Abstract
A novel coronavirus SARS-CoV-2, also called novel coronavirus 2019 (nCoV-19), started to circulate among humans around December 2019, and it is now widespread as a global pandemic. The disease caused by SARS-CoV-2 virus is called COVID-19, which is highly contagious and has an overall mortality rate of 6.96% as of May 4, 2020. There is no vaccine or antiviral available for SARS-CoV-2. In this study, we report our discovery of inhibitors targeting the SARS-CoV-2 main protease (Mpro). Using the FRET-based enzymatic assay, several inhibitors including boceprevir, GC-376, and calpain inhibitors II, and XII were identified to have potent activity with single-digit to submicromolar IC50 values in the enzymatic assay. The mechanism of action of the hits was further characterized using enzyme kinetic studies, thermal shift binding assays, and native mass spectrometry. Significantly, four compounds (boceprevir, GC-376, calpain inhibitors II and XII) inhibit SARS-CoV-2 viral replication in cell culture with EC50 values ranging from 0.49 to 3.37 μM. Notably, boceprevir, calpain inhibitors II and XII represent novel chemotypes that are distinct from known Mpro inhibitors. A complex crystal structure of SARS-CoV-2 Mpro with GC-376, determined at 2.15 Å resolution with three monomers per asymmetric unit, revealed two unique binding configurations, shedding light on the molecular interactions and protein conformational flexibility underlying substrate and inhibitor binding by Mpro. Overall, the compounds identified herein provide promising starting points for the further development of SARS-CoV-2 therapeutics.
Keywords: SARS-CoV-2, COVID-19, main protease, 3CL protease, calpain inhibitors, boceprevir, GC-376
INTRODUCTION
An emerging respiratory disease COVID-19 started to circulate among human in December 2019. Since its first outbreak in China from an unknown origin, it quickly became a global pandemic. As of May 4, 2020, there are 239,740 deaths among 3,442,234 confirmed cases in 215 countries.1 The etiological pathogen of COVID-19 is a new coronavirus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), also called novel coronavirus (nCoV-2019). As the name indicates, SARS-CoV-2 is similar to SARS, the virus that causes severe respiratory symptoms in human and killed 774 people among 8098 infected worldwide in 2003.2 SARS-CoV-2 shares ~82% of sequence identity as SARS and to a less extent for Middle East respiratory syndrome (MERS) (~50%).3, 4 SARS-CoV-2 is an enveloped, positive-sense, single-stranded RNA virus that belongs to the β-lineage of the coronavirus.5 The β-lineage also contains two other important human pathogens, the SARS coronavirus and MERS coronavirus. The mortality rate of SARS-CoV-2 is around 6.96% as of May 4, 2020, which is lower than that of SARS (~10%) and MERS (~34%).2 However, current data indicate that SARS-CoV-2 is more contagious and has a larger R0 value than SARS and MERS,6 resulting in higher overall death tolls than SARS and MERS. The SARS-CoV-2 virus is currently spreading at an alarming speed in Europe and the United States.
There is currently no antiviral or vaccine for SARS-CoV-2. The SARS-CoV-2 viral genome encodes a number of structural proteins (e.g. capsid spike glycoprotein), non-structural proteins (e.g. 3-chymotrypsin-like protease (3CL or main protease), papain-like protease, helicase, and RNA-dependent RNA polymerase), and accessary proteins. Compounds that target anyone of these viral proteins might be potential antiviral drug candidates.7, 8 In this study, we focus on the viral 3CL protease, also called the main protease (Mpro), and aim to develop potent Mpro inhibitors as SAR-CoV-2 antivirals. The Mpro plays an essential role in coronavirus replication by digesting the viral polyproteins at more than 11 sites, and it appears like a high profile target for antiviral drug discovery.9–12 The Mpro has an unique substrate preference for glutamine at the P1 site (Leu-Gln↓(Ser,Ala,Gly)), a feature that is absent in closely related host proteases, suggesting it is feasible to achieve high selectivity by targeting viral Mpro. As such, we developed the Fluorescence Resonance Energy Transfer (FRET)-based enzymatic assay for the SARS-CoV-2 Mpro and applied it to screen a focused library of protease inhibitors. Here we report our findings of several novel hits targeting SARS-CoV-2 Mpro and their mechanism of action. The in vitro antiviral activity of the hits was also evaluated in cell culture using infectious SARS-CoV-2 virus. Overall, our study provides a list of drug candidates for SARS-CoV-2 with a confirmed mechanism of action, and the results might help speed up the drug discovery efforts in combating COVID-19. The compounds identified herein represent one of the most potent and selective SARS-CoV-2 Mpro inhibitors so far with both enzymatic inhibition and cellular antiviral activity.9, 11, 12 The X-ray crystal structure of SARS-CoV-2 Mpro with GC-376 showed that the compound can adapt two configurations R and S, offering a molecular explanation of the high-binding affinity of the aldehyde-containing inhibitors. Significantly, the discovery of calpain II and XII inhibitors as potent SARS-CoV-2 antivirals suggest that it might be feasible to design dual inhibitors against the viral Mpro and the host calpains, both of which are important for viral replication.
RESULTS
Establishing the FRET-based assay for the SARS-CoV-2 main protease (Mpro)
The Mpro gene from SARS-CoV-2 strain BetaCoV/Wuhan/WIV04/2019 was inserted into pET-29a(+) vector and expressed in BL21(DE3) E. Coli. with a His-tag in its C-terminus. The Mpro protein was purified with Ni-NTA column to high purity (Fig. 1A). To establish the FRET assay condition, we designed a FRET based substrate using the sequence between viral polypeptide NSP4-NSP5 junction from SARS-CoV-2: Dabcyl-KTSAVLQ/SGFRKME(Edans). We then tested the Mpro proteolytic activity in buffers with different pH. We found that Mpro displays highest activity in pH 6.5 buffer (Fig. 1B), which contains 20 mM HEPES, 120 mM NaCl, 0.4 mM EDTA, and 4 mM DTT and 20% glycerol. As such, all the following proteolytic assay was conducted using this pH 6.5 buffer. Next, we characterized the enzymatic activity of this SARS-CoV-2 Mpro by measuring the Km and Vmax values. When 100 nM Mpro was mixed with various concentration of FRET substrate (0 to 200 μM), the initial velocity was measured and plotted against substrate concentration. Curve fitting with Michaelis-Menton equation gave the best-fit values of Km and Vmax as 32.8 ± 3.5 μM and 29.4 ± 1.1 RFU/s, respectively (Fig. 1C).
