Abstract
Background:
Postpartum depression (PPD), which affects up to 1 in 5 mothers globally, negatively impacts the health of both mothers and children. Exposure to ambient air pollution has been linked to depressive symptoms in animal models and human studies, but the relationship between air pollution and PPD has not been widely studied.
Methods:
In a birth cohort in Mexico City (509 mothers with available data), we examined the association between exposure to particulate matter ≤2.5 μm in diameter (PM2.5) with symptoms of psychological dysfunction at 1 and 6 months postpartum. Daily PM2.5 estimates were derived from a hybrid satellite-based spatio-temporally resolved model and averaged over pregnancy and the first year postpartum. Edinburgh Postnatal Depression Scale (EPDS) scores at 1 and 6 months were used to assess the relationship between PM2.5 exposure and probable PPD (EPDS score ≥13) using relative risk regression and symptoms of anhedonia, depression, and anxiety (derived from EPDS subscales) using negative binomial regression.
Results:
A 5-μg/m3 increase in average PM2.5 exposure during pregnancy was associated with an increased risk of PPD at 6 months (RR = 1.59; 95% CI: 1.11 to 2.28) and of late-onset PPD (no PPD at 1 month, PPD at 6 months) (RR = 2.58; 95% CI: 1.40 to 4.73) in covariate-adjusted models. No association was observed between PM2.5 exposure in the first year postpartum and PPD. Average PM2.5 exposure during pregnancy was also associated with increased 6-month EPDS subscale symptom scores for anhedonia (p = 0.03) and depression (p = 0.04).
Conclusion:
Our results suggest that in women in Mexico City, particulate matter exposure during pregnancy is positively associated with PPD and symptoms of anhedonia and depression at 6 months postpartum. Future studies should examine mechanisms linking air pollution and other environmental exposures during pregnancy with postpartum psychological functioning.
1. Introduction
Postpartum depression (PPD), also called postnatal depression, greatly impacts both the health of the infant and the mother (Gelaye et al., 2016; Nieto et al., 2017; Surkan et al., 2011; Wisner et al., 2006). PPD is a heterogeneous condition with three underlying dimensions—depressed mood, anxiety, and anhedonia—that may have varying times of onset during the postpartum period (Putnam et al., 2017). The prevalence of depressive postpartum symptoms varies worldwide: overall pooled prevalence is estimated to be 12% (Woody et al., 2017), with estimates ranging from 6 to 38% in developed countries and 20–57% in developing countries (Lara et al., 2015; Norhayati et al., 2015). In low- and middle-income countries, PPD is of particular concern given its high prevalence and the limited resources for its diagnosis and management (Lara et al., 2015; Place et al., 2016; Shrestha et al., 2016). Therefore, identifying modifiable risk factors for PPD is an important public health goal.
Evidence from animal and human studies suggests that ambient air pollution is associated with adverse neuropsychological functioning. In adult mice, exposure to ambient air pollutants has been associated with anxiety and depressive-like symptoms (Costa et al., 2014; Mokoena et al., 2015; Zhang et al., 2015). In human studies, most research has been focused on non-pregnant and aging populations. Increased exposure to particulate matter has been linked to depressive and anxiety symptoms in adults in South Korea (Shin et al., 2018), elderly populations in South Korea (Lim et al., 2012) and the United States (Pun et al., 2017), and in women in the Nurses’ Health Study in the United States (Kioumourtzoglou et al., 2017; Power et al., 2015). Short-term exposure to PM2.5 has also been linked to increased risk of hospitalization for depression in two studies in China (Qiu et al., 2018; Wang et al., 2018).
To our knowledge, only one previous study has examined the association between air pollution in pregnancy and postpartum psychological functioning. Our group reported that PM2.5 exposure in mid-pregnancy was associated with increased postpartum anhedonia symptoms, measured using the Edinburgh Postnatal Depression Scale (EPDS), during the first year postpartum among a diverse sample including Black, Hispanic, and white mothers in Boston; further, after stratifying by race/ethnicity, the strongest associations of PM2.5 with symptoms of depression and anhedonia were seen in the subpopulation of Black mothers (Sheffield et al., 2018). Herein, we leverage an ongoing birth cohort in Mexico City with data on air pollution and maternal psychological functioning to evaluate the association between exposure to PM2.5 in pregnancy and psychological functioning, assessed using the EPDS, at 1 and 6 months postpartum.
2. Methods
2.1. Study population
Women who were pregnant and receiving prenatal care through the Mexican Social Security System (Instituto Mexicano del Seguro Social – IMSS) between July 2007 and February 2011 were recruited in the Programming Research in Obesity, GRowth, Environment and Social Stressors (PROGRESS) study. Women were eligible to participate in the study if they met the following criteria: ≥18 years old, < 20 weeks gestation, planned to stay in Mexico City for the next 3 years, had access to a telephone, had no medical history of heart or kidney disease, did not consume alcohol daily, and did not use any steroid or anti epilepsy medications. Procedures were approved by institutional review boards at the Harvard School of Public Health, Icahn School of Medicine at Mount Sinai, and the Mexican National Institute of Public Health. Women provided written informed consent in Spanish.
