Abstract
Background:
Chlorination is globally used to produce of safe drinking water. Chlorination by-products are easily formed, and there are indications that these are associated with adverse reproductive outcomes.
Objectives:
We conducted a nationwide register-based prospective study to assess whether gestational exposure to the four most common chlorination by-products [total trihalomethanes (TTHMs)] via tap water was associated with risk of small for gestational age (SGA), preterm delivery, and very preterm delivery. To date, this is one of the largest studies assessing drinking water TTHM-associated adverse reproductive outcomes.
Methods:
We included all singleton births 2005–2015 (live and stillbirths) of mothers residing in Swedish localities having inhabitants, operating waterworks, adequate information on chlorination treatment, and a sufficient number of routine TTHM measurements in tap water. Individual maternal second and third trimester exposure was obtained by linking TTHM measurements to residential history, categorized into no chlorination, , 5–15, and TTHM/L. Outcomes and covariates were obtained via the linkage to Swedish health and administrative registers. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated by logistic regression using inverse probability weighting. We stratified the analyses by chlorination treatment (chloramine, hypochlorite).
Results:
Based on approximately 500,000 births, we observed a TTHM dose-dependent association with increased risk of SGA, confined to treatment with hypochlorite, corresponding to a multivariable-adjusted (95% CI: 1.08, 1.33) comparing drinking water TTHM to the unexposed. Similar results were obtained when, instead of unexposed, the lowest exposure category ( TTHM) was used as reference. No clear associations were observed for preterm delivery and very preterm delivery.
Discussion:
Chlorination by-products exposure via drinking water was associated with increased risk of SGA in areas with hypochlorite treatment. https://doi.org/10.1289/EHP6012
Introduction
Chlorine is globally used in drinking water treatment owing to its high efficiency in inactivating microbial pathogens and reducing microbial growth in the distribution system. As a strong oxidant, chlorine reacts with natural organic matter and other substances in the water, generating hundreds of chlorination by-products (CBPs) (Richardson et al. 2007). There is increasing evidence that some CBPs are genotoxic and carcinogenic (Boorman 1999; Richardson et al. 2007) but also indications that CBP exposure, such as trihalomethanes (THMs), may be associated with increased risk of adverse reproductive outcomes (Bove et al. 2002; Colman et al. 2011; Graves et al. 2001; Grellier et al. 2010; Nieuwenhuijsen et al. 2000; Tardiff et al. 2006). From epidemiological studies, the strongest support of an association is for intrauterine growth retardation, resulting in, for example, small for gestational age (SGA) (Cao et al. 2016; Grazuleviciene et al. 2011; Hinckley et al. 2005; Kramer et al. 1992; Levallois et al. 2012; Lewis et al. 2006; Smith et al. 2016; Wright et al. 2003, 2004), but the results are inconclusive, with several studies indicating no association (Hoffman et al. 2008; Ileka-Priouzeau et al. 2015; Iszatt et al. 2014; Jaakkola et al. 2001; Kogevinas et al. 2016; Patelarou et al. 2011; Porter et al. 2005; Rivera-Núñez and Wright 2013; Villanueva et al. 2011). On the other hand, indications of inverse associations—although mostly statistically nonsignificant—have been observed for some other specific adverse reproductive outcomes such as preterm delivery and very preterm delivery (Grellier et al. 2010).
Assessing the association between CBP exposure and adverse reproductive outcomes is challenging because of the potential of exposure misclassification. Given that the formation of CBPs has a seasonal variation, linked to the content of organic matter, a correct timing of the exposure in relation to the relevant gestational effect-window is crucial (Mercier Shanks et al. 2013; Uyak et al. 2008). Moreover, correct residential information is important because there are also spatial variations, and approximately 20% of pregnant women move and often resettle in a new county (Miller et al. 2010). The selection of a suitable unexposed reference area is another challenge. Because chlorination of public drinking water is commonplace, most assigned unexposed reference areas are those with private wells in rural areas, potentially introducing, for example, contextual confounding. The alternative has been to use municipal areas with low, but not zero, CBP exposure as the reference group (Hrudey 2009), resulting in reduced exposure contrast and an increased probability of misclassified exposure.
With strong emphasis on reducing the aforementioned shortcomings, we conducted a nationwide register-based prospective cohort study among singleton births in Sweden during 2005–2015 in order to assess the association between gestational exposure to the four most common CBPs, the total trihalomethanes (TTHMs: chloroform, bromoform, bromodichloromethane, and dibromochloromethane), in drinking water and the risk of SGA, preterm, and very preterm delivery.
Methods
Study Area and Study Population
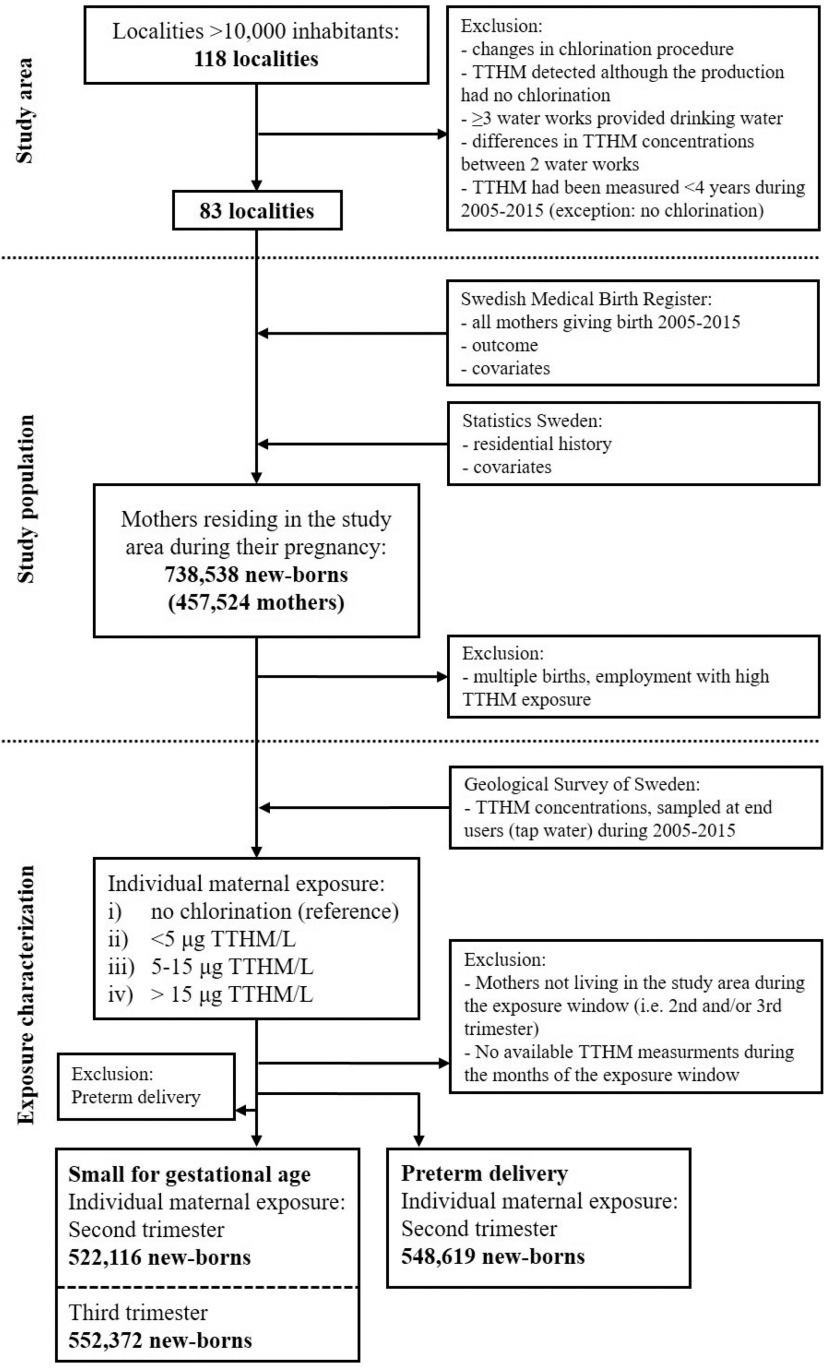
As study area, we selected all localities (coherent and densely populated areas) in Sweden having a population of inhabitants ( of the total population) in order to be able to include a large study population with a wide range of CBP exposure while avoiding inclusion of areas not receiving municipal drinking water. Localities were excluded if a) there were changes in chlorination treatment of the drinking water between 1994 and 2010 (SWWA 1996, 2014); b) CBPs were detected in the drinking water, but no chlorination was reported in the drinking water production; c) three or more water utilities provided drinking water to the locality or within municipal borders; d) two different utilities provided drinking water with differences in mean CBP concentrations ; or e) CBPs were measured for a period of during 2005–2015 (with the exception of utilities with no chlorination). In total, 83 localities were included in the study area ( of the total Swedish population) (Figure 1).
