Abstract
Beta-adrenergic receptors (β-ARs) play a critical role in many diseases. Quantification of β-AR density may have clinical implications in terms of assessing disease severity and identifying patients who could potentially benefit from beta-blocker therapy. Classical methods for β-AR quantification are based on labor-intensive and time-consuming radioligand binding assays. Here, we report optimization of a flow cytometry-based method utilizing a biotinylated β-AR ligand alprenolol as a probe and use of this method to quantify relative receptor expression in healthy controls (HC). Quantum™ MESF beads were used for quantification in absolute fluorescence units. The probe was chemically modified by adding a spacer moiety between biotin and alprenolol to stabilize receptor binding, thus preventing binding decay. Testing of three different standard cell fixation and permeabilization methods (formaldehyde fixation and saponin, Tween-20, or Triton-X 100 permeabilization) showed that the formalde-hyde/Triton-X 100 method yielded the best results. β-AR expression was significantly higher in granulocytes compared to mononuclear cells. These data show that flow cytometric quantification of relative β-AR expression in circulating leukocytes is a suitable technology for large-scale clinical application.
Keywords: beta-adrenergic receptor peripheral blood cells, flow cytometry, quantification
Beta -adrenergic receptors (β-ARs) belong to a family of transmembrane G-protein coupled proteins that are physiologically activated by epinephrine and norepinephrine. Three subgroups of β-ARs—β1, β2, and β3—are distinguished based on differences in interactions with agonists. Stimulation of β-AR activates adenylyl cyclase, resulting in increased intracellular levels of the second messenger cyclic adenosinemonophosphate (cAMP) that signals via cAMP-dependent protein kinase A (PKA) to orchestrate downstream intracellular events (1).
β-ARs, particularly β2-ARs, are expressed on virtually all peripheral blood cells(2). Growing evidence suggests that the sympathetic nervous system interacts with the immune system through the neurotransmitters norepinephrine and epinephrine that are released from noradrenergic nerve endings in primary and secondary lymphoid organs. This cross-talk leads to stimulation of β-ARs on mononuclear cells, which inhibits T-cell proliferation, decreases cytotoxic-T-cell and natural killer cell activity, and regulates immune cell maturation and lymphocyte homing (3–5). Therefore, it is not surprising that many autoimmune diseases have been shown to be associated with β-AR dysregulation. For example, juvenile type 1 diabetes mellitus is characterized by autoimmune destruction of the insulin-producing beta cells in the pancreas and is associated with decreased β-AR expression on granulocytes (6). Multiple sclerosis, an auto-immune disease characterized by axonal loss and demyelination in the central nervous system, is associated with increased cell surface expression of β2-AR in peripheral blood mononuclear cells (7,8). Given the increased expression of β2-ARs in multiple sclerosis, β2-AR agonist salbutamol has been considered as a potential therapeutic option(9). Rheumatoid arthritis, another autoimmune disease, is associated with a downregulation of β2-AR on peripheral blood mononuclear cells that has been correlated to favorable outcomes (10,11). Other autoimmune diseases associated with decreased β-ARs include Crohn’s disease, systemic lupus erythematosus, and myasthenia gravis (12–14).
Additionally, β-ARs have also been suggested to play role in the pathogenesis of several cardiac diseases such as chronic heart failure, coronary artery by-pass grafting, and ischemic heart disease (15,16). The heart possesses high numbers of both β1 and β2-ARs that mediate myocardial contractility and heart rate. In heart failure, the sympathetic system is activated, and beta-blockers (β-receptor antagonists) are used in treatment. As the function of β-ARs on lymphocyte and myocardium has shown to be comparable, quantification of β-ARs on lymphocytes may useful to monitor therapeutic responses to β-receptor antagonist (17,18). However, such analysis is lacking.
Quantification of β-AR expression has the potential to become an important clinical measure in the evaluation of treatment responses, disease activity, and prognosis of several diseases involving beta receptor dysfunction. Fast and easy-touse methods could make β-AR expression assessment accessible for incorporation into routine clinical practice and aid in shedding light on β-AR dysregulation in disease. The current technique to assess β-AR expression is time-consuming and labor-intensive radioligand binding assays (19–23). The β-AR agonist alprenolol is a useful probe to quantify the receptor, but the technique has not been optimized for processing of large-scale clinical samples (19,24). Here, we describe an optimized method that will allow for large-scale flow cytometric quantification of relative β-AR expression on peripheral blood cells in MESF units.
MATERIALS AND METHODS
Study Population
This study was approved by the Cleveland Clinic Institutional Review Board and conducted in accordance with the Declaration of Helsinki. Healthy participants who did not have a history of chronic disease or medication use were enrolled in the study. Peripheral venous blood was drawn into a 5 ml tube containing EDTA as an anticoagulant from all participants and processed within 2 h after collection. Basic demographic and clinical information were retrieved from electronic medical records.
HEK 293 Cells
HEK 293 cells (CRL-1573™) were obtained from American Type Culture Collection (ATCC) (Manassas, VA), and HEK 293 cells stably overexpressing β2-AR were generated in house as previously described (25). [125]-cyanopindalol-binding was performed as reported (25). Cells were cultured in MEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin and processed for staining as described below. Cell viability was >95% as determined by trypan blue exclusion test after harvesting the cells using accutase (Sigma-Aldrich). Alprenolol staining for flow cytometry was performed after fixation and permeabilization using 10% formaldehyde and 0.2% Triton X100 as described for peripheral blood cells.
