Abstract
Alzheimer’s disease (AD) is characterized by memory loss and executive dysfunction, which correspond to structural changes to the medial temporal lobes (MTL) and prefrontal cortex (PFC), respectively. Given the overlap in cognitive deficits between healthy aging and the earliest stages of AD, early detection of AD remains a challenge. The goal of the present study was to study MTL- and PFC-dependent cognitive functioning in middle-aged individuals at genetic risk for AD or cognitive impairment who do not currently manifest any clinical symptoms. Participants (N = 150; aged 40-60 years) underwent genotyping of 47 single nucleotide polymorphisms (SNPs) in five genes previously associated with memory or executive functioning: APOE, SORL1, BDNF, KIBRA, and COMT. They completed two MTL-dependent tasks, the virtual Morris Water Task (vMWT) and transverse patterning discriminations task (TPDT), and the PFC-dependent reversal learning task (RLT). Although age was associated with poorer performance on the vMWT and TPDT within this middle-aged sample, there were no genotype-associated differences in cognitive performance. Although the vMWT and TPDT may be sensitive to age-related changes in cognition, carriers of APOE, SORL1, BDNF, KIBRA, and COMT risk alleles do not exhibit alteration in MTL- and PFC-dependent functioning in middle age compared to non-carriers.
Keywords: Alzheimer’s disease, apolipoproteins E, brain-derived neurotrophic factor, aging, hippocampus, prefrontal cortex, middle age, cognition
Introduction
It is estimated that the incidence of Alzheimer’s disease (AD) will triple by the year 2050, affecting 13.8 million people [1]. Despite decades of research into the best targets for intervention, clinical trials have largely failed to yield effective therapies [2], with current FDA-approved medications for the treatment of early stage AD providing only a modest benefit. This has led to a need for accurate preclinical identification of AD, which may facilitate prevention and early intervention efforts.
One of the difficulties in early identification of those at risk for AD is that both pathological and healthy aging are associated with overlapping changes in cognitive performance, including declines in episodic memory and executive functioning [3]. Although AD eventually results in significant impairment across multiple cognitive domains, distinguishing healthy aging from the earliest stages of pathological decline and at a single time point remains a significant challenge. Traditional neuropsychological measures of memory and executive functioning, the domains prominently affected in AD, often require multiple component processes and may be too general to detect subtle cognitive differences emerging in prodromal or preclinical stages [4]. A more fruitful approach may be to develop cognitive paradigms that more tightly depend on specific structures affected early in the course of AD. For instance, AD-associated atrophy involves the hippocampus and other medial temporal lobe (MTL) structures as well as the prefrontal cortex (PFC) early in the disease course [5]. As MTL and PFC structural integrity is associated with episodic memory [6] and executive functioning [7], respectively, alterations in these brain structures may underlie the major cognitive changes observed in AD. Indeed, longitudinal studies of older adults have demonstrated that greater dysfunction of the MTL and PFC is associated with poorer cognitive and clinical outcomes [8, 9]. Thus, it is critical that we identify biomarkers that are sensitive to detect MTL and PFC dysfunction prior to the onset of clinical symptoms, allowing us to identify those at risk for cognitive decline before the substantial structural damage has occurred.
One method for identifying potential biomarkers of risk for AD is to study individuals who have higher genetic risk for the disease but do not currently manifest symptoms. This strategy has been employed most prominently in studies examining cognitive performance in carriers of the APOE ε4 allele, the best known genetic risk factor for AD [10]. A meta-analysis reported that Caucasian carriers of a single ε4 allele are at a fourfold greater risk of developing AD compared to ε3/ε3 carriers, while ε4/ε4 carriers are at 16-fold higher risk [11]. Another meta-analysis on the association between APOE ε4 and cognition found that healthy, non-demented ε4 carriers perform significantly worse than non-ε4 carriers on measures of global cognitive ability, episodic memory, executive functioning, and perceptual speed, with larger effect sizes with higher age [12]. Furthermore, ε4 carriers with mild cognitive impairment (MCI) are more likely to convert to AD than non-ε4 carriers [13].
Another gene in the low-density lipoprotein receptor family, sortilin-related receptor (SORL1), has also been associated with differential age-related cognitive performance and risk for AD [14]. SORL1 is involved in processing amyloid precursor protein (APP), affecting the accumulation of Aβ plaques [15]. Numerous SNPs in SORL1 have been associated with elevated risk for AD [16], but there have been relatively fewer studies examining associations between SORL1 polymorphism and cognitive functioning. In the 1936 Lothian Birth Cohort Study, SORL1 risk allele carriers had poorer spatial working memory [17], while associations between SORL1 and abstract verbal reasoning were reported in the Framingham Study [64]. Reports of smaller hippocampal volumes among SORL1 risk allele carriers [18, 19] suggest that SORL1 polymorphism may affect memory, which is supported by longitudinal evidence that SORL1 risk allele carriers show greater declines in spatial ability and episodic memory [20]. Given these associations between SORL1 and risk for AD, examining SORL1-associated cognitive performance in healthy, middle-aged individuals who do not show overt cognitive impairment may inform early prevention or intervention efforts.
Genetic studies have also identified genes associated with episodic memory and executive functioning, the core cognitive domains affected in early AD, including brain-derived neurotrophic factor (BDNF), kidney and brain protein (KIBRA), and catechol-O-methyltransferase (COMT). BDNF, a member of the nerve growth factor family, is expressed in the hippocampus and throughout the cerebral cortex [21]. Polymorphism in BDNF has been associated with hippocampal volume and episodic memory performance in both healthy younger and older adults [22-24]. KIBRA is also highly expressed in the hippocampus and plays a role in regulating synaptic plasticity [25]. KIBRA polymorphism has been associated with poorer episodic memory in younger and older populations [26-28] as well as greater risk for AD [29]. COMT encodes an enzyme that degrades catecholamine neurotransmitters in the synaptic cleft. The best-studied COMT polymorphism, Val158Met (rs4680), is associated with altered dopamine levels in the PFC [30]. In younger and older samples, COMT polymorphism has been associated with executive functioning [31-33] and episodic memory [33, 34].
The majority of existing studies examining age-related cognition and gene polymorphisms have focused on either healthy younger or older adults, leaving out middle-aged individuals. As several of these genes may differentially affect cognition at different points in the lifespan [24, 35], studying the middle-aged group is critical to identifying individuals who are vulnerable to pathological aging. Genotype-associated differences in cognitive performance in middle age are likely to be subtle and may not be detectable using traditional neuropsychological measures [36]. Several studies of middle-aged individuals have failed to find significant associations between APOE ε4 and neuropsychological performance [4, 37-39]. This has led to a growing recognition of the need for novel tasks that are more tightly linked to specific circuitry that may be affected in AD. The use of paradigms translated from animal models offers a unique opportunity to probe cognitive functions that have been directly linked to specific brain areas through lesion studies. The purpose of the present study is to capitalize on this extensive animal literature to characterize translational behavioral paradigms that are sensitive to MTL and PFC functioning. As these brain regions are the earliest to be affected by AD neuropathology, using these translational tasks in a human population may reveal subtle dysfunction indicative of preclinical risk for AD. Here, we focus on three translational tasks: a spatial MTL-dependent task, the virtual Morris Water Task (vMWT); a non-spatial MTL-dependent task, the Transverse Patterning Discriminations Task (TPDT); and a PFC-dependent task, the reversal learning task (RLT).
The vMWT [40] is a place learning and memory task in which participants must navigate a virtual pool of water to find a hidden escape platform. In rodents, hippocampal lesions impair ability to encode the platform location [41]. The vMWT is also associated with MTL integrity in humans. Hippocampal amnesic patients have profound impairments on this task [42], while in healthy individuals, poorer task performance is associated with smaller volumes of MTL structures [43-45] and altered hippocampus neurochemistry [44]. Thus, vMWT performance may be sensitive to early aberrations in MTL structure or function that may be indicative of risk for future cognitive decline.
