Highlights
-
•
Technical advances are motivating translational applications of MS-based imaging.
-
•
Tissue imaging by MS enables increased objectivity in disease classification.
-
•
MS-based imaging is useful for toxicological assessment of pharmaceuticals.
-
•
Recent technologies enable interrogation of tissue by MS in the surgical theater.
Tissue imaging by mass spectrometry (MS) combines the sensitivity and molecular specificity of MS with the spatial fidelity of classical histology for analysis of metabolites, lipids and proteins in tissues (Fig. 1). MS-based imaging is label-free, untargeted, sensitive, and specific, thereby enabling application in both basic biomedical research and the clinical laboratory. While all tissue imaging experiments are conceptually similar in their ability to generate spatial molecular data; ionization, data collection, and purpose vary widely. Here, we highlight recent technical advances and efforts that are motivating translational applications of this emerging technology.
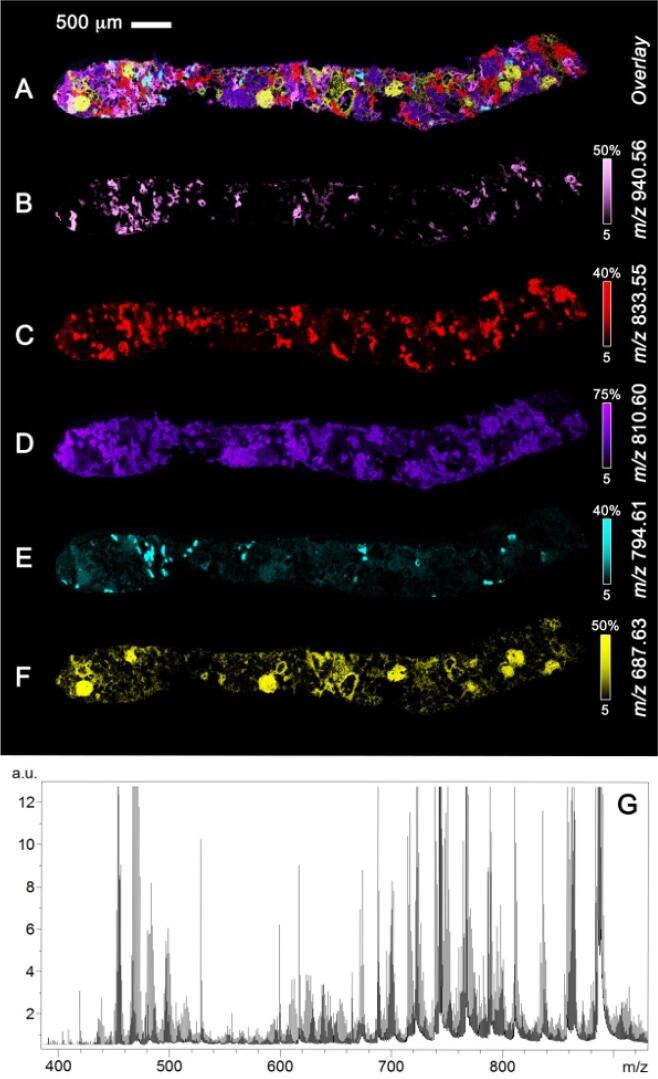
Fig. 1.
MALDI time-of-flight (TOF) imaging MS of a human kidney needle biopsy showing molecular maps for 5 lipid species collected at 10 µm spatial resolution. Overlay of ions (A); m/z 940.56 (B); m/z 833.55 (C); m/z 810.60 (D); m/z 794.61 (E); m/z 687.63 (F) highlight differences in spatial distributions. Spectral data (G) shows the hundreds of ions detected in a single MALDI TOF imaging experiment. Data courtesy of the Spraggins research group and the Mass Spectrometry Research Center at Vanderbilt University.
1. Clinical disease classification
Tissue imaging by MS provides molecular context to classical histological assessment enabling increased objectivity in disease classification. Although histopathology is both accurate and effective in the diagnosis of most diseases, there are some diseases that have a high inter-observer variability and are notoriously difficult to diagnose. Numerous groups have utilized tissue imaging technologies to study and classify various diseases, including different types of cancer. Lazova et. al. [1] used matrix-assisted laser desorption/ionization (MALDI) tissue imaging to analyze melanocytic lesions, demonstrating the ability to distinguish benign Spitz nevi from malignant Spitzoid melanoma. Other studies, such as Sans et. al. [2], have utilized desorption electrospray ionization (DESI) imaging to grade aggressiveness of serous ovarian cancer, in an attempt to provide a novel approach to more accurately determine late stage diagnoses; which occurs in over 70% of ovarian cancer cases. Lou et. al. [3] used MALDI tissue imaging to investigate heterogeneity within different sarcomas, which allowed differentiation of high-grade osteosarcoma, leiomyosarcoma, myxofibrosarcoma, and undifferentiated pleomorphic sarcoma. As these studies have shown, synergistically combining tissue imaging and classical histopathology provides an opportunity to develop next-generation diagnostic tools.