Figure 1.
Study area, study population, and exposure categorization (average of the four most common trihalomethanes). Note: TTHM, total trihalomethanes.
The study population was identified as all mothers giving birth (live and stillbirths) from 1 January 2005 to 31 December 2015 with an official residential registration in any of the localities in the study area during their pregnancy. Data was provided by the Swedish Medical Birth Register (at the National Board of Health and Welfare), linked to the Longitudinal Integration Database for Health Insurance and Labour Market Studies (LISA; Statistics Sweden) and a national register for regional divisions based on real estate (Geografidatabasen; Statistics Sweden) by the personal identification number (a unique 10-digit number assigned to all Swedes) (Figure 1). From the 738,538 newborns (including 2,578 stillbirths) and 457,524 mothers, we excluded nonsingleton births and all mothers with occupational TTHM exposure (registered as professional swimmers or coaches and swimming pool lifeguards). Because this was a register-based study, where the individuals are nonidentifiable after matching, informed consent was not obtained. The study was approved by the Regional Ethics Review Board in Stockholm.
Exposure and Covariates
The CBP drinking water concentrations, sampled at the end users (tap) in the study area, were obtained from a register administered by the Geological Survey of Sweden ( confirmed TTHM analyses). The drinking water utilities in Sweden are obliged to follow the national drinking water regulations [SLVFS 2001:30 (Swedish National Food Agency 2001)], at least measuring four tap water samples yearly at the end user, preferably distributed over time and over the distribution system, with three additional samplings per every of drinking water produced per day (the TTHM should not exceed ). Although a strong effort was made in assigning an appropriate and as robust as possible CBP exposure for each individual pregnancy, no additional collection of household water samples or data on drinking water consumption was performed. For the exposure, we used a multiannual monthly average of TTHM for each locality (i.e., with one or two waterworks). By using a multiannual average for each month and locality, we a) accounted for seasonal variations (Andersson et al. 2019b), b) reduced the weight of single extreme values, and by reducing the influence of locality-specific differences in monitoring programs we also c) minimized the number of pregnancy dropouts. The multiannual monthly average TTHM were then used to estimate a trimester-specific (3 months) average for each single pregnancy.
The third trimester was considered the most relevant effect-window for SGA, but we also assessed exposure during the second trimester because this time period could be relevant (Lewis et al. 2006). For preterm and very preterm delivery, we focused only on the second trimester exposure because the short exposure period in the third trimester for the children born preterm increases the likelihood of missing data on TTHM exposure (dropouts) among the exposed preterm or very preterm delivery cases. Because season highly affects the occurrence of natural organic matter and other substances in raw water in Sweden (the precursor materials for CBP formation), estimating a mean TTHM for the total gestation (all three trimesters) was not considered appropriate.
The individual maternal TTHM exposure was then categorized into a) no chlorination (reference, only mothers living in localities with no chlorination), b) TTHM/L, c) , and d) TTHM/L. We were unable to assess the exposure to single CBPs/THMs due to the too low concentrations (chloroform was the principal THM, whereas other THMs often were below the limit of detection). Due to a combination of seasonal differences in birth rates and the frequency of tap water monitoring, a higher number of pregnancies were assigned third trimester exposure, compared with second trimester exposure. In total, 11% of the pregnant women changed locality during the second or third trimester. In cases where the woman had moved outside the study area or had changed locality during the exposure-relevant effect-window (third or second trimester for SGA, and second trimester for preterm or very preterm delivery), the birth was excluded from that particular analysis (this also applied to women in the reference area). Moving within a locality did not result in exclusion.
We obtained information on the sex and year of birth for each child and the following maternal covariates from the Swedish Medical Birth Register: age, body mass index (BMI), previous miscarriages, parity, smoking (gestational week 30–32), use of drugs with suspected teratogenic effects (Class 3) (Nörby et al. 2013), sick leave/being on disability as reported by the mother, and maternal diagnosis of pregnancy-related conditions categorized according to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10; WHO 2016): preexisting and pregnancy-related diabetes (ICD-10 codes O24.0–24.9), preeclampsia (ICD-10 code O14), preexisting and pregnancy-related hypertension (ICD-10 codes O10 and O13.9), and high weight gain (ICD-10 code O26.0)/low weight gain (ICD-10 code O26.1) as reported by antenatal care. From LISA we obtained information on country of birth, highest attained education, and household income. We also collected locality-related information such as raw water source, size of the locality, and permanent chlorination treatment used. The latter enabled separate assessments of newborns of mothers living in localities with water treatment plants using exclusively chloramine or hypochlorite (the two most commonly used disinfectants in the study area).
Outcomes
The outcomes were term SGA (excluding preterm delivery for both cases and noncases), preterm delivery (born before gestational week 37) and very preterm delivery (born before gestational week 32). SGA was registered at delivery care defined as (SDs) from the average weight at the gestational age and sex at partus (Maršál et al. 1996). Preterm and very preterm delivery were defined according to the registered days of gestation made at antenatal care (calculated based on ultrasound evaluations and/or date for last menstrual period).
Statistical Analyses
Logistic regression was used to estimate the odds ratios (ORs) and corresponding 95% confidence intervals (CIs). To reduce dependency for multiple births by the same mother, we clustered the models (intragroup correlation) by anonymized maternal identification as received by the data provider. Confounders were selected based on current knowledge of risk factors for SGA, preterm, and very preterm delivery and on available data in the registers. We used standardization (inverse probability weight) to adjust for confounding in order to reduce bias due to missing data on covariates (Hernán and Robins 2020). First, models were adjusted for the risk factors considered most relevant (Model 1): maternal age (, , , , ), BMI (at registration to antenatal care: , 18.5–24.9, 25–29.9, ), birth region (Nordic/Europe/Africa/North and South America/Asia/other), attained education (elementary school/secondary education/postsecondary education), household income (yearly quartiles by year of birth), and smoking at gestational week 30–32 (no, 1–9, cigarettes/d). Second, in Model 2, we further adjusted for additional risk factors related to the outcome: previous miscarriages (yes/no), parity (nulliparous, 1, 2, ), sick leave/being on disability, use of teratogenic drugs (yes/no), maternal diabetes (yes/no), preeclampsia (yes/no), maternal hypertension (yes/no), maternal weight gain (high/normal/low weight gain) and year of birth (continuous). Linear trends across categories were tested using the median TTHM concentration within categories as a continuous variable. In a sensitivity analysis, we assigned the TTHM/L exposure category as the reference in order to minimize any impact of possible contextual confounding linked to the localities using no chlorination treatment. All analyses were performed on live and stillbirths combined because conditioning the analyses to live births may introduce a bias via the opening of new pathways through unmeasured confounding. Nevertheless, for some assessments we performed additional analyses excluding stillbirths for comparison. All statistical analyses were performed using Stata (version 14.1; Stata Corporation) and the statistical significance level was set at 0.05.