The second MTL-dependent translational measure, the TPDT [46, 47], is a problem requiring the acquisition of relational associations between visual stimuli. Hippocampal amnesic patients are unable to acquire transverse patterning associations [48, 49], consistent with evidence from non-human animals with MTL lesions [50]. Older adults also have difficulty learning transverse patterning associations [44, 51], and deficits in performance on TPDT have been associated with smaller hippocampal volumes and poorer biochemical integrity [44].
Finally, the PFC-dependent RLT task assesses rapid behavioral adaptation to shifting contingencies. Once individuals have learned a set of visual discriminations to criterion, the contingencies are reversed. Ability to learn the reversed pattern of stimulus-reward discriminations has been associated with PFC integrity, particularly of the orbitofrontal cortex and inferior frontal gyrus, in human [52, 53] and non-human [54, 55] animals. However, no known studies have investigated whether individuals at higher genetic risk for AD have impaired RLT performance.
The purpose of the present study was to examine MTL- and PFC-dependent cognitive functioning in healthy, non-demented, middle-aged individuals who carry genetic risk factors for age-related cognitive impairment. We hypothesized carriers of risk alleles for SNPs in APOE, SORL1, BDNF, KIBRA, and COMT would perform worse on MTL-dependent and PFC-dependent tasks. Identifying genotype-associated cognitive differences in this middle-aged population is of high clinical relevance, as it may improve early detection and prevention efforts.
Method
Participants
Participants (N = 150) were community-dwelling, Caucasian adults aged 40 to 60 years (M = 49.9 years, SD = 6.0; 60 men; M = 15.3 years education, SD = 2.4). Exclusion criteria included history of central nervous system disease (e.g., dementia, stroke, Parkinson’s disease, epilepsy, other neurological disorders), history of severe cardiac disease (e.g., myocardial infarction, coronary bypass surgery, angioplasty), history of metastatic cancer, and history of serious psychiatric disorder or substance use disorder.
Participants were screened for global cognitive impairment and clinically significant symptoms of depression. One participant was excluded based on a Mini-Mental Status Exam (MMSE) [56] score ≤ 24; three participants were excluded based on a Mattis Dementia Rating Scale Second Edition (DRS-2) [57] score ≤ 135; and three participants were excluded based on a Geriatric Depression Scale (GDS) [58] score > 10, indicating clinically elevated symptoms of depression. Four participants were excluded because they did not provide a blood sample for genotyping. All participants provided written informed consent and received financial compensation for their participation. The study was carried out in accordance with guidelines set by the institutional review boards at the University of Wisconsin-Milwaukee and the Medical College of Wisconsin.
Cognitive Measures
Virtual Morris Water Task (vMWT)
vMWT Environment
The vMWT (NeuroInvestigations, Inc., Lethbridge, AB, Canada) was administered on a Dell computer with a 17-inch monitor. The virtual environment consisted of a circular pool located in the center of a square room. Four distal cues (e.g., window, painting) were the only visual features of the virtual environment that could be used to disambiguate spatial locations. Participants began each trial within the pool, which consisted of opaque blue water. They navigated through the environment using the up arrow key to control forward movement and the left and right arrow keys to rotate their position. Backward navigation was not possible. Participants were given a practice trial in a similar virtual environment with different distal cues from the test environment, giving them the opportunity to learn how to use the keys to navigate.
The vMWT consisted of five blocks of learning trials with four trials in each block. At the beginning of each learning trial, participants were placed in a random location in the pool. A square platform was hidden beneath the surface of the opaque water, and participants were instructed to find the platform as quickly as possible. Once they crossed over the platform, it elevated above the pool and remained elevated for 10 seconds until the next trial began. During this time, participants were able to rotate on the platform to view their position within the room, although they were not explicitly instructed to do so. A limit of 60 seconds was allotted for each hidden platform trial. After this time, the platform became visible and participants received a visual message on the screen instructing them to move to the platform as quickly as possible. Latency and path length were calculated for each trial. Heading error toward the platform was calculated as the angular deviation from a straight path to the center of the platform from the participant’s starting position. Heading error was measured at the first occurrence that participant distance was greater than 25% of the pool diameter from the starting position. Total latency, distance, and heading error were summed across learning trials to create summary measures of vMWT performance.
Following the completion of five blocks of four learning trials, participants completed a probe trial in the same environment. During this trial, the participants were unaware that the platform had been removed. Participants were given 60 seconds of time to navigate the environment. The proportion of latency and distance spent in the goal quadrant as well as probe trial heading error were used as measures of the participants’ memory for the platform location.
After completing the probe trial, the hidden platform was made visible and participants were instructed to navigate to the platform as quickly as possible. Two blocks of four trials each were completed. The summed latency to complete visible platform trials was used as a measure of vMWT navigation speed. This was used as a covariate in statistical models for vMWT total latency and probe latency to control for perceptual or motor abilities that may have affected performance.
Transverse Patterning Discriminations Test (TPDT)
The TPDT consisted of six phases conducted in a stepwise fashion. Non-nameable black stimuli were presented on a white background. Each trial began with a fixation cross displayed in the center of the screen for 1 second. Two stimuli appeared on the screen and participants responded by pressing a response key to indicate the left or right stimulus of the displayed pair. Correct responses were indicated by a beeping tone and the word “Correct” displayed in green on the screen. Incorrect responses were indicated by a buzzing tone and the word “Incorrect” displayed in red. The display was then cleared for a 2-second inter-trial interval before the next stimulus pair appeared. Participants were told to try to get as many correct as possible but were not explicitly instructed about the response contingencies.
Phases 1-3 of the TPDT consisted of elemental discriminations. Phase 1 consisted of the stimulus pair A+B-. In phase 2, stimulus pair C+D- was presented in addition to A+B-. Phase 3 consisted of three sets of elemental discriminations: A+B-, C+D-, E+F-. Phases 4-6 of the TPDT consisted of transverse patterning discriminations. Phase 4 consisted of the stimulus pair G+H-. Phase 5 consisted of G+H- and H+I-. In phase 6, the transverse discriminations included G+H-, H+I-, and I+G-. For each phase, training continued until participants achieved 11 correct out of 12 trials. A maximum of 400 trials was allotted to complete all six phases. Dependent variables included total trials to complete transverse patterning discriminations (phases 4-6) and total errors for phases 4-6.
Reversal Learning Task (RLT)
The RLT consisted of six phases conducted in a stepwise fashion. All stimuli were non-nameable fractal images that differed from the stimuli used for the TPDT. Like the TPDT, the first three phases consisted of elemental discriminations (A+B-, C+D-, E+F-). In phases 4-6, these contingencies were reversed. Phase 4 consisted of the stimulus pair A-B+, phase 5 of A-B+ and C-D+, and phase 6 of A-B+, C-D+, and E-F+. For each phase, training continued until participants achieved 11 correct out of 12 trials. A maximum of 400 trials was allotted to complete all six phases. Dependent variables included total trials to complete RLT discriminations (phases 4-6) and total errors for phases 4-6.
Genotyping
Blood was drawn from participants in order to obtain their DNA. Forty-seven SNPs from five genes (APOE, SORL1, COMT, BDNF, and KIBRA) were sequenced (Table 1) at the University of Wisconsin Biotechnology Core Facility. DNA concentration was verified using the Quant-iT™ PicoGreen® dsDNA kit (Life Technologies, Grand Island, NY). DNA samples were first normalized/standardized to 0.5 ng/uL using epMotion 5075 and pH 7.5 10 mM Ultrapure TM Tris-HCl (Life Technologies, Grand Island, NY). KASPar assays were amplified using the Eppendorf Mastercycler pro384 and analyzed using a Synergy 2 (BioTek®) plate reader to measure the fluorescence of the samples using Gen5 TM software. Number of successfully genotyped samples for each SNP is presented in Table 1. All SNPs were in Hardy-Weinberg equilibrium, with the exceptions of KIBRA rs764221, SORL1 rs1131497, and SORL1 rs689021; these SNPs were removed from subsequent analyses.