2.2. PM2.5 levels during pregnancy
Daily exposure to PM2.5 was estimated for each cohort participant during pregnancy. Gestational age was based on last menstrual period (LMP) and by a standardized physical examination to determine gestational age at birth (Capurro et al., 1978) because ultrasounds are not part of routine care in Mexico. If the physical examination assessment of gestational age differed by more than 3 weeks from the gestational age based on LMP, the physical exam was used instead of the gestational age determined by LMP. Daily PM2.5 exposure was then estimated using a hybrid spatio-temporal model that incorporates Moderate Resolution Imaging Spectroradiometer (MODIS) satellite-derived Aerosol Optical Depth (AOD) measurements at a 1 × 1 km spatial resolution (Just et al., 2015). Remote sensing data were calibrated with municipal ground level monitors of PM2.5, land use regression (LUR) variables, and meteorological data to yield estimates of daily residential PM2.5 levels for each participant. The model was run using day-specific calibrations of AOD data calibrated against ground PM2.5 measurements and LUR and meteorological variables (roadway density, temperature, relative humidity, planetary boundary layer and daily precipitation). Mixed effect models with spatial and temporal predictors and day-specific random effects were used to account for temporal variations in the PM2.5–AOD relationship. For days without AOD data, the model was fit with a seasonal smooth function of latitude and longitude and time-varying average incorporating local monitoring. Model performance was assessed using monitor-level leave one-out cross-validation; the model performed well with a cross-validated R2 of 0.724. We calculated the average PM2.5 over pregnancy and clinically defined trimesters (1st trimester: 1–13 weeks, 2nd trimester: 14–27 weeks, 3rd trimester: 28 weeks-delivery).
2.3. Edinburgh Postnatal Depression Scale
Mothers completed the Spanish version of the EPDS at a visit during the second or third trimester of pregnancy. Following birth, 815 mother-child dyads had at least 1 follow-up visit, of which 679 (83%) and 629 (77%) completed the EPDS at 1 and 6 months, respectively (Cox et al., 1987). The Spanish version of the EPDS is validated in Mexican populations (Oquendo et al., 2008; Ortega et al., 2001). The 10-item EPDS asks about symptoms in the past 7 days, including: “1: I have laughed and been able to see the funny side of things,” “2: I have looked forward with enjoyment to things,” “3: I have blamed myself unnecessarily when things went wrong,” “4: I have been anxious or worried for no good reason,” “5: I have felt scared or panicky for no very good reason,” “6: Things have been getting on top of me,” “7: I have been so unhappy that I have had difficulty sleeping,” “8: I have felt sad or miserable,” “9: I have been so unhappy that I have been crying,” and “10: The thought of harming myself has occurred to me.” Participants rated the severity or frequency of each item based on 4 levels scored from 0 (indicating the most favorable condition) to 3 (indicating the least favorable condition) for each item. Total scores can range from 0 to 30.
The EPDS scale was constructed as a unidimensional tool to screen for postpartum depression; however, multiple studies have demon strated that the EPDS identifies multiple dimensions of postpartum psychological functioning, specifically depression, anxiety and anhedonia (Hartley et al., 2014; Matthey et al., 2013; Phillips et al., 2009). As previously reported, the results from an exploratory factor analysis (EFA) and confirmatory factor analysis (CFA) indicated that a three factor model including depressive, anxiety, and anhedonia symptom subscales was the most optimal fit in our sample (Flom et al., 2018). Item 10 (“thought of self-harming”) was omitted from the subscale analysis given the rare frequency of endorsement (0.3% for quite often, 1.8% for sometimes and 2.1% for not very often). The anhedonia subscale includes items 1 and 2; the anxiety subscale includes items 4, 5 and 6; and the depression subscale includes items 7, 8, and 9.
2.4. Covariates
Mother’s age and educational attainment were ascertained at enrollment. The Crisis in Family Systems-Revised (CRISYS) survey, validated in Spanish (Berry et al., 2006), was administered by a trained psychologist during the second or third trimester of pregnancy. The CRISYS survey assesses life events across 11 domains: financial, legal, career, relationship, home safety, neighborhood safety, medical issues (self and others), home, prejudice and authority. Participants rated life events in the past 6 months as positive, negative or neutral. Domains with one or more negative life event were summed into a negative life event (NLE) domain score, with higher scores indicating greater stress. Only 1 mother reported smoking in pregnancy in our current sample; therefore, prenatal exposure to environmental tobacco smoke was included in the models and was defined as report of any smoker in the home during the second or third trimester of pregnancy. Birth season was defined according to weather patterns in Mexico City as dry/cold (January-February; November-December), dry/warm (March-April) and rainy (May-October).
2.5. Statistical analysis
Analyses were conducted on the 509 mothers with complete data (EPDS scores during pregnancy and at 1 and 6 months, and all covariates). Probable PPD (hereafter PPD) was defined as a dichotomous measure using EPDS scores at a clinically-relevant cutoff (EPDS score ≥ 13) (Sit and Wisner, 2009). The association between mean PM2.5 averaged over the full gestational period and PPD was analyzed using the modified Poisson regression with robust error variance approach of Zou (2004), which provides estimates of relative risk. Due to evidence of overdispersion, negative binomial regression was used to examine the association between PM2.5 and EPDS subscale scores. Models were adjusted for maternal age at enrollment, exposure to environmental tobacco smoke (ETS) in pregnancy (yes/no based on the presence of at least one smoker in the household at any point in pregnancy), maternal education, birth season, gestational age and prenatal NLE score. In sensitivity analyses, models were also adjusted for average PM2.5 in the first year of life, restricted to mothers with full-term pregnancies (gestational age ≥ 37 weeks), and run using different cutoffs for PPD (EPDS ≥ 10 and EPDS ≥ 15). Analyses were performed in RStudio version 1.1.383.