Figure 1: SARS-CoV-2 Mpro expression and characterization.
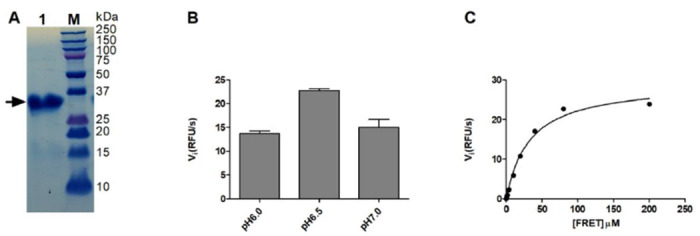
(A) SDS-PAGE of His-tagged-Main protease (Mpro) (lane 1); Lane M, protein ladder; the calculated molecular weight of the His-tagged-Mpro is 34,992 Da. (B) Reaction buffer optimization: 250 nM His-tagged-Mpro was diluted into three reaction buffers with different pH values. (C) Michaelis-Menten plot of 100 nM His-tagged- Mpro with the various concentrations of FRET substrate in pH 6.5 reaction buffer.
Primary screening of a focused protease library against the SARS-CoV-2 Mpro.
With the established FRET assay condition, we screened a collection of protease inhibitors from the Selleckchem bioactive compound library to identify potential SARS-CoV-2 Mpro inhibitors. The protease inhibitors are grouped based on their targets and mechanism of action and include proteasome inhibitors (1-8); HIV protease inhibitors (9-14); γ-secretase inhibitors (15-22); HCV NS3-4A protease inhibitors (23-29); DPP-4 inhibitors (30-35); miscellaneous serine protease inhibitors (36-39); cathepsin and calpain protease inhibitors (40-43); miscellaneous cysteine protease inhibitors (44-48); matrix metalloprotease inhibitors (49-51); and miscellaneous protease inhibitors (52-55). The inhibitors were pre-incubated with 100 nM of Mpro at 30 °C for 30 minutes in the presence of 4 mM 1,4-dithiothreitol (DTT) before the addition of 10 μM FRET substrate. The addition of DTT was to quench non-specific thiol reactive compounds and to ensure the Mpro is in the reducing condition. All compounds were tested at 20 μM, except compound 26, which was tested at 2 μM due to its fluorescent background. Encouragingly, four inhibitors (24, 28, 29 and 43) showed more than 60% inhibition against Mpro at 20 μM. Among the hits, simeprevir (24), boceprevir (28), and narlaprevir (29) are HCV NS3-4A serine protease inhibitors, and compound MG-132 (43) inhibits both proteasome and calpain.
Secondary screening of a focused library of calpain/cathepsin inhibitors and known viral 3CLpro inhibitors
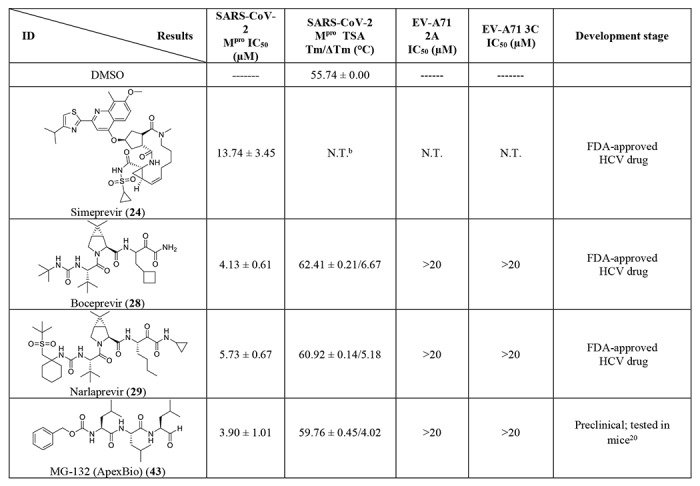
Given the encouraging results from the primary screening, we then further characterized the four hits (24, 28, 29, and 43) in a consortium of assays including dose-response titration, thermal shift binding assay (TSA), and counter screening assays with two other viral cysteine proteases, the enterovirus A71 (EV-A71) 2A and 3C proteases, both of which are cysteine proteases (Table 2). The HCV NS3-4A protease inhibitors boceprevir (28) and narlaprevir (29) inhibited Mpro with IC50 values of 4.13 and 4.73 μM, respectively (Table 2), more potent than simeprevir (24) (IC50 = 13.74 μM). Both compounds 28 and 29 also showed strong binding towards Mpro and shifted the melting temperature of the protein (ΔTm) by 6.67 and 5.18 °C, respectively, at 40 μM. Despite their potent inhibition against the HCV NS3-4A serine protease and the SARS-CoV-2 cysteine Mpro, boceprevir (28) and narlaprevir (29) did not inhibit the EV-A71 2A and 3C proteases (IC50 > 20 μM), suggesting they are not non-specific cysteine protease inhibitors. The calpain inhibitor MG-132 (43) had an IC50 value of 3.90 μM against the Mpro, and was not active against the EV-A71 2A and 3C proteases (IC50 > 20 μM). The binding of MG-132 (43) to Mpro was also confirmed in the TSA assay with a ΔTm of 4.02 °C.
Table 2:
Characterization of HCV and calpain proteases inhibitors against SARS-CoV-2 Mpro using a consortium of secondary assaysa
 |
 |
Value = mean ± S.E. from 3 independent experiments;
N.T. = not tested;
The IC50 of PSI (64) on SARS CoV-2 Mpro was calculated by end point reading of 1 hour digestion, instead of the initial velocity.