Table 1.
Distribution of SNPs of interest.
| Homozygous Minor |
Heterozygous | Homozygous Major |
Total | |
|---|---|---|---|---|
| KIBRA | ||||
| rs17070145 (minor: T) | 16 (11.9%) | 62 (44.3%) | 56 (41.8) | N = 134 |
| rs10475878 (minor: A) | 7 (5.2%) | 58 (43.0%) | 70 (51.9%) | N = 135 |
| rs4320284 (minor: G) | 18 (13.4%) | 71 (53.0%) | 45 (33.6%) | N = 134 |
| rs11740112 (minor: G) | 22 (17.7%) | 60 (48.4%) | 42 (33.9%) | N = 124 |
| rs244904 (minor: A) | 26 (19.0%) | 77 (56.2%) | 34 (24.8%) | N = 137 |
| rs10040267 (minor: A) | 28 (20.9%) | 57 (42.5%) | 49 (36.6%) | N = 134 |
| rs13171394 (minor: G) | 35 (25.9%) | 60 (44.4%) | 40 (29.6%) | N = 135 |
| rs6555802 (minor: A) | 14 (10.1%) | 64 (46.0%) | 61 (43.9%) | N = 139 |
| rs2241368 (minor: A) | 29 (21.3%) | 33 (24.3%) | 74 (54.4%) | N = 136 |
| rs1477306 (minor: A) | 16 (11.9%) | 60 (44.4%) | 59 (43.7%) | N = 135 |
| rs7700355 (minor: T) | 0 (0.0%) | 36 (27.5%) | 95 (72.5%) | N = 131 |
| rs11750709 (minor: A) | 12 (8.6%) | 71 (51.1%) | 56 (40.3%) | N = 139 |
| rs1422422 (minor: A) | 10 (7.8%) | 65 (50.8%) | 53 (41.4%) | N = 128 |
| rs4976592 (minor: T) | 9 (6.7%) | 62 (45.9%) | 64 (47.4%) | N = 135 |
| rs3822660 (minor: A) | 0 (0.0%) | 27 (20.6%) | 104 (79.4%) | N = 131 |
| rs3822659 (minor: C) | 0 (0.0%) | 42 (30.2%) | 97 (69.8%) | N = 139 |
| rs1030182 (minor: A) | 24 (17.6%) | 66 (48.5%) | 46 (33.8%) | N = 136 |
| rs7723533 (minor: A) | 8 (5.8%) | 50 (36.0%) | 81 (58.3%) | N = 139 |
| rs6555791 (minor: T) | 14 (10.1%) | 68 (49.3%) | 56 (40.6%) | N = 138 |
| rs11134509 (minor: A) | 14 (10.1%) | 68 (49.3%) | 56 (40.6%) | N = 138 |
| rs764221 (minor: A)* | 15 (11.3%) | 84 (63.9%) | 33 (24.8%) | N = 133 |
| COMT | ||||
| rs4646316 (minor: T) | 6 (4.4%) | 65 (48.1%) | 64 (47.4%) | N = 135 |
| rs1544325 (minor: A) | 14 (10.8%) | 76 (58.5%) | 40 (30.8%) | N = 130 |
| rs4680 (minor: A) | 32 (27.6%) | 50 (43.1%) | 34 (29.3%) | N = 116 |
| rs4633 (minor: T) | 37 (27.2%) | 56 (41.2%) | 43 (31.6%) | N = 136 |
| rs737865 (minor: C) | 13 (9.4%) | 64 (46.4%) | 61 (44.2%) | N = 138 |
| APOE | ||||
| rs429385 & rs7412 (minor: ε4) | 3 (2.4%) | 33 (27.9%) | 101 (69.7%) | N = 122 |
| rs157580 (minor: G) | 15 (12.5%) | 59 (49.2%) | 46 (38.3%) | N = 120 |
| rs439401 (minor: T) | 19 (15.3%) | 53 (42.7%) | 52 (41.9%) | N = 124 |
| rs405697 (minor: T) | 6 (4.4%) | 54 (39.4%) | 77 (56.2%) | N = 137 |
| rs157582 (minor: A) | 13 (9.4%) | 57 (41.3%) | 68 (49.3%) | N = 138 |
| rs405509 (minor: C) | 34 (25.2%) | 61 (45.2%) | 40 (29.6%) | N = 135 |
| rs8106922 (minor: G) | 18 (13.2%) | 66 (48.5%) | 52 (38.2%) | N = 136 |
| BDNF | ||||
| rs1491850 (minor: C) | 23 (16.5%) | 74 (53.2%) | 42 (30.2%) | N = 139 |
| rs985205 (minor: A) | 1 (0.8%) | 44 (36.4%) | 76 (62.8%) | N = 121 |
| rs11030096 (minor: C) | 28 (20.9%) | 69 (51.5%) | 37 (27.6%) | N = 134 |
| rs6265 (minor: A) | 7 (6.7%) | 25 (24.0%) | 72 (69.2%) | N = 104 |
| SORL1 | ||||
| rs668387 (minor: A) | 24 (17.8%) | 78 (57.8%) | 33 (24.4%) | N = 135 |
| rs1131497 (minor: G)* | 19 (14.6%) | 13 (10.0%) | 98 (75.4%) | N = 130 |
| rs1614735 (minor: G) | 31 (23.3%) | 61 (45.9%) | 41 (30.8%) | N = 133 |
| rs2282649 (minor: T) | 11 (10.6%) | 46 (44.2%) | 47 (45.2%) | N = 104 |
| rs1010159 (minor: G) | 21 (15.6%) | 61 (45.2%) | 53 (39.3%) | N = 135 |
| rs689021 (minor: T)* | 19 (14.1%) | 87 (64.4%) | 29 (21.5%) | N = 135 |
| rs641120 (minor: T) | 23 (17.6%) | 72 (55.0%) | 36 (27.5%) | N = 131 |
| rs1699102 (minor: C) | 19 (13.7%) | 72 (51.8%) | 48 (34.5%) | N = 139 |
| rs3824968 (minor: A) | 16 (11.8%) | 61 (44.9%) | 59 (43.4%) | N = 136 |
Note.
denotes SNPs that were not in Hardy-Weinberg equilibrium. These SNPs were removed from subsequent analysis.
Statistical and Power Analysis
Separate ANCOVAs were constructed for each SNP and dependent variable of interest. For SNPs in which the minor homozygote category had more than 10 individuals, an additive genetic model was tested (i.e., three groups: homozygote minor allele carriers, heterozygotes, and homozygote major allele carriers). For SNPs with fewer than 10 minor allele homozygotes, a dominant genetic model was tested (i.e., minor allele carriers [homozygote or heterozygote] versus major allele homozygotes). As age, sex, and education are associated with the outcome measures of interest, these variables were included as covariates in the model. Each model also tested the age x genotype interaction term.
A power analysis was conducted using G*Power for ANCOVA. Assuming an additive genetic model and α = .05, the study had a power of .91 to detect a moderate-sized effect (d = .3). Thus, the sample size was adequate to power the analyses and detect main effects of genotype on cognitive performance.
Results
Higher age was significantly associated with poorer performance on learning trials of the vMWT, including higher latency, r = .31, p < .001, greater heading error, r = .32, p < .001, and marginally higher distance to find the hidden platform, r = .16, p = .07 (Figure 1). Age was also associated with poorer performance on the vMWT probe trial, including less time spent in the goal quadrant, r = −.33, p < .001, and greater heading error, r = .25, p = .003. On the TPDT, higher age was associated with more trials to complete the task, r = .21, p = .02, and a greater number of errors, r = .17, p = .05. Associations between age and RLT performance were not significant (p = .11).
Figure 1. Age Is Associated with Poorer MTL-Dependent Cognitive Performance.
