ABSTRACT
Background
Vitamin D is important for bone health; in 2014 it was the fifth most commonly ordered laboratory test among Medicare Part B payments.
Objectives
The aim of this study was to describe vitamin D status in the US population in 2011–2014 and trends from 2003 to 2014.
Methods
We used serum 25-hydroxyvitamin D data from NHANES 2011–2014 (n = 16,180), and estimated the prevalence at risk of deficiency (<30 nmol/L) or prevalence at risk of inadequacy (30–49 nmol/L) by age, sex, race and Hispanic origin, and dietary intake of vitamin D. We also present trends between 2003 and 2014.
Results
In 2011–2014, the percentage aged ≥1 y at risk of vitamin D deficiency or inadequacy was 5.0% (95% CI: 4.1%, 6.2%) and 18.3% (95% CI: 16.2%, 20.6%). The prevalence of at risk of deficiency was lowest among children aged 1–5 y (0.5%; 95% CI: 0.3%, 1.1%), peaked among adults aged 20–39 y (7.6%; 95% CI: 6.0%, 9.6%), and fell to 2.9% (95% CI: 2.0%, 4.0%) among adults aged ≥60 y; the prevalence of at risk of inadequacy was similar. The prevalence of at risk of deficiency was higher among non-Hispanic black (17.5%; 95% CI: 15.2%, 20.0%) than among non-Hispanic Asian (7.6%; 95% CI: 5.9%, 9.9%), non-Hispanic white (2.1%; 95% CI: 1.5%, 2.7%), and Hispanic (5.9%; 95% CI: 4.4%, 7.8%) persons; the prevalence of at risk of inadequacy was similar. Persons with higher vitamin D dietary intake or who used supplements had lower prevalences of at risk of deficiency or inadequacy. From 2003 to 2014 there was no change in the risk of vitamin D deficiency; the risk of inadequacy declined from 21.0% (95% CI: 17.9%, 24.5%) to 17.7% (95% CI: 16.0%, 19.7%).
Conclusion
The prevalence of at risk of vitamin D deficiency in the United States remained stable from 2003 to 2014; at risk of inadequacy declined. Differences in vitamin D status by race and Hispanic origin warrant additional investigation.
Keywords: vitamin D status, 25(OH)D, NHANES, supplements, survey, trend, diet
Introduction
In 2014, vitamin D3 concentration [serum 25-hydroxycholecalciferol, 25(OH)D3] was the fifth most common laboratory test ordered among Medicare Part B payments, accounting for $323 million (1). Although the utility of screening in the primary care setting has been questioned (2), population-based nutritional surveillance can be used to describe the diet–disease relations, develop hypotheses for future research (3), and monitor the impact of food-fortification policies (4).
Adequate concentrations of vitamin D are necessary for bone health, immune function, and other health outcomes (5). Dietary sources of vitamin D include fatty fish, fortified foods, such as milk with vitamin D added, and dietary supplements. In addition to obtaining vitamin D from food sources, vitamin D is also made in the body when ultraviolet B rays from sunlight are absorbed by 7-dehydrocholesterol in the skin, which initiates synthesis of vitamin D in the body. Factors that can modify vitamin D synthesis in the body include: season of the year, time of day, length of day, cloud cover, smog, skin pigmentation, and use of sunscreen (6, 7). Vitamin D status is assessed by measuring circulating serum 25-hydroxyvitamin D [25(OH)D] both in clinical settings and from surveillance of the population because 25(OH)D takes into account vitamin D intake (food, beverages, and dietary supplements) and synthesis via the skin. Serum 25(OH)D concentrations are measured in HANES.
Low concentrations of serum 25(OH)D (<30 nmol/L) are associated with clinical pathology, such as rickets and osteomalacia. Higher concentrations of serum 25(OH)D above the deficiency range, yet not sufficient, carry a risk of inadequacy, as they are associated with bone mineral depletion and fractures. Above sufficiency, chronic high concentrations of 25(OH)D (>125 nmol/L) that extend beyond seasonal variation are concerning because they carry no additional health benefits, but have the potential for adverse consequences (8).
This report provides the most recent estimates for the US population of the prevalence of at risk of deficiency or inadequacy of vitamin D by sex, age, race and Hispanic origin, and by intake of vitamin D from food and beverages, and supplement use. For the first time, we present estimates for non-Hispanic Asian individuals. We also present trends from 2003 to 2004 through 2013 to 2014.
Methods
Study design
NHANES is a complex, stratified, multistage probability sample of the US noninstitutionalized population, conducted by the National Center of Health Statistics. Since 1999, NHANES has continuously collected and publicly released data in 2-y cycles. Participants receive a detailed in-home interview, followed by a physical examination, which includes the collection of a blood sample. Participants aged ≥18 y provide consent; children and adolescents aged 7–17 y provide documented assent, and parental permission is obtained for those aged <18 y. Non-Hispanic Asian, non-Hispanic black, and Hispanic individuals were oversampled for the 2011–2014 survey years to obtain reliable estimates for these population subgroups (9). The National Center of Health Statistics Research Ethics Review Board approved the NHANES protocol.
Trend analysis for those ≥1 y started in 2003–2004, the first survey cycle in which NHANES measured 25(OH)D in participants in this age group; however, serum 25(OH)D measurement values are available for participants aged ≥12 y beginning with NHANES III (1988–1994) (10). The unweighted examination response rates for all participants were 76%, 77%, 75%, 77%, 70%, and 69% for the 2003–2004, 2005–2006, 2007–2008, 2009–2010, 2011–2012, and 2013–2014 survey cycles, respectively (11).
