Abstract
Suitable micropumping methods for flow control represent a major technical hurdle in the development of microfluidic systems for point-of-care testing (POCT). Passive micropumping for point-of-care microfluidic systems provides a promising solution to such challenges, in particular, passive micropumping based on capillary force and air transfer based on the air solubility and air permeability of specific materials. There have been numerous developments and applications of micropumping techniques that are relevant to the use in POCT. Compared with active pumping methods such as syringe pumps or pressure pumps, where the flow rate can be well-tuned independent of the design of the microfluidic devices or the property of the liquids, most passive micropumping methods still suffer flow-control problems. For example, the flow rate may be set once the device has been made, and the properties of liquids may affect the flow rate. However, the advantages of passive micropumping, which include simplicity, ease of use, and low cost, make it the best choice for POCT. Here, we present a systematic review of different types of passive micropumping that are suitable for POCT, alongside existing applications based on passive micropumping. Future trends in passive micropumping are also discussed.
I. INTRODUCTION
Point-of-care testing (POCT) is fast and easy to perform, enabling diagnosis near the patient without the need for a clinical laboratory.1–3 The rationale behind POCT is that it will significantly simplify healthcare delivery and improve clinical outcomes, with the potential to shift from curative medicine to predictive or preventive medicine.
POCT usually requires a low volume of sample and reagents, miniaturization of devices, and a fast turnaround time for analysis. These features fit well with the nature of microfluidics, and so it is unsurprising that since the inception of microfluidics, one of its major applications has been POCT.4 In recent years, POCT devices combining both microfluidic cartridges and microelectronic interfaces have been introduced to the market.1,5,6 It is estimated that the market for POCT will be several billion USD.5–7 However, POCT has an additional requirement that limits the adoption of microfluidics: extreme simplicity of use. Especially in developing countries, where there is the greatest need for POCT,8 the technology should be ASSURED (affordable, sensitive, specific, user-friendly, rapid and robust, equipment-free, and deliverable to end-users), according to the World Health Organization.2,9,10 Therefore, the challenge for microfluidics in POCT will be how to maintain the features of microfluidics, such as complex fluid handling and integrated analysis, without cumbersome ancillary equipment.
This challenge has proven to be a major drawback of many otherwise promising microfluidic devices for POCT.6,11–13 Micropumping is one of the most fundamental components in microfluidics, and active micropumping methods using cumbersome external equipment (i.e., syringe pumps and pressure pumps) are still widely seen in microfluidic devices.11,14
There are several general considerations in determining whether a micropumping method used in microfluidics is suitable for POCT: (1) it needs to be a standalone component and meet the requirements of liquid control; (2) it should require as few user interventions and as low a volume of sample liquid as possible; (3) it should be made as cheap as possible while retaining robust performance under various conditions. In microfluidics, micropumping methods can be classified into active or passive types, depending on whether an external power source is needed.14 However, in practice, it is not always clear whether to define a power supply as an external power source or not. The key to this classification is whether the power source can be easily obtained in a low-resource environment. For example, many micropumping methods are based on magnetism. If the device needs to be plugged into a socket to generate the magnetic force, the power source is considered an external one and the method will be classified in the active micropumping category. If only a hand-held or embedded magnet is required, the method does not need an external power source and belongs to the passive micropumping category. Thus, two methods may be classified differently even though the mechanism behind them is same, that is, both are based on magnetism. For active micropumping, the external power source is controlled so that it forms a continuous flow with a controllable flow rate.14,15 However, much progress has been made in making external power sources portable to fit POCT. Examples include sources based on mechanical displacement,16 centripetal force,17 electrical18 or magnetic fields,19,20 and acoustic force.21 The requirement for external power sources inevitably increases the complexity and cost of microfluidic devices.6 However, no external power source is needed for passive micropumping; the source is usually embedded in the device itself. Passive micropumping usually lacks capacity for accurate flow control;22,23 however, it is used in most commercially available POCT products owing to its simplicity.5–7 Much research has focused on improving flow control in passive micropumping,24–27 and this is likely to play an increasingly important part in POCT.28 Therefore, we narrow the scope of this review to passive micropumping and its development for use in microfluidics, as this seems most relevant to POCT. There are already many excellent reviews on microfluidics in POCT and micropumps in microfluidics;1,14–16,29–33 however, to the best of our knowledge, none of them focus on passive micropumping of microfluidics for POCT. Thus, in this paper, we summarize the major categories of passive micropumps suitable for POCT, as illustrated in Fig. 1. We also state our opinions about the future directions of passive micropumps for POCT.
FIG. 1.
Categories of passive micropumping methods that are suitable for POCT.
II. PASSIVE MICROPUMPING UTILIZING CAPILLARY FORCE
Micropumps utilizing capillary force are probably the most successful and widely used micropumping method.14,15,34 This method is quite robust and easy to use, and it does not require any moving components. This type of micropumping is usually only concerned with the filling flow; once the device is filled with liquid, the micropumping will stop. There are two types of micropumps utilizing capillary force according to the type of substrate material: (1) capillaries based on porous materials35 and (2) capillaries based on solid materials36,37 (Fig. 2).
FIG. 2.
Examples of passive micropumping based on capillary force. (a) Passive micropumping utilizing capillaries based on porous materials. (a-1) and (a-2) Paper-based 3D microfluidic device. (a-3) Multi-inlet/single-outlet design based on micropatterned superhydrophobic textile. (a-4) Cotton yarn knots used as microfluidic splitters, mixers, and for controlling mixing ratios. (b) Passive micropumping utilizing capillaries based on solid materials. (b-1) Closed-channel microfluidic device made from silicon for multiparametric immunoassays. (b-2) Suspended-microfluidic device made from plasma-treated PDMS. (b-3) Open-microfluidic device made from poly(methyl methacrylate) (PMMA). (a-1) and (a-2) Reproduced with permission from Martinez et al. Proc. Natl Acad. Sci. U.S.A. 105, 19606–19611 (2008). Copyright 2008 National Academy of Sciences; (a-3) Reproduced with permission from Xing et al., Lab Chip 13(10), 1937–1947 (2013). Copyright 2013 Royal Society of Chemistry. (a-4) Reproduced with permission from Safavieh et al., Lab Chip, 11(15), 2618–2624 (2011). Copyright 2011 Royal Society of Chemistry. (b-1) Reproduced with permission from Gervais et al., Biosens. Bioelectron. 27, 64–70 (2011). Copyright 2011 Elsevier B.V. (b-2) Reproduced with permission from Casavant et al., Proc. Natl. Acad. Sci. U.S.A. 110, 10111–10116 (2013). Copyright 2013 National Academy of Sciences; (b-3) Reproduced with permission from Berry et al., Anal. Methods 11, 4528–4536 (2019). Copyright 2019 Royal Society of Chemistry.
A. Capillaries based on porous materials
Various porous materials including paper,38–42 wool,43 polyester,44 and cotton yarn45,46 have been exploited for capillary-based micropumping, as shown in Fig. 2(a). Based on the porosity and other characteristics of the materials used, the initial filling flow rate can be calculated as follows according to Washburn's equation,47 if evaporation, swelling of the materials, and external body forces such as gravity are not considered:45
| (1) |
| (2) |
where Porosity is the percentage of gaps that can be filled with liquid to the total volume of the porous material, A is the cross-sectional area of the flow path of the porous material, is the interfacial tension, is the contact angle between the liquid and the surface of the porous material used, D is the effective capillary diameter, is the viscosity of the liquid, and k is a mobility parameter, which is related to the characteristic of the materials and dimension of the flow path as illustrated by Eq. (1). The relationship has been validated in microfluidics using micro- and nanocapillaries, especially at short time-scales, which fits the scenario of POCT.
As predicted by Eq. (2), the flow rate of this micropumping method is not constant over time and is set during the design of the device, which means the flow rate is built-in once the devices are made. Also, depending on the porosity and dimensions of the material, the dead volume may vary considerably.35
B. Capillaries based on solid materials
Before the widespread availability of polymers such as polydimethylsiloxane (PDMS),48,49 microfluidics mainly relied on solid materials, including glass50 and silicon,51,52 adopting the appropriate fabrication technologies from microelectronics.53 By nature, both glass and silicon are hydrophilic and thus can be employed directly for capillary micropumping.54,55 Moreover, through either wet chemical56–58 or gas phase59 surface treatment, other solid materials such as polymers used for casting microfluidic devices can also be rendered hydrophilic.24 Figure 2(b) shows examples of capillaries based on solid materials.
Unlike capillaries based on porous materials, where the liquid will always wick in as long as the porous material is philic for the sample liquids, with capillaries based on solid materials this will only occur when the flow path is a small tubing or a closed channel.60 If the channel is open to the air or not fully closed, a criterion derived from Young's law needs to be met before the initial flow can occur,60
| (3) |
where is the perimeter of free or unbounded surfaces (where the liquid is open to air), is the perimeter of wetted surfaces at the cross-section plane of the channel (where the fluid front is), and is the contact angle between the liquid and the surface of the solid material. For example, for a rectangular channel of width w and height h but without a ceiling, the criterion of Eq. (3) can be written as
| (4) |
Therefore, if the channel width w is too large compared with the height h, the flow may not occur even if the solid material is hydrophilic for the sample liquid.