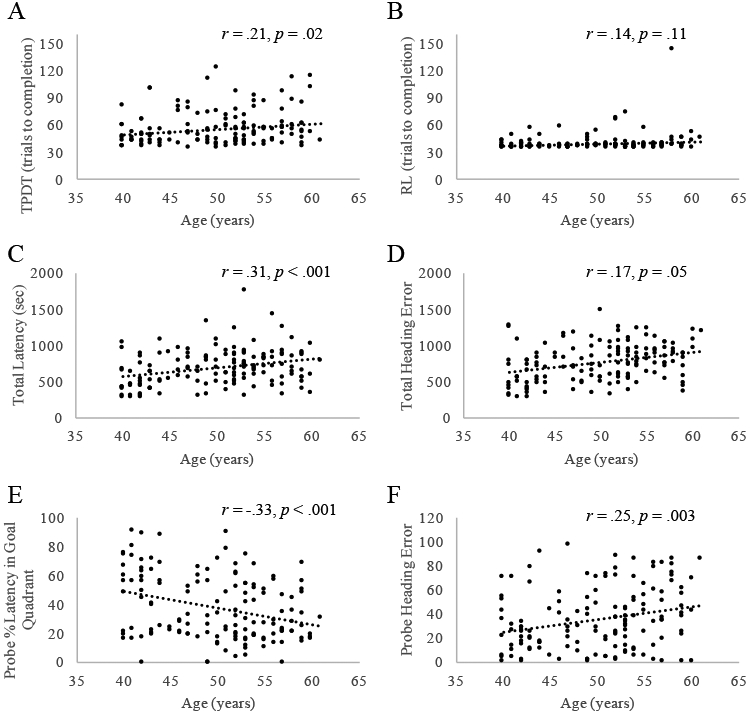
Age was associated with more trials to complete the TPDT (A), greater latency (C) and heading error (D) on vMWT learning trials, and less time spent in the goal quadrant (E) and greater heading error (F) on the vMWT probe trial. Associations between age and RLT performance were not significant (B).
Associations between SNPs and vMWT, TPDT, and RLT summary variables are summarized in Table 2. After controlling for age, sex, and education, poorer vMWT performance was observed in minor allele carriers of KIBRA rs244904, rs11750709, and rs1030182 and APOE rs405509. There was a significant age x genotype interaction for KIBRA rs244904. Follow-up pairwise correlations revealed that the association between age and vMWT performance was significant in homozygous major allele carriers but not heterozygotes or minor allele carriers. There was also a significant age x genotype interaction for KIBRA rs1030182, follow-up correlations significant in heterozygotes and major allele homozygotes.
Table 2.
Associations between SNPs and cognitive performance.
| vMWT Latency | vMWT Distance | TPDT Trials | TPDT Errors | RL Trials | RL Errors | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Statistic | p | Statistic | p | Statistic | p | Statistic | p | Statistic | p | Statistic | p | |
| KIBRA | ||||||||||||
| rs17070145 | F = 1.86 | .16 | F = .71 | .49 | F = 1.41 | .25 | F = .11 | .90 | F = .53 | .59 | F = .52 | .60 |
| rs10475878 | F = 1.38 | .24 | F = .15 | .70 | F = .77 | .38 | F = 1.23 | .27 | F = .62 | .43 | F = .21 | .65 |
| rs4320284 | F = .14 | .87 | F = .24 | .79 | F = 2.07 | .13 | F = .76 | .47 | F = .69 | .51 | F = .35 | .71 |
| rs11740112 | F = 2.81 | .06 | F = 1.15 | .32 | F = .69 | .50 | F = .40 | .67 | F = 1.41 | .25 | F = 2.05 | .13 |
| rs244904 | F = 3.27 | .003 | F = 2.92 | .06 | F = 6.39 | .01 | F = 1.84 | .16 | F = .15 | .86 | F = .12 | .89 |
| rs10040267 | F = .60 | .55 | F = 2.74 | .07 | F = 1.47 | .24 | F = .92 | .40 | F = 1.26 | .29 | F = 1.42 | .25 |
| rs13171394 | F = .14 | .87 | F = .19 | .83 | F = .36 | .70 | F = .43 | .65 | F = 1.03 | .36 | F = 1.81 | .17 |
| rs6555802 | F = 1.81 | .17 | F = .50 | .61 | F = .07 | .93 | F = .29 | .75 | F = 1.69 | .19 | F = 1.72 | .18 |
| rs2241368 | F = .28 | .76 | F = .70 | .50 | F = 2.50 | .09 | F = 3.14 | .05 | F = .89 | .41 | F = .45 | .64 |
| rs1477306 | F = .54 | .59 | F = .48 | .62 | F = 1.29 | .26 | F = .08 | .92 | F = .44 | .64 | F = .38 | .69 |
| rs7700355 | F = .80 | .37 | F = .03 | .87 | F = .40 | .53 | F = .13 | .72 | F < .01 | .99 | F < .01 | .99 |
| rs11750709 | F = .59 | .56 | F = 2.99 | .05 | F = 1.17 | .32 | F = 1.14 | .33 | F = .75 | .48 | F = .52 | .60 |
| rs1422422 | F = 1.13 | .33 | F = 1.67 | .19 | F = 1.80 | .17 | F = 1.69 | .19 | F = .62 | .54 | F = .59 | .56 |
| rs4976592 | F = .01 | .92 | F = .11 | .74 | F = .36 | .55 | F = 1.99 | .16 | F = 1.18 | .28 | F = 1.38 | .24 |
| rs3822660 | F = 1.12 | .29 | F = .70 | .40 | F = 3.08 | .08 | F = 1.52 | .22 | F = .61 | .44 | F = .29 | .59 |
| rs3822659 | F = .39 | .54 | F = 1.23 | .27 | F = 4.19 | .04 | F = 1.61 | .20 | F = 1.66 | .56 | F = 2.15 | .15 |
| rs1030182 | F = 5.02 | .01 | F = 3.18 | .04 | F = .79 | .45 | F = 1.45 | .24 | F = 1.25 | .29 | F = 1.20 | .31 |
| rs7723533 | F = .20 | .65 | F = .09 | .77 | F = .40 | .53 | F = .30 | .59 | F = 1.26 | .56 | F = 1.87 | .17 |
| rs6555791 | F = .14 | .87 | F = .12 | .89 | F = .87 | .42 | F = .66 | .52 | F = 1.19 | .31 | F = 1.29 | .28 |
| rs11134509 | F = .09 | .91 | F = .01 | .99 | F = 1.70 | .19 | F = .46 | .63 | F = .74 | .48 | F = 1.05 | .35 |
| COMT | ||||||||||||
| rs4646316 | F = .04 | .84 | F = .59 | .45 | F = .36 | .55 | F = .03 | .87 | F = 1.74 | .19 | F = 1.84 | .18 |
| rs1544325 | F = .46 | .63 | F = .42 | .66 | F = .11 | .90 | F = .21 | .81 | F = .47 | .63 | F = .66 | .52 |
| rs4680 | F = .04 | .96 | F = .62 | .54 | F = .12 | .88 | F = .77 | .47 | F = .30 | .74 | F = .45 | .64 |
| rs4633 | F = .29 | .75 | F = 1.36 | .26 | F = .44 | .65 | F = .37 | .69 | F = .46 | .63 | F = .35 | .70 |
| rs737865 | F = .23 | .79 | F = .87 | .42 | F = .51 | .60 | F = 1.03 | .36 | F = .44 | .65 | F = .46 | .64 |
| APOE | ||||||||||||
| ε2/ε3/ε4 | F = .34 | .56 | F = .04 | .85 | F = 1.07 | .30 | F = 2.73 | .10 | F = 2.33 | .13 | F = 2.19 | .14 |
| rs157580 | F = .91 | .41 | F = 1.63 | .20 | F = .83 | .36 | F = .22 | .81 | F = 3.16 | .05 | F = 2.85 | .06 |
| rs439401 | F = .14 | .87 | F = .20 | .82 | F = 1.72 | .18 | F = 2.84 | .06 | F = 1.02 | .36 | F = .73 | .48 |
| rs405697 | F = .01 | .94 | F = .29 | .59 | F = 2.12 | .15 | F = .49 | .49 | F = 1.69 | .20 | F = 1.18 | .28 |
| rs157582 | F = 1.45 | .24 | F = .51 | .60 | F = 1.40 | .25 | F = .34 | .71 | F = 1.10 | .34 | F = 1.23 | .30 |
| rs405509 | F = 3.62 | .03 | F = 1.15 | .32 | F = .51 | .60 | F = .69 | .50 | F = .26 | .78 | F = .03 | .98 |
| rs8106922 | F = .48 | .62 | F = .38 | .69 | F = .15 | .86 | F = .08 | .92 | F = .73 | .48 | F = .57 | .57 |
| BDNF | ||||||||||||
| rs1491850 | F = .16 | .85 | F = .84 | .44 | F = 1.39 | .25 | F = 1.65 | .20 | F = .55 | .58 | F = 1.02 | .36 |
| rs985205 | F = 1.26 | .27 | F = .08 | .79 | F = .21 | .65 | F = .70 | .41 | F = 2.00 | .16 | F = 1.98 | .16 |
| rs11030096 | F = .77 | .47 | F = 1.13 | .33 | F = .40 | .67 | F = .83 | .44 | F = 3.00 | .05 | F = 3.14 | .05 |
| rs6265 | F < .01 | .96 | F = 1.03 | .31 | F = .58 | .45 | F = .24 | .63 | F = .57 | .45 | F = .33 | .57 |
| SORL1 | ||||||||||||
| rs668387 | F = .14 | .87 | F = .02 | .98 | F = .34 | .71 | F = 1.35 | .26 | F = .52 | .60 | F = .50 | .61 |
| rs1614735 | F = 1.58 | .21 | F = .45 | .64 | F = .44 | .64 | F = 1.36 | .26 | F = .22 | .80 | F = .25 | .78 |
| rs2282649 | F = 1.42 | .25 | F = 1.93 | .15 | F = .34 | .71 | F = .17 | .84 | F = .35 | .71 | F = .53 | .59 |
| rs1010159 | F = .83 | .44 | F = .54 | .59 | F = 1.59 | .21 | F = .70 | .50 | F = .56 | .57 | F = .52 | .60 |
| rs641120 | F = .05 | .95 | F = .31 | .73 | F = 2.28 | .11 | F = .96 | .39 | F = 2.65 | .08 | F = 2.54 | .08 |