Results
Among 576,483 pregnancies with third trimester exposure information, we ascertained 10,887 cases of term SGA. Among 548,619 pregnancies with second trimester exposure, we ascertained 25,874 and 4,046 cases of preterm and very preterm delivery, respectively (Table 1). We observed some differences in baseline population characteristics across the exposure groups for maternal age, country of birth, attained educational level, household income, and sick leave/disability pension (Table 1). As expected, groundwater was a common source of raw water in localities with no chlorination (the unexposed reference) (Table 2). In localities with the exclusive use of chloramine treatment, groundwater was uncommon, whereas it was the dominating source in areas using hypochlorite. The size of the localities differed between the exposed and the areas with no chlorination, mainly because drinking water producers in large cities generally use chlorination, especially chloramine.
Table 1.
Baseline population characteristics by individual maternal average exposure to the four most common trihalomethanes (TTHMs), during the second () and third trimester (), expressed as proportions (%).
| Variables | Categories | No chlorination | Second trimester | Third trimester | ||||
|---|---|---|---|---|---|---|---|---|
| TTHM/L | TTHM/L | |||||||
| 66,008 | 262,177 | 138,672 | 81,762 | 263,687 | 168,449 | 78,439 | ||
| Average | — | 0.76 | 9.0 | 24 | 0.75 | 9.2 | 24 | |
| Outcome | SGAa | 1.8 | 1.9 | 2.2 | 2.0 | 1.9 | 2.2 | 2.1 |
| Preterm delivery | 5.1 | 3.8 | 4.1 | 4.0 | 3.8 | 4.1 | 4.0 | |
| Very preterm delivery | 0.80 | 0.27 | 0.24 | 0.38 | 0.27 | 0.25 | 0.38 | |
| Sex of child | Female | 51 | 52 | 51 | 51 | 52 | 51 | 51 |
| Year of birth | 2005 | 8.2 | 8.1 | 7.7 | 7.4 | 8.2 | 7.6 | 7.2 |
| 2006 | 8.6 | 8.3 | 8.1 | 7.9 | 8.3 | 8.0 | 7.8 | |
| 2007 | 8.8 | 8.4 | 8.4 | 8.3 | 8.4 | 8.4 | 8.3 | |
| 2008 | 8.9 | 8.9 | 8.7 | 8.8 | 8.8 | 8.8 | 8.9 | |
| 2009 | 9.3 | 8.9 | 9.2 | 9.1 | 8.8 | 9.3 | 9.2 | |
| 2010 | 9.5 | 9.7 | 9.8 | 9.8 | 9.7 | 9.7 | 9.9 | |
| 2011 | 9.3 | 9.4 | 9.4 | 9.6 | 9.4 | 9.4 | 9.6 | |
| 2012 | 9.3 | 9.5 | 9.6 | 9.7 | 9.5 | 9.6 | 9.8 | |
| 2013 | 9.2 | 9.6 | 9.6 | 9.5 | 9.7 | 9.5 | 9.6 | |
| 2014 | 9.3 | 9.7 | 9.8 | 9.9 | 9.7 | 9.8 | 9.9 | |
| 2015 | 9.4 | 9.5 | 9.8 | 10 | 9.5 | 9.8 | 9.8 | |
| Maternal age (y) | 17 | 10 | 11 | 14 | 10 | 12 | 14 | |
| 25–29 | 33 | 26 | 28 | 30 | 26 | 29 | 30 | |
| 30–34 | 32 | 38 | 37 | 35 | 38 | 37 | 35 | |
| 35–39 | 15 | 22 | 19 | 17 | 22 | 18 | 17 | |
| 2.9 | 4.9 | 4.1 | 3.6 | 4.8 | 3.9 | 3.5 | ||
| Maternal BMI () | 2.4 | 2.8 | 2.7 | 2.5 | 2.9 | 2.6 | 2.6 | |
| 59 | 65 | 63 | 61 | 65 | 63 | 61 | ||
| 26 | 22 | 23 | 25 | 22 | 24 | 25 | ||
| 13 | 10 | 11 | 12 | 9 | 11 | 12 | ||
| Prior miscarriages | Yes | 20 | 22 | 21 | 21 | 22 | 21 | 21 |
| Parity | Nulliparous | 44 | 45 | 47 | 45 | 45 | 46 | 45 |
| 1 | 37 | 37 | 37 | 37 | 38 | 37 | 36 | |
| 2 | 13 | 13 | 12 | 13 | 13 | 12 | 13 | |
| 5.6 | 4.7 | 4.7 | 5.9 | 4.7 | 5.0 | 6.1 | ||
| Country of birth | Nordic | 80 | 73 | 73 | 70 | 73 | 73 | 69 |
| Europe, other | 6.3 | 6.4 | 7.1 | 9.1 | 6.3 | 7.0 | 9.3 | |
| Africa | 2.9 | 5.3 | 5.4 | 4.3 | 5.2 | 5.3 | 4.3 | |
| North/South America | 1.0 | 2.5 | 1.8 | 1.5 | 2.5 | 1.6 | 1.5 | |
| Asia | 9.4 | 13 | 13 | 16 | 13 | 13 | 16 | |
| Attained education | Elementary school | 11 | 11 | 11 | 12 | 10 | 11 | 12 |
| Secondary education | 39 | 32 | 32 | 31 | 32 | 32 | 31 | |
| Postsecondary education | 50 | 58 | 58 | 57 | 58 | 57 | 57 | |
| Household income (quartile) | First | 16 | 16 | 17 | 21 | 15 | 17 | 22 |
| Second | 29 | 22 | 25 | 28 | 22 | 26 | 27 | |
| Third | 40 | 30 | 34 | 33 | 30 | 35 | 33 | |
| Fourth | 15 | 33 | 23 | 18 | 33 | 22 | 18 | |
| Sick leave/early retirement | Yes | 0.22 | 0.15 | 0.11 | 0.11 | 0.2 | 0.1 | 0.1 |
| Smoking: gestational week 30–32 (cigarettes/d) | 1–9 | 3.9 | 2.6 | 3.3 | 3.3 | 2.6 | 3.3 | 3.5 |
| 0.94 | 0.94 | 0.87 | 0.95 | 0.69 | 0.89 | 1.0 | ||
| Drugs (suspected teratogens) | Yes | 0.053 | 0.043 | 0.043 | 0.051 | 0.046 | 0.042 | 0.047 |
| Maternal diagnosis | Diabetes | 1.9 | 1.3 | 1.5 | 2.7 | 1.3 | 1.6 | 2.7 |
| Preeclampsia | 2.9 | 2.5 | 2.6 | 2.6 | 2.4 | 2.5 | 2.5 | |
| Hypertension | 1.5 | 1.8 | 1.2 | 1.6 | 1.9 | 1.2 | 1.6 | |
| High weight gain | 0.014 | 0.012 | 0.014 | 0.097 | 0.012 | 0.040 | 0.12 | |
| Low weight gain | 0.015 | 0.0015 | 0.0051 | 0.012 | 0.00076 | 0.0065 | 0.013 | |
Note: —, not applicable; BMI, body mass index; NA, not possible to evaluate the coverage; SGA, small for gestational age, excluding preterm delivery; TTHM, total trihalomethanes.
Excluding preterm delivery: based on for second trimester exposure and for third trimester exposure. There was no missing information except for BMI, sick leave, and smoking (8%, 13%, and 6% respectively).
Table 2.