2. Tissue imaging on the clinical scale
Many research groups have been developing methodologies to allow for imaging of tissues that have been preserved using classical fixation methods. Most histological methods for tissue preservation involve the fixation of tissue in paraformaldehyde followed by paraffin embedding (FFPE). Tissue paraffin blocks can be made from a single tissue or an array of many tissue biopsies, a so-called tissue microarray (TMA), to allow for the rapid analysis of many patients under the same experimental conditions. Although preservation using FFPE is not ideal for MS analysis, globally, tissue banks rely on this type of preservation, which necessitates development of methods for MS-based analysis of FFPE TMAs. Kriegsmann et al. [4] have successfully analyzed tryptic peptides form FFPE TMAs of two different types of lung cancer using a series of organic washes, antigen retrieval, and on-tissue tryptic digestion. In these studies, robust biomolecular signals were observed that could reliably differentiate between both types of lung cancer. The Drake group [5] has developed an alternative method that utilizes an enzyme that cleaves oligosaccharides from glycosylated proteins allowing the imaging of glycans by MS. Development of reliable and reproducible methods for the analysis of vast banks of FFPE tissues will be critical for rapid integration of this technology into clinical settings.
3. Imaging drugs and metabolites
Not only has tissue imaging played an important role in the classification of tumor margins and diseases, it has also proven useful in the toxicological assessment of pharmaceutical compounds and their metabolites. In addition to being able to image drug metabolism in tissue [6], [7], mass spectrometry can be used to image hair and provide a unique insight into prolonged use of pharmaceutical compounds or drugs of abuse. Flinders et al. [8] developed a cutting apparatus to prepare six cm longitudinal sections of hair enabling rapid detection of cocaine abuse. Their method was found to be six times faster than previous methods. These imaging analyses were shown to be in agreement with standard LC-MS/MS results. Rosen et al. [9] have used infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI) imaging to analyze HIV antiretrovirals in single hair strands. The distribution of efavirenz was monitored in a series of hair strands collected from HIV infected, virologically suppressed patients, providing a means by which to evaluate drug accumulation.
4. Expansion into the surgical environment
A variety of technologies have recently been developed that enable direct integration of tissue imaging by mass spectrometry in the surgical theater to provide molecular guidance during surgery, in particular for the evaluation of tumor margins. Balog et al. [10] demonstrated the capabilities of Rapid Evaporative Ionization Mass Spectrometry (REIMS) coupled with electrosurgery using the intelligent knife (iKnife). This technology allows rapid analysis of the aerosol produced during electrosurgical dissection to determine tumor margins. iKnife results from the lab analysis of 302 ex vivo human tissue samples were used to construct a database containing 1624 mass spectra from cancerous tissue regions and 1309 mass spectra from non-cancerous tissue regions. This database was then used as part of the matching algorithm during the direct tissue analysis of 81 cancer resections using the iKnife in the clinical setting.
DESI imaging has also been shown to be effective for rapid assessment of surgical tumor margins through the analysis of tissue sections or tissue smears. The advantage of DESI, relative to other imaging modalities, is that it requires minimal to no sample preparation, making it both fast and amenable to intraoperative applications. The feasibility of DESI imaging as a metabolite profiling tool in surgical tumor margins has been widely demonstrated, including in the recent work of Calligaris et al. [11] for breast cancer margin analysis, and by Jarmusch et al. [12] for brain cancer analysis. Both studies demonstrate the potential of DESI imaging as a surgical tool to support rapid, real-time, accurate, intraoperative decisions.
The MasSpec Pen is the most recent technology in development with the goal of to providing rapid, non-destructive diagnosis of human tissues in the surgical theater. This technology is composed of a handheld device that holds a water droplet onto a tissue surface to rapidly (i.e., 3 s) extract endogenous molecules that could be indicative of cancer. The water is then delivered through PTFE tubing from the pen to the mass spectrometer for analysis. In a recent paper by Zhang et al [13], the MasSpec Pen was used to characterize 253 ex vivo fresh human tissue samples and for in vivo analysis of breast cancer in murine model tissues.
