Abstract
Background
Small cell lung cancer (SCLC) is a very fast growing form of cancer and is characterised by early metastasis. As a result, chemotherapy is the mainstay of treatment. A number of different platinum‐based chemotherapy regimens and non‐platinum‐based chemotherapy regimens have been used for the treatment of SCLC, with varying results. This review was conducted to analyse the data from these studies in order to compare their effectiveness.
Objectives
To determine the effectiveness of platinum chemotherapy regimens compared with non‐platinum chemotherapy regimens in the treatment of SCLC with respect to survival, tumour response, toxicity and quality of life.
Search methods
We searched the biomedical literature databases CENTRAL (TheCochrane Library 2014, Issue 7), MEDLINE, EMBASE and CINAHL from 1966 to August 2014. In addition, we handsearched reference lists from relevant resources.
Selection criteria
All randomised controlled trials involving patients with pathologically confirmed SCLC (including both limited‐stage disease and extensive‐stage disease) and the use of a platinum‐based chemotherapy regimen in at least one treatment arm and a non‐platinum‐based chemotherapy regimen in a separate arm.
Data collection and analysis
We used standard methodological procedures expected by the Cochrane Collaboration. Two authors independently assessed search results. We assessed included studies for methodological quality and recorded the following outcome data: survival, tumour response, toxicity and quality of life. We combined the results of the survival, tumour response and toxicity data in a meta‐analysis. Quality‐of‐life data were analysed individually.
Main results
A total of 32 studies involving 6075 patients with SCLC were included in this systematic review. The majority of studies were multi‐centre randomised controlled trials conducted throughout Europe, North America and Asia with the earliest study publishing data in 1981 and the latest in 2014. The duration of studies ranged from 12 to 72 months with a median of 32 months. The median age of patients in the vast majority of studies was between 60 and 65 years of age. Eighteen studies presented data on extensive‐stage disease. Nine studies presented data on limited‐stage disease. Eleven studies did not present data based on the disease stage. These data were analysed separately in subgroup analyses. Sixteen (50%) studies were of good quality with a low risk of bias and the data from these studies were analysed separately in a heterogeneity analysis.
There was no statistically significant difference between treatment groups in terms of survival at 6 months, 12 months and 24 months. There was also no statistically significant difference in terms of overall tumour response. However, platinum‐based treatment regimens did have a significantly higher rate of complete response. Platinum‐based chemotherapy regimens had significantly higher rates of nausea and vomiting and thrombocytopenia toxicity. Four trials presented quality‐of‐life data, but, due to the different systems used to measure quality of life this data could not be combined in a meta‐analysis.
Authors' conclusions
Platinum‐based chemotherapy regimens did not offer a statistically significant benefit in survival or overall tumour response compared with non‐platinum‐based regimens. However, platinum‐based chemotherapy regimens did increase complete response rates, at the cost of higher adverse events including nausea and vomiting, anaemia and thrombocytopenia toxicity. These data suggest non‐platinum chemotherapy regimens have a more advantageous risk‐benefit profile. This systematic review highlights the lack of quality‐of‐life data in trials involving chemotherapy treatment for SCLC. With poor long‐term survival associated with both treatment groups, the issue of the quality of the survival period takes on even more significance. It would be beneficial for future trials in this area to include a quality‐of‐life assessment.
Plain language summary
A comparison of platinum‐based and non‐platinum‐based chemotherapy regimens for the treatment of small cell lung cancer
Review question
Do patients with small cell lung cancer (SCLC) who receive platinum‐based chemotherapy treatment live longer than those who receive non‐platinum‐based chemotherapy treatment?
Other questions include: do these patients also respond better to treatment, experience fewer side‐effects and have a better quality of life?
Background
SCLC is a type of cancer that originates in the lungs. It is a very aggressive form of cancer that tends to grow and spread throughout the body quickly. As a result, chemotherapy is often the first type of treatment used for this type of cancer. Another type of treatment used for SCLC is radiotherapy, which is often given to the lung or to the brain.
A combination of a number of chemotherapy drugs used together is called a ‘chemotherapy regimen’. Currently, there are two main chemotherapy regimens used for treating SCLC:
• platinum‐based chemotherapy regimens – containing a chemotherapy drug known as a “platinum agent” in combination with other chemotherapy drugs and;
• non‐platinum‐based chemotherapy regimens – containing other chemotherapy drugs without a “platinum agent”.
Over the past years, many studies have been done comparing the use of platinum‐based chemotherapy regimens and non‐platinum‐based chemotherapy regimens in SCLC.
We carried out a study, called a meta‐analysis, which included patients with SCLC who took part in randomised controlled trials comparing platinum‐based chemotherapy regimens and non‐platinum‐based chemotherapy regimens.
Study characteristics
We searched for studies up to 1st August 2014. A total of 32 studies were part of this review and included 6,075 patients in total. The studies were carried out in many different countries throughout Europe, Asia and North America. The studies were conducted between 1981 and 2014.
Key results
The review showed that patients who received platinum‐based chemotherapy were not any more likely to be alive at 6 months, 12 months and 24 months after treatment compared with patients who received non‐platinum‐based chemotherapy.
Platinum‐based chemotherapy, however, showed higher rates of complete tumour response (the complete disappearance of tumours, at least for a period of time after treatment) compared to non‐platinum‐based chemotherapy. Platinum‐based chemotherapy also caused some more side effects, including nausea and vomiting, and low platelets.
Only four studies looked at quality of life but because they each used different methods to measure the effects, their results could not be combined. However, in each study there was no difference in the quality of life between the platinum‐based chemotherapy group and the non‐platinum‐based chemotherapy group.
Background
Lung cancer is one of the most common cancers in the world, both in terms of incidence and mortality (Dela Cruz 2011). Smoking is the biggest risk factor for lung cancer and is associated with 90% of cases (Alberg 2003). Other risk factors include occupational and environmental exposure, for example asbestos (Heintz 2010). Lung cancer is divided into small cell lung cancer (SCLC) and non‐small cell lung cancer (NSCLC) based on histological appearance. SCLC makes up about 20% of lung cancers (Le Pechoux 2004).
SCLC is divided into two stages ‐ limited disease stage and extensive disease stage. Based on the Veterans' Administration Lung Study Group (VALG) classification, The International Association for the Study of Lung Cancer (IASLC) defined limited disease (LD‐SCLC) as: "Disease restricted to one hemithorax with regional lymph node metastases, including hilar, ipsilateral and contralateral mediastinal, and ipsilateral and contralateral supraclavicular nodes and should also include patients with ipsilateral pleural effusion independent of whether cytology is positive or negative" (Micke 2002).
Extensive disease (ED‐SCLC) was given the definition: "All patients with sites of disease beyond the definition of limited disease" (Micke 2002).
In SCLC, long‐term survival is quite poor. Untreated, the median survival is 4 to 12 weeks (Chan 2013). Because SCLC is a very aggressive type of cancer and early metastasis (both local and distant) is common, chemotherapy is the first line treatment (Stinchcombe 2010). Even with treatment, long‐term survival is poor. Median survival is in the order of 15 to 20 months for LD‐SCLC and 8 to 13 months for ED‐SCLC. Two‐ and five‐year survival rates for LD‐SCLC range from 20% to 40% and 10% to 13% respectively. For ED‐SCLC, survival rates are even poorer ‐ less than 5% at two years and 1% to 2% at five years (Glisson 2014).
Chemotherapy is the most common treatment for SCLC because of early metastatic spread. Platinum therapy has been widely used and is regarded as first line treatment, as it has been considered one of the most efficacious agents. It is often combined with the non‐platinum agent etoposide (Stinchcombe 2010). Platinum agents are cytotoxic alkylating agents that are active throughout the cell cycle (Chabner 2010). The most widely used platinum agents in SCLC are cisplatin (cis‐diamine‐dichloroplatinum II) and carboplatin (cis‐diamine‐(1,1‐cyclobutanedicarboxylate) platinum) (Stinchcombe 2010).
Non‐platinum agents for SCLC include vincristine, doxorubicin (Adriamycin), cyclophosphamide and ifosfamide. All of these agents have been shown to have anti‐tumour activity and have also been used in combination regimens in SCLC (Pujol 2000). One of the most common non‐platinum combinations that has been shown to be effective in SCLC is the vincristine, doxorubicin and cyclophosphamide regimen (Stinchcombe 2010).
As the available treatments have varying success rates and the different treatments have various advantages and disadvantages, a systematic review will be useful in determining optimal treatment regimens for SCLC.
Objectives
To determine the effectiveness of platinum chemotherapy regimens compared with non‐platinum chemotherapy regimens in the treatment of small cell lung cancer (SCLC) with respect to survival, tumour response, toxicity and quality of life.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) that compared platinum‐based chemotherapy regimens with other non‐platinum‐based chemotherapy regimens.
Inclusion criteria:
Studies involving only patients with pathologically confirmed (cytological or histological) small cell lung cancer.
Studies with at least one platinum‐based treatment group.
Studies with at least one non‐platinum‐based treatment group.
Exclusion criteria:
Studies with platinum agents used in all treatment groups.
Studies with no platinum agent used in any treatment groups.
Types of participants
Adult patients of either sex with pathologically confirmed (cytological or histological) small cell lung cancer.
Types of interventions
Platinum agents at any dose or for any number of cycles compared with any other chemotherapy regimen [P versus A].
Platinum agents at any dose or for any number of cycles in combination with other chemotherapy regimens versus the same chemotherapy regimen without the platinum agent (i.e. non‐platinum chemotherapy is identical in both interventions) [(P+A) versus A].
Platinum agents at any dose or for any number of cycles in combination with any other chemotherapy regimen versus any other chemotherapy regimen not containing platinum agents [(P+A) versus B].
(Where P = platinum chemotherapy agents, A = non‐platinum chemotherapy regimens and B = non‐platinum chemotherapy regimens (different from A)).
Studies where platinum agents were administered to the control group were excluded from this review.
Radiotherapy
RCTs that involve the use of radiotherapy (RT) were included, provided that RT was planned to be given in an identical way (dose, fractionation, timing and technique) in both treatment arms. If RT was given unequally to a treatment arm, or if the chemotherapy regimen to which patients were randomised routinely affected the way in which the RT was given, then the RCT was excluded.
Types of outcome measures
The primary outcome measure was survival at 6 months, 12 months and 24 months of follow up. Other outcome measures, such as tumour response, treatment‐related toxicity and quality of life were also considered. Tumour response for objective overall response and complete response were defined as per World Health Organization (WHO) guidelines for tumour response evaluation (Park 2003). Toxic events were classified (if they had not already been) according to the WHO scale (WHO 1979) and only grades 3 and 4 of toxicity were analysed. The following toxic events were considered: toxic death, nausea and vomiting, alopecia, infection, anaemia, leukopenia, thrombocytopenia and granulocytopenia. Quality‐of‐life data were recorded qualitatively.
Search methods for identification of studies
Please see Appendix 1 and Appendix 2 for the search strategies used in the original review and current update, respectively
In 2007, we designed a search strategy to search the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2007, Issue 2), MEDLINE (accessed through PubMed), EMBASE and CINAHL (accessed through EBSCO). The date of the search was from 1966 to April 2007. The search strategy was not restricted by date or language.
The search strings used to retrieve studies are reported in Appendix 1
For this update, a search strategy generated by the Cochrane Lung Cancer Group was used to search the following databases:
Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2014, issue 7)
MEDLINE (accessed through PubMed) (1966 to 1 August 2014)
EMBASE (accessed through Ovid) (1966 to 1 August 2014)
The search strings used are reported in Appendix 2.
The strategy was combined with a validated filter to retrieve clinical trials (see section 6.4.11 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011)).
In addition to the search strategy, we handsearched references from relevant studies to identify any further studies. We contacted principal authors to identify any further studies or data that may be relevant to this review.
Data collection and analysis
Two authors (IA and SC) assessed eligibility of articles retrieved via the search strategy from the title and abstract, with differences resolved through the input of a third author (JW). When there was insufficient information for assessment, the authors reviewed the full articles.
Two authors (IA and SC) independently evaluated all RCTs found in the search in order to rule out those that did not meet the inclusion criteria. We evaluated those studies for probable inclusion by critical reading of the whole article. There was no blinding of the author as to the origin or conclusions of the article for eligibility assessment, data extraction or quality assessment.
If necessary, we sought information from the principal investigator of the trial concerned. Two authors (IA and SC) independently extracted the data to ensure validity, and we resolved any discrepancies by an open discussion between all investigators (IA, SC, JW, KF).
To evaluate the methodological quality of selected studies, the authors independently assessed the studies with respect to the criteria set out in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0.(Higgins 2011a)
Each author independently undertook the extraction and registration of data from each study using data and study forms specifically designed for this review. The data extracted included details of the methodology used, the characteristics of the study participants, the type of interventions undertaken, the comparison groups, and the results obtained including the follow‐up period.
We resolved any disagreement by consensus, or with the input of a third member (JW) of the review team.
We analysed all patients initially randomised on an intention‐to‐treat (ITT) basis. The ITT population was defined as randomised patients, irrespective of whether they received any form of treatment. For dichotomous variables, we calculated risk ratio (RR) with a 95% confidence interval (CI). We used a random‐effects model for the pooled analysis. We undertook the meta‐analysis in Review Manager 5.3 (RevMan 2014).
We also calculated the proportion of people alive at 6, 12 and 24 months from both treatment groups. This data was used to generate survival curves for each of the subgroups.
Subgroup analysis
We performed subgroup analyses for the outcomes of survival (at 6 months, 12 months and 24 months) and tumour response. We categorised data from included studies into the subgroups:
'undifferentiated' ‐ if the study did not differentiate between patients with limited disease and extensive disease small cell lung cancer;
LD‐SCLC ‐ if the study presented data specifically from patients with limited disease small cell lung cancer;
ED‐SCLC ‐ if the study presented data specifically from patients with extensive disease small cell lung cancer.
This was undertaken in order to determine if there are differences between the treatment groups depending on the stage of the SCLC.
Heterogeneity assessment
Where substantial heterogeneity occurred, we explored the effect of potential sources of heterogeneity in an attempt to identify the cause of the heterogeneity. Substantial heterogeneity was considered to exist when the I2 value was greater than 50% (Higgins 2003).
We identified the following potential sources of heterogeneity (postulated a priori).
Quality of studies. We deemed studies to be of higher quality if they satisfied the following criteria in the risk of bias assessment:
incomplete outcome data addressed for all assessed outcomes;
free of selective reporting;
free of other bias.
(Please refer to the 'Risk of bias' table for quality assessment).
In the current update, the studies were also assessed for quality using the GRADE approach as described in the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0(Schünemann 2011). Using this method, studies were given a quality rating of High, Moderate, Low or Very Low.
Full article publication versus abstract publication.
Use of radiotherapy as a co‐intervention.
Sensitivity analysis
We performed a sensitivity analysis, systematically excluding studies from the overall analysis based on the potential sources of heterogeneity hypothesised above. This included conducting a sensitivity analysis involving only studies that used radiotherapy treatment in order to explore the potential influence of platinum‐based chemotherapy regimens and the use of radiotherapy on toxicity.
Results
Description of studies
Search results
The search strategies used for this review are outlined in Appendix 1 (strategy for the original review) and Appendix 2 (strategy for the update). The search yielded 3669 search results. We excluded 112 results as they were duplicates. A further 3525 records were excluded by abstract as they did not meet the inclusion criteria. This left 32 studies to be included in the review, including 29 from the original review (Figure 1).
1.
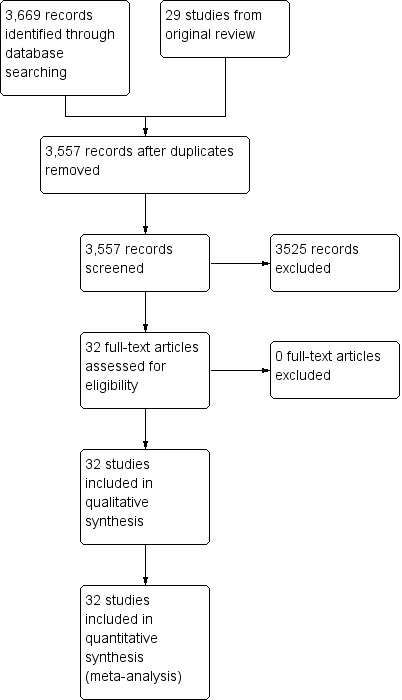
Study flow diagram
Study characteristics
The 32 studies included 6075 participants who were randomised. Of these, 3036 were assigned to platinum‐based chemotherapy and 3039 to non‐platinum‐based chemotherapy regimens. All studies reported ITT analyses, thus recomputation of outcomes was not required.
Of the included studies, nineteen were conducted in centres throughout Europe, nine in North America, three in Asia and one (Quoix 2005) conducted in both Canada and Europe. All studies were one or more years in duration. The shortest study, Wolf 1987, lasted 12 months and the longest study, Urban 1999a, lasted six years.
A summary of the included studies is presented in Table 1. A detailed description of each study is presented in the section 'Characteristics of included studies'.
1. Summary of included studies.
| Study ID | Number of Patients | Radiotherapy | |||
| First Author | Year | Platinum group | Non‐platinum group | Total | |
| Baka | 2008 | 141 | 139 | 280 | PCI, TRT |
| Chahinian | 1989 | 105 | 103 | 208 | PCI |
| Creech | 1982 | 21 | 19 | 40 | ‐ |
| de Jong | 2007 | 101 | 102 | 203 | ‐ |
| Eagan | 1981 | 31 | 31 | 62 | TRT |
| Evans | 1987 | 145 | 144 | 289 | PCI, TRT |
| Farris | 1993 | 56 | 57 | 113 | PCI, TRT |
| Fukuoka | 1986 | 35 | 34 | 69 | TRT |
| Fukuoka | 1991 | 97 | 97 | 194 | TRT |
| Gatzemeier | 1994 | 171 | 173 | 344 | ‐ |
| Goodman | 1990 | 194 | 194 | 388 | PCI, TRT |
| Greco | 2005 | 60 | 60 | 120 | PCI |
| Havemann | 1987 | 150 | 152 | 302 | PCI, TRT |
| Jones | 1993 | 54 | 50 | 104 | PCI, TRT |
| Kanitz | 1992 | 59 | 52 | 111 | ‐ |
| Lyss | 2002 | 12 | 13 | 25 | PCI |
| Postmus | 1992 | 60 | 63 | 123 | PCI |
| Postmus | 1996 | 70 | 73 | 143 | ‐ |
| Quoix | 2005 | 41 | 41 | 82 | ‐ |
| Roth | 1992 | 148 | 146 | 294 | PCI |
| Sculier | 1990 | 95 | 106 | 201 | PCI |
| Sculier | 1993 | 107 | 108 | 215 | PCI, TRT |
| Sekine | 2014 | 30 | 32 | 62 | ‐ |
| Smith | 1991 | 47 | 48 | 95 | PCI |
| Souhami | 1997 | 80 | 75 | 155 | ‐ |
| Sundstrom | 2002 | 218 | 218 | 436 | PCI, TRT |
| Urban | 1999a | 191 | 203 | 394 | PCI, TRT |
| Urban | 1999b | 229 | 228 | 457 | PCI, TRT |
| Veronesi | 1994 | 70 | 66 | 136 | PCI, TRT |
| Wampler | 1991 | 85 | 85 | 170 | ‐ |
| White | 2001 | 60 | 59 | 119 | PCI, TRT |
| Wolf | 1987 | 73 | 68 | 141 | PCI, TRT |
| Total: 32 | ‐ | 3036 | 3039 | 6075 | ‐ |
PCI ‐ Prophylactic Cranial Irradiation
TRT ‐ Thoracic Radiotherapy
Study chemotherapy interventions
The most common platinum agent was cisplatin, used in 24 studies (75%). Carboplatin was used in the remaining eight studies (25%). The most common non‐platinum agents used were etoposide (26 trials, 81.25%), cyclophosphamide (24 trials, 75%), doxorubicin (20 trials, 62.5%) and vincristine (18 trials, 56.25%).
Radiotherapy as a co‐intervention
Of the 32 included studies, seven used only prophylactic cranial irradiation (PCI), three used only thoracic radiotherapy (TRT), and thirteen used both. The remaining nine studies did not involve any form of radiotherapy. The use of radiotherapy by study is presented in Table 1.
Subgroups
The 32 studies were also divided a priori into subgroup comparisons according to disease staging, as indicated in Table 2.
2. Studies by subgroup.
| Undifferentiated | LD‐SCLC | ED‐SCLC | |||
| Creech | 1982 | Eagan | 1981 | Chahinian | 1989 |
| Farris^ | 1993 | Fukuoka | 1986 | Fukuoka | 1986 |
| Fukuoka^ | 1991 | Goodman | 1990 | Gatzemeier | 1994 |
| Postmus | 1992 | Havemann | 1987 | Greco | 2005 |
| Sculier | 1990 | Jones | 1993 | Havemann | 1987 |
| Sculier | 1993 | Sundstrom | 2002 | Jones | 1993 |
| Smith | 1991 | Urban* | 1999a | Kanitz | 1992 |
| Souhami | 1997 | Wolf | 1987 | Lyss | 2002 |
| Urban | 1999b | Baka* | 2008 | Postmus | 1996 |
| Veronesi | 1994 | ‐ | ‐ | Quoix | 2005 |
| White | 2001 | ‐ | ‐ | Roth | 1992 |
| ‐ | ‐ | ‐ | ‐ | Sundstrom | 2002 |
| ‐ | ‐ | ‐ | ‐ | Urban* | 1999a |
| ‐ | ‐ | ‐ | ‐ | Wampler | 1991 |
| ‐ | ‐ | ‐ | ‐ | Wolf | 1987 |
| ‐ | ‐ | ‐ | ‐ | de Jong | 2007 |
| ‐ | ‐ | ‐ | ‐ | Sekine | 2014 |
| Baka* | 2008 | ||||
^Presented undifferentiated survival data; response data sorted by stage. *Presented undifferentiated response data; survival data sorted by stage.
Risk of bias in included studies
We assessed study quality according to the criteria set out in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Higgins 2011b)). The individual assessment for each study is set out in the 'Risk of bias' graph (Figure 2).
2.
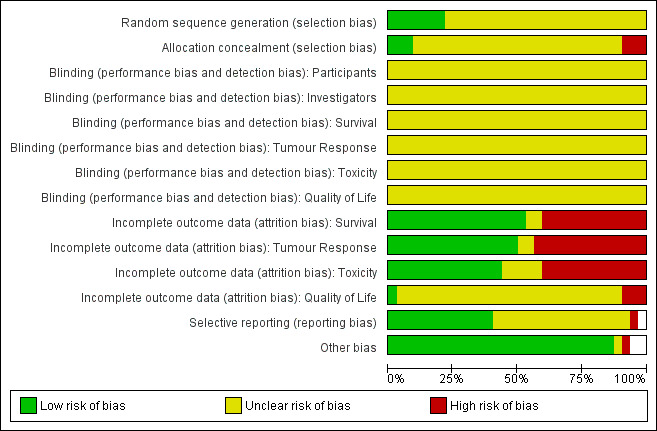
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Twenty‐six of the 32 studies (89.66%) did not report the randomisation process clearly. Hence, they were given an 'Unclear' rating for both sequence generation and allocation concealment. Baka 2008; de Jong 2007; Havemann 1987; Sekine 2014; Urban 1999a; and Urban 1999b, were the studies that did describe the randomisation process. Of these, all adequately described sequence generation, but not allocation concealment.
No study described the use of blinding for any participants, investigators or any of the outcomes.
Seventeen of the 32 studies (53.13%) addressed the issue of incomplete survival data. The same seventeen studies also addressed the issue of incomplete tumour response data ‐ the only exception was Sundstrom 2002 which did not report tumour response data as an outcome. Fourteen of the 32 studies (43.75%) addressed the issue of incomplete toxicity data. No study addressed the issue of incomplete quality of life data.
Fourteen of the 32 studies (43.75%) were judged to be free of selective reporting. Creech 1982 was given a rating of 'high risk' for this criterion because it did not report survival data, an outcome that would be expected from studies in this area. The remaining 17 studies were given a rating of 'Unclear'.