Laboratory measurement of 25(OH)D
From 2000 to 2006, serum total 25(OH)D was measured by radioimmunoassay (RIA; DiaSorin) (12). Beginning in 2007–2008, and through 2013–2014, a fully validated standardized liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was used to measure 25(OH)D3, 25-hydroxyvitamin D2 [25(OH)D2], and the C3 epimer of 25(OH)D3 for all eligible participants. For the LC-MS/MS method, total 25(OH)D was defined as the sum of 25(OH)D3 and 25(OH)D2, excluding the C3 epimer of 25(OH)D3. For the correct interpretation of trends, the NHANES 2003–2006 vitamin D data have been converted, using regression, to equivalent 25(OH)D measurements from the standardized (LC-MS/MS) method. Detailed information on the conversion of RIA data to LC-MS/MS-equivalent data and the Vitamin D Standardization Program can be found elsewhere (13–15).
Cut-points for 25(OH)D (primary outcome variables)
The National Academy of Medicine–recommended cut-points for vitamin D status using serum 25(OH)D were used for these analyses (8). These cutoffs are: at risk of deficiency [serum 25(OH)D <30 nmol/L (<12 ng/mL)], at risk of inadequacy [serum 25(OH)D 30–49 nmol/L (12–19 ng/mL)], sufficiency [serum 25(OH)D 50–125 nmol/L (20–50 ng/mL)], and concentrations of possible concern [serum 25(OH)D >125 nmol/L (>50 ng/mL)] (8).
Vitamin D from foods and beverages
Trained interviewers, who used a computer-assisted dietary interview system that included a multiple-pass format with standardized probes (16), collected type and quantity of all food and beverages consumed in the 24 h previous to the physical examination at the Mobile Exam Center (MEC) (specifically from midnight to midnight). Although a second 24-h dietary interview was collected by telephone 3–10 d later, only the first recall was used in the current analysis and results refer to intake “on a given day.” Random errors and day-to-day variation associated with 24-h recalls will cancel out if the data are collected evenly across days of the week and seasons of the year as is done in NHANES; therefore a single 24-h recall is representative of mean population intake (17).
Proxies, generally a parent, provided dietary recalls for children 1–5 y, and assisted children aged 6–11 y; study participants ≥12 y self-reported their dietary interview. Dietary interviews were marked unreliable if they were incomplete (i.e., all 5 steps in the multiple pass were not finished) or if they included an eating occasion with missing information about the type and quantity of foods consumed (16, 18). We only used records deemed reliable in the current analysis (98%).
The USDA's Food and Nutrient Database for Dietary Studies (19, 20) was used to code dietary intake data and calculate vitamin D intakes from all of the foods and beverages participants reported consuming. Vitamin D first appeared in the Food and Nutrient Database for Dietary Studies in the 2007–2008 NHANES survey cycle.
Vitamin D from dietary supplements
As part of the in-home interview, NHANES participants are asked to provide information on the frequency, duration, and amount of dietary supplements used in the past 30 d (21, 22). Interviewers also examined each dietary supplement container and recorded the complete product information so that each reported dietary supplement could be matched or entered into the NHANES Dietary Supplement Database. For the current analysis, dietary supplement use was defined as reporting the consumption of ≥1 dietary supplement containing vitamin D in the past 30 d.
Statistical analysis
We present overall population estimates for the prevalence of vitamin D sufficiency and the prevalence of concentrations of concern. The prevalence of at risk of deficiency or at risk of inadequacy are presented by gender (male, female), age (1–5, 6–11, 12–19, 20–39, 40–59, ≥60 y), race and Hispanic origin (non-Hispanic white, non-Hispanic black, non-Hispanic Asian, and Hispanic), tertiles of vitamin D intake from foods and beverages (0–2.0, 2.1–5.1, >5.0 μg), and use of a dietary supplement containing vitamin D (no, yes). Race- and Hispanic origin-specific estimates reflect persons reporting only 1 race; those reporting >1 race are included in the total but are not reported separately.
For practical reasons, NHANES collects data in the south during the winter months (November–March) and in the north during the summer months (April–October). Since vitamin D is produced in the skin by sunlight exposure, it can also vary by season. Consequently, we used logistic regression to estimate predicted marginals, adjusted for season to control for differences in the time of year when blood was drawn. Estimates were also adjusted for age. Examination sample weights, which account for the differential probabilities of selection, nonresponse, and poststratification, were incorporated into the estimation process except when the association of dietary sources of vitamin D and serum 25(OH)D were explored. For these analyses, dietary weights, which account for additional nonresponse and differential allocation by weekdays, were used. All variance estimates accounted for the complex survey design by using Taylor series linearization. Statistical hypotheses were tested by using logistic regression at an α level of 0.002, after applying a Bonferroni correction for 30 tests, driven by the number of pairwise tests performed to assess differences by race and Hispanic origin over 3 levels of dietary intake from food and beverages, and 2 categories of use of a dietary supplement containing vitamin D. All comparisons are significant at P < 0.002 unless otherwise noted. Data from NHANES 2011–2014 were used to test for differences between subgroups. In testing for trends in vitamin D status, orthogonal contrasts were used with the six 2-y cycles. Data analyses were conducted with SAS System for Windows version 9.4 (SAS Institute Inc.) and SUDAAN version 11.0 (RTI International).
Results
In NHANES 2011–2014, 18,378 participants aged ≥1 y were interviewed. Of these, 16,180 had their 25(OH)D evaluated and were included in the current analysis (Supplemental Figure 1). For the trend analysis, using data from NHANES 2003–2014, 56,139 participants aged ≥1 y were interviewed and 48,430 had 25(OH)D measured (Supplemental Figure 2).
In 2011–2014, nearly three-quarters (73.0%; 95% CI: 70.2%, 75.6%) of the population aged ≥1 y had a serum 25(OH)D value considered sufficient (data not shown). Almost one-fifth (18.3%; 95% CI: 16.2%, 20.6%) of the population had serum 25(OH)D values categorized as at risk of inadequacy (Table 1). The prevalence of at risk of deficiency was 5.0% (95% CI: 4.1%, 6.2%). A high serum 25(OH)D value, associated with concentrations of concern, was observed in 3.7% (95% CI: 3.1%, 4.4%) of persons aged ≥1 y (data not shown).