Area-specific characteristics by average total trihalomethane (TTHM) exposure (second and third trimester) and chlorination treatments for all births, expressed as proportions (%).
| Variablesa | No chlorination | All | Chloramine | Hypochlorite | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Second trimester | ||||||||||
| 66,008 | 262,177 | 138,672 | 81,762 | 172,320 | 15,240 | 33,996 | 27,803 | 28,768 | 43,893 | |
| Average | — | 0.76 | 9.0 | 24 | 0.67 | 8.0 | 25 | 1.3 | 10 | 23 |
| Water source | ||||||||||
| Surface water | 5 | 87 | 83 | 57 | 97 | 99 | 80 | 17 | 25 | 41 |
| Groundwater | 95 | 13 | 17 | 43 | 3 | 0.0066 | 20 | 83 | 75 | 59 |
| Chlorination treatment | ||||||||||
| Chloramine | 0 | 86 | 17 | 45 | 100 | 100 | 100 | — | — | — |
| Hypochlorite | 0 | 15 | 25 | 55 | — | — | — | 100 | 100 | 100 |
| Chlorine dioxideb | 0 | 1.8 | 60 | 0.18 | — | — | — | — | — | — |
| Chlorine gas | 0 | 0 | 1.6 | 0.0013 | — | — | — | — | — | — |
| Population () | ||||||||||
| 25 | 1.2 | 1.4 | 3.5 | 0.48 | 0 | 0 | 7.9 | 5.1 | 6.3 | |
| 20,000–200,000 | 75 | 60 | 34 | 41 | 71 | 85 | 25 | 92 | 83 | 50 |
| 0 | 39 | 65 | 56 | 28 | 15 | 75 | 0 | 12 | 44 | |
| Third trimester | ||||||||||
| 66,008 | 263,687 | 168,449 | 78,439 | 173,265 | 22,204 | 34,237 | 28,527 | 48,731 | 42,477 | |
| Average | — | 0.75 | 9.2 | 24 | 0.66 | 8.9 | 25 | 1.3 | 10 | 22 |
| Water source | ||||||||||
| Surface water | 5 | 97 | 80 | 56 | 97 | 96 | 74 | 18 | 38 | 40 |
| Groundwater | 95 | 13 | 20 | 44 | 3.2 | 4.5 | 26 | 83 | 62 | 60 |
| Treatment | ||||||||||
| Chloramine | 0 | 86 | 19 | 44 | 100 | 100 | 100 | — | — | — |
| Hypochlorite | 0 | 16 | 34 | 56 | — | — | — | 100 | 100 | 100 |
| Chlorine dioxideb | 0 | 2.0 | 50 | 0.36 | — | — | — | — | — | — |
| Chlorine gas | 0 | 0.0011 | 1.5 | 0.010 | — | — | — | — | — | — |
| Population () | ||||||||||
| 25 | 1.2 | 2.8 | 3.5 | 0.49 | 0.0090 | 0 | 8.2 | 8.2 | 6.1 | |
| 20,000–200,000 | 75 | 60 | 35 | 40 | 72 | 72 | 29 | 92 | 65 | 48 |
| 0 | 39 | 62 | 57 | 28 | 28 | 71 | 0 | 27 | 46 | |
Note: —, not applicable; TTHM, total trihalomethanes.
No missing data.
Used in combination with chlorite or chlorite and chlorine gas.
Small for Gestational Age
Based on exposure during the third trimester, comparing the highest TTHM exposure () to the unexposed for all chlorination treatments, we observed statistically significant associations with increased odds of SGA in the crude and multivariable-adjusted Model 1, corresponding to (95% CI: 1.11, 1.31, ) and (95% CI: 1.04, 1.24, ), respectively. After further multivariable-adjustment (Model 2), the OR for SGA was 1.05 (95% CI: 0.95, 1.16) when comparing the highest exposure to unexposed (Table 3).
Table 3.
Associations between average trihalomethane (TTHM) exposure (third trimester) for full-term small for gestational age expressed as odds ratios (ORs) and 95% confidence intervals (CIs).
| Treatment | Components | Total births () | No chlorine (OR) | [OR (95% CI)] | [OR (95% CI)] | [OR (95% CI)] | a |
|---|---|---|---|---|---|---|---|
| All chlorination treatmentsb | Cases () | 552,372 | 1,100 | 4,712 | 3,484 | 1,592 | |
| Noncases () | 61,404 | 248,494 | 157,913 | 73,674 | |||
| Crude | 1.00 (ref) | 1.06 (0.99, 1.13) | 1.23 (1.15, 1.32) | 1.21 (1.11, 1.31) | |||
| Model 1 | 1.00 (ref) | 1.00 (0.92, 1.08) | 1.18 (1.08, 1.27) | 1.13 (1.04, 1.24) | |||
| Model 2 | 1.00 (ref) | 0.93 (0.85, 1.01) | 1.10 (1.01, 1.20) | 1.05 (0.95, 1.16) | 0.009 | ||
| Chloramine | Cases () | 283,385 | 1,100 | 3,136 | 401 | 667 | |
| Noncases () | 61,404 | 166,953 | 20,646 | 32,214 | |||
| Model 1 | 1.00 (ref) | 1.07 (1.00, 1.15) | 1.03 (0.91, 1.08) | 1.06 (0.91, 1.18) | 0.2 | ||
| Model 2 | 1.00 (ref) | 0.90 (0.82, 0.99) | 0.94 (0.82, 1.08) | 0.91 (0.80, 1.03) | 0.4 | ||
| Model 2 | 1.00 (ref) | 1.01 (0.89, 1.14) | 0.96 (0.86, 1.06) | 0.4 | |||
| Hypochlorite | Cases () | 177,048 | 1,100 | 576 | 988 | 884 | |
| Noncases () | 61,404 | 26,660 | 45,489 | 39,947 | |||
| Model 1 | 1.00 (ref) | 1.11 (1.00, 1.25) | 1.16 (1.06, 1.28) | 1.18 (1.07, 1.30) | |||
| Model 2 | 1.00 (ref) | 1.09 (0.96, 1.23) | 1.14 (1.04, 1.26) | 1.20 (1.08, 1.33) | |||
| Model 2 | 1.00 (ref) | 1.14 (1.03, 1.26) | 1.21 (1.09, 1.35) |
Note: Model 1 was adjusted for maternal age, BMI, household income, attained education, smoking at week 30, and country of birth by inverse probability weighting. Model 2 was as Model 1 additionally adjusted for previous miscarriages, parity, sick leave/early retirement, use of teratogenic drugs, diabetes, preeclampsia, hypertension, weight gain, and year of birth. BMI, body mass index; ref, reference; TTHM, trihalomethanes.
: linear trends across categories were tested using the median TTHM concentration within categories as a continuous variable.
Chloramine, hypochlorite, chlorine gas, chlorine dioxide in single or combined treatment.
In the assessments stratified by chlorination treatment, we observed for hypochlorite statistically significantly increased odds of SGA comparing the highest TTHM exposure group with the unexposed reference, (95% CI: 1.08, 1.33, ). If we excluded stillbirths from these analyses, the results remained very similar, (95% CI: 1.07, 1.32). Excluding the unexposed group from the analysis and instead assigning the lowest TTHM exposure group (i.e., ) as the reference, the OR for the highest TTHM exposure remained essentially the same: (95% CI: 1.09, 1.35, ) (Table 3). When chloramine was used as the disinfection treatment, we observed (95% CI: 0.80, 1.03, ) comparing the highest TTHM exposure group to the unexposed reference (Table 3). These results did not appreciably change when we assigned the lowest TTHM exposure group (i.e., ) as the reference.
Changing the assessment from third to second trimester exposure gave essentially the same results: no overall association with risk of SGA, comparing the highest exposures to the unexposed reference (see Table S1). In agreement with the third trimester exposure, there was a statistically significantly increased risk in areas using hypochlorite: (95% CI: 1.03, 1.28, ). When was used as reference, we observed (95% CI: 0.98, 1.22) for the highest TTHM exposure group. No associations were observed for chloramine.
Preterm and Very Preterm Delivery
For all different chlorination treatments combined, TTHM exposure during the second trimester, the OR for preterm delivery from Model 1 indicated associations with lower risk, comparing the highest TTHM exposure to the unexposed reference [ (95% CI: 0.88, 0.99)] (Table 4). In the further multivariable-adjusted model (Model 2), the (95% CI: 0.88, 1.00). In localities having exclusive treatment with chloramine, the corresponding OR for preterm delivery was 0.90 (95% CI: 0.83, 0.98) (Table 4). However, no association was observed when was assigned as the reference (excluding the unexposed group from the analysis). Likewise, in localities having exclusive treatment with hypochlorite, we observed no association between TTHM exposure and preterm delivery when comparing the highest exposure to [ (95% CI: 0.88, 1.02)]. For very preterm delivery and all chlorination treatments combined, the multivariable-adjusted OR (Model 2) indicated a significant inverse association, (95% CI: 0.69, 0.98, ), comparing the highest TTHM exposure to the unexposed reference (Table 4). However, in the analyses stratified by chlorination treatment (chloramine or hypochlorite), we observed no clear associations, although the number of cases was limited and the confidence intervals wide.