All studies were deemed to be free from other bias, except Souhami 1997. This is due to the skewed treatment protocol used in the study ‐ the non‐platinum treatment arm consisting of a single oral agent compared with the platinum treatment arm consisting of multiple intravenous agents.
It was noted that one study, Sekine 2014, had to be terminated early due to treatment‐related toxicity in the non‐platinum arm.
Effects of interventions
Survival
Survival at six months
Thirty studies involving 5755 participants were included in the six‐month survival analysis. The included studies that did not present survival data were Creech 1982 and Baka 2008. Of the participants, 2874 of 5755 received a platinum‐based chemotherapy regimen and the remaining 2881 received a non‐platinum‐based chemotherapy regimen. At six months, 4066 participants were alive: 2083 from the platinum‐based groups and 1983 from the non‐platinum‐based treatment groups. There was no statistically significant difference between interventions (risk ratio (RR) 1.04, 95% confidence interval (CI) 1.00 to 1.09). There was no substantial heterogeneity present in the data (I2 = 44%).
Subgroup: 'undifferentiated'
Ten studies reported data from six‐month survival comparisons, but did not differentiate between limited and extensive disease. This included 1808 participants, 901 receiving a platinum‐based and 907 receiving a non‐platinum‐based regimen. At six months, 1231 participants were alive: 624 from the platinum‐based arm and 607 from the non‐platinum‐based arm. There was no statistically significant difference between interventions (RR 1.02, 95% CI 0.94 to 1.10). There was no substantial heterogeneity present in the data (I2 = 36%).
Subgroup: LD‐SCLC
Eight studies reported data from six‐month survival comparisons for participants with limited disease, involving 1044 participants. Of these 516 received a platinum‐based and 528 received a non‐platinum‐based regimen. At six months, 926 participants were alive: 459 from the platinum‐based arm and 467 from the non‐platinum‐based arm. There was no statistically significant difference between interventions (RR 1.00, 95% CI 0.94 to 1.07). There was no substantial heterogeneity present in the data (I2 = 45%).
Subgroup: ED‐SCLC
Eighteen studies reported data from six‐month survival comparisons for participants with extensive disease, involving 2903 participants. Of these, 1457 received a platinum‐based and 1446 received a non‐platinum‐based regimen. At six months, 1909 participants were alive: 1000 from the platinum‐based arm and 909 from the non‐platinum‐based arm. In contrast to the other subgroups above, there was a statistically significant effect favouring platinum‐based regimens (RR 1.09, 95% CI 1.02 to 1.17). There was no substantial heterogeneity present in the data (I2 = 37%).
Survival at 12 months
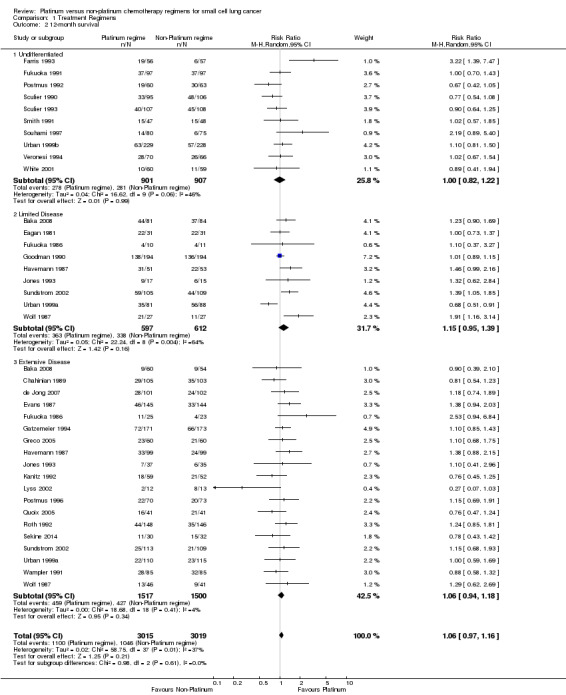
31 studies involving 6034 participants were included in the 12‐month survival analysis. The only included study that did not present survival data was Creech 1982. Of these participants, 3015 received a platinum‐based chemotherapy regimen and 3019 received a non‐platinum‐based chemotherapy regimen. At 12 months, 2146 participants were alive: 1100 from the platinum‐based groups and 1046 from the non‐platinum‐based treatment groups. Similar to six‐month survival, at 12 months there was no statistically significant difference between interventions (RR 1.06, 95% CI 0.97 to 1.16). There was no substantial heterogeneity present in the data (I2 = 37%).
Subgroup: 'undifferentiated'
Ten studies reported data from 12‐month survival comparisons, but did not differentiate between limited and extensive disease. This included 1808 participants, 901 receiving a platinum‐based and 907 receiving a non‐platinum‐based regimen. At 12 months, 559 participants were alive: 278 from the platinum‐based arm and 281 from the non‐platinum‐based arm. There was no statistically significant difference between interventions (RR 1.00, 95% CI 0.82 to 1.22). There was no substantial heterogeneity present in the data (I2 = 46%).
Subgroup: LD‐SCLC
Nine studies reported data from 12‐month survival comparisons for participants with limited disease, involving 1209 participants. Of these, 597 received a platinum‐based and 612 received a non‐platinum‐based regimen. At 12 months, 701 participants were alive: 363 from the platinum‐based arm and 338 from the non‐platinum‐based arm. There was no statistically significant difference between interventions (RR 1.15, 95% CI 0.95 to 1.39). There was substantial heterogeneity present in the data (I2 = 64%).
Subgroup: ED‐SCLC
Nineteen studies reported data from 12‐month survival comparisons for participants with extensive disease, involving 3017 participants. Of these, 1517 received a platinum‐based and 1500 received a non‐platinum‐based regimen. At 12 months, 886 participants were alive: 459 from the platinum‐based arms and 427 from the non‐platinum‐based arm. In contrast to the corresponding subgroup at six months, there was no statistically significant difference between interventions (RR 1.06, 95% CI 0.94 to 1.18). There was no substantial heterogeneity present in the data (I2 = 4%).
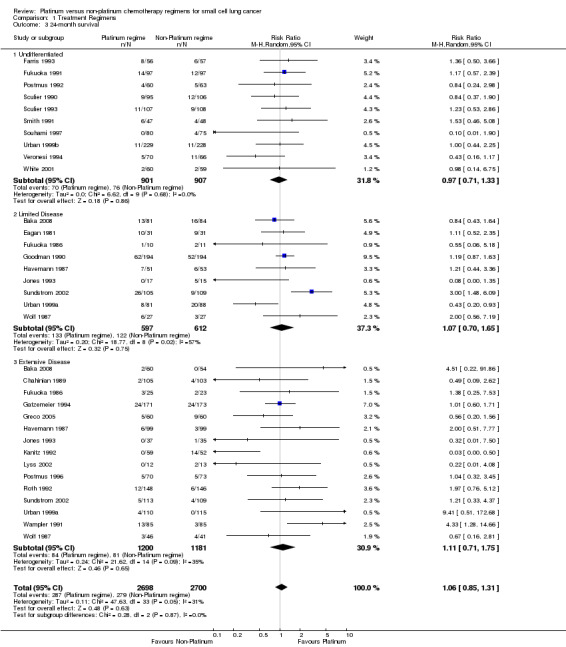
Survival at 24 months
Twenty seven studies involving 5398 participants were included in the 24‐month survival analysis. The four studies that were included in the 12‐month survival analyses, but that did not report 24‐month survival data were Evans 1987, Quoix 2005, de Jong 2007 and Sekine 2014. All were part of the ED‐SCLC subgroup. The remaining 27 studies were the same as those included for 12‐month survival. Of the participants, 2698 of 5398 received a platinum‐based chemotherapy regimen and the remaining 2700 received a non‐platinum‐based chemotherapy regimen. At 24 months, 566 participants were alive: 287 from the platinum‐based groups and 279 from the non‐platinum‐based treatment groups. As with 12‐month survival, there was no statistically significant difference between interventions (RR 1.06, 95% CI 0.85 to 1.31). There was no substantial heterogeneity present in the data (I2 = 31%).
Subgroup: 'undifferentiated'
Ten studies reported data from 24‐month survival comparisons, but did not differentiate between limited and extensive disease. This included 1808 participants, 901 receiving a platinum‐based and 907 receiving a non‐platinum‐based regimen. At 24 months, 146 participants were alive: 70 from the platinum‐based arm and 76 from the non‐platinum‐based arm. There was no statistically significant difference between interventions (RR 0.97, 95% CI 0.71 to 1.33). There was no heterogeneity (I2 = 0%).
Subgroup: LD‐SCLC
Nine studies reported data from 12‐month survival comparisons for participants with limited disease, involving 1209 participants. Of these, 597 received a platinum‐based and 612 received a non‐platinum‐based regimen. At 24 months, 255 participants were alive: 133 from the platinum‐based arm and 122 from the non‐platinum‐based arm. There was no statistically significant difference between interventions (RR 1.07, 95% CI 0.7 to 1.65). There was substantial heterogeneity present in the data (I2 = 57%).
Subgroup: ED‐SCLC
Fifteen studies reported data from 24‐month survival comparisons for participants with extensive disease, involving 2381 participants. Of these, 1200 received a platinum‐based and 1181 received a non‐platinum‐based regimen. At 24 months, 165 participants were alive: 84 from the platinum‐based arms and 81 from the non‐platinum‐based arm. There was no statistically significant difference between interventions (RR 1.11, 95% CI 0.71 to 1.75). There was substantial heterogeneity present in the data (I2 = 35%).
Survival by subgroup
The proportion of people alive at 6, 12 and 24 months from both treatment groups is presented in Table 3. Figure 3 illustrates the survival curves data based on the subgroup data.
3. Survival by subgroup.
| Subgroup | % Survival | ||
| 6 months | 12 months | 24 months | |
| Undifferentiated | 68.09% | 30.92% | 8.08% |
| LD‐SCLC | 88.70% | 57.98% | 21.09% |
| ED‐SCLC | 65.36% | 29.37% | 6.93% |
3.
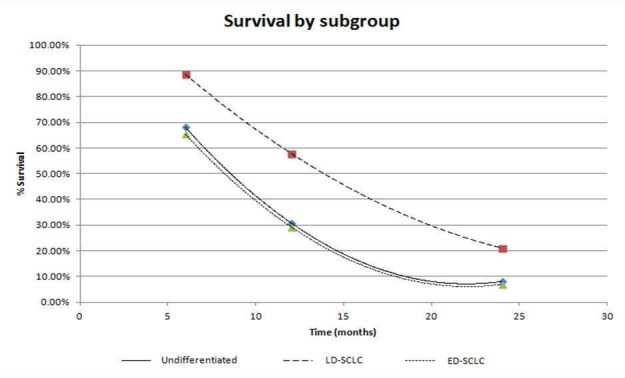
Survival by Subgroup
Tumour response
Overall response
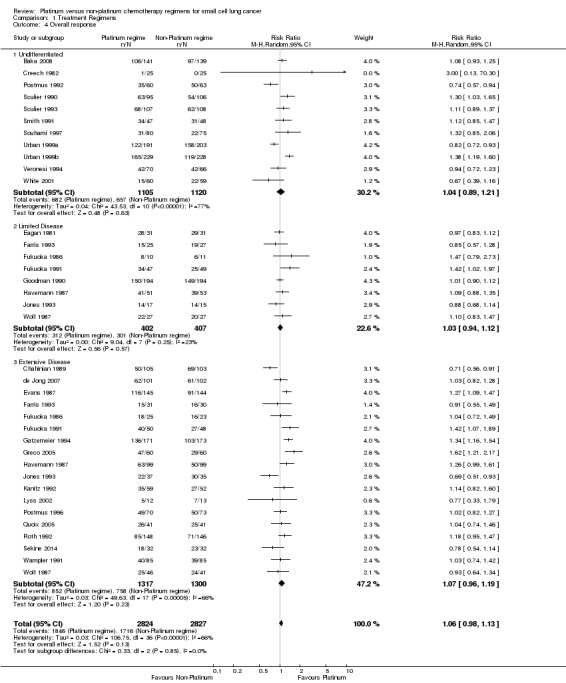
Thirty‐one studies involving 5651 participants were included in the overall response analysis. Of the 31 studies, 30 were the same as for 12‐month survival. The difference was due to the exclusion of Sundstrom 2002 (presented survival data but not response data) and the inclusion of Creech 1982 (presented response data but not survival data). Of the participants, 2824 of 5651 received a platinum‐based chemotherapy regimen and the remaining 2827 received a non‐platinum‐based chemotherapy regimen. The number of participants with a recorded overall response was 3562, consisting of 1846 participants from the platinum arm and 1716 participants from the non‐platinum arm. There was no statistically significant difference between interventions (RR 1.06, 95% CI 0.98 to 1.13). There was substantial heterogeneity present in the data (I2 = 66%).
Subgroup: 'undifferentiated'
Eleven studies reported data from overall response comparisons, but did not differentiate between limited and extensive disease. This included 2225 participants, 1105 receiving a platinum‐based and 1120 receiving a non‐platinum‐based regimen. A total of 1339 participants recorded an overall response, consisting of 682 participants from the platinum arm and 657 participants from the non‐platinum arm. There was no statistically significant difference between interventions (RR 1.04, 95% CI 0.89 to 1.21). There was substantial heterogeneity present in the data (I2 = 77%).
Subgroup: LD‐SCLC
Eight studies reported data from overall response comparisons for limited disease. This included 809 participants, 402 receiving a platinum‐based and 407 receiving a non‐platinum‐based regimen. A total of 613 participants recorded an overall response, consisting of 312 participants from the platinum arm and 301 participants from the non‐platinum arm. There was no statistically significant difference between interventions (RR 1.03, 95% CI 0.94 to 1.12). There was no substantial heterogeneity present in the data (I2 = 23%).
Subgroup: ED‐SCLC
Eighteen studies reported data from overall response comparisons for participants with ED‐SCLC. This subgroup consisted of 2617 participants, with 1317 receiving a platinum‐based chemotherapy regimen and 1300 receiving a non‐platinum‐based chemotherapy regimen. A total of 1610 participants recorded an overall response, consisting of 852 participants from platinum arms and 758 participants from non‐platinum arms. Similar to other subgroups above, there was no statistically significant difference between interventions (RR 1.07, 95% CI 0.96 to 1.19). There was substantial heterogeneity present in the data (I2 = 66%).
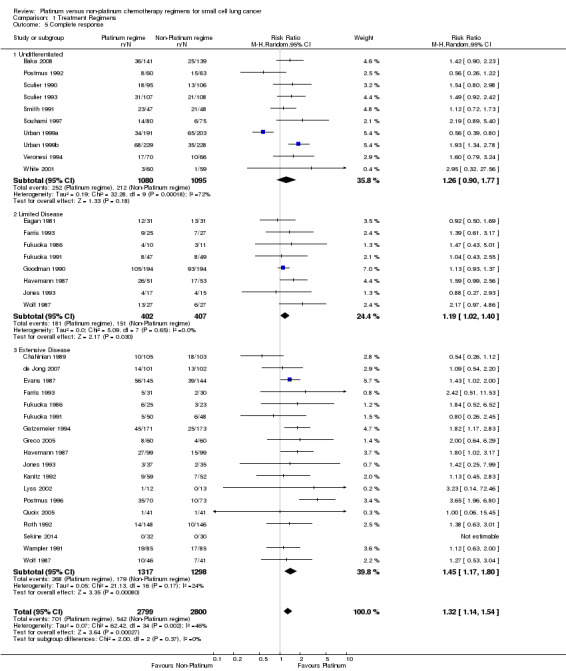
Complete response
Thirty studies involving 5599 participants were included in the complete response analysis. Creech 1982 (included in overall response) was excluded because it did not present complete response data. Of the 5599 participants, 2799 received a platinum‐based chemotherapy regimen and the remaining 2800 participants received a non‐platinum‐based chemotherapy regimen. Of the participants, 1243 recorded a complete response, consisting of 701 participants from the platinum arm and 542 participants from the non‐platinum arm. There was a statistically significant effect favouring platinum‐based chemotherapy regimens (RR 1.32, 95% CI 1.14 to 1.54). There was no substantial heterogeneity present in the data (I2 = 46%).
Subgroup: 'Undifferentiated'
Ten studies reported data from complete response comparisons, but did not differentiate between limited and extensive disease. This included 2175 participants, 1080 receiving a platinum‐based and 1095 receiving a non‐platinum‐based regimen. A total of 427 participants recorded a complete response, consisting of 252 participants from the platinum arm and 212 participants from the non‐platinum arm. In contrast to the other subgroups below, there was no statistically significant difference between interventions (RR 1.26, 95% CI 0.90 to 1.77). There was substantial heterogeneity present in the data (I2 = 72%).
Subgroup: LD‐SCLC
Eight studies reported data from complete response comparisons for limited disease. This included 809 participants, 402 receiving a platinum‐based and 407 receiving a non‐platinum‐based regimen. A total of 332 participants recorded a complete response, consisting of 181 participants from the platinum arm and 151 participants from the non‐platinum arm. There was a statistically significant effect favouring platinum‐based regimens (RR 1.19, 95% CI 1.02 to 1.40). There was no heterogeneity (I2 = 0%).
Subgroup: ED‐SCLC
Eighteen studies reported data from complete response comparisons for extensive disease. This subgroup consisted of 2615 participants, with 1317 receiving a platinum‐based chemotherapy regimen and 1298 receiving a non‐platinum‐based chemotherapy regimen. A total of 447 participants recorded a complete response, consisting of 268 participants from platinum arms and 179 participants from non‐platinum arms. There was a statistically significant effect, favouring platinum‐based chemotherapy regimens (RR 1.45, 95% CI 1.17 to 1.80). There was no substantial heterogeneity present in the data (I2 = 24%).
Toxicity
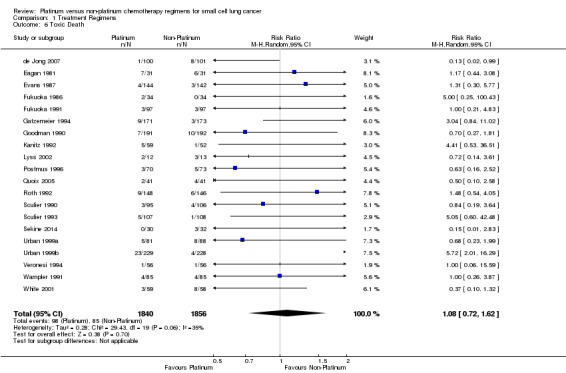
Toxic death
Twenty studies reported data regarding toxic death, involving 3696 (60.83%) participants. Overall, 1840 of these participants were from platinum‐based chemotherapy groups and 1856 from non‐platinum‐based chemotherapy groups. Of these 183 died from a toxicity‐related cause, 98 from platinum‐based groups and 85 from non‐platinum‐based groups. There was no statistically significant difference between interventions (RR 1.08, 95% CI 0.72 to 1.62). There was no substantial heterogeneity present in the data (I2 = 35%).
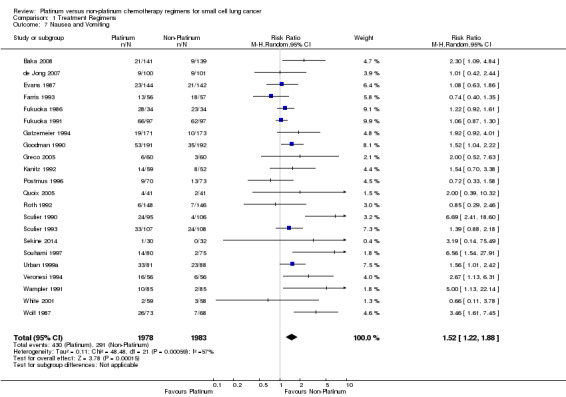
Nausea and vomiting
Twenty‐two studies reported toxicity data regarding nausea and vomiting, involving 3961 (65.20%) participants. Of those, 1978 were from platinum‐based chemotherapy groups and 1983 from non‐platinum‐based chemotherapy groups. Overall, 721 participants experienced nausea and vomiting toxicity, 430 from platinum‐based groups and 291 from non‐platinum‐based groups. There was a statistically significant difference between interventions, with higher rates of nausea and vomiting toxicity in platinum‐based chemotherapy regimens (RR 1.52, 95% CI 1.22 to 1.88). There was substantial heterogeneity present in the data (I2 = 57%).
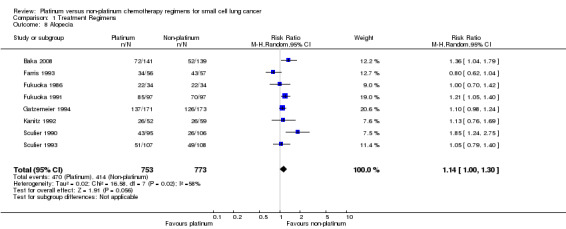
Alopecia
Eight studies reported toxicity data regarding alopecia, involving 1526 (25.12%) participants. Of those, 753 were from platinum‐based chemotherapy groups and 773 from non‐platinum‐based chemotherapy groups. Overall, 884 participants experienced alopecia toxicity, 470 from platinum‐based groups and 414 from non‐platinum‐based groups. There was no statistically significant difference between interventions (RR 1.14, 95% CI 1.00 to 1.30). There was substantial heterogeneity present in the data (I2 = 58%).
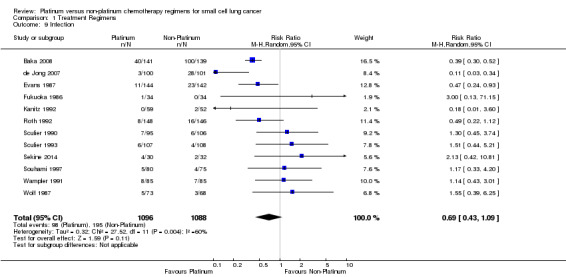
Infection
Twelve studies reported toxicity data regarding infection, involving 2184 (35.95%) participants. Of those, 1096 were from platinum‐based chemotherapy groups and 1088 from non‐platinum‐based chemotherapy groups. Overall, 293 participants experienced infections, 98 from platinum‐based groups and 195 from non‐platinum‐based groups. There was no statistically significant difference between interventions (RR 0.69 95% CI 0.43 to 1.09). There was substantial heterogeneity present in the data (I2 = 60%).
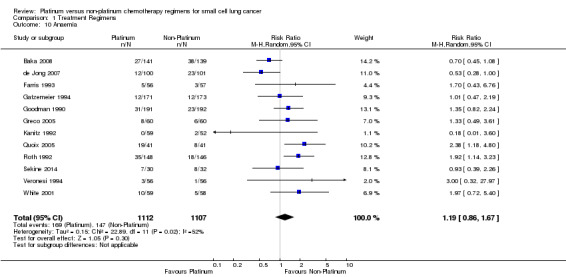
Anaemia
Twelve studies reported toxicity data regarding anaemia, involving 2219 (36.53%) participants. Of these, 1112 were from platinum‐based chemotherapy groups and 1107 from non‐platinum‐based chemotherapy groups. Overall, 316 participants experienced anaemia toxicity, 169 from platinum‐based groups and 147 from non‐platinum‐based groups. There was no statistically significant difference between interventions (RR 1.19, 95% CI 0.86 to 1.67). There was substantial heterogeneity present in the data (I2 = 52%).