Processing of Peripheral Blood Samples
To optimize the quantification of β-AR expression, different fixation and permeabilization methods were tested. In the first method, cells were fixed with 10% formaldehyde before Triton X-100 was used at a concentration of 0.2% to lyse red blood cells and permeabilize cells in a single step (26,27). Two other methods were tested, each starting with lysis of red blood cells using ammonium chloride (8.3 g/l) followed by fixation using 4% formaldehyde. Cells were permeabilized by including either saponin (1 mg/ml) or Tween-20(0.05% by volume) in the buffers used throughout the flow staining process, including washing steps. After a series of final washes with 4% FBS in phosphate buffered saline (PBS), peripheral blood cells were divided into aliquots of approximately 10 × 106 cells and frozen at −80°C in freezing medium consisting of 10% glycerol and 20% FBS in RPMI 1640.
Alprenolol Staining
Labeling was performed by thawing fixed/permeabilized cells at room temperature followed by addition of 3 ml saline and centrifugation at 900g for 5 min to pellet the cells. Following aspiration of supernatants, 2–5 × 106 cells were resuspended in 250 μl Streptavidin blocking buffer (10 μg/ml unconjugated streptavidin in PBS) and incubated for 20 min in the dark. After two washes with dilution buffer (1% bovine serum albumin (BSA)in PBS), cells were aliquoted into two tubes named test and secondary only. Cells in the test tube were stained with 100 μl alprenolol biotin probe (1:200 in dilution buffer, final concentration 25 μg alprenolol/ml), while cells in secondary only tube were resuspended in 100 μl dilution buffer. Both tubes were then covered and incubated on a plate shaker for 40 min at room temperature. After two washes with dilution buffer, 100 μl streptavidin-PE (1:100 in dilution buffer, final concentration 1 μg/ml) were added to both tubes and incubated for 25 min in dark. As a final step, cells were washed and resuspended in 300 μl FACSflow. 100,000 events were acquired for each tube using an LSR II flow cytometer that has five lasers (355 nm, 405 nm, 488 nm, 532 nm, 639 nm; Becton Dickinson, Franklin Lakes, New Jersey). Light scatter and PE fluorescence signals (PE excitation: Green laser (532 nm), filter: 575/26 (emission range: 562–588 nm)) were collected. Data were stored in ListMode files. For confocal microscopy, DyLight 488 streptavidin (1/1,000, final concentration 1 μg/ml) was used a secondary reagent instead of streptavidin-PE due to rapid PE fluorophore photo-bleaching in microscopy. Cells were stained in suspension as described above. A quantity of 50 μl was pipetted onto a Cyto-spin slide and covered with a cover slip. Images were collected with a Leica SP8 confocal microscope ((Leica Microsystems, GmbH, Wetzlar, Germany) using an HC Pl Apo CS2 63x/1.40 oil immersion lens at a zoom of 1.5. Two fluorescence channels were imaged sequentially to eliminate any crosstalk between them. DAPI-stained nuclei were imaged using the 405 nm laser with emitted light collected between 410 and 480 nm. DyLight 488 streptavidin-labeled probe was excited at 488 nm with emission collected between 500 and 550 nm.
Data Analysis
Data analysis was performed using FlowJo software(vX.0.7, Tree Star, OR). Quantum PE MESF (Molecules of Equivalent Soluble Fluorochrome) microsphere kit (Bangs Laboratory, Fishers, IN) was used for absolute fluorescence quantification according to the manufacturer’s instructions. Quantum PE MESF beads were run with each sample batch at identical instrument settings as the probe-labeled samples. The microspheres contained five different intensity peaks, and each peak was gated on a PE histogram. The median fluorescence value of each peak corresponds to a specific PE MESF value provided by the manufacturer in a QuickCalVR Excel template. Entering the median fluorescence value of each peak automatically generated a calibration curve. The PE median fluorescence values derived from the samples were then entered into the Excel spreadsheet and accordingly converted into PE MESF. An example of the QuickCal worksheet is shown in Supporting Information figure 1. MESF units of the samples were obtained for both test (MESFT) and secondary only (MESFS) tubes. Background MESFS was subtracted from MESFT to obtain MESF corresponding with actual β AR binding.
Two-tailed Student’s t test was used for statistical analysis using JMP software (JMP, Version 13. SAS Institute, Cary, NC). Data are expressed as mean (±SE) values.
RESULTS
Stabilization of Biotinylated Alprenolol Probe β-AR Binding by Addition of a Linker Amino Acid
We published previously that biotinylated alprenolol is a β-AR specific probe for flow cytometric detection of β-AR expression on circulating cells. However, instability of binding resulted in significant decay within minutes after labeling of the cells (24). In Figure 1A, we show the chemical structure of the improved probe containing an amino acid linker molecule between the alprenolol and biotin. Comparison of the previous probe and the improved probe showed that the addition of a spacer molecule stabilized binding of biotinylated alprenolol to β-AR (Fig. 1B). The data showed that a linker amino acid between the alprenolol and biotin improved the binding kinetics. Optimal binding concentration of the old probe was 25 μg/ml (24). Similarly, the optimized probe gave the best resolution between mononuclear cells and granulocytes at an optimal binding concentration of 25 μg/ml (Fig. 1C). Both probes were titrated in parallel demonstrating that stabilization of the alprenolol probe did not affect the optimal working concentration (Fig. 1D). Specificity of alprenolol probe binding to β-AR was previously demonstrated by competitive inhibition of receptor binding using isoproterenol, another beta-adrenergic receptor ligand (24). As an additional control, wild type HEK 293 cells and HEK 293 cells overexpressing β AR stained with the optimized probe at the optimal binding concentration of 25 μg/ml showed that at this concentration, the probe was able to distinguish cells with low (wild type) and high (overexpressing) β-AR (Fig. 1E). To further validate that HEK 293 cells specifically overexpress β2-AR, we performed a radioligand binding assay, the gold standard in assessing cell surface receptors. Plasma membranes were isolated, and radioligand binding was performed on them to determine the quantitative receptor expression. The studies were performed with a saturating concentration of [125]-I cyanopindalol (25), and its specificity was assessed by displacement to β2-AR-specific antagonist ICI 118 551. Data shown in Figure 1F confirmed that β-AR were higher in HEK 293 cells overexpressing the receptor. Binding of the old vs.new probes to same HC subjects showed an improved binding of the optimized probe (Fig. 1G) (median fluorescence intensity × 103: old probe 17.7 ± 0.3; new probe 96.6 ± 0.5; P < 0.001, n = 4).