Table 4.
Associations between average trihalomethane (TTHM) exposure (second trimester) for preterm delivery, and very preterm delivery expressed as odds ratios (ORs) and 95% confidence interval (CIs).
| Treatment | Components | Total births () | No chlorine (OR) | [OR (95% CI)] | [OR (95% CI)] | [OR (95% CI)] | a |
|---|---|---|---|---|---|---|---|
| Preterm delivery | |||||||
| All chlorination treatmentsb | Cases () | 548,619 | 3,394 | 12,151 | 6,455 | 3,874 | |
| Noncases () | 62,614 | 250,026 | 132,217 | 77,888 | |||
| Crude | 1.00 (ref) | 0.90 (0.86, 0.93) | 0.90 (0.86, 0.94) | 0.92 (0.87, 0.96) | 0.8 | ||
| Model 1 | 1.00 (ref) | 0.91 (0.87, 0.96) | 0.93 (0.88, 0.98) | 0.93 (0.88, 0.99) | 0.09 | ||
| Model 2 | 1.00 (ref) | 0.92 (0.88, 0.97) | 0.94 (0.89, 1.00) | 0.94 (0.88, 1.00) | 0.1 | ||
| Chloramine | Cases () | 285,586 | 3,394 | 7,810 | 670 | 1,684 | |
| Noncases () | 62,614 | 165,202 | 14,570 | 34,845 | |||
| Model 1 | 1.00 (ref) | 0.90 (0.85, 0.94) | 0.84 (0.76, 0.93) | 0.91 (0.85, 0.99) | 0.1 | ||
| Model 2 | 1.00 (ref) | 0.90 (0.85, 0.95) | 0.85 (0.77, 0.94) | 0.90 (0.83, 0.98) | 0.06 | ||
| Model 2 | 1.00 (ref) | 0.93 (0.85, 1.03) | 1.00 (0.93, 1.07) | 1.0 | |||
| Hypochlorite | Cases () | 166,172 | 3,394 | 1,474 | 1,477 | 2,091 | |
| Noncases () | 62,614 | 26,329 | 27,291 | 41,502 | |||
| Model 1 | 1.00 (ref) | 1.01 (0.94, 1.09) | 1.01 (0.94, 1.09) | 0.93 (0.88, 1.00) | 0.05 | ||
| Model 2 | 1.00 (ref) | 1.04 (0.97, 1.13) | 1.05 (0.97, 1.13) | 0.95 (0.89, 1.02) | 0.2 | ||
| Model 2 | 1.00 (ref) | 1.04 (0.97, 1.13) | 0.95 (0.88, 1.02) | 0.2 | |||
| Very preterm delivery | |||||||
| All chlorination treatmentsb | Cases () | 548,619 | 530 | 1,908 | 1,028 | 580 | |
| Noncases () | 65,478 | 260,269 | 137,644 | 81,182 | |||
| Crude | 1.00 (ref) | 0.91 (0.82, 1.00) | 0.92 (0.83, 1.03) | 0.88 (0.78, 1.00) | 0.4 | ||
| Model 1 | 1.00 (ref) | 0.88 (0.77, 1.00) | 0.91 (0.79, 1.04) | 0.83 (0.71, 0.98) | 0.2 | ||
| Model 2 | 1.00 (ref) | 0.92 (0.80, 1.06) | 0.94 (0.81, 1.09) | 0.82 (0.69, 0.98) | 0.05 | ||
| Chloramine | Cases () | 315,749 | 530 | 1,390 | 139 | 249 | |
| Noncases () | 65,478 | 194,248 | 17,435 | 36,280 | |||
| Model 1 | 1.00 (ref) | 0.86 (0.75, 0.99) | 1.04 (0.83, 1.32) | 0.81 (0.67, 0.99) | 0.2 | ||
| Model 2 | 1.00 (ref) | 0.91 (0.78, 1.06) | 1.13 (0.89, 1.45) | 0.77 (0.62, 0.96) | 0.05 | ||
| Model 2 | 1.00 (ref) | 1.23 (0.99, 1.52) | 0.85 (0.70, 1.02) | 0.06 | |||
| Hypochlorite | Cases () | 166,172 | 530 | 225 | 217 | 315 | |
| Noncases () | 65,478 | 27,578 | 28,551 | 43,278 | |||
| Model 1 | 1.00 (ref) | 0.87 (0.71, 1.07) | 0.83 (0.67, 1.02) | 0.84 (0.70, 1.01) | 0.07 | ||
| Model 2 | 1.00 (ref) | 0.95 (0.76, 1.17) | 0.89 (0.71, 1.10) | 0.88 (0.73, 1.07) | 0.2 | ||
Note: Model 1 was adjusted for maternal age, BMI, household income, attained education, smoking at week 30, and country of birth by inverse probability weighting. Model 2 was as Model 1 additionally adjusted for previous miscarriages, parity, sick leave/early retirement, use of teratogenic drugs, diabetes, preeclampsia, hypertension, weight gain, and year of birth. BMI, body mass index; ref, reference; TTHM, total trihalomethanes.
: Linear trends across categories were tested using the median TTHM concentration within categories as a continuous variable.
Chloramine, hypochlorite, chlorine gas, chlorine dioxide in single or combined treatment.
Discussion
The present nationwide register-based cohort, including singleton births, is one of the largest studies assessing CBP exposure and adverse reproductive outcomes. Based on address and average-trimester–linked TTHM concentrations in tap water, we observed dose-dependent associations with increased risk of SGA, confined to treatment with hypochlorite but not to chloramine. For preterm and very preterm delivery, the results indicated no clear associations, although there were some indications of an inverse association.
Although several epidemiological studies have indicated that CBP exposure is associated with an increased risk of SGA (Tardiff et al. 2006), reproductive and developmental animal studies generally show no indication of adverse reproductive effects, alternatively, showing that the effect is secondary to any adverse toxicological effect occurring in the dams (Colman et al. 2011). However, CBPs formed by the drinking water treatment are numerous, and there is experimental evidence where individual CBPs (e.g., THMs) have caused lower fetal body weight or growth retardations as the primary effect (Schwetz et al. 1974; Smith et al. 1987, 1989, 1992). The toxicity of the majority of the CBPs is likely due to the formation of reactive intermediates (Colman et al. 2011) and linked to the indications that to some CBPs have the potential to pass placental barrier (Christian et al. 2001).
Most previous epidemiological studies assessing CBP exposure and adverse reproductive outcomes did not consider different chlorination treatments. Several studies in which hypochlorite was the only treatment indicated an association between TTHM exposure and increased risk of SGA (Aggazzotti et al. 2004; Grazuleviciene et al. 2011; Källén and Robert 2000), although no association has also been observed (Villanueva et al. 2011). In comparison with previous studies, the TTHM concentrations in the present study were low, irrespective of the type of chlorination treatment. Although the THMs are mainly formed after treatment with hypochlorite, lower concentrations of THMs are also produced by chloramine being hydrolyzed into free chlorine (Hua and Reckhow 2007). Accordingly, in the present study the prevalence of high TTHM exposure () was considerably more common in areas using hypochlorite (44%) as compared with chloramine (15%). In addition to the fact that chloramine is less reactive than hypochlorite, consequently forming fewer CBPs, including TTHMs (Hua and Reckhow 2007), there are also differences in the types of CBPs generated between the two methods (WHO 2000). Thus, our TTHM-associated finding for increased risk of SGA for drinking water treated with hypochlorite, but not chloramine, could be due to quantitative as well as qualitative differences in the formation of CBPs. It could also be due to a lower exposure misclassification in the areas using hypochlorite because it is well established that THMs are reasonably good proxies for total CBPs when this chlorination method is used. Because other CBPs are formed when chloramines are used, TTHMs become less representative of the total CBPs.