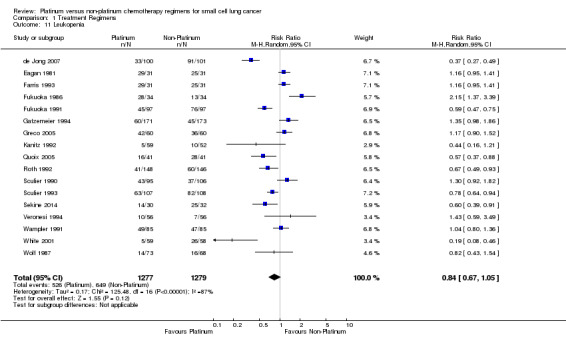
Leukopenia
Seventeen studies reported toxicity data regarding leukopenia, involving 2556 (42.07%) participants. Of these, 1277 were from platinum‐based chemotherapy groups and 1279 from non‐platinum‐based chemotherapy groups. Overall, 1175 participants experienced leukopenia toxicity, 526 from platinum‐based groups and 649 from non‐platinum‐based groups. There was no statistically significant difference between interventions (RR 0.84, 95% CI 0.67 to 1.05). There was substantial heterogeneity present in the data (I2 = 87%)
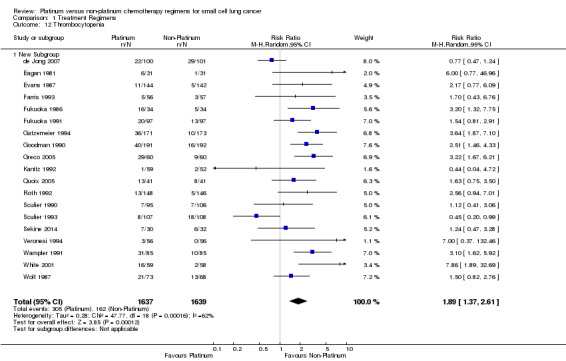
Thrombocytopenia
Nineteen studies reported toxicity data regarding thrombocytopenia, involving 3276 (53.93%) participants. Of those, 1637 were from platinum‐based chemotherapy groups and 1639 from non‐platinum‐based chemotherapy groups. Overall, 467 participants experienced thrombocytopenia toxicity, 305 from platinum‐based groups and 162 from non‐platinum‐based groups. There was a statistically significant difference between interventions, with higher rates of thrombocytopenia toxicity in platinum‐based chemotherapy regimens (RR 1.89, 95% CI 1.37 to 2.61). There was substantial heterogeneity present in the data (I2 = 62%).
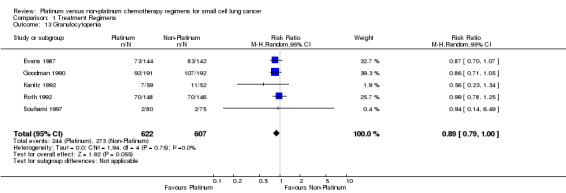
Granulocytopenia
Five studies reported toxicity data regarding granulocytopenia, involving 1229 (22.22%) participants. Of those, 622 were from platinum‐based chemotherapy groups and 607 from non‐platinum‐based chemotherapy groups. Overall, 517 participants experienced granulocytopenia toxicity, 244 from platinum‐based groups and 273 from non‐platinum‐based groups. There was no statistically significant difference between interventions (RR 0.89, 95% CI 0.79 to 1.00). There was no heterogeneity (I2 = 0%).
Quality of life
Data about quality of life (QOL) were available from four studies. It was not possible to combine these in a meta‐analysis because none of the studies reported enough data to calculate standardised mean differences. Results from these studies are discussed individually.
Souhami 1997 measured QOL via two scales: the Rotterdam Symptom Checklist and the daily diary card. The Rotterdam Symptom Checklist measured physical symptoms, lung cancer symptoms, treatment symptoms, physical activity, psychological well‐being and general QOL. It was completed by participants at each treatment cycle and at the first two follow‐up visits. The study reported that there was no significant difference in physical symptoms, psychological well‐being or physical activity, between interventions. Treatment‐related symptoms were significantly worse, but lung cancer symptoms and general QOL were significantly better in the platinum treatment arm. Souhami 1997 was a unique study in that it was the only study to have a non‐intravenous form of delivery: its non‐platinum treatment arm (oral etoposide).
A daily diary card was used to measure acute chemotherapy‐related symptoms. It measured nausea, vomiting, appetite, pain, sleep, mood, general well‐being and physical activity on a daily basis. The findings from this study showed that there was no significant difference in vomiting or physical activity between the two treatment arms. Nausea was found to be worse in the platinum‐based treatment arm. But in all other categories, the non‐platinum‐based treatment arms were significantly worse (Souhami 1997).
Sundstrom 2002 measured QOL via the European Organisation for the Research and Treatment of Cancer (EORTC) quality of life questionnaire C30 (QLQ‐C30). The QLQ‐C30 questionnaire reports five functional scales (physical, role, emotional, cognitive and social functioning), three symptom scales (fatigue, nausea/vomiting and pain), a global health and overall QOL scale and five single item scales (dyspnoea, appetite loss, sleep disturbance, constipation and diarrhoea, and the financial impact of disease and treatment). Results from the questionnaire showed no statistically significant difference between the platinum group and the non‐platinum group for most of the scales.
Quoix 2005 measured QOL using the functional assessment of cancer therapy‐lung (FACT‐L) QOL questionnaire and a separate symptom questionnaire. The questionnaires were completed by participants at baseline and prior to each course of treatment.
The FACT‐L questionnaire assessed physical well‐being, social/family well‐being, emotional well‐being and additional concerns on a five‐point scale. These were used to determine a FACT‐L score, and the scores for physical and functional well‐being were summed to give a trial outcome index (TOI) score. Both the platinum and non‐platinum arm had improvements in FACT‐L and TOI scores compared to baseline scores following six cycles of treatments. However there was no significant difference in FACT‐L or TOI scores between treatment arms (Quoix 2005).
The symptom questionnaire employed by Quoix 2005 assessed shortness of breath, cough, chest pain, haemoptysis, anorexia, insomnia, hoarseness, fatigue and interference with daily activity. The results found both interventions improved all symptoms except haemoptysis, in more than 20% of participants when compared to baseline. However, there was no significant difference between treatments (Quoix 2005).
Sekine 2014 used two scales to assess QOL ‐ lung cancer subscale (LCS) of FACT‐L questionnaire and Euro‐QOL 5‐dimension (EQ‐5D) utility index. Assessments were performed at 3 weeks, 3 months, 6 months and 12 months after the commencement of chemotherapy. The LCS measured a number of symptoms including breathlessness, weight loss, cognitive function, appetite and chest discomfort (Cella 2002). For both the platinum group and the non‐platinum group, there were no significant differences in LCS scores at all of the time points (P values: 0.171 at 3 weeks; 0.08 at 3 months; 0.112 at 6 months; 0.371 at 12 months). The EQ‐5D measured five socially relevant domains ‐ mobility, self‐care, usual activities, pain/discomfort and anxiety/depression (Gusi 2010). For both the platinum group and the non‐platinum group, there were no significant differences in LCS scores at all of the time points (P values: 0.171 at 3 weeks; 0.08 at 3 months; 0.112 at 6 months; 0.371 at 12 months). For both the platinum group and the non‐platinum group, there were no significant differences in EQ‐5D utility index scores at all of the time points (P values: 0.171 at 3 weeks; 0.08 at 3 months; 0.112 at 6 months; 0.371 at 12 months).
Sensitivity analysis
Quality of studies
The effect of the quality of studies was examined by including all studies that were deemed to be of high quality using the GRADE assessment approach as discussed in the Methods. As such, the included studies for the sensitivity analysis of quality were: Baka 2008; de Jong 2007; Evans 1987; Fukuoka 1991; Havemann 1987; Kanitz 1992; Lyss 2002; Postmus 1996; Roth 1992; Sculier 1990; Sculier 1993; Smith 1991; Sundstrom 2002; Urban 1999a; Veronesi 1994 and White 2001, . The results are presented in Table 4.
4. Sensitivity analysis ‐ quality.
| Outcome | All studies | Higher quality studies |
| 6‐month survival | NSSD | NSSD |
| 12‐month survival | NSSD | NSSD |
| 24‐month survival | NSSD | NSSD |
| Overall response | NSSD | P |
| Complete response | P | P |
| Toxic death | NSSD | NSSD |
| Nausea and Vomiting | NP | NP |
| Alopecia | NSSD | NP |
| Infection | NSSD | P |
| Anaemia | NSSD | NSSD |
| Leukopenia | NSSD | P |
| Thrombocytopenia | NP | NSSD |
| Granulocytopenia | NSSD | NSSD |
NSSD ‐ No statistically significant difference between treatment groups.
P ‐ Statistically significant effect favouring platinum‐based regimens.
NP ‐ Statistically significant effect favouring non‐platinum‐based regimens.
It can be seen from this table that there were five differences in outcomes between the sensitivity analysis of quality and the overall analysis. The outcomes affected were overall response, alopecia, infection, leukopenia and thrombocytopenia toxicities. For overall response and infection, there was no significant difference between the two treatment groups, however, when only the higher quality studies were included there was a significant difference favouring the platinum group. There was no significant difference between the two treatment groups in terms of alopecia toxicity. However, in the sensitivity analysis for quality, the platinum‐based treatment group had a significantly higher level of alopecia toxicity. The reverse was true for thrombocytopenia toxicity. There was a significantly higher rate of thrombocytopenia toxicity in the platinum‐based treatment group in the overall analysis, but this difference was not statistically significant in the sensitivity analysis for quality. Finally, for leukopenia toxicity, there was no significant difference between the treatment groups in the overall analysis. However, in the sensitivity analysis for quality, the rate of leukopenia toxicity was significantly higher in the non‐platinum‐based treatment group.
Full article versus abstract publication
Full articles for all studies were available, hence this sensitivity analysis was not conducted.
Use of radiotherapy
The effect of radiotherapy was explored by including all studies that used radiotherapy. Hence, the following studies were included in the analysis: Baka 2008; Chahinian 1989; Eagan 1981; Evans 1987; Farris 1993; Fukuoka 1986; Fukuoka 1991; Goodman 1990; Greco 2005; Havemann 1987; Jones 1993; Lyss 2002; Postmus 1992; Roth 1992; Sculier 1990; Sculier 1993; Smith 1991; Sundstrom 2002; Urban 1999a; Urban 1999b; Veronesi 1994; White 2001 and Wolf 1987. The results are presented in Table 5.
5. Sensitivity analysis ‐ radiotherapy use.
| Outcome | All studies | Only studies using radiotherapy |
| 6‐month survival | NSSD | NSSD |
| 12‐month survival | NSSD | NSSD |
| 24‐month survival | NSSD | NSSD |
| Overall response | NSSD | NSSD |
| Complete response | P | P |
| Toxic death | NSSD | NSSD |
| Nausea and Vomiting | NP | NP |
| Alopecia | NSSD | NSSD |
| Infection | NSSD | NSSD |
| Anaemia | NSSD | NSSD |
| Leukopenia | NSSD | NSSD |
| Thrombocytopenia | NP | NP |
| Granulocytopenia | NSSD | NSSD |
NSSD ‐ No statistically significant difference between treatment groups.
P ‐ Statistically significant effect favouring platinum‐based regimens.
NP ‐ Statistically significant effect favouring non‐platinum‐based regimens.
It can be seen from this table that there is no significant difference between the outcomes of all studies compared with the outcomes of only studies that used radiotherapy.
Heterogeneity analysis
It can be seen from the results that there was substantial heterogeneity associated with a number of outcomes, including overall response, nausea and vomiting, alopecia, leukopenia and thrombocytopenia toxicity. For all of these outcomes, possible reasons for the heterogeneity were explored. No specific causes of heterogeneity were found for any of the outcomes, except for thrombocytopenia toxicity (discussed below). Possible reasons for heterogeneity are mentioned in the Tumour Response and Toxicity sections in the Discussion. It is important to note that the results of all outcomes with substantial heterogeneity must be treated with caution.
Discussion
This systematic review included 32 trials and 6075 patients, which provides adequate data to undertake meaningful meta‐analyses.
Cisplatin and carboplatin are the only platinum‐based agents used in SCLC, with cisplatin more widely used than carboplatin. Of the non‐platinum agents, etoposide (E) and cyclophosphamide (C) are the most commonly used, followed by doxorubicin (D), adriamycin (A) and vincristine (V) usually in the triplet combination of either CEV, CDE or CAV (Abeloff 2004).
Co‐intervention with radiotherapy occurred in approximately 72% of studies indicating that this plays a major role in the treatment of SCLC. It should be noted that the sensitivity analysis to exclude any confounding effects of radiotherapy did not find any statistically significant differences in any of the outcomes, including toxicity, from the overall analysis.
Survival
No statistically significant difference between platinum‐based chemotherapy regimens and non‐platinum‐based chemotherapy regimens was found for survival at 6 months, 12 months or 24 months.
Subgroup analyses demonstrated that survival for the 'undifferentiated' subgroup was similar to that of extensive disease. This is explained by the high proportion of extensive disease‐stage patients in the studies in the 'undifferentiated' group. For example, Urban 1999a had 360 extensive disease‐stage patients and 97 limited disease‐stage patients. The high proportion of extensive disease‐stage participants reflects the fact that SCLC is an aggressive disease that metastasises early and usually presents with extensive disease (Abeloff 2004).
Despite survival being better in limited disease, the slopes of the survival curves (in Figure 3) for all three subgroups are relatively similar. This implies that the effect of chemotherapy does not differ depending on the stage of the disease and survival can be attributed to the disease extent at diagnosis (ACN 2004).
The outcomes show that even with treatment, long‐term survival with SCLC is uncommon; the highest proportions of survivors at 24 months are recorded in the limited disease group (21.09%).
Tumour response
There was no statistically significant difference between platinum‐based chemotherapy regimens and non‐platinum‐based chemotherapy regimens in terms of overall response when the results of all studies were considered. However, the sensitivity analysis revealed that, when only studies of higher quality were included, there was a statistically significant difference in response rates favouring platinum‐based chemotherapy regimens. In addition, platinum‐based chemotherapy regimens did demonstrate a significantly higher complete response than non‐platinum‐based chemotherapy regimens. However this does not translate into improved survival in the short, intermediate or long‐term. These findings are consistent with the knowledge that SCLC is initially responsive to chemotherapy, but relapse is common and further response to chemotherapy is poor (Abeloff 2004; Peckham 1995). This is thought to be due to resistant subpopulations of tumour cells developing over time (Evans 1986).
It should be noted that there was substantial heterogeneity in the overall response analysis. A definite reason for this could not be identified. However, a possible explanation for this is the variation in staging and response assessment methods used. Since this review involved some studies that were conducted decades apart, earlier studies may have used less sensitive staging and response assessment methods than later studies. Similarly, some study centres may not have had the sophisticated resources available for staging and response assessment as other study centres. So while the definition of overall response has remained constant, the assessment methods used may have varied. As such, this variation may have contributed to the heterogeneity in the overall response analysis.
Toxicity
"Given that there was no significant difference in survival between the two types of regimen, the balance between the benefits and risks of treatment is more important. However the adverse events were not well‐reported. This systematic review has shown that platinum‐based regimens result in higher rates of nausea and vomiting, and thrombocytopenia than non‐platinum‐based regimens. There was no statistically significant difference between the two groups with respect to toxic death, alopecia, infection, anaemia, leukopenia and granulocytopenia. Therefore platinum‐based chemotherapy regimens did not result in statistically significant lower rates of toxicity in any of the toxicity analyses.
It should also be noted that there was substantial heterogeneity in many of the toxicity analyses. The cause of this heterogeneity was difficult to identify. This is surprising considering the fact that all studies adhered to the grade 3/4 WHO definitions for each of the toxicity outcomes. One possible reason for the heterogeneity may be differences in the therapies used to prevent or treat toxicities, including antiemetics, antibiotics, cytokines, blood and platelet transfusions and granulocyte‐colony stimulating factor (G‐CSF). In particular, it should be noted that 5‐HT3 antagonists, which are powerful anti‐emetic agents, were only discovered in 1988 and did not come into widespread use until a decade later (Stubblefield 2009) – after the publication date of the majority of studies in this review. This could have resulted in higher grade 3/4 nausea and vomiting toxicity with platinum‐based chemotherapy regimens, than would be seen today with the availability of a wider range of more effective anti‐emetic agents, including 5‐HT3 antagonists.
Quality of life
A major limitation of this systematic review was the insufficient data to conduct a meta‐analysis on quality of life (QOL). A qualitative description of the results from four studies that reported QOL did not indicate substantial differences between platinum‐ and non‐platinum‐based treatment regimens.
While it has been shown by a number of studies that tumour progression is associated with a poorer QOL (Gralla 2004), it is not reasonable to infer that a treatment that produces a tumour response will necessarily improve QOL. While platinum‐based regimens, with the better tumour response may improve QOL through tumour control, this is offset by the higher incidence of some toxicities. Therefore, the only reliable method of determining which treatment regimen is associated with a better QOL is for more RCTs to include QOL in their outcome assessment. This systematic review highlights the current lack of QOL data and the need for future studies to incorporate QOL as an outcome measure.
Limitations
There are a number of limitations associated with this systematic review. Firstly, a systematic review should ideally be conducted using individual patient data (IPD). However, this is rarely done in practice, as it is not considered practical to do so in a large number of cases, largely because IPD from studies are not always easily obtainable (Higgins 2011c. For this systematic review, IPD were not available for the studies, despite written requests to the authors. While this is understandable, considering a significant number of the included studies were conducted more than a decade earlier, it does limit the quality and reliability of this systematic review.
Secondly, the effect of including studies spanning a large time period is not known, and some chemotherapy regimens used in earlier studies may now be considered out of date. This is particularly the case with some non‐platinum‐based regimens. Hence, it should be kept in mind that the results from these studies may have affected the overall results. Thirdly, the effect of co‐intervention with radiotherapy in limited disease is unclear as none of the studies in this subgroup included radiotherapy and thus all were excluded in the sensitivity analysis for this variable.
Finally, although survival graphs were given by a majority of studies, the associated log hazard ratio (HR) and summary statistics were rarely provided. As a result, the survival data from studies could not be combined to obtain an overall HR. Instead, data on survival at pre‐defined time points (6 months, 12 months and 24 months) were used. Because of the lack of HR and summary statistic data, calculating the survival at these pre‐defined time points was considered a reasonable procedure to determine short, intermediate and long‐term survival. In addition, this method of using predefined time intervals has been used in other published systematic reviews exploring survival in SCLC (Pujol 2000).
Authors' conclusions
Implications for practice.
This systematic review has shown that, despite the fact that the lack of individual patient data precluded calculation of a hazard ratio, platinum‐based chemotherapy regimens do not offer significant survival benefit over non‐platinum‐based regimens. There is also no significant difference in terms of overall tumour response, although platinum‐based regimens do have a significantly higher rate of complete response. Platinum‐based regimens are associated with greater nausea and vomiting, and thrombocytopenia toxicity. The effect on quality of life could not be adequately assessed. These data suggest non‐platinum chemotherapy regimens have a more advantageous risk‐benefit profile.
Implications for research.
This systematic review has highlighted the lack of quality of life data in trials involving chemotherapy treatment for small cell lung cancer. With poor long‐term survival despite treatment, the issue of the quality of the survival period takes on even more significance. Future trials in this area should focus of quality of life assessments, given there is no long‐term survival benefit.
What's new
| Date | Event | Description |
|---|---|---|
| 13 June 2015 | New search has been performed | New author for update ‐ Saion Chatterjee |
| 18 May 2015 | New search has been performed | A new search was run in August 2014 |
| 18 May 2015 | New citation required but conclusions have not changed | 3 new studies were identified (Baka 2008; de Jong 2007; Sekine 2014). A meta‐analysis was carried out. Conclusions not changed |
History
Protocol first published: Issue 4, 2007 Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 12 November 2008 | Amended | Contact details updated |
| 8 May 2008 | Amended | Converted to new review format. |
Acknowledgements
Thanks to Matthew Beech for his assistance with identifying studies for inclusion.
Thanks to Wayne Chou and Melanie Sung for their assistance with data extraction.
Thanks to the Australian Lung Foundation for their support of this review.
Appendices
Appendix 1. Search strategy used in original review
MEDLINE (accessed through PubMed)
#1 (carcinoma, small cell AND lung neoplasms[MeSH]) OR SCLC[ti] OR ((lung[ti] OR lungs[ti] OR pulmonary[ti] OR bronchus[ti] OR bronchogenic[ti] OR bronchial[ti] OR bronchoalveolar[ti] OR alveolar[ti]) AND (small‐cell[ti] OR oat‐cell[ti]) AND (cancer*[ti] OR carcinoma*[ti] OR malignan*[ti] OR tumor*[ti] OR tumour*[ti] OR neoplasm*[ti]))
#2 "Platinum Compounds"[MeSH] OR "Cisplatin"[MeSH] OR platinum[tiab] OR cisplatin[tiab] OR Platinol[ti] OR carboplatin[tiab] OR Paraplatin[ti] OR oxaliplatin[tiab] OR Eloxatin*[ti]
#3 combined searches #1 AND #2 above
EMBASE (accessed through Ovid)
#1 ('small cell carcinoma' AND 'lung tumor') OR SCLC:ti OR ((lung:ti OR lungs:ti OR pulmonary:ti OR bronchus:ti OR brochogenic:ti OR bronchial:ti OR bronchoalveolar:ti OR alveolar:ti) AND (small‐cell:ti OR oat‐cell:ti) AND (cancer*:ti OR carcinoma*:ti OR adenocarcinoma*:ti OR malignan*:ti OR tumor*:ti OR tumour*:ti OR neoplasm*:ti))
#2 'platinum derivative' OR 'cisplatin' OR platinum:ti,ab OR cisplatin:ti,ab OR Platinol:ti OR carboplatin:ti,ab OR Paraplatin:ti OR oxaliplatin:ti,ab OR Eloxatin*:ti
#3 combined searches #1 AND #2 above
CINAHL (accessed through EBSCO)
#1 (carcinoma, small cell AND lung neoplasms) OR SCLC OR ((lung OR lungs OR pulmonary OR bronchus OR brochogenic OR bronchial OR broncho alveolar OR alveolar) AND (small‐cell OR oat‐cell) AND (cancer* OR carcinoma* OR adenocarcinoma* OR malignan* OR tumor* OR tumour* OR neoplasm*))
#2 "Platinum Compounds" OR "Cisplatin" OR platinum OR cisplatin OR Platinol OR carboplatin OR Paraplatin OR oxaliplatin OR Eloxatin*
#3 combined searches #1 AND #2 above
The Cochrane Central Register of Controlled Trials (CENTRAL)
#1 (carcinoma, small cell AND lung neoplasms[MeSH]) OR SCLC[ti] OR ((lung[ti] OR lungs[ti] OR pulmonary[ti] OR bronchus[ti] OR brochogenic[ti] OR bronchial[ti] OR bronchoalveolar[ti] OR alveolar[ti]) AND (small‐cell[ti] OR oat‐cell[ti]) AND (cancer*[ti] OR carcinoma*[ti] OR adenocarcinoma*[ti] OR malignan*[ti] OR tumor*[ti] OR tumour*[ti] OR neoplasm*[ti]))
#2 "Platinum Compounds"[MeSH] OR "Cisplatin"[MeSH] OR platinum[tiab] OR cisplatin[tiab] OR Platinol[ti] OR carboplatin[tiab] OR Paraplatin[ti] OR oxaliplatin[tiab] OR Eloxatin*[ti]
#3 combined searches #1 AND #2 above
Appendix 2. Search strategy used in current update
MEDLINE (accessed through PubMed; 01.08.2014)
#1 "carcinoma, small cell"[MeSH] AND lung neoplasms[MeSH]
#2 SCLC[tiab]
#3 lung[ti] OR lungs[ti] OR pulmonary[ti] OR bronchus[ti] OR bronchogenic[ti] OR bronchial[ti] OR bronchoalveolar[ti] OR alveolar[ti]
#4 (small‐cell[ti] OR oat‐cell[ti]) AND (cancer*[ti] OR carcinoma*[ti] OR malignan*[ti] OR tumor*[ti] OR tumour*[ti] OR neoplasm*[ti])
#5 #3 AND #4
#6 #1 OR #2 OR #5
#7 "Platinum Compounds"[MeSH]
#8 "Cisplatin"[MeSH]
#9 platinum[tiab]
#10 cisplatin[tiab]
#11 platinol[ti]
#12 carboplatin[tiab]
#13 paraplatin[ti]
#14 oxaliplatin[tiab]
#15 eloxatin*[ti]
#16 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
#17 #6 AND #16
#18 (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT (animals[mh] NOT (humans[mh] AND animals[mh]))
#19 #17 AND #18
#20 #17 AND #18 AND ("2007/01/01"[PDAT] : "3000/12/31"[PDAT])
EMBASE (accessed through Ovid < 1980 to 2014 Week 30>; 01.08.2014)
1 exp lung small cell cancer/ (
2 SCLC.ti.
3 lung*.ti.