| rs1699102 | F = .09 | .92 | F = .76 | .47 | F = 1.03 | .36 | F = .88 | .42 | F = .62 | .54 | F = .76 | .47 |
| rs3824968 | F = .52 | .60 | F = .55 | .58 | F = 2.03 | .14 | F = .34 | .71 | F = .10 | .91 | F = .10 | .90 |
Note. Only vMWT total latency and distance are included in the table for brevity; other vMWT outcome variables were not significantly associated with SNPs. *Denotes SNPs for which a dominant genetic model was used (minor allele carriers [homozygote or heterozygote] vs. major allele carriers). All other SNPs were analyzed using an additive model.
Poorer TPDT performance was observed in minor allele carriers of KIBRA rs244904, rs3822659, and rs2241368. There was a significant age x genotype interaction for KIBRA rs3822659, with follow-up tests showing that the association between age and TPDT performance was significant in homozygous major allele carriers.
Poorer RLT performance was observed in minor allele carriers of APOE rs157580 and BDNF rs11030096. The age x genotype interactions were significant for both of these SNPs. For both SNPs, follow-up tests showed that the association between age and RLT performance was significant for homozygous major allele carriers.
After adjusting for multiple comparisons (FDR correction; q = .05), there were no significant SNP-associated differences in performance on the vMWT, TPDT, or RLT and no significant age x genotype interactions.
Discussion
To the best of our knowledge, this is the first study to investigate associations between genetic risk factors and performance on translational MTL- or PFC-dependent tasks in middle age. We report a pattern of poorer cognitive performance in minor allele carriers of SNPs in genes associated with risk for cognitive impairment (i.e, APOE, BDNF, and KIBRA) in a healthy, non-demented middle-aged sample. Although these associations did not survive correction for multiple comparisons, given our a priori hypotheses based on existent literature, our findings provide potential candidates for future replication studies. Although we did not observe significant associations between genotype and performance on translational cognitive measures, older individuals within this middle-aged sample exhibited poorer performance on MTL-dependent tasks (i.e., vMWT and TPDT), consistent with the literature in older adults [43, 44, 59, 60]. Taken together, these results suggest that middle-aged individuals carrying single SNPs that elevate risk for age-related cognitive impairment have largely preserved cognitive performance. This presents challenges for preclinical detection of AD and suggests that complex, interacting effects of genes, age, and environmental factors may be needed for accurate prediction of risk for age-related cognitive decline.
SNP-Associated Cognitive Performance in Middle Age
Our finding of negligible differences in cognitive performance between carriers and non-carriers of APOE, SORL1, COMT, BDNF, and KIBRA risk alleles is not entirely unexpected. Although APOE ε4 is the largest genetic risk factor for AD, numerous cross-sectional studies have reported that middle-aged ε4 carriers do not differ from non-ε4 carriers on standardized neuropsychological tests [4, 37-39]; however, greater ε4-associated decline may be observed longitudinally [61, 62]. Our findings are congruent with these results, suggesting that APOE ε4 carriers may have preserved cognitive performance during middle age. One alternative explanation is related to evidence that the APOE ε4 allele confers a cognitive advantage in young adulthood before becoming deleterious during the aging process [35, 63]. Cross-sectional studies in middle age, such as the present investigation, may capture some individuals maintaining the ε4-associated advantage while others begin to undergo decline.
Although polymorphism in SORL1, COMT, BDNF, and KIBRA has been associated with age-related cognitive impairment [17, 26, 64, 65], there is considerably less research focusing on middle-aged individuals. No known studies have characterized the cognitive phenotypes of SORL1 variants in younger or middle adulthood. For KIBRA, the first study to report an association between KIBRA polymorphism and memory performance included both younger and middle-aged individuals [26]; however, the authors did not investigate an age by genotype interaction or separate middle-aged from younger adults. To our knowledge, no subsequent investigations have focused on this middle-aged group. We report several significant associations between KIBRA SNPs and cognitive performance, although these did not survive corrections for multiple comparisons. COMT studies investigating the most well-characterized SNP, rs4680 (Val158Met), have yielded conflicting results. Some find that younger met/met carriers have better executive functioning than val carriers [66-69], while other have failed to find an effect or have documented the reverse pattern [70-73]. Among older adults, findings are also equivocal, leading some to propose that interactions between age, sex, and COMT genotype may contribute to the lack of consistent associations [74].
Reports of differential cognition with BDNF polymorphism are also complicated to interpret, with studies of the Val66Met polymorphism reporting a memory advantage among younger val carriers [22, 75] but older val carriers performing worse across cognitive domains [76] and having higher risk for AD [77]. A longitudinal study reported that older val carriers outperformed met carriers at baseline but experienced a greater reduction in executive functioning over a 10-year follow-up period [78]. We did not observe an association between BDNF Val66Met polymorphism and cognitive performance even in models uncorrected for multiple comparisons. As with COMT polymorphism, it is possible that age and sex interact with genotype to affect cognitive performance across the lifespan. Thus, despite the unique advantages of studying a middle-aged population, focusing on this age range may have limited our power to detect SNP-associated cognitive differences.