Figure 1.
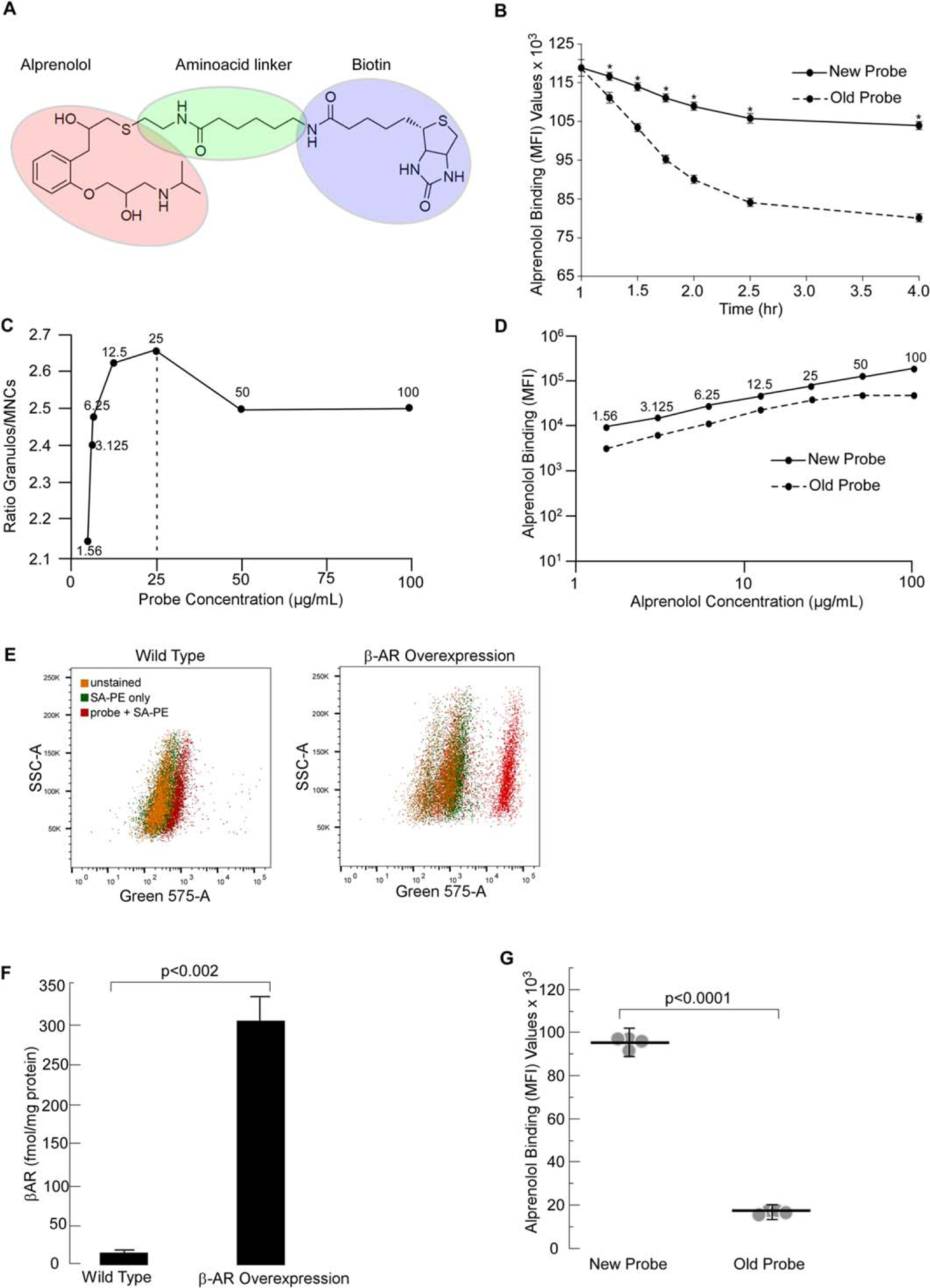
Optimization of Aprenolol Probe. Molecular structure of the new alprenolol probe is depicted in (A). This optimized probe was obtained by adding an aminoacid linker between alprenolol and biotin. Graph comparing the decaying times of old and new alprenolol probes is shown in (B). The decay times of the new alprenolol probe is significantly less over time compared to the old probe. Mean ± SE values of 4 independent experiments are shown. * indicates P < 0.001. (C). Granulocyte/mononuclear median fluorescence intensity ratio illustrating best separation between the two subsets at 25 μg/ml. (D) Titration curves of the old and new alprenolol probes. (E) Ability of the optimized probe to distinguish between cells with low and high β-AR expressing cells at the optimal binding concentration of 25 μg/ml. (F) Quantification of β-AR levels in HEK 293 cells using radioligand binding assay (n = 3). (G) Comparison of the old vs.new probe binding on leukocytes in HC subjects showed improved binding of the optimized probe. Mean and SE bars are shown.