4 pulmonary.ti.
5 bronch*.ti.
6 bronchoalveolar.ti.
7 alveolar.ti.
8 2 or 3 or 4 or 5 or 6 or 7
9 (small cell or oat cell).ti.
10 (cancer* or carcinoma* or adenocarcinoma* or malignan* or tumor* or tumour* or neoplasm*).ti.
11 9 and 10
12 8 and 11
13 1 or 2 or 12
14 exp platinum derivative/
15 exp cisplatin/
16 (platinum or cisplatin or Platinol or carboplatin or Paraplatin or oxaliplatin or Eloxatin*).ti,ab.
17 14 or 15 or 16
18 13 and 17
19 (double‐blind: or placebo:).mp. or blind:.tw.
20 18 and 19
21 limit 20 to yr="2007 ‐Current"
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library: Issue 7 of 12, July 2014; 01.08.2014)
#1 MeSH descriptor: [Small Cell Lung Carcinoma] explode all trees
#2 SCLC:ti,ab
#3 (lung or lungs or pulmonary or bronchus or bronchogenic or bronchial or bronchoalveolar or alveolar):ti
#4 (small‐cell or oat‐cell):ti
#5 (cancer* or carcinoma* or malignan* or tumor* or tumour* or neoplasm*):ti
#6 #4 and #5
#7 #3 and #6
#8 #1 or #2 or #7
#9 MeSH descriptor: [Platinum Compounds] explode all trees
#10 MeSH descriptor: [Cisplatin] explode all trees
#11 platinum:ti,ab
#12 cisplatin:ti,ab
#13 Platinol:ti
#14 carboplatin:ti,ab
#15 paraplatin:ti
#16 oxaliplatin:ti,ab
#17 eloxatin*:ti
#18 #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17
#19 #8 and #18
#20 #8 and #18 Publication Year from 2012 to 2014 267
Data and analyses
Comparison 1. Treatment Regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 6‐month survival | 30 | 5755 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [1.00, 1.09] |
| 1.1 Undifferentiated | 10 | 1808 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.94, 1.10] |
| 1.2 Limited Disease | 8 | 1044 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.07] |
| 1.3 Extensive Disease | 18 | 2903 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [1.02, 1.17] |
| 2 12‐month survival | 31 | 6034 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.97, 1.16] |
| 2.1 Undifferentiated | 10 | 1808 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.82, 1.22] |
| 2.2 Limited Disease | 9 | 1209 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.95, 1.39] |
| 2.3 Extensive Disease | 19 | 3017 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.94, 1.18] |
| 3 24‐month survival | 27 | 5398 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.85, 1.31] |
| 3.1 Undifferentiated | 10 | 1808 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.33] |
| 3.2 Limited Disease | 9 | 1209 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.70, 1.65] |
| 3.3 Extensive Disease | 15 | 2381 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.71, 1.75] |
| 4 Overall response | 31 | 5651 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.98, 1.13] |
| 4.1 Undifferentiated | 11 | 2225 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.89, 1.21] |
| 4.2 Limited Disease | 8 | 809 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.94, 1.12] |
| 4.3 Extensive Disease | 18 | 2617 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.96, 1.19] |
| 5 Complete response | 30 | 5599 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.14, 1.54] |
| 5.1 Undifferentiated | 10 | 2175 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.90, 1.77] |
| 5.2 Limited Disease | 8 | 809 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.02, 1.40] |
| 5.3 Extensive Disease | 18 | 2615 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.17, 1.80] |
| 6 Toxic Death | 20 | 3696 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.72, 1.62] |
| 7 Nausea and Vomiting | 22 | 3961 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [1.22, 1.88] |
| 8 Alopecia | 8 | 1526 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.00, 1.30] |
| 9 Infection | 12 | 2184 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.43, 1.09] |
| 10 Anaemia | 12 | 2219 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.86, 1.67] |
| 11 Leukopenia | 17 | 2556 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.67, 1.05] |
| 12 Thrombocytopenia | 19 | 3276 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [1.37, 2.61] |
| 12.1 New Subgroup | 19 | 3276 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [1.37, 2.61] |
| 13 Granulocytopenia | 5 | 1229 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 1.00] |
1.1. Analysis.
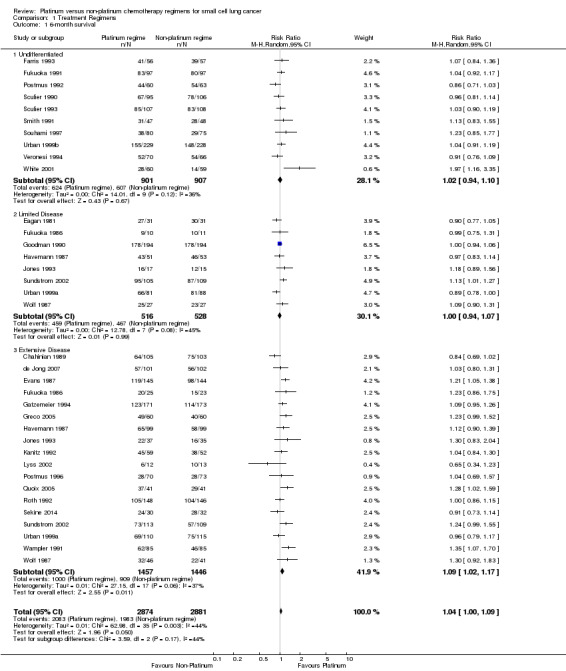
Comparison 1 Treatment Regimens, Outcome 1 6‐month survival.
1.2. Analysis.
Comparison 1 Treatment Regimens, Outcome 2 12‐month survival.
1.3. Analysis.
Comparison 1 Treatment Regimens, Outcome 3 24‐month survival.
1.4. Analysis.
Comparison 1 Treatment Regimens, Outcome 4 Overall response.
1.5. Analysis.
Comparison 1 Treatment Regimens, Outcome 5 Complete response.
1.6. Analysis.
Comparison 1 Treatment Regimens, Outcome 6 Toxic Death.
1.7. Analysis.
Comparison 1 Treatment Regimens, Outcome 7 Nausea and Vomiting.
1.8. Analysis.
Comparison 1 Treatment Regimens, Outcome 8 Alopecia.
1.9. Analysis.
Comparison 1 Treatment Regimens, Outcome 9 Infection.
1.10. Analysis.
Comparison 1 Treatment Regimens, Outcome 10 Anaemia.
1.11. Analysis.
Comparison 1 Treatment Regimens, Outcome 11 Leukopenia.
1.12. Analysis.
Comparison 1 Treatment Regimens, Outcome 12 Thrombocytopenia.
1.13. Analysis.
Comparison 1 Treatment Regimens, Outcome 13 Granulocytopenia.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baka 2008.
| Methods |
STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: United Kingdom, 2 DURATION OF STUDY: April 1999 – February 2005 CONCEALMENT OF ALLOCATION: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: No METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Appropriate METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: N/A DESCRIPTION OF WITHDRAWALS/DROPOUTS: Adequate GRADE ASSESSMENT QUALITY RATING: High TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT COMPLIANCE: CONFOUNDERS: |
|
| Participants |
INCLUSION CRITERIA: Previously untreated patients with histologically or cytologically proven SCLC and a maximum of two adverse prognostic factors (extensive stage disease, PS >2, raised LDH, serum sodium <130mmol/l:, Alk Phos >1.25 ULN) were eligible. Other eligibility criteria included age >18 years, normal blood count, serum bilirubin <35 mmol/l and creatinine clearance >50 ml/min. In patients with impaired renal function, that is, creatinine clearance >30 ml/min but <50 ml/min and/or patients with significant cardiovascular disease, carboplatin could be substituted for cisplatin in the first or subsequent cycles. EXCLUSION CRITERIA: A CT brain scan was not routinely performed, but patients with known brain metastases were not eligible. N SCREENED: Unknown N RANDOMISED: 280 (ACE – 139; PE – 141) N COMPLETED: 143 (ACE – 52; PE – 91) ASSESS STAGE: Yes (N LIMITED): 165 (ACE – 84; PE 81) (N EXTENSIVE): 114 (ACE – 54; PE – 60) M: 142 (ACE – 67; PE – 75) F: 138 (ACE – 72; PE – 66) MEAN AGE: Median – ACE – 66 (38‐81); PE – 65 (39‐89) BASELINE DETAILS: Physical examination, Histological diagnosis, CT scan of thorax and abdomen, blood tests Tumour stage was assessed with CT scan of thorax and abdomen. Disease measurement was performed within 4 weeks before the start of treatment. |
|
| Interventions |
TYPE: Chemotherapy REGIMENS, DOSE, DELIVERY: ACE ‐ (doxorubicin 50 mg/m2 i.v., cyclophosphamide 1 g/m2 i.v. and etoposide 120 mg/m2 i.v. on day 1, followed by etoposide 240 mg/m2 orally for 2 days) PE ‐ (cisplatin 80 mg/m2 and etoposide 120 mg/m2 i.v. on day 1, followed by etoposide 240 mg/m2 orally for 2 days every 3 weeks). For patients where cisplatin was not suitable, carboplatin was substituted at an AUC of 6, calculated according to the Calvert formula (ie, carboplatin dose 1⁄4 target AUC of 6 (glomerular filtration rate þ 25 mg), where glomerular filtration rate was based on EDTA or measured creatinine clearance). CYCLES: 6 cycles every 3 weeks CO‐INTERVENTIONS PERMITTED: Thoracic radiotherapy CO‐INTERVENTIONS: Thoracic radiotherapy was given to patients with limited stage disease achieving a complete or partial response to chemotherapy, beginning 3 weeks after the last cycle of chemotherapy (30 Gy in 10 daily fractions). Patients with ED SCLC received thoracic irradiation only if they had thoracic symptoms amenable to palliation with radiotherapy after completion of chemotherapy. Prophylactic cranial irradiation was considered for all LD patients achieving a complete response; suitable patients received 25 Gy in 10 daily fractions after completion of chemotherapy. |
|
| Outcomes |
OUTCOMES MEASURED: Primary – 1 year survival Secondary – 2 year survival, median survival, response rate, toxicity FOLLOW‐UP ASSESSMENT POINTS: During chemotherapy, patients were assessed on days 1 and 15 with physical examination, and weekly, with blood count, biochemistry and WHO Performance Status. A chest X‐ray (CXR) was carried out after every second cycle of treatment, but assessment of response was made according to the WHO criteria by CT scanning at the end of chemotherapy unless progressive disease was detected in the interim by CXR. Toxicities were graded according to the National Cancer Institute Common Toxicity Grading Criteria version December 1994 (revised). OUTCOMES INCLUDED IN ANALYSES: Survival Response Rate Toxicity TYPE: Chemotherapy REGIMENS: ACE ‐ (doxorubicin 50 mg/m2 i.v., cyclophosphamide 1 g/m2 i.v. and etoposide 120 mg/m2 i.v. on day 1, followed by etoposide 240 mg/m2 orally for 2 days) PE ‐ (cisplatin 80 mg/m2 and etoposide 120 mg/m2 i.v. on day 1, followed by etoposide 240 mg/m2 orally for 2 days every 3 weeks). For patients where cisplatin was not suitable, carboplatin was substituted at an AUC of 6, calculated according to the Calvert formula (ie, carboplatin dose 1⁄4 target AUC of 6 (glomerular filtration rate þ 25 mg), where glomerular filtration rate was based on EDTA or measured creatinine clearance). CYCLES: 6 cycles every 3 weeks DOSE: DELIVERY: IV CO‐INTERVENTIONS PERMITTED: Thoracic radiotherapy CO‐INTERVENTIONS: Thoracic radiotherapy was given to patients with limited stage disease achieving a complete or partial response to chemotherapy, beginning 3 weeks after the last cycle of chemotherapy (30 Gy in 10 daily fractions). Patients with ED SCLC received thoracic irradiation only if they had thoracic symptoms amenable to palliation with radiotherapy after completion of chemotherapy. Prophylactic cranial irradiation was considered for all LD patients achieving a complete response; suitable patients received 25 Gy in 10 daily fractions after completion of chemotherapy. |
|
| Notes |
SUB‐GROUPS INDENTIFIED: Limited disease stage Extensive disease stage |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described and appropriate |
| Allocation concealment (selection bias) | Low risk | Not described |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not described |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not described |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not described |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not described |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not described |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Survival | Low risk | All randomised patients accounted for; withdrawals and dropouts adequately described |
| Incomplete outcome data (attrition bias) Tumour Response | Low risk | All randomised patients accounted for; withdrawals and dropouts adequately described |
| Incomplete outcome data (attrition bias) Toxicity | Low risk | All randomised patients accounted for; withdrawals and dropouts adequately described |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | Not assessed |
Chahinian 1989.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES: 20 Cancer and Leukaemia Group B (CALGB) main member institutions
DURATION OF STUDY: February 1981 to May 1984
CONCEALMENT OF ALLOCATION: D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND:
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Not described GRADE ASSESSMENT QUALITY RATING: Low TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY
INCLUSION CRITERIA:
CALGB performance status of 0 to 3
Informed consent
One measurable tumour EXCLUSION CRITERIA: Prior chemotherapy Myocardial infarction within 6 months Cardiac failure Serious arrhythmia not directly attributable to metastatic small cell lung cancer Patients with any contraindications to the use of warfarin Patients already on warfarin N RANDOMISED: 294 ASSESS STAGE: Yes (N LIMITED): 0 (N EXTENSIVE): 294 M: 199 (MACC ‐ 62; MACC + W ‐ 70; MEPH/MACC ‐ 67) F: 95 (MACC ‐ 24; MACC + W ‐ 33; MEPH/MACC ‐ 38 AGE: |
|
| Interventions | TYPE: Chemotherapy
REGIMENS:
MACC ‐ methotrexate (30 mg/m2 intravenously [IV]), doxorubicin (40mg/m2 IV), cyclophosphamide (400 mg/m2 IV) and lomustine (30 mg/m2 orally) given once every 3 weeks. Doxorubicin was discontinued after a total cumulative dose of 450 mg/m2 MACC + W ‐ methotrexate (30 mg/m2 intravenously [IV]), doxorubicin (40 mg/m2 IV), cyclophosphamide (400 mg/m2 IV), lomustine (30 mg/m2 orally) and warfarin sodium (single oral daily dose of 10 mg starting on day 1) given once every 3 weeks. Doxorubicin was discontinued after a total cumulative dose of 450 mg/m2. MEPH/MACC ‐ Mitomycin (7 mg/m2 IV on day 1), etoposide (40 mg/m2 IV on days 1 to 3), cisplatin (50 mg/m2 IV on day 1) and hexamethylmelamine (100 mg/m2 orally from day 3 to 17). This was the MEPH regimen. On day 35 (week 6) of each cycle, patients received MACC chemotherapy as described above. On day 56 (week 9), MEPH component was given again 3 weeks after MACC. The two regimens were alternated in this fashion (MEPH/MACC) CO‐INTERVENTIONS: Radiotherapy CLASSIFICATION OF INTERVENTION (ADJUVANT/NEO‐ADJUVANT/PALLIATIVE): Palliative |
|
| Outcomes | OUTCOMES MEASURED:
Tumour response
Overall survival
Toxicity FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Tumour response Overall survival |
|
| Notes | Only data from arms MACC + W and MEPH/MACC were considered for this systematic review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | N/A |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | Unclear risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Tumour Response | Unclear risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Toxicity | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
Creech 1982.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES:
DURATION OF STUDY: September 1978 to October 1979
CONCEALMENT OF ALLOCATION: D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Yes GRADE ASSESSMENT QUALITY RATING: Moderate TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | INCLUSION CRITERIA: Each patient had to be refractory to standard chemotherapy; have measurable or evaluable disease; an Eastern Cooperative Oncology Group performance status of 0 to 2; a white blood cell count >= 4000/mm3, a platelet count >= 100,000/mm3, a BUN <= 25 mg/100 mL, a creatinine < 1.5 mg/100 mL, and a bilirubin <= 2.0 mg/100 mL. All patients gave informed written consent prior to participation in this study. EXCLUSION CRITERIA: N SCREENED: 73 (58 evaluable) N RANDOMISED: 58 (cisplatin ‐ 21; maytansine ‐ 19; chlorozotocin ‐ 18) N COMPLETED: ASSESS STAGE: No (N LIMITED): (N EXTENSIVE): M: F: AGE: | |
| Interventions | TYPE: Chemotherapy
REGIMENS:
Cisplatin ‐ 75 mg/m2 intravenous (IV) Cisplatin every 21 days after adequate hydration and diuresis.
Maytansine ‐ maytansine 1.5 mg/m2 IV every 21 days.
Chlorozotocin ‐ chlorozotocin 120 mg/m2 IV every 42 days. Each drug was given until there was clinical evidence of progressive disease, after which patients were not eligible for treatment with the other study drugs. CO‐INTERVENTIONS: |
|
| Outcomes | OUTCOMES MEASURED:
Tumour response
Survival
Toxicity FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Tumour response Survival Toxicity |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | N/A |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | N/A |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Tumour Response | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Toxicity | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | High risk | No mention of survival ‐ an expected outcome |
| Other bias | Low risk | Adequate |
de Jong 2007.
| Methods |
STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: Not described DURATION OF STUDY: Feb 1999 – Feb 2005 CONCEALMENT OF ALLOCATION: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Not described METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Yes METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: N/A DESCRIPTION OF WITHDRAWALS/DROPOUTS: Appropriate GRADE ASSESSMENT QUALITY RATING: High TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT COMPLIANCE: CONFOUNDERS: |
|
| Participants |
ELIGIBILITY INCLUSION CRITERIA: Patients were included if they met all the following criteria: age over 18 years, histologically or cytologically proven ED SCLC with measurable or evaluable lesions, no prior chemotherapy or radiotherapy except for symptomatic brain metastases, Eastern Cooperative Oncology Group (ECOG) performance score 0–2, adequate haematological, renal and hepatic functions (absolute neutrophil count (ANC) P2.0 x 10^9/L, platelet count P100 · 109/L, bilirubin 61.25 · upper normal limit, creatinine clearance according to Cockroft formula P60 ml/min). EXCLUSION CRITERIA: N SCREENED: Unknown N RANDOMISED: 203 N COMPLETED: 203 (CDE ‐ 102; CP – 101) ASSESS STAGE: Yes (N LIMITED): N/A (N EXTENSIVE): 203 M: 118 (CDE – 52; CP – 63) F: 85 (CDE – 47; CP – 38) MEDIAN AGE: CDE – 61.7; CP – 62.7 BASELINE DETAILS: History, physical examination, ECOG performance status, complete blood cell count (CBC), electrolytes, liver enzymes, serum creatinine and electrocardiography (ECG) |
|
| Interventions |
TYPE: Chemotherapy REGIMENS, DOSE, DELIVERY: CDE ‐ Cyclophosphamide (1000 mg/m2 i.v) on day 1, doxorubicin (45 mg/m2 i.v) on day 1, and etoposide (100 mg/m2 i.v) on days 1, 2, and 3. Carboplatin (AUC 7 using the Calvert formula, i.v) followed by paclitaxel (175 mg/m2 i.v) as a 3‐hour infusion both on day 1 CYCLES: Maximum of 5 cycles every 3 weeks CO‐INTERVENTIONS PERMITTED: Nil CO‐INTERVENTIONS: Nil |
|
| Outcomes |
OUTCOMES MEASURED: Primary – Progression free survival, Secondary – Overall survival, tumour response rates, toxicities FOLLOW‐UP ASSESSMENT POINTS: On day 14 of each cycle and on clinical indications, a CBC was performed in a similar way in both arms. Tumour evaluations were performed with a computed tomography (CT) scan of the chest and repeated after two cycles and at the end of treatment. Tumour response was defined according to the WHO criteria. Follow‐up after treatment was every 4–6 weeks with a CBC, liver enzymes, chest X‐ray, or additional tests if clinically indicated. Toxicity was scored before each cycle according to the National Cancer Institute Common Toxicity Criteria (CTC), version 2.0. OUTCOMES INCLUDED IN ANALYSES: Progression‐free survival Overall survival Tumour response rates Toxicities SUB‐GROUPS INDENTIFIED: Nil |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described and appropriate |
| Allocation concealment (selection bias) | Low risk | Described and appropriate |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not described |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not described |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not described |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not described |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not described |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Survival | Low risk | All randomised patients accounted for; withdrawals and dropouts adequately described |
| Incomplete outcome data (attrition bias) Tumour Response | Low risk | All randomised patients accounted for; withdrawals and dropouts adequately described |
| Incomplete outcome data (attrition bias) Toxicity | Low risk | All randomised patients accounted for; withdrawals and dropouts adequately described |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | Not assessed |
| Selective reporting (reporting bias) | Low risk | Adequate |
Eagan 1981.
| Methods | STUDY DESIGN: Parallel group
LOCATION, NUMBER OF CENTRES:
DURATION OF STUDY: July 1976 to July 1978
CONCEALMENT OF ALLOCATION: D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Not described GRADE ASSESSMENT QUALITY RATING: Low TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY
INCLUSION CRITERIA: Previously untreated limited stage small cell lung cancer EXCLUSION CRITERIA: Patients with significant cardiac disease or serum creatinines > 1.5 mg/dl N RANDOMISED: 62 N COMPLETED: ASSESS STAGE: Yes (N LIMITED): 62 (VOCA ‐ 31; VOCAP ‐ 31) (N EXTENSIVE): 0 M: 45 (VOCA ‐ 22; VOCAP ‐ 23) F: 17 (VOCA ‐ 9; VOCAP ‐ 8) AGE: Median VOCA ‐ 58 (39 to 74); VOCAP ‐ 59 (38 to 77) |
|
| Interventions | TYPE: Chemotherapy
REGIMENS:
VOCA ‐ VP‐16 50 mg/m2 IV on days 1, 2 and 3; vincristine (Oncovin) 1.4 mg/m2 IV on day 1; cyclophosphamide 150 mg/m2 IV on days 1, 2 and 3; and Adriamycin 15 mg/m2 IV on days 1, 2 and 3. VOCAP ‐ VP‐16 50 mg/m2 IV on days 1, 2 and 3; vincristine (Oncovin) 1.4 mg/m2 IV on day 1; cyclophosphamide 150 mg/m2 IV on days 1, 2 and 3; Adriamycin 15 mg/m2 IV on days 1, 2 and 3; and cisplatin 40 mg/m2 IV on day 1. Both treatment arms were administered for 8 cycles CO‐INTERVENTIONS: Thoracic radiation therapy was administered concomitantly with the third and fourth courses of chemotherapy CLASSIFICATION OF INTERVENTION: (ADJUVANT/NEO‐ADJUVANT/PALLIATIVE): Palliative |
|
| Outcomes | OUTCOMES MEASURED:
Regression rates
Time to progression
Survival
Tumour response
Toxicity FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Survival Tumour response Toxicity |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Tumour Response | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Toxicity | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Adequate |
Evans 1987.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES: Canada, multicentric
DURATION OF STUDY: November 1982 to April 1985
CONCEALMENT OF ALLOCATION: D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Adequate
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Yes GRADE ASSESSMENT QUALITY RATING: High TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY INCLUSION CRITERIA: histologically/cytologically proven SCLC; no prior chemotherapy or radiotherapy; evidence of extensive disease (ED); evidence of good bone marrow function (leukocyte count > 3.0 × 109/L, granulocytes > 1.5 × 109/L, platelet count > 75 × 109/L) and satisfactory liver function (bilirubin < 35 µmol/L) and renal function (serum creatinine < 120 µmol/L, creatinine clearance > 3.3 mL/S, blood urea nitrogen < 10 mmol/L); measurable or evaluable disease; patients with malignant pleural effusions were eligible if, in addition to the effusion, they had other measurable or evaluable disease; patients had to give written and informed consent and be available for follow up. EXCLUSION CRITERIA: patients were ineligible if central nervous system metastases were present; they had prior radiotherapy or chemotherapy or had a performance status lower than 3 on the Eastern Cooperative Oncology Group scale. Patients with a prior history of malignant disease, except for nonmelanoma skin tumours, were excluded unless they had been without evidence of disease for at least 5 years. Patients with a recent myocardial infarction (less than 3 months from the date of diagnosis) or congestive cardiac failure or arrhythmia requiring medical treatment were excluded, as were patients over 80 years of age, or those who lived too far from the treatment centre. N RANDOMISED: 289 (standard ‐ 144; alternating ‐ 145) ASSESS STAGE: No (N LIMITED): (N EXTENSIVE): M: 193 F: 96 AGE: Mean: standard ‐ 61.1; alternating ‐ 61.5 (range: standard ‐ 34 to 79; alternating ‐ 41 to 79) | |
| Interventions | TYPE: chemotherapy
REGIMENS:
Standard ‐ cyclophosphamide (1000 mg/m2 body surface area), doxorubicin 50 mg/m2 and vincristine 2 mg by intravenous (IV) bolus every 3 weeks for 6 cycles.