3. Results
3.1. Sample characteristics
Table 1 shows the distribution of exposure, outcomes, and covariates across our sample. The majority of participants (76%) had a high school education or less, and 36% were exposed to environmental tobacco smoke during pregnancy. The median PM2.5 exposure during pregnancy was 22.9 μg/m3 (IQR: 3.8 μg/m3) and 18% of participants were classified as likely suffering from PPD (EPDS scores ≥ 13) at the 1 and 6 months postpartum visits. At 6 months, EPDS subscale scores were highest for anxiety, followed by anhedonia and depression.
Table 1.
Descriptive characteristics (N = 509).
| Characteristics | Level/Unit | Median (IQR) or N (%) |
|---|---|---|
| Demographic | ||
| Maternal age at enrollment | Years | 27.4 (23.3, 31.7) |
| ETS exposure in pregnancya | Yes | 180 (35%) |
| Maternal education | < High school | 211 (41%) |
| High School | 178 (35%) | |
| > High School | 120 (24%) | |
| Birth season | Dry/cold | 188 (37%) |
| Dry/warm | 103 (20%) | |
| Rainy | 218 (43%) | |
| Gestational age | Weeks | 39 (38, 39) |
| NLEs during pregnancyb | Score | 3 (2, 5) |
| Air pollution | ||
| Average PM2.5 in pregnancy | μg/m3 | 22.9 (20.6, 24.4) |
| Average PM2.5 in 1st year postpartum | μg/m3 | 22.8 (20.8, 24.0) |
| Depression symptoms | ||
| EPDS during pregnancy, total | Score | 8 (4, 13) |
| EPDS at 1 month, total | Score | 6(2, 11) |
| EPDS at 6 months, total | Score | 5 (2, 10) |
| Anhedonia subscale | Score | 0 (0, 2) |
| Anxiety subscale | Score | 2 (1, 5) |
| Depression subscale | Score | 1 (0, 4) |
| Probable depression, pregnancy | EPDS ≥ 13 | 136 (27%) |
| Probable PPD, 1 month | EPDS ≥ 13 | 93 (18%) |
| Probable PPD, 6 months | EPDS ≥ 13 | 90 (18%) |
Presence of at least one smoker in the household during pregnancy.
Sum of negative life events during pregnancy assessed by the CRISYS survey.
3.2. PM2.5 in pregnancy and PPD at 6 months
Table 2 presents the fully-adjusted models for average PM2.5 over pregnancy predicting PPD at 1 and 6 months. While PM2.5 was not associated with PPD at 1 month (RR [95% CI], 0.85 [0.61, 1.19]), a 5-μg/m3 increase in average PM2.5 in pregnancy was associated with 1.59 (95% CI: 1.11, 2.28) times the risk of PPD at 6 months. Results at 6 months remained unchanged after inclusion of the average PM2.5 levels during the first year of life (RR [95% CI], 1.60 [1.10, 2.33]), and exposure during this period was not independently associated with PPD at 6 months (RR [95% CI] for a 5-μg/m3 change in average PM2.5 in the 1st year postpartum, 0.98 [0.63, 1.51]).
Table 2.
Relative risksa of PPD (EPDS ≥ 13) at 1 and 6 months postpartum (N = 509).
| Variable | Level/unit | PPD, 1 month RR (95% CI) | P | PPD, 6 months RR (95% CI) | P |
|---|---|---|---|---|---|
| PM2.5 in pregnancy | 5-μg/m3 change | 0.85 (0.61, 1.19) | 0.35 | 1.59 (1.11, 2.28) | 0.01 |
| Maternal age at enrollment | 1-yr change | 0.96 (0.93, 1.00) | 0.04 | 0.99 (0.96, 1.02) | 0.51 |
| Tobacco exposure in pregnancy | Yes | 0.78 (0.53, 1.15) | 0.22 | 1.03 (0.71, 1.49) | 0.88 |
| Maternal education | < High School | Ref. | - | Ref. | - |
| High School | 0.95 (0.63, 1.44) | 0.82 | 0.85 (0.56, 1.28) | 0.42 | |
| > High School | 0.61 (0.36, 1.02) | 0.06 | 0.57 (0.34, 0.93) | 0.03 | |
| Birth season | Dry/cold | Ref. | - | Ref. | - |
| Dry/warm | 1.72 (1.07, 2.76) | 0.03 | 1.17 (0.71, 1.95) | 0.54 | |
| Rainy | 1.18 (0.76, 1.81) | 0.46 | 1.00 (0.66, 1.53) | 0.99 | |
| Gestational age | 1-week change | 0.95 (0.83, 1.08) | 0.42 | 0.87 (0.78, 0.98) | 0.02 |
| Pregnancy NLE score | 1-event change | 1.21 (1.13, 1.30) | 9 × 10−8 | 1.21 (1.12, 1.31) | 1 × 10−6 |
Estimated from modified Poisson regression models with robust error variance, adjusted for all variables listed in table.