In the present study, SGA was predefined in the Swedish Medical Birth Register according to below the sex- and gestational age–specific average weight at partus (corresponding to the 2.3rd percentile). Although this cutoff is more stringent, capturing mainly moderate-to-severe cases of SGA (increasing the specificity—less of a threat to validity) as compared with those studies using the 10th percentile as cutoff, we may not capture the full effect of the CBP exposure.
We observed no clear associations between TTHM and preterm or very preterm delivery in the present study although there were some indications of inverse associations, as also suggested in some previous epidemiological studies (Grellier et al. 2010). Because maternal infections may induce preterm delivery (Pararas et al. 2006), it has been hypothesized that chlorination of drinking water may cause a lower occurrence of maternal infections (Jaakkola et al. 2001), which could potentially explain the inverse associations. Yet, no studies have confirmed this link. An alternative explanation for these previous inverse findings could be selection bias introduced by restricting the analyses to only live births (Hernán et al. 2002; Lewis et al. 2007). Although we were able to include the stillbirths in the present study, we were not able to include pregnancies resulting in spontaneous abortion. Because we observed no clear dose–response and because the results were highly dependent on the choice of reference area, the most reasonable interpretation of our findings is that TTHM, or chlorination per se, was not linked to preterm or very preterm delivery at the TTHM concentrations appearing in the present study.
As in any epidemiological study, the present work suffered from limitations. Foremost, we consider exposure misclassification as the most important issue, which also highlights the complexity of the assessment. First, we were unable to assess the drinking water consumption or the exposure via any other relevant routes. We observed in a previous survey that the vast majority of the adult population in Sweden is consuming unheated tap water (99.8%) (Säve-Söderbergh et al. 2018). On the other hand, the THMs are highly volatile and therefore ingestion, inhalation, and dermal exposure could be equally important routes of exposure (Backer et al. 2000; Xu et al. 2002), indicating that nonconsumers of tap water are also exposed to the THMs. Some studies have tried to reduce the misclassification by collecting information on water consumption and on showering/bathing habits (Danileviciute et al. 2012; Grazuleviciene et al. 2013), and others measured the blood concentrations of THMs (Smith et al. 2016; Zhou et al. 2018). However, because THMs are easily absorbed and have a short half-life, the blood concentrations fluctuate, with high peaks of the most volatile CBPs seen shortly after showering, complicating the use of biomarkers of exposure (Backer et al. 2008). Second, we applied the same average TTHM concentration to the whole locality. Because most of the water treatment plants were small ( distributed drinking water to localities with more inhabitants), with a more rapid water distribution turnover, the spatial variation in exposure within the network is likely less pronounced. Third, to avoid the impact of missing CBP tap water data for a specific month and locality, we assigned the trimester average exposure for each pregnancy. Although a more precise exposure period could have been preferable, the knowledge is still limited on the gestational age for the specific effect-windows for the outcomes evaluated in the present study, resulting in the trimester-specific average being the most appropriate. Fourth, we cannot exclude that CBPs other than the four most common THMs were responsible for the observed association because the concentrations and composition of CBPs in Sweden are dependent on several raw water–related factors (Andersson et al. 2019a; Lavonen et al. 2013).
Besides exposure misclassification, confounding needs to be reflected upon. Although we have good information on many important risk factors and have used inverse probability weighting to account for unequal distribution of risk factors, we cannot fully exclude that some unmeasured confounding may have affected our results. For example, differences in study area–specific characteristics such as larger localities in exposed areas could not be controlled for in the analyses. However, because the results remained essentially the same by changing the reference area from no chlorination to the lowest exposure category (), this potential impact seemed limited.
Several important strengths need to be highlighted. This is one of the largest prospective studies, assessing adverse reproductive outcomes in relation to TTHM exposure, and we were able to adjust for most relevant confounders at an individual level. The Swedish Medical Birth Register and the Statistics Sweden registers have a high coverage, close to 100% (Källén and Källén 2003; Ludvigsson et al. 2016), which, for example, reduced the risk of a biased selection of the pregnancies included. Moreover, because the maternal and delivery care is publicly funded in Sweden, there is likely no bias introduced by mothers not visiting the antenatal care because of their economic situation, resulting in similar data quality of the information gathered during pregnancy across the regions. Despite the aforementioned shortcomings linked to the exposure assessment, strong effort was made to accurately classify the exposure. The individual maternal TTHM exposure assigned was based on residential and temporal information at an exposure-relevant effect-window. In contrast to most previous studies, we were able to include an unexposed reference population that was served by municipal drinking water and not by private wells. This substantially improved the comparability with the exposed populations and minimized the risk of introducing contextual confounding related mainly to differences between urban and rural areas. Nevertheless, potential remaining contextual confounding related to differences in locality characteristics, such as size, cannot be fully excluded. In any case, the sensitivity analysis performed, based on the lowest exposure () as the reference, supported our conclusion. Moreover, based on detailed information on drinking water production, we were able to stratify our analyses by the chlorination treatment, something that few previous studies have been able to consider.
In conclusion, the results of the present study provide the evidence that CBP exposure via drinking water is associated with increased risk of SGA in areas with hypochlorite treatment, but not chloramine, potentially due to CBP formation differences. There was no clear association for preterm or very preterm delivery.
Supplementary Material
Acknowledgments
This study was funded by the Swedish Research Council Formas (grant 942-2015-425).
References
- Aggazzotti G, Righi E, Fantuzzi G, Biasotti B, Ravera G, Kanitz S, et al. 2004. Chlorination by-products (CBPs) in drinking water and adverse pregnancy outcomes in Italy. J Water Health 2(4):233–247, PMID: 15666965, 10.2166/wh.2004.0021. [DOI] [PubMed] [Google Scholar]
- Andersson A, Ashiq MJ, Shoeb M, Karlsson S, Bastviken D, Kylin H. 2019a. Evaluating gas chromatography with a halogen-specific detector for the determination of disinfection by-products in drinking water. Environ Sci Pollut Res Int 26(8):7305–7314, PMID: 29492811, 10.1007/s11356-018-1419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson A, Harir M, Gonsior M, Hertkorn N, Schmitt-Kopplin P, Kylin H, et al. 2019b. Waterworks-specific composition of drinking water disinfection by-products. Environ Sci Water Res Technol 5(5):861–872, 10.1039/C9EW00034H. [DOI] [Google Scholar]