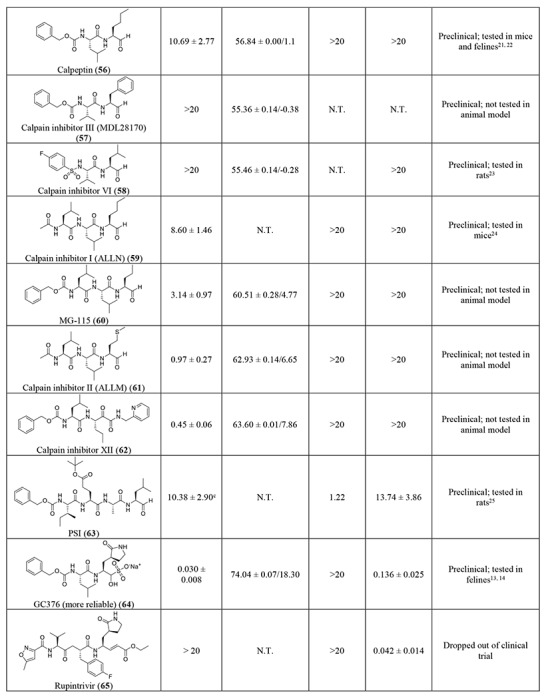
In light of the promising results of the calpain inhibitor MG-132 (43), we then pursued to testing other calpain and cathepsin inhibitors that are commercially available (56-63) (Table 2). These compounds were not included in the initial library because they have not been advanced to clinical studies. Among this series of analogs, calpain inhibitor II (61) and XII (62) are the most potent Mpro inhibitors with IC50 values of 0.97 and 0.45 μM, respectively. Binding of compounds 61 and 62 to Mpro shifted the melting curve of the protein by 6.65 and 7.86 °C, respectively. Encouragingly, both compounds 61 and 62 did not inhibit the EV-A71 2A and 3C proteases (IC50 > 20 μM). Calpain inhibitor I (59) and MG-115 (60) also showed potent inhibition against Mpro with IC50 values of 8.60 and 3.14 μM, respectively. Calpeptin (56) and PSI (63) had moderate activity against Mpro with IC50 values of 10.69 and 10.38 μM, respectively. In contrast, calpain inhibitors III (57) and VI (58) were not active (IC50 > 20 μM).
We also included two well-known viral 3CL protease inhibitors GC-376 (64) and rupintrivir (65) in the secondary screening. GC-376 (64) is an investigational veterinary drug that is being developed for feline infectious peritonitis (FIP).13, 14 GC-376 (64) was designed to target the viral 3CL protease and had potent antiviral activity against multiple viruses including MERS, FIPV, and norovirus.13, 15 Rupintrivir (65) was developed as a rhinovirus antiviral by targeting the viral 3CL protease, but it was discontinued in clinical trials due to side effects.16 In our study, we found that GC-376 (64) was the most potent Mpro inhibitor with an IC50 value of 0.03 μM. It shifted the melting curve of Mpro by 18.30 °C upon binding. In contrast, rupintrivir (65) was not active against Mpro (IC50 > 20 μM). Previous report also showed that rupintrivir was not active against the SARS-CoV 3CLpro (Mpro) (IC50 > 100 μM).17 Both compounds 64 and 65 were not active against the EV-A71 2A protease, but showed potent inhibition against the EV-A71 3C protease, which is consistent with previously reported results.15, 18, 19
When plotting the IC50 values (log scale) of the inhibitors against Mpro from the FRET enzymatic assay with the melting temperature shifts (ΔTm) from thermal shift binding assay (Fig. 3A), a linear correlation was observed, and the r2 of the linear regression fitting is 0.94. This suggests that there is a direct correlation between the enzymatic inhibition and protein binding: a more potent enzyme inhibitor also binds to the protein with higher affinity. The stabilization of the Mpro against thermal denaturation was also compound concentration dependent (Fig. 3B).
Figure 3: Binding of inhibitors to SARS-CoV-2 Mpro using thermal shift binding assay and native mass spectrometry.
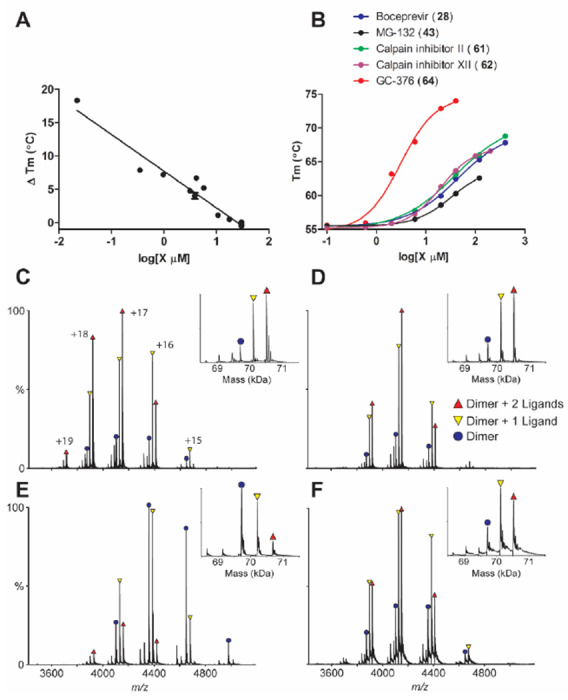
(A) Correlation of inhibition efficacy (IC50) with ΔTm from thermal shift binding assay. Data in Table 2 were used for the plot. The r2 of fitting is 0.94. (B) Dose-dependent melting temperature (Tm) shift. Native MS reveals binding of SARS-CoV-2 Mpro to (C) GC-376 (64), (D) Calpain inhibitor II (61), (E) calpain inhibitor XII (62), and (F) Boceprevir (28). All ligand concentrations are 12.5 μM except E, which is 25 μM. Peak are annotated for dimer (blue circle), dimer with one bound ligand, (yellow down triangle), and dimer with two bound ligands (red up triangle). Other minor signals are truncated dimers, which bind ligands at the same ratios. Charge states are annotated in C, and insets show the deconvolved zero-charge mass distribution.
The binding of four most potent inhibitors boceprevir (28), calpain inhibitors II (61), XII (62), and GC-376 (64) to SARS-CoV-2 Mpro was further characterized by native mass spectrometry (MS) (Figs. 3C–F). Native MS analysis showed that the Mpro formed a dimer complex with a mass of 69,722 Da, indicating a cleaved N-terminal methionine (Fig. S1). A small amount of monomer and dimer with a single C-terminal truncation of the His-tag was also observed, but the intact dimer was the predominant signal (Fig. S1). Addition of all four ligands tested, boceprevir (28), calpain inhibitors II (61), XII (62), and GC-376 (64), showed binding of up to two ligands per dimer (Fig. 3C–F), suggesting a binding stoichiometry of one drug per monomer.