Based on this result, we additionally examined 6-month PPD subtypes according to the presence of depression at 1 month postpartum. As shown in Table 3, a 5-μg/m3 increase in PM2.5 exposure during pregnancy was associated with 2.58 (95% CI: 1.40, 4.73) times the risk of late-onset PPD at 6 months, while no association was observed for “chronic” PPD (PPD at 1 and 6 months: RR [95% CI], 1.06 [0.63, 1.76]). When we used depression during pregnancy (EPDS ≥ 13 in pregnancy) along with 1-month PPD to categorize the 6-month PPD onset groups, the strongest association between PM2.5 exposure during pregnancy and PPD at 6 months was observed among women without depression both during pregnancy and at 1 month postpartum (RR [95% CI], 3.12 [1.67, 5.86]) (Supplementary Table 1).
Table 3.
Relative risksa of PPD (EPDS ≥ 13) onset subtypes at 6 months postpartum for 5-μg/m3 change in PM2.5 exposure during pregnancy (N = 509).
| 6-month PPD subtype | No. of cases | RR (95% CI) | P |
|---|---|---|---|
| Late postpartum onset (no PPD at 1 month + PPD at 6 months) | 45 | 2.58 (1.40, 4.73) | 0.002 |
| Chronic postpartum (PPD at 1 and 6 months) | 45 | 1.06 (0.63, 1.76) | 0.83 |
Estimated from modified Poisson regression models with robust error variance, adjusted for maternal age at enrollment, tobacco exposure during pregnancy, maternal education, birth season, gestational age, and NLE score during pregnancy.
3.3. PM2.5 and depression subtypes at 6 months
Fig. 1 shows results from negative binomial regression models examining associations between PM2.5 exposure over pregnancy and EPDS subscale scores at 6 months. With an increase in average PM2.5 over pregnancy (5-μg/m3 change), we observed an increase in subscale scores for anhedonia (IRR [95% CI]: 1.28 [1.02, 1.60]) and depression (IRR [95% CI]: 1.29 [1.01, 1.64]), while no association between PM2.5 and anxiety scores was observed (IRR [95% CI]: 1.04 [0.89, 1.22]). No associations were observed between PM2.5 in pregnancy and EPDS subtypes at 1 month (p > 0.40 for anhedonia, anxiety, and depression; data not shown).
Fig. 1.
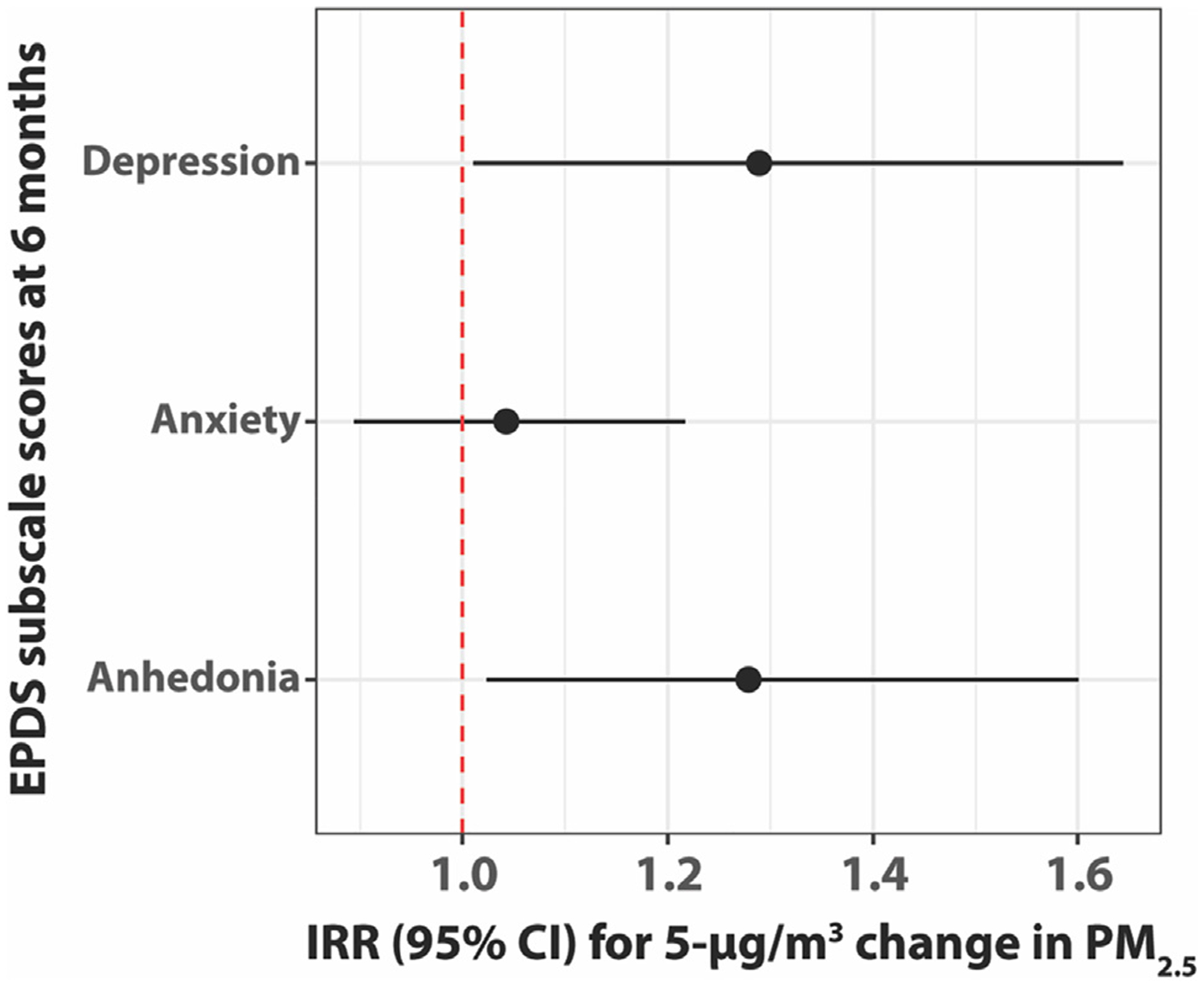
Negative binomial regression models for average PM2.5 exposure during pregnancy predicting EPDS subscale scores at 6 months postpartum (N = 509). Models adjusted for maternal age at enrollment, tobacco exposure during pregnancy, maternal education, birth season, gestational age, and NLE score during pregnancy.