TABLE 1.
Prevalence of at risk of deficiency [serum 25(OH)D <30 nmol/L] or at risk of inadequacy [serum 25(OH)D 30–49 nmol/L] for US persons aged ≥1 y, by dietary and demographic variables, NHANES 2011–20141
| n | At risk of deficiency | At risk of inadequacy | |
|---|---|---|---|
| <30 nmol/L | 30–49 nmol/L | ||
| All (≥1 y) | 16,180 | 5.0 (4.1, 6.2) | 18.3 (16.2, 20.6) |
| Gender2 | |||
| Male | 8,013 | 4.4 (3.5, 5.5) | 18.7 (16.3, 21.4) |
| Female | 8,167 | 5.7 (4.5, 7.0) | 17.8 (15.8, 20.1) |
| Age,3 y | |||
| 1–5 | 1,438 | 0.5 (0.3, 1.1)* | 6.6 (5.2, 8.3)* |
| 6–11 | 2,060 | 1.4 (1.1, 1.9) | 12.3 (10.0, 15.0) |
| 12–19 | 2,355 | 4.8 (3.5, 6.4) | 22.7 (19.3, 26.4) |
| 20–39 | 3,564 | 7.6 (6.0, 9.6) | 23.8 (20.9, 27.0) |
| 40–59 | 3,496 | 5.7 (4.6, 7.0) | 18.6 (15.9, 21.6) |
| ≥60 | 3,267 | 2.9 (2.0, 4.0) | 12.3 (10.7, 14.1) |
| Race and Hispanic origin2,4 | |||
| Non-Hispanic white | 5,603 | 2.1 (1.5, 2.7)a | 11.8 (10.0, 13.8) a |
| Non-Hispanic black | 3,929 | 17.5 (15.2, 20.0)b | 35.8 (33.3, 38.5)b |
| Non-Hispanic Asian | 1,845 | 7.6 (5.9, 9.9)c | 29.1 (26.4, 32.0)c |
| Hispanic | 4,145 | 5.9 (4.4, 7.8)c | 26.3 (23.0, 29.8)c |
| Vitamin D from foods and beverages,2,5 μg | |||
| 0.0–2.0 | 4,765 | 7.1 (5.7, 8.9)** | 21.0 (18.6, 23.7)** |
| 2.1–5.1 | 4,713 | 4.7 (3.4, 6.5) | 18.0 (15.5, 20.7) |
| >5.1 | 5,213 | 2.6 (2.0, 3.4) | 15.7 (13.4, 18.6) |
| Vitamin D from supplements2,5 | |||
| No | 9,975 | 6.9 (5.6, 8.5)a | 24.2 (21.7, 26.9)a |
| Yes | 4,716 | 1.1 (0.8, 1.6)b | 7.6 (6.2, 9.3)b |
Source: CDC/National Center for Health Statistics. Unweighted sample size, weighted proportions, and 95% CI. Unless otherwise noted, MEC weights used. Within logistic models, pairwise differences were evaluated using t-tests and orthogonal contrast matrices were used to test for linear and quadratic trends. Different lowercase letters within a column and covariate category indicate significant differences, after adjustment with the Bonferroni method for multiple comparisons, P < 0.002. *Significant quadratic trend by age, P < 0.002. **Significant linear trend by tertiles of vitamin D intake from food and beverages, P < 0.002. Vitamin D intake unit conversion: 1 μg is 40 IU; the 0.0–2.0, 2.1–5.1, and >5.1 μg groups are >80, 80–204, and >204 IU, respectively. MEC, Mobile Examination Center; 25(OH)D, 25-hydroxyvitamin D.
Estimates are adjusted for age and season.
Estimates are adjusted for season.
Estimates for non-Hispanic persons reporting >1 race are not shown separately, but are included in the total.
Data do not sum to 16,180 due to missing values. Dietary weights used for analysis.
The age- and season-adjusted prevalence of at risk of deficiency was lower for males (4.4%; 95% CI: 3.5%, 5.5%) than for females (5.7%; 95% CI: 4.5%, 7.0%; P = 0.005); however, after Bonferroni adjustment this difference was no longer statistically significant. There was no significant difference by sex in the risk of vitamin D inadequacy (males: 18.7%; 95% CI: 16.3%, 21.4% and females: 17.8%; 95% CI: 15.8%, 20.1%). Both the prevalence of at risk of deficiency and at risk of inadequacy by age followed a quadratic trend. The season-adjusted percentage of at risk of deficiency was 0.5% (95% CI: 0.3%, 1.1%) among children 1–5 y, 7.6% (95% CI: 6.0%, 9.6%) among adults aged 20–39 y, and 2.9% (95% CI: 2.0%, 4.0%) among adults aged ≥60 y. The equivalent season-adjusted percentages of persons at risk of vitamin D inadequacy were 6.6% (95% CI: 5.2%, 8.3%), 23.8% (95% CI: 20.9%, 27.0%), and 12.3% (95% CI: 10.7%, 14.1%).
Non-Hispanic white persons had a significantly lower (2.1%; 95% CI: 1.5%, 2.7%) age- and season-adjusted percentage of at risk of deficiency than non-Hispanic black (17.5%; 95% CI: 15.2%, 20.0%), non-Hispanic Asian (7.6%; 95% CI: 5.9%, 9.9%), and Hispanic (5.9%; 95% CI: 4.4%, 7.8%) persons. Non-Hispanic black persons also had a significantly higher prevalence of at risk of deficiency than non-Hispanic Asian and Hispanic persons. We observed a similar pattern by race and Hispanic origin in the age- and season-adjusted prevalence of at risk of inadequacy, i.e., non-Hispanic white, 11.8% (95% CI: 10.0%, 13.8%); non-Hispanic black, 35.8% (95% CI: 33.3%, 38.5%); non-Hispanic Asian, 29.1% (95% CI: 26.4%, 32.0%); and Hispanic, 26.3% (95% CI: 23.0%, 29.8%).