The flow rate for capillaries based on solid materials can be calculated using Eq. (2), as described earlier, although parameter k needs to be calculated differently for an open channel; it is derived by balancing the free energy of the system as the liquid advances with the hydrodynamic viscous friction.36 Thus, the calculation is usually more complicated compared with the case of closed channels or capillaries based on porous materials, but the relationship still holds.37
The flow rate for micropumping using capillaries based on solid materials is again not constant, as predicted by Eq. (2), and cannot be changed once the devices are made. However, there are advantages compared with capillaries based on porous materials: (1) the dead volume or the minimum required sample volume can be reduced substantially,61 (2) in a closed channel, contamination from outside can be minimized, (3) flow rates are more reproducible among devices owing to the more uniform surface.60
C. Challenges for passive micropumping utilizing capillary force
As mentioned previously, the biggest challenge for passive micropumping utilizing capillary is flow control and maintaining a constant flow rate.62 The major approach currently for micropumping based on capillary force is to vary the dimensions of flow pathways to gain more control of the flow.37,63 Using partial surface treatments to fine-tune the capillary force64 and smart designs,62,65,66 sequential flows can be achieved automatically in immunoassays24,37,67,68 and molecular tests69–72 (Fig. 3). Also, combinations of capillaries based on both porous materials and solid materials have been exploited:73–82 closed microfluidic channels are made with solid materials to take advantage of the more controllable and repeatable flow, whereas porous materials are used at the outlet to absorb more liquids, thereby extending the total duration of the micropumping (Fig. 4).
FIG. 3.
Examples of sequential flows enabled by micropumping based on capillary force. (a) Shaped-paper microfluidic device for multi-step sequential flows. (b) PDMS microfluidic device capable of pre-programmed sequential flows. (a) Reproduced with permission from Lutz et al., Lab Chip 11, 4274 (2011). Copyright 2011 Royal Society of Chemistry. (b) Reproduced with permission from Safavieh and Juncker, Lab Chip 13, 4180–4189 (2013). Copyright 2013 Royal Society of Chemistry.
FIG. 4.
Passive micropumping utilizing capillary force based on both porous and solid materials. (a) Microfluidic device using capillaries based on both solid and porous materials for patterning proteins and immunoassays. (b) Passive pumps to generate controllable whole blood flow using capillaries based on both solid and porous materials. (a) Reproduced with permission from Pla-Roca and Juncker, Biological Microarrays Methods and Protocols. Copyright 2011 Humana Press. Springer Science+Business Media, LLC. (b) Reproduced with permission from Sotoudegan et al., Lab Chip 19, 3787–3795 (2019). Copyright 2019 Royal Society of Chemistry.
However, in both cases, the flow rate is still not constant and cannot be changed once the devices are made. It should also be noted that evaporation may become increasingly important after the initial filling with liquids; evaporation is related to the open area of liquids, ambient temperature, and moisture level. Thus, it is quite difficult to characterize flow rates based on evaporation, although some attempts have been made to do so in micropumping83,84 and continuous flow.44,85
III. PASSIVE MICROPUMPING UTILIZING AIR TRANSFER
The foundation of micropumping utilizing air transfer is an appropriate material: such a material must have either good air solubility to store vacuum or high pressure, or good air permeability to allow vacuum or high pressure to be transferred to microfluidic channels in a controllable manner (Fig. 5). To be more specific, on the one hand, if the material used has good air solubility, the air dissolved inside this material can be degassed when it is placed in a vacuum and, once the air pressure of the environment increases, this degassed material can reabsorb the air around it. With proper design, it is possible to generate a vacuum inside the microfluidic channel, and vice versa for high pressure. Thus, vacuum or high pressure can be stored in the air soluble material and used later for micropumping. On the other hand, if the material has good air permeability, air trapped inside the microfluidic channel can diffuse into the adjacent pneumatic chamber where a vacuum is stored; thus, negative pressure is generated inside the microfluidic channel as well, and vice versa for high pressure. The rationale for adding an air-permeable membrane between the microfluidic channel and the stored pressure in the pneumatic chamber instead of directly connecting them is that the flow rate can be tuned by the geometry and air permeability of the membrane instead of adjusting the pressure levels. Also, as there is a maximum flux rate for air transferred through the membrane, further increase of the pressure difference above a certain value will not increase the flow rate. Thus, the membrane also functions as a flow regulator, damping the flow variations due to the fluctuation of pressure.86 There is already a review paper about vacuum based microfluidics using PDMS;87 here, we will focus more on recent progress and the more general concept of using any suitable material for air transfer-based passive micropumping.
FIG. 5.
Passive micropumping utilizing air transfer. (a) Air transfer based on air solubility. (b) Air transfer based on air permeability. Both (a) and (b) show general device configurations for vacuum-based micropumping and one for high-pressure-based micropumping.
A. Air transfer based on air solubility
For passive micropumping utilizing air solubility, if the whole microfluidic device is made of a material with good air solubility, such as PDMS, it can itself function as a self-powered vacuum or high-pressure source. Normally, the device is degassed in a vacuum chamber for the appropriate time (>15 min) before usage to ensure dissolved air is driven out. Once the degassed device is exposed to air again, it starts to reabsorb air from all surfaces, including embedded microfluidic channels, resulting in a vacuum that will draw sample liquids along the microfluidic channels. The flow rate depends on the geometry of the device, including the channel surface area and the volume of the device, as well as the waiting time before liquids are added and the total time since the device was exposed to the atmosphere,87 and the same effects are also applied if high pressure is stored in the device.88 Using Fick's second law of diffusion,88 we can obtain an approximation of the flow rate, assuming the sample liquid is added immediately after the degassed or pressurized device is exposed to air and the flow is on a microfluidic scale,
| (5) |
where S is the total surface area of the device over which the air can diffuse, w is the distance between the surface of the device and the embedded microfluidic channel, and is a time constant related to the geometry of the microfluidic channel and the air diffusion coefficient of the specific material used.
As illustrated by Eq. (5), the flow rate decays immediately after the device is exposed to the atmosphere, which makes it difficult to achieve a stable and constant flow. Moreover, the waiting time before liquids are added depends on the operator and will inevitably vary, leading to a variable flow rate among tests. Thus, the major applications of micropumping based on air solubility are in cases where a constant or repeatable flow is not critical. Here, we have summarized recent progress about this type of passive micropumping. Tottori and Nisisako89 reported a degassing-driven microfluidic device with deterministic lateral displacement, which had two different configurations. The first configuration was a single-input device; the characteristics of the degassing-driven flow through micro-pillars were investigated, and it was used for selective enrichment of fluorescent polymer particles based on sizes. The second configuration was a sheath-input device, which was successfully used for separation of white blood cells from red blood cells, demonstrating a high separation efficiency of ∼96% and purity of ∼87% [Fig. 6(a-1)]. Shin et al.90 also used an air solubility-based PDMS micropump for plasma separation [Fig. 6(a-2)]. The proposed device functioned as a fully integrated microfluidic diagnostic device with an integrated pneumatic microfluidic circuit. It could pump whole blood autonomously, sort blood plasma simultaneously, and enable blood plasma proteomic analysis on-site by quantifying thrombin in blood samples using an aptamer beacon within 5 min of sample injection. Fu et al.91 reported a device utilizing micropumping based on air solubility to fill an array of microcompartments for the digital Polymerase Chain Reaction (dPCR). The chip was constructed by sandwiching a PDMS layer containing 10 000 microcompartments of 0.785 nl each between two glass slides. Chatzimichail et al.92 reported a new fabrication process by which metal electrode patterning could be achieved by vacuum-filling microfluidic channel geometries with liquid metals. Liu et al.88 demonstrated a microfluidic device that used high pressure stored in PDMS for micropumping [Fig. 6(b)]. Compared with micropumping based on vacuumed PDMS, it could generate a higher flow rate, as the maximum pressure difference was no longer limited to one atmosphere as is the case for vacuum-based micropumping. A viscometer based on micropumping utilizing pressurized PDMS was also demonstrated in that paper.
FIG. 6.
Passive micropumping utilizing air transfer based on air solubility of the materials. (a) Microfluidic devices utilizing vacuum-based micropumping based on air solubility. (a-1) Microfluidic device using degassing-driven deterministic lateral flow for particle separation. (a-2) Blood analysis device employing passive micropumping based on the air solubility of PDMS. (b) Positive pressure-driven micropump based on the air solubility of PDMS. (a-1) Reproduced with permission from Tottori and Nisisako, Anal. Chem. 91, 3093–3100 (2019). Copyright 2019 American Chemical Society. (a-2) Reproduced with permission from Shin et al., Biosens. Bioelectron. 133, 169–176 (2019). Copyright 2019 Elsevier B.V. (b) Reproduced with permission from Gervais et al., Biosens. Bioelectron. 27, 64–70 (2011). Copyright 2011 Springer-Verlag GmbH Germany.
Gas solubility-based PDMS micropumps gradually lose their pumping ability once the degassed PDMS is exposed to the air. For instance, a micropump based on this method was reported to only last for around 60 min after air exposure in a typical device made of PDMS.93 Thus, there is a need to increase the duration of micropumping. Liu et al.93 reported on using wax coatings on the surface of PDMS to increase the duration of micropumping. In their design, only the inlets of the device were exposed to the atmosphere. The encapsulated devices retained their micropumping ability for more than 3 weeks without any vacuum source or additional packaging. Song et al.94 demonstrated another method using Parylene C as the coating layer. Compared with wax, Parylene C has much lower air permeability. Thus, the coated device was able to maintain its micropumping ability for more than 30 days. Moreover, the vacuum could be directly stored in a sealed pouch containing the microfluidic device.95 In this case, the sample liquid is added on top of sealed inlets and then a needle is used to pierce holes at the inlets through the sample liquid. Thus, the vacuum in the microfluidic channels will draw the flow toward the outlets. As the whole pouch is used for vacuum storage, the material of the microfluidic device is no longer required to have good air solubility and, as no surface is exposed to air during the flow process, the duration of the vacuum-based micropumping will be theoretically infinite or last until the whole device is filled with liquid. However, the flow rate will still not be constant and, depending on the timing of hole piercing among different inlets, variations may still exist between tests.