3.4. Sensitivity analyses
Results for PPD and EPDS subscales were unchanged after additional adjustment for average PM2.5 in the 1st year postpartum and in the subset of mothers with full-term pregnancies (gestational age ≥ 37 weeks) (see Supplementary Materials). Results were also robust to changes in the PPD cutoff to EPDS ≥ 10 or EPDS ≥ 15 (Supplementary Materials).
4. Discussion
We leveraged data from PROGRESS, an ongoing Mexico-based cohort, to examine the association between exposure to fine particulate matter during pregnancy and postpartum psychological functioning. Our findings suggest that at 6 months postpartum, a 5-μg/m3 increase in average PM2.5 exposure during pregnancy (a unit change well within the range of average PM2.5 exposures during pregnancy in our study of 16.4 μg/m to –29.3 μg/m3) is associated with an 83% increase in risk of PPD and a 158% increase in risk of late-onset PPD (PPD at 6 months with no PPD at 1 month). EPDS subscale analyses also revealed that higher PM2.5 in pregnancy was associated with increased symptoms of anhedonia and depression at 6 months. PM2.5 exposure in the first year postpartum was not independently associated with PPD or subscale scores, and adjustment for postnatal exposure did not mitigate the association with prenatal PM2.5 exposure. In sum, our findings suggest that pregnancy is a vulnerable window for the detrimental impacts of PM2.5 exposure on postpartum depression and anhedonia in Mexican women, which may not occur until several months following birth.
The current results are in line with other published work in non-pregnant populations. A recent analysis in the Nurses’ Health Study found an association between higher PM2.5, estimated using a spatio-temporal model, in the past year and an increased hazard of depression diagnosis and antidepressant use (Kioumourtzoglou et al., 2017). In a combined analysis of data from 6 low- and middle-income countries that included Mexico, a 10 μg/m3 increase in 3-year average PM2.5 was associated with increased prevalence of depressive symptoms in the past 12 months (Lin et al., 2017). A recent meta-analysis of observational studies reported a significant increase in the pooled odds for the association between long-term exposure to PM2.5, defined as exposure time greater than 1 year, and depression and depressive symptoms (Zeng et al., 2019).
Our findings indicate that particulate matter exposure during pregnancy may not influence PPD until several months postpartum. Historically, PPD has been defined as the onset of major depression within the first 4 weeks after childbirth (Pearlstein et al., 2009), but recent evidence suggests that women are susceptible to PPD far beyond that window, with risks extending well into the first year postpartum (Stewart and Vigod, 2016). Indeed, half of women in our study with PPD at 6 months postpartum did not meet criteria for PPD at 1 month. PPD onset subtypes are characterized by different risk factors, with a study by Wikman et al. (2019) showing that Swedish women with later onset of PPD (at 6 months) were more likely to experience sleep deficits, lack of support from partners, and issues with bonding. Late onset PPD may also be triggered by weaning from breastfeeding and the subsequent recommencement of menstrual cycles, which could result in changes in the levels of oxytocin and other hormones that affect mood (Burke et al., 2019). While beyond the scope of the current analysis, we plan to investigate how the relationship between PM2.5 and PPD risk may be modified by maternal factors such as stress, social support, and breastfeeding duration.
While the etiology of PPD is unclear, a proposed mechanism involves the dysregulation of the maternal hypothalamic-pituitary-adrenal (HPA) axis (Jolley et al., 2007). The maternal neuroendocrine system undergoes dramatic changes during pregnancy, largely due to the endocrine effects of the developing placenta (Duthie and Reynolds, 2013; Glynn et al., 2013). Disruption of normal HPA axis activity during pregnancy, possibly through stress-induced elevations in maternal cortisol levels, may extend the postpartum HPA refractory period, thereby increasing risk of depression (Glynn et al., 2013). Given that fine particulate matter has been shown to be associated with alterations in cortisol and other HPA axis hormones in studies in adults (Li et al., 2017; Niu et al., 2018), future studies should investigate the roles of cortisol and HPA axis functioning in the etiology of PM2.5-induced PPD.
Mechanistic data suggest that inflammation may also contribute to the impact of fine particulate matter on depression. Mice exposed to PM2.5 for 10 months had higher upregulation of pro-inflammatory cytokines in the hippocampus—which are thought to play an important role in the pathophysiology of depression (Raison et al., 2006)—and were more likely to exhibit depressive-like responses when compared to mice who received filtered air (Fonken et al., 2011). Another study in mice also found that 3-month exposure to PM triggered depressive-like response in mice and increased pro-inflammatory cytokine production in the brain (Liu et al., 2018). It is important to note that these studies were conducted using male mice only; thus, findings may not be directly translatable to females, particularly during the antepartum and postpartum periods. Additional studies in female mice and in human cohorts are warranted.