Mechanism of action of hits
To elucidate the mechanism of action of hits against SARS-CoV-2 Mpro, we focus on five most potent compounds prioritized from the primary and secondary screenings including boceprevir (28), MG-132 (43), calpain inhibitor II (61), calpain inhibitor XII (62), and GC-376 (64). For this, we performed enzyme kinetic studies with different concentrations of inhibitors (Fig. 4). A biphasic enzymatic progression curve in the presence but not in the absence of inhibitor is typically a hallmark for a slow covalent binding inhibitor. In the Fig. 4, left column shows the progression curves up to 4 hours. Biphasic progression curves were observed for all 5 inhibitors at high drug concentrations. Significant substrate depletion was observed when the proteolytic reaction proceeded beyond 90 minutes, we therefore chose the first 90 minutes of the progression curves for curve fitting (Fig. 4 middle column). We fit the progression curves in the presence different concentrations of GC-376 (64) with the two-step Morrison equation (equation 3 in methods section). GC-376 (64) binds to SARS-CoV-2 Mpro with an equilibrium dissociation constant for the inhibitor (KI) of 59.9 ± 21.7 nM in the first step. After initial binding, a slower covalent bond is formed between GC-376 (64) and Mpro with the second reaction rate constant (k2) being 0.00245 ± 0.00047 s−1, resulting an overall k2/KI value of 4.08 x 104 M−1 s−1 (Fig. 4A). However, when we tried to fit the proteolytic progression curves for boceprevir (28), MG-132 (43), calpain inhibitors II (61) and XII (62) using the same two-step reaction mechanism, we could not obtain accurate values for the second rate constant k2. This is presumably due to significant substrate depletion before the equilibrium between EI and EI*, leading to very small values of k2. Accordingly, for these four inhibitors 28, 43, 61, and 62, only the dissociation constant KI values from the first step were determined (Figs. 4B–4E). The inhibition constants (KI) for boceprevir (28), MG-132 (43), calpain inhibitors II (61) and XII (62) are 1.18 ± 0.10 μM, 1.57 ± 0.13 μM, 0.40 ± 0.02 μM, and 0.13 ± 0.02 μM, respectively.
Figure 4: Proteolytic reaction progression curves of Mpro in the presence or the absence of compounds.
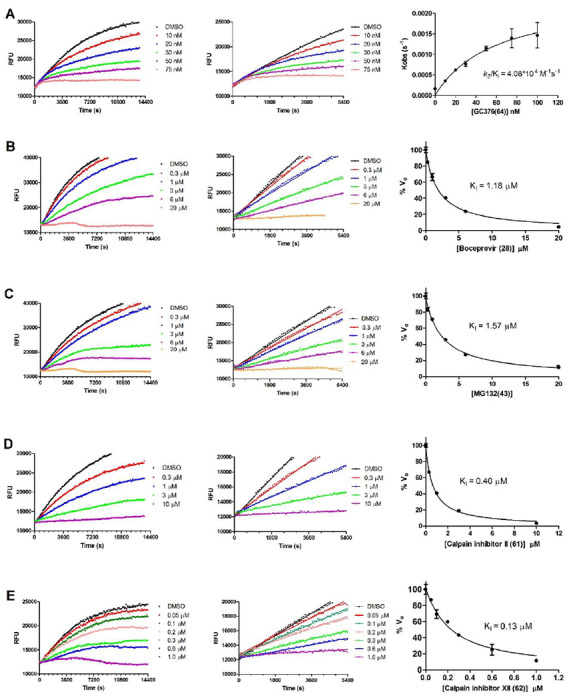
In the kinetic studies, 5 nM Mpro was added to a solution containing various concentrations of protease inhibitors and 20 μM FRET substrate to initiate the reaction, the reaction was then monitored for 4 hrs. Left column shows the reaction progression up to 4 hrs; middle column shows the progression curves for the first 90 minutes, which were used for curve fitting to generate the plot shown in the right column. Detailed methods were described in the Method section. (A) GC-376 (64); (B) Boceprevir (28); (C) MG-132 (43); (D) Calpian inhibitor II (61); (E) Calpain inhibitor XII (62).
Cellular antiviral activity and cytotoxicity of hits
To test the hypothesis that inhibiting the enzymatic activity of Mpro will lead to the inhibition of SARS-CoV-2 viral replication, we performed cellular antiviral assays for the five promising hits 64, 28, 43, 61, and 62 against SARS-CoV-2. For this, we first tested the cellular cytotoxicity of these compounds in multiple cell lines (Table S1). GC-376 (64), boceprevir (28), and calpain inhibitor II (61) were well tolerated and had CC50 values of over 100 μM for all the cell lines tested. MG-132 (43) was cytotoxic to all the cells with CC50 values less than 1 μM except A549 cells. Calpain inhibitor XII (62) had acceptable cellular cytotoxicity with CC50 values above 50 μM for all the cell lines tested (Table S1).
Next, we chose four compounds boceprevir (28), calpain inhibitors II (61), XII (62), and GC-376 (64) for the antiviral assay with infectious SARS-CoV-2. MG-132 (43) was not included due to its cytotoxicity. Gratifyingly, all four compounds showed potent antiviral activity against SARS-CoV-2 in the primary viral cytopathic effect (CPE) assay with EC50 values ranging from 0.49 to 3.37 μM (Table 3). Their antiviral activity was further confirmed in the secondary viral yield reduction (VYR) assay. The most potent compound was calpain inhibitor XII (62), which showed EC50 of 0.49 μM in the primary CPE assay and EC90 of 0.45 μM in the secondary VYR assay. In comparison, remdesivir was reported to inhibit SARS-CoV-2 in the VYR assay with an EC50 of 0.77 μM.26 None of the compounds inhibited the unrelated influenza virus A/California/07/2009 (H1N1) virus (EC50 > 20 μM) (Table S1), suggesting the antiviral activity of the four compounds (boceprevir, calpain inhibitors II, XII, and GC-376) against SARS-CoV-2 is specific. In comparison with recently reported SARS-CoV-2 Mpro inhibitors (Table 3), the hits identified herein represent one of the most potent and selective drug candidates with broad chemical diversity.
Table 3:
Antiviral activity of hits against SARS-CoV-2 and the comparison with recently reported Mpro inhibitors.