B. Air transfer based on air permeability
As discussed in Sec. III A, passive micropumping based on air solubility leads to various problems, including inconsistent and variable flow rates among tests. One way to solve these problems is micropumping utilizing air transfer based on air permeability. For this type of micropumping, a thin membrane made of a material with good air permeability, such as PDMS, is used to allow air transfer across the membrane. Generally, the microfluidic channels are on one side of the membrane, and on the other side is a pneumatic chamber with a vacuum or high pressure. If a vacuum is stored in the pneumatic chamber, air will diffuse from the microfluidic channel through the membrane into the vacuum chamber, and micropumping based on vacuum occurs (and vice versa if high pressure is stored in the pneumatic chamber). The flow rate of this type of micropumping can be deduced from Fick's law,96
| (6) |
where k is an empirical factor including the viscous effect of the pumped liquid flow; is the steady-state air flux diffusing into the vacuum chamber; and are the air concentrations in the membrane and the pneumatic chamber, respectively;is the air concentration of the atmosphere; is the total surface area that allows air to diffuse out of the microfluidic channels, denoted the diffusion area; and w is the thickness of the membrane between the microfluidic channel and the pneumatic chamber. Note that here the volume of the microfluidic channels is negligible compared with that of the pneumatic chamber used for pressure storage, and thus the amount of air transferred from the microfluidic channels to the pneumatic chamber is also negligible. As a result, and can be considered constant during the micropumping process. Therefore, as predicted by Eq. (6), if the diffusion area S and membrane thickness w are fixed, the flow rate Q is constant most of the time during liquid filling of the microfluidic channels.97
There are various ways to store pressure in the pneumatic chamber at one side of the membrane; here, we summarized recent progress in this area. Wang et al.98 reported an ad hoc type of micropumping based on a vacuum, in which a separated pneumatic chamber with embedded PDMS membrane could be added on top of the microfluidic outlet [Fig. 7(a)]. The flow rate was constant most of the time during sample liquid filling, and this micropumping method could be easily adapted for use in almost any type of microfluidic device. Wu et al.99 demonstrated a microfluidic PCR device utilizing micropumping based on high pressure, with a gas-permeable conduit as the membrane [Fig. 7(b)]. Note that here the flow rate is not constant and decreases with the decreases of the high pressure generated in the pneumatic chamber; in this case, the syringe is designed to leak from the conduit as well. A spiral channel with radius reducing from outer circles to inner circles is used, so that the flow takes the same amount of time to fill each circle. Therefore, PCR based on thermocycling can be realized.
FIG. 7.
Passive micropumping utilizing air transfer based on the air permeability of the materials. (a) Vacuum-driven micropumping device based on the air permeability of PDMS membrane. (b) High pressure-driven micropumping device based on the air permeability of a silicone tubing for PCR. (a) From Wang et al., Micromachines 10, 543 (2019) Copyright 2012 Author(s), licensed under a Creative Commons Attribution (CC BY) license; (b) Reproduced with permission from Wu et al., Analyst 137, 983–990 (2012). Copyright 2012 Royal Society of Chemistry.
Similar to the case of micropumping using air solubility, the pneumatic chamber for pressure storage in this type of micropumping needs to be sealed properly to prevent loss of pressure in long-term storage. However, by employing a hand-held syringe,97–99 the packaging step can be avoided. Moreover, this type of micropumping does not suffer problems such as short pumping duration and variable flow rates. Most important of all, as predicted by Eq. (6), the flow rate for this type of passive micropumping is constant most of the time during liquid filling of the microfluidic channels, which is difficult to achieve in other types of passive micropumping.
C. Challenges for passive micropumping utilizing air transfer
As discussed previously, in micropumping based on air solubility, the flow rate is not constant and is difficult to control. This is similar to the problems encountered in micropumping based on capillary force. The advantage of air transfer methods is that the surface does not need to be hydrophilic, which is important when PDMS is used as the building material. However, an additional degassing step is required, as is suitable packaging to prevent pressure leakage during long-term storage. These problems can be solved by micropumping based on air permeability, especially when a portable pressure source like a hand-held syringe is used. As illustrated by Eq. (6), the flow rate is constant and can be easily adjusted by varying the diffusion area or the thickness of the membrane. One drawback, which is also quite common in passive micropumping, is that the flow rate is set once the devices are made. Moreover, as predicted by Eq. (6), the flow rate is not the same for all types of liquids; it varies based on properties of the liquids such as viscosity and surface tension.
IV. PASSIVE MICROPUMPING UTILIZING PRESSURE DIRECTLY WITHOUT AIR TRANSFER
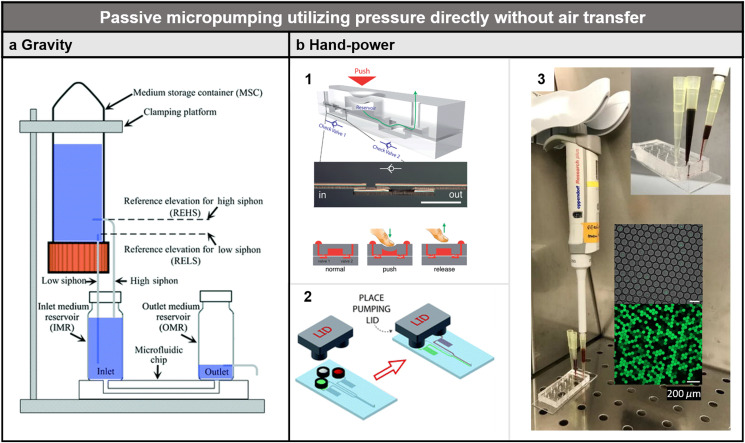
Besides the previously discussed methods that utilize air transfer to provide a controllable flow rate, pressure can be used directly for passive micropumping based on the gravity of liquids, surface tension of liquids, a pressure source stored in a sealed chamber such as a balloon, and pressure applied by the user's hands (Fig. 8).
FIG. 8.
Passive micropumping utilizing pressure directly without air transfer. (a) Microfluidic device using hydrostatic pressure-driven micropumping. (b) Microfluidic devices based on hand power for passive micropumping. (b-1) Finger-controlled microfluidic flow network device. (b-2) Pumping lid made with 3D printing to generate positive and negative pressures for microfluidic applications. (b-3) Micropipette-powered droplet-based microfluidics. Scale bar is 200 μm. (a) Reproduced with permission from Wang et al., Lab Chip 18, 2167–2177 (2018). Copyright 2018 Royal Society of Chemistry. (b-1) Reproduced with permission from Li et al., Lab Chip 12, 1587–1590 (2012). Copyright 2012 Royal Society of Chemistry. (b-2) Reproduced with permission from Begolo et al., Lab Chip 14, 4616–4628 (2014). Copyright 2014 Royal Society of Chemistry. (b-3) Reproduced with the permission from Langer et al., Biomicrofluidics 12(4), 044106 (2018). Copyright 2018 AIP Publishing LLC.
Gravity exists everywhere and it is quite intuitive to employ gravity of liquids or hydrostatic pressure directly for passive micropumping. The challenge is that for microfluidics, the volume of samples is usually quite small (nanoliter- to microliter-scale), which usually makes the gravity of liquids negligible compared with other forces such as surface tension. However, by smart design or combination with other methods, gravity has been exploited for passive micropumping: by fine-tuning the height and increasing the volume of liquid at the inlet, various flow rates can be achieved100–102 [Fig. 8(a)]. Similarly, by adjusting the tilting angle of the microfluidic device, different flow rates can be generated; researchers have tried to use this approach for immunoassays.103 Moreover, gravity has been combined with capillary force in immunoassays such as ELISA, using a standard 96-well format.104
Surface tension, which depends on the size of the droplet, can also be utilized for passive micropumping:105,106 if two droplets are connected by a closed microfluidic channel filled with liquid, a droplet with a smaller diameter will have a higher surface tension compared with a larger droplet at the other end of the channel. This difference in surface tension between the droplets will drive the flow from the smaller droplet to the larger one. Although the pumping force generated in this way is relatively small, it has been used for cell culturing.107
Last but not the least, smart designs enable the user to apply pressure for passive micropumping using their hand [Fig. 8(b)]. SlipChip, where liquids are moved manually by sliding two glass chips containing microchambers against each other, has been demonstrated to be a powerful tool for immunoassays, molecular tests, and various chemical tests.108–110 Similarly, “squeeze-chip,” where the flow is powered by using the fingers to push a soft microfluidic chamber, has been used for complicated flow control111 [Fig. 8(b-1)]. Even squeezing a balloon filled with air as a high-pressure source can achieve controllable flow in microfluidic channels.112 Besides high pressure, a vacuum can also be generated to withdraw flow by manually compressing a rubber chamber with a one-way passive valve.113 Micropumping based on either vacuum or high pressure has been demonstrated by pulling or pushing a three-dimensional (3D)-printed lid of an air-tight chamber114 [Fig. 8(b-2)]. Micropipettes can also be used to make droplets directly without the need for sophisticated pumps115 [Fig. 8(b-3)].
V. PASSIVE MICROPUMPING UTILIZING OTHER METHODS
Besides the previously discussed methods, there are many other passive micropumping methods based on various mechanisms. In general, these methods are not as good as those described above, but they have their own niches, and they may inspire readers to consider new ways of achieving passive micropumping for POCT.