The median intake of vitamin D from food and beverage sources was 3.5 μg (95% CI: 3.4 μg, 3.6 μg); the mean intake was 4.9 μg (95% CI: 4.8 μg, 5.1 μg) (data not shown). A significant linear decrease was observed in the prevalence of at risk of deficiency as vitamin D intake from food and beverage sources increased, as measured in tertiles of intake: 7.1% (95% CI: 5.7%, 8.9%), 4.7% (95% CI: 3.4%, 6.5%), and 2.6% (95% CI: 2.0%, 3.4%) for 0–2.0, 2.1–5.1, and >5.1 μg (Table 1) (P < 0.002). A similar decrease was observed in the prevalence of at risk of inadequacy with increasing tertiles of vitamin D intake from foods and beverages (P < 0.002).
Overall, 37.4% (95% CI: 35.6%, 39.3%) of the population reported taking a dietary supplement that contained vitamin D (data not shown). Dietary supplement use varied by race and Hispanic origin: use was higher among non-Hispanic white (43.7%; 95% CI: 41.6%, 46.0%) and non-Hispanic Asian individuals (41.2%; 95% CI: 37.2%, 45.4%) than among non-Hispanic black (24.8%; 95% CI: 23.0%, 26.8%) and Hispanic individuals (22.0%; 95% CI: 19.8%, 24.3%) (P < 0.002) (data not shown). The prevalence of at risk of deficiency was lower for those who reported the use of ≥1 dietary supplement containing vitamin D: 6.9% (95% CI: 5.6%, 8.5%) compared with 1.1% (95% CI: 0.8%, 1.6%), (P < 0.002). The prevalence of at risk of inadequacy was also lower for those reporting the use of a dietary supplement containing vitamin D.
Table 2 presents the prevalence of at risk of deficiency or at risk of inadequacy by race and Hispanic origin, and by dietary sources of vitamin D. For all race and Hispanic-origin groups, except non-Hispanic white individuals, as vitamin D intake from foods and beverages increased, the prevalence of at risk of deficiency risk decreased linearly. For example, among the non-Hispanic black subgroup, the age- and season-adjusted prevalence of vitamin D deficiency decreased from 21.4% (95% CI: 18.1%, 25.2%) to 15.1% (95% CI: 12.1%, 18.6%) to 10.5% (95% CI: 8.0%, 13.8%), as the intake of vitamin D from foods and beverages increased from 0–2.0 to 2.1–5.1 to >5.1 μg.
TABLE 2.
Prevalence of at risk of deficiency [serum 25(OH)D <30 nmol/L] or at risk of inadequacy [serum 25(OH)D 30–49 nmol/L] for US persons aged ≥1 y, by race and Hispanic origin and dietary sources of vitamin D, NHANES 2011–20141
| Tertiles of vitamin D intake from food and beverage sources | Use of a dietary supplement that contains vitamin D | ||||||
|---|---|---|---|---|---|---|---|
| 0.0–2.0 μg | 2.1–5.1 μg | >5.1 μg | P 2 | No | Yes | P 3 | |
| n = 4,765 | n = 4,713 | n = 5,213 | n = 9,975 | n = 4,716 | |||
| Risk of deficiency (<30 nmol/L) | |||||||
| Non-Hispanic white | 2.2 (1.5, 3.4)a | 2.2 (1.2, 3.9)a | 1.0 (0.6, 1.9)a | 0.029 | 2.7 (1.9, 3.9)a | 0.5 (0.3, 0.9)a | <0.001 |
| Non-Hispanic black | 21.4 (18.1, 25.2)b | 15.1 (12.1, 18.6)b | 10.5 (8.0, 13.8)b | <0.001 | 21.4 (18.2, 25.0)b | 3.6 (2.6, 4.9)b | <0.001 |
| Non-Hispanic Asian | 9.8 (6.7, 14.0)c | 7.9 (5.0, 12.2)c | 3.1 (1.7, 5.8)a | <0.001 | 10.8 (7.8, 14.7)c | 1.3 (0.4, 3.9)a | <0.001 |
| Hispanic | 10.2 (7.1, 14.4)c | 5.6 (3.4, 9.1)a,c | 2.8 (2.0, 4.1)a | 0.001 | 7.4 (5.4, 10.0)c | 2.5 (1.2, 5.2)a | <0.001 |
| Risk of inadequacy (30–49 nmol/L) | |||||||
| Non-Hispanic white | 14.2 (11.5, 17.3)a | 11.3 (9.2, 13.7)a | 9.5 (7.3, 12.4)a | <0.001 | 16.9 (14.3, 19.8)a | 4.2 (3.2, 5.6)a | <0.001 |
| Non-Hispanic black | 36.4 (32.9, 40.0)b | 38.1 (33.9, 42.5)b | 33.5 (29.5, 37.9)b | 0.289 | 40.7 (37.8, 43.7)b | 24.2 (20.7, 28.1)b | <0.001 |
| Non-Hispanic Asian | 30.4 (25.2, 36.2)b,c | 29.0 (22.5, 36.4) b,c | 24.2 (19.5, 29.5)a | 0.326 | 37.6 (32.1, 43.5)b,c | 13.8 (10.3, 18.2)c | <0.001 |
| Hispanic | 27.4 (23.1, 32.1)c | 26.5 (22.1, 31.4)c | 24.7 (20.3, 29.7)a | 0.107 | 30.6 (26.6, 34.9)c | 13.3 (9.8, 17.9)c | <0.001 |
Source: CDC/National Center for Health Statistics. Values are weighted proportions and 95% CI. Dietary weights used. Within logistic models adjusted for age and season, pairwise differences were evaluated through the use of t-tests and orthogonal contrast matrices were used to test for linear and quadratic trends, P < 0.002. Different lowercase letters within a column and covariate category indicate significant differences, after adjustment with the Bonferroni method for multiple comparisons, P < 0.002. Vitamin D intake unit conversion: 1 μg is 40 IU; the 0.0–2.0, 2.1–5.1, and >5.1 μg groups are >80, 80–204, and >204 IU, respectively. MEC, Mobile Examination Center; 25(OH)D, 25-hydroxyvitamin D.