Consistent with our group’s previous study in Boston (Sheffield et al., 2018), particulate matter exposure during pregnancy was associated with increased postpartum symptoms of anhedonia. Anhedonia, or the reduction in the ability to experience pleasure (i.e., lack of positive affect) (Winer et al., 2019), may arise, in part, from inflammatory processes. For example, increased inflammation has been shown to induce symptoms of anhedonia in animal models (De La Garza, 2005; Yirmiya, 1996) and randomized controlled trials of inflammatory challenge (Eisenberger et al., 2010), with effects most pronounced among women (Moieni et al., 2019). Future research should explore whether inflammation during pregnancy, particularly through fine particulate matter exposure, may be a contributing factor to the development of anhedonia after birth.
There are two notable differences between our group’s previous and current studies on PM2.5 exposure and postpartum psychological functioning. First, our previous study in Boston did not find that PM2.5 exposure in mid-pregnancy was associated with depression and anhedonia among the subpopulation of Hispanic women (Sheffield et al., 2018). However, the population of Hispanic women in our Boston study was largely born in the United States, with few women born in Mexico or of Mexican origin, while the cohort in the current study was entirely Mexican-born. Second, our previous study did not find that PM2.5 averaged over the entire pregnancy was associated with psychological functioning (Sheffield et al., 2018). This discrepancy may be attributable to the differences in PM2.5 exposure levels: the median PM2.5 exposure during pregnancy was markedly lower in Boston compared to Mexico City (16.5 μg/m3 vs. 22.9 μg/m3), which may explain the stronger effects observed in the current study. Nonetheless, findings from the two studies together lend support to the link between air pollution exposure during pregnancy and postpartum psychological functioning, which may vary according to the level of PM2.5 exposure and across sociodemographic groups.
Our study had several strengths, including a well-characterized, longitudinal cohort with a large sample size; the high spatial resolution of the PM2.5 estimates; and the examination of different aspects of postpartum psychological functioning (depression, anxiety, and anhedonia) using EPDS subscale score loadings previously validated in our sample (Flom et al., 2018). We also acknowledge some limitations. The PM2.5 exposure estimates from the spatiotemporal model were based on the address of the maternal residence, which do not account for exposures when the women were away from their homes and also do not take into account PM2.5 exposures from indoor sources. Notably, adjustment for environmental tobacco smoke, a major contributor to indoor air pollution, did not mitigate the observed effects of ambient PM2.5. Further, we do not have information on other air pollutants that may co-occur with PM2.5 that could potentially confound our associations. While we adjusted for several other variables that may confound the association between PM2.5 and PPD, including maternal age, maternal education, and stress, we cannot dismiss the possibility that there are unknown confounders that may explain our associations. Future studies should be designed to better disentangle the effects of potential confounders, such as proximity to roadways or traffic density, on PPD through indirect pathways involving noise pollution and/or sleep deficits. We do not have data on previous PPD or mental health status, an important predictor of future PPD, in our participants. The original eligibility criteria for inclusion in the study, including access to a telephone, might have led to the exclusion of populations particularly at risk for depressive outcomes. Finally, while the homogenous makeup of the PROGRESS cohort provides excellent internal validity, our findings may not be generalizable to non-Mexican populations.
In conclusion, we found that among women in Mexico City, increased particulate matter exposure during pregnancy was a risk factor for PPD and symptoms of depression and anhedonia at 6 months postpartum. Our findings add to the growing body of literature implicating air pollution exposure in neuropsychological dysfunction in mothers and in other populations. Future studies should examine mechanisms of inflammation and HPA axis dysregulation that may underlie these associations.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health/National Institute of Environmental Health Sciences [R01 ES014930, R01 ES013744, P30 ES023515, R00 ES027496] and the National Institute of Public Health/Ministry of Health of Mexico. We thank the ABC (American British Cowdray Medical Center) in Mexico for providing research facilities.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of Competing Interest
The authors declare no actual or potential conflicts of interest.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2019.105325.