Comparison of Different RBC Lysis, Fixation, and Permeabilization Strategies to Quantify β-AR Expression in White Blood Cells
Three different methods were compared to determine the most effective fixation/permeabilization strategy for the quantification of total (cell surface and intracellular) β-AR expression. Triton X-100 and saponin permeabilization gave better results than Tween-20. Median Fluorescence Intensity per cell: saponin 40,791 ± 938; Triton X-100: 39,964 ± 574; Tween-20: 15,759 ± 163, ANOVA P < 0.001 (n = 4). Because the Triton X-100 method is a one-step RBC-lysis/fixation/permeabilization, in contrast to the saponin method that requires separate RBC-lysis, fixation, and permeabilization steps, we choose the Triton X-100 protocol for our pilot study of HC samples. Confocal microscopy imaging showed that the alprenolol probe penetrated the cell using this method (Fig. 2).
Figure 2.
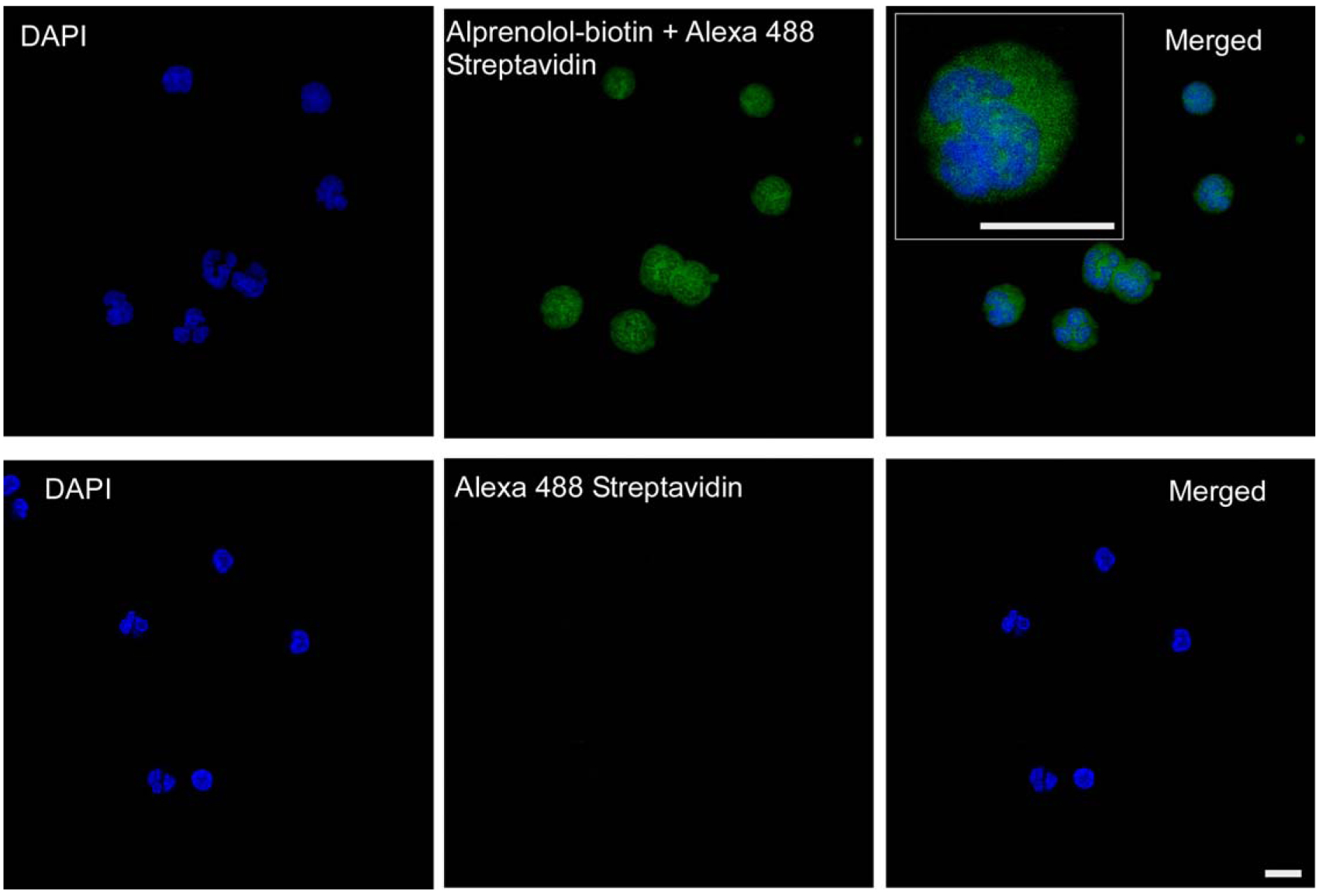
Imaging of Alprenolol Probe Binding. Confocal microscopy was used to image peneration and intracellular binding of the alprenolol probe. Upper panels show images obtained from DAPI and alprenolol probe channels and both channels merged. Inset shows a high-power image of a cell. Lower panels show staining with DAPI and secondary reagent only. Scale bar = 10 μm.
β-AR Receptor Expression in Healthy White Blood Cells
Using the improved probe and one-step RBC-lysis/fixation/permeabilization method, we quantified β-AR expression in HC. Nine HC were included in the study. All the participants were Caucasian, consisting of 7 females and 2 males. Average age was 46.4 years (± 9.5, range: 36–61). Standard light scatter gating was used to distinguish granulocytes and mononuclear cells as reported previously (26,27). A detailed gating strategy is shown in Figure 3. Total β-AR expression, in MESF PE units per cell, was 9.0 ± 1.0 on granulocytes, 4.9 ± 0.41 on mononuclear cells, and 7.6 ± 0.61 on total white blood cell fraction (Table 1). β-AR expression was significantly higher in granulocytes compared to mononuclear cell fraction (P ≤ 0.0001). The β-AR number was comparable between males and females (P = 0.73 for granulocytes and 0.24 for mononuclear and total white blood cells) and did not show any correlations with age (P = 0.63, R = −0.18 for granulocytes, P = 0.68, R = −0.15 for mononuclear cells, P = 0.69, R = −0.15 for total white blood cell fraction).