One example is micropumping utilizing chemicals, as described in some good review papers29,116 [Fig. 9(a)]. Here, we only summarize the chemistry-based passive micropumping methods that are suitable for POCT. Good examples include micropumping methods based on diffusion and osmosis pressure, where chemicals are embedded directly in the flow channels.117,118 Catalysts and enzymes can also be employed to further enhance the flow119–123 [Fig. 9(a-1)]. Alternatively, if the stored chemicals react and generate heat under certain conditions, they can be used to form thermogradients to drive a circulating flow between the heat source and the cold end.124,125 If the chemical reactions can generate gas, they can be used as a high-pressure source to push the flow of sample liquids forward126–128 [Fig. 9(a-2)]. As well as the chemical-based methods mentioned here, there is a good review of micropumping using gas bubbles.129 In addition, if electricity can be generated at the same time as either heat or gas, as in the case of fuel cells, then, electrical measurements and analysis can be performed as well as micropumping, using the electrical power generated130,131 [Fig. 9(a-3)]. Similarly, electrophoresis, electroosmosis, and even electrowetting based on batteries can also be considered to be micropumping methods utilizing chemicals, but they are not passive methods. Some good existing review papers cover these areas.18,132 The general requirements for this type of micropumping to be suitable for POCT are: (1) the chemicals are safe and remain stable before use and (2) the chemicals can be packed small enough to fit in the devices.
FIG. 9.
Passive micropumping utilizing other methods. (a) Microfluidic devices based on chemistry for passive micropumping. (a-1) Self-organization of fluids in a multi-enzymatic pump system. (a-2) Chemical reaction-based micropump that uses gas for portable microfluidics. (a-3) Fuel cell-powered microfluidic platform that uses chemical reaction for micropumping based on gas and measurements based on electricity. (b) Microfluidic devices using a magnet for passive micropumping. (b-1) 3D-printed microfluidic preconcentrator using magnetic force. (b-2) Microfluidic device based on magnetophoretic control of water droplets in bulk ferrofluid. (a-1) Reproduced with permission from Maiti et al., Langmuir 35, 3724–3732 (2019). Copyright 2019 American Chemical Society. (a-2) Reproduced with permission from Good et al. Lab Chip 6, 659–666 (2006). Copyright 2006 Royal Society of Chemistry. (a-3) Reproduced with permission from Esquivel et al., Lab Chip 12, 74–79 (2012). Copyright 2012 Royal Society of Chemistry. (b-1) Reproduced with permission from Park et al., J. Microbiol. Methods 132, 128–133 (2017). Copyright 2017 Elsevier B.V. (b-2) Reproduced with permission from Katsikis et al., Soft Matter 14, 681–692 (2018). Copyright 2018 Royal Society of Chemistry.
Another example is passive micropumping using a magnetic field [Fig. 9(b)]. If the sample liquids are premixed with magnetic particles, a magnet can be placed along the flow pathway to attract magnetic particles, which in turn will drag the liquid flow forward133,134 [Fig. 9(b-1)]. If the carrier phase is made with ferrofluid oil and is immiscible with sample liquids, the movement of the ferrofluid oil can be used for sample liquid control135 [Fig. 9(b-2)]. Alternatively, a magnet can be moved manually or automatically to control the movement of liquids, as described in two good review papers.136,137
VI. DISCUSSION AND OUTLOOK
Table I gives a brief comparison of the major passive micropumping methods. Numerous other methods have been designed and tested for POCT, as discussed in Sec. IV. Compared with active pumping methods such as syringe pumps or pressure pumps, where the flow rate can be well-tuned independent of the design of the microfluidic devices or the property of the liquids, most passive micropumping methods still suffer flow-control problems. For example, the flow rate may be set once the device has been made, and the properties of liquids may affect the flow rate. However, the advantages of passive micropumping, which include simplicity, ease of use, and low cost, make it the best choice for POCT. In this section, we summarize important aspects of the applications of passive micropumping for POCT and provide a brief overview of the outlook.
TABLE I.
Comparison of passive micropumping methods utilizing capillary force, air transfer based on air solubility and air permeability, and direct pressure without air transfer, respectively.
| Passive micropumping method | Integration | Operation | Flow rate control | Constant flow |
|---|---|---|---|---|
| Capillary force | Easy | Easy | Hard | No |
| Air solubility | Easy | Easy | Medium | No |
| Air permeability | Medium | Easy to medium | Easy | Yes |
| Direct pressure | Medium | Medium | Medium | No |
A. Backflow prevention at inlets
Backflow is a unique problem that occurs when small volumes of different types of liquids are loaded directly at more than two inlets of a microfluidic device. Besides backflow, flow crosstalk and asynchronous pumping may also occur. All of these problems are caused by the pressure difference at each inlet, which depends on the properties of liquids, including volume loaded, surface tension, viscosity, and density.138,139 Based on the equations for surface tension and gravity, the built-in pressure of a liquid at a specific inlet can be written as139
| (7) |
where is the surface tension of the liquid loaded at the inlet, h is the height of the convex meniscus of the liquid at the inlet, and r is the radius of the spherical cap of the liquid, which can be assumed to be equal to the radius of the inlet reservoir. As predicted by Eq. (7), in order to prevent backflow, a pressure balancing design is needed to achieve reliable sample loadings in passive micropumping. Kim et al.140 reported pressure-balancing structures that directly connected two sample inlets with a tiny bridge structure. Zhai et al.141 utilized capillary-driven flow for pressure balance and introduced a robust micromixing approach for micropumping with a stored vacuum. The robust sample loading and portable micropumping of the device were verified by its ability to deal with both similar liquids such as water samples and dissimilar liquids such as simulated blood and antibody serum for blood type testing. Wu et al.142 reported a pressure balance scheme in which oils were directly applied to every sample inlet to offset pressure differences without affecting samples. Their work used a silicone oil-based pressure balancing strategy to ensure identical and stable gradient formation in each of eight chemotaxis units.
B. Flow control
The flow rate usually varies with the type of liquid, depending on the property for passive micropumping. Smart designs can be exploited to reduce this variation. Il et al.143 reported a good example, in which the microfluidic channel had a variable fluidic resistance depending on the pressure of the micropumping through a passive microfluidic structure. If the flow rate is too high, the fluidic resistance of the channel will increase, which in turn will reduce the flow rate, and vice versa. Based on similar ideas, various flow regulators for microfluidic devices have been designed.144–146 Interestingly, adding passive pillar structures in microfluidic channels was sufficient to control flow rates of two streams following Braess's paradox, independent of pumping pressure.147
C. Durability
To fulfill the ASSURED criteria for POCT, the durability of devices must be considered. Especially for devices that rely on complex chemical assays, proper fabrication and storage are essential to maintain functional assays and pumping mechanisms for field applications. Reagent degradation is one of the main causes of unreliable test results, and it is largely dependent on factors such as light exposure, storage temperature, moisture, and chemical additives. Previous successful applications such as glucose meters and pregnancy test strips have demonstrated techniques that the passive pumping microfluidic community could adopt. These techniques include printing and laminating to prevent light or moisture exposure, surface chemistry and substrates for accurate signal generation, chemical stabilizers for maintaining reagent function, and selection of proper signal readout methods.148 Of course, innovations have already been introduced to increase the durability of chemical reagents in microfluidics. For example, SHERLOCK (Specific High-Sensitivity Enzymatic Reporter UnLOCKing), a CRISPR (clustered regularly interspaced short palindromic repeats) lateral flow microfluidic platform for nucleic acid detection, used freeze-drying in liquid nitrogen to immobilize reaction reagents on the device instead of using conventional spray-drying to ensure the stability of the reagents.149,150 This freeze-drying technology also enabled cold-chain independence and long-term storage. This platform has been successfully used for detection of the Zika virus and dengue virus in patient samples in resource-limited areas.151
Different passive micropump-based devices also require specific storage conditions to maintain their pumping functions. Especially for self-powered devices based on the air solubility property of PDMS, the degassed or pressurized PDMS must be sealed to retain its function until use. For example, the SIMPLE (self-powered integrated microfluidic point-of-care low-cost enabling) chip, developed by Luke Lee's group, must be sealed in aluminum vacuum packs immediately after fabrication and vacuum charged.152 The SIMPLE chips could pump liquid normally after being packed in vacuum seals for at least 3 years.
D. Multiphase microfluidics
Unlike continuous flow systems, multiphase microfluidics or droplet microfluidics focus on creating tiny discrete volumes with the use of immiscible phases. Droplet microfluidics enables a large number of reactions to be carried out in parallel within nanoliter- to femtoliter-scale droplets. Its application in single-cell analysis153,154 and digital detection155 has revolutionized chemical and biological studies. However, the majority of multiphase microfluidics devices are operated using active external controls, such as a syringe pump,156 centrifugation,157 pressure,158 dielectric electrowetting,159 or pneumatic valves.160 Some progress has been made in the use of passive micropumping for droplet generation. For example, Kim et al.161 reported the generation of droplets using the gas permeability of PDMS. They successfully generated droplets in approximately 180 min, with a maximum volumetric rate of 14 nl/s and a droplet generation rate of 6 Hz at 45 min when the microfluidic device was degassed for 1 h. Figure 8(b) shows another example of passive micropumps in multiphase microfluidics, where micropipettes are used to utilize pressure directly without air transfer. The throughput and uniformity of the generated droplet are similar to those obtained with traditional pressure pumping methods. However, the challenge here is not only how to generate droplets passively but also how to use the passively generated droplets for applications without an external power source. In digital PCR155 and single-cell RNA sequencing,153,154 where droplet microfluidics is the foundation, the analysis of droplets requires an external power source to generate heat precisely or to read out the signal with complicated setups. To the best of our knowledge, there are few reports on how to use droplets passively for POCT applications. Researchers focusing on passive micropump-based microfluidics are encouraged to devote more efforts to bring multiphase microfluidics into the area of POCT.
AUTHORS’ CONTRIBUTIONS
L.X. and K.W.O. conceived of the idea and outline of the paper. L.X., A.W., and X.L. wrote the manuscript. A.W. and X.L. contributed to the writing of Secs. III, IV, and VI. All authors read and approved the manuscript.