Taken together, our findings are consistent with the resource modulation hypothesis, which states that genetic differences exert larger effects on cognition as resources deplete during the course of brain aging [79]. Thus, our inability to detect substantial SNP-associated differences in age-sensitive cognitive tasks during middle age may be due to relatively preserved brain resources within this age range. Our findings are also congruent with the Gompertz Law, which classically models mortality with an age-independent component as well as an age-dependent component that increases exponentially with age. When applied to cognition during aging, the Gompertz function predicts an extended period of preserved cognition during middle adulthood, followed by a steep apparent decline in cognition as the age-dependent loss-of-function component exponentially compounds, and then a flattening of the curve during oldest age when there is little cognitive function left to decline [80]. Thus, the Gompertz Law, like the resource modulation hypothesis, predict relative preservation of cognitive functions during middle adulthood. Indeed, in a well-controlled longitudinal study of age-related atrophy across the lifespan, accelerating regional shrinkage in the hippocampus and prefrontal cortex was noted to begin in the mid-50s [81]. This is at the higher end of the age range of the present sample (40-60 years), suggesting that relative preservation of neural resources in our sample may have limited our ability to detect genotype-associated differences in MTL- and PFC-dependent cognitive performance.
Establishing Behavioral Biomarkers of Risk for Age-Related Cognitive Impairment
Although the tasks used in the present investigation did not discriminate between carriers and non-carriers of risk alleles after correcting for multiple comparisons, they may still be effective behavioral biomarkers of risk for age-related cognitive impairment. One of the limitations of traditional neuropsychological tests is that they often require several component cognitive processes, providing more global assessments of cognitive domains. Researchers have proposed that behavioral assays associated with particular brain regions or networks may be more sensitive to preclinical detection of AD [36]. For example, in the BIOCARD study (mean age 59 years), APOE genotype was associated with performance on behavioral assays of spatial attention and spatial working memory, despite no observed differences on traditional neuropsychological measures [4].
In the present study, higher age was associated with significantly poorer performance on the vMWT and TPDT tasks, even within the relatively restricted age range of our middle-aged sample. This is consistent with evidence from several samples suggesting that age-related differences in vMWT [43, 59] and TPDT (Blujus et al., unpublished data) performance may begin to emerge by middle age. As these MTL-dependent tasks appear to be sensitive early to aging effects, they remain a potentially fruitful avenue for exploration as potential biomarkers of age-related cognitive impairment. In addition, understanding the moderating effects of health, lifestyle, and neurologic factors as well as the longitudinal trajectory of SNP-associated task performance will be important areas for future research.
Study Limitations
This study is not without limitations. Although the study was adequately powered to detect SNP-associated differences in cognitive performance, we lacked the power to explore gene x gene or gene x environment interactions. Additionally, the study is limited by its cross-sectional design. Longitudinal evidence is needed to capture intra-individual declines in task performance over time as well as to determine which risk allele carriers actually will go on to develop cognitive impairment.
Finally, candidate gene studies have been criticized for failing to generate robust findings that can be replicated in independent samples [82]. Critics of candidate gene studies often point to the risk of inflated type I error, resulting in false positives. Our failure to identify significant associations between SNPs and cognitive performance after appropriate corrections for multiple comparisons argues against inflated type I error in the present study. One possible explanation for our findings is that while we targeted SNPs from genes previously associated with memory and executive functioning in older adults, it is possible that other gene polymorphisms may be more strongly associated with cognitive performance in middle age. Additionally, genotype-associated cognitive deficits are likely to be subtle healthy, non-demented middle-aged individuals. The large number of statistical comparisons in the present study may have limited our power to detect significant effects after correcting for multiple comparisons. Carefully designed candidate gene studies are needed to replicate and extend the information gathered from genome-wide association studies and the uncorrected associations reported in this sample.
Summary and Conclusions
In summary, we report modest differences in MTL- and PFC-dependent cognitive performance between carriers and non-carriers of APOE, SORL1, COMT, BDNF, and KIBRA risk alleles, with no gene-associated differences in cognitive performance that survived correction for multiple comparisons. This is the first known study to investigate associations between genetic risk factors and performance on translational MTL- or PFC-dependent tasks in middle age. The negligible differences between carriers and non-carriers of risk alleles suggests that detecting very early dysfunction in brain areas vulnerable to AD neuropathology remains a challenge. However, the vMWT and TPDT, translational tasks tapping MTL integrity, may be sensitive behavioral biomarkers for future investigations of age-related cognitive decline.
Acknowledgments
This work was supported by National Institute on Aging (NIA) R00-AG032361 (Driscoll) and F31-AG050407 (Korthauer).
Footnotes
Conflict of Interest/Disclosure Statement
The authors have no conflict of interest to report.
References
- [1].Alzheimer’s Association (2015) 2015 Alzheimer's disease facts and figures, Alzheimers Dement. 11, 332–84. [DOI] [PubMed] [Google Scholar]
- [2].Cummings JL, Morstorf T, Zhong K, (2014) Alzheimer's disease drug-development pipeline: few candidates, frequent failures Alzheimers Res Ther. 6, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7, 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Greenwood PM, Lambert C, Sunderland T, Parasuraman R (2005) Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: results From the National Institute of Mental Health's BIOCARD study. Neuropsychology 19, 199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McDonald CR, McEvoy LK, Gharapetian L, Fennema-Notestine C, Hagler DJ, Holland D, Koyama A, Brewer JB, Dale AM, Alzheimer’s Disease Neuroimaging Initiative (2009) Regional rates of neocortical atrophy from normal aging to early Alzheimer disease. Neurology 73, 457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].LR Squire, Zola-Morgan S (1991) The medial temporal lobe memory system. Science 253,1380–6. [DOI] [PubMed] [Google Scholar]
- [7].Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD (2000) The unity and diversity of executive functions and their contributions to complex "Frontal Lobe" tasks: a latent variable analysis. Cogn Psychol 41, 49–100. [DOI] [PubMed] [Google Scholar]
- [8].Apostolova LG, Dutton RA, Dinov ID, Hayashi KM, Toga AW, Cummings JL, Thompson PM (2006) Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol 63, 693–9. [DOI] [PubMed] [Google Scholar]
- [9].Drzezga A, Lautenschlager N, Siebner H, Riemenschneider M, Willoch F, Minoshima S, Schwaiger M, Kurz A (2003). Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer's disease: a PET follow-up study. Eur J Nucl Med Mol Imaging 30, 1104–13. [DOI] [PubMed] [Google Scholar]
- [10].Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM (1997) Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: A meta-analysis. JAMA 278, 1349–56. [PubMed] [Google Scholar]