Figure 3.
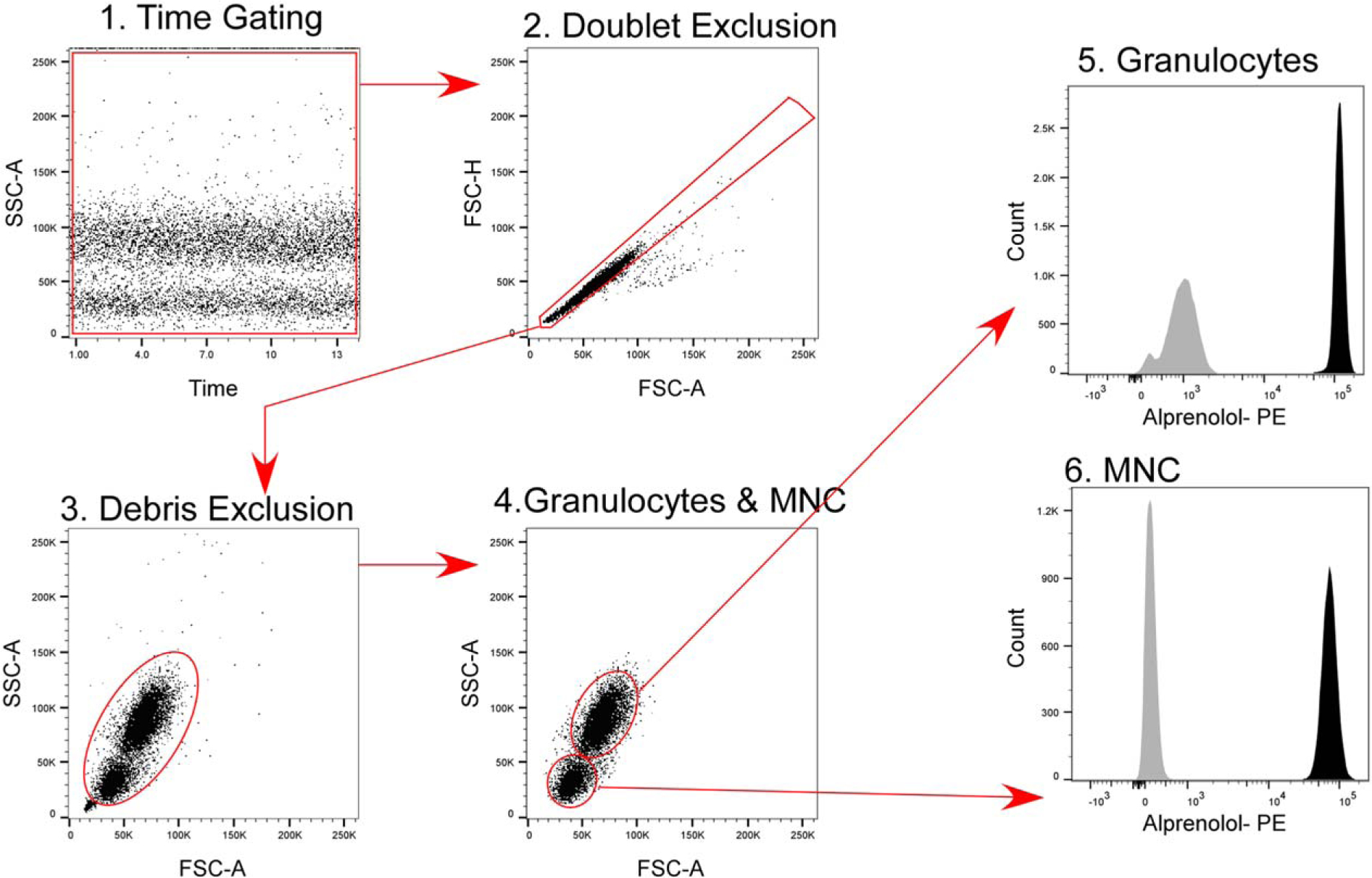
Gating Strategy. Time gating was performed to evaluate fluidic disturbances in the flow cel. Aggregates were excluded on an FSC-A/ FSC-H dot plot, followed by cell debris exclusion on an FSC-A/SSC-A dot plot. Granulocytes and mononuclear cells were gated based on standard light scatter characteristics (26,28), and median fluorescence intensity of each subset was determined using the statistics tool in FlowJo. Histograms show staining with alprenolol probe (black) and secondary reagent only (gray).
Table 1.