ACKNOWLEDGMENTS
We thank AIP Publishing for editing our review paper.
Contributor Information
Linfeng Xu, Email: .
Kwang W. Oh, Email: .
DATA AVAILABILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Sia S. K. and Kricka L. J., “Microfluidics and point-of-care testing,” Lab Chip 8(12), 1982–1983 (2008). 10.1039/b817915h [DOI] [PubMed] [Google Scholar]
- 2.Wang P. and Kricka L. J., “Current and emerging trends in point-of-care technology and strategies for clinical validation and implementation,” Clin. Chem. 64(10) 1439–1452 (2018). 10.1373/clinchem.2018.287052 [DOI] [PubMed] [Google Scholar]
- 3.Yager P. et al. , “Microfluidic diagnostic technologies for global public health,” Nature 442(7101), 412–418 (2006). 10.1038/nature05064 [DOI] [PubMed] [Google Scholar]
- 4.David Issadore R. M. W., “Point-of-care diagnostics on a chip,” in Biological and Medical Physics, Biomedical Engineering (Springer, Berlin, 2013). [Google Scholar]
- 5.Gervais L., De Rooij N., and Delamarche E., “Microfluidic chips for point-of-care immunodiagnostics,” Adv. Mater. 23(24), H151–H176 (2011). 10.1002/adma.201100464 [DOI] [PubMed] [Google Scholar]
- 6.Chin C. D., Linder V., and Sia S. K., “Commercialization of microfluidic point-of-care diagnostic devices,” Lab Chip 12(12), 2118–2134 (2012). 10.1039/c2lc21204h [DOI] [PubMed] [Google Scholar]
- 7.Vashist S. K., “Point-of-care diagnostics: Recent advances and trends,” Biosensors 7(4), 62 (2017). 10.3390/bios7040062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buser J. R., Holstein C. A., and Yager P., “Microfluidic diagnostics for Low-resource settings: Improving global health without a power cord,” in Microfluidics for Medical Applications (The Royal Society of Chemistry, 2015), Chap. 8, pp. 151–190. [Google Scholar]
- 9.Chin C. D., Linder V., and Sia S. K., “Lab-on-a-chip devices for global health: Past studies and future opportunities,” Lab Chip 7(1), 41–57 (2007). 10.1039/B611455E [DOI] [PubMed] [Google Scholar]
- 10.Chen H. et al. , “Point of care testing for infectious diseases,” Clin. Chim. Acta 493, 138–147 (2019). 10.1016/j.cca.2019.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nayak S. et al. , “Integrating user behavior with engineering design of point-of-care diagnostic devices: Theoretical framework and empirical findings,” Lab Chip 19(13), 2241–2255 (2019). 10.1039/C9LC00188C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St John A. and Price C. P., “Existing and emerging technologies for point-of-care testing,” Clin. Biochem. Rev. 35(3), 155–167 (2014). [PMC free article] [PubMed] [Google Scholar]
- 13.Hitzbleck M. and Delamarche E., “Reagents in microfluidics: An “in” and “out” challenge,” Chem. Soc. Rev. 42(21), 8494 (2013). 10.1039/c3cs60118h [DOI] [PubMed] [Google Scholar]
- 14.Laser D. J. and Santiago J. G., “A review of micropumps,” J. Micromech. Microeng. 14(6), R35–R64 (2004). 10.1088/0960-1317/14/6/R01 [DOI] [Google Scholar]
- 15.Wang Y.-N. and Fu L.-M., “Micropumps and biomedical applications—A review,” Microelectron. Eng. 195, 121–138 (2018). 10.1016/j.mee.2018.04.008 [DOI] [Google Scholar]
- 16.Das P. K. and Hasan A. B. M. T., “Mechanical micropumps and their applications: A review,” AIP Conf. Proc. 1851(1), 020110 (2017). 10.1063/1.4984739 [DOI] [Google Scholar]
- 17.Strohmeier O. et al. , “Centrifugal microfluidic platforms: Advanced unit operations and applications,” Chem. Soc. Rev. 44(17), 6187–6229 (2015). 10.1039/C4CS00371C [DOI] [PubMed] [Google Scholar]
- 18.Samiei E., Tabrizian M., and Hoorfar M., “A review of digital microfluidics as portable platforms for lab-on a-chip applications,” Lab Chip 16(13), 2376–2396 (2016). 10.1039/C6LC00387G [DOI] [PubMed] [Google Scholar]
- 19.Yang J. et al. , “Detection platforms for point-of-care testing based on colorimetric, luminescent and magnetic assays: A review,” Talanta 202, 96–110 (2019). 10.1016/j.talanta.2019.04.054 [DOI] [PubMed] [Google Scholar]
- 20.den Dulk R. C. et al. , “Magneto-capillary valve for integrated purification and enrichment of nucleic acids and proteins,” Lab Chip 13(1), 106–118 (2013). 10.1039/C2LC40929A [DOI] [PubMed] [Google Scholar]
- 21.Schmid L. et al. , “Novel surface acoustic wave (SAW)-driven closed PDMS flow chamber,” Microfluid. Nanofluid. 12(1–4), 229–235 (2012). 10.1007/s10404-011-0867-5 [DOI] [Google Scholar]
- 22.Nabavi M., “Steady and unsteady flow analysis in microdiffusers and micropumps: A critical review,” Microfluid. Nanofluid. 7(5), 599–619 (2009). 10.1007/s10404-009-0474-x [DOI] [Google Scholar]
- 23.Noh H. and Phillips S. T., “Metering the capillary-driven flow of fluids in paper-based microfluidic devices,” Anal. Chem. 82(10), 4181–4187 (2010). 10.1021/ac100431y [DOI] [PubMed] [Google Scholar]
- 24.Safavieh R. and Juncker D., “Capillarics: Pre-programmed, self-powered microfluidic circuits built from capillary elements,” Lab Chip 13(21), 4180–4189 (2013). 10.1039/c3lc50691f [DOI] [PubMed] [Google Scholar]
- 25.Lutz B. R. et al. , “Two-dimensional paper networks: Programmable fluidic disconnects for multi-step processes in shaped paper,” Lab Chip 11(24), 4274 (2011). 10.1039/c1lc20758j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gökçe O. et al. , “Self-coalescing flows in microfluidics for pulse-shaped delivery of reagents,” Nature 574(7777), 228–232 (2019). 10.1038/s41586-019-1635-z [DOI] [PubMed] [Google Scholar]
- 27.Jung W. et al. , “An innovative sample-to-answer polymer lab-on-a-chip with on-chip reservoirs for the POCT of thyroid stimulating hormone (TSH),” Lab Chip 13(23), 4653–4662 (2013). 10.1039/c3lc50403d [DOI] [PubMed] [Google Scholar]
- 28.Derda R. et al. , “Enabling the development and deployment of next generation point-of-care diagnostics,” PLoS Neglect. Trop. D. 9(5), e0003676 (2015). 10.1371/journal.pntd.0003676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou C. et al. , “Chemistry pumps: A review of chemically powered micropumps,” Lab Chip 16(10), 1797–1811 (2016). 10.1039/C6LC00032K [DOI] [PubMed] [Google Scholar]
- 30.Iverson B. D. and Garimella S. V., “Recent advances in microscale pumping technologies: A review and evaluation,” Microfluid. Nanofluid. 5(2), 145–174 (2008). 10.1007/s10404-008-0266-8 [DOI] [Google Scholar]
- 31.Lagally E., Microfluidics and Nanotechnology Biosensing to the Single Molecule Limit (CRC Press, 2014). [Google Scholar]
- 32.Tsai N.-C. and Sue C.-Y., “Review of MEMS-based drug delivery and dosing systems,” Sens. Actuators A 134(2), 555–564 (2007). 10.1016/j.sna.2006.06.014 [DOI] [Google Scholar]
- 33.De Volder M. and Reynaerts D., “Pneumatic and hydraulic microactuators: A review,” J. Micromech. Microeng. 20(4), 043001 (2010). 10.1088/0960-1317/20/4/043001 [DOI] [Google Scholar]
- 34.Zimmermann M. et al. , “Capillary pumps for autonomous capillary systems,” Lab Chip 7(1), 119–125 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Berejnov V., Djilali N., and Sinton D., “Lab-on-chip methodologies for the study of transport in porous media: Energy applications,” Lab Chip 8(5), 689 (2008). 10.1039/b802373p [DOI] [PubMed] [Google Scholar]
- 36.Yang D. et al. , “Dynamics of capillary-driven flow in open microchannels,” J. Phys. Chem. C 115(38), 18761–18769 (2011). 10.1021/jp2065826 [DOI] [Google Scholar]
- 37.Olanrewaju A. et al. , “Capillary microfluidics in microchannels: From microfluidic networks to capillaric circuits,” Lab Chip 18(16), 2323–2347 (2018). 10.1039/C8LC00458G [DOI] [PubMed] [Google Scholar]
- 38.Yetisen A. K., Akram M. S., and Lowe C. R., “Paper-based microfluidic point-of-care diagnostic devices,” Lab Chip 13(12), 2210–2251 (2013). 10.1039/c3lc50169h [DOI] [PubMed] [Google Scholar]
- 39.Tian T. et al. , “Integrated paper-based microfluidic devices for point-of-care testing,” Anal. Methods 10(29), 3567–3581 (2018). 10.1039/C8AY00864G [DOI] [Google Scholar]
- 40.Yamada K. et al. , “Toward practical application of paper-based microfluidics for medical diagnostics: State-of-the-art and challenges,” Lab Chip 17(7), 1206–1249 (2017). 10.1039/C6LC01577H [DOI] [PubMed] [Google Scholar]
- 41.Holstein C. A. et al. , “Immobilizing affinity proteins to nitrocellulose: A toolbox for paper-based assay developers,” Anal. Bioanal. Chem. 408(5), 1335–1346 (2016). 10.1007/s00216-015-9052-0 [DOI] [PubMed] [Google Scholar]