References
- Berry CA, Quinn KA, Portillo N, Shalowitz MU, 2006. Reliability and validity of the Spanish version of the crisis in family systems-revised. Psychol. Rep 98, 123–132. [DOI] [PubMed] [Google Scholar]
- Burke CS, Susser LC, Hermann AD, 2019. Gabaa dysregulation as an explanatory model for late-onset postpartum depression associated with weaning and resumption of menstruation. Archives Women’s Mental Health 22, 55–63. [DOI] [PubMed] [Google Scholar]
- Capurro H, Konichezky S, Fonseca D, Caldeyro-Barcia R, 1978. A simplified method for diagnosis of gestational age in the newborn infant. J. Pediatrics 93, 120–122. [DOI] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roque P, 2014. Neurotoxicants are in the air: convergence of human, animal, and in vitro studies on the effects of air pollution on the brain. Biomed. Res. Int 2014, 736385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R, 1987. Detection of postnatal depression. Development of the 10-item edinburgh postnatal depression scale. Br. J. Psychiatry 150, 782–786. [DOI] [PubMed] [Google Scholar]
- De La Garza R 2nd, 2005. Endotoxin- or pro-inflammatory cytokine-induced sickness behavior as an animal model of depression: focus on anhedonia. Neurosci. Biobehav. Rev 29, 761–770. [DOI] [PubMed] [Google Scholar]
- Duthie L, Reynolds RM, 2013. Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: influences on maternal and fetal outcomes. Neuroendocrinology 98, 106–115. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR, 2010. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol. Psychiatry 68, 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flom JD, Chiu YM, Tamayo-Ortiz M, Schnaas L, Curtin PC, Wright RJ, et al. , 2018. Subconstructs of the edinburgh postpartum depression scale in a postpartum sample in mexico city. J. Affect. Disord 238, 142–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Xu X, Weil ZM, Chen G, Sun Q, Rajagopalan S, et al. , 2011. Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol. Psychiatry 16 (987–995), 973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelaye B, Rondon MB, Araya R, Williams MA, 2016. Epidemiology of maternal depression, risk factors, and child outcomes in low-income and middle-income countries. Lancet Psychiatry 3, 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LM, Davis EP, Sandman CA, 2013. New insights into the role of perinatal hpaaxis dysregulation in postpartum depression. Neuropeptides 47, 363–370. [DOI] [PubMed] [Google Scholar]
- Hartley CM, Barroso N, Rey Y, Pettit JW, Bagner DM, 2014. Factor structure and psychometric properties of english and spanish versions of the edinburgh postnatal depression scale among hispanic women in a primary care setting. J. Clin. Psychol 70, 1240–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolley SN, Elmore S, Barnard KE, Carr DB, 2007. Dysregulation of the hypothalamic-pituitary-adrenal axis in postpartum depression. Biol. Res. Nurs 8, 210–222. [DOI] [PubMed] [Google Scholar]
- Just AC, Wright RO, Schwartz J, Coull BA, Baccarelli AA, Tellez-Rojo MM, et al. , 2015. Using high-resolution satellite aerosol optical depth to estimate daily pm2.5 geographical distribution in mexico city. Environ. Sci. Technol 49, 8576–8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioumourtzoglou MA, Power MC, Hart JE, Okereke OI, Coull BA, Laden F, et al. , 2017. The association between air pollution and onset of depression among middle-aged and older women. Am. J. Epidemiol 185, 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara MA, Navarrete L, Nieto L, Martin JP, Navarro JL, Lara-Tapia H, 2015. Prevalence and incidence of perinatal depression and depressive symptoms among mexican women. J. Affect. Disord 175, 18–24. [DOI] [PubMed] [Google Scholar]
- Li H, Cai J, Chen R, Zhao Z, Ying Z, Wang L, et al. , 2017. Particulate matter exposure and stress hormone levels: a randomized, double-blind, crossover trial of air purification. Circulation 136, 618–627. [DOI] [PubMed] [Google Scholar]
- Lim YH, Kim H, Kim JH, Bae S, Park HY, Hong YC, 2012. Air pollution and symptoms of depression in elderly adults. Environ. Health Perspect 120, 1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HL, Guo YF, Kowal P, Airhihenbuwa CO, Di Q, Zheng Y, et al. , 2017. Exposure to air pollution and tobacco smoking and their combined effects on depression in six low- and middle-income countries. Brit. J. Psychiat 211, 157-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Qian X, Xing J, Wang J, Sun Y, Wang Q, et al. , 2018. Particulate matter triggers depressive-like response associated with modulation of inflammatory cytokine homeostasis and brain-derived neurotrophic factor signaling pathway in mice. Toxicol. Sci 164, 278–288. [DOI] [PubMed] [Google Scholar]
- Matthey S, Fisher J, Rowe H, 2013. Using the edinburgh postnatal depression scale to screen for anxiety disorders: conceptual and methodological considerations. J. Affect. Disord 146, 224–230. [DOI] [PubMed] [Google Scholar]
- Moieni M, Tan KM, Inagaki TK, Muscatell KA, Dutcher JM, Jevtic I, et al. , 2019. Sex differences in the relationship between inflammation and reward sensitivity: a randomized controlled trial of endotoxin. Biol. Psychiatry: Cognitive Neurosci. Neuroimag [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokoena ML, Harvey BH, Viljoen F, Ellis SM, Brink CB, 2015. Ozone exposure of flinders sensitive line rats is a rodent translational model of neurobiological oxidative stress with relevance for depression and antidepressant response. Psychopharmacology 232, 2921–2938. [DOI] [PubMed] [Google Scholar]
- Nieto L, Lara MA, Navarrete L, 2017. Prenatal predictors of maternal attachment and their association with postpartum depressive symptoms in Mexican women at risk of depression. Matern. Child Health J 21, 1250–1259. [DOI] [PubMed] [Google Scholar]