Total (Cell Surface and Intercellular) β-AR on different white cell populations in HC
| β-AR (UNITS OF MESF PE PER CELL × 104) | |||||
|---|---|---|---|---|---|
| PARTICIPANT NO. | AGE | GENDER | MONONUCLEAR CELLS | GRANULOCYTES | TOTAL WHITE CELLS |
| 1 | 38 | Female | 4.3 | 7.7 | 5.9 |
| 2 | 61 | Female | 3.6 | 6.8 | 6.0 |
| 3 | 36 | Female | 5.1 | 9.4 | 8.4 |
| 4 | 51 | Female | 3.6 | 6.8 | 6.0 |
| 5 | 36 | Male | 6.9 | 16.4 | 10.8 |
| 6 | 42 | Male | 4.5 | 7.5 | 6.9 |
| 7 | 47 | Female | 4.6 | 8.6 | 7.8 |
| 8 | 47 | Female | 4.4 | 7.0 | 6.4 |
| 9 | 60 | Female | 6.9 | 11.2 | 10.0 |
| Mean ± SE | 4.9 ± 0.41 | 9.0 ± 1.0 | 7.6 ± 0.61 | ||
DISCUSSION
The present study, for the first time, demonstrates a modern and fast flow cytometric method to quantify relative β-AR expression on peripheral blood cells in a robust and reproducible way that is amenable to high-throughput clinical testing. We have measured the expression of both intracellular and membrane-bound β-ARs by rendering the cells permeable to biotinylated alprenolol probe. Alprenolol binds to all β-ARs and has been utilized as a probe to quantify β-AR expression in the cell membrane fraction in radioligand assays (19–23). We tested three different cell membrane permeabilization agents and found that Triton X-100 was the fastest method compared to saponin and Tween-20. In contrast to saponin and Tween-20, which requires an extra step for red blood cell lysis, Triton X-100 allows for one-step lysis of red blood cells and white blood cell permeabilization. The formaldehyde fixation/Triton X-100 permeabilization method allows immunophenotyping in combination with β-AR detection (24). It is critical to test first that antibodies of interest recognize formaldehyde-fixed and denatured antigens. Titration of antibodies using formaldehyde fixed/Triton X-100 permeabilized samples is also important to determine the optimal labeling concentration for this application. Intracellular β-ARs are internalized in cell membrane vesicles that may not have been sufficiently permeabilized by Tween-20 to allow binding of the alprenolol probe to β-ARs. We previously showed that the binding between alprenolol probe without a spacer molecule and β-AR decays over time(24). Radioligand binding studies showed the need for a substantial chain length between alprenolol and the biotin moieties to preserve the affinity for β-AR (28). Based on this principle, we optimized the alprenolol probe for flow cytometry by introducing an amino acid link between alprenolol and biotin moie-ties, resulting in a more stable alprenolol probe allowing more accurate determination of β-AR expression. Alprenolol binding in MESF PE per cell could easily be converted into absolute receptor number per cell with knowledge of the correct ratio of PE-conjugated streptavidin binding to biotin. Based on published literature, one biotin molecule binds four streptavidin(29). However, we cannot exclude that PE conjugation of streptavidin or thousand molecules of biotinylated alprenolol per cell may alter the binding ratio.
To date, the radioligand-binding assay has been the most commonly used method for receptor quantification. Although it is a sensitive method, it is labor intensive as it is associated with multiple steps requiring a ligand labeled with a radioisotope such as tritium [3H] or iodine [125I]. In short, the first step of the assay is the incubation of a radioligand with the sample (either intact cells, plasma membrane, or endosome fractions) in a buffer. At the end of the incubation, a small fraction of the radioligand binds to the specific target receptor, while the rest of the radioligand stays free in solution or binds nonspecifically to other receptors. The next step involves the separation of bound radioligands from free radioligands by harvesting the sample using vacuum filtration onto special filter papers. Finally, the last step involves measurement of the radiation emitted from the radioisotopes by appropriate scintillation cocktails and counters. Results are obtained as counts per minute that are converted to disintegration per minute and later transformed into molar concentrations. Type of receptor preparation, radioisotope and separation technique, incubation temperature and duration, and assay conditions (e.g., pH, ions) may differ in different assay protocols, which might account for the different results in different studies (30).
The quantification of absolute receptor numbers may be helpful and desired in some cases but was not the purpose of the method reported here. Absolute quantification requires very high probe concentrations. Apart from economic cost, high concentrations increased background staining as demonstrated by reduced resolution between mononuclear and granulocyte subsets. To allow comparison of relative expression under different conditions, the highest concentration with maximal resolution 25 μg/ml was chosen.
Radioligand binding is a widely accepted and well-regarded technique that allows precise determination of membrane-bound receptor expression; however, its high cost, high levels of radioactivity requiring careful handling, storage and disposal precautions, long duration, and requirement for large quantities of receptor preparation make it less desirable to use in studies and potentially in routine clinical use in the future (Table 2). Moreover, flow cytometry also offers the possibility to assess receptor densities in different cell populations by gating strategies based on scatter parameters. In this study, we have determined β-AR expression in granulocytes and mononuclear cells.
Table 2.
Comparison of radioligand binding assay and flow cytometry for the quantification of β-AR density
| RADIOLIGAND BINDING ASSAY | FLOW CYTOMETRY |
|---|---|
| Requires large quantities of cells | Allows use of limited number of cells |
| Unable to discriminate receptor heterogeneity among subsets | Able to discriminate receptor expression by different cell populations |
| Requires use of hazardous radioactive isotopes | No need to use radioactive isotopes |
| High cost | Cost effective |
| Time consuming | Fast |
| Not practical for large-scale samples | Allows high-throughput |
| Quantifies receptors localized in the cell membrane | Allows analysis of both cell surface and internalized receptors |
Studies have shown that many different factors can affect the absolute number of β-ARs in various cells of the human body. For instance, several studies have proposed that the number of β-ARs correlates with age (20,22). However, our study showed no correlation between age and β-AR number. Comparisons of physically active and inactive persons have shown differences in the number of β-ARs, with active persons having fewer β-ARs on lymphocytes as well as higher levels of adrenaline (22). Other studies propose that the number of β-ARs shows not only individual differences but also within-person changes depending on the time of the day and physical activity level. Anstead et al. questioned this variability further by investigating whether it was related to the differential distribution of β-ARs in different lymphocyte subsets(31). It is well known that CD41 T cells have lower expression of β2ARs than CD81 T cells. This study showed that β-AR expression had a positive correlation with CD81 lymphocyte count and negative correlation with CD41 lymphocyte count, which means that higher β-AR expression may be simply due to lower CD41/CD81 lymphocyte ratio (31). Absolute number of β-ARs reported in the literature ranges from 680 to 14,000 per cell, which might be explained by differences in the assay protocol, timing of blood draw, and characteristics of the study population such as age, physical activity, and lymphocyte subset differential (Table 3) (19–21). We measured both intracellular and cell surface levels of β-AR rather than only membrane-bound β-ARs measured the radioligand binding assays. Furthermore, we quantified expression of β-AR not only on mononuclear cells but also on granulocytes. Similar to the findings of the previous studies, granulocytes had a significantly higher number of β-ARs than mononuclear cells(32). This may be attributed to the unique role of granulocytes in the first line of host defense against bacterial, fungal, and parasitic infections as part the innate immune system, which necessitates a large number of intracellular granules and cell surface receptors to recognize pathogens, play pivotal role in inflammatory response, and activate the adaptive immune response.