- Backer LC, Ashley DL, Bonin MA, Cardinali FL, Kieszak SM, Wooten JV. 2000. Household exposures to drinking water disinfection by-products: whole blood trihalomethane levels. J Expo Anal Environ Epidemiol 10(4):321–326, PMID: 10981726, 10.1038/sj.jea.7500098. [DOI] [PubMed] [Google Scholar]
- Backer LC, Lan Q, Blount BC, Nuckols JR, Branch R, Lyu CW, et al. 2008. Exogenous and endogenous determinants of blood trihalomethane levels after showering. Environ Health Perspect 116(1):57–63, PMID: 18197300, 10.1289/ehp.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman GA. 1999. Drinking water disinfection byproducts: review and approach to toxicity evaluation. Environ Health Perspect 107(suppl 1):207–217, PMID: 10229719, 10.1289/ehp.99107s1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove F, Shim Y, Zeitz P. 2002. Drinking water contaminants and adverse pregnancy outcomes: a review. Environ Health Perspect 110(suppl 1):61–74, PMID: 11834464, 10.1289/ehp.02110s161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao WC, Zeng Q, Luo Y, Chen HX, Miao DY, Li L, et al. 2016. Blood biomarkers of late pregnancy exposure to trihalomethanes in drinking water and fetal growth measures and gestational age in a Chinese cohort. Environ Health Perspect 124(4):536–541, PMID: 26340795, 10.1289/ehp.1409234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian MS, York RG, Hoberman AM, Diener RM, Fisher LC, Gates GA. 2001. Biodisposition of dibromoacetic acid (DBA) and bromodichloromethane (BDCM) administered to rats and rabbits in drinking water during range-finding reproduction and developmental toxicity studies. Int J Toxicol 20(4):239–253, PMID: 11563419, 10.1080/109158101750408064. [DOI] [PubMed] [Google Scholar]
- Colman J, Rice GE, Wright JM, Hunter ES III, Teuschler LK, Lipscomb JC, et al. 2011. Identification of developmentally toxic drinking water disinfection byproducts and evaluation of data relevant to mode of action. Toxicol Appl Pharmacol 254(2):100–126, PMID: 21296098, 10.1016/j.taap.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Danileviciute A, Grazuleviciene R, Vencloviene J, Paulauskas A, Nieuwenhuijsen MJ. 2012. Exposure to drinking water trihalomethanes and their association with low birth weight and small for gestational age in genetically susceptible women. Int J Environ Res Public Health 9(12):4470–4485, PMID: 23222181, 10.3390/ijerph9124470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves CG, Matanoski GM, Tardiff RG. 2001. Weight of evidence for an association between adverse reproductive and developmental effects and exposure to disinfection by-products: a critical review. Regul Toxicol Pharmacol 34(2):103–124, PMID: 11603954, 10.1006/rtph.2001.1494. [DOI] [PubMed] [Google Scholar]
- Grazuleviciene R, Kapustinskiene V, Vencloviene J, Buinauskiene J, Nieuwenhuijsen MJ. 2013. Risk of congenital anomalies in relation to the uptake of trihalomethane from drinking water during pregnancy. Occup Environ Med 70(4):274–282, PMID: 23404756, 10.1136/oemed-2012-101093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grazuleviciene R, Nieuwenhuijsen MJ, Vencloviene J, Kostopoulou-Karadanelli M, Krasner SW, Danileviciute A, et al. 2011. Individual exposures to drinking water trihalomethanes, low birth weight and small for gestational age risk: a prospective Kaunas cohort study. Environ Health 10:32, PMID: 21501533, 10.1186/1476-069X-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grellier J, Bennett J, Patelarou E, Smith RB, Toledano MB, Rushton L, et al. 2010. Exposure to disinfection by-products, fetal growth, and prematurity: a systematic review and meta-analysis. Epidemiology 21(3):300–313, PMID: 20375841, 10.1097/EDE.0b013e3181d61ffd. [DOI] [PubMed] [Google Scholar]
- Hernán MA, Hernández-Díaz S, Werler MM, Mitchell AA. 2002. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol 155(2):176–184, PMID: 11790682, 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- Hernán MA, Robins JM. 2020. Causal Inference: What If. Boca Raton: Chapman & Hall/CRC. [Google Scholar]
- Hinckley AF, Bachand AM, Reif JS. 2005. Late pregnancy exposures to disinfection by-products and growth-related birth outcomes. Environ Health Perspect 113(12):1808–1813, PMID: 16330369, 10.1289/ehp.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman CS, Mendola P, Savitz DA, Herring AH, Loomis D, Hartmann KE, et al. 2008. Drinking water disinfection by-product exposure and fetal growth. Epidemiology 19(5):729–737, PMID: 18633330, 10.1097/EDE.0b013e3181812bd4. [DOI] [PubMed] [Google Scholar]
- Hrudey SE. 2009. Chlorination disinfection by-products, public health risk tradeoffs and me. Water Res 43(8):2057–2092, PMID: 19304309, 10.1016/j.watres.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Hua G, Reckhow DA. 2007. Comparison of disinfection byproduct formation from chlorine and alternative disinfectants. Water Res 41(8):1667–1678, PMID: 17360020, 10.1016/j.watres.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Ileka-Priouzeau S, Campagna C, Legay C, Deonandan R, Rodriguez MJ, Levallois P. 2015. Women exposure during pregnancy to haloacetaldehydes and haloacetonitriles in drinking water and risk of small-for-gestational-age neonate. Environ Res 137:338–348, PMID: 25601737, 10.1016/j.envres.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Iszatt N, Nieuwenhuijsen MJ, Bennett JE, Toledano MB. 2014. Trihalomethanes in public drinking water and stillbirth and low birth weight rates: an intervention study. Environ Int 73:434–439, PMID: 25244706, 10.1016/j.envint.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Jaakkola JJ, Magnus P, Skrondal A, Hwang BF, Becher G, Dybing E. 2001. Foetal growth and duration of gestation relative to water chlorination. Occup Environ Med 58(7):437–442, PMID: 11404447, 10.1136/oem.58.7.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källén B, Källén K. 2003. The Swedish Medical Birth Register - A Summary of Content and Quality. Report no. 2003-112-3. Stockholm, Sweden: Socialstyrelsen; http://lup.lub.lu.se/record/1127699 [accessed 5 May 2020]. [Google Scholar]
- Källén BA, Robert E. 2000. Drinking water chlorination and delivery outcome-a registry-based study in Sweden. Reprod Toxicol 14(4):303–309, PMID: 10908833, 10.1016/S0890-6238(00)00086-1. [DOI] [PubMed] [Google Scholar]
- Kogevinas M, Bustamante M, Gracia-Lavedán E, Ballester F, Cordier S, Costet N, et al. 2016. Drinking water disinfection by-products, genetic polymorphisms, and birth outcomes in a European mother–child cohort study. Epidemiology 27:903–911, PMID: 27468006, 10.1097/EDE.0000000000000544. [DOI] [PubMed] [Google Scholar]
- Kramer MD, Lynch CF, Isacson P, Hanson JW. 1992. The association of waterborne chloroform with intrauterine growth retardation. Epidemiology 3(5):407–413, PMID: 1391132, 10.1097/00001648-199209000-00005. [DOI] [PubMed] [Google Scholar]
- Lavonen EE, Gonsior M, Tranvik LJ, Schmitt-Kopplin P, Köhler SJ. 2013. Selective chlorination of natural organic matter: identification of previously unknown disinfection byproducts. Environ Sci Technol 47(5):2264–2271, PMID: 23373647, 10.1021/es304669p. [DOI] [PubMed] [Google Scholar]
- Levallois P, Gingras S, Marcoux S, Legay C, Catto C, Rodriguez M, et al. 2012. Maternal exposure to drinking-water chlorination by-products and small-for-gestational-age neonates. Epidemiology 23(2):267–276, PMID: 22317810, 10.1097/EDE.0b013e3182468569. [DOI] [PubMed] [Google Scholar]
- Lewis C, Suffet IH, Hoggatt K, Ritz B. 2007. Estimated effects of disinfection by-products on preterm birth in a population served by a single water utility. Environ Health Perspect 115(2):290–295, PMID: 17384780, 10.1289/ehp.9394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C, Suffet IH, Ritz B. 2006. Estimated effects of disinfection by-products on birth weight in a population served by a single water utility. Am J Epidemiol 163(1):38–47, PMID: 16282238, 10.1093/aje/kwj009. [DOI] [PubMed] [Google Scholar]