Linear test for trend as vitamin D intake from food and beverages increases.
Pairwise test for differences between those who used a supplement and those who did not use a supplement that contained vitamin D.
Within the lowest tertile of vitamin D dietary intake, differences by race and Hispanic origin were identical to the pattern observed in the total population. At higher intakes of vitamin D from foods and beverages, the magnitude of difference between race and Hispanic-origin groups decreased and differences between some race and Hispanic-origin groups were no longer significant. For example, with intakes of vitamin D from foods and beverages >5.1 μg, non-Hispanic black (10.5%; 95% CI: 8.0%, 13.8%) individuals had a significantly higher age- and season-adjusted percentage at risk of deficiency than all other race and Hispanic-origin groups, and no other differences by race and Hispanic origin were observed [non-Hispanic white, 1.0% (95% CI: 0.6%, 1.9%); non-Hispanic Asian, 3.1% (95% CI: 1.7%, 5.8%); and Hispanic, 2.8% (95% CI: 2.0%, 4.1%)]. The pattern of differences by race and Hispanic origin and vitamin D intake from food and beverage sources for vitamin D inadequacy was similar, but was attenuated and did not reach statistical significance for some groups, compared with the prevalence of at risk of deficiency.
Use of a dietary supplement that contains vitamin D decreased the prevalence of at risk of deficiency significantly for all race and Hispanic-origin groups (P < 0.001 for all). The pattern of differences by race and Hispanic origin was similar to the total population among nonusers of supplements. With the use of a supplement, non-Hispanic black individuals had a significantly higher prevalence of at risk of vitamin D deficiency than all other race and Hispanic-origin groups, and no other differences were observed. It should be noted that the prevalence of at risk of deficiency fell to <5% for all race and Hispanic- origin groups. Among supplement users, the prevalence of at risk of deficiency was 0.5% (95% CI: 0.3%, 0.9%) for non-Hispanic white; 3.6% (95% CI: 2.6%, 4.9%) for non-Hispanic black; 2.5% (95% CI: 1.2%, 5.2%) for Hispanic; and 1.3% (95% CI: 0.4%, 3.9%) for non-Hispanic Asian individuals. The prevalence of at risk of inadequacy decreased for all race and Hispanic-origin groups, similar to the prevalence of at risk of deficiency (P < 0.001 for all). The pattern of race and Hispanic origin differences was similar among both supplement users and nonusers, and, as expected, the prevalence of at risk of inadequacy was lower among supplement users than among nonusers.
From 2003 to 2004 through 2013 to 2014, there was no change in the percentage of persons at risk of deficiency (Figure 1) [6.8% (95% CI: 4.9%, 9.2%) to 5.0% (95% CI: 4.0%, 6.2%)], whereas there was a significant linear decline in the percentage of persons at risk of inadequacy from 21.0% (95% CI: 17.9%, 24.5%) to 17.7% (95% CI: 16.0%, 19.7%) (P = 0.001).
FIGURE 1.
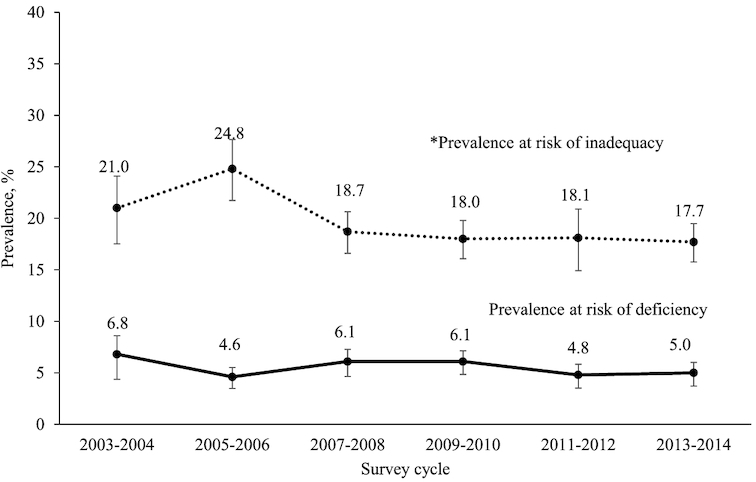
Trends in prevalence of at risk of deficiency [serum 25(OH)D <30 nmol/L] or at risk of inadequacy [serum 25(OH)D 30–49 nmol/L] among persons aged ≥1 y and over (NHANES, 2003–2014, n = 48,430). Asterisk (*) indicates statistically significant decreasing linear trend, determined with the use of orthogonal contrasts with six 2-y cycles in logistic regression models adjusted for age and season; significant difference P ≤ 0.002. 25(OH)D, 25-hydroxyvitamin D.
Discussion
Using data from 2011 to 2014, we found that 5% of the US population aged ≥1 y was at risk of vitamin D deficiency and 18% was at risk of inadequacy. There were no observed differences in the prevalence of at risk of deficiency or of at risk of inadequacy by sex. Similar to previous publications, we found that vitamin D status differed by age with the prevalence of at risk of deficiency or inadequacy, increasing from childhood to adults aged 20–39 y and then decreasing among older adults (23, 24).