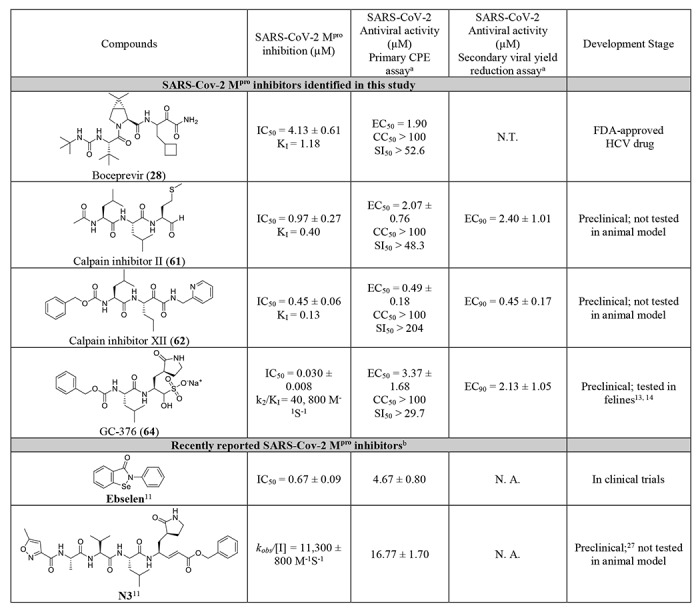 |
 |
Complex crystal structure of SARS-CoV-2 Mpro with GC-376 (64)
The crystal structure of the SARS-CoV-2 Mpro in complex with GC-376 (64) was solved in the p3221 space group at 2.15 Å resolution (Table S2). There are three monomers per asymmetric unit (ASU), with two constituting a biological dimer and the third forming a dimer with a crystallographic symmetry related neighboring protomer (Fig. S2). The presence of three monomers in our crystal structure allowed us to capture different binding configurations of GC-376 (64) (Fig. 5), a unique feature that was not observed in previous X-ray crystal structures 9, 11, 12. The pairwise r.m.s.d among the monomer backbone Cα atoms ranges from 0.435 Å to 0.564 Å. Previously, SARS-CoV Mpro and SARS-CoV-2 Mpro crystal structures have been solved most frequently as a monomer per ASU, and occasionally a dimer 9, 11, 12, 28, 29. In its native state, Mpro requires dimerization to become catalytically active 30, 31, which is supported by our native MS data (Fig. S1). In our crystal structures, all three protomers appear catalytically competent, with the third protomer activated by the N-finger from an adjacent asymmetric unit (Fig. S2B).
Figure 5: Molecular recognition of GC-376 (64) by SARS-CoV-2 Mpro.
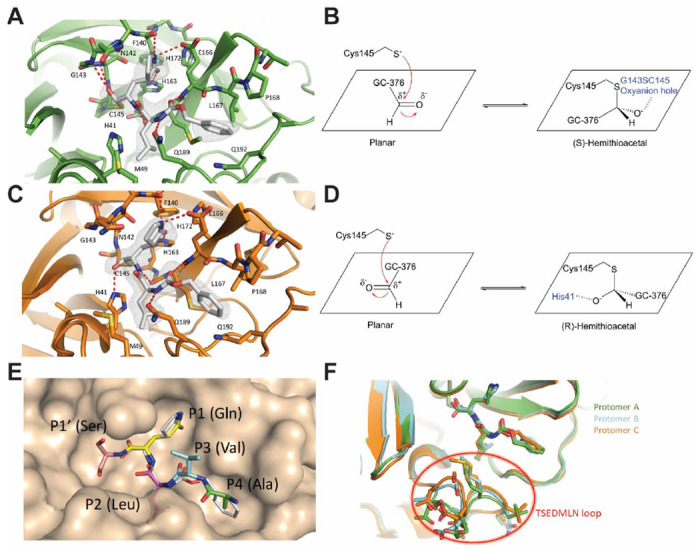
Complex of SARS-CoV-2 Mpro and GC-376 (64) with (A, B) protomer A and (C, D) protomer C. Unbiased Fo-Fc map, shown in grey, is contoured at 2 σ. Hydrogen bonds are shown as red dashed lines. (E) Surface representation of SARS-CoV-2 Mpro + GC-376 (64) (white) superimposed with the SARS-CoV Mpro natural, N-terminal substrate (PDB ID 2Q6G, with residues P1’-P4 in different colors). The SARS-CoV-2 Mpro cleaves between the P1’ and P1 residues. (F) Superimposition of the three protomers in the asymmetric subunit of SARS-CoV-2 Mpro with GC-376 (64). Significant conformational flexibility is observed, particularly in the TSEDMLN loop.
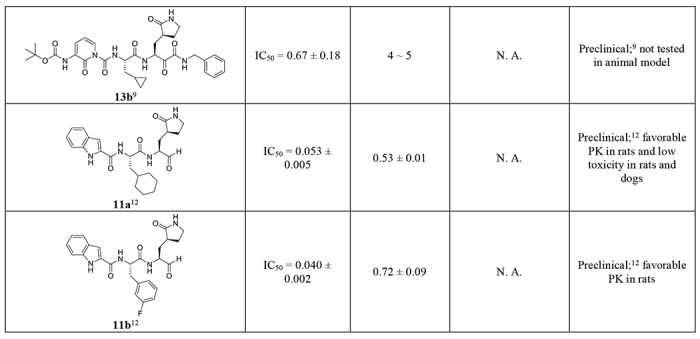
GC-376 (64) forms an extensive network of hydrogen bonds with the active site while also exhibiting excellent geometric complementarity (Fig. 5). These interactions are coupled with the thermodynamic payoff of covalent adduct formation between the aldehyde bisulfite warhead and Cys145, making GC-376 (64) one of the most potent SARS-CoV-2 Mpro inhibitors in vitro with an IC50 of 0.030 ± 0.008 μM (Table 3). Along with other known Mpro inhibitors N3, 13b, 11a and 11b (Table 3), GC-376 (64) mimics the peptide substrate that is cleaved by this enzyme (Fig. 5E) 9, 27, 29, 32. The glutamine surrogate γ-lactam ring is a cyclized derivative of the P1 glutamine side chain that normally occupies the S1 site; here it forms hydrogen bonds with the His163 and Glu166 side chains and the main chain of Phe140 (Figs. 5A & C). An amide bond connects the γ-lactam side chain to an isobutyl moiety that embeds itself in the hydrophobic S2 site formed by His41, Met49, and Met169. Normally, this S2 site in SARS-CoV-2 Mpro can accommodate a variety of hydrophobic substitutions such as isobutyl in GC-376 (64) and N3, cyclopropyl in 13b, cyclohexyl in 11a, and 3-fluorophenyl in 11b (Table 3) 33, 34. A carbamate bond in GC-376 (64), which forms hydrogen bonds with the main chain of Glu166 and the side chain of Gln189, connects the P2 isobutyl group to a phenylmethyl ester that interacts with the aliphatic S4 site. Compared with previous inhibitors, the phenylmethyl ester of GC-376 exhibits high complementarity with the S4 site, and the extensive non-polar interactions may contribute significantly to the potency of this compound (Fig. 5E).