- [11].Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE (2007) Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet 39, 17–23. [DOI] [PubMed] [Google Scholar]
- [12].Wisdom NM, Callahan JL, Hawkins KA (2011) The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiol Aging 32, 63–74. [DOI] [PubMed] [Google Scholar]
- [13].Elias-Sonnenschein LS, Viechtbauer W, Ramakers IH, Verhey FR, Visser PJ (2011) Predictive value of APOE-ε4 allele for progression from MCI to AD-type dementia: a meta-analysis. J Neurol Neurosurg Psychiatry 82, 1149–56. [DOI] [PubMed] [Google Scholar]
- [14].Kölsch H, Jessen F, Wiltfang J, Lewczuk P, Dichgans M, Kornhuber J, Frölich L, Heuser I, Peters O, Schulz JB, Schwab SG, Maier W (2008) Influence of SORL1 gene variants: association with CSF amyloid-beta products in probable Alzheimer's disease. Neurosci Lett 440, 68–71. [DOI] [PubMed] [Google Scholar]
- [15].Offe K, Dodson SE, Shoemaker JT, Fritz JJ, Gearing M, Levey AI, Lah JJ (2006) The lipoprotein receptor LR11 regulates amyloid beta production and amyloid precursor protein traffic in endosomal compartments. J Neurosci 26, 1596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Webster JA, Myers AJ, Pearson JV, Craig DW, Hu-Lince D, Coon KD, Zismann VL, Beach T, Leung D, Bryden L, Halperin RF, Marlowe L, Kaleem M, Huentelman MJ, Joshipura K, Walker D, Heward CB, Ravid R, Rogers J, Papassotiropoulos A, Hardy J, Reiman EM, Stephan DA (2008) Sorl1 as an Alzheimer's disease predisposition gene? Neurodegener Dis 5, 60–4. [DOI] [PubMed] [Google Scholar]
- [17].Houlihan LM, Harris SE, Luciano M, Gow AJ, Starr JM, Visscher PM, Deary IJ (2009) Replication study of candidate genes for cognitive abilities: the Lothian Birth Cohort 1936. Genes Brain Behav 8, 238–47. [DOI] [PubMed] [Google Scholar]
- [18].Bralten J, Arias-Vásquez A, Makkinje R, Veltman JA, Brunner HG, Fernández G, Rijpkema M, Franke B (2011) Association of the Alzheimer's gene SORL1 with hippocampal volume in young, healthy adults. Am J Psychiatry 168, 1083–9. [DOI] [PubMed] [Google Scholar]
- [19].Assareh AA, Piguet O, Lye TC, Mather KA, Broe GA, Schofield PR, Sachdev PS, Kwok JB (2014) Association of SORL1 gene variants with hippocampal and cerebral atrophy and Alzheimer's disease. Curr Alzheimer Res 11, 558–63. [DOI] [PubMed] [Google Scholar]
- [20].Reynolds CA, Zavala C, Gatz M, Vie L, Johansson B, Malmberg B, Ingelsson E, Prince JA, Pedersen NL (2013) Sortilin receptor 1 predicts longitudinal cognitive change. Neurobiol Aging 34, 1710.e11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Murer MG, Boissiere F, Yan Q, Hunot S, Villares J, Faucheux B, Agid Y, Hirsch E, Raisman-Vozari R (1999) An immunohistochemical study of the distribution of brain-derived neurotrophic factor in the adult human brain, with particular reference to Alzheimer's disease. Neuroscience 88,1015–32. [DOI] [PubMed] [Google Scholar]
- [22].Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR (2003) Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci 23, 6690–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kennedy KM, Reese ED, Horn MM, Sizemore AN, Unni AK, Meerbrey ME, Kalich AG, Rodrigue KM (2015) BDNF val66met polymorphism affects aging of multiple types of memory. Brain Res 1612, 104–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Voineskos AN, Lerch JP, Felsky D, Shaikh S, Rajji TK, Miranda D, Lobaugh NJ, Mulsant BH, Pollock BG, Kennedy JL (2011) The brain-derived neurotrophic factor Val66Met polymorphism and prediction of neural risk for Alzheimer disease. Arch Gen Psychiatry 68, 198–206. [DOI] [PubMed] [Google Scholar]
- [25].Johannsen S, Duning K, Pavenstädt H, Kremerskothen J, Boeckers TM (2008) Temporal-spatial expression and novel biochemical properties of the memory-related protein KIBRA. Neuroscience 155, 1165–73. [DOI] [PubMed] [Google Scholar]
- [26].Papassotiropoulos A, Stephan DA, Huentelman MJ, Hoerndli FJ, Craig DW, Pearson JV, Huynh KD, Brunner F, Corneveaux J, Osborne D, Wollmer MA, Aerni A, Coluccia D, Hänggi J, Mondadori CR, Buchmann A, Reiman EM, Caselli RJ, Henke K, de Quervain DJ (2006) Common Kibra alleles are associated with human memory performance. Science 314, 475–8. [DOI] [PubMed] [Google Scholar]
- [27].Bates TC, Price JF, Harris SE, Marioni RE, FG Fowkes, Stewart MC, Murray GD, Whalley LJ, Starr JM, Deary IJ (2009) Association of KIBRA and memory. Neurosci Lett 458, 140–3. [DOI] [PubMed] [Google Scholar]
- [28].Witte AV, Köbe T, Kerti L, Rujescu D, Flöel A (2016) Impact of KIBRA Polymorphism on Memory Function and the Hippocampus in Older Adults. Neuropsychopharmacology 41, 781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Corneveaux JJ, Liang WS, Reiman EM, Webster JA, Myers AJ, Zismann VL, Joshipura KD, Pearson JV, Hu-Lince D, Craig DW, Coon KD, Dunckley T, Bandy D, Lee W, Chen K, Beach TG, Mastroeni D, Grover A, Ravid R, Sando SB, Aasly JO, Heun R, Jessen F, Kölsch H,Rogers J, Hutton ML, Melquist S, Petersen RC, Alexander GE, Caselli RJ, Papassotiropoulos A, Stephan DA, Huentelman MJ (2010) Evidence for an association between KIBRA and late-onset Alzheimer's disease. Neurobiol Aging 31, 901–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR (2004) Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet 75, 807–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Barnett JH, PB Jones, Robbins TW, Müller U. (2007) Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Mol Psychiatry 12, 502–9. [DOI] [PubMed] [Google Scholar]
- [32].Wishart HA, Roth RM, Saykin AJ, Rhodes CH, Tsongalis GJ, Pattin KA, Moorev JH, McAllister TW (2011) COMT Val158Met Genotype and Individual Differences in Executive Function in Healthy Adults. J Int Neuropsychol Soc 17, 174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Papenberg G, Bäckman L, Nagel IE, Nietfeld W, Schröder J, Bertram L, Heekeren HR, Lindenberger U, Li SC (2013) COMT Polymorphism and Memory Dedifferentiation in Old Age, Psychol Aging. [DOI] [PubMed] [Google Scholar]
- [34].de Frias CM, Annerbrink K, Westberg L, Eriksson E, Adolfsson R, Nilsson LG (2004) COMT gene polymorphism is associated with declarative memory in adulthood and old age, Behav Genet 34, 533–9. [DOI] [PubMed] [Google Scholar]
- [35].Tuminello ER, Han SD (2011) The apolipoprotein e antagonistic pleiotropy hypothesis: review and recommendations. Int J Alzheimers Dis 2011, 726197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lowndes G, Savage G (2007) Early detection of memory impairment in Alzheimer's disease: a neurocognitive perspective on assessment. Neuropsychol Rev 17, 193–202. [DOI] [PubMed] [Google Scholar]
- [37].Sager MA, Hermann B, La Rue A (2005) Middle-aged children of persons with Alzheimer's disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer's Prevention. J Geriatr Psychiatry Neurol 18, 245–9. [DOI] [PubMed] [Google Scholar]
- [38].Greenwood PM, Espeseth T, Lin MK, Reinvang I, Parasuraman R (2014) Longitudinal change in working memory as a function of APOE genotype in midlife and old age. Scand J Psychol 55, 268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D (1996) Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med 334, 752–8. [DOI] [PubMed] [Google Scholar]
- [40].Astur RS, , Ortiz ML, Sutherland RJ (1998) A characterization of performance by men and women in a virtual Morris water task: a large and reliable sex difference. Behav Brain Res 93, 185–90. [DOI] [PubMed] [Google Scholar]
- [41].Morris RG, , Garrud P, Rawlins JN, O'Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297, 681–3. [DOI] [PubMed] [Google Scholar]
- [42].Astur RS, , Taylor LB, Mamelak AN, Philpott L, Sutherland RJ (2002) Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task, Behav Brain Res 132, 77–84. [DOI] [PubMed] [Google Scholar]
- [43].Korthauer LE, , Nowak NT, Moffat SD, An Y, Rowland LM, Barker PB, Resnick SM, Driscoll I (2016) Correlates of virtual navigation performance in older adults. Neurobiol Aging 39, 118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Driscoll I, , Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, Sutherland RJ (2003) The aging hippocampus: cognitive, biochemical and structural findings. Cereb Cortex 13, 1344–51. [DOI] [PubMed] [Google Scholar]