Alternating ‐ same doses as the standard regimen alternating with etoposide 100 mg/m2 infused over 30 to 60 minutes, and cisplatin 25 mg/m2 in an IV bolus, daily for 3 consecutive days.
Treatment cycles were repeated at 3‐week intervals in the alternating study arm. The duration of treatment was 18 weeks for both arms of the study. |
|
| Outcomes | OUTCOMES MEASURED: Tumour response (best response to chemotherapy) Survival Toxicity FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Tumour response (best response to chemotherapy) Survival Toxicity | |
| Notes | At the end of the 6 cycles, patients were seen every 6 weeks for 6 months and every 3 months thereafter or as judged appropriate by their physician. Patients receiving the etoposide and cisplatin regimen had to drink 6 to 8 glasses of water on the mornings of treatment. The etoposide was diluted in normal saline in a volume sufficient to achieve a concentration of 0.4 mg/mL or less. Blood pressure was measured every 15 minutes during the etoposide infusion, at least during the first treatment. Cisplatin was given at the end of the etoposide infusion. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Tumour Response | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Toxicity | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
Farris 1993.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES:
DURATION OF STUDY:
CONCEALMENT OF ALLOCATION: D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Not described GRADE ASSESSMENT QUALITY RATING: Low TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY
INCLUSION CRITERIA: Histologically confirmed undifferentiated small oat cell carcinoma EXCLUSION CRITERIA: N RANDOMISED: 113 (CEV ‐ 57, CDDP‐VP16/C‐E ‐ 57) ASSESS STAGE: Yes (N LIMITED): 52 (CEV ‐ 27; CDDP‐VP16/C‐E ‐ 25) (N EXTENSIVE): 61 (CEV ‐ 30; CDDP‐VP16/C‐E ‐ 31) M: 100 (CEV ‐ 50; CDDP‐VP16/C‐E ‐ 50) F: 13 (CEV ‐ 7; CDDP‐VP16/C‐E ‐ 6) AGE: Overall: median 61 (range: 43 to 74); CEV: 62 (45 to 74), CDDP‐VP16/C‐E: 60 (43 to 69) |
|
| Interventions | TYPE: Chemotherapy
REGIMENS:
CEV ‐ cyclophosphamide 1000 mg/m2, epirubicin 60 mg/m2 and vincristine 1 mg/m2 all administered on day 1 and cycles repeated every 21 days (all given IV) CDDP‐VP16/C‐E ‐ cisplatin 50 mg/m2 on days 1 and 2, etoposide 200 mg/m2 on days 3, 4 and 5 alternating every 28 days with cyclophosphamide 1000 mg/m2 on day 1 and epirubicin 60 mg/m2 on day 1 (all given IV, except for etoposide, which was given orally) CO‐INTERVENTIONS: Patients with limited disease experiencing complete remission received chest irradiation (45 Gy in 15 fractions over 3 weeks) and prophylactic irradiation of the skull. CLASSIFICATION OF INTERVENTION: (ADJUVANT/NEO‐ADJUVANT/PALLIATIVE): Palliative |
|
| Outcomes | OUTCOMES MEASURED:
Tumour response
Survival
Toxicity FOLLOW UP ASSESSMENT POINTS: After the end of chemotherapy, assessable patients were re‐checked monthly for the first 3 months and then every 3 months thereafter. At each re‐check visit, each patient received a complete physical examination, a chest X‐ray and routine haematological and biochemistry tests OUTCOMES INCLUDED IN ANALYSES: Tumour response Survival Toxicity |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Tumour Response | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Toxicity | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Adequate |
Fukuoka 1986.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES: Osaka Prefectural Habikino Hospital, single centre.
DURATION OF STUDY: August 1982 to April 1985
CONCEALMENT OF ALLOCATION: D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Not described GRADE ASSESSMENT QUALITY RATING: Low TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY
INCLUSION CRITERIA: Histologically or cytologically confirmed SCLC, age <= 79 years, a performance status (ECOG scale) of 0 to 3, with no prior therapy, with measurable or evaluable disease, with adequate bone marrow function (white blood cells [WBC] >= 4000/mm3, platelets >= 10 x 104/mm3), serum creatinine <1.5 mg/dl, serum glutamine oxaloacetic transaminase and glutamine pyruvic transaminase < 2 x normal, with normal cardiac function and no other malignant disease. EXCLUSION CRITERIA: N SCREENED: 71 (69 eligible) N RANDOMISED: 69 (continuous ‐ 34; alternating ‐ 35) N COMPLETED: ASSESS STAGE: Yes (N LIMITED): 21 (N EXTENSIVE): 48 M: 55 F: 14 AGE: mean: continuous ‐ 61.5; alternating ‐ 62.1 (range: continuous ‐ 40 to 77, alternating ‐ 36 to 74) |
|
| Interventions | TYPE: Chemotherapy
REGIMENS:
The continuous regimen (CONP) consisted of nimustine hydrochloride (ACNU: 70 mg/m2 IV on day 1), cyclophosphamide (700 mg/m2 IV on day 2), oncovin (0.7 mg/m2 IV on day 2) and procarbazine (100 mg/m2 body surface‐area orally on days 1 to 7). This regimen was repeated every 4 weeks.
The alternating regimen (CONPVAD) consisted of CONP treatment as described above, followed by treatment with VAD, which contained etoposide (VP‐16) (60 mg/m2 IV on days 1 to 4), Adriamycin (30 mg/m2 IV on day 1), and cisplatin (DDP: 60 mg/m2 IV with 2600 ml hydration and diuresis on day 1). Thus, CONP was alternated with VAD at 4‐week intervals. CO‐INTERVENTIONS: Radiation therapy was performed on primary lesions and the mediastinum at the time of expected maximum response to all patients with LD |
|
| Outcomes | OUTCOMES MEASURED:
Tumour response
Response duration
Survival
Toxicity FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Tumour response Survival Toxicity |
|
| Notes | Other: Patients with pleural effusion were excluded from LD (limited disease) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Tumour Response | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Toxicity | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Adequate |
Fukuoka 1991.
| Methods | STUDY DESIGN: Parallel group
LOCATION, NUMBER OF CENTRES:
DURATION OF STUDY: April 1985 to May 1988
CONCEALMENT OF ALLOCATION: D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Adequate
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Yes GRADE ASSESSMENT QUALITY RATING: High TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | INCLUSION CRITERIA: Histologically or cytologically proven SCLC; no prior therapy; signs of measurable or evaluable disease; a performance status of 0 to 3 on the Eastern Cooperative Oncology Group (ECOG) scale; age less than 75 years; adequate bone marrow reserve (leukocyte count >= 4000/mm3 and platelet count >= 100,000/mm3); adequate liver function (bilirubin <= 1.5 mg/dL and alkaline phosphatase, aspartate aminotransferase and alanine aminotransferase no greater than twice the upper limit of normal); adequate renal function (serum creatinine <= 1.5 mg/dL and blood urea nitrogen <= 25 mg/dL) and informed consent. EXCLUSION CRITERIA: Ineligible if patients had another active malignant disease, a history of myocardial infarction within the previous 3 months or other cardiac disease requiring medical treatment. N SCREENED: 300 (288 eligible) N RANDOMISED: 288 (CAV ‐ 97; PE ‐ 97; CAV/PE ‐ 94) N COMPLETED: 279 ASSESS STAGE: Yes (N LIMITED): CAV ‐ 49; PE ‐ 47; CAV/PE ‐ 50 (N EXTENSIVE): CAV ‐ 48; PE ‐ 50; CAV/PE ‐ 44 M: CAV ‐ 81; PE ‐ 78; CAV/PE ‐ 76 F: CAV ‐ 16; PE ‐ 19; CAV/PE ‐ 18 AGE: median: CAV ‐ 63; PE ‐ 64; CAV/PE ‐ 64 (range: CAV ‐ 40 to 74; PE ‐ 37to 74; CAV/PE ‐ 31 to 74) |
|
| Interventions | TYPE: Chemotherapy
REGIMENS:
CAV ‐ cyclophosphamide at a dose of 800 mg/m2 given intravenously (IV) on day 1, doxorubicin at 50 mg/m2 IV on day 1 and vincristine at 1.4 mg/m2 (maximum 2.0 mg) IV on day 1.
PE ‐ cisplatin at 80 mg/m2 IV on day 1 and etoposide at 100 mg/m2 IV on days 1, 3 and 5.
(Cisplastin was given with adequate prehydration, posthydration, diuretics and antiemetic agents) CAV/PE ‐ CAV alternating with PE Treatments were repeated every 3 to 4 weeks CO‐INTERVENTIONS: All patients with limited disease received thoracic irradiation after restaging. Thoracic irradiation consisted of 200‐cGy fractions given daily 5 days per week for 4 to 5 weeks. |
|
| Outcomes | OUTCOMES MEASURED:
Tumour response (classified according to World Health Organization criteria)
Survival
Toxicity FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Tumour response (classified according to World Health Organization criteria) Survival Toxicity |
|
| Notes | Other: There were 3 cancellations due to patient refusal before therapy started; 9 patients were considered ineligible: 4 had histologies other than SCLC, 3 were 75 years of age or older, one had congestive heart failure and one had a simultaneous squamous cell carcinoma of the lung. If patients on the CAV arm or PE arm did not respond after 2 cycles of chemotherapy, they were crossed over to the other regimen (restaging). Restaging was carried out at the completion of 4 cycles of chemotherapy to evaluate the response. Cisplastin was discontinued if renal failure occurred. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Tumour Response | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Toxicity | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
Gatzemeier 1994.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES:
DURATION OF STUDY:
CONCEALMENT OF ALLOCATION: D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Not described GRADE ASSESSMENT QUALITY RATING: Low TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY INCLUSION CRITERIA: Histologic diagnosis of SCLC; good performance status [Eastern Coorperative Oncology Group (ECOG) 0 to 2]; no evidence of central nervous system metastases; age between 18 and 75 years; life expectancy of at least 3 months; adequate bone marrow, renal and hepatic functions; and extensive disease (ED). EXCLUSION CRITERIA: N SCREENED: 344 N RANDOMISED: 317 (CEV ‐ 156; EV ‐ 161) ASSESS STAGE: Yes ED only (N LIMITED): (N EXTENSIVE): 317 M: 235 (CEV ‐ 117; EV ‐ 118) F: 82 (CEV ‐ 39; EV ‐ 43) AGE: median: CEV ‐ 58.5; EV ‐ 62 (range: CEV ‐ 33 to 76; EV ‐ 18 to 75) | |
| Interventions | TYPE: Chemotherapy
REGIMENS:
CEV ‐ carboplatin 300 mg/m2 on day 1, etoposide 140 mg/m2 on days 1 to 3 and vincristine 1.4 mg/m2 on days 1, 8 and 15
EV ‐ etoposide 200 mg/m2 on days 1 to 3, and vincristine 1.4 mg/m2 on days 1 and 8. Chemotherapy cycles in each treatment arm were repeated every 4 weeks. CO‐INTERVENTIONS: |
|
| Outcomes | OUTCOMES MEASURED:
Tumour response
Survival
Toxicity FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Tumour response Survival Toxicity |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Tumour Response | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Toxicity | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Adequate |
Goodman 1990.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES:
DURATION OF STUDY: September 1982 to September 1984
CONCEALMENT OF ALLOCATION: D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Not described GRADE ASSESSMENT QUALITY RATING: Low TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY
INCLUSION CRITERIA:
Patients with previously untreated limited disease SCLC EXCLUSION CRITERIA: Patients with extensive disease SCLC N RANDOMISED: 388 ASSESS STAGE: Yes (LD only) (N LIMITED): 388 (N EXTENSIVE): M: 247 F: 141 AGE: Median 61 in both arms |
|
| Interventions | TYPE: Chemotherapy
REGIMENS:
EVAC ‐ VP‐16, 75 mg/m2 IV on days 1, 2 and 3, vincristine 1.0 mg/m2 IV on days 1 and 8, Adriamycin 40 mg/m2 IV on day 1, cyclophosphamide 750 mg/m2 IV on day 1
Treatment was repeated every 3 weeks for 6 cycles VP‐16/CDDP alternating with VAC ‐ VP‐16 100 mg/m2 IV on days 1, 2 and 3, CDDP 100 mg/m2 IV on day 1, vincristine 1.0 mg/m2 IV on days 22 and 29, Adriamycin 50 mg/m2 IV on day 22, cyclophosphamide, 750 mg/m2 on day 22 Treatment was repeated every 6 weeks for a total of 6 cycles CO‐INTERVENTIONS: Chest irradiation Prophylactic cranial irradiation CLASSIFICATION OF INTERVENTION (ADJUVANT/NEO‐ADJUVANT/PALLIATIVE): Palliative |
|
| Outcomes | OUTCOMES MEASURED:
Survival
Tumour response
Toxicity FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Survival Tumour response Toxicity |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Tumour Response | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Toxicity | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
Greco 2005.
| Methods | STUDY DESIGN: Parallel group
LOCATION, NUMBER OF CENTRES: 29 affiliate Minnie Pearl Cancer Research Network participating sites DURATION OF STUDY: CONCEALMENT OF ALLOCATION: D DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: No METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Not described GRADE ASSESSMENT QUALITY RATING: Low TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY
INCLUSION CRITERIA: Patients with previously untreated histologically confirmed SCLC; age > 18 years; Easter Cooperative Oncology Group (ECOG) performance status score of 0 or 1; ANC > 1500/µl; platelet count > 100,000/µl; serum bilirubin <= 1.5 mg/dl, for those without known hepatic metastases, and < 2.5 mg/dl for those with known hepatic metastases; serum creatinine <= 1.5 mg/dl; no previous treatment for small cell lung cancer; no history of prior malignancy within 5 years, with the exception of nonmelanoma skin cancer or cervical carcinoma in situ; and no history of congestive heart failure or myocardial infarction within 3 months. EXCLUSION CRITERIA: Patients with mixed histology lung cancer. N SCREENED: N RANDOMISED: 120 (PCE ‐ 60; PT ‐ 60) N COMPLETED: 120 ASSESS STAGE: Yes (N LIMITED): 0 (N EXTENSIVE): 120 M: PCE ‐ 39; PT ‐ 29 F: PCE ‐ 21; PT ‐ 31 AGE: median age (range): PCE ‐ 60 (42 to 78); PT 62 (38 to 79) |
|
| Interventions | TYPE: Chemotherapy
REGIMENS:
PCE ‐ paclitaxel, carboplatin, etoposide
PT ‐ paclitaxel, topotecan Up to 8 cycles After the completion of 4 courses of chemotherapy, patients responding well to treatment and tolerating treatment with no grade 4 toxicity could continue on treatment for a maximum of 8 courses at the discretion of the treating physician PCE ‐ paclitaxel at a dose of 200 mg/m2 IV over 1 hour on day 1; carboplatin dosing was based on the Calvert formula with a target area under the concentration‐time curve of 6 given IV over 1 hour on day 1; etoposide given orally with a total dose of 50 mg, alternating with 100 mg, on a daily basis on days 1 to 10. Repeated every 21 days. PT ‐ paclitaxel was administered at a dose of 175 mg/m2 IV over 1 hour on day 1, and topotecan was given at a dose of 1.5 mg/m2 IV over 1 hour on days 1, 2 and 3. Repeated every 21 days. CO‐INTERVENTIONS: Patients with brain metastases were treated with whole‐brain radiotherapy concurrently with the beginning of chemotherapy. Cytokines were used at the discretion of each investigator; however, they were not used during the first course of therapy. All patients received standard supportive care, including blood and platelet transfusions, antiemetics and antibiotics. |
|
| Outcomes | OUTCOMES MEASURED:
Primary: tumour response rate; time to progression
Secondary: overall survival; toxicity (haematologic and non‐haematologic) FOLLOW UP ASSESSMENT POINTS: Tumour status was assessed after 2 courses of chemotherapy (6 weeks), and thereafter every 2 courses until the completion of treatment (4 to 8 courses). OUTCOMES INCLUDED IN ANALYSES: Tumour response rate Overall survival Toxicity |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Tumour Response | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Toxicity | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Adequate |
Havemann 1987.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES: Germany, multicentre
DURATION OF STUDY: July 1981 to November 1983.
CONCEALMENT OF ALLOCATION: C
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Yes GRADE ASSESSMENT QUALITY RATING: High TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | INCLUSION CRITERIA: Histologic proof of SCLC ‐ positive cytologic findings were not considered histologic confirmation; signs of measurable or evaluable disease; performance status of 50% or more according to the Karnofsky scale; age of 70 years or younger; and informed patient consent. EXCLUSION CRITERIA: prior radiotherapy, chemotherapy or surgical treatment received; existence of an accessory malignant disease; evidence of renal dysfunction (creatinine > 1.5 mg/dL), chronic haptic disease (bilirubin > 2.0 mg/dL); or advanced respiratory or cardiac insufficiency. N RANDOMISED: 306 (302 evaluable) (sequential ‐ 155, alternating ‐ 151) ASSESS STAGE: Yes (N LIMITED): 104 (N EXTENSIVE): 198 M: 254 F: 48 AGE: | |
| Interventions | TYPE: Chemotherapy
REGIMENS:
Sequential chemotherapy consisted of 8 cycles of CAV ‐ cyclophosphamide 1000 mg/m2 intravenously (IV) on day 1, Adriamycin 50 mg/m2 IV on day 1, and vincristine 2 mg IV on day 1, all administered in 3‐week intervals. Alternating chemotherapy consisted of 3 cycles of EVI (cycles 1, 3 and 5) ‐ etoposide 80 mg/m2 IV on days 1 to 3, vindesine 3 mg/m2 IV on day 1, and iphosphamide 1500 mg/m2 IV on days 1 to 5 alternating with 3 cycles (cycles 2, 4 and 6) of PAV ‐ cisplatin 90 mg/m2 IV on day 1, Adriamycin 60 mg/m2 IV on day 1, and vincristine 2 mg IV on day 1, all administered in 3‐week intervals and followed by application of CMC ‐ cyclophosphamide 1000 mg/m2 IV on days 1 and 22, methotrexate 15 mg/m2 orally on days 1, 4, 8 and 11; and CCNU 100 mg/m2 orally on day 1 in a 6‐week cycle. CO‐INTERVENTIONS: Responsive patients received prophylactic cranial irradiation after 3 cycles. Patients without distant metastases after 8 cycles of chemotherapy received chest irradiation. Patients with brain metastases received cranial irradiation immediately. Accessory painful metastatic sites were irradiated as necessary. |
|
| Outcomes | OUTCOMES MEASURED: Tumour response (defined as best response at any time during treatment) Survival Progression‐free survival Tumour progression or relapse Toxicity FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Tumour response (defined as best response at any time during treatment) Survival Toxicity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation reported and adequate |
| Allocation concealment (selection bias) | High risk | Allocation concealment not adequate |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | N/A |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Tumour Response | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Toxicity | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
Jones 1993.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES:
DURATION OF STUDY: August 1987 to April 1990
CONCEALMENT OF ALLOCATION:D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Not described GRADE ASSESSMENT QUALITY RATING: Low TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY INCLUSION CRITERIA: Previously untreated patients; histologically or cytologically confirmed SCLC; World Health Organization performance status EXCLUSION CRITERIA: N RANDOMISED: 104 (CVM ‐ 54; ACE ‐ 50) ASSESS STAGE: Yes (N LIMITED): 32 (CVM ‐ 17; ACE ‐ 15) (N EXTENSIVE): 72 (CVM ‐ 37; ACE ‐ 35) M: 61 F: 43 AGE: median: CVM ‐ 67; ACE ‐ 64 (range: CVM ‐ 33 to 79; ACE ‐ 47 to 75) | |
| Interventions | TYPE: Chemotherapy
REGIMENS:
CVM ‐ carboplatin 300 mg/m2 in 500 ml (Dextrose 5% in Water solution) delivered intravenously (IV) over 60 minutes, on day 1, vinblastine 6 to 10 mg/m2 IV on day 1, and methotrexate 30 to 50 mg/m2 IV on day 1.
The course was repeated every 28 days. Leucovorin was administered orally 24 hours after methotrexate to reverse the latter agent's toxic effects (15 mg every 6 hours × 6 doses) ACE ‐ doxorubicin 40 mg/m2 IV on day 1, cyclophosphamide 600 mg/m2 IV on day 1 and etoposide 100 mg/m2 days 1 to 3 (outpatient treatment: 200 mg/m2 orally. days 2 and 3). The course was repeated every 21 days CO‐INTERVENTIONS: Some limited disease (LD) patients who did not respond to chemotherapy were given thoracic radiotherapy Prophylactic cranial radiotherapy was also given to some patients |
|
| Outcomes | OUTCOMES MEASURED:
Tumour response
Toxicity FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Tumour response Toxicity |
|
| Notes | To prevent vomiting dexamethasone and metoclopramide (8 and 20 mg respectively) were administered IV prior to the first chemotherapy course and orally after chemotherapy (4 and 10 to 20 mg respectively, every 4 hours over 48 hours). Thereafter the doses of the antiemetics were modified as needed. If platelet and leukocyte counts had not returned to at least 3 × 109/L and 100 × 109/L, respectively, by the time of the next treatment cycle, an additional week of no treatment was added before the cycle began to allow blood count recovery. Doses of all chemotherapy agents were reduced by 25% for the duration of the study if 2 treatment cycle delays occurred. During part of the selection period, patients with limited disease and a good performance status (grade 1 to 2) had the option of undergoing intensive therapy with other protocols. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Tumour Response | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Toxicity | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Adequate |
Kanitz 1992.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES: Czechoslovakia, Hungary, Poland and Yugoslavia; multicentric
DURATION OF STUDY:
CONCEALMENT OF ALLOCATION: D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Adequate GRADE ASSESSMENT QUALITY RATING: High TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY INCLUSION CRITERIA: Histologically or cytologically confirmed SCLC; measurable and/or evaluable extensive disease; no prior chemotherapy and no prior radiotherapy except for brain metastases; adequate bone marrow, renal, liver and cardiac function; age up to 70 years; any infection controlled; and informed patient's consent. EXCLUSION CRITERIA: N RANDOMISED: 111 (HD ‐ EDX + CPA: 52; HD ‐ EDX + DDP: 59) ASSESS STAGE: Yes (ED only) (N LIMITED): (N EXTENSIVE): 111 M: 94 (HD ‐ EDX + CPA: 42; HD ‐ EDX + DDP: 52) F: 17 (HD ‐ EDX + CPA: 10; HD ‐ EDX + DDP: 7) AGE: median: HD ‐ EDX + CPA: 55; HD ‐ EDX + DDP: 56 (range: HD ‐ EDX + CPA: 33 to 69; HD ‐ EDX + DDP: 33 to 70) | |
| Interventions | TYPE: Chemotherapy
REGIMENS:
HD ‐ EDX + CPA: High dose epirubicin 120 mg/m2 intravenously (IV) on day 1, as a bolus injected into a running infusion in combination with cyclophosphamide 800 mg/m2 IV in 500 mL infusion.