Three copies of GC-376 (64) were found in the crystal structure, one in each protomer active site (Figs. 5A, 5C, S3). The configurations of G-C376 (64) were consistent in protomers A and B, where the thioacetal hydroxide is positioned in the “oxyanion hole” formed by the backbone amides of Gly143, Ser144, and Cys145 (Figs. 5A & S3), resulting in the (S)-configuration. It is noted that aldehydes 11a and 11b also bind in the active site of SARS-CoV-2 Mpro in the (S)-configuration (PDB: 6M0K and 6LZE) (Figs. S4A).12 In protomer C, however, the same hydroxide group orients outwards from the oxyanion hole, forming hydrogen bonds with His41 (Fig. 5C), which gives the (R)-configuration. This (R)-configuration is consistent with the binding mode of α-ketoamide 13b in the active site of SARS-CoV-2 Mpro (PDB: 6Y2F) (Fig. S4B).9 These two unique configurations R and S might be a result of the Cys145 thiol nucleophilic attacking the aldehyde from two different faces (Figs. 5B & D). The fact that GC-376 (64) can adapt two different configurations R and S upon binding to the active site might explain its high binding affinity towards the target.
An additional difference between the configurations of GC-376 (64) in A, B and C is observed in the orientation of the phenylmethyl ester. In protomer C, the CH2 of the phenylmethyl points towards the main chain of Leu167 in a ‘cis’ conformation (Fig. 5C), whereas in protomers A and B this same CH2 points downwards in a ‘trans’ conformation (Figs. 5A & S3). Consequently, this influences the rotameric configuration of the Leu167 isobutyl moiety, where a rotational adjustment of 180° occurs at its “β” carbon. Furthermore, large rearrangements are observed in the flexible TSEDMLN loop consisting of residues 45-51 (TAEDMLN in SARS-CoV-2 Mpro) that form the S2 and S3’ subsites (Fig. 5F), explaining the broad substrate scope in the P2 site (Table 3). The loop conformations in protomers B and C may be influenced by crystal packing interactions with protomers from adjacent asymmetric units and resemble the conformations in previously determined structures 11, 12. Meanwhile, the conformation of protomer A is less restrained and exhibits the most significant conformational divergence. The different loop conformations offer a glimpse of the protein plasticity that allows Mpro to accommodate peptides with differing amino acid composition, and underscores the importance of considering this flexibility when analyzing and modeling protein-ligand interactions for Mpro.
DISCUSSION
Coronaviruses have caused three epidemics/pandemics in the past twenty years including SARS, MERS, and COVID-19. With the ongoing pandemic of COVID-19, scientists and researchers around the globe are racing to find effective vaccines and antiviral drugs.7 The viral polymerase inhibitor remdesivir holds the greatest promise and it is currently being evaluated in several clinical trials.35, 36 The HIV drug combination lopinavir and ritonavir recently failed in a clinical trial for COVID-19 with no significant therapeutic efficacy was observed.37 To address this unmet medical need, we initiated a drug repurposing screening to identify potent inhibitors against the SARS-CoV-2 Mpro from a collection of FDA-approved protease inhibitors. The Mpro has been shown to be a validated antiviral drug target for SARS and MERS.38 As the SARS-CoV-2 Mpro shares a high sequence similarity with SARS and to a less extent with MERS, we reasoned that inhibiting the enzymatic activity of SARS-CoV-2 Mpro will similarly prevent viral replication.9, 11
Noticeable findings from our study include: 1) Boceprevir (28), an FDA-approved HCV drug, inhibits the enzymatic activity of Mpro with IC50 of 4.13 μM, and has an EC50 of 1.90 μM against the SARS-CoV-2 virus in the cellular viral cytopathic effect assay. The therapeutic potential of boceprevir (28) should be further evaluated in relevant animal models and human clinic trials. Since boceprevir (28) is a FDA-approved drug, the dose, toxicity, formulation, and pharmacokinetic properties are already known, which will greatly speed up the design of follow up studies; 2) GC-376 (64), an investigational veterinary drug, showed promising antiviral activity against the SARS-CoV-2 virus (EC50 = 3.37 μM ). It has the highest enzymatic inhibition against the Mpro with an IC50 value of 0.03 μM. This compound has promising in vivo efficacy in treating cats infected with FIP, and has favorable in vivo pharmacokinetic properties. Therefore, GC-376 (64) is ready to be tested in relevant animal models of SARS-CoV-2 when available. Importantly, the X-ray crystal structure of SARS-CoV-2 Mpro in complex with GC-376 (64) provides a molecular explanation of the high binding affinity of aldehyde-containing compounds as they can adapt two configurations R and S. The conformational flexibility at the TSEDMLN loop explains the broad substrate scope at the P2 position of Mpro inhibitors; 3) Three calpain/cathepsin inhibitors, MG-132 (43), calpain inhibitors II (61) and XII (62), are potent inhibitors of Mpro and inhibit SARS-CoV-2 with single-digit to submicromolar efficacy in the enzymatic assay. Calpain inhibitors II (61) and XII (62) also inhibit SARS-CoV-2 in the CPE assay with EC50 values of 2.07 and 0.49 μM, respectively. This result suggests that calpain/cathepsin inhibitors are rich sources of drug candidates for SARS-CoV-2. Indeed, previous studies have shown that calpain and cathepsin are required for the proteolytic processing of the coronavirus S protein, a step that is essential for the viral fusion and genome release during the early stage of viral replication.39 Calpain and cathepsin inhibitors such as MDL28170 (calpain inhibitor III)39, MG-13240, calpain inhibitor VI41 have been shown to inhibit SARS-CoV replication in cell culture. Other than the increased potency of targeting both Mpro and calpain/cathepsin, an additional benefit of such dual inhibitors might be their high genetic barrier to drug resistance. A significant number of calpain/cathepsin inhibitors have been developed over the years for various diseases including cancer, neurodegeneration disease, kidney diseases, and ischemia/reperfusion injury.42 Given our promising results of calpain inhibitors II (61) and XII (62) in inhibiting the SARS-CoV-2 Mpro and their potent antiviral activity in cell culture, it might be worthwhile to repurposing them as antivirals for SARS-CoV-2.