For the first time, we present national estimates of vitamin D status for non-Hispanic Asian persons (prevalence of at risk of deficiency: 7.6%) in the United States. The prevalence of at risk of deficiency for this subgroup was most similar to that of Hispanic (5.6%) individuals. Both of these groups had a lower prevalence of at risk of deficiency than that of non-Hispanic black (17.5%) individuals, but higher prevalences than that of non-Hispanic white (2.1%) individuals. The same pattern was observed in the prevalence of at risk of vitamin D inadequacy. Differences in vitamin D status by ethnicity have been documented in Europe (25), the United Kingdom (26, 27), the United States (23, 24, 28), and Canada (29). Across locations, studies found that dark-skinned ethnic groups had a higher prevalence of at risk of deficiency than white groups. Many of these studies did not have sufficiently large samples to test for differences within dark-skinned ethnic groups; but for those that did, the pattern of increased prevalence of at risk of deficiency with increasing skin pigmentation was not always found. Cashman et al. (25) summarized results across Europe and found the prevalence of deficiency risk [25(OH)D <30 nmol/L] among Kurdish and Somali adults living in Finland to be quite different: 50.4% compared with 28.0%. Also, albeit with a small sample size, the National Diet and Nutrition Survey among participants aged ≥1 y in the United Kingdom found 35.7% of black respondents and 59.6% of Asian respondents at risk of deficiency. Clearly, skin pigmentation is not the sole driver of vitamin D status; other nonmodifiable lifestyle factors, such as latitude and cultural clothing practices, play a role (30), as well as modifiable factors such as diet.
The mean population intake from food and beverage sources in the current study was 4.9 μg/d, roughly equivalent to 200 IU. The Estimated Average Requirement for vitamin D is 400 IU (8), suggesting that vitamin D intake from food and beverage sources in the United States is low. Despite the low intake, the prevalence of at risk of deficiency and of at risk of inadequacy decreased with increasing intake. Similar decreases were also observed with the use of dietary supplements that contained vitamin D; however, the magnitude of decrease was larger with the use of dietary supplements. The larger decrease could be attributed to the larger dose of vitamin D from supplements compared with foods and beverages; among those who took a supplement containing vitamin D, the median average daily intake of vitamin D was 12.4 μg/d (mean 25.8 μg/d).
We also examined the prevalence of at risk of deficiency and at risk of inadequacy by race and Hispanic origin, and by dietary sources of vitamin D. It is noteworthy that the use of a dietary supplement dramatically reduced the prevalence of being at risk of deficiency for all race and Hispanic-origin groups, ranging from a reduction by half among non-Hispanic white and more than three-quarters among non-Hispanic black persons. With the use of a dietary supplement, the prevalence of at risk of deficiency was 3.6% among non-Hispanic blacks and 0.5% among non-Hispanic whites. This demonstrates that the use of a dietary supplement significantly attenuates the effect of race and Hispanic origin on vitamin D status. In support of this finding, a Canadian study found a reduction in the prevalence of 25(OH)D concentrations <50 nmol/L (in the winter) among non-white participants aged 6–79 y in the Canadian Health Measures Survey—Cycle 1, from 60.7% to 36.3% with the use of supplements (29).
The relationship between vitamin D status, defined by categories of total serum 25(OH)D and bone health, has also been shown to vary by race and Hispanic origin (31). NHANES data can now be used to investigate any such association among non-Hispanic Asian persons. Previous research has found that non-Hispanic black persons have lower concentrations of total serum 25(OH)D (23, 32), but paradoxically, higher bone mineral density and lower risk of facture compared with their non-Hispanic white counterparts, who have higher concentrations of total serum 25(OH)D (33–36).
The prevalence of at risk of deficiency in the US population did not change between 2003 and 2004 through 2013 and 2014. There was a decrease in the percentage of the US population at risk of inadequacy over the same period, from 21.0% to 17.7%. Previous estimates reported no change in the prevalence of deficiency risk or of inadequacy risk from 2001 to 2002 through 2005 to 2006 in the US population aged ≥12 y (24). In a sensitivity analysis (data not shown), we examined the risk of inadequacy in the US population aged ≥12 y from 2003 to 2004 through 2013 to 2014 and still found a significant decreasing trend: 22.6% (95% CI: 19.4%, 26.1%) in 2003–2004 to 18.9% (95% CI: 17.0%, 21.0%) in 2013–2014 (P = 0.0005). This decrease is therefore unlikely to be related to the inclusion of children and adolescents.
This study has several limitations. The trend analysis uses LC-MS/MS-equivalent data for 2003–2006, i.e., it was measured by RIA, and thus there is potential for slight error arising from the conversion, such as a slight underestimation of the prevalence of deficiency and of the standard error (37). Vitamin D was not included in the nutrient or supplement databases until 2007, so we were unable to look at the association between dietary sources of vitamin D over time. It is well documented that self-reported measures of dietary intake are subject to random and systematic error due to day-to-day differences in intake, systematic underreporting, difficulty remembering foods, and errors in the food and nutrient database to which intakes are linked (17, 38). Despite this, the 24-h recall is the recommended measurement tool to assess dietary intake in a population (3) and the method used has been shown to be accurate and reduce bias (16, 39). We also included both dietary supplement and food and beverage sources of vitamin D and their association with vitamin D status in a racially diverse population. Another strength of the study is that estimates for 2011–2014 are based on 25(OH)D measured directly by LC-MS/MS, the state-of-the-art method (14, 40–43). An additional strength of this analysis is that NHANES is nationally representative and we present national estimates for various subgroups of the US population, including the first estimates for non-Hispanic Asian individuals.