Table 3.
Number of β-AR cells in different studies using radioligand methods
| NUMBER OF β-AR IN CELL. MHMBRANK FRACTION | RADIOLIGAND BINDING ASSAY |
|---|---|
| ~2,000 binding sites per lymphocyte in HC | 3H-alprenolol (19) |
| ~8,000 sites per lymphocyte in young and 14,000 old HC | 3H-dihydro-alprenolol (20) |
| 801 ± 114 sites per lymphocyte in elderly, 680 ± 47 in young HC | 3H-dihydro-alprenolol (21) |
| 2,230 ± 482 membrane β-AR per lymphocyte in inactive persons, 1,743 ± 85 per lymphocyte in active persons | 125I-cyanopindolol (22) |
| 2,499 ± 1,140 in untrained individuals, 2,366 ± 765 in baseball players, 1,634 ± 1,380 in short-distance runners, 1,446 ± long distance runner HC | 125I-cyanopindolol (23) |
In conclusion, dysregulation of β-AR has been shown to play roles in the pathobiology of a variety of autoimmune and cardiovascular diseases including diabetes mellitus, multiple sclerosis, and congestive heart failure. Modern, fast, and effective tools are needed to measure β-AR expression to further scrutinize the role of β-AR in these diseases for research purposes as well as for routine clinical testing. The present study has demonstrated, for the first time, the use of flow cytometry in relative quantification of β-AR expression of peripheral blood cells. This method using flow cytometry has many advantages over conventional radioligand binding assay techniques and would allow for facile, high-throughput testing in both research and clinical settings.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Eric Schultz and Joe Gerow from the LRI Flow Cytometry Core for instrument QC and setup, and Dr. Judy Drazba from the LRI Imaging Core for assistance with confocal microscopy. We thank Allison Janocha for editing the manuscript.
Grant sponsor: National Institutes of Health, Grant numbers: HL115008; HL60917 and HL125177
Grant sponsor: National Center for Advancing Translational Sciences, Grant number: UL1TR000439
Footnotes
Additional supporting information may be found in the online version of this article.
LITERATURE CITED
- 1.Naga Prasad SV, Nienaber J, Rockman HA. Beta-adrenergic axis and heart disease. Trends Genet 2001;17:S44–S49. [DOI] [PubMed] [Google Scholar]
- 2.Marino F, Cosentino M. Adrenergic modulation of immune cells: An update. Amino Acids 2013;45:55–71. [DOI] [PubMed] [Google Scholar]
- 3.Feldman RD, Hunninghake GW, McArdle WL. Betaadrenergic-receptor-mediated suppression of interleukin 2 receptors in human lymphocytes. J Immunol 1987;139: 3355–3359. [PubMed] [Google Scholar]
- 4.Elliott L, Brooks W, Roszman T. Inhibition of anti-CD3 monoclonal antibody-induced T-cell proliferation by dexamethasone, isoproterenol, or prostaglandin E2 either alone or in combination. Cell Mol Neurobiol 1992;12:411–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartik MM, Brooks WH, Roszman TL. Modulation of T cell proliferation by stimulation of the beta-adrenergic receptor: Lack of correlation between inhibition of T cell proliferation and cAMP accumulation. Cell Immunol 1993;148:408–421. [DOI] [PubMed] [Google Scholar]
- 6.Schwab KO, Bartels H, Martin C, Leichtenschlag EM. Decreased beta 2-adrenoceptor density and decreased isoproterenol induced c-AMP increase in juvenile type I diabetes mellitus: An additional cause of severe hypoglycaemia in childhood diabetes?. Eur J Pediatr 1993;152:797–801. [DOI] [PubMed] [Google Scholar]
- 7.Arnason BG, Brown M, Maselli R, Karaszewski J, R A. Blood lymphocyte beta-adrenergic receptors in multiple sclerosis. Ann N Y Acad Sci 1988;540:585–588. [DOI] [PubMed] [Google Scholar]
- 8.Karaszewski JW, Reder AT, Maselli R, Brown M, Arnason BG. Sympathetic skin responses are decreased and lymphocyte beta-adrenergic receptors are increased in progressive multiple sclerosis. Ann Neurol 1990;27:366–372. [DOI] [PubMed] [Google Scholar]
- 9.Makhlouf K, Weiner HL, Khoury SJ. Potential of beta2-adrenoceptor agonists as add-on therapy for multiple sclerosis: Focus on salbutamol (albuterol). CNS Drugs 2002;16:1–8. [DOI] [PubMed] [Google Scholar]
- 10.Baerwald C, Graefe C, von Wichert P, Krause A. Decreased density of beta-adrenergic receptors on peripheral blood mononuclear cells in patients with rheumatoid arthritis. J Rheumatol 1992;19:204–210. [PubMed] [Google Scholar]
- 11.Baerwald CG, Laufenberg M, Specht T, von Wichert P, Burmester G, Krause A. Impaired sympathetic influence on the immune response in patients with rheumatoid arthritis due to lymphocyte subset-specific modulation of beta 2-adrenergic receptors. Br J Rheumatol 1997;36:1262–1269. [DOI] [PubMed] [Google Scholar]