- 42.Martinez A. W., Phillips S. T., and Whitesides G. M., “Three-dimensional microfluidic devices fabricated in layered paper and tape,” Proc. Natl Acad. Sci. U.S.A. 105(50), 19606–19611 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nilghaz A., Ballerini D. R., and Shen W., “Exploration of microfluidic devices based on multi-filament threads and textiles: A review,” Biomicrofluidics 7(5), 051501 (2013). 10.1063/1.4820413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lynn N. S. and Dandy D. S., “Passive microfluidic pumping using coupled capillary/evaporation effects,” Lab Chip 9(23), 3422 (2009). 10.1039/b912213c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Safavieh R., Zhou G. Z., and Juncker D., “Microfluidics made of yarns and knots: From fundamental properties to simple networks and operations,” Lab Chip 11(15), 2618–2624 (2011). 10.1039/c1lc20336c [DOI] [PubMed] [Google Scholar]
- 46.Xing S., Jiang J., and Pan T., “Interfacial microfluidic transport on micropatterned superhydrophobic textile,” Lab Chip 13(10), 1937–1947 (2013). 10.1039/c3lc41255e [DOI] [PubMed] [Google Scholar]
- 47.Washburn E. W., “The dynamics of capillary flow,” Phys. Rev. 17(3), 273–283 (1921). 10.1103/PhysRev.17.273 [DOI] [Google Scholar]
- 48.Duffy D. C. et al. , “Rapid prototyping of microfluidic systems in poly(dimethylsiloxane),” Anal. Chem. 70(23), 4974–4984 (1998). 10.1021/ac980656z [DOI] [PubMed] [Google Scholar]
- 49.Berry S. B. et al. , “Droplet incubation and splitting in open microfluidic channels,” Anal. Methods 11(35), 4528–4536 (2019). 10.1039/C9AY00758J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng S. B. et al. , “Development of a multichannel microfluidic analysis system employing affinity capillary electrophoresis for immunoassay,” Anal. Chem. 73(7), 1472–1479 (2001). 10.1021/ac0007938 [DOI] [PubMed] [Google Scholar]
- 51.Juncker D. et al. , “Autonomous microfluidic capillary system,” Anal. Chem. 74(24), 6139–6144 (2002). 10.1021/ac0261449 [DOI] [PubMed] [Google Scholar]
- 52.Hitzbleck M., Gervais L., and Delamarche E., “Controlled release of reagents in capillary-driven microfluidics using reagent integrators,” Lab Chip 11(16), 2680–2685 (2011). 10.1039/c1lc20282k [DOI] [PubMed] [Google Scholar]
- 53.Nguyen N.-T., Huang X., and Chuan T. K., “MEMS-Micropumps: A review,” J. Fluids Eng. 124(2), 384–392 (2002). 10.1115/1.1459075 [DOI] [Google Scholar]
- 54.Gervais L., Hitzbleck M., and Delamarche E., “Capillary-driven multiparametric microfluidic chips for one-step immunoassays,” Biosens. Bioelectron. 27(1), 64–70 (2011). 10.1016/j.bios.2011.06.016 [DOI] [PubMed] [Google Scholar]
- 55.Gervais L. and Delamarche E., “Toward one-step point-of-care immunodiagnostics using capillary-driven microfluidics and PDMS substrates,” Lab Chip 9(23), 3330–3337 (2009). 10.1039/b906523g [DOI] [PubMed] [Google Scholar]
- 56.Zhang C. et al. , “PCR microfluidic devices for DNA amplification,” Biotechnol. Adv. 24(3), 243–284 (2006). 10.1016/j.biotechadv.2005.10.002 [DOI] [PubMed] [Google Scholar]
- 57.Wu Z. and Hjort K., “Surface modification of PDMS by gradient-induced migration of embedded pluronic,” Lab Chip 9(11), 1500–1503 (2009). 10.1039/b901651a [DOI] [PubMed] [Google Scholar]
- 58.Sun X. et al. , “A general microchip surface modification approach using a spin-coated polymer resist film doped with hydroxypropyl cellulose,” Lab Chip 9(7), 949–953 (2009). 10.1039/B815069A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jönsson C. et al. , “Silane–dextran chemistry on lateral flow polymer chips for immunoassays,” Lab Chip 8(7), 1191–1197 (2008). 10.1039/b800297e [DOI] [PubMed] [Google Scholar]
- 60.Casavant B. P. et al. , “Suspended microfluidics,” Proc. Natl. Acad. Sci. U.S.A. 110(25), 10111–10116 (2013). 10.1073/pnas.1302566110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kokalj T. et al. , “Self-powered imbibing microfluidic pump by liquid encapsulation: SIMPLE,” Lab Chip 14(22), 4329–4333 (2014). 10.1039/C4LC00920G [DOI] [PubMed] [Google Scholar]
- 62.Vestad T., Marr D. W. M., and Oakey J., “Flow control for capillary-pumped microfluidic systems,” J. Micromech. Microeng. 14(11), 1503–1506 (2004). 10.1088/0960-1317/14/11/010 [DOI] [Google Scholar]
- 63.Lutz B. R. et al. , “Two-dimensional paper networks: Programmable fluidic disconnects for multi-step processes in shaped paper,” Lab Chip 11(24), 4274–4278 (2011). 10.1039/c1lc20758j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suk J. W. and Cho J.-H., “Capillary flow control using hydrophobic patterns,” J. Micromech. Microeng. 17(4), N11–N15 (2007). 10.1088/0960-1317/17/4/N01 [DOI] [Google Scholar]
- 65.Fu E. and Paul Yager B. L., “Two-Dimensional paper networks for automated multistep processes in point-of-care diagnostics,” in Microfluidics and Nanotechnology, edited by Lagally E. (CRC Press, Boca Raton, 2014). [Google Scholar]
- 66.Jafry A. T. et al. , “Flexible time–temperature indicator: A versatile platform for laminated paper-based analytical devices,” Microfluid. Nanofluid. 21(3), 57 (2017). 10.1007/s10404-017-1883-x [DOI] [Google Scholar]
- 67.Ramachandran S. et al. , “A rapid, multiplexed high-throughput flow-through membrane immunoassay: A convenient alternative to ELISA,” Diagnostics 3(2), 244–260 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khodayari Bavil A. and Kim J., “A capillary flow-driven microfluidic system for microparticle-labeled immunoassays,” Analyst 143(14), 3335–3342 (2018). 10.1039/C8AN00898A [DOI] [PubMed] [Google Scholar]
- 69.Mauk M. et al. , “Miniaturized devices for point of care molecular detection of HIV,” Lab Chip 17(3), 382–394 (2017). 10.1039/C6LC01239F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Byrnes S. A., Bishop J. D., and Yager P., “Enabling lateral transport of genomic DNA through porous membranes for point-of-care applications,” Anal. Methods 9(23), 3450–3463 (2017). 10.1039/C7AY00293A [DOI] [Google Scholar]
- 71.Lafleur L. K. et al. , “A rapid, instrument-free, sample-to-result nucleic acid amplification test,” Lab Chip 16(19), 3777–3787 (2016). 10.1039/C6LC00677A [DOI] [PubMed] [Google Scholar]
- 72.Toley B. J. et al. , “Isothermal strand displacement amplification (iSDA): A rapid and sensitive method of nucleic acid amplification for point-of-care diagnosis,” Analyst 140(22), 7540–7549 (2015). 10.1039/C5AN01632K [DOI] [PubMed] [Google Scholar]
- 73.Kokalj T. et al. , “Self-powered imbibing microfluidic pump by liquid encapsulation: SIMPLE,” Lab Chip 14(22), 4329–4333 (2014). 10.1039/C4LC00920G [DOI] [PubMed] [Google Scholar]
- 74.Dal Dosso F. et al. , “Creasensor: SIMPLE technology for creatinine detection in plasma,” Anal. Chim. Acta 1000, 191–198 (2018). 10.1016/j.aca.2017.11.026 [DOI] [PubMed] [Google Scholar]
- 75.Dosso D. and et al F., “SIMPLE analytical model for smart microfluidic chip design,” Sens. Actuators A 287, 131–137 (2019). 10.1016/j.sna.2019.01.005 [DOI] [Google Scholar]
- 76.Wang X., Hagen J. A., and Papautsky I., “Paper pump for passive and programmable transport,” Biomicrofluidics 7(1), 014107 (2013). 10.1063/1.4790819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dosso D. and et al F., “Self-powered infusion microfluidic pump for ex vivo drug delivery,” Biomed. Microdevices 20(2), 44 (2018). 10.1007/s10544-018-0289-1 [DOI] [PubMed] [Google Scholar]
- 78.Cummins B. M. et al. , “Modular pumps as programmable hydraulic batteries for microfluidic devices,” Technology 05(01), 21–30 (2017). 10.1142/S2339547817200011 [DOI] [Google Scholar]
- 79. Dal Dosso F. et al. , “Innovative hydrophobic valve allows complex liquid manipulations in a self-powered channel-based microfluidic device,” ACS Sensors 4(3), 694–703 (2019). 10.1021/acssensors.8b01555 [DOI] [PubMed] [Google Scholar]
- 80.Sotoudegan M. S. et al. , “Paper-based passive pumps to generate controllable whole blood flow through microfluidic devices,” Lab Chip 19(22), 3787–3795 (2019). 10.1039/C9LC00822E [DOI] [PubMed] [Google Scholar]
- 81.Rich M. et al. , “Characterization of glass frit capillary pumps for microfluidic devices,” Microfluid. Nanofluid. 23(5), 70 (2019). 10.1007/s10404-019-2238-6 [DOI] [Google Scholar]