HD ‐ EDX + DDP: High dose epirubicin 120 mg/m2 IV on day 1, as a bolus injected into a running infusion, in combination with cisplatin 60 mg/m2 IV in infusion of at least 1.5 L of saline followed by 500 mL of 10% mannitol if needed. Both regimens were repeated every 4 weeks CO‐INTERVENTIONS: |
|
| Outcomes | OUTCOMES MEASURED: Tumour response (judged according to World Health Organization criteria) Survival Toxicity FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Tumour response (judged according to World Health Organization criteria) Survival Toxicity | |
| Notes | Other: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Tumour Response | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Toxicity | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Adequate |
Lyss 2002.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES:
DURATION OF STUDY: April 1995 to [not reported]
CONCEALMENT OF ALLOCATION: D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Yes GRADE ASSESSMENT QUALITY RATING: High TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | INCLUSION CRITERIA: Extensive stage histologically or cytologically documented small cell carcinoma of the bronchus; included patients with disease that was not considered to be limited stage. Patients were allowed to have evaluable or measurable disease. Patients had to have a CALGB performance status of 0 to 2 (except for patients in Arm 4); have a life expectancy >= 2 months and lack other serious comorbidity. Patients were also required to be at least 2 weeks since major surgery; at least 3 weeks from cranial irradiation; and were not allowed to have received prior pelvic or mediastinal radiotherapy, systemic chemotherapy or to have ongoing need for corticosteroid administration. Each patient had to be aware of the nature of his/her disease and willingly give written consent to participate in the study after being informed of the experimental nature of the therapy, alternatives, potential benefits, side effects, risks and discomfort. EXCLUSION CRITERIA: patients with a prior or concomitant malignancy, other than curatively treated carcinoma in situ of the cervix, basal cell carcinoma of the skin or other primary cancer that had been completely resected more than 5 years ago (without radiotherapy or chemotherapy). Pregnant people and patients who were < 16 years of age were excluded. Patients could not have had serious medical or psychiatric illness that would prevent informed consent or intensive treatment or would limit survival to less than 2 years. N RANDOMISED: 95 ASSESS STAGE: Yes (ED only) (N LIMITED): (N EXTENSIVE): M: 66 F: 24 AGE: mean: Arm 1 ‐ 60.8; Arm 2 ‐ 64.7; Arm 3 ‐ 58.0; Arm 4 ‐ 61.1 | |
| Interventions | TYPE: Chemotherapy
REGIMENS:
Chemotherapy was given every 21 days for 6 cycles in patients with CR, PR or stable disease.
Arm 1 ‐ consisted of cisplatin 75 mg/m2 intravenously (IV) on day 1 prior to topotecan 1 mg/m2 IV on days 1 to 5.
Arm 2 ‐ cisplatin 75 mg/m2 IV on day 1 prior to paclitaxel 230 mg/m2 IV over 3 hours on day 1.
Arm 3 ‐ paclitaxel 230 mg/m2 IV over 3 hours on day 1 prior to topotecan 1 mg/m2 IV on days 1 to 5.
Arm 4 ‐ paclitaxel 175 mg/m2 IV over 3 hours on day 1 prior to topotecan 1 mg/m2 IV on days 1 to 5. CO‐INTERVENTIONS: G‐CSF was given for all patients until white blood cell count was 10,000/L after day 15 Dexamethasone for patients who received paclitaxel. Thirty minutes prior to paclitaxel administration they also received cimetidine, diphenhydramine and dexamethasone. For patients who attained a CR from chemotherapy, prophylactic cranial irradiation was allowed at the discretion of the investigator. |
|
| Outcomes | OUTCOMES MEASURED: Tumour response Survival Toxicity FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Tumour response Survival Toxicity | |
| Notes | Other: Only Arm 1 and Arm 3 were considered for this meta‐analysis Arm 3 was added in October 1997 after early termination of Arms 1 and 3 due to toxicity and after completion of accrual to Arm 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Tumour Response | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Toxicity | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Adequate |
Postmus 1992.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES: Multicentre
DURATION OF STUDY: April 1986 to June 1987
CONCEALMENT OF ALLOCATION: D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Not described GRADE ASSESSMENT QUALITY RATING: Low TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY INCLUSION CRITERIA: Newly diagnosed patients with histologically or cytologically proven SCLC; no previous chemotherapy; normal renal function (creatinine clearance > 60 ml/min); normal bilirubin (< 25 µmol/L); ECOG performance score <= 3; age <= 70 years; normal number of leukocytes (> 3.0 x 109/L) and platelets (> 100 x 109/L). In the case of bone marrow metastases, all values of leukocytes and platelets were acceptable. Informed consent was obtained from all patients. EXCLUSION CRITERIA: N RANDOMISED: 178 (CDE ‐ 63; IMP ‐ 55; VP ‐ 60) ASSESS STAGE: Yes (N LIMITED): 78 (N EXTENSIVE): 100 M: 142 F: 36 AGE: median: CDE ‐ 59; IMP ‐ 59; VP ‐ 57 (range: CDE ‐ 39 to 70; IMP ‐ 38 to 69; VP ‐ 39 to 70) | |
| Interventions | TYPE: Chemotherapy
REGIMENS:
CDE ‐ cyclophosphamide 1 g/m2 IV on day 1, doxorubicin 45 mg/m2 IV on day 1, and etoposide 100 mg/m2 IV on days 1, 3 and 5. Maximally 5 courses were given at 3‐week intervals between courses. IMP ‐ carboplatin 400 mg/m2 dissolved in 250 ml dextrose 5% and given as a 30 min IV infusion, and ifosfamide 5 g/m2 given as a 24‐hour infusion. Mesna 0.6 g/m2 was given as an IV bolus with 200 ml mannitol (20%) before the ifosfamide infusion. During the ifosfamide infusion and the following 12 hours 3.75 g/m2 mesna was given as a continuous infusion. Forced diuresis was established by giving 6 L of dextrose/saline in 38 hours. Maximally 5 courses were given at 4‐week intervals. VP ‐ carboplatin 400 mg/m2 dissolved in 250 ml dextrose 5% and given as a 30 min IV infusion, and vincristine 2 mg IV bolus on day 1 and 8. Maximally 5 courses were given at 4‐week intervals. CO‐INTERVENTIONS: Patients with a complete response after chemotherapy received prophylactic cranial irradiation 12 x 2.5 Gy |
|
| Outcomes | OUTCOMES MEASURED:
Tumour response (defined according to standard criteria)
Survival
Toxicity FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Tumour response (defined according to standard criteria) Survival Toxicity |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Tumour Response | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Toxicity | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Adequate |
Postmus 1996.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES:
DURATION OF STUDY: September 1988 to February 1992
CONCEALMENT OF ALLOCATION: D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Yes GRADE ASSESSMENT QUALITY RATING: High TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY INCLUSION CRITERIA: Histologically or cytologically proven SCLC; no previous chemotherapy, normal renal function (creatinine clearance > 60 ml/min); normal bilirubin levels (< 25 µmol/L); an ECOG (Easter Cooperative Oncology Group) performance score <= 3; age < 75 years; normal numbers of leukocytes (> 3 × 109/L) and platelets (> 100 × 109/L); extensive disease; and informed consent obtained from all patients. EXCLUSION CRITERIA: N SCREENED: 148 (143 eligible) N RANDOMISED: 143 (CDE ‐ 73; CDE + VIMP ‐ 70) N COMPLETED: ASSESS STAGE: Yes (ED only) (N LIMITED): (N EXTENSIVE): M: 117 F: 26 AGE: median: CDE ‐ 61; CDE + VIMP ‐ 61 (range: CDE ‐ 41 to 73; CDE + VIMP ‐ 29 to 74) | |
| Interventions | TYPE: Chemotherapy
REGIMENS:
Standard therapy (CDE) ‐ 1 g/m2 cyclophosphamide intravenously (IV) on day 1, 45 mg/m2 doxorubicin IV on day 1 and 100 mg/m2 etoposide IV on days 1, 3 and 5. A maximum of 5 courses were given at 3‐week intervals between courses.
Alternating therapy (CDE + VIMP) ‐ 2 mg vincristine IV as a bolus dose on days 1 and 8, 400 mg/m2 carboplatin dissolved in 250 ml 5% dextrose and given as a 30 min IV infusion, 5 g/m2 ifosfamide given as a 24‐hour infusion and 0.6 g/m2 mesna given as an IV bolus with 200 ml mannitol (20%) before infusion with ifosfamide. During the ifosfamide infusion and over the following 12 hours, 3.75 g/m2 of mesna was given as a continuous infusion. Forced diuresis was established by giving 6 L of dextrose/saline in 38 hours. CDE was given during courses 1, 3 and 5 and VIMP during courses 2 and 4. The interval between CDE and VIMP was 3 weeks and between VIMP and CDE was 4 weeks. CO‐INTERVENTIONS: |
|
| Outcomes | OUTCOMES MEASURED: Tumour response (defined according to standard criteria of the World Health Organization) Toxicity Survival FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Tumour response Toxicity Survival | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Tumour Response | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Toxicity | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Adequate |
Quoix 2005.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES: Canada and Europe; multicentric
DURATION OF STUDY:
CONCEALMENT OF ALLOCATION: D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Not described GRADE ASSESSMENT QUALITY RATING: Low TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY INCLUSION CRITERIA: Aged at least 18 years; histologically proven ED SCLC; no prior chemotherapy or immunotherapy; written informed consent from patients, in accordance with local and national guidelines for the centre; at least one non‐ CNS indicator lesion, which was bi‐dimensionally measurable; an Eastern Cooperative Oncology Group performance status score ? 2; life expectancy of at least 3 months; adequate bone marrow reserves (haemoglobin >= 9.0 g/dL, white blood cells >= 3.5 x 109/L, neutrophils >= 1.5 x 109/L, platelets >= 100 x 109/L) and adequate renal and hepatic function (creatinine <= 1.5 mg/dL, creatinine clearance >= 60 mL/min, serum bilirubin <= 2.0 mg/ dL, aspartate transaminase [AST], alanine transaminase [ALT] and alkaline phosphatase <= 2 times the upper limit of normal, or <= 5 times the upper limit of normal if liver metastases were present.) Patients with brain and/or leptomeningeal metastases scan were included, provided they were asymptomatic on neurological examination and were not receiving corticosteroid therapy. EXCLUSION CRITERIA: N RANDOMISED: 82 (T/C ‐ 41; T/E ‐ 41) ASSESS STAGE: Yes (ED only) (N LIMITED): (N EXTENSIVE): 82 M: 57 F: 25 AGE: median: 61 (range: T/C ‐ 33 to 78; T/E ‐ 25 to 78) | |
| Interventions | TYPE: Chemotherapy
REGIMENS:
T/C‐ topotecan 1.25 mg/m2 as a 30 min intravenous (IV) infusion daily for 5 consecutive days, followed by cisplatin 50 mg/m2 as a 3‐hour infusion after the 5th dose of topotecan. Patients also received fluids for hydration (before and for 24 hours after cisplatin) and antiemetic prophylaxis.
T/E ‐ topotecan 0.75 mg/m2 as a 30‐minute IV infusion daily for 5 consecutive days and etoposide 60 mg/m2 as a 30 to 60‐minute IV infusion, daily before each topotecan infusion. Treatment was repeated every 21 days CO‐INTERVENTIONS: Use of therapeutic G‐CSF was allowed but not prophylactically |
|
| Outcomes | OUTCOMES MEASURED:
Tumour response (evaluated according by strict radiological criteria)
Disease progression
Survival
Toxicity
Quality of life FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Tumour response (evaluated according by strict radiological criteria) Survival Toxicity Quality of life |
|
| Notes | Patients who required delays in treatment of more than 2 weeks were considered for alternative cytotoxic therapy off‐protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) Survival | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Tumour Response | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Toxicity | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Quality of Life | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Adequate |
Roth 1992.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES: United States of America; multicentric
DURATION OF STUDY: February 1985 to April 1989
CONCEALMENT OF ALLOCATION: D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Adequate GRADE ASSESSMENT QUALITY RATING: High TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY INCLUSION CRITERIA: Age >= 18 years; histologic or cytologic confirmation of SCLC of the lung; no prior chemotherapy or radiotherapy; patients had extensive disease (ED) which is defined as disease spread beyond the primary site, mediastinum and ipsilateral supraclavicular lymph nodes; patients with brain metastases were eligible; adequate bone marrow reserve was required (total leukocyte count >= 4000 / µL and platelet count >= 100 000/ µL) although patients with documented bone marrow involvement were eligible if their leukocyte and platelet count were greater than 3000 /µL and greater than 50 000 /µL, respectively; adequate hepatic function, defined as serum total bilirubin level less than 2.9 mg/dL; adequate renal function with a serum creatinine level <= 1.8 mg/dL for men or <= 1.5 mg/dL for women; a Zubrod performance status of 0 to 3 was required; all patients gave written consent before entry. EXCLUSION CRITERIA: Patients with malignant pleural effusions as their only site of metastatic disease; patients with previous or concomitant malignancy other than adequately treated squamous cell or basal cell carcinoma or in situ carcinoma of the uterine cervix; patients with clinical congestive cardiac failure, cardiac arrhythmias requiring medical therapy or who had myocardial infarction within 3 months of diagnosis. N RANDOMISED: 437 (EP ‐ 148; CAV ‐ 146; CAV/EP ‐ 143) ASSESS STAGE: Yes (ED only) (N LIMITED): (N EXTENSIVE): 437 M: 370 F: 107 AGE: median EP ‐ 62.2; CAV ‐ 61.7; CAV/EP ‐ 62.6 | |
| Interventions | TYPE: Chemotherapy
REGIMENS:
Regimen A: EP ‐ cisplatin 20 mg/m2/d IV for 5 days in combination with etoposide 80 mg/m2/d IV for 5 days, repeated every 3 weeks for 4 cycles (12 weeks) Regimen B: CAV ‐ cyclophosphamide 1000 mg/m2, doxorubicin 40 mg/m2 and vincristine 1 mg/m2 (maximum 2.0 mg), all given IV on day 1 and repeated every 3 weeks for 6 cycles (18 weeks) CAV/EP ‐ CAV in the same dosages as in regimen B was given on day 1 and EP given in the same dosages as in regimen A on days 22 to 26, with cycles repeated every 6 weeks for 3 cycles (18 weeks) CO‐INTERVENTIONS: Patients with brain metastases were required to have concomitant whole brain radiotherapy and chemotherapy at the initiation of the study |
|
| Outcomes | OUTCOMES MEASURED:
Tumour response
Survival
Toxicity FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Tumour response Survival Toxicity |
|
| Notes | In the cisplatin containing arms, sufficient hydration to ensure a urinary output of at least 100 cc/h before and for 4 hours after the infusion of cisplatin was required | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Tumour Response | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Toxicity | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
Sculier 1990.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES:
DURATION OF STUDY:
CONCEALMENT OF ALLOCATION: D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Yes GRADE ASSESSMENT QUALITY RATING: High TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY
INCLUSION CRITERIA: Patients with pathologically proven SCLC (histology was checked by 2 independent pathologists according to the WHO classification) had to have received no prior therapy (radiotherapy, chemotherapy or surgery), have an evaluable or measurable lesion, a Kamofsky performance status of at least 50, no history of prior malignant tumour except non‐melanoma skin cancer or in situ carcinoma of the cervix, and no demonstrated brain metastasis. In addition, they had to have adequate haematological (white blood cells >= 3000/mm3 and platelets >= 100,000/mm3), renal (serum creatinine < 1.5 mg/dl) and hepatic (serum bilirubin < 1.5 mg/dl) functions, be aged <= 75 years, have no uncontrolled infectious disease, be accessible for follow up and provide informed consent. EXCLUSION CRITERIA: N SCREENED: 221 (201 eligible) N RANDOMISED: 201 (CEV ‐ 95; EV ‐ 106) N COMPLETED: ASSESS STAGE: Yes (N LIMITED): 99 (N EXTENSIVE): 102 M: 181 F: 20 AGE: median: CEV ‐ 61; EV ‐ 62 (range: CEV ‐ 37 to 75; EV ‐ 35 to 74) |
|
| Interventions | TYPE: Chemotherapy
REGIMENS:
CEV ‐ cisplatin 60 mg/m2 on day 1; etoposide 120 mg/m2 on days 1, 2 and 3; vindesine 3 mg/m2 on day 1
EV ‐ etoposide 120 mg/m2 on days 1, 2 and 3; vindesine 3 mg/m2 on day 1 All drugs were IV administered. Cisplatin was given by IV drip over 15 minutes, after prehydration with 1000 ml of 5% dextrose in half normal saline for 1½ hours. Another litre of the same solution was then infused over 2 hours. Patients received 40 mg IV of furosemide during prehydration and immediately after cisplatin administration. Vindesine was given by IV bolus also after the cisplatin administration and prior to the infusion of etoposide. Etoposide was diluted in 500 ml of saline and given over 1 hour. Administration of high doses of metoclopamide was recommended for control of emesis. Courses were repeated every 3 weeks, according to haematologic status, for a total of 8 courses. |
|
| Outcomes | OUTCOMES MEASURED: Tumour response Toxicity Survival FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Tumour response Toxicity Survival | |
| Notes | Other: 20 patients were ineligible because of having: non‐small cell lung cancer (6), brain metastases initially (5), no brain CT scan at initial workup (5), a history of prior malignant disease (2), thrombocytopenia (1), an incomplete initial workup (1). Of the 201 eligible patients, 18 were not evaluable for response to chemotherapy for the following reasons: loss to follow up (4), treatment refusal (2), workup refusal at evaluation (1), death prior to receiving chemotherapy (1), drug dosage violation (2), and early death considered not due to SCLC or toxicity (8). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Tumour Response | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Toxicity | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Adequate |
Sculier 1993.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES:
DURATION OF STUDY:
CONCEALMENT OF ALLOCATION: D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Yes GRADE ASSESSMENT QUALITY RATING: High TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | INCLUSION CRITERIA: Patients with pathologically proven SCLC (using World Health Organization (WHO) classification); no prior therapy (radiotherapy, chemotherapy or surgery); Karnofsky performance status of at least 60; no history of prior malignant tumour except non melanoma skin cancer or in situ carcinoma of the cervix adequate hematologic (WBC count >= 3000/µL and platelet count >= 100,000/ µL), renal (serum creatinine < 1.5 mg/dL) and hepatic (serum bilirubin < 1.5 mg/dL) functions; age less than 75 years; no recent myocardial infarction (< 3 months before date of diagnosis); no congestive cardiac failure or cardiac arrhythmia requiring medical treatment and no uncontrolled hypertension; no uncontrolled infectious disease; patients had to be accessible for follow up and provide informed consent. EXCLUSION CRITERIA: N RANDOMISED: 215 (MDC ‐ 107; SC ‐ 108) ASSESS STAGE: Yes (N LIMITED): 95 (N EXTENSIVE): 120 M: 193 F: 22 AGE: median‐ 61 (range: MDC ‐ 35 to 74; SC ‐ 33 to 74) | |
| Interventions | TYPE: Chemotherapy REGIMENS: MDC ‐ Adriamycin 25 mg/m2 on day 1, cyclophosphamide 500 mg/m2 on day 1, etoposide 120 mg/m2 on day 1, cisplatin 60 mg/m2 on day 8, vindesine 3 mg/m2 on day 8, vincristine 2 mg total dose on day 15, methotrexate 100 mg/m2 on day 15 with leucovorin. SC ‐ Adriamycin 50 mg/m2 on day 1, cyclophosphamide 1 g/m2 on day 1, etoposide 80 mg/m2 on days 1 to 3. All drugs were IV administered. Adriamycin, cyclophosphamide, vindesine, vincristine and methotrexate were administered by bolus. Etoposide was diluted in 250 mL of saline and administered over 1 hour. Cisplastin 60 mg/m2 was administered by IV drip over 15 minutes in 250 mL of saline, after prehydration with 1000 mL of 5% dextrose in half normal saline for 1 hour. Another 1 L of the same solution was infused after cisplatin injection over 1 hour. Patients received 40 mg of IV furosemide during prehydration. Leucovorin (factor citrovorum) was administered 24 hours after methotrexate infusion at 15 mg oral total dose every 6 hours for 2 days. Administration of high dose metoclopramide was recommended for control of emesis. Courses were repeated every 3 weeks, according to haematologic status, for a total of 6 courses. CO‐INTERVENTIONS: Those with complete response (CR) received prophylactic cranial irradiation in a dosage of 30 Gy delivered in 10 fractions over 2 weeks, as soon as CR was documented. Chest irradiation was performed, but not for patients with limited disease (LD). | |
| Outcomes | OUTCOMES MEASURED: Tumour response (evaluated during regular meetings of the group by at least 3 independent observers) Dose‐intensity Survival Toxicity FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Tumour response (evaluated during regular meetings of the group by at least 3 independent observers) Survival Toxicity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Tumour Response | Low risk | Reasons for withdrawals, dropouts and exclusions reported |
| Incomplete outcome data (attrition bias) Toxicity | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Adequate |
Sekine 2014.