In conclusion, the prevalence of the US population at risk of vitamin D deficiency has remained fairly stable, ∼5%, over the past decade, from 2003 to 2004 through 2013 to 2014. The prevalence of at risk of vitamin D inadequacy decreased from 21% to 17.7%. The percentage of non-Hispanic Asian persons at risk of low vitamin D status was higher than that of non-Hispanic whites and lower than that of non-Hispanic blacks. Dietary intake, both from dietary supplements and foods and beverages, reduced the prevalence of persons at risk of deficiency and at risk of inadequacy. Our work demonstrates that differences in vitamin D status by race and Hispanic origin include differences between non-Hispanic Asian individuals and both non-Hispanic white and non-Hispanic black individuals. Strategies to reduce vitamin D deficiency may include food- and dietary supplement-based solutions. Future work could also investigate whether differences in vitamin D status by race and Hispanic origin extend to outcomes associated with vitamin D status, such as bone health.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—all authors had full access to all data in this study; KAH and JA: were responsible for the integrity of the data and accuracy of the data analysis; KAH: was responsible for drafting the manuscript; KAH, RJS, JJG, NP, RLS, and CMP: participated in the study design and interpretation of the data; all authors were responsible for critical revision of the manuscript; and all authors: have read and approved the final manuscript as submitted and agree to be accountable for all aspects of the work. The authors have no conflicts of interest to disclose.
Notes
This work was performed under employment of the US Federal government and the authors did not receive any outside funding. The findings and conclusions in this report are those of the authors and not necessarily the official position of the CDC.
Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: LC-MS/MS, liquid chromatography-tandem mass spectrometry; MEC, Mobile Exam Center; RIA, radioimmunoassay; 25(OH)D, 25-hydroxyvitamin D; 25(OH)D2, 25-hydroxyvitamin D2; 25(OH)D3, 25-hydroxyvitamin D3.
REFERENCES
- 1. Department of Health and Human Services Office of Inspector General Medicare payments for clinical laboratory tests in 2014: baseline data [Internet]. Available from: https://oig.hhs.gov/oei/reports/oei-09-15-00210.pdf (cited June 28, 2018). [Google Scholar]
- 2. Lin KW. Vitamin D screening and supplementation in primary care: time to curb our enthusiasm. Am Fam Physician. 2018;97(4):226–7. [PubMed] [Google Scholar]
- 3. Ahluwalia N, Dwyer J, Terry A, Moshfegh A, Johnson C. Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr. 2016;7(1):121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dwyer JT, Woteki C, Bailey R, Britten P, Carriquiry A, Gaine PC, Miller D, Moshfegh A, Murphy MM, Smith Edge M. Fortification: new findings and implications. Nutr Rev. 2014;72(2):127–41. [DOI] [PubMed] [Google Scholar]
- 5. Newberry SJ CM, Shekelle PG, Booth MS, Liu JL, Maher AR, Motala A, Cui M, Perry T, Shanman R, Balk EM. Vitamin D and Calcium: A Systematic Review of Health Outcomes (update). Evidence Report/technology Assessment no. 217. Rockville (MD): Agency for Healthcare Research and Quality; 2014. [DOI] [PubMed] [Google Scholar]
- 6. Office of Dietary Supplements. [Internet]. Available from: https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ (cited March 1, 2018). [Google Scholar]
- 7. Holick MF. Sunlight, UV-radiation, vitamin D and skin cancer: how much sunlight do we need?. Adv Exp Med Biol. 2008;624:1–15. [DOI] [PubMed] [Google Scholar]
- 8. Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC), The National Academies Press; 2011. [PubMed] [Google Scholar]
- 9. Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National Health and Nutrition Examination Survey: Sample design, 2011–2014. National Center for Health Statistics. Vital Health Stat 2, 2014;(162):1–33. [PubMed] [Google Scholar]
- 10. National Center for Health Statistics. NHANES III (1988–1994) [Internet]. Available from: https://wwwn.cdc.gov/nchs/nhanes/nhanes3/default.aspx (cited October 2018). [Google Scholar]
- 11. National Center for Health Statistics. [Internet]. Available from: http://www.cdc.gov/nchs/nhanes/response_rates_CPS.htm (cited May 11, 2017). [Google Scholar]
- 12. National Center for Health Statistics Laboratory Procedure Manual: 25-Hydroxyvitamin D [Internet]. Available from: https://wwwn.cdc.gov/Nchs/Data/Nhanes/2003-2004/LabMethods/VID_C_met_Vitamin_D.pdf (cited April 4, 2018). [Google Scholar]
- 13. CDC/National Center for Health Statistics. Analytical Note for 25-Hydroxyvitamin D Data Analysis using NHANES III (1988–1994) NHANES 2001–2006, and NHANES 2007–2010 (October 2015). [Internet]. Available from: https://wwwn.cdc.gov/nchs/nhanes/VitaminD/AnalyticalNote.aspx (cited April 4, 2018). [Google Scholar]
- 14. NIH/Office of Dietary Supplements. [Internet]. Available from: https://ods.od.nih.gov/Research/vdsp.aspx (cited April 4, 2018). [Google Scholar]
- 15. Schleicher RL, Sternberg MR, Lacher DA, Sempos CT, Looker AC, Durazo-Arvizu RA, Yetley EA, Chaudhary-Webb M, Maw KL, Pfeiffer CMet al. A method-bridging study for serum 25-hydroxyvitamin D to standardize historical radioimmunoassay data to liquid chromatography-tandem mass spectrometry. Natl Health Stat Report. 2016;(93):1–16. [PubMed] [Google Scholar]
- 16. Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuczynski KJ, Ingwersen LAet al. The US Department of Agriculture automated multiple-pass method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88(2):324–32. [DOI] [PubMed] [Google Scholar]
- 17. Gibson RS. Principles of Nutritional Assessement. 2nd ed. New York: Oxford University Press; 2005. [Google Scholar]
- 18. National Center for Health Statistics. National Health and Nutrition Examination Survey. Dietary Interview—Total Nutrient Intakes 2011–2012. [Internet]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/DR1TOT_G.htm (accessed November 11, 2016). [Google Scholar]