- [45].Moffat SD, , Elkins W, Resnick SM (2006) Age differences in the neural systems supporting human allocentric spatial navigation. Neurobiol Aging 27, 965–72. [DOI] [PubMed] [Google Scholar]
- [46].Spence KW, (1952) The nature of the response in discrimination learning. Psychol Rev 59, 89–93. [DOI] [PubMed] [Google Scholar]
- [47].Alvarado MC, , Rudy JW (1992) Some properties of configural learning: an investigation of the transverse-patterning problem. J Exp Psychol Anim Behav Process 18, 145–53. [DOI] [PubMed] [Google Scholar]
- [48].Reed JM, , Squire LR (1999) Impaired transverse patterning in human amnesia is a special case of impaired memory for two-choice discrimination tasks. Behav Neurosci 113, 3–9. [DOI] [PubMed] [Google Scholar]
- [49].Rickard TC, , Grafman J (1998) Losing their configural mind. Amnesic patients fail on transverse patterning. J Cogn Neurosci 10, 509–24. [DOI] [PubMed] [Google Scholar]
- [50].Alvarado MC, , Bachevalier J (2005) Comparison of the effects of damage to the perirhinal and parahippocampal cortex on transverse patterning and location memory in rhesus macaques. J Neurosci 25, 1599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ostreicher ML, , Moses SN, Rosenbaum RS, Ryan JD (2010) Prior experience supports new learning of relations in aging. J Gerontol B Psychol Sci Soc Sci 65, 32–41. [DOI] [PubMed] [Google Scholar]
- [52].Hornak J, , O'Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, Polkey CE (2004) Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. J Cogn Neurosci 16, 463–78. [DOI] [PubMed] [Google Scholar]
- [53].Budhani S, , Marsh AA, Pine DS, Blair RJ (2007) Neural correlates of response reversal: considering acquisition. Neuroimage 34, 1754–65. [DOI] [PubMed] [Google Scholar]
- [54].McAlonan K, , Brown VJ (2003) Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res 146, 97–103. [DOI] [PubMed] [Google Scholar]
- [55].Boulougouris V, , Dalley JW, Robbins TW (2007) Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res 179, 219–28. [DOI] [PubMed] [Google Scholar]
- [56].Folstein MF, , Folstein SE, McHugh PR (1975) Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–98. [DOI] [PubMed] [Google Scholar]
- [57].Jurica PJ, , Leitten CL, Mattis S (2004) DRS-2 Dementia rating scale-2: Professional manual. Psychological Assessment Resources. [Google Scholar]
- [58].Yesavage JA, , Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO (1982) Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 17, 37–49. [DOI] [PubMed] [Google Scholar]
- [59].Moffat SD, , Resnick SM (2002) Effects of age on virtual environment place navigation and allocentric cognitive mapping. Behav Neurosci 116, 851–9. [DOI] [PubMed] [Google Scholar]
- [60].Driscoll I, , Hamilton DA, Yeo RA, Brooks WM, Sutherland RJ. (2005) Virtual navigation in humans: the impact of age, sex, and hormones on place learning. Horm Behav 47, 326–35. [DOI] [PubMed] [Google Scholar]
- [61].Caselli RJ, , Reiman EM, Osborne D, Hentz JG, Baxter LC, Hernandez JL, Alexander GG (2004) Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology 62, 1990–5. [DOI] [PubMed] [Google Scholar]
- [62].Blair CK, , Folsom AR, Knopman DS, Bray MS, Mosley TH, Boerwinkle E, ARiCAS Investigators (2005) APOE genotype and cognitive decline in a middle-aged cohort. Neurology 64, 268–76. [DOI] [PubMed] [Google Scholar]
- [63].Han SD, , Drake AI, Cessante LM, Jak AJ, Houston WS, Delis DC, Filoteo JV, Bondi MW (2007) Apolipoprotein E and traumatic brain injury in a military population: evidence of a neuropsychological compensatory mechanism? J Neurol Neurosurg Psychiatry 78, 1103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Seshadri S, , DeStefano AL, Au R, Massaro JM, Beiser AS, Kelly-Hayes M, Kase CS, D'Agostino RB, Decarli C, Atwood LD, Wolf PA (2007) Genetic correlates of brain aging on MRI and cognitive test measures: a genome-wide association and linkage analysis in the Framingham Study. BMC Med Genet 8, S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Egan MF, , Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112, 257–69. [DOI] [PubMed] [Google Scholar]
- [66].Egan MF, , Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR (2001) Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A 98, 6917–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Joober R, , Gauthier J, Lal S, Bloom D, Lalonde P, Rouleau G, Benkelfat C, Labelle A (2002) Catechol-O-methyltransferase Val-108/158-Met gene variants associated with performance on the Wisconsin Card Sorting Test. Arch Gen Psychiatry 59, 662–3. [DOI] [PubMed] [Google Scholar]
- [68].Malhotra AK, , Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D (2002) A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry 159, 652–4. [DOI] [PubMed] [Google Scholar]
- [69].Goldberg TE, , Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR (2003) Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry 60, 889–96. [DOI] [PubMed] [Google Scholar]
- [70].Barnett JH, , Scoriels L, Munafò MR (2008) Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biol Psychiatry 64, 137–44. [DOI] [PubMed] [Google Scholar]
- [71].Bruder GE, , Keilp JG, Xu H, Shikhman M, Schori E, Gorman JM, Gilliam TC (2005) Catechol-O-methyltransferase (COMT) genotypes and working memory: associations with differing cognitive operations. Biol Psychiatry 58, 901–7. [DOI] [PubMed] [Google Scholar]
- [72].MacDonald AW, , Carter CS, Flory JD, Ferrell RE, Manuck SB (2007) COMT val158Met and executive control: a test of the benefit of specific deficits to translational research. J Abnorm Psychol 116, 306–12. [DOI] [PubMed] [Google Scholar]
- [73].Tsai SJ, , Yu YW, Chen TJ, Chen JY, Liou YJ, Chen MC, Hong CJ. (2003) Association study of a functional catechol-O-methyltransferase-gene polymorphism and cognitive function in healthy females. Neurosci Lett 338, 123–6. [DOI] [PubMed] [Google Scholar]
- [74].O'Hara R, , Miller E, Liao CP, Way N, Lin X, Hallmayer J (2006) COMT genotype, gender and cognition in community-dwelling, older adults. Neurosci Lett 409, 205–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ho BC, , Milev P, O'Leary DS, Librant A, Andreasen NC, Wassink TH (2006) Cognitive and magnetic resonance imaging brain morphometric correlates of brain-derived neurotrophic factor Val66Met gene polymorphism in patients with schizophrenia and healthy volunteers. Arch Gen Psychiatry 63, 731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Harris SE, , Fox H, Wright AF, Hayward C, Starr JM, Whalley LJ, Deary IJ (2006) The brain-derived neurotrophic factor Val66Met polymorphism is associated with age-related change in reasoning skills. Mol Psychiatry 11, 505–13. [DOI] [PubMed] [Google Scholar]
- [77].Ventriglia M, , Bocchio Chiavetto L, Benussi L, Binetti G, Zanetti O, Riva MA, Gennarelli M (2002) Association between the BDNF 196 A/G polymorphism and sporadic Alzheimer's disease. Mol Psychiatry 7, 136–7. [DOI] [PubMed] [Google Scholar]
- [78].Erickson KI, , Kim JS, Suever BL, Voss MW, Francis BM, Kramer AF (2008) Genetic contributions to age-related decline in executive function: a 10-year longitudinal study of COMT and BDNF polymorphisms. Front Hum Neurosci 2, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lindenberger U, , Nagel IE, Chicherio C, Li SC, Heekeren HR, Bäckman L (2008) Age-related decline in brain resources modulates genetic effects on cognitive functioning. Front Neurosci 2, 234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sas AA, , Snieder H, Korf J. (2012) Gompertz' survivorship law as an intrinsic principle of aging. Med Hypotheses 78, 659–63. [DOI] [PubMed] [Google Scholar]
- [81].Raz N, , Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD (2005) Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex 15, 1676–89. [DOI] [PubMed] [Google Scholar]
- [82].Jorgensen TJ, , Ruczinski I, Kessing B, Smith MW, Shugart YY, Alberg AJ (2009) Hypothesis-driven candidate gene association studies: practical design and analytical considerations. Am J Epidemiol 170, 986–93. [DOI] [PMC free article] [PubMed] [Google Scholar]