5. Conclusion
In conclusion, imaging by mass spectrometry has become a prominent technology for clinical use through its versatility with numerous applications including, for example, surgical margin evaluation, disease diagnosis, and detection of prolonged drug abuse. However, there are still challenges associated with the direct analysis of biological samples. Tissue specimens are extremely complex containing many thousands of chemically diverse analytes expressed in concentrations ranging many orders of magnitude. In most cases, this range of analyte concentrations far exceeds the dynamic range of the even the most advanced mass spectrometers available today. To overcome this challenge, innovations in sample preparation and instrumentation will be critical for improving molecular coverage and novel computation approaches will be required to mine these increasingly complex data. Many research groups are taking on these challenges and the ongoing technological advancements in throughput, sensitivity, dynamic range, and ease-of-use are driving the transition of imaging mass spectrometry to the clinical laboratory.
Acknowledgments
Acknowledgements
The authors thank Drs. deCaestecker and Harris (Vanderbilt University Medical Center) for providing kidney tissue needle biopsy tissue and William Perry and Marissa Jones (Vanderbilt University) for assistance editing this manuscript. Example MALDI imaging data were provided by J.M.S. and supported by the National Institutes of Health (NIH) under awards P41 GM103391-07 and 1U54 DK120058-01. R.M.A.H. acknowledges the Dutch Province of Limburg for financial support through the LINK program. L.S.E. acknowledges support from the National Cancer Institute of the NIH under Award Number R00CA190783.
Conflict of interest
None of the authors has any conflicts of interest to disclose.
References
- 1.Lazova R., et al. Imaging mass spectrometry assists in the classification of diagnostically challenging atypical Spitzoid neoplasms. J. Am. Acad. Dermatol. 2016;75(6):1176–1186e4. doi: 10.1016/j.jaad.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sans M., et al. Metabolic markers and statistical prediction of serous ovarian cancer aggressiveness by ambient ionization mass spectrometry imaging. Cancer Res. 2017;77(11):2903–2913. doi: 10.1158/0008-5472.CAN-16-3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lou S., et al. High-grade sarcoma diagnosis and prognosis: biomarker discovery by mass spectrometry imaging. Proteomics. 2016;16(11–12):1802–1813. doi: 10.1002/pmic.201500514. [DOI] [PubMed] [Google Scholar]
- 4.Kriegsmann M., et al. Reliable entity subtyping in non-small cell lung cancer by matrix-assisted laser desorption/ionization imaging mass spectrometry on formalin-fixed paraffin-embedded tissue specimens. Mol. Cell Proteomics. 2016;15(10):3081–3089. doi: 10.1074/mcp.M115.057513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powers T.W., et al. MALDI imaging mass spectrometry profiling of N-glycans in formalin-fixed paraffin embedded clinical tissue blocks and tissue microarrays. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruinen A.L., et al. Mass spectrometry imaging of drug related crystal-like structures in formalin-fixed frozen and paraffin-embedded rabbit kidney tissue sections. J. Am. Soc. Mass Spectrom. 2016;27(1):117–123. doi: 10.1007/s13361-015-1254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleinridders A., et al. Regional differences in brain glucose metabolism determined by imaging mass spectrometry. Mol. Metab. 2018;12:113–121. doi: 10.1016/j.molmet.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flinders B., et al. Mass spectrometry imaging of drugs of abuse in hair. Methods Mol. Biol. 2017;1618:137–147. doi: 10.1007/978-1-4939-7051-3_12. [DOI] [PubMed] [Google Scholar]
- 9.Rosen E.P., et al. Analysis of antiretrovirals in single hair strands for evaluation of drug adherence with infrared-matrix-assisted laser desorption electrospray ionization mass spectrometry imaging. Anal. Chem. 2016;88(2):1336–1344. doi: 10.1021/acs.analchem.5b03794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balog J., et al. Intraoperative tissue identification using rapid evaporative ionization mass spectrometry. Sci. Transl. Med. 2013;5(194):p. 194ra93. doi: 10.1126/scitranslmed.3005623. [DOI] [PubMed] [Google Scholar]
- 11.Calligaris D., et al. Application of desorption electrospray ionization mass spectrometry imaging in breast cancer margin analysis. Proc. Natl. Acad. Sci. U.S.A. 2014;111(42):15184–15189. doi: 10.1073/pnas.1408129111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarmusch A.K., et al. Lipid and metabolite profiles of human brain tumors by desorption electrospray ionization-MS. Proc. Natl. Acad. Sci. U.S.A. 2016;113(6):1486–1491. doi: 10.1073/pnas.1523306113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J., et al. Nondestructive tissue analysis for ex vivo and in vivo cancer diagnosis using a handheld mass spectrometry system. Sci. Transl. Med. 2017;9(406) doi: 10.1126/scitranslmed.aan3968. [DOI] [PMC free article] [PubMed] [Google Scholar]