075 | 87 | Female | 2016 | PEGylated liposomal | Yes |
| 14542996 | 43 | Male | 2016 | PEGylated liposomal | Yes |
| 13265967 | 89 | Female | 2017 | PEGylated liposomal | No |
| 13376730 | N/A | N/A | 2017 | Human albumin | No |
| 1423988 | 37 | Male | 2017 | PEGylated liposomal | Yes |
| 14373279 | 55 | Female | 2017 | Human albumin | Yes |
| 14542977 | 58 | Male | 2017 | PEGylated liposomal | Yes |
| 14542978 | 63 | Female | 2017 | PEGylated liposomal | Yes |
| 14542979 | 50 | Male | 2017 | PEGylated liposomal | No |
| 14542982 | 57 | Male | 2017 | PEGylated liposomal | Yes |
| 14542987 | 58 | Female | 2017 | PEGylated liposomal | Yes |
| 14542992 | 62 | Female | 2017 | PEGylated liposomal | No |
| 14542999 | 92 | Male | 2017 | PEGylated liposomal | Yes |
| 14543018 | 57 | Female | 2017 | PEGylated liposomal | Yes |
| 14543024 | 34 | Male | 2017 | PEGylated liposomal | Yes |
| 15244981 | 57 | Female | 2018 | PEGylated liposomal | Yes |
| 15985388 | 47 | Female | 2018 | PEGylated liposomal | Yes |
| 15985389 | 55 | Female | 2018 | PEGylated liposomal | Yes |
| 15985398 | 91 | Male | 2018 | PEGylated liposomal | Yes |
| 15985401 | 50 | Male | 2018 | PEGylated liposomal | Yes |
| 15985402 | 63 | Male | 2018 | PEGylated liposomal | Yes |
| 15985408 | 76 | Male | 2018 | PEGylated liposomal | Yes |
| 15985412 | 48 | Male | 2018 | PEGylated liposomal | Yes |
| 15985419 | N/A | N/A | 2018 | PEGylated liposomal | No |
| 15985424 | 56 | Female | 2018 | PEGylated liposomal | Yes |
| 15985445 | 69 | Male | 2018 | PEGylated liposomal | No |
| 15985448 | 84 | Female | 2018 | PEGylated liposomal | Yes |
| 15985453 | 55 | Male | 2018 | PEGylated liposomal | Yes |
| 15985454 | 61 | Male | 2018 | PEGylated liposomal | Yes |
| 15985458 | 70 | Female | 2018 | PEGylated liposomal | Yes |
| 15985471 | 65 | Male | 2018 | PEGylated liposomal | No |
| 15985480 | 75 | Female | 2018 | PEGylated liposomal | Yes |
| 15985483 | N/A | Male | 2018 | PEGylated liposomal | Yes |
| 15914392 | 57 | Male | 2019 | PEGylated liposomal | No |
| 15942179 | 67 | Female | 2019 | PEGylated liposomal | No |
| 15985392 | N/A | N/A | 2019 | PEGylated liposomal | No |
| 16043833 | 75 | Female | 2019 | PEGylated liposomal | Yes |
Data marked as N/A indicate that the information was not contained in the primary report to the FDA.
Clinical Implications: IgE-mediated macrogol allergy is a potential mechanism for anaphylaxis to PEGylated liposomal perflutren. In patients with a history of severe macrogol allergy, high molecular weight PEG-containing drugs should be avoided in favor of alternatives.
Acknowledgments
Funding:
Dr. Stone received funding support related to this project from NIH/NIGMS T32 GM007569 and 1K12HS026395–01.
Dr. Liu received funding related to this project from: NIH/NCI CA 142665, CA 21765, and NIH/NIGMS GM 115279
Dr. Phillips receives funding from National Institutes of Health (1P50GM115305, R34 AI136815, R21 AI139021, 1R01HG010863, National Health and Medical Research Foundation of Australia
IRB: This study was done under IRB approved protocols from Vanderbilt University IRB #161455
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors declare that they have no conflicts of interest to disclose.
References
- 1.Stone CA, Liu Y, Relling MV, Krantz MS, Pratt AL, Abreo A, et al. Immediate Hypersensitivity to Polyethylene Glycols and Polysorbates: More Common Than We Have Recognized. J Allergy Clin Immunol Pract 2019; 7:1533–40.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Main ML, Goldman JH, Grayburn PA. Thinking outside the “box”-the ultrasound contrast controversy. J Am Coll Cardiol 2007; 50:2434–7. [DOI] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration, prescribing information for Optison on September 16, 2016] Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/020899s018s019lbl.pdf. Accessed on November 13, 2019.
- 4.US Food and Drug Administration, revised prescribing information for Definity on April 2008.] Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021064s009lbl.pdf. Accessed on November 13, 2019.
- 5.Wei K, Mulvagh SL, Carson L, Davidoff R, Gabriel R, Grimm RA, et al. The safety of deFinity and Optison for ultrasound image enhancement: a retrospective analysis of 78,383 administered contrast doses. J Am Soc Echocardiogr 2008; 21:1202–6. [DOI] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration, revised prescribing information for Definity on August 2011.] Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021064s011lbl.pdf. Accessed on November 13, 2019.
- 7.Szebeni J. Complement activation-related pseudoallergy: a new class of drug-induced acute immune toxicity. Toxicology 2005; 216:106–21. [DOI] [PubMed] [Google Scholar]
- 8.Blokh I, Ganem A, Bonaros EP, Stephen BD, Steinberg B, Rosen SE. Hemodynamic instability after receiving intravenous Perflutren for contrast echocardiography in an elderly female. Echocardiography 2004; 21:613–5. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A, Harris S, Naina H. Perflutren-Induced Angioedema. Echocardiography 2015; 32:1329. [DOI] [PubMed] [Google Scholar]