- 19. US Department of Agriculture: Agricultural Research Service. USDA Food and Nutrient Database for Dietary Studies 2013–2014 [Internet]. Food Surveys Research Group Home Page, Available from: http://www.ars.usda.gov/nea/bhnrc/fsrg. 2016. [Google Scholar]
- 20. US Department of Agriculture: Agricultural Research Service. USDA Food and Nutrient Database for Dietary Studies 2011–. 2012 [Internet]. Food Surveys Research Group Home Page, Available from: http://www.ars.usda.gov/ba/bhnrc/fsrg. 2014. [Google Scholar]
- 21. National Center for Health Statistics. National Health and Nutrition Examination Survey—Dietary Supplement use 30-day—Total Dietary Supplement 2011–2012. [Internet]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/DSQTOT_G.htm (accessed May 2018). [Google Scholar]
- 22. National Center for Health Statistics. National Health and Nutrition Examination Survey—Dietary Supplement use 30-day—Total Dietary Supplement 2013–2014. [Internet]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/DSQTOT_H.htm (accessed May 2018). [Google Scholar]
- 23. Schleicher RL, Sternberg MR, Lacher DA, Sempos CT, Looker AC, Durazo-Arvizu RA, Yetley EA, Chaudhary-Webb M, Maw KL, Pfeiffer CMet al. The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin D shows recent modest increases. Am J Clin Nutr. 2016;104(2):454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. Vitamin D status: United States, 2001–2006. NCHS Data Brief. 2011;(59):1–8. [PubMed] [Google Scholar]
- 25. Cashman KD, Dowling KG, Skrabakova Z, Gonzalez-Gross M, Valtuena J, De Henauw S, Moreno L, Damsgaard CT, Michaelsen KF, Molgaard Cet al. Vitamin D deficiency in Europe: pandemic?. Am J Clin Nutr. 2016;103(4):1033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kift R, Berry JL, Vail A, Durkin MT, Rhodes LE, Webb AR. Lifestyle factors including less cutaneous sun exposure contribute to starkly lower vitamin D levels in U.K. South Asians compared with the white population. Br J Dermatol. 2013;169(6):1272–8. [DOI] [PubMed] [Google Scholar]
- 27. Farrar MD, Kift R, Felton SJ, Berry JL, Durkin MT, Allan D, Vail A, Webb AR, Rhodes LE. Recommended summer sunlight exposure amounts fail to produce sufficient vitamin D status in UK adults of South Asian origin. Am J Clin Nutr. 2011;94(5):1219–24. [DOI] [PubMed] [Google Scholar]
- 28. Schleicher RL, Sternberg MR, Looker AC, Yetley EA, Lacher DA, Sempos CT, Taylor CL, Durazo-Arvizu RA, Maw KL, Chaudhary-Webb Met al. National estimates of serum total 25-hydroxyvitamin D and metabolite concentrations measured by liquid chromatography-tandem mass spectrometry in the US Population during 2007–2010. J Nutr. 2016;146(5):1051–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Whiting SJ, Langlois KA, Vatanparast H, Greene-Finestone LS. The vitamin D status of Canadians relative to the 2011 Dietary Reference Intakes: an examination in children and adults with and without supplement use. Am J Clin Nutr. 2011;94(1):128–35. [DOI] [PubMed] [Google Scholar]
- 30. O'Neill CM, Kazantzidis A, Kiely M, Cox L, Meadows S, Goldberg G, Prentice A, Kift R, Webb AR, Cashman KD. A predictive model of serum 25-hydroxyvitamin D in UK white as well as black and Asian minority ethnic population groups for application in food fortification strategy development towards vitamin D deficiency prevention. J Steroid Biochem Mol Biol. 2017;173:245–52. [DOI] [PubMed] [Google Scholar]
- 31. Aloia J, Mikhail M, Dhaliwal R, Shieh A, Usera G, Stolberg A, Ragolia L, Islam S. Free 25(OH)D and the vitamin D paradox in African Americans. J Clin Endocrinol Metab. 2015;100(9):3356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SAet al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369(21):1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aloia JF. African Americans, 25-hydroxyvitamin D, and osteoporosis: a paradox. Am J Clin Nutr. 2008;88(2):S545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cauley JA, Lui LY, Ensrud KE, Zmuda JM, Stone KL, Hochberg MC, Cummings SR. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA. 2005;293(17):2102–8. [DOI] [PubMed] [Google Scholar]
- 35. Gutierrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2011;22(6):1745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brown LL, Cohen B, Tabor D, Zappala G, Maruvada P, Coates PM. The vitamin D paradox in Black Americans: a systems-based approach to investigating clinical practice, research, and public health—expert panel meeting report. BMC Proceedings. 2018;12(Suppl 6):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sternberg M. Multiple imputation to evaluate the impact of an assay change in national surveys. Stat Med. 2017;36(17):2697–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Willett W. Nutritional Epidemiology. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- 39. Blanton CA, Moshfegh AJ, Baer DJ, Kretsch MJ. The USDA automated multiple-pass method accurately estimates group total energy and nutrient intake. J Nutr. 2006;136(10):2594–9. [DOI] [PubMed] [Google Scholar]
- 40. Stepman HC, Vanderroost A, Van Uytfanghe K, Thienpont LM. Candidate reference measurement procedures for serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 by using isotope-dilution liquid chromatography-tandem mass spectrometry. Clin Chem. 2011;57(3):441–8. [DOI] [PubMed] [Google Scholar]
- 41. Mineva EM, Schleicher RL, Chaudhary-Webb M, Maw KL, Botelho JC, Vesper HW, Pfeiffer CM. A candidate reference measurement procedure for quantifying serum concentrations of 25-hydroxyvitamin D(3) and 25-hydroxyvitamin D(2) using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2015;407(19):5615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Phinney KW, Bedner M, Tai SS, Vamathevan VV, Sander LC, Sharpless KE, Wise SA, Yen JH, Schleicher RL, Chaudhary-Webb Met al. Development and certification of a standard reference material for vitamin D metabolites in human serum. Anal Chem. 2012;84(2):956–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tai SS, Bedner M, Phinney KW. Development of a candidate reference measurement procedure for the determination of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem. 2010;82(5):1942–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