- Ludvigsson JF, Almqvist C, Bonamy AKE, Ljung R, Michaëlsson K, Neovius M, et al. 2016. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 31(2):125–136, PMID: 26769609, 10.1007/s10654-016-0117-y. [DOI] [PubMed] [Google Scholar]
- Maršál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. 1996. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr 85(7):843–848, PMID: 8819552, 10.1111/j.1651-2227.1996.tb14164.x. [DOI] [PubMed] [Google Scholar]
- Mercier Shanks C, Sérodes JB, Rodriguez MJ. 2013. Spatio-temporal variability of non-regulated disinfection by-products within a drinking water distribution network. Water Res 47(9):3231–3243, PMID: 23582352, 10.1016/j.watres.2013.03.033. [DOI] [PubMed] [Google Scholar]
- Miller A, Siffel C, Correa A. 2010. Residential mobility during pregnancy: patterns and correlates. Matern Child Health J 14(4):625–634, PMID: 19568920, 10.1007/s10995-009-0492-z. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuijsen MJ, Toledano MB, Eaton NE, Fawell J, Elliott P. 2000. Chlorination disinfection byproducts in water and their association with adverse reproductive outcomes: a review. Occup Environ Med 57(2):73–85, PMID: 10711274, 10.1136/oem.57.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nörby U, Källén K, Eiermann B, Korkmaz S, Winbladh B, Gustafsson LL. 2013. Drugs and Birth Defects: a knowledge database providing risk assessments based on national health registers. Eur J Clin Pharmacol 69(4):889–899, PMID: 23011015, 10.1007/s00228-012-1399-y. [DOI] [PubMed] [Google Scholar]
- Pararas MV, Skevaki CL, Kafetzis DA. 2006. Preterm birth due to maternal infection: causative pathogens and modes of prevention. Eur J Clin Microbiol Infect Dis 25(9):562–569, PMID: 16953371, 10.1007/s10096-006-0190-3. [DOI] [PubMed] [Google Scholar]
- Patelarou E, Kargaki S, Stephanou EG, Nieuwenhuijsen M, Sourtzi P, Gracia E, et al. 2011. Exposure to brominated trihalomethanes in drinking water and reproductive outcomes. Occup Environ Med 68(6):438–445, PMID: 20952554, 10.1136/oem.2010.056150. [DOI] [PubMed] [Google Scholar]
- Porter CK, Putnam SD, Hunting KL, Riddle MR. 2005. The effect of trihalomethane and haloacetic acid exposure on fetal growth in a Maryland county. Am J Epidemiol 162(4):334–344, PMID: 16014784, 10.1093/aje/kwi211. [DOI] [PubMed] [Google Scholar]
- Richardson SD, Plewa MJ, Wagner ED, Schoeny R, DeMarini DM. 2007. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat Res 636(1–3):178–242, PMID: 17980649, 10.1016/j.mrrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Rivera-Núñez Z, Wright JM. 2013. Association of brominated trihalomethane and haloacetic acid exposure with fetal growth and preterm delivery in Massachusetts. J Occup Environ Med 55(10):1125–1134, PMID: 24064786, 10.1097/JOM.0b013e3182a4ffe4. [DOI] [PubMed] [Google Scholar]
- Säve-Söderbergh M, Toljander J, Mattisson I, Åkesson A, Simonsson M. 2018. Drinking water consumption patterns among adults—SMS as a novel tool for collection of repeated self-reported water consumption. J Expo Sci Environ Epidemiol 28(2):131–139, PMID: 28612838, 10.1038/jes.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwetz BA, Leong BK, Gehring PJ. 1974. Embryo- and fetotoxicity of inhaled chloroform in rats. Toxicol Appl Pharmacol 28(3):442–451, PMID: 4851839, 10.1016/0041-008X(74)90229-4. [DOI] [PubMed] [Google Scholar]
- Smith MK, George EL, Zenick H, Manson JM, Stober JA. 1987. Developmental toxicity of halogenated acetonitriles: drinking water by-products of chlorine disinfection. Toxicology 46(1):83–93, PMID: 3660423, 10.1016/0300-483X(87)90140-5. [DOI] [PubMed] [Google Scholar]
- Smith MK, Randall JL, Read EJ, Stober JA. 1989. Teratogenic activity of trichloroacetic acid in the rat. Teratology 40(5):445–451, PMID: 2623633, 10.1002/tera.1420400506. [DOI] [PubMed] [Google Scholar]
- Smith MK, Randall JL, Read EJ, Stober JA. 1992. Developmental toxicity of dichloroacetate in the rat. Teratology 46(3):217–223, PMID: 1523579, 10.1002/tera.1420460305. [DOI] [PubMed] [Google Scholar]
- Smith RB, Edwards SC, Best N, Wright J, Nieuwenhuijsen MJ, Toledano MB. 2016. Birth weight, ethnicity, and exposure to trihalomethanes and haloacetic acids in drinking water during pregnancy in the Born in Bradford cohort. Environ Health Perspect 124(5):681–689, PMID: 26340797, 10.1289/ehp.1409480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedish National Food Agency. 2001. SLVFS 2001:30 Statens livsmedelsverks författningssamling ISSN 0346-119X Statens livsmedelsverks föreskrifterom dricksvatten [SLVFS 2001:30 Swedish National Food Agency’s statutory collection ISSN 0346-119X Stats food regulations for drinking water]. [In Swedish.] https://www.livsmedelsverket.se/globalassets/om-oss/lagstiftning/dricksvatten---naturl-mineralv---kallv/slvfs-2001-30-hela_foreskriften.pdf [accessed 5 May 2020].
- SWWA (Swedish Water and Wastewater Association). 1996. Analysdata. 1994-Uppgifter över bakteriologisk och kemisk beskaffenhet hos råvatten och dricksvatten vid kommunala vattenverk [Analysis data 1994. Information on the bacteriological and chemical characteristics of raw water and drinking water at municipal water utilities]. [In Swedish.] Report no. VAV AD94. Stockholm, Sweden: SWWA. [Google Scholar]
- SWWA. 2014. Mikrobiologiska säkerhetsbarriärer-Lägesrapport efter uppdatering av databas 2014 [Microbiological safety barriers-Situation report after database update in 2014]. [In Swedish.] Report no. 2014-12-10. Uppsala, Sweden: SWWA; https://www.svensktvatten.se/globalassets/dricksvatten/vattenverk-och-reningsprocesser/mikrobiologiska-barriarer.pdf [accessed 5 May 2020]. [Google Scholar]
- Tardiff RG, Carson ML, Ginevan ME. 2006. Updated weight of evidence for an association between adverse reproductive and developmental effects and exposure to disinfection by-products. Regul Toxicol Pharmacol 45(2):185–205, PMID: 16624462, 10.1016/j.yrtph.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Uyak V, Ozdemir K, Toroz I. 2008. Seasonal variations of disinfection by-product precursors profile and their removal through surface water treatment plants. Sci Total Environ 390(2–3):417–424, PMID: 17997473, 10.1016/j.scitotenv.2007.09.046. [DOI] [PubMed] [Google Scholar]
- Villanueva CM, Gracia-Lavedán E, Ibarluzea J, Santa Marina L, Ballester F, Llop S, et al. 2011. Exposure to trihalomethanes through different water uses and birth weight, small for gestational age, and preterm delivery in Spain. Environ Health Perspect 119(12):1824–1830, PMID: 21810554, 10.1289/ehp.1002425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2000. Disinfectants and Disinfectant By-Products. Environmental Health Criteria 216. Geneva: WHO, International Programme on Chemical Safety; http://inchem.org/documents/ehc/ehc/ehc216.htm [accessed 5 May 2020]. [Google Scholar]
- WHO. 2016. International Statistical Classification of Diseases and Related Health Problems, 10th Revision. http://apps.who.int/classifications/icd10/browse/2016/en [accessed 28 April 2020].
- Wright JM, Schwartz J, Dockery DW. 2003. Effect of trihalomethane exposure on fetal development. Occup Environ Med 60(3):173–180, PMID: 12598663, 10.1136/oem.60.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JM, Schwartz J, Dockery DW. 2004. The effect of disinfection by-products and mutagenic activity on birth weight and gestational duration. Environ Health Perspect 112(8):920–925, PMID: 15175183, 10.1289/ehp.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Mariano TM, Laskin JD, Weisel CP. 2002. Percutaneous absorption of trihalomethanes, haloacetic acids, and haloketones. Toxicol Appl Pharmacol 184(1):19–26, PMID: 12392965, 10.1006/taap.2002.9494. [DOI] [PubMed] [Google Scholar]
- Zhou B, Yang P, Gong YJ, Zeng Q, Lu WQ, Miao XP. 2018. Effect modification of CPY2E1 and GSTZ1 genetic polymorphisms on associations between prenatal disinfection by-products exposure and birth outcomes. Environ Pollut 243(pt B):1126–1133, PMID: 30253304, 10.1016/j.envpol.2018.09.083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.