| Methods |
STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: Japan DURATION OF STUDY: July 2006 – September 2007; December 2007 – April 2008 CONCEALMENT OF ALLOCATION: Not described DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: No METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Yes METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: N/A DESCRIPTION OF WITHDRAWALS/DROPOUTS: Yes GRADE ASSESSMENT QUALITY RATING: Low TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT COMPLIANCE: CONFOUNDERS: |
|
| Participants |
INCLUSION CRITERIA: The eligibility criteria were histologically or cytologically proven SCLC; no previous chemotherapy; measurable disease; age >= 70 years; Eastern Cooperative Oncology Group performance status (PS) of 0 to 2; life expectancy of >= 2 months; adequate bone marrow function (white blood cell count of 4.0 x 10^9 to 12 x 10^9/L, neutrophil count >= 2.0x10^9/L, hemoglobin >=9.5 g/dL, and platelet count >=100x10^9/L); adequate liver function (aspartate aminotransferase and alanine aminotransferase <= 2.5 times the upper limit of the normal range and total bilirubin < 1.5 mg/dL); adequate renal function (serum creatinine <= 1.5 mg/dL and glomerular filtration rate [GFR] calculated using the Cockcroft‐Gault method >= 30 mL/min); adequate pulmonary function (PaO2 >= 60 Torr under room air); adequate cardiac function (electrocardiogram without abnormal findings requiring treatment and left ventricular ejection fraction measured using echocardiography >= 60%); and written informed consent. Patients who received radiation or surgery for metastatic sites other than the primary site were eligible if they received these treatments two weeks or more before registration for this study. EXCLUSION CRITERIA: Patients were excluded if they had symptomatic brain metastases; pleural or pericardial effusion or ascites that required drainage; superior vena cava syndrome; abnormal cardiac function that required treatment or a history of this condition; interstitial pneumonitis or lung fibrosis identified on chest radiograph; severe infection; serious syndrome of inappropriate secretion of antidiuretic hormone or uncontrolled diabetes mellitus; gastric or duodenal ulcer; or active prior malignancies with a disease‐free interval of less than five years, except for carcinoma in situ. Pregnant or lactating women, men who had no intention of using contraception, and patients who had participated in registration‐directed clinical trials in the previous six months were also ineligible. N SCREENED: 62 N RANDOMISED: 62 (32 in Arm A ‐ non‐platinum (amrubicin) and 30 in Arm B ‐ platinum (carboplatin/etoposide) N COMPLETED: 61 (31 for amrubicin and 30 carboplatin/etoposide) ASSESS STAGE: Yes (N LIMITED): NA (N EXTENSIVE): 62 M: 24 (amrubicin), 24 carboplatin/etoposide F: 8 (amrubicin), 6 (carboplatin/etoposide) MEAN AGE: Median Age: [Amrubicin –76years (70‐88); EP ‐ 75 years (70‐82)] |
|
| Interventions |
TYPE: Chemotherapy REGIMENS AND DOSE: The patients were randomly assigned to receive amrubicin monotherapy (arm A) or carboplatin/etoposide (arm B). In arm A, amrubicin dissolved in 20 mL normal saline was administered once intravenously as a 5‐minute infusion on days 1 to 3, every 3 weeks. At the start of the study, the dose of amrubicin was set at 45 mg/m2/ d for 3 days in patients aged < 75 years and at 40 mg/m2/d for 3 days in patients aged >= 75 years. However, 2 of the first 21 patients in arm A who received amrubicin at 45 mg/m2/d died of severe infection associated with serious myelosuppression, and dose reduction was also required in subsequent cycles in 4 of 8 patients who started at 45 mg/m2/d. CYCLES: In both arms chemotherapy was repeated every 3 weeks for a total of 4‐6 cycles DELIVERY: IV CO‐INTERVENTIONS PERMITTED: Nil CO‐INTERVENTIONS: Nil |
|
| Outcomes |
OUTCOMES MEASURED: Primary endpoint: overall survival Secondary endpoints: objective response rate, toxicity, time to progression and quality of life. FOLLOW‐UP ASSESSMENT POINTS: Objective tumor response was evaluated based on the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.0, using CT or MRI for target and nontarget lesions performed every 4 weeks, and every 2 months after the best tumor response was established. OUTCOMES INCLUDED IN ANALYSES: Survival Response rate Toxicity Quality of Life SUB‐GROUPS INDENTIFIED: Nil – Extensive Stage SCLC (ED‐SCLC) only |
|
| Notes | Trial terminated early due to treatment‐related deaths in non‐platinum (amrubicin) arm Between July 4, 2006, and September 5, 2007, 21 and 22 patients were enrolled in arms A and B, respectively. Two patients in arm A treated with amrubicin at 45 mg/m2/d died from severe infection associated with grade 4 neutropenia (sepsis in the first cycle in one patient and pneumonia in the third cycle in the other). There were no treatment‐related deaths in arm B. The dose of amrubicin was reduced to 40 mg/m2/d in subsequent cycles in 4 of 8 patients who started at 45 mg/m2/d. After a recommendation from the Data Monitoring Committee (DMC), the protocol was amended and amrubicin was administered at 40 mg/m2/d in all patients registered in arm A thereafter. From December 2007 to April 2008, 11 and 8 patients were added to arms A and B, respectively. Of these patients, one in arm A died of amrubicin‐ induced pneumonitis. Enrollment of patients was then terminated early after a DMC recommendation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described and appropriate |
| Allocation concealment (selection bias) | Low risk | Described and appropriate |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not described |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not described |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not described |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not described |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not described |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Survival | Low risk | All randomised patients accounted for; withdrawals and dropouts adequately described |
| Incomplete outcome data (attrition bias) Tumour Response | Low risk | All randomised patients accounted for; withdrawals and dropouts adequately described |
| Incomplete outcome data (attrition bias) Toxicity | Low risk | All randomised patients accounted for; withdrawals and dropouts adequately described |
| Incomplete outcome data (attrition bias) Quality of Life | Low risk | All randomised patients accounted for; withdrawals and dropouts adequately described |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Unclear risk | Trial initially interrupted (to allow for a dose reduction) and then terminated early due to treatment related deaths in non‐platinum (amrubicin) arm |
Smith 1991.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES:
DURATION OF STUDY:
CONCEALMENT OF ALLOCATION: D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Yes GRADE ASSESSMENT QUALITY RATING: High TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY INCLUSION CRITERIA: Histologically confirmed small cell lung cancer. EXCLUSION CRITERIA: N RANDOMISED: 95 (VACE ‐ 48; CVACE ‐ 47) N COMPLETED: 78 ASSESS STAGE: Yes (N LIMITED): 60 (N EXTENSIVE): 35 M: 62 F: 33 AGE: median: VACE ‐ 61; CVACE ‐ 60 (range: 37 to 75; CVACE ‐ 46 to 72) | |
| Interventions | TYPE: Chemotherapy
REGIMENS:
VACE ‐ 6 cycles of the VACE combination; vincristine 1.2 mg/m2, doxorubicin 40 mg/m2 and cyclophosphamide 700 mg/m2 on day 1, plus etoposide 110 mg/m2 daily for 3 days. This cycle was repeated at 3‐weekly intervals.
CVACE ‐ etoposide 110 mg/m2 for 3 days plus cisplatin 100 mg/m2 with mannitol diuresis on the second day for the first 2 3‐weekly cycles.
VACE in the above doses was administered for the next 2 cycles; cycles 5 and 6, if given, consisted of etoposide and cisplatin in the same doses as before. The effects of the treatments were assessed formally at the end of cycle 4. CO‐INTERVENTIONS: 12 complete responders after cycle 6 received prophylactic cranial irradiation. Partial responders received further chemotherapy (with mitomycin C and procarbazine) or radiotherapy or no further treatment depending on individual circumstances. |
|
| Outcomes | OUTCOMES MEASURED: Tumour response Survival Cause of death Effect of prophylactic cranial irradiation FOLLOW‐UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Tumour response Survival | |
| Notes | Patient withdrawal: of the VACE regimen ‐ 4 withdrew due to leukopenia; 1 due to severe vomiting, and of the CVACE regimen ‐ 1 due to renal failure, 4 due to nausea and vomiting or leukopenia and 1 due to cerebral metastases developed after 2 cycles of treatment. Patients who had not responded were withdrawn 3 weeks after cycle 4. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | N/A |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Tumour Response | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Toxicity | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Adequate |
Souhami 1997.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES: United Kingdom
DURATION OF STUDY: February 1993 to December, 1995
CONCEALMENT OF ALLOCATION: D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Not described GRADE ASSESSMENT QUALITY RATING: Low TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY
INCLUSION CRITERIA:
Untreated SCLC based on biopsy findings or on cytologic criteria
WHO performance status grade 2 or 3 or serum alkaline phosphatase levels greater than 1.5 times the upper limit of normal according to the participating institution.
Patients over the age of 75 with any stage SCLC EXCLUSION CRITERIA: Patients who were less than 75 years of age with limited SCLC and performance status 0 or 1 Patients with a medical contraindication to chemotherapy N RANDOMISED: 155 ASSESS STAGE: Yes (N LIMITED): 11 (N EXTENSIVE): 144 M: 84 F: 71 AGE: IV group ‐ 67 (49 to 80); oral group ‐ 66 (50 to 86) |
|
| Interventions | TYPE: Chemotherapy
REGIMENS:
Intravenous chemotherapy group ‐ alternating cycles of:
1) PE ‐ cisplatin (60 mg/m2 on day 1) and etopside (120 mg/m2 IV on day 1, 100 mg given orally twice a day on days 2 and 3)
2) CAV ‐ cyclophosphamide (750 mg/m2), doxorubicin (50 mg/m2) and vincristine (2 mg/m2) all given on day 1 3 alternating cycles of PE and CAV were given at 21‐day intervals Oral etoposide group 100 mg of oral etopside was given twice daily for 5 days every 212 days for 6 cycles CO‐INTERVENTIONS: Palliative therapy was permitted to control symptoms associated with disease progression or for persisting symptoms such as pain CLASSIFICATION OF INTERVENTION (ADJUVANT/NEO‐ADJUVANT/PALLIATIVE): Palliative |
|
| Outcomes | OUTCOMES MEASURED:
Survival
Progression‐free survival
Tumour response
Toxicity
Quality of life FOLLOW‐UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Survival Tumour response Toxicity |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as randomised |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) Survival | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Tumour Response | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Toxicity | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Quality of Life | High risk | Data incomplete |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | High risk | Compared a single oral agent versus multiple intravenous agents |
Sundstrom 2002.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES: Norway, multicentric
DURATION OF STUDY: January 1989 to August 1994
CONCEALMENT OF ALLOCATION:D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Adequate
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Adequate GRADE ASSESSMENT QUALITY RATING: High TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY INCLUSION CRITERIA: Histologically/cytologically confirmed SCLC; age of 18 to 75 years; and Eastern Cooperative Oncology Group (ECOG) performance status <= 2; adequate bone marrow and renal function (WBC count >= 3000/µL, platelet count >= 125,000/ µL, serum creatinine level < 125 µmol/L); no serious cardiovascular disease, and no previous or other concomitant malignant disease with the exception of basal cell carcinoma or in situ carcinoma of the cervix. Patients with evidence of brain metastases were eligible. EXCLUSION CRITERIA: N SCREENED: 440 N RANDOMISED: 436 (EP ‐ 218; CEV ‐ 218) N COMPLETED: ASSESS STAGE: Yes (N LIMITED): 214 (N EXTENSIVE): 222 M: 281 F: 155 AGE: median ‐ 64, range (EP ‐ 43 to 75; CEV ‐ 44 to 75) | |
| Interventions | TYPE: Chemotherapy
REGIMENS:
EP ‐ etoposide 100 mg/m2 followed by cisplatin 75 mg/m2, both administered intravenously (IV) on day 1. Daily oral etoposide 200 mg/m2 was given on days 2 to 4 (on an empty stomach). Standard prehydration and posthydration procedures were followed in conjunction with cisplatin administration. CEV ‐ epirubicin 50 mg/m2, cyclophosphamide 1000 mg/m2, and vincristine 2 mg, all IV on day 1. For both, chemotherapy was administered every 3 weeks to a maximum of 5 courses. Before the third course, all patients were evaluated for a response. CO‐INTERVENTIONS: Patients who achieved complete remission during the treatment period received prophylactic cranial irradiation. ED ‐ SCLC patients did not receive radiotherapy as part of routine therapy. But chest or cranial irradiation was optional if severe symptoms could not be palliated by chemotherapy. |
|
| Outcomes | OUTCOMES MEASURED:
Tumour response (according to World Health Organization response criteria)
Relapse pattern and palliative treatment
Survival
Toxicity
Quality of life FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Tumour response (according to World Health Organization response criteria) Relapse pattern and palliative treatment Survival Toxicity Quality of life |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | N/A |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | N/A |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) Survival | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Tumour Response | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Toxicity | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Quality of Life | High risk | Data incomplete |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Adequate |
Urban 1999a.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES: France
DURATION OF STUDY: October 1 1985 to April 30 1988
CONCEALMENT OF ALLOCATION: C
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Yes GRADE ASSESSMENT QUALITY RATING: High TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY INCLUSION CRITERIA: Patients with proven small cell lung cancer. There were no eligibility restrictions in terms of age, sex, performance status or extent of disease. EXCLUSION CRITERIA: Patients with associated neoplasia, heart failure or renal failure and patients previously treated by surgery, chemotherapy or radiation were eligible for this trial. N RANDOMISED: 394 (standard ‐ 203; alternating ‐ 191) ASSESS STAGE: Yes (N LIMITED): 169 (N EXTENSIVE): 225 M: 361 F: 33 AGE: median: standard ‐ 59 ± 11, alternating ‐ 60 ± 19 (range: standard ‐ 28 to 79; alternating ‐ 29 to 81) | |
| Interventions | TYPE: Chemotherapy
REGIMENS:
Standard regimen ‐ (CCVAP 16) consisted of CCNU 80 mg orally, cyclophosphamide 1000 mg/m2 IV d1, doxorubicin 45 mg/m2 IV d1, and VP 16 225 mg/m2 IV d1.
Alternating regimen ‐ consisted of sequence B with CCNU 80 mg orally d1, cyclophosphamide 1000 mg/m2 IV d1, and doxorubicin 45 mg/m2 IV d1 alternating at 4‐weekly intervals with sequence C, consisting of cisplatin 80 mg/m2 IV d1, vindesine 3 mg/m2 IV d1, and VP 16 225 mg/m2 IV d1. Courses of therapy were administered every 28 days in the 2 groups. CO‐INTERVENTIONS: Thoracic irradiation was scheduled for patients with limited forms of disease after 2 cycles of chemotherapy in the case of no response. Thoracic irradiation delivered 45 to 50 Grays equivalent. Cephalic irradiation was administered to patients with cerebral metastases ‐ 40 Grays equivalent or recommended prophylactically ‐ 24 to 30 Grays equivalent in the case of complete response. |
|
| Outcomes | OUTCOMES MEASURED: Survival Tumour response (defined as best response for at least 1 month at any time during treatment) Toxicity FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Survival Tumour response (defined as best response for at least 1 month at any time during treatment) Toxicity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation reported and adequate |
| Allocation concealment (selection bias) | High risk | Allocation concealment not adequate |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Tumour Response | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Toxicity | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
Urban 1999b.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES: Multicentre, 39 French centres
DURATION OF STUDY: May 1988 to May 1994
CONCEALMENT OF ALLOCATION: C
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Appropriate
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Not described GRADE ASSESSMENT QUALITY RATING: Moderate TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY
INCLUSION CRITERIA:
Biopsy confirmed limited or extensive disease SCLC EXCLUSION CRITERIA: Patients who had undergone thoracic surgery to remove the tumour Patients who had been treated previously with chemotherapy and radiotherapy Patients for whom close follow up would be unlikely Age >70 years, renal or hepatic disease, serious cardiac disease, history of prior malignant tumour in the past 5 years Patients who did not meet the standard criteria for haematological status N RANDOMISED: 457 ASSESS STAGE: Yes (N LIMITED): 97 (N EXTENSIVE): 360 M:37 F: 420 AGE: Mean CDE ‐ 57 +/‐ 9 years; PCDE ‐ 56 +/‐ 10 years |
|
| Interventions | TYPE: Chemotherapy
REGIMENS:
CDE ‐ Cyclophosphamide (1000 mg/m2 on day 1), doxorubicin (45 mg/m2 on day 1) and etoposide (150 mg/m2 on days 1 and 2) PCDE ‐ Cisplatin (100 mg/mm2 on day 1), cyclophosphamide (1000 mg/m2 on day 1), doxorubicin (45 mg/m2 on day 1) and etoposide (150 mg/m2 on days 1 and 2) Chemotherapy was administered every 4 weeks for a total of 6 courses CO‐INTERVENTIONS: Thoracic radiotherapy Prophylactic brain irradiation Brain irradiation CLASSIFICATION OF INTERVENTION: (ADJUVANT/NEO‐ADJUVANT/PALLIATIVE): Palliative |
|
| Outcomes | OUTCOMES MEASURED:
Survival
Tumour response
Haematologic toxicity
Mortality related to toxic events FOLLOW UP ASSESSMENT POINTS: Monthly during treatment 2‐monthly after treatment up to 1 year 3‐monthly from 1 year after treatment OUTCOMES INCLUDED IN ANALYSES: Survival Tumour response Haematologic toxicity Mortality related to toxic events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation reported and adequate |
| Allocation concealment (selection bias) | High risk | Allocation concealment not adequate |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Tumour Response | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Toxicity | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
Veronesi 1994.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES: Italy, multicentric
DURATION OF STUDY: September 1986 to December 1991
CONCEALMENT OF ALLOCATION: D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Adequate GRADE ASSESSMENT QUALITY RATING: High TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY
INCLUSION CRITERIA:
Histologically‐proven small cell lung cancer
Age < 75 years
Karnofsky Performance status > 40
Normal serum creatinine values
Adequate cardiac function
Adequate liver function EXCLUSION CRITERIA: Brain metastases Previous treatment N SCREENED: 139 N RANDOMISED: 136 ASSESS STAGE: Yes (N LIMITED): 55 (CEV ‐ 33; PE 22) (N EXTENSIVE): 81 (CEV ‐ 33; PE ‐ 48) M: 119 (CEV ‐ 59; PE ‐ 60) F: 17 (CEV ‐ 7; PE ‐ 10) AGE: Median: CEV ‐ 60 (41 to 70); PE ‐ 61 (41 to 70) |
|
| Interventions | TYPE: Chemotherapy
REGIMENS:
CEV ‐ cyclophosphamide 1000 mg/m2 IV, epirubicin 70 mg/m2 IV, vincristine 1.2 mg/m2 IV every 3 weeks. This was repeated for 6 cycles. PE ‐ cisplatin 20 mg/m2 IV for 5 consecutive days, every 3 weeks, and etoposide 75 mg/m2 IV given as a 45‐min IV infusion on the same days, plus 1000ml of IV fluids with 100 g of mannitol daily. This was repeated for 6 cycles. CO‐INTERVENTIONS: After 3 cycles, responding patients received radiotherapy to the chest (45 Gy/15 sessions) and to the brain (30 Gy/10 sessions ‐ only in patients with limited disease achieving complete remission). CLASSIFICATION OF INTERVENTION (ADJUVANT/NEO‐ADJUVANT/PALLIATIVE): Palliative |
|
| Outcomes | OUTCOMES MEASURED:
Overall survival
Tumour response
Duration of response
Toxicity FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Overall survival Tumour response Toxicity |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Tumour Response | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Toxicity | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
Wampler 1991.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES:
DURATION OF STUDY: November 1983 to November 1987
CONCEALMENT OF ALLOCATION: D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Not described GRADE ASSESSMENT QUALITY RATING: Low TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY
INCLUSION CRITERIA:
Patients with histologically or cytologically documented extensive disease SCLC without prior treatment
A performance status of 0, 1, 2 or 3
Adequate marrow, renal and hepatic function EXCLUSION CRITERIA: N RANDOMISED: 167 ASSESS STAGE: Yes (ED only) (N LIMITED): (N EXTENSIVE): 167 M: 113 F: 54 AGE: |
|
| Interventions | TYPE: Chemotherapy
REGIMENS:
CAV ‐ 3‐week cycle of cyclophosphamide (1200 mg/m2), doxorubicin (45 mg/m2), vincristine (1.4 mg/m2, maximum dose 2 mg) all on day 1 MEP/CAV ‐ 3‐weekly alternating cycles of MEP ‐ methotrexate (40 mg/m2 on day 1), etopside (100 mg/m2 on days 1, 2, 3) and cisplatin (100 mg/m2 on day 3) CAV ‐ cyclophosphamide (1200 mg/m2), doxorubicin (45 mg/m2), vincristine (1.4 mg/m2, maximum dose 2 mg) all on day 1 CO‐INTERVENTIONS: CLASSIFICATION OF INTERVENTION (ADJUVANT/NEO‐ADJUVANT/PALLIATIVE): Palliative |
|
| Outcomes | OUTCOMES MEASURED:
Survival
Time to progression
Tumour response
Toxicity FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Survival Tumour response Toxicity |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Tumour Response | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Toxicity | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
White 2001.
| Methods | STUDY DESIGN: Parallel group
LOCATION, NUMBER OF CENTRES:
DURATION OF STUDY: June 1996 to January 1999
CONCEALMENT OF ALLOCATION: D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Adequate GRADE ASSESSMENT QUALITY RATING: High TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY
INCLUSION CRITERIA: Patients with histologically or cytologically proven SCLC were eligible for inclusion in the study. They were required to have either a Karnofsky Performance status <= 50 and/or at least 2 of the following adverse prognostic indicators: Karnofsky Performance status < 60; extensive disease; and elevated alkaline phosphatase, lactic dehydrogenase, or low sodium. Patients had received no previous chemotherapy, surgery, or radiotherapy for SCLC, and no prior malignancy was permitted except basal cell carcinoma of the skin or in situ carcinoma of the cervix. A creatinine clearance of at least 30 mL per minute was required, and patients with liver derangement were only eligible if their plasma bilirubin was < 35 mol/L. EXCLUSION CRITERIA: N SCREENED: N RANDOMISED: 119 (CAV ‐ 59; Carboplatin ‐ 60) N COMPLETED: ASSESS STAGE: Yes (N LIMITED): (CAV ‐ 17; Carbo ‐ 21) (N EXTENSIVE): (CAV ‐ 42; Carbo ‐ 39) M: (CAV ‐ 34; Carbo ‐ 29) F: (CAV ‐ 25; Carbo ‐ 31) MEAN AGE: CAV ‐ median 70 (46 to 85); Carbo 70 (54 to 76) |
|
| Interventions | REGIMENS:
Control arm ‐ in the control arm, CAV was administered on day 1 of a 3‐week cycle for 4 cycles. This consisted of cyclophosphamide 750 mg/m2 IV, vincristine 1.3 mg/m2 IV (to a maximum of 2 mg), and doxorubicin 40 mg/m2 IV, all given as a bolus on day 1. Experimental arm ‐ patients who were allocated to the experimental arm received 4 courses of single‐agent carboplatin IV infused over 1 hour and administered at 4‐week intervals. The dosage was derived from the formula of Calvert giving an AUC of 7. Dose reductions were not performed. Chemotherapy was delayed if there was evidence of sepsis or persisting myelosuppression (white blood cells < 3000/mm3, neutrophils < 1500/mm3, and platelets < 100,000/mm3) at the time of scheduled retreatment. Control arm ‐ In the event of significant peripheral neuropathy, vincristine was omitted. CO‐INTERVENTIONS: Patients who had limited disease and an objective response to treatment could be considered for consolidation thoracic and prophylactic cranial irradiation. Erythropoietin and growth factors were permitted at the individual physician's discretion. |
|
| Outcomes | OUTCOMES MEASURED:
Primary: toxicity
Secondary: objective response rate; survival; quality of life FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Toxicity Objective response rate Survival Quality of life |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Tumour Response | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Toxicity | Low risk | Reasons for withdrawals, drop‐outs and exclusions reported |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Adequate |
Wolf 1987.
| Methods | STUDY DESIGN: Parallel study
LOCATION, NUMBER OF CENTRES: 14 German hospitals
DURATION OF STUDY: December 1983 to December 1984
CONCEALMENT OF ALLOCATION: D
DESCRIBED AS RANDOMISED: Yes
DESCRIBED AS DOUBLE BLIND: No
METHOD OF RANDOMISATION WELL‐DESCRIBED/APPROPRIATE: Not described
METHOD OF BLINDING WELL‐DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROP‐OUTS: Not described GRADE ASSESSMENT QUALITY RATING: Low TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT |
|
| Participants | ELIGIBILITY
INCLUSION CRITERIA:
Histologic proof of small cell lung cancer
Signs of measurable or evaluable disease
Karnofsky Performance Status of 50% of more
Informed patient's consent EXCLUSION CRITERIA: Age > 70 years Prior treatment (chemotherapy, radiotherapy or surgery) Existence of accessory malignant disease Evidence of renal dysfunction Chronic hepatic disease Advanced respiratory or cardiac insufficiency N RANDOMISED: 141 ASSESS STAGE: Yes (N LIMITED): 54 (PE ‐ 27; IE ‐ 27) (N EXTENSIVE): 87 (PE ‐ 46; IE ‐ 41) M: 127 (PE ‐ 65; IE ‐ 62) F: 14 (PE ‐ 8; IE ‐ 6) AGE: |
|
| Interventions | TYPE: Chemotherapy
REGIMENS:
PE ‐ cisplatin 80 mg/m2, IV on day 1 and etoposide 150 mg/m2, IV on days 3 through 5. IE ‐ ifosfamide, 1500 mg/m2 IV on days 1 through 5 and etoposide 120 mg/m2 IV on days 3 through 5. A maximum of 6 cycles as administered in 3‐week intervals CO‐INTERVENTIONS: In both arms, second‐line therapy consisted of CAV ‐ cyclophosphamide 600 mg/m2 IV on days 1 and 2, Adriamycin 50 mg/m2 IV on day 1 and vincristine 2 mg IV on day 1. Chest irradiation was applied to patients in both arms. In limited stage, 45 Gy was administered. Patients achieving a complete response received prophylactic cranial irradiation after the third cycle of chemotherapy respectively after the onset of complete response. Patients with cranial metastases received brain irradiation immediately. Painful metastases were irradiated as necessary. CLASSIFICATION OF INTERVENTION (ADJUVANT/NEO‐ADJUVANT/PALLIATIVE): Palliative |
|
| Outcomes | OUTCOMES MEASURED:
Tumour response
Survival
Toxicity FOLLOW UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES: Survival Tumour response |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Investigators | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Survival | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Tumour Response | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Toxicity | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Quality of Life | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) Survival | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Tumour Response | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Toxicity | High risk | Reasons for withdrawals, drop‐outs and exclusions not reported |
| Incomplete outcome data (attrition bias) Quality of Life | Unclear risk | N/A |
| Selective reporting (reporting bias) | Low risk | Adequate |
| Other bias | Low risk | Adequate |
ED = extensive disease IV = intravenous LD = limited disease SCLC = small cell lung cancer
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Artel‐Cortes 2004 | Platinum agents used in all treatment groups |
| Blackstock 2005 | Does not compare chemotherapy regimens |
| De Marinis 2005 | Platinum agents used in all treatment groups |
| Eckardt 2006 | Platinum agents used in all treatment groups |
| Hanna 2006 | Platinum agents used in all treatment groups |
| Lara 2006 | Platinum agents used in all treatment groups |
| McClay 2005 | Platinum agents used in all treatment groups |
| Niell 2005 | Platinum agents used in all treatment groups |
| Paccagnella 2004 | Platinum agents used in all treatment groups |
| Pathak 2005 | Study disease is non‐small cell lung cancer |
| Schild 2004 | Does not compare chemotherapy regimens |
| Seifart 2005 | Platinum agents used in all treatment groups |
Differences between protocol and review
We conducted a new search in August 2014 (search strategy outlined in Appendix 2). This yielded a further 3 studies bringing the total number of studies included in the review to 32 (29 studies were included in the original review). This is illustrated in the study flow diagram (Figure 1). A meta‐analysis was carried out and the conclusions not changed.