- 12.Krause A, Henrich A, Beckh K, Von Wichert P, Baerwald C. Correlation between density of beta 2-adrenergic receptors on peripheral blood mononuclear cells and serum levels of soluble interleukin-2 receptors in patients with chronic inflammatory diseases. Eur J Clin Invest 1992;22:47–51. [PubMed] [Google Scholar]
- 13.del Rey A, Besedovsky HO. Sympathetic nervous system-immune interactions in auto-immune lymphoproliferative diseases. Neuroimmunomodulation 2008;15:29–36. [DOI] [PubMed] [Google Scholar]
- 14.Xu BY, Pirskanen R, Lefvert AK. Antibodies against beta1 and beta2 adrenergic receptors in myasthenia gravis. J Neuroimmunol 1998;91:82–88. [DOI] [PubMed] [Google Scholar]
- 15.Chello M, Mastroroberto P, Romano R, Cirillo F, Marchese AR. Improved beta-adrenergic receptor function after coronary artery bypass grafting in patients with congestive heart failure. Coron Artery Dis 1995;6:957–963. [PubMed] [Google Scholar]
- 16.Townend JN, Virk SJ, Qiang FX, Lawson N, Bain RJ, Davies MK. Lymphocyte beta adrenoceptor upregulation and improved cardiac response to adrenergic stimulation following converting enzyme inhibition in congestive heart failure. Eur Heart J 1993; 14:243–250. [DOI] [PubMed] [Google Scholar]
- 17.Brodde OE, Kretsch R, Ikezono K, Zerkowski HR, Reidemeister JC. Human beta-adrenoceptors: Relation of myocardial and lymphocyte beta-adrenoceptor density. Science 1986;231:1584–1585. [DOI] [PubMed] [Google Scholar]
- 18.Gordon EP, Bristow MR, Laser JA, Minobe WA, Fowler MB, Savin WM. Correlation between beta-adrenergic receptors in human lymphocytes and heart (abstract). Circulation 1983;68:111. [Google Scholar]
- 19.Williams LT, Snyderman R, Lefkowitz RJ. Identification of beta-adrenergic receptors in human lymphocytes by (−) (3H) alprenolol binding. J Clin Invest 1976;57:149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schocken DD, Roth GS. Reduced beta-adrenergic receptor concentrations in ageing man. Nature 1977;267:856–858. [DOI] [PubMed] [Google Scholar]
- 21.Abrass IB, Scarpace PJ. Human lymphocyte beta-adrenergic receptors are unaltered with age. J Gerontol 1981;36:298–301. [DOI] [PubMed] [Google Scholar]
- 22.Krawietz W, Klein EM, Unterberg C, Ackenheil M. Physical activity decreases the number of beta-adrenergic receptors on human lymphocytes. Klin Wochenschr 1985;63:73–78. [DOI] [PubMed] [Google Scholar]
- 23.Fujii N, Homma S, Yamazaki F, Sone R, Shibata T, Ikegami H, Murakami K, Miyazaki H. Beta-adrenergic receptor number in human lymphocytes is inversely correlated with aerobic capacity. Am J Physiol 1998;274:E1106–E1112. [DOI] [PubMed] [Google Scholar]
- 24.Rose JA, Wanner N, Cheong HI, Queisser K, Barrett P, Park M, Hite C, Naga Prasad SV, Erzurum S, Asosingh K. Flow cytometric quantification of peripheral blood cell β-adrenergic receptor density and urinary endothelial cell-derived microparticles in pulmonary arterial hypertension. PLoS One 2016;11:e0156940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naga Prasad SV, Barak LS, Rapacciuolo A, Caron MG, Rockman HA. Agonist-dependent recruitment of phosphoinositide 3-kinase to the membrane by beta-adrenergic receptor kinase 1. A role in receptor sequestration. J Biol Chem 2001;276: 18953–18959. [DOI] [PubMed] [Google Scholar]
- 26.Chow S, Hedley D, Grom P, Magari R, Jacobberger JW, Shankey TV. Whole blood fixation and permeabilization protocol with red blood cell lysis for flow cytometry of intracellular phosphorylated epitopes in leukocyte subpopulations. Cytometry A 2005;67A:4–17. [DOI] [PubMed] [Google Scholar]
- 27.Chow S, Hedley D, Shankey TV. Whole blood processing for measurement of signaling proteins by flow cytometry. Curr Protoc Cytom 2008;Chapter 9:Unit 9.27. [DOI] [PubMed] [Google Scholar]
- 28.Meier KE, Ruoho AE. Formation of complexes between avidin and beta-adrenergic receptors using biotinyl-alprenolol derivatives. Biochim Biophys Acta 1983;761: 257–261. [DOI] [PubMed] [Google Scholar]
- 29.Chaiet L, Wolf FJ. The properties of streptavidin, a biotin-binding protein produced by streptomycetes. Arch Biochem Biophys 1964;106:1–5. [DOI] [PubMed] [Google Scholar]
- 30.Keen M The problems and pitfalls of radioligand binding. Methods Mol Biol 1995;41:1–16. [DOI] [PubMed] [Google Scholar]
- 31.Anstead M, Hunt T, Carlson S, Burki N. Variability of peripheral blood lymphocyte beta-2-adrenergic receptor density in humans. Am J Respir Crit Care Med 1998;157: 990–992. [DOI] [PubMed] [Google Scholar]
- 32.Davis PB, Dieckman L, Boat TF, Stern RC, Doershuk CF. Beta adrenergic receptors in lymphocytes and granulocytes from patients with cystic fibrosis. J Clin Invest 1983;71:1787–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