- Niu Y, Chen R, Xia Y, Cai J, Ying Z, Lin Z, et al. , 2018. Fine particulate matter constituents and stress hormones in the hypothalamus-pituitary-adrenal axis. Environ. Int 119, 186–192. [DOI] [PubMed] [Google Scholar]
- Norhayati MN, Hazlina NH, Asrenee AR, Emilin WM, 2015. Magnitude and risk factors for postpartum symptoms: a literature review. J. Affect. Disord 175, 34–52. [DOI] [PubMed] [Google Scholar]
- Oquendo M, Lartigue T, Gonzalez-Pacheco I, Mendez S, 2008. Validez y seguridad de la escala de depresión perinatal de edinburgh como prueba de tamiz para detectar depresión perinatal [validity and confidence of edinburgh perinatal depression scale as screening tool for detecting perinatal depression]. Perinatol. Reprod. Hum 22, 195–202. [Google Scholar]
- Ortega L, Lartigue T, Figueroa ME, 2001. Prevalencia de depresión, a través de la escala de depresión perinatal de edinburgh (epds), en una muestra de mujeres mexicanas embarazadas [prevalence of depression, ascertained through the edinburgh perinatal depression scale, in a sample of pregnant mexican women]. Perinatol. Reprod. Hum 15, 11–20. [Google Scholar]
- Pearlstein T, Howard M, Salisbury A, Zlotnick C, 2009. Postpartum depression. Am. J. Obstet. Gynecol 200, 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J, Charles M, Sharpe L, Matthey S, 2009. Validation of the subscales of the edinburgh postnatal depression scale in a sample of women with unsettled infants. J. Affect. Disord 118, 101–112. [DOI] [PubMed] [Google Scholar]
- Place JM, Billings DL, Frongillo EA, Blake CE, Mann JR, deCastro F, 2016. Policy for promotion of women’s mental health: insight from analysis of policy on postnatal depression in mexico. Adm. Policy Ment. Health 43, 189–198. [DOI] [PubMed] [Google Scholar]
- Power MC, Kioumourtzoglou MA, Hart JE, Okereke OI, Laden F, Weisskopf MG, 2015. The relation between past exposure to fine particulate air pollution and prevalent anxiety: observational cohort study. BMJ – Brit. Med. J 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun VC, Manjourides J, Suh H, 2017. Association of ambient air pollution with depressive and anxiety symptoms in older adults: results from the nshap study. Environ. Health Perspect 125, 342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam KT, Wilcox M, Robertson-Blackmore E, Sharkey K, Bergink V, Munk-Olsen T, et al. , 2017. Clinical phenotypes of perinatal depression and time of symptom onset: analysis of data from an international consortium. Lancet Psychiatry 4, 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, Zhu X, Wang L, Pan J, Pu X, Zeng X, et al. , 2018. Attributable risk of hospital admissions for overall and specific mental disorders due to particulate matter pollution: a time-series study in Chengdu, China. Environ. Res 170, 230–237. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH, 2006. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 27, 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield PE, Speranza R, Chiu Y-HM, Hsu H-HL, Curtin PC, Renzetti S, et al. , 2018. Association between particulate air pollution exposure during pregnancy and postpartum maternal psychological functioning. PLoS ONE 13, e0195267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Park JY, Choi J, 2018. Long-term exposure to ambient air pollutants and mental health status: a nationwide population-based cross-sectional study. PLoS ONE 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha SD, Pradhan R, Tran TD, Gualano RC, Fisher JR, 2016. Reliability and validity of the edinburgh postnatal depression scale (epds) for detecting perinatal common mental disorders (pcmds) among women in low-and lower-middle-income countries: a systematic review. BMC Pregnancy Childbirth 16, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit DKY, Wisner KL, 2009. Identification of postpartum depression. Clin. Obstet. Gynecol 52, 456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DE, Vigod S, 2016. Postpartum depression. N. Engl. J. Med 375, 2177–2186. [DOI] [PubMed] [Google Scholar]
- Surkan PJ, Kennedy CE, Hurley KM, Black MM, 2011. Maternal depression and early childhood growth in developing countries: systematic review and meta-analysis. Bull. World Health Organ 89, 608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Liu H, Li H, Liu J, Guo X, Yuan J, et al. , 2018. Ambient concentrations of particulate matter and hospitalization for depression in 26 chinese cities: a case crossover study. Environ. Int 114, 115–122. [DOI] [PubMed] [Google Scholar]
- Wikman A, Axfors C, Iliadis SI, Cox J, Fransson E, Skalkidou A, 2019. Characteristics of women with different perinatal depression trajectories. J. Neurosci. Res [DOI] [PubMed] [Google Scholar]
- Winer ES, Jordan DG, Collins AC, 2019. Conceptualizing anhedonias and implications for depression treatments. Psychol. Res. Behav. Manage 12, 325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner KL, Chambers C, Sit DK, 2006. Postpartum depression: a major public health problem. JAMA 296, 2616–2618. [DOI] [PubMed] [Google Scholar]
- Woody CA, Ferrari AJ, Siskind DJ, Whiteford HA, Harris MG, 2017. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J. Affect. Disord 219, 86–92. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, 1996. Endotoxin produces a depressive-like episode in rats. Brain Res. 711, 163–174. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Lin R, Liu L, Liu Y, Li Y, 2019. Ambient air pollution exposure and risk of depression: a systematic review and meta-analysis of observational studies. Psychiatry Res. 276, 69–78. [DOI] [PubMed] [Google Scholar]
- Zhang X, Xu Y, Zhou L, Zhang C, Meng Q, Wu S, et al. , 2015. Sex-dependent depression-like behavior induced by respiratory administration of aluminum oxide nanoparticles. Int. J. Environ. Res. Public Health 12, 15692–15705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou G, 2004. A modified poisson regression approach to prospective studies with binary data. Am. J. Epidemiol 159, 702–706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