A new author joined the team for this update : Saion Chatterjee
Contributions of authors
IUA: protocol writing, data extraction, analysis, review writing ‐ 1st version and update SC: protocol writing, data extraction, analysis, review writing ‐ update JAEW: protocol writing, data extraction, analysis, review writing ‐ 1st version and update RWB: supervisor, protocol writing, review writing ‐ 1st version KMF: supervisor, review writing ‐ 1st version and update
Sources of support
Internal sources
No sources of support supplied
External sources
The Australian Lung Foundation / Lung Cancer Consultative Group Cochrane Review Scholarship, Australia.
Declarations of interest
IUA: none known SC: none known JAEW: none known RWB: none known KMF: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Baka 2008 {published data only}
- Baka S, Califano R, Ferraldeschi R, Ashcroft L, Thatcher N, Taylor P, et al. Phase III randomised trial of doxorubicin‐based chemotherapy compared with platinum‐based chemotherapy in small‐cell lung cancer. British Journal of Cancer 2008;99(3):442‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chahinian 1989 {published data only}
- Chahinian AP, Propert KJ, Ware JH, Zimmer B, Perry MC, Hirsh V, et al. A randomized trial of anticoagulation with warfarin and of alternating chemotherapy in extensive small‐cell lung cancer by the Cancer and Leukemia Group B. Journal of Clinical Oncology 1989 Aug;7(8):993‐1002. [DOI] [PubMed] [Google Scholar]
Creech 1982 {published data only}
- Creech RH, Stanley K, Vogl SE, Ettinger DS, Bonomi PD, Salazar O. Phase II study of cisplatin, maytansine, and chlorozotocin in small cell lung carcinoma (EST 2578). Cancer Treatment Reports 1982 Jun;66(6):1417‐9. [PubMed] [Google Scholar]
de Jong 2007 {published and unpublished data}
- Jong WK, Groen HJ, Koolen MG, Biesma B, Willems LN, Kwa HB, et al. Phase III study of cyclophosphamide, doxorubicin, and etoposide compared with carboplatin and paclitaxel in patients with extensive disease small‐cell lung cancer. European Journal of Cancer 2007;43:2345–2350. [DOI] [PubMed] [Google Scholar]
Eagan 1981 {published data only}
- Eagan RT, Lee RE, Frytak S, Fleming TR, Ingle JN, Creagan ET, et al. An evaluation of low‐dose cisplatin as part of combined modality therapy of limited small cell lung cancer. Cancer Clinical Trials 1981;4(3):267‐71. [PubMed] [Google Scholar]
Evans 1987 {published data only}
- Evans WK, Feld R, Murray N, Willan A, Coy P, Osoba D, et al. Superiority of alternating non‐cross‐resistant chemotherapy in extensive small cell lung cancer. A multicenter, randomized clinical trial by the National Cancer Institute of Canada. Annals of Internal Medicine 1987 Oct;107(4):451‐8. [DOI] [PubMed] [Google Scholar]
Farris 1993 {published data only}
- Farris A, Bisail M, Sarobba MG, Sanna G, Scotto T, Valzelli S, et al. Cisplatin‐VP16 alternating with cyclophosphamide‐epirubicin versus cyclophosphamide‐epirubicin‐vincristine in small cell lung cancer. Journal of Chemotherapy 1993 Oct;5(5):344‐7. [DOI] [PubMed] [Google Scholar]
Fukuoka 1986 {published data only}
- Fukuoka M, Takada M, Negoro S, Kusunoki Y, Matsui K, Ryu S, et al. Alternating non‐cross resistant chemotherapy for small cell lung cancer. Japanese Journal of Clinical Oncology 1986 Sep;16(3):261‐70. [PubMed] [Google Scholar]
Fukuoka 1991 {published data only}
- Fukuoka M, Furuse K, Saijo N, Nishiwaki Y, Ikegami H, Tamura T, et al. Randomized trial of cyclophosphamide, doxorubicin, and vincristine versus cisplatin and etoposide versus alternation of these regimens in small‐cell lung cancer. Journal of the National Cancer Institute 1991 Jun 19;83(12):855‐61. [DOI] [PubMed] [Google Scholar]
Gatzemeier 1994 {published data only}
- Gatzemeier U, Pawel JV, Laumen R, Hossfeld DK, Neuhauss R. Etoposide/vincristine‐based chemotherapy with or without carboplatin in extensive‐stage small cell lung cancer: a prospective randomized phase III trial. Seminars in Oncology 1994 Jun;21(3 Suppl 6):31‐5. [PubMed] [Google Scholar]
Goodman 1990 {published data only}
- Goodman GE, Crowley JJ, Blasko JC, Livingston RB, Beck TM, Demattia MD, et al. Treatment of limited small‐cell lung cancer with etoposide and cisplatin alternating with vincristine, doxorubicin, and cyclophosphamide versus concurrent etoposide, vincristine, doxorubicin, and cyclophosphamide and chest radiotherapy: a Southwest Oncology Group Study. Journal of Clinical Oncology 1990 Jan;8(1):39‐47. [DOI] [PubMed] [Google Scholar]
Greco 2005 {published data only}
- Greco FA, Thompson DS, Morrissey LH, Erland JB, Burris HA, Spigel DR, et al. Paclitaxel/carboplatin/etoposide versus paclitaxel/topotecan for extensive‐stage small cell lung cancer: a Minnie Pearl Cancer Research Network randomized, prospective phase II trial. Oncologist 2005 Oct;10(9):728‐33. [DOI] [PubMed] [Google Scholar]
Havemann 1987 {published data only}
- Havemann K, Wolf M, Holle R, Gropp C, Drings P, Manke HG, et al. Alternating versus sequential chemotherapy in small cell lung cancer. A randomized German multicenter trial. Cancer 1987 Mar 15;59(6):1072‐82. [DOI] [PubMed] [Google Scholar]
Jones 1993 {published data only}
- Jones AL, Holborn J, Ashley S, Smith IE. CVM versus ACE in the treatment of small cell lung cancer. Oncology 1993 Nov;50 Suppl 2:10‐5. [DOI] [PubMed] [Google Scholar]
Kanitz 1992 {published data only}
- Kanitz E, Kolaric K, Jassem J, Mechl Z, Pawlicki M, Ringwald G, et al. Randomized phase II trial of high‐dose 4'‐epi‐doxorubicin + cyclophosphamide versus high‐dose 4'‐epi‐doxorubicin + cisplatin in previously untreated patients with extensive small cell lung cancer. Oncology 1992;49(5):327‐32. [DOI] [PubMed] [Google Scholar]
Lyss 2002 {published data only}
- Lyss AP, Herndon JE, Lynch TJ, Turrisi AT, Watson DM, Grethlein SJ, et al. Novel doublets in extensive‐stage small‐cell lung cancer: a randomized phase II study of topotecan plus cisplatin or paclitaxel (CALGB 9430). Clinical Lung Cancer 2002 Feb;3(3):205‐10; discussion 11‐2. [DOI] [PubMed] [Google Scholar]
Postmus 1992 {published data only}
- Postmus PE, Splinter TA, Palmen FM, Carney DN, Festen J, Burghouts JT, et al. Comparison of two carboplatin‐containing regimens with standard chemotherapy for small cell lung cancer in a randomised phase II study. The EORTC Lung Cancer Cooperative group. European Journal of Cancer 1992;28(1):96‐100. [DOI] [PubMed] [Google Scholar]
Postmus 1996 {published data only}
- Postmus PE, Scagliotti G, Groen HJ, Gozzelino F, Burghouts JT, Curran D, et al. Standard versus alternating non‐cross‐resistant chemotherapy in extensive small cell lung cancer: an EORTC Phase III trial. European Journal Cancer 1996 Aug;32A(9):1498‐503. [DOI] [PubMed] [Google Scholar]
Quoix 2005 {published data only}
- Quoix E, Breton JL, Gervais R, Wilson J, Schramel F, Cardenal F, et al. A randomised phase II study of the efficacy and safety of intravenous topotecan in combination with either cisplatin or etoposide in patients with untreated extensive disease small‐cell lung cancer. Lung Cancer 2005 Aug;49(2):253‐61. [DOI] [PubMed] [Google Scholar]
Roth 1992 {published data only}
- Roth BJ, Johnson DH, Einhorn LH, Schacter LP, Cherng NC, Cohen HJ, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small‐cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. Journal of Clinical Oncology 1992 Feb;10(2):282‐91. [DOI] [PubMed] [Google Scholar]
Sculier 1990 {published data only}
- Sculier JP, Klastersky J, Libert P, Ravez P, Thiriaux J, Lecomte J, et al. A randomized study comparing etoposide and vindesine with or without cisplatin as induction therapy for small cell lung cancer. EORTC Lung Cancer Working Party. Annals of Oncology 1990;1(2):128‐33. [DOI] [PubMed] [Google Scholar]
Sculier 1993 {published data only}
- Sculier JP, Paesmans M, Bureau G, Dabouis G, Libert P, Vandermoten G, et al. Multiple‐drug weekly chemotherapy versus standard combination regimen in small‐cell lung cancer: a phase III randomized study conducted by the European Lung Cancer Working Party. Journal of Clinical Oncology 1993 Oct;11(10):1858‐65. [DOI] [PubMed] [Google Scholar]
Sekine 2014 {published data only}
- Sekine I, Okamoto H, Horai T, Nakagawa K, Ohmatsu H, Yokoyama A, et al. A randomized phase III study of single‐agent amrubicin vs. carboplatin/etoposide in elderly patients with extensive‐disease small‐cell lung cancer. Clinical Lung Cancer 2014;15(2):96‐102. [DOI] [PubMed] [Google Scholar]
Smith 1991 {published data only}
- Smith AP, Anderson G, Chappell G, Bowen DR. Does the substitution of cisplatin in a standard four drug regimen improve survival in small cell carcinoma of the lung? A comparison of two chemotherapy regimens. Thorax 1991 Mar;46(3):172‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Souhami 1997 {published data only}
- Souhami RL, Spiro SG, Rudd RM, Ruiz de Elvira MC, James LE, Gower NH, et al. Five‐day oral etoposide treatment for advanced small‐cell lung cancer: randomized comparison with intravenous chemotherapy. Journal of the National Cancer Institute 1997 Apr 16;89(8):577‐80. [DOI] [PubMed] [Google Scholar]
Sundstrom 2002 {published data only}
- Sundstrom S, Bremnes RM, Kaasa S, Aasebo U, Hatlevoll R, Dahle R, et al. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small‐cell lung cancer: results from a randomized phase III trial with 5 years' follow‐up. Journal of Clinical Oncology 2002 Dec 15;20(24):4665‐72. [DOI] [PubMed] [Google Scholar]
Urban 1999a {published data only}
- Urban T, Baleyte T, Chastang CL, Jeannin L, Delaval P, Zaegel M, et al. Standard combination versus alternating chemotherapy in small cell lung cancer: a randomised clinical trial including 394 patients. 'Petites Cellules' Group. Lung Cancer 1999 Aug;25(2):105‐13. [DOI] [PubMed] [Google Scholar]
Urban 1999b {published data only}
- Urban T, Chastang C, Lebas FX, Duhamel JP, Adam G, Darse J, et al. The addition of cisplatin to cyclophosphamide‐doxorubicin‐etoposide combination chemotherapy in the treatment of patients with small cell lung carcinoma: A randomized study of 457 patients. 'Petites Cellules' Group. Cancer 1999 Dec 1;86(11):2238‐45. [PubMed] [Google Scholar]
Veronesi 1994 {published data only}
- Veronesi A, Cartei G, Crivellari D, Magri MD, Della Valentina M, Foladore S, et al. Cisplatin and etoposide versus cyclophosphamide, epirubicin and vincristine in small cell lung cancer: a randomised study. European Journal of Cancer 1994;30A(10):1474‐8. [DOI] [PubMed] [Google Scholar]
Wampler 1991 {published data only}
- Wampler GL, Heim WJ, Ellison NM, Ahlgren JD, Fryer JG. Comparison of cyclophosphamide, doxorubicin, and vincristine with an alternating regimen of methotrexate, etoposide, and cisplatin/cyclophosphamide, doxorubicin, and vincristine in the treatment of extensive‐disease small‐cell lung carcinoma: a Mid‐Atlantic Oncology Program study. Journal of Clinical Oncology 1991 Aug;9(8):1438‐45. [DOI] [PubMed] [Google Scholar]
White 2001 {published data only}
- White SC, Lorigan P, Middleton MR, Anderson H, Valle J, Summers Y, et al. Randomized phase II study of cyclophosphamide, doxorubicin, and vincristine compared with single‐agent carboplatin in patients with poor prognosis small cell lung carcinoma. Cancer 2001 Aug 1;92(3):601‐8. [DOI] [PubMed] [Google Scholar]
Wolf 1987 {published data only}
- Wolf M, Havemann K, Holle R, Gropp C, Drings P, Hans K, et al. Cisplatin/etoposide versus ifosfamide/etoposide combination chemotherapy in small‐cell lung cancer: a multicenter German randomized trial. Journal of Clinical Oncology 1987 Dec;5(12):1880‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Artel‐Cortes 2004 {published data only}
- Artel‐Cortes A, Gomez‐Codina J, Gonzalez‐Larriba JL, Barneto I, Carrato A, Isla D, et al. Prospective randomized phase III trial of etoposide/cisplatin versus high‐dose epirubicin/cisplatin in small‐cell lung cancer. Clinical Lung Cancer 2004 Nov;6(3):175‐83. [DOI] [PubMed] [Google Scholar]
Blackstock 2005 {published data only}
- Blackstock AW, Bogart JA, Matthews C, Lovato JF, McCoy T, Livengood K, et al. Split‐course versus continuous thoracic radiation therapy for limited‐stage small‐cell lung cancer: final report of a randomized phase III trial. Clinical Lung Cancer 2005 Mar;6(5):287‐92. [DOI] [PubMed] [Google Scholar]
De Marinis 2005 {published data only}
- Marinis F, Nelli F, Lombardo M, Ferrau F, Barbera S, Bertetto O, et al. A multicenter, randomized, Phase II study of cisplatin, etoposide, and gemcitabine or cisplatin plus gemcitabine as first‐line treatment in patients with poor‐prognosis small cell lung carcinoma. Cancer 2005 Feb 15;103(4):772‐9. [DOI] [PubMed] [Google Scholar]
Eckardt 2006 {published data only}
- Eckardt JR, Pawel J, Papai Z, Tomova A, Tzekova V, Crofts TE, et al. Open‐label, multicenter, randomized, phase III study comparing oral topotecan/cisplatin versus etoposide/cisplatin as treatment for chemotherapy‐naive patients with extensive‐disease small‐cell lung cancer. Journal of Clinical Oncology 2006 May 1;24(13):2044‐51. [DOI] [PubMed] [Google Scholar]
Hanna 2006 {published data only}
- Hanna N, Bunn PA, Langer C, Einhorn L, Guthrie T, Beck T, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive‐stage disease small‐cell lung cancer. Journal of Clinical Oncology 2006 May 1;24(13):2038‐43. [DOI] [PubMed] [Google Scholar]
Lara 2006 {published data only}
- Lara PN Jr, Gandara DR, Natale RB. Randomized phase III trial of cisplatin/irinotecan versus cisplatin/etoposide in patients with extensive‐stage small‐cell lung cancer. Clinical Lung Cancer 2006 Mar;7(5):353‐6. [DOI] [PubMed] [Google Scholar]
McClay 2005 {published data only}
- McClay EF, Bogart J, Herndon JE, Watson D, Evans L, Seagren SL, et al. A phase III trial evaluating the combination of cisplatin, etoposide, and radiation therapy with or without tamoxifen in patients with limited‐stage small cell lung cancer: Cancer and Leukemia Group B Study (9235). American Journal of Clinical Oncology 2005 Feb;28(1):81‐90. [DOI] [PubMed] [Google Scholar]
Niell 2005 {published data only}
- Niell HB, Herndon JE, Miller AA, Watson DM, Sandler AB, Kelly K, et al. Randomized phase III intergroup trial of etoposide and cisplatin with or without paclitaxel and granulocyte colony‐stimulating factor in patients with extensive‐stage small‐cell lung cancer: Cancer and Leukemia Group B Trial 9732. Journal of Clinical Oncology 2005 Jun 1;23(16):3752‐9. [DOI] [PubMed] [Google Scholar]
Paccagnella 2004 {published data only}
- Paccagnella A, Favaretto A, Oniga F, Barbieri F, Ceresoli G, Torri W, et al. Cisplatin versus carboplatin in combination with mitomycin and vinblastine in advanced non small cell lung cancer. A multicenter, randomized phase III trial. Lung Cancer 2004 Jan;43(1):83‐91. [DOI] [PubMed] [Google Scholar]
Pathak 2005 {published data only}
- Pathak AK, Bhutani M, Guleria R, Bal S, Mohan A, Mohanti BK, et al. Chemotherapy alone vs. chemotherapy plus high dose multiple antioxidants in patients with advanced non small cell lung cancer. Journal of the American College of Nutrition 2005 Feb;24(1):16‐21. [DOI] [PubMed] [Google Scholar]
Schild 2004 {published data only}
- Schild SE, Bonner JA, Shanahan TG, Brooks BJ, Marks RS, Geyer SM, et al. Long‐term results of a phase III trial comparing once‐daily radiotherapy with twice‐daily radiotherapy in limited‐stage small‐cell lung cancer. International Journal of Radiation, Oncology, Biology, Physics 2004 Jul 15;59(4):943‐51. [DOI] [PubMed] [Google Scholar]
Seifart 2005 {published data only}
- Seifart U, Jensen K, Ukena J, Mueller C, Schroder M, Fuhr HG, et al. Randomized phase II study comparing topotecan/cisplatin administration for 5 days versus 3 days in the treatment of extensive stage small cell lung cancer (SCLC). Lung Cancer 2005 Jun;48(3):415‐22. [DOI] [PubMed] [Google Scholar]
Additional references
Abeloff 2004
- Abeloff MD. Clinical oncology. 3rd Edition. Philadelphia, Pa: Elsevier Churchill Livingstone, 2004. [Google Scholar]
ACN 2004
- ACN. Clinical practice guidelines for the prevention, diagnosis and management of lung cancer. Clinical practice guidelines for the prevention, diagnosis and management of lung cancer. Sidney: Cancer Council Australia, 2004. [Google Scholar]
Alberg 2003
- Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest Jan 2003;123(1 Suppl):21S‐49S. [DOI] [PubMed] [Google Scholar]
Cella 2002
- Cella D, Eton DT, Fairclough DL, Bonomi P, Heyes AE, Silberman C, et al. What is a clinically meaningful change on the FACT‐L Questionnaire? Results of ECOG study 5592. J Clin Epidemiology 2002;55(3):285‐95. [DOI] [PubMed] [Google Scholar]
Chabner 2010
- Chabner BA, Longo DL. Cancer Chemotherapy and Biotherapy: Principles and Practice (Chabner, Cancer Chemotherapy and Biotherapy). 5. LWW, December 2010. [Google Scholar]
Chan 2013
- Chan BA, Coward JI. Chemotherapy advances in small‐cell lung cancer. Journal of Thoracic Disease October 2013;5(Supplement 5):S565‐S578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dela Cruz 2011
- Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clinics in Chest Medicine December 2011;32(4):605‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 1986
- Evans WK, Feld R, Murray N, Pater J, Shelley W, Willan A, et al. The use of VP‐16 plus cisplatin during induction chemotherapy for small‐cell lung cancer. Seminars in Oncology 1986;13(3 (Suppl 3)):10‐6. [PubMed] [Google Scholar]
Glisson 2014
- Glisson BS, Byers LA. Pathobiology and staging of small cell carcinoma of the lung. UpToDate. Waltham, MA: UpToDate, Nov 2014. [Google Scholar]
Gralla 2004
- Gralla RJ. Quality‐of‐life considerations in patients with advanced lung cancer: effect of topotecan on symptom palliation and quality of life. Oncologist 2004;9 Suppl 6:14‐24. [DOI] [PubMed] [Google Scholar]
Gusi 2010
- Gusi N, Olivares PR, Rajendram R. The EQ‐5D Health‐Related Quality of Life Questionnaire. Handbook of Disease Burdens and Quality of Life Measures. Springer New York, 2010:87‐99. [Google Scholar]
Heintz 2010
- Heintz NH, Janssen‐Heininger YM, Mossman BT. Asbestos, lung cancers, and mesotheliomas: from molecular approaches to targeting tumor survival pathways. American Journal of Respiratory Cell and Molecular Biology Feb 2010;42(2):133‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ (editors). Chapter 7. Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Chichester, UK: John Wiley & Sons for The Cochrane Collaboration..
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Le Pechoux 2004
- Pechoux C, Dhermain F, Bretel JJ, Laplanche A, Dunant A, Tarayre M, et al. Modalities of radiotherapy in small cell lung cancer: thoracic radiotherapy and prophylactic cerebral irradiation. Revue de Pneumologie Clinique 2004;3S:91‐103. [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Micke 2002
- Micke P, Faldum A, Metz T, Beeh K‐M, Bittinger F, Hengstler J‐G, Buhl R. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer ‐ what limits limited disease?. Lung Cancer September 2002;37(3):271‐276. [DOI] [PubMed] [Google Scholar]
Park 2003
- Park JO, Lee SI, Song SY, Kim K, Kim WS, Jung CW, et al. Measuring response in solid tumors: comparison of RECIST and WHO response criteria. Japanese Journal of Clinical Oncology September 2003;33(10):533‐537. [DOI] [PubMed] [Google Scholar]
Peckham 1995
- Peckham MJ, Pinedo HM, Veronesi U. Oxford Textbook of Oncology. Oxford University Press, 1995. [Google Scholar]
Pujol 2000
- Pujol JL, Carestia L, Daures JP. Is there a case for cisplatin in the treatment of small‐cell lung cancer? A meta‐analysis of randomized trials of a cisplatin‐containing regimen versus a regimen without this alkylating agent. British Journal of Cancer 2000;83(1):8‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Stinchcombe 2010
- Stinchcombe TE, Gore EM. Limited‐Stage Small Cell Lung Cancer: Current Chemoradiotherapy Treatment Paradigms. The Oncologist Feb 2010;15(2):187‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stubblefield 2009
- Stubblefield M, O'Dell M. Cancer Rehabilitation: Principles and Practice. New York City: Demos Medical Publishing, Apr 22, 2009. [Google Scholar]
WHO 1979
- World Health Organization. WHO handbook for reporting results of cancer treatment. WHO offset publication no 48.. Geneva, Switzerland: World Health Organization, 1979. [Google Scholar]
References to other published versions of this review
Amarasena 2008
- Amarasena IU, Walters JA, Wood‐Baker R, Fong K. Platinum versus non‐platinum chemotherapy regimens for small cell lung cancer. Cochrane Database of Systematic Reviews 2008, Issue Oct 8;(4):CD006849. [DOI: 10.1002/14651858.CD006849.pub2] [DOI] [PubMed] [Google Scholar]