- 82.Pla-Roca M. and Juncker D., “PDMS microfluidic capillary systems for patterning proteins on surfaces and performing miniaturized immunoassays,” in Biological Microarrays Methods and Protocols, edited by Khademhosseini A., Suh K.-Y., and Zourob M. (Humana Press, Totowa, NJ, 2011), pp. 177–194. [DOI] [PubMed] [Google Scholar]
- 83.Guha R. et al. , “Modulation of spatiotemporal particle patterning in evaporating droplets: Applications to diagnostics and materials science,” ACS Appl. Mater. Interfaces 9(49), 43352–43362 (2017). 10.1021/acsami.7b13675 [DOI] [PubMed] [Google Scholar]
- 84.Li J. M. et al. , “A micropump based on water potential difference in plants,” Microfluid. Nanofluid. 11(6), 717–724 (2011). 10.1007/s10404-011-0837-y [DOI] [Google Scholar]
- 85.Guan Y. et al. , “The use of a micropump based on capillary and evaporation effects in a microfluidic flow injection chemiluminescence system,” Talanta 68(4), 1384–1389 (2006). 10.1016/j.talanta.2005.08.021 [DOI] [PubMed] [Google Scholar]
- 86.Lamberti A., Marasso S. L., and Cocuzza M., “PDMS membranes with tunable gas permeability for microfluidic applications,” RSC Adv. 4(106), 61415–61419 (2014). 10.1039/C4RA12934B [DOI] [Google Scholar]
- 87.Xu L. et al. , “Vacuum-driven power-free microfluidics utilizing the gas solubility or permeability of polydimethylsiloxane (PDMS),” Lab Chip 15(20), 3962–3979 (2015). 10.1039/C5LC00716J [DOI] [PubMed] [Google Scholar]
- 88.Liu B. et al. , “A positive pressure-driven PDMS pump for fluid handling in microfluidic chips,” Microfluid. Nanofluid. 22(9), 94 (2018). 10.1007/s10404-018-2112-y [DOI] [Google Scholar]
- 89.Tottori N. and Nisisako T., “Degas-driven deterministic lateral displacement in poly(dimethylsiloxane) microfluidic devices,” Anal. Chem. 91(4), 3093–3100 (2019). 10.1021/acs.analchem.8b05587 [DOI] [PubMed] [Google Scholar]
- 90.Shin S. et al. , “Integrated microfluidic pneumatic circuit for point-of-care molecular diagnostics,” Biosens. Bioelectron. 133, 169–176 (2019). 10.1016/j.bios.2019.03.018 [DOI] [PubMed] [Google Scholar]
- 91.Fu Y. et al. , “A microfluidic chip based on surfactant-doped polydimethylsiloxane (PDMS) in a sandwich configuration for low-cost and robust digital PCR,” Sens. Actuators B 245, 414–422 (2017). 10.1016/j.snb.2017.01.161 [DOI] [Google Scholar]
- 92.Chatzimichail S. et al. , “Micropatterning of planar metal electrodes by vacuum filling microfluidic channel geometries,” Sci. Rep. 8(1), 14380 (2018). 10.1038/s41598-018-32706-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu B. et al. , “Hermetic encapsulation of negative-pressure-driven PDMS microfluidic devices using paraffin wax and glass,” Microsyst. Technol. 24(4), 2035–2043 (2018). 10.1007/s00542-017-3600-9 [DOI] [Google Scholar]
- 94.Song J. S. et al. , “Improved biocompatibility of parylene-C films prepared by chemical vapor deposition and the subsequent plasma treatment,” J. Appl. Polym. Sci. 112(6), 3677–3685 (2009). 10.1002/app.29774 [DOI] [Google Scholar]
- 95.Lee C.-J. and Hsu Y.-H., “Vacuum pouch microfluidic system and its application for thin-film micromixers,” Lab Chip 19(17), 2834–2843 (2019). 10.1039/C8LC01286E [DOI] [PubMed] [Google Scholar]
- 96.Fick A., “Ueber diffusion,” Annal. Phys. Chem. 170(1), 59–86 (1855). 10.1002/andp.18551700105 [DOI] [Google Scholar]
- 97.Xu L., Lee H., and Oh K. W., “Syringe-assisted point-of-care micropumping utilizing the gas permeability of polydimethylsiloxane,” Microfluid. Nanofluid. 17(4), 745–750 (2014). 10.1007/s10404-014-1356-4 [DOI] [Google Scholar]
- 98.Wang A. et al. , “A compact, syringe-assisted, vacuum-driven micropumping device,” Micromachines 10(8), 543 (2019). 10.3390/mi10080543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu W., Trinh K. T. L., and Lee N. Y., “Hand-held syringe as a portable plastic pump for on-chip continuous-flow PCR: Miniaturization of sample injection device,” Analyst 137(4), 983–990 (2012). 10.1039/C2AN15860D [DOI] [PubMed] [Google Scholar]
- 100.Shin J.-H. et al. , “A stand-alone pressure-driven 3D microfluidic chemical sensing analytic device,” Sens. Actuators B 230, 380–387 (2016). 10.1016/j.snb.2016.02.085 [DOI] [Google Scholar]
- 101.Wang X. et al. , “A hydrostatic pressure-driven passive micropump enhanced with siphon-based autofill function,” Lab Chip 18(15), 2167–2177 (2018). 10.1039/C8LC00236C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chuang C.-H. and Chiang Y.-Y., “Bio-O-pump: A novel portable microfluidic device driven by osmotic pressure,” Sens. Actuators B 284, 736–743 (2019). 10.1016/j.snb.2019.01.020 [DOI] [Google Scholar]
- 103.Rossier J. S. et al. , “GRAVI: Robotized microfluidics for fast and automated immunoassays in low volume,” J. Assoc. Lab. Autom. 13(6), 322–329 (2008). 10.1016/j.jala.2008.09.001 [DOI] [Google Scholar]
- 104.Kai J. et al. , “A novel microfluidic microplate as the next generation assay platform for enzyme linked immunoassays (ELISA),” Lab Chip 12(21), 4257–4262 (2012). 10.1039/c2lc40585g [DOI] [PubMed] [Google Scholar]
- 105.Walker G. M. and Beebe D. J., “A passive pumping method for microfluidic devices,” Lab Chip 2(3), 131–134 (2002). 10.1039/b204381e [DOI] [PubMed] [Google Scholar]
- 106.Berthier E. and Beebe D. J., “Flow rate analysis of a surface tension driven passive micropump,” Lab Chip 7(11), 1475–1478 (2007). 10.1039/b707637a [DOI] [PubMed] [Google Scholar]
- 107.Yu J. et al. , “Reconfigurable open microfluidics for studying the spatiotemporal dynamics of paracrine signalling,” Nat. Biomed. Eng. 3(10), 830–841 (2019). 10.1038/s41551-019-0421-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu W. et al. , “Slipchip for immunoassays in nanoliter volumes,” Anal. Chem. 82(8), 3276–3282 (2010). 10.1021/ac100044c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shen F. et al. , “Nanoliter multiplex PCR arrays on a SlipChip,” Anal. Chem. 82(11), 4606–4612 (2010). 10.1021/ac1007249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Du W. et al. , “Slipchip,” Lab Chip 9(16), 2286–2292 (2009). 10.1039/b908978k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li W. et al. , “Squeeze-chip: A finger-controlled microfluidic flow network device and its application to biochemical assays,” Lab Chip 12(9), 1587–1590 (2012). 10.1039/c2lc40125h [DOI] [PubMed] [Google Scholar]
- 112.Thurgood P. et al. , “A self-sufficient pressure pump using latex balloons for microfluidic applications,” Lab Chip 18(18), 2730–2740 (2018). 10.1039/C8LC00471D [DOI] [PubMed] [Google Scholar]
- 113.Laksanasopin T. et al. , “A smartphone dongle for diagnosis of infectious diseases at the point of care,” Sci. Transl. Med. 7(273), 273re1–273re1 (2015). 10.1126/scitranslmed.aaa0056 [DOI] [PubMed] [Google Scholar]
- 114.Begolo S. et al. , “The pumping lid: Investigating multi-material 3D printing for equipment-free, programmable generation of positive and negative pressures for microfluidic applications,” Lab Chip 14(24), 4616–4628 (2014). 10.1039/C4LC00910J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Langer K. et al. , “Micropipette-powered droplet based microfluidics,” Biomicrofluidics 12(4), 044106 (2018). 10.1063/1.5037795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dey K. K. and Sen A., “Chemically propelled molecules and machines,” J. Am. Chem. Soc. 139(23), 7666–7676 (2017). 10.1021/jacs.7b02347 [DOI] [PubMed] [Google Scholar]
- 117.Niu R. et al. , “Microfluidic pumping by micromolar salt concentrations,” Soft Matter 13(7), 1505–1518 (2017). 10.1039/C6SM02240E [DOI] [PubMed] [Google Scholar]
- 118.Gregory D. A. and Ebbens S. J., “Symmetrical catalytically active colloids collectively induce convective flow,” Langmuir 34(14), 4307–4313 (2018). 10.1021/acs.langmuir.8b00310 [DOI] [PubMed] [Google Scholar]
- 119.Kline T. R. et al. , “Catalytic micropumps: microscopic convective fluid flow and pattern formation,” J. Am. Chem. Soc. 127(49), 17150–17151 (2005). 10.1021/ja056069u [DOI] [PubMed] [Google Scholar]
- 120.Shklyaev O. E., Shum H., and Balazs A. C., “Using chemical pumps and motors to design flows for directed particle assembly,” Acc. Chem. Res. 51(11), 2672–2680 (2018). 10.1021/acs.accounts.8b00234 [DOI] [PubMed] [Google Scholar]
- 121.Zhang H. et al. , “Self-Powered glucose-responsive micropumps,” ACS Nano 8(8), 8537–8542 (2014). 10.1021/nn503170c [DOI] [PubMed] [Google Scholar]