All potent SARS-CoV-2 Mpro inhibitors contain reactive warheads such as α-ketoamide (boceprevir (28), calpain inhibitor XII (62)) or aldehyde (MG-132 (43), calpain inhibitor II (61)) or aldehyde prodrug, the bisulfite (GC-376 (64)). This result suggests that reactive warheads might be essential for SARS-CoV-2 Mpro inhibition. The compounds identified in this study represent one of the most potent and selective hits reported so far, and are superior than recently reported SARS-CoV-2 Mpro inhibitors ebselen, N3, and 13b (Table 3). Calpain inhibitor XII (62) had similar potency as the recently disclosed compounds 11a and 11b (Table 3).12 Notably, calpain inhibitor II (61) and XII (62) have different chemical scaffolds as GC-376 (64), N3, 13b, 11a, and 11b, therefore providing new opportunities for designing more potent and selective SARS-CoV-2 Mpro inhibitors.
Aside from the above positive results, we also showed that ritonavir (9) and lopinavir (10) failed to inhibit the SARS-CoV-2 Mpro (IC50 > 20 μM, Fig. 2), which might explain their lack efficacy in clinical trials for COVID-19.37 Camostat (39) was recently proposed to inhibit SARS-CoV-2 entry through inhibiting the host TMPRSS2, a host serine protease that is important for viral S protein priming.43 However, the antiviral activity of camostat has not been confirmed with infectious SARS-CoV-2 virus. In our study, we found camostat (39) has no inhibition against the SARS-CoV-2 Mpro (IC50 > 20 μM).
Figure 2: Screening of known protease inhibitors against SARS-CoV-2 Mpro using the FRET assay.
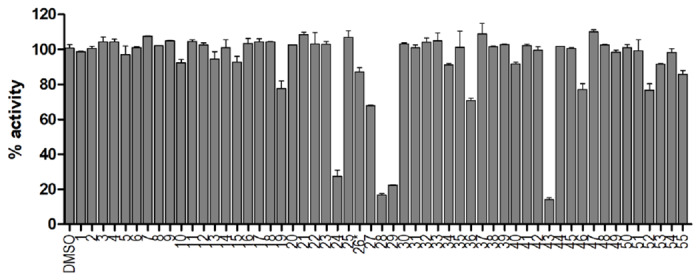
20 μM of compounds (26 was tested at 2 μM) was pre-incubated with 100 nM of SARS-CoV-2 Mpro for 30 minutes at 30 °C, then 10 μM FRET substrate was added to reaction mixture to initiate the reaction. The reaction was monitored for 2 hours. The initial velocity was calculated by linear regression using the data points from the first 15 minutes of the reaction. The calculated initial velocity with each compound was normalized to DMSO control. The results are average ± standard deviation of two repeats.
In summary, this study identified several potent SARS-CoV-2 Mpro inhibitors with potent enzymatic inhibition as well as cellular antiviral activity. Further development based on these hits might lead to clinically useful COVID-19 antivirals. They can be used either alone or in combination with polymerase inhibitors such as remdesivir as a means to achieve potential synergic antiviral effect as well as to suppress drug resistance.
MATERIALS AND METHODS
Details of materials and methods can be found in the supporting information.
Supplementary Material
Table 1.
List of protease inhibitors tested against SARS-CoV-2 Mpro in the primary FRET assay.
 |
 |
ACKNOWLEDGEMENTS
This research was partially supported by the National Institutes of Health (NIH) (Grant AI147325) and the Arizona Biomedical Research Centre Young Investigator grant (ADHS18-198859) to J. W. B. H. and B. T. thanks for the support from the Respiratory Diseases Branch, National Institute of Allergy and Infectious Diseases, NIH, USA (Contract N01-AI-30048). J. A. T. and M. T. M. were funded by the National Institute of General Medical Sciences and National Institutes of Health (Grant R35 GM128624 to M. T. M.). We thank Michael Kemp for assistance with crystallization and X-ray diffraction data collection. We also thank the staff members of the Advanced Photon Source of Argonne National Laboratory, particularly those at the Structural Biology Center (SBC), with X-ray diffraction data collection. SBC-CAT is operated by UChicago Argonne, LLC, for the U.S. Department of Energy, Office of Biological and Environmental Research under contract DE-AC02-06CH11357.
Footnotes
DATA AVAILABILITY. The structure for SARS-CoV-2 Mpro has been deposited in the Protein Data Bank with accession number 6WTT.
Supplementary information accompanies this paper at
Competing interests
J. W. and C. M. are inventors of a pending patent that claims the use of the identified compounds for COVID-19.
REFERENCES
- 1. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 2.Mahase E. Coronavirus: covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ 2020; 368:m641. [DOI] [PubMed] [Google Scholar]
- 3.Lu R, Zhao X, Li J et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020; 395:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu A, Peng Y, Huang B et al. Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China. Cell Host Microbe 2020; 27:325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coronaviridae Study Group of the International Committee on Taxonomy of V. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 2020; 5:536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang B, Bragazzi NL, Li Q, Tang S, Xiao Y, Wu J. An updated estimation of the risk of transmission of the novel coronavirus (2019-nCov). Infect Dis Model 2020; 5:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov 2020; 19:149–150. [DOI] [PubMed] [Google Scholar]
- 8.Xia S, Liu MQ, Wang C et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res 2020; 30:343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Lin D, Sun X et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved alpha-ketoamide inhibitors. Science 2020; 368:409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science 2003; 300:1763–1767. [DOI] [PubMed] [Google Scholar]
- 11.Jin Z, Du X, Xu Y et al. Structure of M(pro) from COVID-19 virus and discovery of its inhibitors. Nature 2020, asap. [Google Scholar]
- 12.Dai W, Zhang B, Su H et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science 2020, asap. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedersen NC, Kim Y, Liu H et al. Efficacy of a 3C-like protease inhibitor in treating various forms of acquired feline infectious peritonitis. J Feline Med Surg 2018; 20:378–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y, Liu H, Galasiti Kankanamalage AC et al. Reversal of the Progression of Fatal Coronavirus Infection in Cats by a Broad-Spectrum Coronavirus Protease Inhibitor. PLoS Pathog 2016; 12:e1005531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim Y, Lovell S, Tiew KC et al. Broad-spectrum antivirals against 3C or 3C-like proteases of picornaviruses, noroviruses, and coronaviruses. J Virol 2012; 86:11754–11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayden FG, Turner RB, Gwaltney JM et al. Phase II, randomized, double-blind, placebo-controlled studies of ruprintrivir nasal spray 2-percent suspension for prevention and treatment of experimentally induced rhinovirus colds in healthy volunteers. Antimicrob Agents Chemother 2003; 47:3907–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shie JJ, Fang JM, Kuo TH et al. Inhibition of the severe acute respiratory syndrome 3CL protease by peptidomimetic alpha,beta-unsaturated esters. Bioorg Med Chem 2005; 13:5240–5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musharrafieh R, Ma C, Zhang J et al. Validating Enterovirus D68-2A(pro) as an Antiviral Drug Target and the Discovery of Telaprevir as a Potent D68-2A(pro) Inhibitor. J Virol 2019; 93, e02221–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuo CJ, Shie JJ, Fang JM et al. Design, synthesis, and evaluation of 3C protease inhibitors as anti-enterovirus 71 agents. Bioorg Med Chem 2008; 16:7388–7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonuccelli G, Sotgia F, Schubert W et al. Proteasome inhibitor (MG-132) treatment of mdx mice rescues the expression and membrane localization of dystrophin and dystrophin-associated proteins. Am J Pathol 2003; 163:1663–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mani SK, Shiraishi H, Balasubramanian S et al. In vivo administration of calpeptin attenuates calpain activation and cardiomyocyte loss in pressure-overloaded feline myocardium. Am J Physiol Heart Circ Physiol 2008; 295:H314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng S, Kuang Z, Zhang Y, Xu H, Cheng Q. The protective effects and potential mechanism of Calpain inhibitor Calpeptin against focal cerebral ischemia-reperfusion injury in rats. Mol Biol Rep 2011; 38:905–912. [DOI] [PubMed] [Google Scholar]
- 23.Akdemir O, Ucankale M, Karaoglan A et al. Therapeutic efficacy of SJA6017, a calpain inhibitor, in rat spinal cord injury. J Clin Neurosci 2008; 15:1130–1136. [DOI] [PubMed] [Google Scholar]
- 24.Li SZ, Zhang HH, Zhang JN et al. ALLN hinders HCT116 tumor growth through Bax-dependent apoptosis. Biochem Biophys Res Commun 2013; 437:325–330. [DOI] [PubMed] [Google Scholar]
- 25.Delli Pizzi S, Rossi C, Di Matteo V et al. Morphological and metabolic changes in the nigro-striatal pathway of synthetic proteasome inhibitor (PSI)-treated rats: a MRI and MRS study. PLoS One 2013; 8:e56501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Cao R, Zhang L et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020; 30:269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xue X, Yu H, Yang H et al. Structures of two coronavirus main proteases: implications for substrate binding and antiviral drug design. J Virol 2008; 82:2515–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang H, Yang M, Ding Y et al. The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor. Proc Natl Acad Sci U S A 2003; 100:13190–13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muramatsu T, Takemoto C, Kim Y-T et al. SARS-CoV 3CL protease cleaves its C-terminal autoprocessing site by novel subsite cooperativity. Proc Natl Acad Sci U S A 2016; 113:12997–13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C, Qi Y, Teng X et al. Maturation mechanism of severe acute respiratory syndrome (SARS) coronavirus 3C-like proteinase. J Biol Chem 2010; 285:28134–28140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anand K, Palm GJ, Mesters JR, Siddell SG, Ziebuhr J, Hilgenfeld R. Structure of coronavirus main proteinase reveals combination of a chymotrypsin fold with an extra alpha-helical domain. EMBO J 2002; 21:3213–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Lin D, Kusov Y et al. alpha-Ketoamides as Broad-Spectrum Inhibitors of Coronavirus and Enterovirus Replication: Structure-Based Design, Synthesis, and Activity Assessment. J Med Chem 2020, asap. [DOI] [PubMed] [Google Scholar]
- 33.Chuck CP, Chong LT, Chen C, Chow HF, Wan DC, Wong KB. Profiling of substrate specificity of SARS-CoV 3CL. PloS one 2010; 5:e13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hegyi A, Ziebuhr J. Conservation of substrate specificities among coronavirus main proteases. J Gen Virol 2002; 83:595–599. [DOI] [PubMed] [Google Scholar]
- 35.Grein J, Ohmagari N, Shin D et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Eng J Med 2020, asap. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holshue ML, DeBolt C, Lindquist S et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med 2020; 382:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao B, Wang Y, Wen D et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Eng J Med 2020, asap. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zumla A, Chan JF, Azhar El, Hui DS, Yuen KY. Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov 2016; 15:327–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simmons G, Gosalia DN, Rennekamp AJ, Reeves JD, Diamond SL, Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc Natl Acad Sci U S A 2005; 102:11876–11881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider M, Ackermann K, Stuart M et al. Severe acute respiratory syndrome coronavirus replication is severely impaired by MG132 due to proteasome-independent inhibition of M-calpain. J Virol 2012; 86:10112–10122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barnard DL, Hubbard VD, Burton J et al. Inhibition of severe acute respiratory syndrome-associated coronavirus (SARSCoV) by calpain inhibitors and beta-D-N4-hydroxycytidine. Antivir Chem Chemother 2004; 15:15–22. [DOI] [PubMed] [Google Scholar]
- 42.Ono Y, Saido TC, Sorimachi H. Calpain research for drug discovery: challenges and potential. Nat Rev Drug Discov 2016; 15:854–876. [DOI] [PubMed] [Google Scholar]
- 43.Hoffmann M, Kleine-Weber H, Schroeder S et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181:271–280 e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