- 122.Ortiz-Rivera I. et al. , “Convective flow reversal in self-powered enzyme micropumps,” PNAS 113(10), 2585–2590 (2016). 10.1073/pnas.1517908113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maiti S. et al. , “Self-organization of fluids in a multienzymatic pump system,” Langmuir 35(10), 3724–3732 (2019). 10.1021/acs.langmuir.8b03607 [DOI] [PubMed] [Google Scholar]
- 124.Valdez L. et al. , “Solutal and thermal buoyancy effects in self-powered phosphatase micropumps,” Soft Matter 13(15), 2800–2807 (2017). 10.1039/C7SM00022G [DOI] [PubMed] [Google Scholar]
- 125.Tan Z., Yang M., and Ripoll M., “Microfluidic pump driven by anisotropic phoresis,” Phys. Rev. Appl. 11(5), 054004 (2019). 10.1103/PhysRevApplied.11.054004 [DOI] [Google Scholar]
- 126.Good B. T., Bowman C. N., and Davis R. H., “An effervescent reaction micropump for portable microfluidic systems,” Lab Chip 6(5), 659–666 (2006). 10.1039/b601542e [DOI] [PubMed] [Google Scholar]
- 127.Guler M. T. et al. , “Self-powered disposable prothrombin time measurement device with an integrated effervescent pump,” Sens. Actuators B 273, 350–357 (2018). 10.1016/j.snb.2018.06.042 [DOI] [Google Scholar]
- 128.Choi Y. H., Son S. U., and Lee S. S., “A micropump operating with chemically produced oxygen gas,” Sens. Actuators A 111(1), 8–13 (2004). 10.1016/j.sna.2003.10.005 [DOI] [Google Scholar]
- 129.Meng D. D. and Kim C.-J. C., “Micropumping of liquid by directional growth and selective venting of gas bubbles,” Lab Chip 8(6), 958–968 (2008). 10.1039/b719918j [DOI] [PubMed] [Google Scholar]
- 130.Esquivel J. P. et al. , “Fuel cell-powered microfluidic platform for lab-on-a-chip applications,” Lab Chip 12(1), 74–79 (2012). 10.1039/C1LC20426B [DOI] [PubMed] [Google Scholar]
- 131.Esquivel J. P. et al. , “Single-use paper-based hydrogen fuel cells for point-of-care diagnostic applications,” J. Power Sources 342, 442–451 (2017). 10.1016/j.jpowsour.2016.12.085 [DOI] [Google Scholar]
- 132.Meng Gao L. G., “Electroosmotic flow pump,” in Advances in Microfluidics—New Applications in Biology, Energy, and Materials Sciences, edited by Yu X.-Y. (IntechOpen, 2016). [Google Scholar]
- 133.Park C. et al. , “3D-printed microfluidic magnetic preconcentrator for the detection of bacterial pathogen using an ATP luminometer and antibody-conjugated magnetic nanoparticles,” J. Microbiol. Methods 132, 128–133 (2017). 10.1016/j.mimet.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 134.Kahkeshani S. and Di Carlo D., “Drop formation using ferrofluids driven magnetically in a step emulsification device,” Lab Chip 16(13), 2474–2480 (2016). 10.1039/C6LC00645K [DOI] [PubMed] [Google Scholar]
- 135.Katsikis G. et al. , “Synchronous magnetic control of water droplets in bulk ferrofluid,” Soft Matter 14(5), 681–692 (2018). 10.1039/C7SM01973D [DOI] [PubMed] [Google Scholar]
- 136.Zhang Y. and Nguyen N.-T., “Magnetic digital microfluidics—A review,” Lab Chip 17(6), 994–1008 (2017). 10.1039/C7LC00025A [DOI] [PubMed] [Google Scholar]
- 137.Van Reenen A. et al. , “Integrated lab-on-chip biosensing systems based on magnetic particle actuation—A comprehensive review,” Lab Chip 14(12), 1966–1986 (2014). 10.1039/C3LC51454D [DOI] [PubMed] [Google Scholar]
- 138.Lee Y., Choi M., and Kim S.-J., “Method to prevent backflow in a capillarity network for bioassays: Exploiting time constant ratios,” Sens. Actuators B 255, 3630–3635 (2018). 10.1016/j.snb.2017.09.093 [DOI] [Google Scholar]
- 139.Lee Y., Seder I., and Kim S.-J., “Influence of surface tension-driven network parameters on backflow strength,” RSC Adv. 9(18), 10345–10351 (2019). 10.1039/C8RA09756A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim S.-J. et al. , “Passive microfluidic control of Two merging streams by capillarity and relative flow resistance,” Anal. Chem. 77(19), 6494–6499 (2005). 10.1021/ac0504417 [DOI] [PubMed] [Google Scholar]
- 141.Zhai Y. et al. , “A robust, portable and backflow-free micromixing device based on both capillary- and vacuum-driven flows,” Lab Chip 18(2), 276–284 (2018). 10.1039/C7LC01077J [DOI] [PubMed] [Google Scholar]
- 142.Wu J. et al. , “A radial microfluidic platform for higher throughput chemotaxis studies with individual gradient control,” Lab Chip 18(24), 3855–3864 (2018). 10.1039/C8LC00981C [DOI] [PubMed] [Google Scholar]
- 143.Doh I. and Cho Y.-H., “Passive flow-rate regulators using pressure-dependent autonomous deflection of parallel membrane valves,” Lab Chip 9(14), 2070–2075 (2009). 10.1039/b821524c [DOI] [PubMed] [Google Scholar]
- 144.Johansson S. B., Stemme G., and Roxhed N.. A novel constant flow regulation principle for compact breath diagnostics in 2014 IEEE 27th International Conference on Micro Electro Mechanical Systems (MEMS) (2014). [Google Scholar]
- 145.Glick C. C. et al. Single-layer microfluidic current source via optofluidic lithography in 28th IEEE International Conference on Micro Electro Mechanical Systems (MEMS) (IEEE, 2015). [Google Scholar]
- 146.Zhang X. et al. , “Passive flow regulator for precise high-throughput flow rate control in microfluidic environments,” RSC Adv. 6(38), 31639–31646 (2016). 10.1039/C6RA01093H [DOI] [Google Scholar]
- 147.Case D. J. et al. , “Braess’s paradox and programmable behaviour in microfluidic networks,” Nature 574(7780), 647–652 (2019). 10.1038/s41586-019-1701-6 [DOI] [PubMed] [Google Scholar]
- 148.Gubala V. et al. , “Point of care diagnostics: Status and future,” Anal. Chem. 84(2), 487–515 (2012). 10.1021/ac2030199 [DOI] [PubMed] [Google Scholar]
- 149.Gootenberg J. S. et al. , “Nucleic acid detection with CRISPR-Cas13a/C2c2,” Science 356(6336), 438–442 (2017). 10.1126/science.aam9321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gootenberg J. S. et al. , “Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6,” Science 360(6387), 439–444 (2018). 10.1126/science.aaq0179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Myhrvold C. et al. , “Field-deployable viral diagnostics using CRISPR-Cas13,” Science 360(6387), 444–448 (2018). 10.1126/science.aas8836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yeh E. C. et al. , “Self-powered integrated microfluidic point-of-care low-cost enabling (SIMPLE) chip,” Sci. Adv. 3(3), e1501645 (2017). 10.1126/sciadv.1501645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Macosko E. Z. et al. , “Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets,” Cell 161(5), 1202–1214 (2015). 10.1016/j.cell.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Klein A. M. et al. , “Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells,” Cell 161(5), 1187–1201 (2015). 10.1016/j.cell.2015.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Diehl F. et al. , “BEAMing: Single-molecule PCR on microparticles in water-in-oil emulsions,” Nat. Methods 3(7), 551–559 (2006). 10.1038/nmeth898 [DOI] [PubMed] [Google Scholar]
- 156.Thorsen T. et al. , “Dynamic pattern formation in a vesicle-generating microfluidic device,” Phys. Rev. Lett. 86(18), 4163–4166 (2001). 10.1103/PhysRevLett.86.4163 [DOI] [PubMed] [Google Scholar]
- 157.Chen Z. T. et al. , “Centrifugal micro-channel array droplet generation for highly parallel digital PCR,” Lab Chip 17(2), 235–240 (2017). 10.1039/C6LC01305H [DOI] [PubMed] [Google Scholar]
- 158.Mavrogiannis N. et al. , “Microfluidics made easy: A robust low-cost constant pressure flow controller for engineers and cell biologists,” Biomicrofluidics 10(3), 034107 (2016). 10.1063/1.4950753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pollack M. G., Fair R. B., and Shenderov A. D., “Electrowetting-based actuation of liquid droplets for microfluidic applications,” Appl. Phys. Lett. 77(11), 1725–1726 (2000). 10.1063/1.1308534 [DOI] [Google Scholar]
- 160.Li X. P., Hu J., and Easley C. J., “Automated microfluidic droplet sampling with integrated, mix-and-read immunoassays to resolve endocrine tissue secretion dynamics,” Lab Chip 18(19), 2926–2935 (2018). 10.1039/C8LC00616D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kim D. Y., Jin S. H., and Lee C. S., “Spontaneous generation of emulsion droplets by autonomous fluid-pumping using the gas permeability of poly(dimethylsiloxane) (PDMS),” J. Dispersion Sci. Technol. 38(2), 194–198 (2017). 10.1080/01932691.2016.1154862 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.